Abstract
The recently identified bfl-1 gene (also known as A1 or GRS), a homologue of bcl-2, encodes an antiapoptotic protein that suppresses apoptosis induced by the p53 tumor suppressor protein and exhibits proliferative and potent cooperative transforming activities. We show that elevated levels of bfl-1 mRNA are a feature of Epstein-Barr virus (EBV)-immortalized B-cell lines and Burkitt's lymphoma cell lines expressing the full spectrum of EBV latent proteins. Using an EBV-negative Burkitt's lymphoma cell line in which the expression of EBV latent membrane protein 1 (LMP1) is inducibly regulated by tetracycline, we demonstrate that LMP1 expression coincides with a dramatic increase in the level of bfl-1 mRNA. Also in this system, an increase in the level of Bcl-2 protein was seen to occur earlier than that of bcl-2 mRNA, suggesting that both transcriptional and translational mechanisms are involved in the control of Bcl-2 expression by LMP-1. We show that elevated bfl-1 mRNA stability can contribute to this effect of LMP-1, thus providing evidence of a novel mechanism of gene regulation by this EBV protein. Upregulation of bfl-1 by LMP1 was not observed in the T-cell line Jurkat or the epithelial cell line C33A. Ectopic expression of Bfl-1 in an EBV-positive cell line exhibiting a latency type I infection protects against apoptosis induced by growth factor deprivation, thereby providing a functional role for Bfl-1 in this cellular context and adding Bfl-1 to the list of antiapoptotic proteins whose expression is modulated by EBV. This is the first report of the regulation of bfl-1 expression by a viral protein, and this novel finding may thus represent an important link between the EBV oncoprotein LMP1 and its cellular growth-transforming properties.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus with oncogenic potential. EBV is associated with infectious mononucleosis and a spectrum of malignant diseases including African endemic Burkitt's lymphoma (BL), anaplastic nasopharyngeal carcinoma, Hodgkin's disease, and lymphoproliferative disorders in immunodeficient individuals. Following primary infection, whether symptomatic or silent, the virus persists in the host for life through mechanisms that are not fully understood. In vitro, EBV is exceptionally efficient at transforming and immortalizing resting human B lymphocytes, leading to the outgrowth of transformed and immortalized lymphoblastoid cell lines (LCLs) displaying elevated levels of several cellular activation antigens and adhesion molecules (25). In an LCL, viral gene expression is generally restricted to a limited number of latent genes which encode six Epstein-Barr nuclear antigens (EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, and EBNA-LP), three integral membrane proteins (LMP1, LMP2A, and LMP2B), and two small nuclear RNAs (EBERs) (12). It is now generally accepted that the EBV latent gene products are responsible for the activation of resting B cells, the induction of continuous cell proliferation, and the replication of EBV episomal DNA. LMP1 is a key effector of EBV-mediated transformation of B cells (24), and it is a classical oncoprotein as defined by its ability to transform rodent fibroblast cell lines and render them tumorigenic (4, 52). Freshly obtained EBV-positive BL cells and early-passage cell lines express EBNA1 as the sole viral protein (group I BL phenotype/type 1 latency). When group I BL cells are serially passaged in vitro, they “drift” to express all the known latency-associated viral proteins (group III BL phenotype/type 3 latency) and acquire many of the phenotypic characteristics of the blast-like LCLs (16).
A central component of the overall EBV strategy and its role in the development of related malignant disease is the ability of the viral latent proteins to suppress the cellular apoptotic program (3, 27). Group I BL cell lines and many EBV-negative BL lines can readily be triggered into apoptosis, whereas group III BL cell lines, like LCLs, are relatively resistant to a variety of triggers of apoptosis including growth factor withdrawal, Ca2+ ionophore treatment, and overexpression of the p53 tumor suppressor gene (19, 38). EBV-negative BL cells converted to the type 3 latency state by infection with the B95-8 strain of EBV also display elevated thresholds of resistance to apoptotic stimuli (17). Bcl-2 is the prototype of a family of related proteins which can be categorized as either apoptotic death agonists or antagonists. Transfection of individual EBV latent genes into EBV-negative BL cell lines has shown that upregulation of Bcl-2 expression correlates with the expression of LMP1 (19, 31, 46) and possibly EBNA2 (13) and EBNA3B (49). The significance of elevated levels of Bcl-2 in BL cells has been demonstrated by experiments in which group I BL cells that are stably transfected with the bcl-2 gene exhibit a degree of resistance to apoptosis proportional to the level of Bcl-2 protein expressed (36). Several lines of evidence, however, point to additional bcl-2-independent survival mechanisms being important in BL cells and during B-cell differentiation within germinal centers (36, 41, 46). LMP1 has also been shown to upregulate the expression of two other host stress response proteins that prevent apoptosis, A20 and Mcl-1 (15, 28, 53), with the latter probably serving as a rapidly inducible, transient, and short-term effector of cell viability before Bcl-2 induction.
This study set out to investigate EBV-associated changes in the expression of a panel of bcl-2-related genes in LCLs and BL-derived cell lines by comparing mRNA levels transcribed from the bclx-L, bclx-S, bfl-1, bik, bak, bax, bcl-2, and mcl-1 genes by using a multiprobe RNase protection assay (RPA). DG75 and BL41 are EBV-negative BL cell lines (47), BL41-B95-8 is a derivative of BL41 infected with the B95-8 strain of EBV, IARC-171 is an LCL established from the same patient from whom the BL41 cell line was derived, MUTU-I and MUTU-III are isogenic stable group I/latency 1 and group III/latency 3 cell lines, respectively (16), and Rael (34) and Kem-BL (45) are EBV-positive BL lines that have retained the group I/latency 1 phenotype. Cell lines were maintained as suspension cultures in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (HyClone), 2 mM glutamine, 100 μg of streptomycin per ml, and 100 IU of penicillin per ml at 37°C in a humidified atmosphere containing 5% carbon dioxide. Total cellular RNA was prepared using RNA isolator solution (Genosys) essentially as specified by the manufacturer. RPAs were performed using the Riboquant multiprobe RPA system as described by the manufacturer (Pharmingen). The multiprobe hAPO-2 template set and T7 RNA polymerase were used to synthesize 32P-labeled antisense riboprobes complementary to portions of transcripts from the apoptosis-related genes bclx-L, bclx-xS, bfl-1, bik, bak, bax, bcl-2, and mcl-1 and the housekeeping genes L32 and GAPDH. The labeled probe set (2 × 105 cpm/μl) was hybridized to 15 μg of total RNA sample in solution, and RNase-protected probe fragments were then resolved on 6% denaturing polyacrylamide gels and detected by autoradiography. Band intensities were quantified by densitometric scanning of autoradiograms.
