Abstract
We have measured in 22 asymptomatic human immunodeficiency virus type 1-infected patients (10 rapid progressors and 12 slow progressors) the proviral load of CD4+ T cells homogeneously superinfected by the same dose of a non-syncytium-inducing virus in the presence or in the absence of autologous CD8+ T cells. We demonstrated that the antiviral activity of CD8+ T cells was highly predictive of the rate of peripheral CD4+ T-cell decline.
Cytotoxic CD8+ T lymphocytes (CTLs) are thought to be important in the control of human immunodeficiency virus (HIV) infection (3, 6). During the asymptomatic phase of HIV infection, an inverse correlation between CTL activity and viral load levels has been shown (4, 14), and CTL activity was also shown to be correlated with disease outcome (5, 15). Recently, the frequency of CD8+ T cells specific for the A2Gag epitope measured by a sensitive assay using HLA-peptide tetrameric complexes was demonstrated to be associated with CTL activity and inversely correlated with plasma viral load (13). However, these two studies do not bring any direct information on the antiviral role of CD8+ T cells on infected CD4+ cells.
In the present study, the antiviral activity of autologous CD8+ T cells was measured by comparing the concentrations of HIV provirus in CD4+ T cells homogeneously superinfected by the same dose of a non-syncytium-inducing virus in the presence or the absence of CD8+ T cells. Such an assay has the advantage to monitor the global antiviral activity of CD8+ T cells toward the natural targets of HIV infection.
To address this issue, cryopreserved peripheral blood mononuclear cells from 22 untreated asymptomatic HIV-1-infected patients with >200 CD4+ T cells per μl were identified in our computerized files. Ten of them were chosen because they were rapid progressors (RP) (CD4+-cell decline of >60% within the 3 years following cryopreservation), and the 12 others were chosen because they were slow progressors (SP) (CD4+-cell decline of <20% within the same period of time after cryopreservation). Demographic and viro-immunologic characteristics of both groups are given in Table 1. Six healthy donors (HD) with a normal blood count also made voluntary blood donations after having been informed of the purpose of this study.
TABLE 1.
Characteristics of the 22 asymptomatic HIV-infected patients participating in the studya
| Characteristic | SP | RP | P value for SP and RP |
|---|---|---|---|
| Male/female | 9/3 | 7/3 | |
| First year tested seropositive (mean ± SD) | 1,988 ± 4 | 1,988 ± 2 | 0.464 |
| Antiretroviral treatment | No | No | |
| Age (yr) | |||
| Mean ± SD | 34 ± 7 | 35 ± 7 | 0.400 |
| Range | 25–46 | 25–44 | |
| CD4+ cell count (cells/mm3)b | |||
| Mean ± SD | 594 ± 209 | 701 ± 311 | 0.172 |
| Range | 205–830 | 418–911 | |
| CD8+ cell count (cells/mm3)b | |||
| Mean ± SD | 1,210 ± 401 | 1,588 ± 1,157 | 0.150 |
| Range | 743–1,875 | 538–4,298 | |
| Plasma viremia (log10 RNA copies/ml)c | |||
| Mean ± SD | 3.8 ± 0.7 | 4.7 ± 0.5 | 0.002 |
| Range | 2.0–4.8 | 3.7–5.6 |
Twenty-two HIV-1-infected patients were divided into two groups, according to the CD4+-cell decrease observed within the 3 years following their entry in the present study. RP had a CD4+-cell decline level of >60% and SP had a CD4+-cell decline level of <20%. P values were calculated by the Student t test; bold value indicates significance.
Cells were quantified by flow cytometry.
Plasma viremia was determined by Nucleic Acid Sequence Based Amplification (NASBA; Organon Teknika, Boxtel, The Netherlands).
Peripheral blood mononuclear cells were defrosted and then were separated in CD4+-enriched or CD8+-enriched subsets using anti-CD4 and anti-CD8 immunomagnetic beads (Dynal, Great Neck, N.Y.). Bound cells were separated using a magnet and removed from beads with Detach-a-Bead product (Dynal). CD4+/CD3+ cell populations as well as CD8+/CD3+/CD56− cell populations were >90% pure, as determined by flow cytofluorometry analysis.
