Abstract
Study Objectives:
Sleep laboratory polysomnography is the gold standard for obstructive sleep apnea (OSA) diagnosis in infants, but its access remains limited. Oximetry-capnography is another simple and widely used tool that can provide information on the presence of desaturations and alveolar hypoventilation. However, its reliability is debated. This study aimed at examining its use in determining OSA severity in infants.
Methods:
This retrospective study was conducted in a sleep unit in a tertiary hospital in infants < 4 months old with clinical signs of OSA or Pierre Robin sequence who underwent a 1-night polysomnography coupled with oximetry-capnography.
Results:
Among the 78 infants included (median [interquartile range] age: 61 [45–89] days at polysomnography), 44 presented with Pierre Robin sequence and 34 presented with isolated airway obstruction. The clinical, sleep, and respiratory characteristics were not significantly different between the 2 subgroups. In the entire cohort, 63.5% had severe OSA. The median obstructive apnea-hypopnea index was 14.5 (7.4–5.9) events/h, peripheral oxygen saturation (SpO2) was 97.4% (96.5–98.1%), and transcutaneous carbon dioxide pressure (PtcCO2) was 41.1 mmHg (38.3–44.9). The optimal threshold to predict an obstructive apnea-hypopnea index > 10 events/h was 6 events/h for an oxygen desaturation index ≥ 3% (sensitivity, 95.7%; specificity, 51.9%) and 2 events/h for an oxygen desaturation index ≥ 4% (sensitivity, 95.7%; specificity, 48.1%).
Conclusions:
Whereas transcutaneous capnography does not appear to be sufficient in predicting severe OSA in infants < 4 months old with Pierre Robin sequence or clinical signs of OSA, oximetry may be a useful alternative for the screening of severe OSA in infants in the absence of polysomnography.
Citation:
Gyapay R, Ioan I, Thieux M, et al. Gas exchange parameters for the prediction of obstructive sleep apnea in infants. J Clin Sleep Med. 2024;20(7):1059–1067.
Keywords: oximetry, index desaturation, infant, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Gas exchange, assessed by oximetry, combined or not with capnography, is a simple, economic, and noninvasive tool, widely used as an alternative to polysomnography. However, its reliability in infants is debated.
Study Impact: The results of this retrospective study show that oximetry could be a helpful alternative to predict severe obstructive sleep apnea in infants < 4 months of age. The desaturation index evaluated by oximetry might be useful to guide the therapeutic management when access to a sleep unit is not possible.
INTRODUCTION
Over the last decade, special attention has been given to pediatric obstructive sleep apnea (OSA), which is a form of sleep-disordered breathing that occurs in infants, children, and adolescents.1 OSA is an obstruction of the airway (ie, repeated episodes of complete or partial obstruction) resulting in increased breathing effort and inadequate ventilation, which can compromise physiological sleep patterns.2 The prevalence of OSA in infants remains known. Using questionnaires in 12,447 infants, Bonuck et al3 reported a prevalence of habitual snoring of approximately 4%, without, however, confirming the presence of OSA by polysomnography (PSG). The main complications of OSA are feeding difficulties, obstruction of the upper airway with growth failure, cardiovascular outcomes, apparent life-threatening events, risk of sudden death, and neurological impairment.1,4–9 The clinical signs suggestive of OSA in infants are classical: snoring, labored breathing, respiratory irregularities, apneas, neck hyperextension during sleep, night sweats, irritability, and growth failure.10 Nevertheless, the presentation of OSA is heterogeneous and varies from no clinical symptoms to acute respiratory distress.10 Thus, clinical signs and physical examination are essential but do not provide a definitive diagnosis.11 In infants, the etiology of OSA also varies, encompassing adenoidal hypertrophy, choanal atresia, laryngomalacia, syndromic craniosynostosis with or without midface hypoplasia, cleft lip or palate, mandibular hypoplasia (eg, Pierre Robin sequence [PRS]), neuromuscular disorders (eg, cerebral palsy, mitochondrial disorders, spinal muscular atrophy), and complex disorders (eg, achondroplasia, Down syndrome, Prader-Willi syndrome, Beckwith-Wiedemann syndrome, mucopolysaccharidoses, Chiari malformation).10 The degree of airway obstruction is variable and the treatment can be either conservative or surgical depending on the severity of the symptoms and the patient’s history.10,12 Early diagnosis and management of OSA in the neonatal period are essential to avoid complications.13
Only sleep recordings enable the diagnosis and evaluation of the severity of OSA. Various techniques can be used: PSG in dedicated centers (type 1), PSG performed in the ambulatory setting (type 2), respiratory polygraphy (type 3), and oximetry (type 4) combined or not with capnography. Type 1 constitutes the gold-standard method but its access is limited due to an increasing demand, the lack of availability in some regions, its cost, and the time required for the recordings and their interpretation.14 To face these challenges, easier, cheaper, and more accessible methods such as type 4 are often used before an eventual complete evaluation.15 The severity of OSA is graded according to the obstructive apnea-hypopnea index (OAHI) when using PSG or respiratory polygraphy.16 For type 4 studies, several teams calculate the McGill oximetry score (MOS).10 However, frequent artifacts and the occurrence of nonhypoxic obstructive events in infants can limit its use. For instance, motion impairs the oximeter’s ability to discern pulsatile blood flow, resulting in artifacts in the form of desaturations. Similarly, nonobstructive events, such as central events, may also result in desaturations. Moreover, there is no consensus regarding the use of oximetry coupled with capnography and little is known about its validity in screening OSA in infants. The main objective of this study was to examine the use of nocturnal oximetry-capnography obtained by PSG in determining OSA severity in a cohort of infants younger than 4 months old. The secondary objective was to determine the diagnostic value of oximetry in terms of sensitivity, specificity, and positive predictive values (PPVs) and negative predictive values (NPVs) for the identification of severe OSA.