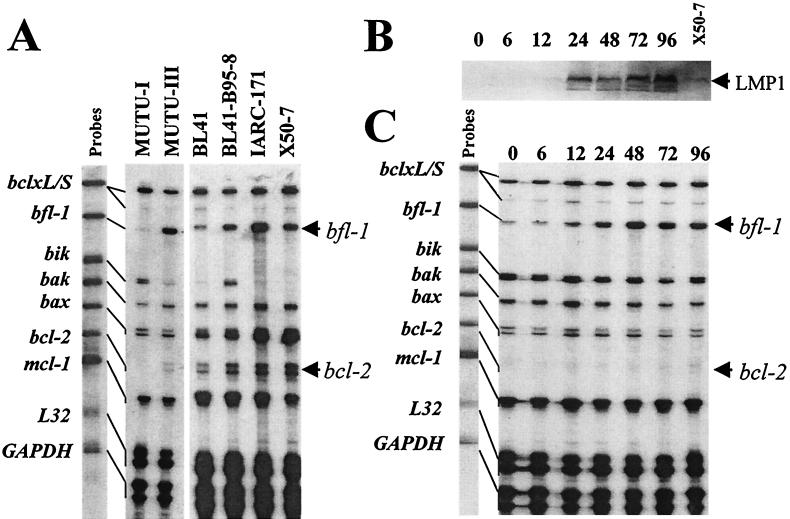
It can be seen from the RPA that significantly elevated steady-state levels of bfl-1 and bcl-2 mRNAs were present in BL cells expressing a group III phenotype (MUTU-III and BL41-B95-8) and LCLs (IARC-171 and X50-7) relative to those of the BL group I phenotype (MUTU-I and BL41; also Rael and Kem-BL [data not shown]) (Fig. 1A). Quantitation of the levels of bfl-1 transcript (after normalizing for GAPDH and L32 mRNA levels) indicated an approximately 20-fold-greater level in MUTU-III than in its isogenic counterpart, MUTU-I. The observation of an elevated level of bcl-2 mRNA in MUTU-III relative to MUTU-I is consistent with the findings of others (19). Transcript from the gene encoding the proapoptotic protein Bik is not detectable in the two LCLs, an interesting observation in that it is also downregulated in MUTU-BL upon drift to the group III phenotype. This does not, however, appear to be a general feature of BL cells exhibiting a group III phenotype, in that the level of bik mRNA is significantly greater in BL41-B95-8 cells than in BL41 cells. Additionally, although elevated levels of bak mRNA are seen in MUTU-III relative to MUTU-I, this effect is also inconsistent in that it is not observed in the BL41/BL41-B95-8 pair. We then assessed the contribution of LMP1 to the regulation of bfl-1 and bcl-2 mRNA levels by RPA using an established tightly regulatable tetracycline-based system to express LMP1 in the EBV-negative BL cell line DG75 (DG75-tTA-LMP1 [14]). LMP1 levels were determined by Western blotting using the anti-LMP1 CS1-4 antibody cocktail as described elsewhere (44). Immunocomplexes were detected with alkaline phosphatase-conjugated sheep anti-mouse immunoglobulin G (Promega) and visualized with 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (BCIP-NBT) liquid substrate (as were all other Western blots in this study). In this experiment, LMP1 was detectable at 12 h postinduction and its level rose to several times the level observed in a reference control LCL (X50-7) by 96 h (Fig. 1B). Total RNA prepared at the same time during the same experiment was used for RPA, and the result is shown in Fig. 1C. The steady-state level of mRNA from the bfl-1 gene rose significantly in response to LMP1 induction. bfl-1 upregulation was dramatic and was detectable at 12 h postinduction (when the levels of LMP1 were comparable to those seen in X50-7), and its increased level was maintained for at least 96 h. Densitometric scanning indicated that the degree of induction by 48 h was about 15-fold (relative to the two internal control mRNAs). In the same experiment, the bcl-2 mRNA level also rose, although in this case much lower levels of transcript were present in the cell and an increase was not clearly evident until after 48 h postinduction upon prolonged exposure of the autoradiogram (data not shown). The effects on bfl-1 and bcl-2 mRNA expression in this system were specific to LMP1, since the level of expression of these genes in the parental cell line DG75-tTA remained unchanged in the absence of tetracycline (data not shown).
FIG. 1.
LMP1 expression correlates with increased steady-state levels of bfl-1 mRNA in a B-cell background. (A) RPA autoradiogram (18-h exposure) in which mRNA levels from the apoptosis-related genes bclx-L, bclx-S, bfl-1, bik, bak, bax, bcl-2, and mcl-1 were analyzed in a range of BL cell lines and LCLs. Unprotected 32P-labeled antisense riboprobes (5,000 cpm) (indicated as Probes) were loaded alongside RPA-processed samples and are shown linked to their smaller RNase-protected fragments which correspond to the steady-state levels of the corresponding mRNA in the sample. The names of the cell lines used are given above each track. The locations of protected fragments derived from bcl-2 and bfl-1 mRNAs are indicated by arrows. The MUTU-I and MUTU-III tracks are from a different RPA from the other four tracks. (B) Western blot of DG75-tTA-LMP1 cells induced to express LMP1 by reculturing cells in the absence of tetracycline. Cells were harvested and analyzed for LMP1 expression at various time points (indicated above each lane); also included was the reference LCL X50-7. (C) RPA performed using RNA samples from the same experiment as that in panel B. Exposure to film was for 18 h. The numbers above the lanes correspond to the times (in hours) after LMP1 induction at which RNA was harvested. Increases in the steady-state levels of bcl-2 and bfl-1 mRNAs are seen upon LMP1 induction.
