Abstract
Study Objectives:
The reduction in daytime sleep during early life is considered one of the indicators of the maturation of sleep patterns, which is closely associated with cognitive development. The current study aims to analyze the relationships between daytime sleep duration (DSD) during infancy and cognitive development at 6 and 10 years.
Methods:
The study included 262 mothers with their newborns from the Shanghai Sleep Birth Cohort Study, spanning 11 follow-ups from 42 days to 10 years. Sleep parameters were assessed using parent-reported questionnaires at each follow-up, and cognitive development was evaluated with the Wechsler Intelligence Scale for Children, fourth edition at 6 and 10 years.
Results:
Two distinct DSD trajectories in early childhood were identified: “typical DSD” (66.7%) and “infancy excessive DSD” (33.3%). Children in the “infancy excessive DSD” trajectory exhibited lower working memory scores than those in the “typical DSD” trajectory at 6 years (mean difference = 5.90, 95% confidence interval [1.83, 9.96], P = .005) and 10 years (mean difference = 4.37, 95% confidence interval [0.26, 8.48], P = .037). Additional analysis in a relatively homogeneous sample consistently showed correlations between DSD trajectories and working memory performance. No consistent significant differences were found in other domains of cognitive development.
Conclusions:
Excessive daytime sleep during infancy may serve as an early indicator for poor working memory at school age. These findings raise concerns about the long-term cognitive development of infants with excessive DSD.
Citation:
Lin J, Jiang Y, Xiao X, et al. Daytime sleep duration during infancy as an indicator for cognitive development at school age: a prospective cohort study. J Clin Sleep Med. 2024;20(7):1069–1077.
Keywords: daytime sleep duration, trajectory, early childhood, cognitive development, working memory
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous studies have highlighted the individual differences in daytime sleep and their association with cognitive development among infants and preschoolers. Considering that higher cognitive functions continue to develop into adulthood and children’s cognitive development at school age predicts later academic performance, the current study aims to examine the relationships between daytime sleep duration in early life and children’s cognitive development at 6 and 10 years.
Study Impact: Our study indicates that excessive daytime sleep during infancy may serve as an early indicator for poor working memory at school age. These findings raise concerns about the cognitive development of infants with relatively longer sleep during the daytime, emphasizing the importance of age-appropriate daytime sleep scheduling for children.
INTRODUCTION
Sleep patterns undergo rapid changes in early life, marked by the consolidation of nighttime sleep and a reduction in daytime sleep.1 The maturation of sleep patterns leads to a decrease in total sleep duration, primarily attributed to the cessation of daytime sleep.2 Most infants transition from multiple naps to 1 or 2 naps between birth and 18 months, eventually adopting an adult-like pattern with no naps (monophasic sleep) before reaching school age.2 Previous studies have highlighted individual differences in daytime sleep during early life. Specifically, children’s daytime sleep duration (DSD) follows distinct trajectories, with some children experiencing more DSD than others in the first few months.3
Early infancy imposes significant demands on learning and memory, taxing the brain. Frequent naps during this period serve to unload synapses across the cortex in a timely manner, facilitating continuous learning and memorization.4 As the cortex and memory system develop, the necessity for substantial daytime sleep to consolidate memories diminishes.5 Given that variations in DSD may reflect the development of the nervous system, several studies have explored the association between DSD in early life and cognitive development. For instance, Cao et al discovered that longer DSD at 1 and 12 months correlated with poor neurocognitive development at 2 years.6 Similarly, Franco et al observed that longer DSD at birth and 6 months were negatively linked to children’s general intellectual ability at 3 years.7 Furthermore, a recently published study from the Growing Up in Singapore Toward healthy Outcomes (GUSTO) cohort indicated that, compared to children with a short, consistent DSD trajectory from 3 to 24 months, a long variable DSD trajectory was associated with lower cognition scores at 4.5 years.8
Although several studies have provided evidence elucidating the relationship between DSD and cognition, the cognitive development follow-ups in the majority of prior studies have been confined to infants, toddlers, and preschoolers.9 Considering that higher cognitive functions continue to develop into adulthood and that a child’s cognitive development at school age predicts later academic progress and achievements, the importance of cognitive assessment during the school-age period is underscored.10 Thus, these findings inspire long-term and comprehensive investigations extending to school-aged children with more mature brain development and higher cognitive functions. Therefore, the current study was undertaken to explore whether DSD in early life could serve as an indicator for cognitive development at school age.
METHODS
Participants
The Shanghai Sleep Birth Cohort Study (SSBCS) is an ongoing mother–child birth cohort designed to investigate the relationship between perinatal and early life environmental and behavioral factors and child physical growth and cognitive development. Detailed methods have been outlined in a published cohort profile.11 In summary, the SSBCS was conducted in the eastern division of Renji Hospital and Shanghai Children’s Medical Center in Pudong New District, Shanghai. A total of 277 healthy pregnant women carrying a single baby during the third trimester were recruited from May 2012–July 2013, and 262 mothers with their full-term newborns were included in the follow-up process. Follow-up visits for children were scheduled at the age of 42 days, 3, 6, 9, 12, 18, and 24 months, and 3, 4, 6, and 10 years.