METHODS
Population
Infants were selected from the ongoing DYSROBIN prospective cohort study, which focuses on the evaluation of brainstem dysfunction in PRS by comparing respiratory sleep events in infants with PRS with those of infants with OSA without PRS and those of healthy infants within the first year of life (Commission Nationale de l’Informatique et des Libertés [CNIL] number 17_0816). We obtained permission to access the data of the first PSG report. The study protocol was approved by the local ethics committee (Ethics Committee 21_629) and the national data protection agency (CNIL number 21_5623). According to the most recent French law, informed consent was obtained. The control group from the DYSROBIN study, which comprised healthy infants, was not included herein as the number of infants was not sufficient at the time of the study (< 10). The infants included in the present study were aged < 4 months old, presented clinical symptoms suggestive of OSA or a diagnosis of PRS (ie, a genetic syndrome known to be responsible for OSA), and underwent a PSG between May 2018 and September 2021 at the sleep unit of the Pediatric University Hospital of Lyon (Hospices Civils de Lyon, France). Infants with PRS were grouped as “PRS’’ and infants with isolated airway obstruction were grouped as “AWO”. Infants diagnosed with PRS were included regardless of symptoms because OSA in this population is widely present.7 Moreover, in our hospital, all infants with PRS undergo an early PSG as part of their management. Patients with neuromuscular, complex genetic disorders or neurological disorders known to cause central sleep apnea as well as patients born prematurely were not included.17
eData collection
Clinical characteristics (ie, sex, age, gestational birth rounded to the nearest completed week, diagnosis, weight, respiratory signs, nutrition support, and respiratory support) were collected from the medical files.
Nocturnal PSG
PSG was performed during 1 night in the presence of the child’s parent, without tilting the mattress, and with continuous monitoring by trained pediatric nurses, using a Morpheus recorder (Micromed, Mogliano Veneto, Italy). The Morpheus recorder was coupled with a NONIN oximeter (Nonin Medical, Plymouth, MN) set up with an averaging time of 3 seconds, and the sampling rate was set at 8 Hz.
To avoid hospitalizing the patient for several nights, most infants were studied in different sleep positions depending on the patient’s referring physician’s objectives: supine position, prone position, and lateral position. As it is the usual procedure in this sleep unit, the night recording was split into several parts, each corresponding to different positions, in order to compare them and choose the best position to prevent airway obstruction. The sequence and choice of positions were planned by the patient’s referring physician; the first position was usually the supine position. If a position was not well tolerated, the nurses were instructed to move the infant to the next position. The nurses performed sleep position changes during the night, if possible, and for identical durations for each position. In this way, they split the recording into different parts. Only the parts of the recording that were in the supine position were considered for this study, as this is the recommended position for the prevention of sudden infant death syndrome. PSG data (ie, portions or full night) recorded with ventilatory support or with a palatal plate were also excluded from the analysis.
The PSG recording used frontal, central, and occipital leads (FP1, FP2, C3, C4, O1, O2, A1, and A2), 2 electrooculogram channels, 1 chin electromyogram, 1 electrocardiogram, inductance plethysmography of chest and abdominal respiratory movements, as well as nasal cannula, oronasal thermistor, and saturation values. A video recording was conducted simultaneously with the PSG. Sleep and respiratory events were manually scored by a single expert physician (L.C.) using sleepRT software (OSG, Rumst, Belgium).
Sleep analysis
Rapid eye movement and non–rapid eye movement sleep stages were scored according to the 2014 pediatric American Academy of Sleep Medicine (AASM) guidelines.18 Total sleep time (TST), sleep efficiency (TST/time in bed × 100), sleep efficacy (TST/duration of sleep × 100), arousal index, and respiratory arousal index were calculated.
Respiratory analysis
Respiratory parameters were manually established by a single expert physician following the AASM 2014 guidelines.18 No automatic computerized analysis was performed prior to the analysis by the expert physician. The OAHI, obstructive apnea index, obstructive hypopnea index, central apnea index (CAI), and mixed apnea index were established. Mean peripheral oxygen saturation (SpO2) values, mean time spent with SpO2 values below 90%, oxygen desaturation index (ODI) ≥ 3% (ODI3%), and ODI ≥ 4% (ODI4%) were collected by the NONIN oximeter. Indexes of events were expressed as the mean number of events per hour. The respiratory analysis was performed for the total duration of sleep spent in the supine position and without ventilatory support and then analyzed according to sleep stages.
Nocturnal carbon dioxide monitoring
Transcutaneous carbon dioxide pressure (PtcCO2) values were obtained over the entire PSG recording period using a coupled SenTec system (SenTec Digital Monitor, Therwil, Switzerland). The mean and maximal PtcCO2, as well as the proportion of time spent with PtcCO2 over 50 mmHg, were calculated. A PtcCO2 > 50 mmHg during ≥ 2% of nocturnal sleep time was chosen for the definition of nocturnal hypercapnia since this value was used to initiate noninvasive ventilation in the European Respiratory Society statement published in 2022 on long-term noninvasive respiratory support in the pediatric population.19
Respiratory scoring rules
Mild OSA is defined as an OAHI ≥ 1 event/h and < 5 events/h of sleep, moderate OSA as an OAHI ≥ 5 events/h and < 10 events/h of sleep, and severe OSA as an OAHI ≥ 10 events/h. Sleep fragmentation is defined as an index of arousals > 11 or an index of respiratory arousals > 1 event/h of sleep. The oxygen desaturation indexes, ODI3%, and ODI4%, are considered abnormal if > 1 event/h, as well as if any oxygen saturation values drop below a threshold of 90%. Arousal was scored if it lasted > 3 seconds and < 15 seconds and awakening was scored if it lasted > 15 seconds.