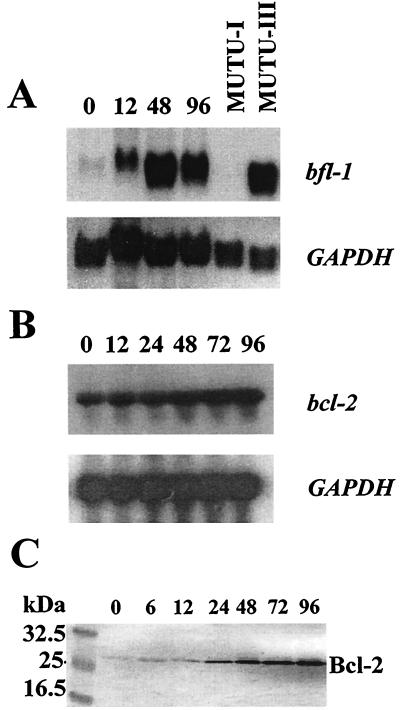
Since RPA gives no indication about the size of a particular mRNA species, the same bfl-1 and bcl-2 riboprobes were used for Northern blotting with RNA samples from the same experiment. Samples (30 μg) of total RNA were size fractionated in a 1.3% formaldehyde–agarose gel and then transferred to nitrocellulose (BDH). Labeled riboprobes were synthesized as described above by in vitro transcription followed by size fractionation in denaturing polyacrylamide gels. Probes were located by autoradiography and eluted from the polyacrylamide gel in 0.5 M ammonium acetate–10 mM magnesium acetate–1 mM EDTA (pH 8.0)–0.1% sodium dodecyl sulfate (SDS) by the crush-and-soak method (32). Filters were hybridized to 32P-labeled probes (106 cpm/ml) in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–50% formamide–1% SDS–0.1% Tween 20–100 μg of Escherichia coli tRNA for 16 to 24 h at 55°C. The filters were washed twice in 1× SSC–0.1% SDS at room temperature for 30 min and then twice in 1 × SSC–0.1% SDS at 65°C for 30 min prior to exposure to X-ray film at −70°C. It can be seen in this experiment (Fig. 2A and B) that (i) only one bfl-1 mRNA, of 0.8 to 0.85 kb, was expressed in all cases, in agreement with the previously reported size of the transcript from this gene (26); (ii) the level of bfl-1 mRNA in MUTU-III was at least twice as high as that detected in DG75-tTA-LMP1 cells at 48 h after induction of LMP1 (normalized to GAPDH mRNA levels), indicating that other EBV latent proteins may serve to further enhance bfl-1 expression; and (iii) the level of bcl-2 mRNA again rose in response to LMP1 expression, and this did not occur until 48 h after induction of the EBV protein. In this case, densitometric scanning indicated that the extent of upregulation by 96 h was threefold. We were unable to extend this work to include an analysis of Bfl-1 protein due to the lack of a suitable antibody for use in Western blotting. Analysis of the levels of Bcl-2 protein in the same experiment showed that upregulation was detectable at 24 h and that the levels rose further thereafter for the duration of the experiment (Fig. 2C). The observation that the upregulation of Bcl-2 protein occurred earlier than that of bcl-2 mRNA suggests that both transcriptional and translational mechanisms may be involved in the control of Bcl-2 expression by LMP1. We did not detect upregulation of bfl-1 mRNA levels in either a tetracycline-regulated LMP1-expressing clone (M. Rowe, unpublished data) derived from the Jurkat T-cell line (6; our unpublished data) or a stably transfected LMP1-expressing clone of the epithelial cell line C33A (references 15 and 35 and data not shown), indicating that the LMP1 effect may be specific to B cells.
FIG. 2.
(A) Northern blot analysis of bfl-1 mRNA levels upon induction of LMP1 in DG75-tTA-LMP1 cells (upper panel). Total RNA was prepared at various times after LMP1 induction (indicated in hours above each lane for all blots in this figure) and also from MUTU-I and MUTU-III cells. RNA samples (30 μg) were loaded onto the gel, which was then blotted and probed with antisense bfl-1 riboprobe as described in the text. The blot was exposed to film for 20 h. The lower panel shows the same blot stripped and reprobed with a GAPDH antisense riboprobe. (B) Northern blot analysis of bcl-2 mRNA levels after LMP1 induction (upper panel). The blot was exposed to film for 24 h. The lower panel shows the same blot stripped and reprobed with GAPDH riboprobe. (C) Western blot analysis of Bcl-2 levels at various times after LMP1 induction. The blot was probed with anti-Bcl-2 antibodies (Bcl-2 100/124) (19).