Sleep assessment
We conducted 10 follow-ups in children from birth to 6 years of age (at 42 days after birth, 3, 6, 9, 12, 18, and 24 months, and 3, 4, and 6 years of age) to assess their sleep conditions. Sleep assessment tools included the Brief Infant Sleep Questionnaire12 (for those under 3 years), Children’s Chronotype Questionnaire13 (at 4 years), and Children’s Sleep Habit Questionnaire14 (at 6 years). The Brief Infant Sleep Questionnaire gathered information on DSD and nighttime sleep duration (NSD) by asking “How much total time does your child spend sleeping during the day (8:00–19:00)?” and “How much total time does your child spend sleeping during the night (19:00–8:00)?” Additionally, parameters such as the nighttime longest sleep period, number of night wakings, and duration of night wakings were investigated as sleep consolidation measures. The Children’s Chronotype Questionnaire assessed sleep duration on scheduled and free days. For children taking regular naps, parents reported the number of days and duration of each nap to calculate DSD. In the Children’s Sleep Habit Questionnaire, DSD was collected similarly to Children’s Chronotype Questionnaire, and NSD on weekdays and weekends was determined using the reported times of “fall asleep” and “wake up.” Average NSD in the Children’s Chronotype Questionnaire and Children’s Sleep Habit Questionnaire was calculated as [(scheduled days’ NSD × 5) + (free days’ NSD × 2)]/7. Total sleep duration (TSD) was calculated as the sum of DSD and NSD.
Cognitive development
Cognitive development was assessed twice using the Wechsler Intelligence Scale for Children, fourth edition (WISC-IV) at the ages of 6 and 10 years, respectively.15 Raw scores were converted into standard scores (mean = 100, standard deviation = 15). The WISC-IV evaluates a child’s intellectual abilities across 4 broad domains: Verbal Comprehension Index, Perceptual Reasoning Index, Working Memory Index, and Processing Speed Index. The Verbal Comprehension Index measures a child’s ability to access and apply acquired word knowledge. The Perceptual Reasoning Index assesses nonverbal reasoning abilities, the capacity to organize visual–spatial information, solve complex problems involving visual patterns, and coordinate visual and motor processes. The Working Memory Index evaluates a child’s ability to register, maintain, and manipulate visual and auditory information in conscious awareness, requiring attention, concentration, and visual and auditory discrimination. The Processing Speed Index assesses a child’s speed and accuracy in visual identification, decision-making, and decision implementation.
Covariates
Demographic data, encompassing maternal age, maternal education level, and family annual income, were gathered through questionnaires during the antenatal period. Infant-related information, such as sex, birth weight, gestational age, and other birth outcomes, was extracted from medical records at birth. When infants were 12 months old, the Bayley Scales of Infant Development were employed to assess their mental development,16 with a higher Mental Development Index indicating more favorable mental development.
Statistical analysis
To achieve our objectives, we followed a multistep statistical approach. First, group-based trajectory models were employed to identify distinctive DSD trajectories from 42 days after birth to 6 years.17 Only children with a minimum of 3 (ranging from 3–10) DSD data points were included. For accuracy, the best-fitting model was selected based on sample-size-adjusted Bayesian information criterion (ssBIC), entropy, and ensuring each group constituted at least 5% of the samples. Model adequacy was assessed with an average posterior probability of assignment (AvePP) of at least 0.7 and odds of correct classification (OCC) exceeding 5.0.
Second, children’s characteristics were described, presenting mean (standard deviation) for continuous variables and frequency (percentage) for categorical variables. Differences between DSD groups were assessed using independent t tests and chi-squared tests, as appropriate. Additionally, differences in children’s characteristics between the finally included and excluded samples were explored using analysis of variance and chi-squared tests.
Third, the association between DSD trajectories and WISC-IV scores was examined using independent t tests and linear regression models. For consideration of confounding factors, we utilized DAGitty to draw and analyze causal diagrams based on Bayesian networks (directed acyclic graph) for variable selection, in order to minimize bias in empirical studies in epidemiology.18 Following a suggested principle of confounder selection,19 only 1 factor—maternal education—correlated with both children’s sleep (Table 1) and cognitive development (Table S6 (535.9KB, pdf) and Table S7 (535.9KB, pdf) in the supplemental material) and was considered a confounder. Because of the relatively small sample size and over 70% of children had mothers with undergraduate education, stratified regression analysis focused on a relatively homogeneous sample (ie, children of mothers with undergraduate education).
Table 1.