Statistical analysis
First, a descriptive analysis was conducted on the entire population and the 2 groups of infants: those with PRS and those with AWO. Then, respiratory and sleep characteristics were compared between the 2 groups using Wilcoxon tests for continuous variables and Fisher’s exact tests for dichotomous variables, according to the results of the Shapiro-Wilk test. Finally, a sensitivity analysis was performed to find an optimal cutoff value of ODI4% and ODI3% to predict an OAHI > 10 events/h. This analysis allowed the calculation of the PPV, NPV, sensitivity, and specificity reported as percentages. Receiver operating characteristic (ROC) curves were also performed and area under the ROC curves (AUC) and their 95% confidence interval reported. Data are presented as median and interquartile range. Statistical significance was set at a P value < .001 to account for multiple testing. The analyses were performed using R software (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Descriptive analysis
PSG recordings were performed on 78 infants (43 girls, 35 boys). Among these, 34 infants (44%) presented with PRS and 44 (56%) presented with clinical signs of OSA (AWO). Among the 78 infants, 4 infants were recorded only under ventilatory support and were thus not included in the respiratory and sleep analysis. Among the PRS group, 26 had isolated PRS and 8 had associated or syndromic PRS. Among the AWO group, 30 had laryngomalacia diagnosed by nasofibroscopy performed by otolaryngologist physicians, 2 had another malformation (1 congenital stenosis of the piriform orifices, 1 laryngeal diplegia), and 12 had no facial, nose, or throat malformation and were only diagnosed with isolated gastroesophageal reflux disease based on clinical presentation associated with 24-hour impedance pH testing. Clinical characteristics are reported in Table 1.
Table 1.
Characteristics of infants included in the study.
| Entire Cohort (n = 78) | PRS (n = 34) | AWO (n = 44) | P | |
|---|---|---|---|---|
| Sex (female/male), n (%) | 43/35 (55.1%/43.9%) | 21/13 (62%/38%) | 22/22 (50%/50%) | .362 |
| Median GA at birth, wk | 39.5 [39–40] | 40 [39–40] | 39 [39–40] | .963 |
| Median birth weight, g | 3,160 [2,875–3,576] | 3,192 [2,685–3,580] | 3,160 [2,965–3,516] | .892 |
| Median age at PSG, d | 61 [45–89] | 50.5 [41–68.5] | 74 [56–92] | .017 |
| Median weight at PSG, g | 4,674 [4,082–5,373] | 4,395 [3,840–4,720] | 5,062 [4,357–5,662] | .001 |
| Calorie-fortified nutrition, n (%) | 13 (16.7%) | 11 (32.4%) | 2 (4.5%) | <.001 |
| Feeding tube, n (%) | 15 (19.2%) | 13 (38.2%) | 2 (4.5%) | <.001 |
| Clinical respiratory characteristics, n (%) | ||||
| At least 1 of the following OSA signs | 73 (93.6%) | 29 (85.3%) | 44 (100%) | .013 |
| Snoring | 42 (53.8%) | 20 (58.8%) | 22 (50%) | .497 |
| Apneas | 36 (46.2%) | 17 (50%) | 19 (43.2%) | .648 |
| Life-threatening event | 16 (20.5%) | 9 (26.5%) | 7 (15.9%) | .273 |
| Poor sleep quality perceived by parents | 19 (24.4%) | 9 (26.5%) | 10 (22.7%) | .793 |
| Stridor | 41 (52.6%) | 13 (38.2%) | 28 (63.6%) | .039 |
| Frequent desaturations if monitored before PSG | 41 (52.6%) | 17 (50%) | 12 (27.3%) | .058 |
| Respiratory effort | 29 (37.2%) | 14 (41.2%) | 15 (34.1%) | .638 |
| Respiratory treatments, n (%) | ||||
| Prone positioning | 27 (34.6%) | 20 (58.8%) | 7 (15.9%) | <.001 |
| Lateral positioning | 10 (12.8%) | 6 (17.6%) | 4 (9.1%) | .317 |
| CPAP | 23 (29.5%) | 18 (52.9%) | 5 (11.4%) | <.001 |
| BPAP | 3 (3.8%) | 2 (5.9%) | 1 (2.3%) | .577 |
| Intubation | 4 (5.1%) | 3 (8.8%) | 1 (2.3%) | .312 |
| Surgery before PSG, n (%) | ||||
| TNP | 6 (7.7%) | 6 (17.6%) | 0 (0%) | .005 |
| Glossoplexy | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Palate surgery | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Palatal plate | 13 (16.7%) | 13 (38.2%) | 0 (0%) | <.001 |
Data are expressed as numbers and percentages or median and interquartile range. P values < .001 were considered statistically significant. AWO = isolated airway obstruction, BPAP = bilevel positive airway pressure, CPAP = continuous positive airway pressure, GA = gestational age, OSA = obstructive sleep apnea, PRS = Pierre Robin sequence, PSG = polysomnography, TNP = Nasopharyngeal Tube.
Comparability between infants with PRS and AWO
Clinical characteristics
The infants with PRS tended to have undergone a PSG at a younger age (median: 50.5 days) than the infants with AWO (74 days). The median birth weight was not significantly different between the 2 groups, whereas weight at PSG was significantly higher in the AWO group. The number of infants presenting clinical symptoms suggestive of OSA was lower in infants with PRS (85.4%) than in infants with AWO (100%) (Table 1).
Sleep characteristics
In the entire cohort, the median TST spent in the supine position without ventilatory support was 133.5 minutes; infants with PRS had a lower TST than infants with AWO. Sleep efficiency and sleep efficacy were lower in infants with PRS (69.9% and 76%, respectively) compared with infants with AWO (80.2% and 81.3%, respectively). Infants with PRS tended to have a higher median arousal and awakening index (37.4 events/h) than infants with AWO (27.9 events/h) as well as a higher median respiratory arousal index (9 events/h for PRS and 4.9 events/h for AWO) (Table 2).
Table 2.