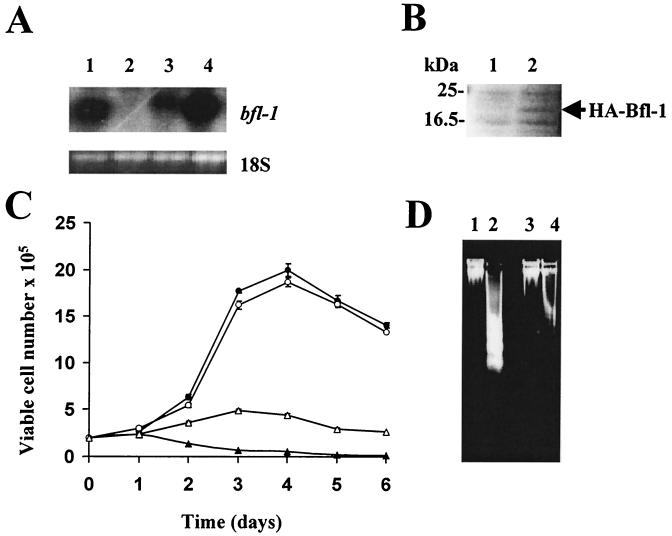
To investigate the functional role of Bfl-1 in BL cells, MUTU-I cells expressing hemagglutinin (HA)-tagged Bfl-1 (HABfl-1) or HA were established by electroporation of 10 μg of pcDNA3HABfl-1 (11) or pcDNA3HA (constructed by excising the bfl-1 cDNA from pcDNA3HABfl-1 with BamHI and XhoI, conversion of the restricted sites to blunt ends, and subsequent religation of the vector) at 270 V in 500 μl of growth medium in a 0.4-mm cuvette and selection with 1,400 μg of active G418 per ml. Antibiotic selection was continued for 21 days until all of the mock-transfected cells were killed. The MUTU-I cell line was chosen for this purpose because it expresses very low levels of bfl-1 mRNA (Fig. 1A) and is sensitive to apoptosis (17). Northern blot analysis showed that bfl-1 mRNA was expressed only in the pcDNA3HABfl-1-transfected pool (Fig. 3A). HA-tagged Bfl-1 was then detected by Western blotting as follows. Cells were lysed at 4°C for 30 min in a buffer containing 1% NP-40, 0.5% sodium deoxycholate, 1% Triton X-100, 150 mM NaCl, 50 mm Tris (pH 8.0), 1 mM sodium orthovanadate, 100 μg of phenylmethylsulfonyl fluoride per ml, and 2 μg of leupeptin per ml. Lysates were clarified by centrifugation at 13,000 rpm in a microcentrifuge (Biofuge 13; Heraeus) for 15 min at 4°C. Protein from 106 cells was separated by discontinuous SDS-polyacrylamide gel electrophoresis (5 to 15% polyacrylamide) and blotted onto a nitrocellulose filter. The filter was probed overnight at 4°C with rabbit polyclonal antibodies to HA diluted 1:1,000 in Blotto. In this experiment, a novel band at 20 kDa, corresponding to that expected for HABfl-1, was visible only in the pool transfected with pcDNA3HABfl-1 (Fig. 3B). The growth kinetics of the Bfl-1-transfected and control-transfected pools were then compared under optimal (10% fetal bovine serum [FBS]) and suboptimal (0.1% FBS) culture conditions (Fig. 3C). In 10% FBS, after an initial lag phase, both pools of transfected cells exhibited rapid growth to a high saturation density followed by a slight fall in viability. However, in 0.1% FBS, the behavior of the two pools differed significantly in that while the control cells showed a rapid decline in viability with time, the Bfl-1-expressing cells exhibited a slight increase in proliferation over the first 72 h in culture followed by a gradual decline in viability. The decline in viability of the control cells cultured in the presence of 0.1% FBS was due to apoptotic death, since there was a marked increase in DNA fragmentation when the cells were grown under low-serum conditions (Fig. 3D, compare lanes 1 and 2) (17). In contrast, the HA-Bfl-1-transfected cells exhibited much less DNA fragmentation when grown under low-serum conditions (compare lanes 2 and 4). The ability of Bfl-1 to promote cell survival during growth factor deprivation was further supported by the observation that while >80% of the control cells exhibited chromatin condensation on staining with acridine orange (final concentration, 5 μg/ml) after 72 h of culture in 0.1% FBS, approximately 35% of HABfl-1-transfected cells contained condensed chromatin under similar culture conditions (17). In 10% FBS, approximately 7% of the cells in both pools of transfectants exhibited chromatin condensation. Based on these observations, it is clear that Bfl-1 can protect BL cells from apoptotic death induced by serum deprivation. The fact that a minority of cells in the HABfl-1-expressing pool continue to undergo apoptotic death in response to serum deprivation is likely to reflect heterogeneity in HABfl-1 levels between individual transfected cells, some of which may express this protein at very low levels or not at all. Indeed, the overall bfl-1 mRNA level in the transfected pool was lower than that in MUTU-III or IARC-171 cells (Fig. 3A). In addition to promoting cell survival, Bfl-1 has cell proliferation properties (10), and this may explain the significant increase in cell numbers seen in the Bfl-1 expressing pool over the first 72 h at the lower serum concentration.
FIG. 3.
Functional analysis of Bfl-1 expression in MUTU-I BL cells. MUTU-I cells were transfected with either pcDNA3HABfl-1-expressing HA-tagged Bfl-1 or control vector (pcDNA3HA) and subjected to selection for 21 days with G418. Total RNA and proteins were then prepared for analysis. (A) The upper panel shows a Northern blot analysis of bfl-1 mRNA in IARC-171 (lane 1), cell pools transfected with pcDNA3HA (lane 2) and pcDNA3HABfl-1 (lane 3), and MUTU-I (lane 4). The lower panel is a photograph of the 18S rRNA band from the same ethidium bromide-stained gel used for blotting. The location of bfl-1 mRNA is indicated. (B) Western blot analysis using polyclonal anti-HA antibodies to detect HABfl-1 expression in cell extracts from the same transfected MUTU-I cell pools (lane 1, pcDNA3HA; lane 2, pcDNA3HABfl-1) as shown in panel A. The location of HABfl-1 is indicated. (C) Growth curves of MUTU-I cells transfected with either control (pcDNA3HA [solid symbols]) or HABfl-1 expression vector (pcDNA3HABfl-1 [open symbols]). Cells from exponentially growing MUTU-I parent cultures transfected with these plasmids were seeded at 2 × 105 cells per ml of growth medium containing either 10% FBS (circles) or 0.1% FBS (triangles) and assessed at daily intervals for viable-cell numbers by trypan blue exclusion. (D) DNA fragmentation assay carried out on transfected MUTU-I cells from panel C above and cultured in medium containing either 10% FBS (lanes 1 and 3) or 0.1% FBS (lanes 2 and 4) for 72 h; lanes 1 and 2 contain cells transfected with control plasmid (pcDNA3HA), and lanes 3 and 4 contain cells transfected with pcDNA3HABfl-1.