Characteristics of the children in the two DSD trajectories.
| Daytime Sleep Duration Trajectories | t/χ2 | P | ||
|---|---|---|---|---|
| Typical (n = 172) | Infancy Excessive (n = 86) | |||
| Sex, n (%) | ||||
| Male | 94 (54.7) | 37 (43.0) | 3.10 | .078 |
| Female | 78 (45.4) | 49 (57.0) | ||
| Birth weight (kg), mean (SD) | 3.39 (0.39) | 3.32 (0.40) | 1.28 | .202 |
| Gestational age (weeks), mean (SD) | 39.73 (1.00) | 39.48 (0.93) | 1.98 | .048 |
| Maternal age (years), mean (SD) | 28.72 (3.19) | 29.64 (3.30) | −2.17 | .031 |
| Maternal education, n (%) | ||||
| High school or below | 12 (7.0) | 11 (12.8) | 8.18 | .017 |
| Undergraduate | 124 (72.1) | 68 (79.1) | ||
| Master’s or above | 36 (20.9) | 7 (8.1) | ||
| Family annual income (CNY), n (%) | ||||
| < 140,000 | 63 (36.6) | 37 (43.0) | 1.19 | .551 |
| 140,000–199,000 | 51 (29.7) | 21 (24.4) | ||
| ≥ 200,000 | 58 (33.7) | 28 (32.6) | ||
| MDI at 12 months of age | 113.38 (12.48) | 111.88 (11.67) | 0.89 | .377 |
CNY = Chinese yuan renminbi, DSD = daytime sleep duration, MDI = Bayley Scales of Infant Development–Mental Development Index, SD = standard deviation.
Finally, to verify the results’ stability, DSD trajectories were conducted with a subset of children completing the WISC-IV test at the follow-ups at both 6 and 10 years of age. Subsequently, the association between DSD trajectories and WISC-IV scores was reanalyzed as in the third step. Statistical analysis used Stata 15.1 (StataCorp LLC, College Station, Texas). All tests were two-sided, and P < .05 was considered statistically significant.
Ethical approval
The study protocol and informed consent procedure underwent review and approval by the Ethics Committees of Shanghai Children’s Medical Center (SCMCIRB-2012033, SCMCIRB-K2018041, and SCMCIRB-K2022140-1). Prior to participation, the parents of all infants provided their consent by signing the informed consent form.
RESULTS
Trajectories of DSD
In total, 258 children (50.8% males) with at least 3 DSD data points were included in the trajectory analysis (Figure 1). The trajectory of children’s DSD demonstrated a decrease as they aged, approaching 0 at 6 years of age (Figure 2). Among the 258 children analyzed, we identified 2 distinct DSD trajectories based on the best-fitting model criteria (ssBIC = −3296.27, entropy = 0.798), both following quartic shapes (Table S1 (535.9KB, pdf) and Table S2 (535.9KB, pdf) ); 66.7% of children were characterized by the “typical DSD” trajectory (AvePP = 0.95, OCC = 9.93) and 33.3% of children were assigned to the “infancy excessive DSD” trajectory (AvePP = 0.91, OCC = 20.55) (Figure 2). Children in the “infancy excessive DSD” trajectory had slightly shorter gestational ages. Their mothers were older (P = .031) and had lower educational levels (P = .017) (Table 1).
Figure 1. Flow chart of the follow-up process.
BISQ = Brief Infant Sleep Questionnaire, CCTQ = Children’s Chronotype Questionnaire, CSHQ = Children’s Sleep Habit Questionnaire, DSD = daytime sleep duration, SSBCS = Shanghai Sleep Birth Cohort Study, WISC-IV = Wechsler Intelligence Scale for Children, fourth edition.
Figure 2. Daytime sleep duration trajectories from 42 days–6 years of age (n = 258).

DSD at each time point for the 2 DSD trajectories is outlined in Table S3 (535.9KB, pdf) . Children following the “infancy excessive DSD” trajectory exhibited significantly longer DSD during the first 18 months (P < .05) and even longer TSD before 24 months of age (P < .05; Table S4 (535.9KB, pdf) ). The most substantial difference in DSD was 3.41 hours at 42 days of age, gradually reducing to 1.29 hours and 0.22 hours at 6 and 18 months of age, respectively. Specifically, at 42 days of age, children in the “infancy excessive DSD” trajectory slept 8.59 ± 1.45 hours during the daytime, exceeding their NSD (DSD/TSD = 0.54). In contrast, children in the “typical DSD” trajectory predominantly slept at night (DSD/TSD = 0.40, P < .001; Table S4 (535.9KB, pdf) ). However, no significant differences were observed in NSD and other sleep consolidation parameters at most time points between the 2 DSD trajectories (Table S4 (535.9KB, pdf) and Table S5 (535.9KB, pdf) ).
The association between DSD trajectories and cognitive development
At the follow-ups at 6 and 10 years of age, a total of 179 and 175 children, respectively, completed the WISC-IV test; 156 of them completed the test at both time points. No significant demographic differences were found between the subgroups of children included in the DSD trajectories and those who completed the WISC-IV test at each time point (Table 2).
