During the past decade, our rapidly escalating understanding of immune surveillance and an appreciation of the mechanisms by which tumours escape its notice have led to promising new strategies against cancer. This paper reviews the concepts behind current research into cellular immunotherapy for cancer, presents data from clinical trials, and discusses the potential of this treatment as an adjunct to conventional modes of cancer treatment.
Methods
All three authors are involved in research into cellular immunotherapy and gene therapy. We searched PubMed and Medline databases using the terms “cancer vaccines,” “dendritic cells,” and “lymphocyte therapy.”
The rationale for cellular immunotherapy of cancer
The importance of the interaction between the immune system and cancer cells was recognised in the 1890s when William Coley used streptococcal cultures to treat patients with advanced sarcoma. These attempts to activate general immunity led to clinical responses. More recently, antibodies and T cells that identify tumour antigens have been isolated from patients with cancer. It is clear that the immune system is capable of recognising tumour cells.
Cellular immunotherapy consists of giving the patient cells that stimulate antitumour activity in the patient (tumour and dendritic cell vaccines) or that have intrinsic antitumour activity (autologous and allogeneic lymphocytes). The aim is to harness potent immunological weapons to destroy cancer cells.
The immune response to cancer
Cytotoxic T lymphocytes are one of the critical effector cells that are able to lyse tumour cells. Receptors on the surface of T cells recognise antigens presented as peptide fragments on the surface of the class I major histocompatibility complex. Recognition of an antigen by a naive T cell bearing an appropriate T cell receptor is insufficient in itself to trigger activation of the T cell—the antigen must be encountered in conjunction with a costimulatory signal. In the absence of this, T cells become tolerant to the antigen.
Predicted developments
Clinical trials of tumour cell vaccines in patients with minimal residual disease at high risk of relapse
Translation of cellular approaches into reproducible clinical benefit
Ability to assay more precisely the clinical and immunological response to cellular treatment
Definitions of the most potent combinations of effector cells and cytokine enhancers
Cellular orchestrators of T cell activation are professional antigen presenting cells (dendritic cells) that possess a remarkable ability to stimulate the immune response. These highly specialised cells capture and process antigens that are released during tumour cell breakdown and present them to antigen specific T cells. Once activated, the T cells, including CD4 T helper cells, proliferate and secrete cytokines such as interleukin 2 and granulocyte-macrophage colony stimulating factor. These cytokines are potent stimulators of T cell proliferation and activation and give rise to a cascade of immune effector cells (fig 1).
Figure 1.
Antitumour immune response. Dendritic cells capture antigens released by cancer cells. After intracellular processing, antigenic peptides are loaded onto major histocompatibility complex (MHC) molecules on the surface of the dendritic cell. Specific T cells encounter these MHC–peptide complexes in conjunction with a costimulatory signal. The activated T cells proliferate and secrete cytokines, resulting in the production of a cascade of immune effector cells (IL-2=interleukin 2; GM-CSF=granulocyte-macrophage colony stimulating factor)
Despite these highly developed responses, effective immunity against cancer frequently fails to develop—in effect, the immune system becomes blinded to the tumour. The ultimate aim of cellular immunotherapy is to overcome the failed immune response and get the immune system to effectively destroy the tumour cells.
Reasons for the failure of immune responses against tumours
Impaired tumour recognition by immune cells
Variable expression of tumour antigens
Loss of expression of class I major histocompatibility complex, resulting in T cells failing to recognise tumours
Poor tumour immunogenicity
Many tumour antigens are self antigens and as such are poorly recognised by T cells
Lack of costimulatory molecules on tumour cells, which results in failure to stimulate T cells
Tumour “counterattack”
Tumour cells secrete immunosuppressive cytokines (transforming growth factor β or interleukin 10, for example)
Molecules expressed on the surface of tumour cells (for example, Fas ligand) may induce lymphocyte death
Evolution of variants of tumour cells that do not express antigens
Tumour antigens
As a target for cancer immunotherapy, the ideal tumour antigen is immunogenic and expressed exclusively on tumour cells. Tumour specific antigens include viral antigens and mutated gene products (table). Most known tumour antigens are expressed, to some degree, on normal tissues, and they are therefore “tumour associated” rather than truly tumour specific.