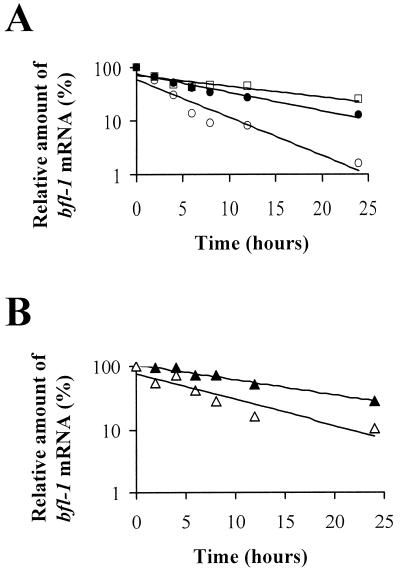
bfl-1 has already been classified as an immediate-early response gene (22, 30, 37). Since many early response genes are regulated at the level of mRNA stability, we investigated whether this was a factor in regulating bfl-1 expression in B cells with a latency group III phenotype and in response to LMP1 expression. The rate of decay of bfl-1 mRNA was monitored by Northern blotting after treatment of cells seeded at 106 cells per ml with an inhibitor of RNA synthesis, actinomycin D (5 μg/ml; Sigma). bfl-1 mRNA levels in treated cells were determined by densitometric analysis of autoradiograms after Northern blotting, and values were corrected for variations due to loading after estimation of the 18S rRNA levels in each lane (as determined by densitometric analysis of ethidium bromide-stained gels). Values thus obtained were expressed as a percentage of the bfl-1 mRNA level seen in untreated cells (taken as 100%). The half-life of bfl-1 mRNA was then determined from the best-fit semilogarithmic line of the graph of these values plotted against time (hours) of exposure to actinomycin D (Fig. 4). The half-life of bfl-1 mRNA in BL41 cells was found to be approximately 1 h, which was increased to 7.4 h in BL41-B95.8 cells and 5.2 h in the corresponding LCL IARC-171 (Fig. 4A). Under similar experimental conditions, the stability of bfl-1 mRNA in uninduced DG75-tTA-LMP1 cells was 4.25 h, and this subsequently increased to 11.5 h on induction of LMP1 (Fig. 4B). It is not clear why the stability of bfl-1 mRNA in uninduced DG75-tTA-LMP1 cells was higher than that in BL41 cells, although both are EBV-negative BL cell lines. It is possible that the rate of inhibition of RNA synthesis by actinomycin D differs between the two cell lines. Nevertheless, against a similar cellular background, EBV infection or LMP1 expression resulted in significant increases in the stability of bfl-1 mRNA. A major determinant in the stability of several mRNAs, including those from c-fos and c-myc, which are known to be controlled at this level, has been shown to reside in their 3′ untranslated regions. In particular, such genes contain few to several copies of an AUUUA motif which serve as binding sites for proteins that regulate mRNA stability (7). An examination of the 3′ untranslated region of the human bfl-1 gene failed to reveal the presence of an AUUUA motif and therefore excludes this element as a target for control of bfl-1 mRNA stability.
FIG. 4.
Stability of bfl-1 mRNA. The graphs show the best-fit semilogarithmic lines generated from the relative amounts of bfl-1 mRNA as determined from Northern blots of total RNA extracted at 0, 2, 4, 6, 8, 12, and 24 h after exposure of cells to actinomycin D and plotted against time of exposure to actinomycin D. All values were normalized for loading based on the intensity of the 18S rRNA band on the corresponding ethidium bromide-stained gels. (A) Relative levels of bfl-1 mRNA as determined in BL41 (○), BL41B95.8 (□), and IARC-171 (●). (B) Relative levels of bfl-1 transcript in uninduced DG75-tTA-LMP-1 (▵) and at 36 h after LMP1 induction 1 (▴).
The human bfl-1 gene was identified by computer analysis of expressed sequenced tag databases (8). Although bfl-1 has 72% sequence homology to its murine homolog, A1, currently available data suggest that they differ in their expression in tissues; while the expression of the murine gene is restricted to hematopoietic tissue, the expression of the human homolog would appear to have a more widespread distribution (22, 26, 30). Expression of the bfl-1 gene is upregulated in cultured endothelial and leukemic cells by phorbol ester and the inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1, suggesting a protective role for Bfl-1 during inflammation (22, 23, 37). bfl-1 expression is also upregulated during differentiation of leukemic cells to granulocytes and macrophages (37). bfl-1 encodes a 175-amino-acid protein that shares the highly conserved Bcl homology 1 (BH1), BH2, and BH3 domains with other members of the Bcl-2 family of proteins. Bfl-1 suppresses p53-mediated apoptosis, and it exhibits cell proliferation and transforming activities in vitro (10, 11). Furthermore, functional dissection of the protein suggests that its antiapoptotic and transforming activities may be linked (10). Northern analysis has revealed elevated levels of expression of bfl-1 in a significant proportion of stomach and colon cancers (8, 40); however, in situ hybridization studies have indicated that expression in tumor tissues is preferentially detected in infiltrating inflammatory cells rather than in cancer cells (21). This discrepancy may explain the low rate of expression of bfl-1 observed in established stomach and colon cancer cell lines (40). However, it appears that cell lines derived from leukemias and lymphomas exhibit high levels of expression of bfl-1 (8, 26). Our results extend this list to include EBV-positive BL cell lines exhibiting a group III phenotype and provide the first evidence for the control of expression of bfl-1 by a viral protein. Furthermore, the ability of LMP1 to regulate gene expression at the level of mRNA stability is also a novel finding. A 1.4-kb DNA sequence from the 5′ transcriptional regulatory region of the human bfl-1 gene has recently been cloned and demonstrated to be responsive to TNF-α in promoter-reporter assays (55). Our preliminary results indicate that LMP1 can also stimulate reporter gene expression driven from this promoter fragment by approximately three- to fivefold in transient-transfection assays of EBV-negative BL cell lines including DG75. Furthermore, coexpression of a dominant IκBα mutant (29) completely abolished trans-activation, suggesting that the LMP1-mediated increase in bfl-1 promoter activity is NF-κB dependent. It is interesting that the receptors for two cytokines, TNF-α and interleukin-1, that can regulate bfl-1 mRNA expression belong to a superfamily of proteins that includes LMP1 (5, 50). One common feature of members of this family is their ability to activate the transcription factor NF-κB. A role for NF-κB in mediating the upregulation of bfl-1 expression by TNF-α and lipopolysaccharide has been demonstrated (20, 55).