Table 2.
Characteristics of the children who completed the WISC-IV test at follow-ups at 6 and 10 years of age.
| Total (n = 258) | 6 years of age (n = 179) | 10 years of age (n = 175) | 6 and 10 years of age (n = 156) | F/χ2 | P | |
|---|---|---|---|---|---|---|
| Sex, n (%) | ||||||
| Male | 131 (50.8) | 89 (49.7) | 88 (50.3) | 77 (49.4) | 0.09 | .993 |
| Female | 127 (49.2) | 90 (50.3) | 87 (49.7) | 79 (50.6) | ||
| Birth weight (kg), mean (SD) | 3.37 (0.40) | 3.36 (0.42) | 3.36 (0.42) | 3.36 (0.43) | 0.03 | .993 |
| Gestational age (week), mean (SD) | 39.65 (0.98) | 39.62 (1.00) | 39.63 (1.00) | 39.60 (1.02) | 0.08 | .969 |
| Maternal age (years), mean (SD) | 29.02 (3.25) | 29.26 (3.36) | 29.13 (3.43) | 29.24 (3.48) | 0.23 | .878 |
| Maternal education, n (%) | ||||||
| High school or below | 23 (8.9) | 14 (7.8) | 16 (9.1) | 11 (7.1) | 1.06 | .983 |
| Undergraduate | 192 (74.4) | 133 (74.3) | 126 (72.0) | 116 (74.4) | ||
| Master’s or above | 43 (16.7) | 32 (17.9) | 33 (18.9) | 29 (18.6) | ||
| Family annual income (CNY), n (%) | ||||||
| < 140,000 | 100 (38.8) | 66 (36.9) | 63 (36.0) | 56 (35.9) | 1.55 | .956 |
| 140,000–199,000 | 72 (27.9) | 49 (27.4) | 56 (32.0) | 46 (29.5) | ||
| ≥ 200,000 | 86 (33.3) | 64 (35.8) | 56 (32.0) | 54 (34.6) | ||
| MDI at 12-month-old | 112.89 (12.22) | 113.66 (12.19) | 113.53 (12.25) | 113.94 (12.32) | 0.26 | .853 |
| DSD trajectories, n (%) | ||||||
| Typical | 172 (66.7) | 116 (64.8) | 111 (63.4) | 102 (65.4) | 0.50 | .919 |
| Infancy excessive | 86 (33.3) | 63 (35.2) | 64 (36.6) | 54 (34.6) |
CNY = Chinese yuan renminbi, DSD = daytime sleep duration, MDI = Bayley Scales of Infant Development–Mental Development Index, SD = standard deviation, WISC-IV = Wechsler Intelligence Scale for Children, fourth edition.
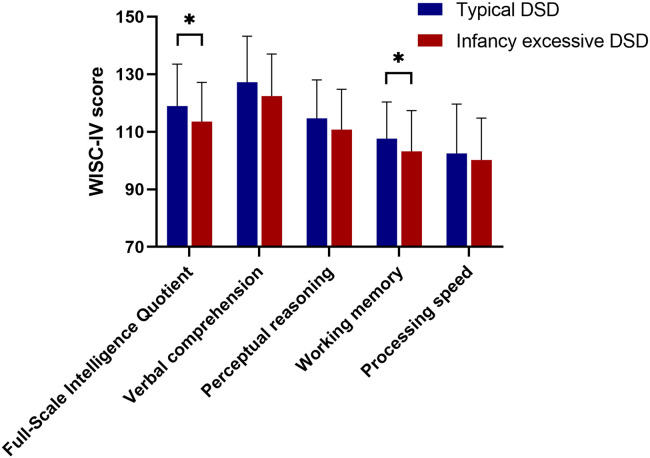
The WISC-IV scores of children at 6 and 10 years of age in different DSD trajectories are depicted in Figure 3 and Figure 4. Although no significant difference was found in the Full-Scale Intelligence Quotient, children in the “infancy excessive DSD” trajectory exhibited lower scores in working memory than children in the “typical DSD” trajectory at 6 years (mean difference = 5.90, 95% confidence interval [CI] [1.83, 9.96], P = .005). Similarly, at 10 years of age, children in the “infancy excessive DSD” trajectory scored lower in working memory than their counterparts in the “typical DSD” trajectory (mean difference = 4.37, 95% CI [0.26, 8.48], P = .037). Furthermore, at 10 years of age, the Full-Scale Intelligence Quotient of children in the “infancy excessive DSD” trajectory was also lower than that in the “typical DSD” trajectory (mean difference = 5.43, 95% CI [1.04, 9.82], P = .016). However, no significant differences were found in other dimensions.
Figure 3. WISC-IV score of children at 6 years of age (n = 179).
*P < .05. DSD = daytime sleep duration, WISC-IV = Wechsler Intelligence Scale for Children, fourth edition.