Tumour cell vaccines
Whole tumour cell vaccines
Whole tumour cells, rendered safe by irradiation and mixed with an immunological adjuvant, were one of the earliest forms of cellular therapy. This approach avoids the need for tumour antigens to be identified before treatment and allows all of the relevant antigens to be included in the vaccine. Initial clinical studies showed the safety of this approach, with side effects mainly limited to erythematous reactions at the site of the vaccine.
Whole tumour vaccines are now entering phase III trials. One research group vaccinated patients with Dukes's type B and C colorectal cancer with autologous tumour cell vaccines mixed with BCG vaccine.1 Although there was no difference in overall survival, significant improvements were seen in recurrence free survival in vaccinated patients, with the most benefit seen in patients with the lowest tumour burden. In patients with melanoma and renal cell carcinoma, the results from phase I and II trials have shown possible survival benefits when compared with those from historical controls.2,3
Gene modified vaccines
A more recent approach is the use of vaccines containing genetically modified cells—gene modified vaccines—in which genes encoding key components of the immune response can be introduced into the tumour cells in vitro to increase the immunogenicity of the vaccine (fig 2). The most common gene modified vaccines use cytokines—the cytokine is produced in high concentrations in the vicinity of the tumour cells, where it alters the local immunological environment and enhances the activities of antigen presenting cells and the activation of tumour specific T cells. This approach avoids the side effects associated with systemic treatment with cytokines.
Figure 2.
Tumour cell vaccines. Immunogenicity of tumour cell vaccines can be improved by transducing the tumour cell with genes that encode key components of the immune response (cytokines such as granulocyte-macrophage colony stimulating factor (GM-CSF) and costimulatory molecules)
A phase I trial evaluated a tumour vaccine that secretes autologous granulocyte-macrophage colony stimulating factor in patients with metastatic renal cell cancer. This trial provided preliminary evidence of the benefits of this technique, with significant tumour regression in one patient and minimal side effects related to the vaccine.4
Generation of autologous tumour vaccines is expensive and labour intensive, and not all primary tumours can be expanded to produce enough cells for use in vaccine therapy. An alternative strategy uses gene transduced tumour cell lines as generic vaccines. In these, antigens common to the cell line and the patient's tumour act as targets for an immune response. Tumour antigens are presented to T cells by the host's (the patient's) antigen presenting cells in association with the host's major histocompatibility complex; compatibility of major histocompatibility complex between the patient and the vaccine is therefore unnecessary.5
Dendritic cell vaccines
Immunity produced by vaccines depends largely on the efficiency of the antigen presenting cell that initially processes and presents the antigen. Dendritic cells are probably the means by which most vaccines work; they possess an extraordinary capacity to capture and process antigen and contain all that is needed to stimulate T cell immunity, including high levels of major histocompatibility complex, costimulatory molecules, and adhesion molecules. These properties, coupled with the fact that it is now possible to generate, ex vivo, large numbers of functional dendritic cells from a patient's peripheral blood monocytes or CD34 haemopoietic stem cells, have led to considerable interest in the use of dendritic cell vaccines as a means to induce antitumour immunity.
Dendritic cells loaded with tumour antigens in the form of peptide fragments (fig 3), whole antigens, or tumour cell lysates are beginning to enter clinical trials, with some encouraging results. Patients with metastatic melanoma have been vaccinated with dendritic cells loaded with a cocktail of tumour specific peptides or tumour lysates, together with a chemical adjuvant to boost the immune response. In 16 patients, three had complete responses and two had partial responses.6 Metastatic renal cell carcinoma has been a target for vaccination with a hybrid cell vaccine consisting of autologous tumour cells fused to dendritic cells. Despite the poor prognosis for such patients, objective clinical responses, including four complete remissions, were seen in 7 of 17 (41%) patients.7 Ongoing clinical trials are using dendritic cells in renal cell carcinoma, prostate cancer, and melanoma.8
Figure 3.