LMP1 upregulates the expression of several other antiapoptotic proteins such as Bcl-2, A20, and Mcl-1. In contrast to bcl-2, the induction of bfl-1 mRNA appears to be an immediate and direct effect of LMP1 function, as is the case with A20 (28). The observation that LMP1 regulates bcl-2 expression by both transcriptional and translational mechanisms is a novel finding. Indeed, there is evidence for the action of both of these mechanisms in the control of bcl-2 expression during normal B-cell development and differentiation. Changes in the rate of transcription regulate bcl-2 mRNA levels during B-cell development (48, 54). Also, in hematopoietic tissue in vivo, although Bcl-2 protein can be detected in the mantle zone but not in the germinal center, its mRNA can be detected in both of these regions, implying that translational mechanisms can also contribute to the regulation of bcl-2 expression (2, 9). In addition, a cis-acting element within the 5′ untranslated region of the bcl-2 gene is necessary for regulating bcl-2 expression at the level of translation (18). Elevated levels of several antiapoptotic proteins would increase the range of apoptotic stimuli against which the host cell can protect itself, since the overlap in the range of apoptotic stimuli to which different antiapoptotic proteins can respond is not always complete. For instance, although Bcl-2 and A20 can independently protect cells against a number of different apoptotic stimuli, Bcl-2 but not A20 is effective against glucocorticoid-induced apoptosis (39). In addition to protecting against p53-mediated apoptosis, Bfl-1 protects against apoptosis induced by serum deprivation and TNF-α-induced cytotoxicity (11, 23, 55). p53 is known to be an important regulator of apoptosis in BL cells. Wild-type p53 appears to function by arresting or slowing cell proliferation and induces apoptosis when introduced into BL cells carrying mutant p53 (43). The fact that LMP1 can upregulate all these three antiapoptotic proteins in the same cellular context questions the relative contribution made by these proteins in protecting against p53-mediated apoptosis. Additionally, in the case of Bfl-1, upregulation may involve the recruitment of both antiapoptotic and cell proliferation functions.
In lymphoid follicles, bfl-1 transcript has been detected in the germinal centers, which are the sites of B-cell proliferation and differentiation (21). Bcl-2 protein has the opposite expression pattern, in that it is absent from the proliferating Ki-67-positive germinal center B cells but detectable in the follicular mantle zone (41). The observation that Bfl-1 expression can protect MUTU-I cells from apoptosis lends further support to a role for Bfl-1 in promoting the survival of germinal center B cells during the process of antigen-driven selection, since such BL lines exhibit phenotypic features of germinal center B cells. Although resting B cells already express a high level of Bcl-2, which is only slightly elevated following infection with EBV (33), the Bfl-1 status of these cells before and after infection remains unknown and therefore merits investigation. Since mitogenic activation of lymphocytes in vitro results in decreased Bcl-2 expression (1), it is possible that depending on the type of B cell that the virus infects, the expression of LMP1 may serve to transiently upregulate or maintain Bfl-1 and Bcl-2 levels during the EBV-associated mitogenic stimulation that cells undergo upon infection and during the critical period prior to entry of the virus-infected cell into the long-lived memory B-cell pool. The induction of bfl-1 expression by LMP1 in B lymphocytes has implications for the biology of EBV. LMP1-mediated upregulation of bfl-1 expression may contribute to the survival of EBV-infected B cells, since cells similar to LCLs are present in the circulation during primary infection by EBV (42, 51). Additionally, the effect on bfl-1 may contribute to the development of EBV-associated B-cell malignancies such as posttransplantation lymphoproliferative disorders and BL tumor metastases in which LMP1 is expressed. Mechanistic studies of the contribution of Bfl-1 to cell survival will provide important information about both normal B-cell development and potential routes to B-cell and non-B-cell malignancy.
Acknowledgments
We thank N. Raab-Traub for the C33A cell line and its derivatives, G. Chinnadurai for the pcDNA3HABfl-1 construct, C. Gelinas for the bfl-1 promoter-reporter construct, and D. Mason for the anti-Bcl-2 antibodies used in this study. We are grateful to D. Kelleher, A. Long, and L. O'Neill for helpful discussions.
This research was supported by grants from the Irish Health Research Board (HRB), the Enterprise Ireland/British Council collaborative research scheme, and the Adhesion Molecule Research Unit of the HRB. B. D'Souza was the recipient of a postgraduate studentship from BioResearch Ireland.