Figure 4. WISC-IV score of children at 10 years of age (n = 175).
*P < .05. DSD = daytime sleep duration, WISC-IV = Wechsler Intelligence Scale for Children, fourth edition.
As outlined in Table 3, in the further analysis focusing on children whose mothers held undergraduate degrees (a relatively homogeneous sample), infancy excessive DSD remained significantly associated with poor working memory performance at 6 (coefficient = −5.86, 95% CI [−10.13, −1.60], P = .007) and 10 years (coefficient = −5.04, 95% CI [−9.68, −0.40], P = .033). Additionally, at the 10-year-old follow-up, children with infancy excessive DSD exhibited lower scores in verbal comprehension (coefficient = −5.82, 95% CI [−11.31, −0.32], P = .038). Furthermore, a borderline significant difference in Full-Scale Intelligence Quotient was observed between the two DSD trajectory groups (coefficient = −5.03, 95% CI [−10.25, 0.20], P = .059). However, no significant differences were found between the two DSD trajectories in other domains of cognitive development among children whose mothers were undergraduates (P > .05).
Table 3.
Linear regression of children’s DSD trajectory groups and WISC-IV scores at 6 and 10 years of age (stratified analysis by maternal education).
| High School or Below | Undergraduate | Master’s or Above | ||||
|---|---|---|---|---|---|---|
| Coef. (95% CI) | P | Coef. (95% CI) | P | Coef. (95% CI) | P | |
| 6 years of age | ||||||
| Full-Scale Intelligence Quotient | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | 4.21 (−8.47, 16.88) | .483 | −3.16 (−7.58, 1.26) | .160 | 8.95 (−2.20, 20.09) | .111 |
| Verbal comprehension | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | 7.96 (−4.78, 20.69) | .198 | −1.93 (−6.26, 2.39) | .379 | 6.19 (−4.72, 17.11) | .256 |
| Perceptual reasoning | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | −2.96 (−21.09, 15.17) | .728 | −0.99 (−5.83, 3.85) | .685 | 10.93 (−2.36, 24.23) | .103 |
| Working memory | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | 3.75 (−8.71, 16.21) | .524 | −5.86 (−10.13, −1.60) | .007 | 0.41 (−14.72, 15.54) | .957 |
| Processing speed | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | 4.92 (−7.29, 17.13) | .398 | −1.65 (−6.33, 3.03) | .487 | 6.63 (−3.43, 16.69) | .188 |
| 10 years of age | ||||||
| Full-Scale Intelligence Quotient | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | −6.60 (−18.72, 5.51) | .262 | −5.03 (−10.25, 0.20) | .059 | 1.30 (−10.48, 13.07) | .824 |
| Verbal comprehension | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | 0.81 (−17.49, 19.11) | .926 | −5.82 (−11.31, −0.32) | .038 | 4.06 (−9.50, 17.62) | .546 |
| Perceptual reasoning | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | −16.51 (−25.27, −7.74) | .001 | −1.46 (−6.52, 3.60) | .568 | −2.89 (−14.25, 8.47) | .608 |
| Working memory | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | −0.10 (−9.50, 9.31) | .983 | −5.04 (−9.68, −0.40) | .033 | 5.48 (−7.06, 18.03) | .379 |
| Processing speed | ||||||
| Typical DSD | Ref. | Ref. | Ref. | |||
| Infancy excessive DSD | −1.24 (−15.19, 12.71) | .852 | −2.54 (−8.59, 3.51) | .408 | −0.37 (−14.98, 14.24) | .959 |
CI = confidence interval, Coef. = coefficient, DSD = daytime sleep duration, Ref. = reference, WISC-IV = Wechsler Intelligence Scale for Children, fourth edition.
Sensitivity analysis
We further characterized the DSD trajectories with the 156 children who completed the WISC-IV test at both the follow-ups at 6 and 10 years of age (Figure S1 (535.9KB, pdf) ). Similar to the previous analysis, 2 trajectories were determined (Table S8 (535.9KB, pdf) and Table S9 (535.9KB, pdf) ). Children with infancy excessive DSD exhibited scores 5.71 (95% CI [1.32, 10.11], P = .011) and 4.03 (95% CI [−0.32, 8.39], P = .069) lower in working memory than children with a typical DSD trajectory at 6 and 10 years of age, respectively (Figure S2 (535.9KB, pdf) and Figure S3 (535.9KB, pdf) ). The further analysis in children whose mothers had undergraduate degrees also showed similar results (Table S10 (535.9KB, pdf) ).
DISCUSSION
To the best of our knowledge, this is the first prospective cohort study suggests that the infancy DSD may serve as an indicator for working memory performance in school children aged 6 and 10 years. We identified 2 distinct DSD trajectories, named “typical DSD” and “infancy excessive DSD” trajectories, revealing a significant difference of over half an hour within the first 12 months of life. Subsequently, we established that children experiencing infancy excessive DSD exhibited poorer cognitive performance at both 6 and 10 years, particularly in the domain of working memory.