In order to concentrate our efforts on the specific anti-HIV role of CD8+ cells without being disturbed by the various ranges of replication of endogenous virus in patients CD4+ T cells (associated with the different rates of disease progression of these patients), we superinfected the phytohemagglutinin (1 μg/ml; Murex) activated CD4+ cell samples of the 22 patients participating in this study with the same dose (100 50% tissue culture infective doses) of a non-syncytium-inducing (NSI) HIV isolate. CD4+ cells from 6 HD were also infected by the same NSI HIV isolate at the same dose. Cell cultures were first performed in the absence of CD8+ cells. On day 8 of the culture, when HIV p24 release in the culture fluid was high, supernatants were collected, filtered, and assayed for viral RNA by using a quantitative reverse transcription-PCR (RT-PCR) (7). On the other hand, at the same time point, CD4+ cells were removed and monitored for cell viability as well as for concentration of HIV-1 Gag proviral DNA by quantitative PCR (9). Results of PCR assays were expressed as log10 proviral HIV DNA copies/106 CD4+ cells, and those of RT-PCR assays were expressed in log10 HIV RNA copies/milliliter of culture supernatants.
After 8 days of culture, the three groups (HD, RP, and SP) had similar HIV proviral DNA concentrations (HD versus RP, P = 0.165; RP versus SP, P = 0.999) and similar supernatant HIV RNA concentrations (HD versus RP, P = 0.986; RP versus SP, P = 0.977) (Fig. 1). Of note is the fact that we previously checked that at the dose used, our superinfecting NSI isolate yielded a peak of HIV p24 release in culture supernatant at day 8. Typically, endogenous virus from asymptomatic individuals is not released into culture medium at appreciable levels before 12 days poststimulation (8, 11). Moreover, the HIV proviral DNA concentration measured after 12 h of culture was 6.3-fold higher in superinfected cells (4.1 ± 0.4 log10 copies [mean ± standard deviation], range of 3.6 to 4.5) than in naturally infected cells (3.3 ± 0.4 log10 copies, range of 2.9 to 3.9). It is thus unlikely that the endogenous virus of patient CD4+ T cells could influence the antiviral activity of CD8+ T cells toward the peptides of the superinfecting virus (12).
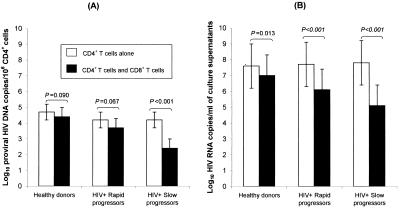
FIG. 1.
Quantitative analysis of antiviral activity of CD8+ T cells toward autologous HIV-infected CD4+ T cells from different groups of donating individuals. Open rectangles, mean (± standard deviation) results in cultures of CD4+ T cells without CD8+ T cells; solid rectangles, mean (± standard deviation) results of cocultures of CD4+ T cells with CD8+ T cells. These measurements were taken in samples from six healthy donors, 10 HIV+-infected rapid-progressor patients, and 12 HIV+-infected slow-progressor patients (for definition of rapid and slow progressors, see Materials and Methods). Provirus HIV DNA concentrations in CD4+ cells and HIV RNA release levels in culture supernatants were measured by PCR (A) and RT-PCR (B).
Having observed that CD4+ T cells, whether they were acutely infected or superinfected, produced similar proviral loads and viral releases, we next examined the ability of autologous uncultured CD8+ T cells to modify HIV proviral DNA concentrations in such infected or superinfected CD4+ T cells. For this purpose, HIV-1-(super)infected CD4+ T cells were cocultured with or without CD8+ T cells. A CD8+ cell-to-CD4+ cell ratio of 2:1 was taken as a representative value because this ratio represented the mean peripheral CD8+-to-CD4+ cell ratio from our patients (Table 2). At day 8, CD4+ T cells were collected and HIV proviral DNA was quantified by PCR; at the same time point, supernatants were harvested and viral RNA was quantified by RT-PCR.
TABLE 2.