Sleep characteristics obtained by 1-night polysomnography.
| Entire Cohort (n = 74) | PRS (n = 31) | AWO (n = 43) | P | |
|---|---|---|---|---|
| TST whole PSG, min | 381 [269–456] | 293 [216–423] | 414 [356–481] | <.001 |
| TST (supine support free, min | 133.5 [99.5–186.5] | 105 [82.5–128.5] | 168 [114–215.5] | .001 |
| Sleep efficacy, % | 77 [67.1–88.1] | 76 [60.4–81.9] | 81.3 [69.2–89.7] | .162 |
| Sleep efficiency, % | 73.8 [62.3–84.6] | 69.9 [56.8–78.2] | 80.2 [66–87.8] | .033 |
| Awakening and arousal index, events/h | 31.3 [24.5–43] | 37.4 [27.1–48.1] | 27.9 [23.7–40.4] | .042 |
| Arousal, events/h | 29.4 [20.1–35.2] | 30.3 [20.4–37.7] | 22.9 [20–30.3] | .178 |
| Respiratory arousal index, events/h | 6.8 [3–16.3] | 9 [3.7–16.6] | 4.9 [2.6–10.8] | .189 |
| Proportion of sleep stages, %TST | ||||
| REM sleep | 40.5% [30.1–49.1%] | 45.1% [30.4–53.9%] | 36.3% [48.4–90.5%] | .237 |
| NREM sleep | 59.5% [47.3–68.8%] | 55% [41.2–69.3%] | 63% [51.9–68.3%] | .209 |
Data were not available for 4 patients (see Methods). Data are expressed as median and interquartile range. P values < .001 were considered statistically significant. AWO = isolated airway obstruction, NREM = non–rapid eye movement, PRS = Pierre Robin sequence, PSG = polysomnography, REM = rapid eye movement, TST = total sleep time.
Respiratory outcomes
In the entire cohort, all infants had OSA. The median OAHI was 14.5 events/h and 63.5% of infants had severe OSA. Infants with PRS tended to have a higher median OAHI than infants with AWO (16.8 events/h and 12.4 events/h, respectively). The median CAI was 0 events/h in the entire cohort. No significant difference was found for the other respiratory parameters between the 2 groups, except that the infants with PRS had higher mean and maximum PtcCO2 values than the infants with AWO (Table 3).
Table 3.
Respiratory characteristics obtained by 1-night polysomnography.
| Entire Cohort (n = 74) | PRS (n = 31) | AWO (n = 43) | P | |
|---|---|---|---|---|
| OAHI, events/h | 14.5 [7.4–25.9] | 16.8 [10–35.1] | 12.4 [7.2–22.1] | .124 |
| OAHI ≥ 1 and < 5 events/h, n (%) | 12 (16.2%) | 5 (16.1%) | 7 (16.3%) | |
| OAHI ≥ 5 and < 10 events/h, n (%) | 15 (20.3%) | 3 (9.7%) | 12 (27.9%) | |
| OAHI ≥ 10 events/h, n (%) | 47 (63.5%) | 23 (74.2%) | 24 (55.8%) | |
| OAHI REM, events/h | 22.9 [11.2–46.5] | 27.6 [12.3–54.1] | 20.8 [11.1–43.2] | .226 |
| OAHI NREM, events/h | 7.8 [3.6–12.8] | 12.4 [5.9–20.3] | 5.5 [3.3–10.7] | .019 |
| OAI, events/h | 8.9 [3.8–19.2] | 10.2 [4.4–25.5] | 7.8 [3.4–15.7] | .230 |
| OAI REM, events/h | 10.1 [3.8–20.9] | 17.1 [5.4–26.9] | 8.3 [3.8–16.4] | .082 |
| OAI NREM, events/h | 4 [1.2–9.2] | 5.1 [1.6–17.9] | 3.1 [1–5.2] | .078 |
| OHI, events/h | 0.8 [0–5.4] | 0.6 [0–5.8] | 0.8 [0–4.6] | .591 |
| OHI REM, events/h | 1.3 [0–11.2] | 0 [0–12.1] | 1.9 [0–9.4] | .600 |
| OHI NREM, events/h | 0 [0–2.2] | 0 [0–3.9] | 0.1 [0–1.8] | .777 |
| CAI, events/h | 0 [0–0.8] | 0 [0–0] | 0.4 [0–1.7] | <.001 |
| CAI REM, events/h | 0 [0–1.1] | 0 [0–0] | 0.6 [0–3.2] | <.001 |
| CAI NREM, events/h | 0 [0–0.5] | 0 [0–0] | 0 [0–0.9] | .006 |
| MAI, events/h | 0.7 [0–1.9] | 0 [0–0.7] | 1.3 [0–2.3] | <.001 |
| MAI REM, events/h | 0.5 [0–3.2] | 0 [0–0] | 2.5 [0–4] | <.001 |
| MAI NREM, events/h | 0 [0–1] | 0 [0–0.5] | 0.5 [0–1.3] | .049 |
| Mean O2% | 97.4 [96.5–98.1] | 97.4 [97–98] | 97.4 [96.2–98.1] | .822 |
| Time with O2 < 90%, % | 0.1 [0–1] | 0.1 [0–0.6] | 0.1 [0–1.1] | .647 |
| ODI3%, events/h | 16.4 [7.2–30.3] | 15.9 [7.4–26.7] | 16.6 [7–34.8] | .767 |
| ODI4%, events/h | 7.9 [2.9–18.1] | 8.8 [3–17.5] | 7.3 [2.8–17.8] | .974 |
| Mean PtcCO2, mmHg | 41.1 [38.3–44.9] | 44 [40.2–46.4] | 40.1 [37.5–43.9] | .010 |
| Maximum PtcCO2, mmHg | 45 [36.7–65.5] | 47.5 [43.5–49.8] | 43.9 [36.6–63.7] | .016 |
| Time spent with PtcCO2 > 50 mmHg, % | 0 [0–0] | 0 [0–2.5] | 0 [0–0] | .445 |
Data were not available for 4 patients (see Methods). Data are expressed as number and percentage or median and interquartile range. P values < .001 were considered statistically significant. AWO = isolated airway obstruction, CAI = central apnea index, MAI = mixed apnea index, NREM = non–rapid eye movement, OAHI = obstructive apnea-hypopnea index, OAI = obstructive apnea index, ODI3% = oxygen desaturation index ≥ 3%, ODI4% = oxygen desaturation index ≥ 4%, OHI = obstructive hypopnea index, PRS = Pierre Robin sequence, PtcCO2, transcutaneous carbon dioxide pressure, REM = rapid eye movement.