In our study, we identified 2 DSD trajectories. In comparison to children in the “typical DSD” trajectory, those in the “infancy excessive DSD” trajectory exhibited extended daytime sleep until 18 months of age, even inverting their day–night patterns. Although specific DSD recommendations for early childhood are lacking, the children in our study experienced more DSD than reported in a meta-analysis for predominantly-Asian countries/regions: 6.32 (95% CI [5.24, 7.41]), 3.59 (95% CI [3.30, 3.88]), and 2.30 (95% CI [2.10, 2.51]) hours in newborns, infants, and toddlers, respectively.1 Additionally, comparing our data with Zurich Longitudinal Studies, the “typical DSD” trajectory aligns with the 50th percentile, whereas the “infancy excessive DSD” trajectory surpasses the 98th percentile.20 Consequently, it is reasonable to infer that children categorized in the “infancy excessive DSD” group in our study experienced longer DSD compared to the majority of children.
Several preceding studies have delineated diverse DSD trajectories in infants and preschoolers. A Chinese cohort study identified 3 distinct DSD trajectories from 1–24 months: including “short daytime sleepers (49.6%),” “long and decreased daytime sleepers (41.0%),” and “decreased slowly daytime sleepers (9.4%).” The durations and trends of the first 2 trajectories (comprising over 90% of participants) were analogous to our current study.6 Additionally, the GUSTO cohort outlined 4 DSD trajectories in children aged 3 to 54 months, where the “long variable” (21%) and “long consistent” (20%) trajectories mirrored the duration and trends of our “infancy excessive DSD” trajectory. Similarly, the “moderate consistent” (34%) and “short consistent” (25%) trajectories bore similarities to our “typical DSD” trajectory.3 Our study’s limited sample size may have precluded the identification of more trajectory patterns, yet overall, DSD patterns and trends remained consistent across studies.
The current study suggests that, among all cognitive dimensions assessed by WISC-IV, working memory appears particularly susceptible to the effects of excessive DSD in infancy. Our findings were consistent with those of Bernier et al indicating that children with a higher night/total sleep ratio in infancy exhibited improved performance on executive functions, a subdomain of cognitive capacity where working memory is a core subcomponent, at 18 months, 26 months, and 4 years of age.21,22 Several underlying mechanisms could elucidate these findings. Working memory, crucial for learning and executive functioning, involves the short-term retention and processing of information in the brain, requiring the involvement of the prefrontal cortex.23 Studies indicate a significant increase in working memory capacity between 6 and 8 months of age, with infants responding well to procedures involving the retention of approximately 3 items, a capacity similar to that of adults.24 The development of working memory depends on the maturation of a system possibly involving the temporal cortex, dorsolateral prefrontal cortex, thalamic, and hippocampal structures, of which the hippocampus is a key limbic region involved in higher-order cognitive processes including learning and memory.25 A previous study demonstrated robust local functional connectivity of the hippocampus with nearby subcortical, limbic, and other network regions during the first year of age, contributing to an adult-like topology of hippocampal functional connectivity that predicts working memory performance in later childhood.26
Concerning the maturation of memory function and its correlation with the development of sleep–wake patterns, studies suggest underlying common mechanisms. Circadian rhythm development commences in utero and extends into early life postbirth.27 By the first 2–3 years of life, neonatal suprachiasmatic nucleus achieves adult levels of neurons expressing arginine vasopressin and vasoactive intestinal polypeptide.28 During this critical developmental window, the suprachiasmatic nucleus may be susceptible to maternal and environmental zeitgebers, such as light exposure and breastfeeding, influencing circadian rhythm maturation.29 As the circadian system matures, pacemaker cells in the suprachiasmatic nucleus establish functional connectivity with the hippocampus and prefrontal cortex, influencing neuron number and morphology in these regions to affect memory processing.30 Disruptions to circadian rhythms can affect memory-related signaling cascades that undergo time-of-day-dependent cycling, regulating synaptic plasticity and ultimately affecting memory and cognitive development negatively.31 Consequently, the significant difference in DSD during circadian maturation in infancy may serve as an indicator for later memory performance, reflecting nervous system development. To enhance optimal cognitive development through sleep arrangements in early childhood, further analyses and studies are encouraged to elucidate potential mechanisms underlying circadian rhythms and memory processing.