Changes in provirus HIV DNA concentration in CD4+ T cells and in HIV RNA release level in culture supernatants in the presence or the absence of CD8+ T cells
| Status of individuals donating CD8+ T cellsa | Provirus HIV DNA (log10 proviral HIV DNA copies/106 CD4+ T cells)b
|
HIV RNA release level (log10 HIV RNA copies/ml in culture supernatants)c
|
||||
|---|---|---|---|---|---|---|
| Without CD8+ T cells | With CD8+ T cells | Change | Without CD8+ T cells | With CD8+ T cells | Change | |
| Healthy donors | 4.2 | 4.1 | −0.1 | 8.1 | 7.5 | −0.6 |
| 4.6 | 4.6 | NCd | 8.0 | 7.8 | −0.2 | |
| 4.3 | 4.4 | +0.1 | 9.1 | 8.9 | −0.2 | |
| 5.0 | 4.6 | −0.4 | 6.2 | 5.0 | −1.2 | |
| 4.8 | 3.9 | −0.9 | 7.0 | 6.0 | −1.0 | |
| 5.1 | 5.0 | −0.1 | 7.1 | 6.9 | −0.2 | |
| HIV+ rapid progressors | 4.8 | 3.8 | −1.0 | 7.5 | 6.9 | −0.6 |
| 3.2 | 2.3 | −0.9 | 5.2 | 3.5 | −1.7 | |
| 4.2 | 3.2 | −1.0 | 5.6 | 4.4 | −1.2 | |
| 4.2 | 4.4 | +0.2 | 4.4 | 3.7 | −0.7 | |
| 4.1 | 3.8 | −0.3 | 9.5 | 8.7 | −0.8 | |
| 4.8 | 3.0 | −1.8 | 9.2 | 6.2 | −3.2 | |
| 3.8 | 3.9 | −0.1 | 9.6 | 7.2 | −2.4 | |
| 3.9 | 4.6 | +0.7 | 9.3 | 7.5 | −1.8 | |
| 4.1 | 4.8 | +0.7 | 8.6 | 6.6 | −2.0 | |
| 4.4 | 3.3 | −1.1 | 8.2 | 5.8 | −2.4 | |
| HIV+ nonprogressors | 4.2 | 3.0 | −1.2 | 8.1 | 5.2 | −2.9 |
| 5.0 | 3.2 | −1.8 | 7.7 | 4.2 | −3.5 | |
| 4.5 | 3.0 | −1.5 | 8.1 | 4.9 | −3.2 | |
| 4.6 | 2.6 | −2.0 | 8.7 | 4.9 | −3.8 | |
| 3.3 | 1.3 | −2.0 | 8.9 | 5.2 | −3.7 | |
| 3.5 | 2.2 | −1.3 | 8.4 | 3.8 | −4.6 | |
| 4.7 | 3.1 | −1.6 | 6.0 | 4.4 | −2.4 | |
| 4.5 | 2.3 | −2.2 | 6.6 | 5.4 | −1.2 | |
| 4.4 | 2.4 | −2.0 | 9.2 | 6.6 | −2.6 | |
| 4.2 | 2.2 | −2.0 | 8.7 | 6.0 | −2.7 | |
| 3.2 | 0.9 | −2.3 | 7.3 | 5.7 | −1.6 | |
| 3.7 | 2.7 | −1.0 | 6.4 | 5.1 | −1.3 | |
See Table 1 for explanation of the status of individuals donating CD8+ T cells.
Provirus HIV DNA concentration in CD4+ cells was assessed on day 8 postactivation by PCR.
HIV RNA release level in coculture supernatant was assessed on day 8 postactivation by RT-PCR.
NC, no change.
We observed a significant decrease in the proviral DNA burden (expressed as log10 copies/106 CD4+ cells) of CD4+ cells cocultured with CD8+ cells from SP (mean [± standard deviation] change, −1.8 ± 0.4 log10 copies, P < 0.001), whereas the decrease in proviral burden which was observed when CD4+ cells and CD8+ cells of RP were cocultured did not reach significance (mean change, −0.5 ± 0.8 log10 copies, P = 0.067). This was also the case when acutely infected CD4+ cells of HD were cocultured with autologous CD8+ cells (mean change, −0.3 ± 0.4 log10 copies, P = 0.090) (Fig. 1A). Overall, HD and RP had no significant decrease in their proviral burden (P = 0.764); in contrast, a strong difference was observed between the decrease of proviral burden of RP and SP (P < 0.001) under the influence of autologous CD8+ T cells. These results demonstrate that CD8+ cells from SP strongly reduced CD4+ T-cell-associated proviral DNA, whereas CD8+ cells of RP and HD had virtually no such reducing capacities. These results were confirmed by the strong correlation which was observed between proviral DNA decrease under the influence of autologous CD8+ T cells and the rate of peripheral CD4+ cell decline observed in this group of 22 patients over the three following years (R2 = 0.561, P < 0.001). Of note is the fact that such a correlation was stronger than that observed between the plasma viral load of our 22 patients and their rate of peripheral CD4+-cell decline (R2 = 0.259, P = 0.016). On the other hand, although there was a significant difference between the levels of plasma viremia of RP and SP, we did not find any correlation between the antiviral capacities of CD8+ cells of these 22 patients and their plasma viral load levels (R2 = 0.072, P = 0.226).
