Abstract
BACKGROUND:
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions are rare oncogenic drivers prevalent in 0.3% of solid tumors. They are most common in salivary gland cancer (2.6%), thyroid cancer (1.6%), and soft-tissue sarcoma (1.5%). Currently, there are 2 US Food and Drug Administration–approved targeted therapies for NTRK gene fusions: larotrectinib, approved in 2018, and entrectinib, approved in 2019. To date, the real-world uptake of tyrosine receptor kinase inhibitor (TRKi) use for NTRK-positive solid tumors in academic cancer centers remains largely unknown.
OBJECTIVE:
To describe the demographics, clinical and genomic characteristics, and testing and treatment patterns of patients with NTRK-positive solid tumors treated at US academic cancer centers.
METHODS:
This was a retrospective chart review study conducted in academic cancer centers in the United States. All patients diagnosed with an NTRK fusion–positive (NTRK1, NTRK2, NTRK3) solid tumor (any stage) and who received cancer treatment at participating sites between January 1, 2012, and July 1, 2023, were included in this study. Patient demographics, clinical characteristics, genomic characteristics, NTRK testing data, and treatment patterns were collected from electronic medical records and analyzed using descriptive statistics as appropriate.
RESULTS:
In total, 6 centers contributed data for 55 patients with NTRK-positive tumors. The mean age was 49.3 (SD = 20.5) years, 51% patients were female, and the majority were White (78%). The median duration of time from cancer diagnosis to NTRK testing was 85 days (IQR = 44-978). At the time of NTRK testing, 64% of patients had stage IV disease, compared with 33% at cancer diagnosis. Prevalent cancer types in the overall cohort included head and neck (15%), thyroid (15%), brain (13%), lung (13%), and colorectal (11%). NTRK1 fusions were most common (45%), followed by NTRK3 (40%) and NTRK2 (15%). Across all lines of therapy, 51% of patients (n = 28) received a TRKi. Among TRKi-treated patients, 71% had stage IV disease at TRKi initiation. The median time from positive NTRK test to initiation of TRKi was 48 days (IQR = 9-207). TRKis were commonly given as first-line (30%) or second-line (48%) therapies. Median duration of therapy was 610 (IQR = 182-764) days for TRKi use and 207.5 (IQR = 42-539) days for all other first-line therapies.
CONCLUSIONS:
This study reports on contemporary real-world NTRK testing patterns and use of TRKis in solid tumors, including time between NTRK testing and initiation of TRKi therapy and duration of TRKi therapy.
Plain language summary
This study examined patients with cancer that have a rare gene mutation, called neurotrophic tyrosine receptor kinase (NTRK) gene fusions, and how they are treated. Approximately half of these patients received a type of medication called a tyrosine receptor kinase inhibitor (TRKi). TRKis target and block the abnormal growth of cells that have NTRK gene fusion mutations. This study describes the cancer characteristics and treatment characteristics of patients who were treated with a TRKi.
Implications for managed care pharmacy
Following the approval of TRKis (eg, larotrectinib and entrectinib), there was a shift away from systemic chemotherapy to targeted therapy for first-line treatment of NTRK fusion–positive solid tumors. For payers, understanding this evolving treatment landscape is crucial for effective formulary management and resource allocation to optimize patient outcomes.
Chromosomal translocations or rearrangements are well-known drivers in hematologic and solid malignancies.1-4 Under normal conditions, tyrosine receptor kinase (TRK) plays an important role in neural development. Fusion of the neurotrophic tyrosine receptor kinase (NTRK) genes promotes oncogenic signaling through downstream signaling pathways.1-4 The prevalence of NTRK fusions is generally low across both adult (0.31%) and pediatric (0.34%) solid tumors.5,6 However, the incidence of NTRK fusions varies widely across specific cancers, including particularly high rates in infantile fibrosarcoma, secretory carcinoma of the salivary gland, and the secretory type of breast cancer (>80%).5 Few treatment options are available for many of these solid tumor types.7
TRK inhibitors (TRKis) are effective for treating advanced solid tumors among patients with an NTRK gene fusion.8 Larotrectinib was the first TRKi to receive US Food and Drug Administration (FDA) approval in November 2018 for the treatment of advanced solid tumors with an NTRK gene fusion, followed by entrectinib in 2019.9 Larotrectinib is indicated for adult and pediatric patients with metastatic disease, for patients who are nonsurgical candidates, or for those who have progressed following other lines of therapy.9 Results from a pooled analysis of three phase 1-2 clinical trials demonstrated an investigator-assessed objective response rate of 79% with a partial response rate of 63% and complete response rate of 16% among NTRK fusion–positive patients treated with larotrectinib (n = 153).8 Similar outcomes have been observed with entrectinib, with 45.5% of patients showing a partial response and 15.7% with a complete response from phase 1/2 trials (n = 121).10 Given the efficacy of TRKis, identification and incorporation of TRKis into real-world practice is important for the utility of these therapies and subsequent clinical benefit of affected patients.