REFERENCES
- 1.Aiello, Delia A D, Borrello M G, Biassoni D, Giardini R, Fontanella E, Pezzella F, Pulford K, Pierotti M, Della-Porta G. Flow cytometric detection of the mitochondrial Bcl-2 protein in normal and neoplastic human lymphoid cells. Cytometry. 1992;13:502–509. doi: 10.1002/cyto.990130509. [DOI] [PubMed] [Google Scholar]
- 2.Akagi T, Kondo E, Yoshino T. Expression of Bcl-2 protein and Bcl-2 mRNA in normal and neoplastic lymphoid tissues. Leuk Lymphoma. 1994;13:81–87. doi: 10.3109/10428199409051655. [DOI] [PubMed] [Google Scholar]
- 3.Allday M J. Apoptosis and Epstein-Barr virus in B cells. Epstein-Barr Virus Rep. 1996;3:55–65. [Google Scholar]
- 4.Baichwal V R, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988;2:461–467. [PubMed] [Google Scholar]
- 5.Baker S J, Reddy E P. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 6.Brattsand G, Cantrell D A, Ward S, Ivars F, Gulberg M. Signal transduction through the T-cell receptor-CD3 complex: evidence for heterogeneity in receptor coupling. J Immunol. 1990;144:3651–3658. [PubMed] [Google Scholar]
- 7.Chen C-Y A, Shyu A-B. AU-rich elements: characterisation and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 8.Choi S S, Park I C, Yun J W, Sung Y C, Hong S I, Shin H S. A novel Bcl-2 related gene, Bfl-1, is overexpressed in stomach cancer and preferentially expressed in bone marrow. Oncogene. 1995;11:1693–1698. [PubMed] [Google Scholar]
- 9.Chleq-Deschamps C M, LeBrun D P, Huie P, Besnier D P, Warnke R A, Sibley R K, Cleary M L. Topographical dissociation of Bcl-2 messenger RNA and protein expression in human lymphoid tissues. Blood. 1993;81:293–298. [PubMed] [Google Scholar]
- 10.D'Sa-Eipper C, Chinnadurai G. Functional dissection of bfl-1, a bcl-2 homolog: anti-apoptosis, oncogene cooperation and cell proliferation activities. Oncogene. 1998;16:3105–3114. doi: 10.1038/sj.onc.1201851. [DOI] [PubMed] [Google Scholar]
- 11.D'Sa-Eipper C, Subramanian T, Chinnadurai G. bfl-1, a bcl-2 homologue, suppresses p53-induced apoptosis and exhibits potent cooperative transforming activity. Cancer Res. 1996;56:3879–3882. [PubMed] [Google Scholar]
- 12.Farrell P. Epstein-Barr virus immortalizing genes. Trends Microbiol. 1995;3:105–109. doi: 10.1016/s0966-842x(00)88891-5. [DOI] [PubMed] [Google Scholar]
- 13.Finke J, Fritzen R, Ternes P, Trivedi P, Bross K J, Lange W, Mertelsman R, Dolken G. Expression of bcl-2 in Burkitt's lymphoma cell lines: induction by latent Epstein-Barr virus genes. Blood. 1992;80:459–469. [PubMed] [Google Scholar]
- 14.Floettmann J E, Ward K, Rickinson A B, Rowe M. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B cell lines. Virology. 1996;223:29–40. doi: 10.1006/viro.1996.0452. [DOI] [PubMed] [Google Scholar]
- 15.Fries K L, Miller W E, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory C D, Rowe M, Rickinson A B. Different Epstein-Barr virus–B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 17.Gregory C D, Dive C, Henderson S, Smith C A, Williams G T, Gordon J, Rickinson A B. Activation of Epstein-Barr virus latent gene products protects human B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- 18.Harigai M, Miyashita T, Hanada M, Reed J C. A cis-acting element in the bcl-2 gene controls expression through translational mechanisms. Oncogene. 1996;12:1369–1374. [PubMed] [Google Scholar]
- 19.Henderson S, Rowe M, Gregory C D, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A B. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Yee E, Harlan J M, Wong F, Karsan A. Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood. 1998;92:2759–2765. [PubMed] [Google Scholar]
- 21.Jung-Ha H, Kim D, Lee S-B, Hong S-I, Park S-Y, Huh J, Kim C-W, Kim S-S, Lee Y, Choi S S, Shin H-S. Expression of Bfl-1 in normal and tumour tissues: Bfl-1 overexpression in cancer is attributable to its preferential expression in infiltrating inflammatory cells. Hum Pathol. 1998;29:723–728. doi: 10.1016/s0046-8177(98)90282-9. [DOI] [PubMed] [Google Scholar]
- 22.Karsan A, Yee E, Kaushansky K, Harlan J M. Cloning of a human Bcl-2 homologue: inflammatory cytokines induce human A1, in cultured endothelial cells. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- 23.Karsan A, Yee E, Harlan J M. Endothelial cell death induced by tumour necrosis factor-α is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996;271:27201–27204. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- 24.Kaye K M, Izumi K M, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keiff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 26.Kenny J J, Knobloch T J, Augustus M, Carter K C, Rosen C A, Lang J C. GRS, a novel member of the Bcl-2 gene family, is highly expressed in multiple cancer cell lines and in normal leukocytes. Oncogene. 1997;14:997–1001. doi: 10.1038/sj.onc.1200898. [DOI] [PubMed] [Google Scholar]
- 27.Klein G. Epstein-Barr virus strategy in normal and neoplastic B cells. Cell. 1994;77:791–793. doi: 10.1016/0092-8674(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 28.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP-1 gene product induces A20 zinc finger protein expression by activating nuclear factor-κB. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 29.Liljeholm S, Hughes K, Grundstorm T, Brodin P. NF-kappaB only partially mediates Epstein-Barr virus latent membrane protein 1 activation of B cells. J Gen Virol. 1998;79:2117–2125. doi: 10.1099/0022-1317-79-9-2117. [DOI] [PubMed] [Google Scholar]