Our study has limitations. First, due to long-term follow-up, some participants dropped out, resulting in a relatively small sample size that may have limited the ability to detect consistent associations. Second, participants were exclusively from Shanghai, China, mainly from highly educated families, limiting the generalizability of findings. Analysis focused on children of mothers with undergraduate degrees, further reducing representativeness. Future studies need larger, more diverse samples, varied maternal education levels, and extended follow-ups. Third, reliance on caregiver-reported questionnaires for assessing children’s sleep may introduce reporting bias, especially for preschoolers in kindergartens. Objective tools should be employed for more accurate assessment. In spite of this, as the primary caregivers, mothers tend to provide relatively accurate reports of infants’ DSD during their waking hours, compared to nighttime sleep conditions. Although our study lacks objective measurements, the data reported by parents still hold a degree of credibility. Fourth, working memory was evaluated only at 6 and 10 years of age, leaving the existence of the DSD-working memory relationship in early life unstudied. Rigorous studies, including eye-tracking paradigms, are needed for confirmation. Finally, although we have attempted to consider as many confounding factors as possible, given the relatively small sample size of our study and avoiding overadjusting, we only selected the measured variables that were significantly associated with the exposure (infant’s sleep) and outcome (cognitive development at school age) in our database as confounding factors (ie, maternal education). Despite strict covariate selection, unmeasured factors (eg, parent–child interaction, childhood illness) may influence results, warranting consideration.
Our study indicates that children’s DSD follows distinct trajectories, and excessive DSD in infancy may serve as an early indicator of poor working memory in school-age children. These findings underscore concerns about the cognitive development of infants with excessive DSD, emphasizing the importance of age-appropriate daytime sleep scheduling for children. However, further research is necessary to elucidate potential genetic and environmental mechanisms influencing the association between sleep pattern maturation and working memory. Moreover, ongoing follow-ups in our cohort design will be crucial to assess the long-term impact of early DSD on cognitive development in adolescents and adults.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Department of Developmental and Behavioral Pediatrics, Pediatric Translational Medicine Institute, National Children’s Medical Center, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China. The study was supported by the National Natural Science Foundation of China (82073568, 81773443, 82071493), the Science and Technology Commission of Shanghai Municipality (2018SHZDZX05, 21Y11907400), the Shanghai Municipal Education Commission (2022you1-2, D1502), the Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZDCX20211900, SHSMU-ZDCX20211100), and the Shanghai Municipal Health Commission (2020CXJQ01, 2022XD056). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all the children and families who participated in the Shanghai Sleep Birth Cohort Study and all the research nurses, technicians, and secretaries who cooperated with us and made this study possible. The data that support the findings of this study are available from the corresponding author upon reasonable request.
ABBREVIATIONS
- AvePP
average posterior probability of assignment
- CI
confidence interval
- DSD
daytime sleep duration
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- NSD
nighttime sleep duration
- OCC
odds of correct classification
- SSBCS
Shanghai Sleep Birth Cohort Study
- ssBIC
sample-size-adjusted Bayesian information criterion
- TSD
total sleep duration
- WISC-IV
Wechsler Intelligence Scale for Children, fourth edition
REFERENCES
- 1. Lin QM , Spruyt K , Leng Y , et al . Cross-cultural disparities of subjective sleep parameters and their age-related trends over the first three years of human life: a systematic review and meta-analysis . Sleep Med Rev. 2019. ; 48 : 101203 . [DOI] [PubMed] [Google Scholar]
- 2. Staton S , Rankin PS , Harding M , et al . Many naps, one nap, none: a systematic review and meta-analysis of napping patterns in children 0-12 years . Sleep Med Rev. 2020. ; 50 : 101247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tham EKH , Xu HY , Fu X , et al . Variations in longitudinal sleep duration trajectories from infancy to early childhood . Sleep Health. 2021. ; 7 ( 1 ): 56 – 64 . [DOI] [PubMed] [Google Scholar]
- 4. Tononi G , Cirelli C . Sleep function and synaptic homeostasis . Sleep Med Rev. 2006. ; 10 ( 1 ): 49 – 62 . [DOI] [PubMed] [Google Scholar]
- 5. Spencer RMC , Riggins T . Contributions of memory and brain development to the bioregulation of naps and nap transitions in early childhood . Proc Natl Acad Sci USA. 2022. ; 119 ( 44 ): e2123415119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao S , Liu J , Li Y , Xu S , Xia W . Associations of sleep duration with neurocognitive development in the first 2 years of life . J Sleep Res. 2022. ; 31 ( 5 ): e13554 . [DOI] [PubMed] [Google Scholar]