Several studies have characterized patients with NTRK fusion–positive solid tumors in the real-world setting. A retrospective study within the Veterans Affairs database characterized genomic markers, clinical characteristics, and outcomes within a population of TRKi-treated patients (n = 12); another study used a clinicogenomic database within the United States to describe characteristics and treatment outcomes among patients with NTRK fusion–positive solid tumors treated with standard-of-care (non-TRKi) therapies (n = 28).11,12 A retrospective study among patients with NTRK fusion–positive solid tumors treated by 19 community oncologists across the United States described real-world treatment patterns for the 110 included patients.13 Existing data within academic institutions have been limited to single-center studies.14,15 To date, no multicenter studies have assessed the real-world treatment patterns of patients with NTRK fusion–positive cancers treated within academic cancer centers in the United States. To address this gap, this multisite cohort study used patient-level health records to characterize clinical and cancer-gene factors associated with NTRK fusion positivity. Further, this study aimed to characterize the impact of TRKi approval on the therapeutic landscape of NTRK fusion–positive cancers by comparing treatment patterns before first TRKi approval (November 2018) and after. Understanding the real-world profile of patients with NTRK fusion–positive cancer and utilization of TRKis at cancer centers in the United States will help to (1) identify the treatment gap that TRKis can address and (2) delineate clinical factors that guide the use of TRKis.
Methods
STUDY DESIGN
This was a retrospective observational study conducted at 6 academic cancer centers in the United States.
STUDY POPULATION
Patients of all ages, diagnosed with an NTRK fusion–positive solid tumor at any stage, who received cancer-related treatment at participating sites between January 1, 2012, and July 1, 2023, were included in the study. Patients receiving an investigational TRKi under clinical trial protocol were excluded. NTRK fusion positivity was determined by positive next-generation sequencing, reverse transcription polymerase chain reaction, or fluorescence in situ hybridization.
DATA COLLECTION
Six academic cancer centers participated in this study: Mayo Clinic, University of California Los Angeles Jonsson Comprehensive Cancer Center, Moffitt Cancer Center, Huntsman Cancer Institute, Medical College of Wisconsin, and Rutgers Cancer Institute of New Jersey. Prior to data collection, institutional review board approval of the study protocol at each participating site was required. Data were collected via electronic health records (EHRs), pharmacy records, and cancer registries from each participating institution using a standardized case report form (CRF) developed by University of Utah Health (UHealth) researchers. Chart abstractors at each site were trained on CRF and data collection by UHealth study personnel to ensure consistency. Deidentified data were transferred from participating sites to UHealth study personnel for quality control. UHealth maintained aggregate deidentified data from all participating sites to facilitate data analysis.
DATA ANALYSIS
Study results were analyzed with descriptive statistics, using counts and percentages, and medians and IQRs. Demographics, clinical characteristics, and genetic characteristics were stratified by exposure to TRKi. Treatment patterns were evaluated by line of therapy and were stratified by FDA approval of larotrectinib (November 2018) into a pre-TRKi and post-TRKi approval period to characterize the impact of TRKis on treatment of NTRK fusion–positive solid tumors. Duration of therapy was assessed using Kaplan-Meier methodology; patients who were still on therapy at final follow-up were censored.
Results
STUDY COHORT
In total, 55 patients were included across all participating sites between January 1, 2012, and July 1, 2023.
DEMOGRAPHICS AND CLINICAL CHARACTERISTICS
Among the 55 included patients, the mean age at cancer diagnosis was 49.3 (SD = 20.5) years, and 51% (n = 28) of patients were female (Table 1). The majority of patients were White at 78% (n = 43), followed by 4% (n = 2) Black and 11% (n = 6) unknown; 16% (n = 9) of patients reported Hispanic ethnicity. Most patients had commercial insurance coverage (58%, n = 32) at cancer diagnosis, followed by Medicare (24%) and Medicaid (5%). Diabetes and chronic pulmonary disease were the most prevalent comorbidities at 11% and 7%, respectively.