Diagnostic performance of ODI3% and ODI4%
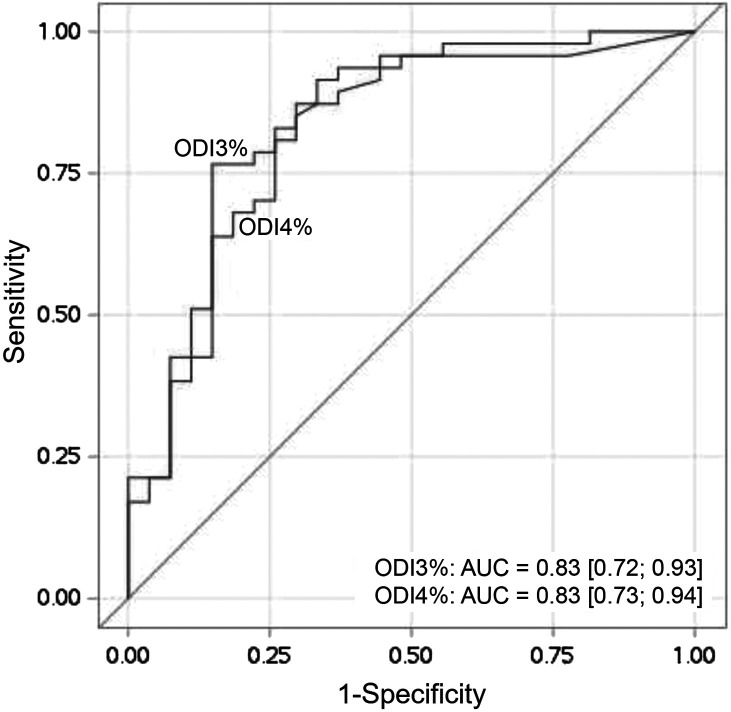
The optimal cutoff for ODI3% to predict OAHI > 10 events/h was 6 events/h, with a sensitivity of 95.7%, a specificity of 51.9%, a PPV of 77.6%, and an NPV of 87.5%. The optimal cutoff for ODI4% to predict OAHI > 10 events/h was 2 events/h, with a sensitivity of 95.7%, a specificity of 48.1%, a PPV of 76.3%, and an NPV of 86.6% (Table 4). The ROC curves for ODI3% and ODI4%, presented in Figure 1, showed similar AUC values—0.83 (0.72; 0.93) for ODI3% and 0.83 (0.73; 0.94) for ODI4%.
Table 4.
Diagnostic performance of ODI3% and ODI4% to predict severe OSA (OAHI ≥ 10 events/h).
| ODI Threshold (events/h) | Sensitivity | Specificity | PPV | NPV | ||||
|---|---|---|---|---|---|---|---|---|
| ODI3% | ODI4% | ODI3% | ODI4% | ODI3% | ODI4% | ODI3% | ODI4% | |
| ≥ 2 | 97.9 | 95.7 | 25.9 | 48.1 | 69.7 | 76.3 | 87.5 | 86.6 |
| ≥ 3 | 97.9 | 91.5 | 25.9 | 55.6 | 69.7 | 78.2 | 87.5 | 78.9 |
| ≥ 4 | 97.9 | 87.2 | 25.9 | 62.9 | 69.7 | 80.4 | 87.5 | 73.9 |
| ≥ 5 | 95.7 | 80.9 | 44.4 | 70.4 | 75.0 | 82.6 | 85.7 | 67.9 |
| ≥ 6 | 95.7 | 78.7 | 51.9 | 77.8 | 77.6 | 86.0 | 87.5 | 67.7 |
| ≥ 7 | 93.6 | 72.3 | 55.6 | 85.2 | 78.6 | 89.5 | 83.3 | 63.9 |
| ≥ 8 | 91.5 | 70.2 | 66.7 | 85.2 | 82.7 | 89.2 | 81.8 | 62.2 |
NPV = negative predictive value, OAHI = obstructive apnea-hypopnea index, ODI = oxygen desaturation index, ODI3% = oxygen desaturation index ≥ 3%, ODI4% = oxygen desaturation index ≥ 4%, OSA = obstructive sleep apnea, PPV = positive predictive value.
Figure 1. Receiver operating characteristic (ROC) curves for desaturation indexes ODI3% and ODI4%.
AUC = area under the receiver operating characteristic curve, ODI3% = oxygen desaturation index ≥ 3%, ODI4% = oxygen desaturation index ≥ 4%.
DISCUSSION
In this relatively large cohort of infants, although all the patients presented with OSA, capnography measurements, mean SpO2, and time spent with SpO2 < 90% were normal. ODI4% and ODI3%, however, appeared to be possible screening tools for OSA. The ODI4% and ODI3% respective thresholds of 6 events/h and 2 events/h were found to be good candidates to predict severe OSA (OAHI > 10 events/h) in both infants with PRS and AWO.
The underlying etiology of OSA appeared to impact infants differently. Those with PRS showed more failure to thrive, as depicted by a lower weight at the time of PSG and more nutrition support than those with AWO, confirming the results of Daniel et al20 and Dorise et al.21 This could be explained, in part, by the tendency toward a higher OAHI in infants with PRS. The fact that infants with PRS tended to have a PSG at a younger age than those with AWO could be due to the management approach used in the sleep unit, which includes a routine PSG before 6 weeks of age in infants with PRS, regardless of clinical symptoms. Infants presenting with OSA without underlying disease are explored by PSG later than 6 weeks because of the time required for parents’ clinical observation, pediatric evaluation, and sleep laboratory access.