- 30.Lin E Y, Orlofsky A, Berger M S, Prystowsky M B. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol. 1996;151:1979–1988. [PubMed] [Google Scholar]
- 31.Liu Y-J, Mason D Y, Johnson G D, Abbot S, Gregory C D, Hardie D L, Gordon J, MacLennan I C M. Germinal center cells express Bcl-2 protein after activation by signals which prevent entry to apoptosis. Eur J Immunol. 1991;21:1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- 32.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 33.Martin J M, Veis D, Korsmeyer S J, Sugden B. Latent membrane protein of Epstein-Barr virus induces cellular phenotypes independently of expression of Bcl 2. J Virol. 1993;67:5269–5278. doi: 10.1128/jvi.67.9.5269-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massuci M G, Contreras-Salazar B, Ragnar E, Falk K, Minarovits J, Ernberg I, Klein G. 5-azacytidine upregulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt's lymphoma cell line Rael. J Virol. 1989;63:3135–3141. doi: 10.1128/jvi.63.7.3135-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller W E, Earp H S, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of epidermal growth factor receptor. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milner A E, Johnson G D, Gregory C D. Prevention of programmed cell death in Burkitt lymphoma cell lines by bcl-2-dependent and -independent mechanisms. Int J Cancer. 1992;52:636–644. doi: 10.1002/ijc.2910520424. [DOI] [PubMed] [Google Scholar]
- 37.Moreb J S, Schweder M. Human A1, a bcl-2-related gene, is induced in leukemic cells by cytokines as well as differentiating factors. Leukemia. 1997;11:998–1004. doi: 10.1038/sj.leu.2400719. [DOI] [PubMed] [Google Scholar]
- 38.Okan I, Wang Y, Chen F, Imreh S, Klein G, Wilman C G. The EBV-encoded LMP1 protein inhibits p53-triggered apoptosis but not growth arrest. Oncogene. 1995;11:1027–1031. [PubMed] [Google Scholar]
- 39.Opipari A W, Hu H M, Yabkovitz R, Dixit V. The A20 zinc finger protein protects cells from tumour necrosis factor cytotoxicity. J Biol Chem. 1992;267:12424–12427. [PubMed] [Google Scholar]
- 40.Park I-C, Lee S H, Whang D Y, Hong W S, Choi S S, Shin H S, Choe T-B, Hong S I. Expression of a novel bcl-2 related gene, bfl-1, in various human cancers and cancer cell lines. Anticancer Res. 1997;17:4619–4622. [PubMed] [Google Scholar]
- 41.Pezzella F, Tse A G D, Cordell J L, Pulford K A F, Gatter K C, Mason D Y. Expression of the bcl-2 oncogene protein is not specific for the 14:18 chromosomal translocation. Am J Pathol. 1990;137:225–232. [PMC free article] [PubMed] [Google Scholar]
- 42.Qu L, Rowe M. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramqvist T, Magnusson K P, Wang Y S, Szekely L, Klein G, Wiman K G. Wild-type p53 induces apoptosis in a Burkitt lymphoma (BL) line that carries mutant p53. Oncogene. 1993;8:1495–1500. [PubMed] [Google Scholar]
- 44.Rowe M, Evans H S, Young L S, Hennessy K, Kieff E, Rickinson A B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987;68:1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- 45.Rowe M, Khanna R, Jacob C A, Argaet V, Kelly A, Powis S, Belich M, Croom Carter D, Lee S, Burrows S R, Trowsdale J, Moss D J, Rickinson A B. Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein-Barr virus latent membrane protein-1: coordinate upregulation of peptide transporters and HLA-classI antigen expression. Eur J Immunol. 1995;25:1374–1384. doi: 10.1002/eji.1830250536. [DOI] [PubMed] [Google Scholar]
- 46.Rowe M, Peng-Pilon M, Huen D S, Hardy R, Croom-Carter D, Lundgren E, Rickinson A B. Upregulation of bcl-2 by Epstein-Barr virus latent membrane protein LMP1: a B-cell-specific response that is delayed relative to NF-kappa B activation and to induction of cell surface markers. J Virol. 1994;68:5602–5612. doi: 10.1128/jvi.68.9.5602-5612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowe M, Rooney C M, Edwards C F, Lenoir G M, Rickinson A B. Epstein-Barr virus status and tumour cell phenotype in sporadic Burkitt's lymphoma. Int J Cancer. 1986;37:367–373. doi: 10.1002/ijc.2910370307. [DOI] [PubMed] [Google Scholar]
- 48.Seto M, Jaeger U, Hockett R D, Graninger W, Bennett S, Goldman P, Korsmeyer S J. Alternative promoters and exons, somatic mutation and deregulation of the bcl2-Ig fusion gene in lymphoma. EMBO J. 1988;7:123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silins S L, Sculley T B. Burkitt's lymphoma cells are resistant to programmed cell death in the presence of the Epstein-Barr virus latent antigen EBNA-4. Int J Cancer. 1995;60:65–72. doi: 10.1002/ijc.2910600110. [DOI] [PubMed] [Google Scholar]
- 50.Tewari M, Dixit V M. Recent advances in tumour necrosis factor and CD40 signaling. Current Opin Genet Dev. 1996;6:39–44. doi: 10.1016/s0959-437x(96)90008-8. [DOI] [PubMed] [Google Scholar]
- 51.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D, Leibowitz D, Kieff E. An EBV membrane protein expressed in immortalised lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Rowe M, Lundgren E. Expression of the Epstein-Barr virus transforming protein LMP1 causes a rapid and transient stimulation of the bcl-2 homologue mcl-1 levels in B cell lines. Cancer Res. 1996;56:4610–4613. [PubMed] [Google Scholar]
- 54.Young R L, Korsmeyer S J. A negative regulatory element in the bcl-2 5′-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol. 1993;13:3686–3697. doi: 10.1128/mcb.13.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zong W-X, Edelstein L C, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNF-α-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]