TABLE 1.
Baseline Demographics and Clinical Characteristics
| Variable | Non-TRKi (n = 27) | TRKi (n = 28) | All (N = 55) |
|---|---|---|---|
| Mean age (SD), years | 49.6 (18.8) | 49.0 (22.5) | 49.3 (20.5) |
| Age group, years, n (%) | |||
| 18-30 | 5 (19) | 8 (29) | 13 (24) |
| 31-45 | 6 (22) | 2 (7) | 8 (15) |
| 46-64 | 12 (44) | 11 (39) | 23 (42) |
| 65-79 | 2 (7) | 6 (21) | 8 (15) |
| ≥80 | 7 (26) | 1 (4) | 8 (15) |
| Sex, n (%) | |||
| Female | 14 (52) | 14 (50) | 28 (51) |
| Male | 13 (48) | 14 (50) | 27 (49) |
| Ethnicity, n (%) | |||
| Hispanic | 4 (15) | 5 (18) | 9 (16) |
| Non-Hispanic | 23 (85) | 23 (82) | 46 (84) |
| Race, n (%) | |||
| White | 24 (89) | 19 (68) | 43 (78) |
| Black | 0 (0) | 2 (7) | 2 (4) |
| Other | 2 (7) | 4 (14) | 6 (11) |
| Unknown | 1 (4) | 1 (4) | 2 (4) |
| Plan type at diagnosis, n (%)a | |||
| Commercial | 19 (70) | 13 (46) | 32 (58) |
| Medicaid | 1 (4) | 2 (7) | 3 (5) |
| Medicare | 3 (11) | 10 (36) | 13 (24) |
| Uninsured/Self-pay | 0 (0) | 1 (4) | 1 (2) |
| Other | 2 (7) | 2 (7) | 4 (7) |
| Unknown | 2 (7) | 1 (4) | 3 (5) |
| Comorbidities, n (%) | |||
| Myocardial infarction | 1 (4) | 0 (0) | 1 (2) |
| Congestive heart failure | 1 (4) | 1 (4) | 2 (4) |
| Chronic pulmonary disease | 2 (7) | 2 (7) | 4 (7) |
| Dementia | 1 (4) | 0 (0) | 1 (2) |
| Diabetes without chronic complications | 5 (19) | 1 (4) | 6 (11) |
| Hemiplegia or paraplegia | 0 (0) | 1 (4) | 1 (2) |
| Renal disease | 1 (4) | 1 (4) | 2 (4) |
| Mild liver disease | 0 (0) | 2 (7) | 2 (4) |
| Peptic ulcer disease | 1 (4) | 0 (0) | 1 (2) |
| Rheumatologic disease | 1 (4) | 0 (0) | 1 (2) |
| None | 18 (67) | 21 (75) | 39 (71) |
a Plan types were also collected at NTRK testing and TRKi initiation and were similar across all timepoints.
NTRK = neurotrophic tyrosine receptor kinase; TRKi = tyrosine receptor kinase inhibitor.
Approximately half the patients (51%, n = 28) were treated with a TRKi (TRKi cohort) during follow-up, and 49% (n = 27) received no TRKi (non-TRKi cohort). There was no TRKi use in the preapproval time period. In the non-TRKi cohort, the mean age was 49.6 (SD = 18.8) years, 52% were female, and 89% were White. In the TRKi cohort, the mean age was 49 (SD = 22.5) years, 50% were female, and 68% were White. At diagnosis, commercial health plans were the most common in both the non-TRKi (70%) and TRKi (46%) cohorts.
CANCER CHARACTERISTICS
In the non-TRKi group, colorectal was the most prevalent cancer type at 15% (n = 4) (Table 2). Common cancers observed in the TRKi cohort were brain, salivary, lung, and thyroid (18% each, n = 5). Cancer staging data were collected at initial cancer diagnosis, NTRK testing, and TRKi initiation (if applicable). Across all patients, 33% (n = 18) had stage IV cancer at diagnosis; at the time of NTRK testing, this proportion almost doubled to 64% (n = 35). Within the TRKi cohort, 71% (n = 20) had stage IV cancer at initiation of TRKi. Lung or liver metastases were observed in 19% and 15% of patients in the non-TRKi cohort and 36% and 29% of patients in the TRKi cohort, respectively.