All infants studied herein presented with OSA and, in more than half of the cases, the OSA was severe. The median OAHI for infants with PRS found herein was similar to the 15.7 events/h reported by Lee et al22 in a cohort of 141 patients with PRS under 12 months of age. Moreover, the median OAHI reported herein for infants with AWO is also in line with a study by Verkest et al,23 who found a median OAHI of 8.9 events/h in a cohort of 44 patients with laryngomalacia under 12 months of age. However, although all infants had evidence of OSA on PSG, nearly one-fifth of those with PRS exhibited no clinical respiratory signs of OSA. This result underlines that clinical status can be misleading in infants with PRS, as pointed out by Coutier et al,24 who found that 30% of patients in a cohort of 20 infants with PRS under 4 months of age were symptom-free.
Although several clinical scoring tools have been developed, none can accurately predict a diagnosis of OSA.25 Recently, Coutier et al24 showed that, although PSG remains the gold standard for OSA evaluation, respiratory polygraphy seems to be a useful alternative to measure OSA in infants with PRS. Of note, the absence of distinction between awake and sleep periods in respiratory polygraphy induces false obstructive events, but this can be balanced by an underestimation of the number of hypopneas associated with arousals due to the lack of sleep analysis (electroencephalography). Thus, sleep studies should systematically be indicated for all infants with PRS younger than 4 months of age.
It is important to note that, in contrast to older children, young infants do not have a specific cutoff for OAHI according to the latest AASM guidelines,26 This is because the majority of studies have reported cutoffs that vary depending on whether hypopneas were categorized as central or obstructive, and did not always use the latest definitions.26,27 The infants in the present study did not show alveolar hypoventilation or hypoxemia but did have repeated desaturations. Mean SpO2, time spent with SpO2 below 90%, and PtcCO2 values thus seem insufficient to evaluate OSA, contrary to oxygen desaturation indexes. The thresholds of ODI3% ≥ 6 events/h and ODI4% ≥ 2 events/h retained herein as showing good sensitivity to predict severe OSA are in line with previous studies. In young infants undergoing PSG, ODI4% was shown to have 100% sensitivity to predict an OAHI ≥ 5 events/h in 38 infants with OSA,28 while the ODI3% was found to have 91% sensitivity for predicting OAHI ≥ 2% in 53 infants with laryngomalacia.29 In older children, Thavagnanam et al30 found a sensitivity of 97.6% for ODI4% ≥ 2 events/h for diagnosing OSA in children aged 1–18 years old, and Hsieh et al31 estimated that ODI3% ≥ 6 events/h could predict severe pediatric OSA in a cohort of children aged 5–12 years (sensitivity, 90%; specificity, 72%). Among children aged 3 to 12 years, Tsai et al32 found that the optimal ODI4% cutoff value for predicting the occurrence of severe OSA was 4.15 events/h (sensitivity, 89.1%; specificity, 86.0%).
New generations of oximeters, which allow short averaging times and the exclusion of motion artifacts, are increasingly being used by pediatricians to wean infants with bronchopulmonary dysplasia from supplemental oxygen treatment. Using this new system, Evans et al33 found a mean ODI4% of 8.2 events/h and a mean ODI3% of 13.9 events/h in 38 presumably healthy infants aged 3–4 months old. However, the authors did not use PSG to assess the presence or absence of OSA in their cohort, and did not provide median values. Overall, it is difficult to compare all of these studies as the setting, devices, and respiratory indexes used to define OSA differed. One score commonly used is the MOS, established by Nixon et al,34 based on the number and depth of desaturation events to facilitate prioritization of adenotonsillectomy surgery in children older than 12 months. However, the latter has been shown to lack sensitivity, especially in infants less than 1 year and was therefore not used herein.35 Very recently, Song et al36 developed a Modified Laryngomalacia Oximetry Score using clusters of desaturation with bradycardic events, which was significantly associated with worse clinical severity in a cohort of 172 patients with laryngomalacia and OSA aged 4 months old. However, it is important to note that pulse oximetry is not as sensitive as electrocardiogram to detect bradycardia.
With regard to gas exchange, PtcCO2 herein was within the normal range. These findings are in agreement with the data of Amaddeo et al37 and Pautrat et al,38 who showed that PtcCO2 values were poorly correlated with apnea-hypopnea index in children aged at least 3 years old with a suspicion of OSA. PtcCO2 might thus be useful as a supplemental index to monitor sleep breathing disorders and search for alveolar hypoventilation but does not appear to be sufficient to assess OSA presence or severity. Similarly, neither time spent with SpO2 < 90% nor mean SpO2 appears to be abnormal in infants with PRS or AWO. This result, which was found in the entire cohort and in both groups, corroborates the findings of Khayat et al,39 who also showed a strong correlation between ODI3% and OAHI in infants with PRS. Intermittent hypoxemia could thus represent a useful tool for suspicion of the presence of OSA. Future studies evaluating the potential correlation between ODI and OAHI could be helpful.
The present study has limitations. First, the oximetry parameters studied herein were obtained coupled with the PSG with continuous monitoring and analyzed by an expert physician. They are thus not representative of what could be obtained at home, using a different device and without the possibility to exclude artifact-related desaturations and awake periods, which can modify the oxygen desaturation indexes reported. Another technical aspect to note is that portable oximeters used at home do not always have the same settings as PSG saturation recordings: some home-based oximetry devices are set with longer averaging times to limit artifacts related to motion, which can lead to missing very short duration events. In the future, it would be interesting to compare the results obtained by oximeters coupled with PSG manually scored from those obtained with a new device using the same averaging time but with software that would automatically extract artifacts. Second, the TST studied herein was relatively short since the periods of sleep recorded in the prone/lateral position and with ventilatory support were excluded from the analysis. However, a previous study showed a good correlation between ODI3% or ODI4% and apnea-hypopnea index when PSG during naptime and 24-hour oximetry were performed on 30 preterm infants ready for discharge.40
CONCLUSIONS
Although PSG remains the gold standard for the diagnosis and early management of OSA in infants, the present study found that the ODI3% ≥ 6 events/h and ODI4% ≥ 2 events/h have good sensitivity to predict severe OSA in infants with PRS or with clinical signs of OSA, while capnography measurements seem to be insufficient. Given the simple, economic, and noninvasive nature of oximetry, its reliability in predicting severe OSA in infants should be further studied in a larger and more heterogenous population of infants.