In the same experiments, we controlled that the different antiviral capacities (in terms of proviral DNA concentration change) of CD8+ cells, according to the different rates of disease progression of SP and RP, had their counterparts in cell culture supernatant viral release inhibition. As expected, viral release (expressed as log10 RNA copies/milliliter) inhibition was very high in SP (−2.8 ± 1.0 log10 copies, P < 0.001), whereas it was much lower in RP patients (−1.7 ± 0.9 log10 copies, P < 0.001) and HD (−0.6 ± 0.4 log10 copies, P = 0.013) (Fig. 1B). These low but significant levels of viral release inhibition occurred in HD and RP without significant change in CD4+ cell proviral burden; they could be the result of the contribution of CD8+-cell-emitted soluble factors which were shown to inhibit, by approximately 0.5 log10 copies, viral replication (R. Salerno-Gonçalves, W. Lu, and J. M. Andrieu, unpublished observations) at a posttranscriptional level (10; Salerno-Gonçalves et al., unpublished). Overall, our results demonstrate that an important driving force controlling HIV replication is the capacity of CD8+ T cells to reduce proviral DNA-bearing CD4+ cells, thus preventing them from releasing their virions. Such CD8+ T-cell capacities were highly predictive of the rate of CD4+-cell decline observed over the three subsequent years (R2 = 0.561, P < 0.001).
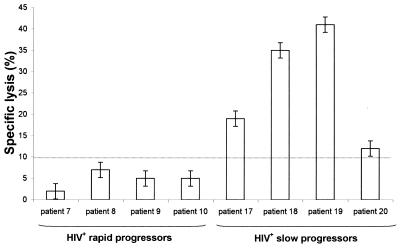
In order to examine the relationship between the antiviral capacities of CD8+ cells toward HIV-infected CD4+ cells and the classical HIV-1-specific CTL activity of the same CD8+ cells, we performed a 51Cr-release assay on 8 of the 22 HIV-infected patients (4 SP and 4 RP). Effector cells were CD8+ T cells isolated from the coculture at day 8, and target cells were autologous Epstein-Barr virus-transformed B lymphoblastoid cell lines (B-LCLs). These target cells have been previously infected with a recombinant vaccinia virus containing an HIV-1 Gag gene or with a wild-type vaccinia virus as control (16). An effector-to-target ratio of 10:1 was taken as a representative value. A specific gag lysis (>10%) was observed in the four SP, whereas no significant gag lysis was noted in the 4 RP (Fig. 2). Although the number of patients examined in this 51Cr-release assay was small, the difference between SP and RP in gag-engineered B-LCL lysis was highly significant (P = 0.007). However, we did not find any correlation between gag-specific CTL lysis and the antiviral activity of CD8+ cells we measured by quantitative DNA PCR (R2 = 0.306, P = 0.155).
FIG. 2.
Analysis of HIV-1 gag-specific cytotoxic CD8+ lymphocyte activity against autologous Epstein-Barr virus-transformed B-LCL targets infected with recombinant vaccinia virus containing a gag gene of HIV-1. These measurements were taken in samples from 8 asymptomatic HIV+-infected patients (4 rapid progressors and 4 slow progressors) by using a 51Cr-release cytotoxicity assay. Percent specific lysis was calculated by subtracting the specific 51Cr-release of wild-type vaccinia virus-infected targets (controls). Each result shown is the mean (± standard deviation) percent specific lysis from each target at an effector ratio of 10:1.
Previous studies have demonstrated that peptide-specific responses of cytotoxic CD8+ T cells were generally directed at Gag epitopes (2) and that Gag-specific cytotoxic responses were associated with better HIV-1 infection clinical outcome (5, 15). In this study, there is also a significant difference between the SP and RP in the cytotoxic activity of CD8+ T cells against autologous target cells expressing HIV-1 gag peptides, but no correlation was found between CTL activity and the global antiviral activity of CD8+ T cells, as measured by proviral DNA PCR. Moreover, we did not find any correlation between antiviral activity of CD8+ T cells of our 22 patients and their plasma viral load. This is in apparent contrast with the results obtained by Ogg et al. (13), who found a strong correlation between the percentage of pol- and/or gag-specific CD8+ cells and plasma viral load. Our system allows a global approach of the functional antiviral activity of CD8+ T cells toward infected CD4+ T cells, whereas the chromium-release assay measures the specific lysis of B-LCLs expressing HIV peptides and the HLA-peptide tetrameric complex assay (1) gives the frequency of CD8+ T cells specific for a given epitope. However, these two assays do not give any information on the functional antiviral activity of CD8+ T cells toward infected CD4+ T cells.