TABLE 2.
Cancer Characteristics
| Non-TRKi (n = 27) | TRKi (n = 28) | All (N = 55) | |
|---|---|---|---|
| Cancer type, n (%) | |||
| Brain | 2 (7) | 5 (18) | 7 (13) |
| Astrocytoma | 1 (50) | 0 (0) | 1 (14) |
| Glioblastoma multiforme | 0 (0) | 5 (100) | 5 (71) |
| Oligodendroglioma | 1 (50) | 0 (0) | 1 (14) |
| Breast, ductal | 3 (11) | 0 (0) | 3 (5) |
| Colorectal, adenocarcinoma | 4 (15) | 2 (7) | 6 (11) |
| Endometrial, adenocarcinoma/endometrioid | 0 (0) | 1 (4) | 1 (2) |
| Gastric, adenocarcinoma | 1 (4) | 0 (0) | 1 (2) |
| Salivary gland | 3 (11) | 5 (18) | 8 (15) |
| Salivary gland | 1 (33) | 4 (80) | 5 (62) |
| Acinic cell carcinoma | 0 (0) | 2 (50) | 2 (40) |
| Mammary analogue secretory carcinoma | 0 (0) | 1 (25) | 1 (20) |
| Secretory carcinoma | 1 (100) | 0 (0) | 1 (20) |
| Unknown | 0 (0) | 1 (25) | 1 (20) |
| Parotid Gland | 2 (67) | 0 (0) | 2 (25) |
| Unknown | 0 (0) | 1 (20) | 1 (12) |
| Lung, non–small cell | 2 (7) | 5 (18) | 7 (13) |
| Adenocarcinoma | 1 (50) | 3 (60) | 4 (57) |
| Large-cell carcinoma | 1 (50) | 0 (0) | 1 (14) |
| Squamous cell carcinoma | 0 (0) | 1 (20) | 1 (14) |
| Poorly differentiated NSCLC NOS | 0 (0) | 1 (40) | 1 (14) |
| Melanoma | 3 (11) | 0 (0) | 3 (5) |
| Superficial spreading | 1 (33) | 0 (0) | 1 (33) |
| Favor atypical spitz tumor | 1 (33) | 0 (0) | 1 (33) |
| Unknown | 1 (33) | 0 (0) | 1 (33) |
| Ovarian | 0 (0) | 2 (7) | 2 (4) |
| Epithelial cancer | 0 (0) | 1 (50) | 1 (50) |
| Unknown | 0 (0) | 1 (50) | 1 (50) |
| Pancreatic | 1 (4) | 0 (0) | 1 (2) |
| Prostate, adenocarcinoma | 1 (4) | 0 (0) | 1 (2) |
| Sarcoma, soft-tissue | 3 (11) | 2 (7) | 5 (9) |
| Liposarcoma | 1 (33) | 0 (0) | 1 (20) |
| Malignant peripheral nerve sheath tumor | 1 (33) | 0 (0) | 1 (25) |
| Infantile fibrosarcoma | 0 (0) | 1 (50) | 1 (25) |
| Mullerian adenosarcoma | 0 (0) | 1 (50) | 1 (25) |
| Uterine leiomyosarcoma | 1 (33) | 0 (0) | 1 (25) |
| Thyroid | 3 (11) | 5 (18) | 8 (15) |
| Papillary | 3 (100) | 4 (80) | 7 (88) |
| Unknown | 0 (0) | 1 (20) | 1 (12) |
| Other | 1 (4) | 1 (4) | 2 (4) |
| Adnexal adenocarcinoma of skin | 1 (100) | 0 (0) | 1 (50) |
| Neuroblastoma | 0 (0) | 1 (100) | 1 (50) |
| Stage at cancer diagnosis, n (%) | |||
| I | 8 (30) | 5 (19) | 13 (24) |
| II | 4 (15) | 3 (11) | 7 (13) |
| III | 6 (22) | 5 (19) | 11 (20) |
| IV | 7 (26) | 11 (41) | 18 (33) |
| Unknown | 2 (7) | 3 (11) | 5 (9) |
| Stage at NTRK testing, n (%) | |||
| I | 4 (15) | 1 (4) | 5 (9) |
| II | 3 (11) | 1 (4) | 4 (7) |
| III | 4 (15) | 4 (14) | 8 (15) |
| IV | 16 (59) | 19 (68) | 35 (64) |
| Unknown | 0 (0) | 3 (11) | 3 (5) |
| Stage at TRKi initiation, n (%) | |||
| I | NA | 1 (4) | NA |
| II | NA | 1 (4) | NA |
| III | NA | 3 (11) | NA |
| IV | NA | 20 (71) | NA |
| Unknown | NA | 3 (11) | NA |
| ECOG performance score at initial cancer diagnosis, n (%) | |||
| 0 | 13 (48) | 14 (50) | 27 (49) |
| 1 | 6 (22) | 9 (32) | 15 (27) |
| 2 | 2 (7) | 2 (7) | 4 (7) |
| Unknown | 6 (22) | 3 (11) | 9 (16) |
| Site of metastases, n (%) | |||
| Lung | 5 (19) | 10 (36) | 15 (27) |
| Liver | 4 (15) | 8 (29) | 12 (22) |
| Brain | 5 (19) | 4 (14) | 9 (16) |
| Bone | 5 (19) | 7 (25) | 12 (22) |
| Other | 9 (33) | 9 (32) | 18 (33) |
| None | 9 (33) | 6 (21) | 15 (27) |
ECOG = Eastern Cooperative Oncology Group; NSCLC NOS = non–small cell lung cancer not otherwise specified; NTRK = neurotrophic tyrosine receptor kinase; TRKi = tyrosine receptor kinase inhibitor.