- 7. Franco P , Guyon A , Stagnara C , et al . Early polysomnographic characteristics associated with neurocognitive development at 36 months of age . Sleep Med. 2019. ; 60 : 13 – 19 . [DOI] [PubMed] [Google Scholar]
- 8. Cai S , Tham EKH , Xu HY , et al . Trajectories of reported sleep duration associate with early childhood cognitive development . Sleep. 2023. ; 46 ( 2 ): zsac264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thorpe K , Staton S , Sawyer E , Pattinson C , Haden C , Smith S . Napping, development and health from 0 to 5 years: a systematic review . Arch Dis Child. 2015. ; 100 ( 7 ): 615 – 622 . [DOI] [PubMed] [Google Scholar]
- 10. Grantham-McGregor S , Cheung YB , Cueto S , Glewwe P , Richter L , Strupp B ; International Child Development Steering Group . Developmental potential in the first 5 years for children in developing countries . Lancet. 2007. ; 369 ( 9555 ): 60 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin J , Sun W , Song Y , et al . Cohort profile: The Shanghai Sleep Birth Cohort Study . Paediatr Perinat Epidemiol. 2021. ; 35 ( 2 ): 257 – 268 . [DOI] [PubMed] [Google Scholar]
- 12. Mindell JA , Gould RA , Tikotzy L , Leichman ES , Walters RM . Norm-referenced scoring system for the Brief Infant Sleep Questionnaire - Revised (BISQ-R) . Sleep Med. 2019. ; 63 : 106 – 114 . [DOI] [PubMed] [Google Scholar]
- 13. Werner H , Lebourgeois MK , Geiger A , Jenni OG . Assessment of chronotype in four- to eleven-year-old children: reliability and validity of the Children’s Chronotype Questionnaire (CCTQ) . Chronobiol Int. 2009. ; 26 ( 5 ): 992 – 1014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Owens JA , Spirito A , McGuinn M . The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children . Sleep. 2000. ; 23 ( 8 ): 1043 – 1051 . [PubMed] [Google Scholar]
- 15. Chen H , Keith TZ , Weiss L , Zhu J , Li Y . Testing for multigroup invariance of second-order WISC-IV structure across China, Hong Kong, Macau, and Taiwan . Pers Individ Dif. 2010. ; 49 ( 7 ): 677 – 682 . [Google Scholar]
- 16. Yi S , Luo X , Yang Z , Wan G . The revising of the Bayley Scales of Infant Development (BSID) in China . Chin J Clin Psychol. 1993. ; 1 ( 2 ): 71 – 75 . [Google Scholar]
- 17. Nagin DS . Group-Based Modeling of Development. London: : Harvard University Press; ; 2005. . [Google Scholar]
- 18. Textor J , van der Zander B , Gilthorpe MS , Liskiewicz M , Ellison GT . Robust causal inference using directed acyclic graphs: the R package ‘dagitty’ . Int J Epidemiol. 2016. ; 45 ( 6 ): 1887 – 1894 . [DOI] [PubMed] [Google Scholar]
- 19. VanderWeele TJ . Principles of confounder selection . Eur J Epidemiol. 2019. ; 34 ( 3 ): 211 – 219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iglowstein I , Jenni OG , Molinari L , Largo RH . Sleep duration from infancy to adolescence: reference values and generational trends . Pediatrics. 2003. ; 111 ( 2 ): 302 – 307 . [DOI] [PubMed] [Google Scholar]
- 21. Bernier A , Carlson SM , Bordeleau S , Carrier J . Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning . Child Dev. 2010. ; 81 ( 6 ): 1739 – 1752 . [DOI] [PubMed] [Google Scholar]
- 22. Bernier A , Beauchamp MH , Bouvette-Turcot AA , Carlson SM , Carrier J . Sleep and cognition in preschool years: specific links to executive functioning . Child Dev. 2013. ; 84 ( 5 ): 1542 – 1553 . [DOI] [PubMed] [Google Scholar]
- 23. Baddeley A . Working memory . Curr Biol. 2010. ; 20 ( 4 ): R136 – R140 . [DOI] [PubMed] [Google Scholar]
- 24. Cowan N . Working memory maturation: can we get at the essence of cognitive growth? Perspect Psychol Sci. 2016. ; 11 ( 2 ): 239 – 264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Káldy Z , Sigala N . The neural mechanisms of object working memory: what is where in the infant brain? Neurosci Biobehav Rev. 2004. ; 28 ( 2 ): 113 – 121 . [DOI] [PubMed] [Google Scholar]
- 26. Liu J , Chen Y , Stephens R , et al . Hippocampal functional connectivity development during the first two years indexes 4-year working memory performance . Cortex. 2021. ; 138 : 165 – 177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong SD , Wright KP Jr , Spencer RL , et al . Development of the circadian system in early life: maternal and environmental factors . J Physiol Anthropol. 2022. ; 41 ( 1 ): 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aton SJ , Colwell CS , Harmar AJ , Waschek J , Herzog ED . Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons . Nat Neurosci. 2005. ; 8 ( 4 ): 476 – 483 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sadeh A , Tikotzky L , Scher A . Parenting and infant sleep . Sleep Med Rev. 2010. ; 14 ( 2 ): 89 – 96 . [DOI] [PubMed] [Google Scholar]
- 30. Page TL . Circadian regulation of learning and memory . Curr Opin Insect Sci. 2015. ; 7 : 87 – 91 . [DOI] [PubMed] [Google Scholar]
- 31. Gerstner JR , Yin JC . Circadian rhythms and memory formation . Nat Rev Neurosci. 2010. ; 11 ( 8 ): 577 – 588 . [DOI] [PMC free article] [PubMed] [Google Scholar]