In conclusion, our findings bring the demonstration of a strong correlation between the antiviral activity of CD8+ T cells of HIV-infected patients (as measured by the CD4+ T-cell proviral DNA decrease) and the rate of peripheral CD4+ T-cell count decline in the next 3 years. It is not excluded that such an activity could be the sum of various HIV-specific CD8+ T-cell activities, but we did not find any correlation with the Gag-specific CTL activity. Our method is a relatively simple one which could be helpful to monitor the antiviral activity of CD8+ T cells along the course of HIV infection and could be a useful tool to test the activity of candidate vaccines.
Acknowledgments
We thank all patients who allowed us to collect data for this study. We also thank Denise Eme and the staff from the Blood Bank of the Laennec hospital for help in collecting blood samples.
This work was supported by grants from SIDACTION, Agence Nationale de Recherche sur le SIDA (ANRS), and Association pour la recherche sur les maladies tumorales et virales (AREMAS).
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Buseyne F, McChesney M, Porrot F, Kovarik S, Guy B, Riviere Y. Gag-specific cytotoxic T lymphocytes from human immunodeficiency virus type 1-infected individuals: Gag epitopes are clustered in three regions of the p24gag protein. J Virol. 1993;67:694–702. doi: 10.1128/jvi.67.2.694-702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg P D, Riddell S R. Deficient cellular immunity—finding and fixing the defects. Science. 1999;285:546–551. doi: 10.1126/science.285.5427.546. [DOI] [PubMed] [Google Scholar]
- 4.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 5.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein M R, van der Burg S H, Pontesilli O, Miedema F. Cytotoxic T lymphocytes in HIV-1 infection: a killing paradox? Immunol Today. 1998;19:317–324. doi: 10.1016/s0167-5699(98)01288-2. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Cao L, Ty L, Arlie M, Andrieu J M. Equivalent amplification of intrinsically variable nucleic acid sequences by multiple-primer-induced overlapping amplification assay: applications for universal detection and quantitation. Nat Med. 1999;5:1081–1085. doi: 10.1038/12520. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Manolikakis G, Andrieu J M. Relationship between frequency of infectious human immunodeficiency virus type 1-harboring cells and kinetics of viral replication: a simple procedure for quantitation of infectious virus-carrying cells in blood samples. J Clin Microbiol. 1992;30:2535–2538. doi: 10.1128/jcm.30.10.2535-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu W, Salerno-Goncalvez R, Yuan J, Doré S, Han D S, Andrieu J M. Glucocorticoids rescue CD4+ T lymphocytes from activation-induced apoptosis triggered by HIV-1: implications for pathogenesis and therapy. AIDS. 1995;9:35–42. doi: 10.1097/00002030-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Mackewicz C E, Blackbourn D J, Levy J A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci USA. 1995;92:2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackewicz C E, Garovoy M R, Levy J A. HLA compatibility requirements for CD8(+)-T-cell-mediated suppression of human immunodeficiency virus replication. J Virol. 1998;72:10165–10170. doi: 10.1128/jvi.72.12.10165-10170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier U C, Klenerman P, Griffin P, James W, Koppe B, Larder B, McMichael A, Phillips R. Cytotoxic T lymphocyte lysis inhibited by viable HIV mutants. Science. 1995;270:1360–1362. doi: 10.1126/science.270.5240.1360. [DOI] [PubMed] [Google Scholar]
- 13.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldo C R, Jr, Beltz L A, Huang X L, Gupta P, Fan Z, Torpey D J., III Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res Hum Retrovir. 1995;11:481–489. doi: 10.1089/aid.1995.11.481. [DOI] [PubMed] [Google Scholar]
- 15.Riviere Y, McChesney M B, Porrot F, Tanneau-Salvadori F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S, et al. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retrovir. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 16.Salerno-Goncalves R, Lu W, Achour A, Andrieu J M. Lysis of CD4+ T cells expressing HIV-1 gag peptides by gag-specific CD8+ cytotoxic T cells. Immunol Lett. 1998;64:71–77. doi: 10.1016/s0165-2478(98)00084-4. [DOI] [PubMed] [Google Scholar]