Dendritic cell vaccines. Dendritic cells generated ex vivo from a patient's peripheral blood monocytes or CD34 haemopoietic stem cells can be loaded with tumour antigens and reinfused into the patient with the aim of generating effective antitumour immunity. Loading of antigen can be achieved by a variety of methods, including pulsing cells with antigenic peptides or infecting the cells with recombinant viral vectors (MHC=major histocompatibility complex)
Gene therapy techniques can be applied to dendritic cell vaccines; such techniques use recombinant viral vectors that are incapable of replication to provide efficient and reliable means of gene transfer. Genetic material is introduced into dendritic cells to provide them with a renewable source of antigen for presentation; this should lead to more sustained expression of antigen. The expression of viral (and therefore foreign) genes may boost the immune response, but this antiviral immunity primed by dendritic cells may cause the immune system to destroy dendritic cells rapidly in subsequent rounds of immunisation. One solution may be to use viral vectors that do not result in the expression of viral genes, such as retroviruses or “gutless” adenoviral vectors.
Autologous T lymphocyte therapy
The use of interleukin 2 in the treatment of renal cell cancer and melanoma proved that an immunological treatment is capable, in some cases, of inducing long term regression of metastatic tumours. The mechanism by which these remissions occur is believed to be through the stimulatory effects of interleukin 2 on T lymphocytes.9 Further research showed that tumour infiltrating lymphocytes, isolated from tumour samples and grown in interleukin 2, could also induce remissions in these disease groups. Disappointingly, in patients receiving interleukin 2, the infusion of these cells did not improve response or survival rates significantly compared with those receiving interleukin 2 alone.10
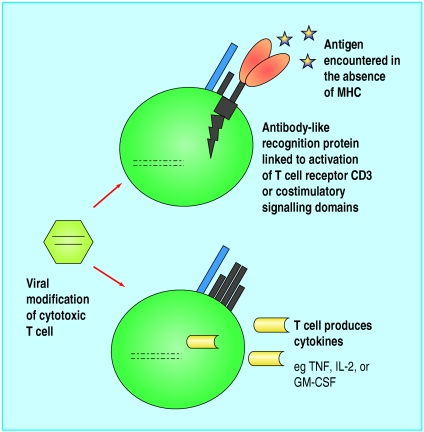
More recently, advances in the ex vivo use of gene transfer technology to genetically modify lymphocytes have made it possible to increase their effectiveness. One strategy involves fusing the antigen recognition domains of specific antitumour antibodies with intracellular T cell receptor signalling chains to form “chimeric” T cell receptors (fig 4). Cytotoxic T lymphocytes modified to express such receptors are specifically activated on contact with tumour antigen, without the need for tumour expression of major histocompatibility complex. T cells genetically modified in this way have been used successfully to treat human ovarian cancer cells in immunodeficient mice,11 and clinical trials are ongoing.
Figure 4.
Gene modified T lymphocyte therapy. Lymphocytes can be transduced with genes that encode chimeric receptors consisting of extracellular antitumour antibody fragments linked to intracellular T cell receptors11 or costimulatory signalling chains.12 Contact with the tumour leads to proliferation and activation of the antigen specific T cells. Lymphocyte survival and antitumour efficacy may also be improved by the use of genes encoding various cytokines (MHC=major histocompatibility complex; TNF=tumour necrosis factor; IL-2=interleukin 2; GM-CSF=granulocyte-macrophage colony stimulating factor)
Other approaches being studied include increasing antitumour efficacy by modifying lymphocytes to secrete antitumour cytokines, such as tumour necrosis factor, and improving in vivo T cell survival through the autocrine production of growth factors such as interleukins.