DISCLOSURE STATEMENT
Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Véréna Landel for proofreading the manuscript. Authorship contributions: Conception and study design—Romane Gyapay, Aurore Guyon, Patricia Franco, Laurianne Coutier; patient data collection— Sonia Ayari, Eglantine Hullo, Aurore Guyon, Romane Gyapay; polysomnographic analysis—Laurianne Coutier; statistical analysis—Marine Thieux; interpretation of results—Marine Thieux, Romane Gyapay, Laurianne Coutier, Patricia Franco; manuscript preparation—Iulia Ioan, Romane Gyapay, Laurianne Coutier, Patricia Franco; final revision—Iulia Ioan, Eglantine Hullo, Laurianne Coutier, Patricia Franco.
ABBREVIATIONS
- AWO
isolated airway obstruction
- CAI
central apnea index
- NPV
negative predictive value
- OAHI
obstructive apnea-hypopnea index
- ODI
oxygen desaturation index
- ODI3%
oxygen desaturation index ≥ 3%
- ODI4%
oxygen desaturation index ≥ 4%
- OSA
obstructive sleep apnea
- PPV
positive predictive value
- PRS
Pierre Robin sequence
- PSG
polysomnography
- PtcCO2
transcutaneous carbon dioxide pressure
- SpO2
peripheral oxygen saturation
- TST
total sleep time
REFERENCES
- 1. Kaditis AG , Alonso Alvarez ML , Boudewyns A , et al . Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management . Eur Respir J. 2016. ; 47 ( 1 ): 69 – 94 . [DOI] [PubMed] [Google Scholar]
- 2. Thorpy MJ . Classification of sleep disorders . Neurotherapeutics. 2012. ; 9 ( 4 ): 687 – 701 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonuck KA , Chervin RD , Cole TJ , et al . Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study . Sleep. 2011. ; 34 ( 7 ): 875 – 884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biggs SN , Walter LM , Jackman AR , et al . Long-term cognitive and behavioral outcomes following resolution of sleep disordered breathing in preschool children . PLoS One. 2015. ; 10 ( 9 ): e0139142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruni O , Ferri R . Neurocognitive deficits in pediatric obstructive sleep apnea: a multifaceted pathogenetic model . Sleep Med. 2009. ; 10 ( 2 ): 161 – 163 . [DOI] [PubMed] [Google Scholar]
- 6. Chan KC , Au CT , Hui LL , Wing YK , Li AM . Childhood OSA is an independent determinant of blood pressure in adulthood: longitudinal follow-up study . Thorax. 2020. ; 75 ( 5 ): 422 – 431 . [DOI] [PubMed] [Google Scholar]
- 7. Côté A , Fanous A , Almajed A , Lacroix Y . Pierre Robin sequence: review of diagnostic and treatment challenges . Int J Pediatr Otorhinolaryngol. 2015. ; 79 ( 4 ): 451 – 464 . [DOI] [PubMed] [Google Scholar]
- 8. Ottaviani G , Buja LM . Pathology of unexpected sudden cardiac death: obstructive sleep apnea is part of the challenge . Cardiovasc Pathol. 2020. ; 47 : 107221 . [DOI] [PubMed] [Google Scholar]
- 9. Lin MT , Lin HH , Lee PL , et al . Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis . Sleep Breathing. 2015. ; 19 ( 3 ): 809 – 817 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaditis AG , Alonso Alvarez ML , Boudewyns A , et al . ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children . Eur Respir J. 2017. ; 50 ( 6 ): 1700985 . [DOI] [PubMed] [Google Scholar]
- 11. Mitchell RB , Garetz S , Moore RH , et al . The use of clinical parameters to predict obstructive sleep apnea syndrome severity in children: the Childhood Adenotonsillectomy (CHAT) study randomized clinical trial . JAMA Otolaryngol Head Neck Surg. 2015. ; 141 ( 2 ): 130 – 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bull MJ , Givan DC , Sadove AM , Bixler D , Hearn D . Improved outcome in Pierre Robin sequence: effect of multidisciplinary evaluation and management . Pediatrics. 1990. ; 86 ( 2 ): 294 – 301 . [PubMed] [Google Scholar]
- 13. Marcus CL , Brooks LJ , Draper KA , et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2012. ; 130 ( 3 ): 576 – 584 . [DOI] [PubMed] [Google Scholar]
- 14. Haute Autorité de Santé (HAS) . Place et Conditions de Réalisation de la Polysomnographie et de la Polygraphie Respiratoire dans les Troubles du Sommeil Argumentaire. France: The French National Authority for Health; 2012. .