Allogeneic lymphocyte therapy
A potent graft versus leukaemia effect may be mediated by donor T cells that recognise disparities between donor's and host's tissue histocompatibility antigens as well as tumour antigens. Infusions of allogeneic donor leucocytes led to clinical responses in 60-80% of patients with chronic myeloid leukaemia who had relapsed after allogeneic transplantation. Recent reports suggest that a graft versus tumour response may be successfully induced against solid tumours such as renal cell carcinoma.13
Unfortunately, use of allogeneic lymphocytes is frequently accompanied by graft versus host disease, in which donor T cells recognise the host tissue as “foreign.” Novel approaches are being used to separate the graft versus leukaemia effect from the graft versus host effect, which should make giving donor leucocytes safer. Donor lymphocytes can be genetically modified to express genes that sensitise cells to specific drugs that can be administered to trigger cell death. This may confer the ability to eliminate effector T cells in the instance of toxic graft versus host disease.14
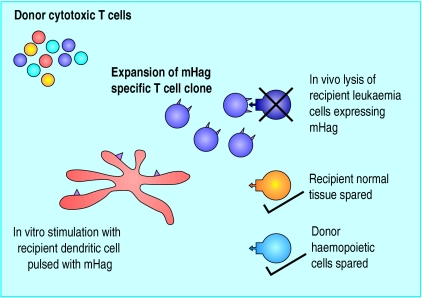
Specifically selected allogeneic donor cytotoxic T lymphocytes offer the prospect of an antileukaemia effect in the absence of graft versus host disease. One exciting approach may be the expansion ex vivo of those allogeneic cytotoxic T lymphocytes that are able to selectively recognise those minor histocompatibility antigens whose expression is restricted to recipient haemopoietic (and therefore leukaemic) cells (fig 5).15 The Wilms's tumour gene WT1 is expressed at increased levels on the blast cells of patients with acute myeloid leukaemia and chronic myelogenous leukaemia. Current approaches are looking at the potential for exploiting WT1 as a target molecule, in order to selectively direct cytotoxic T lymphocytes against leukaemic blast cells.16
Figure 5.
Adoptive immunotherapy with cytotoxic T lymphocytes specific for minor histocompatibility antigens restricted to the haemopoietic system. Responder lymphocytes from the allogeneic donor (who may be a sibling or unrelated matched donor) are stimulated by dendritic cells pulsed with antigens expressed specifically by haemopoietic cells from the recipient. This leads to the expansion of a specific T cell clone that is able to lyse recipient haemopoietic cells but is unable to attack the recipient tissues, which are susceptible to graft versus host disease. (mHag=minor histocompatibility antigens)
Limitations of cellular therapy
One concern with cellular immunotherapy is the induction of autoimmunity—vitiligo developed in 20% of melanoma patients who responded to interleukin 2.17 Other evidence of autoimmune disease has not been seen in any of the cancer vaccine trials to date, but is a possibility. Inducing autoimmunity against organs for which replacement therapy is available, such as the pancreas, may be acceptable to patients who otherwise face the possibility of dying from their disease, but a more widespread autoimmune reaction could limit the use of some cancer vaccines.
We have discussed small pilot studies performed in specialist units, but it is important to prove clinical benefit in large, randomised studies. Cellular therapy is expensive, time consuming, and complex, and adopting this approach on a large scale will be challenging.
The future
Most clinical trials to date have vaccinated patients with advanced disease. These patients will have some degree of immunosuppression, from the cancer itself and as a result of previous treatment. Immunisation strategies are likely to be most beneficial when applied to patients with minimal levels of disease and tumour types known to be particularly immunogenic, such as melanoma and renal cell carcinoma. Safety issues must be evaluated in patients where no conventional treatment is proved to be successful; however, as we move from the realm of pilot studies, it will be critical to design future trials to tackle the subject of residual disease burden, which may occur after surgery. Preliminary research suggests that these therapies will be less toxic than more conventional modes of treatment.
Cellular therapy is a rapidly evolving field, with incremental technological advances in cellular manipulation and genetic modification. As we attain a deeper understanding of the power of the immune response, we may be able to exploit this system and use it as a platform on which to build a successful therapeutic strategy to fight cancer.
Additional educational resources
Websites
CancerNet (www.cancernet.nci.nih.gov)
Information about cancer vaccine trials that are currently recruiting
Gene therapy advisory committee (www.doh.gov.uk/genetics/gtac)
UK gene therapy trials approved by the gene therapy advisory committee
Review articles
Pardoll D. Cancer vaccines. Nat Med 1998;4:525-31.
Greten TF, Jaffee EM. Cancer vaccines. J Clin Oncol 1999;17:1047-60.
Table.
Potential sources of tumour antigens
| Category of antigen | Example | Associated tumour |
|---|---|---|
| Expression of oncofetal antigens | Carcinoembryonic antigen | Colorectal cancer |
| MAGE gene family products* | Melanoma and breast cancer | |
| Tissue specific differentiation antigens | MART-1† | Melanoma |
| Glycoprotein gp100 | Melanoma | |
| Oncogene/tumour suppressor gene products | p53 | Many cancers |
| HER-2/neu oncogene | Breast and ovarian cancer | |
| bcr/abl | Chronic myeloid leukaemia | |
| Viral proteins | Human papilloma virus | Cervical cancer |
| Epstein-Barr virus | Burkitt's lymphoma Hodgkin's disease | |
| Hepatitis B virus | Hepatocellular cancer |
MAGE=melanoma antigen.
MART-1=melanoma antigen recognised by T cells; also known as Melan A.
Footnotes
Funding: ACA is a clinical research fellow funded by the Kay Kendall Leukaemia Fund. DE and JCE are clinical research fellows funded by the Cancer Research Campaign.
References
- 1.Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 2.Morton DL, Hoon DS, Nizze JA, Foshag LJ, Famatiga E, Wanek LA, et al. Polyvalent melanoma vaccine improves survival of patients with metastatic melanoma. Ann N Y Acad Sci. 1993;690:120–134. doi: 10.1111/j.1749-6632.1993.tb44002.x. [DOI] [PubMed] [Google Scholar]
- 3.Repmann R, Wagner S, Richter A. Adjuvant therapy of renal cell carcinoma with active-specific-immunotherapy (ASI) using autologous tumor vaccine. Anticancer Res. 1997;17:2879–2882. [PubMed] [Google Scholar]
- 4.Simons JW, Jaffee EM, Weber CE, Levitsky HI, Nelson WG, Carducci MA, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 5.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 6.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 7.Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 8.CancerNet. www.cancernet.nci.nih.gov. (Accessed 26 October 2001)
- 9.Jeal W, Goa KL. Aldesleukin (recombinant interleukin-2) a review of its pharmacological properties, clinical efficacy and tolerability in patients with renal cell carcinoma. BioDrugs. 1997;7:285–317. doi: 10.2165/00063030-199707040-00005. [DOI] [PubMed] [Google Scholar]
- 10.Figlin RA, Thompson JA, Bukowski RM, Vogelzang NJ, Novick AC, Lange P, et al. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J Clin Oncol. 1999;17:2521–2529. doi: 10.1200/JCO.1999.17.8.2521. [DOI] [PubMed] [Google Scholar]
- 11.Hwu P, Yang JC, Cowherd R, Treisman J, Shafer GE, Eshhar Z, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T cell receptor genes. Cancer Res. 1995;55:3369–3373. [PubMed] [Google Scholar]
- 12.Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol. 1996;26:2304–2309. doi: 10.1002/eji.1830261006. [DOI] [PubMed] [Google Scholar]
- 13.Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 14.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 15.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93:2336–2341. [PubMed] [Google Scholar]
- 16.Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 17.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]