- 15. Garde A , Dehkordi P , Wensley D , Ansermino JM , Dumont GA . Pulse oximetry recorded from the Phone Oximeter for detection of obstructive sleep apnea events with and without oxygen desaturation in children. Presented at: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 25–29 August 2015, Milan, Italy. 2015. . [DOI] [PubMed]
- 16. Iber C , Ancoli-Israel S , Chesson AL Jr , Quan SF ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed . Westchester, IL: : American Academy of Sleep Medicine; ; 2007. . [Google Scholar]
- 17. Erickson G , Dobson NR , Hunt CE . Immature control of breathing and apnea of prematurity: the known and unknown . J Perinatol. 2021. ; 41 ( 9 ): 2111 – 2123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sateia MJ . International Classification of Sleep Disorders-third edition: highlights and modifications . Chest. 2014. ; 146 ( 5 ): 1387 – 1394 . [DOI] [PubMed] [Google Scholar]
- 19. Fauroux B , Abel F , Amaddeo A , et al . ERS statement on paediatric long-term noninvasive respiratory support . Eur Respir J. 2022. ; 59 ( 6 ): 2101404 . [DOI] [PubMed] [Google Scholar]
- 20. Daniel M , Bailey S , Walker K , et al . Airway, feeding and growth in infants with Robin sequence and sleep apnoea . Int J Pediatr Otorhinolaryngol. 2013. ; 77 ( 4 ): 499 – 503 . [DOI] [PubMed] [Google Scholar]
- 21. Dorise B , Trivedi A , Galea C , Walker K , Mehta B . Feeding practices and growth of infants with Pierre Robin sequence . Int J Pediatr Otorhinolaryngol. 2019. ; 118 : 11 – 14 . [DOI] [PubMed] [Google Scholar]
- 22. Lee JJ , Thottam PJ , Ford MD , Jabbour N . Characteristics of sleep apnea in infants with Pierre-Robin sequence: is there improvement with advancing age? Int J Pediatr Otorhinolaryngol. 2015. ; 79 ( 12 ): 2059 – 2067 . [DOI] [PubMed] [Google Scholar]
- 23. Verkest V , Verhulst S , Van Hoorenbeeck K , Vanderveken O , Saldien V , Boudewyns A . Prevalence of obstructive sleep apnea in children with laryngomalacia and value of polysomnography in treatment decisions . Int J Pediatr Otorhinolaryngol. 2020. ; 137 : 110255 . [DOI] [PubMed] [Google Scholar]
- 24. Coutier L , Bierme P , Thieux M , et al . The role of sleep laboratory polygraphy in the evaluation of obstructive sleep apnea syndrome in Robin infants . Sleep Med. 2020. ; 72 : 59 – 64 . [DOI] [PubMed] [Google Scholar]
- 25. Patel AP , Meghji S , Phillips JS . Accuracy of clinical scoring tools for the diagnosis of pediatric obstructive sleep apnea . Laryngoscope. 2020. ; 130 ( 4 ): 1034 – 1043 . [DOI] [PubMed] [Google Scholar]
- 26. Kaditis A , Gozal D . Sleep studies for clinical indications during the first year of life: infants are not small children . Children (Basel). 2022. ; 9 ( 4 ): 523 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daftary AS , Jalou HE , Shively L , Slaven JE , Davis SD . Polysomnography reference values in healthy newborns . J Clin Sleep Med. 2019. ; 15 ( 3 ): 437 – 443 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ehsan Z , He S , Huang G , Hossain MM , Simakajornboon N . Can overnight portable pulse oximetry be used to stratify obstructive sleep apnea risk in infants? A correlation analysis . Pediatr Pulmonol. 2020. ; 55 ( 8 ): 2082 – 2088 . [DOI] [PubMed] [Google Scholar]
- 29. Makhout S , Boudewyns A , Van Hoorenbeeck K , Verhulst S , Van Eyck A . Nocturnal pulse oximetry as a possible screening method for obstructive sleep apnea in infants with laryngomalacia . Sleep Med. 2022. ; 90 : 91 – 95 . [DOI] [PubMed] [Google Scholar]
- 30. Thavagnanam S , H’ng SY , Nathan AM , et al . WRISTOX2 is a reliable tool to diagnose obstructive sleep apnoea syndrome . Int J Pediatr Otorhinolaryngol. 2021. ; 151 : 110930 . [DOI] [PubMed] [Google Scholar]
- 31. Hsieh HS , Kang CJ , Chuang HH , et al . Screening severe obstructive sleep apnea in children with snoring . Diagnostics (Basel, Switzerland). 2021. ; 11 ( 7 ): 1168 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsai CM , Kang CH , Su MC , et al . Usefulness of desaturation index for the assessment of obstructive sleep apnea syndrome in children . Int J Pediatr Otorhinolaryngol. 2013. ; 77 ( 8 ): 1286 – 1290 . [DOI] [PubMed] [Google Scholar]
- 33. Evans HJ , Karunatilleke AS , Grantham-Hill S , Gavlak JC . A cohort study reporting normal oximetry values in healthy infants under 4 months of age using Masimo technology . Arch Dis Child. 2018. ; 103 ( 9 ): 868 – 872 . [DOI] [PubMed] [Google Scholar]
- 34. Jonas C , Thavagnanam S , Blecher G , Thambipillay G , Teng AY . Comparison of nocturnal pulse oximetry with polysomnography in children with sleep disordered breathing . Sleep Breathing. 2020. ; 24 ( 2 ): 703 – 707 . [DOI] [PubMed] [Google Scholar]
- 35. Nixon GM , Kermack AS , Davis GM , Manoukian JJ , Brown KA , Brouillette RT . Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oximetry . Pediatrics. 2004. ; 113 ( 1 Pt 1 ): e19 – e25 . [DOI] [PubMed] [Google Scholar]
- 36. Song JS , Sloychuk J , El-Hakim H , Isaac A . A novel sleep oximetry scoring tool for pediatric laryngomalacia . Int J Pediatr Otorhinolaryngol. 2022. ; 160 : 111220 . [DOI] [PubMed] [Google Scholar]
- 37. Amaddeo A , Fauroux B . Oxygen and carbon dioxide monitoring during sleep . Paediatr Respir Rev. 2016. ; 20 : 42 – 44 . [DOI] [PubMed] [Google Scholar]
- 38. Pautrat J , Khirani S , Boulé M , Ramirez A , Beydon N , Fauroux B . Carbon dioxide levels during polygraphy in children with sleep-disordered breathing . Sleep Breathing. 2015. ; 19 ( 1 ): 149 – 157 . [DOI] [PubMed] [Google Scholar]
- 39. Khayat A , Bin-Hassan S , Al-Saleh S . Polysomnographic findings in infants with Pierre Robin sequence . Ann Thorac Med. 2017. ; 12 ( 1 ): 25 – 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roberts T , Campbell A , Larsen P , Elder D . Preterm infants at discharge: nap polysomnography vs 24-hour oximetry . Acta Paediatr. 2017. ; 106 ( 11 ): 1754 – 1759 . [DOI] [PubMed] [Google Scholar]