NTRK TESTING
The median time from cancer diagnosis to sample collection used in NTRK testing was 27 days (0-497) for the overall cohort (n = 55). Median time from cancer diagnosis to actual NTRK testing was 85 days (44-978) (Figure 1). Among patients who received first-line (1L) therapy (n = 46), 43% received NTRK testing prior to initiating 1L therapy.
FIGURE 1.
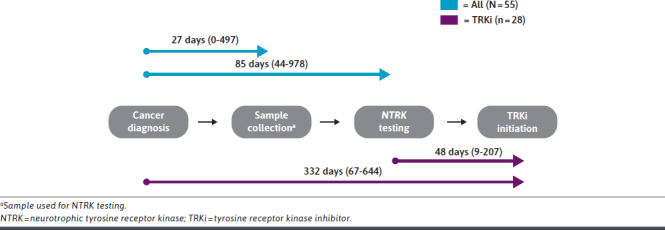
Timing of NTRK Testing
Next-generation sequencing was the primary modality for NTRK testing (n = 54, 98%) (Table 3).
TABLE 3.
NTRK Testing
| Variable | Non-TRKi (n = 27) | TRKi (n = 28) | All (N = 55) |
|---|---|---|---|
| NTRK test type, n (%) | |||
| NGS | 27 (100) | 27 (96) | 54 (98) |
| Caris Life Science | 0 (0) | 7 (26) | 7 (13) |
| MI Tumor Profile | 0 (0) | 4 (67) | 4 (67) |
| MI Intelligence Profile | 0 (0) | 1 (17) | 1 (17) |
| Unknown | 0 (0) | 1 (17) | 1 (17) |
| Foundation Medicine | 13 (48) | 5 (19) | 18 (33) |
| FoundationOne CDx | 8 (62) | 6 (86) | 14 (70) |
| FoundationOne Heme | 3 (23) | 1 (14) | 4 (20) |
| FoundationOne NOS | 2 (15) | 0 (0) | 2 (10) |
| NeoGenomics Laboratories | 1 (4) | 1 (4) | 2 (4) |
| Tempus | 7 (26) | 6 (22) | 13 (24) |
| Xt Targeted Panel | 6 (86) | 6 (86) | 12 (86) |
| Tempus xF | 1 (14) | 1 (100) | 2 (100) |
| Other | 6 (22) | 7 (26) | 13 (24) |
| RT-PCR | 0 (0) | 1 (4) | 1 (2) |
| FISH | 0 (0) | 2 (7) | 2 (4) |
| NTRK fusion type, n (%)a | |||
| NTRK1 | 14 (52) | 11 (39) | 25 (45) |
| NTRK2 | 5 (19) | 3 (11) | 8 (15) |
| NTRK3 | 9 (33) | 13 (46) | 22 (40) |
| NTRK1 fusion gene partner, n (%) | |||
| TPM3 | 5 (36) | 2 (20) | 7 (29) |
| LMNA | 4 (29) | 0 (0) | 4 (17) |
| TPR | 0 (0) | 2 (20) | 2 (8) |
| TP53 | 1 (7) | 0 (0) | 1 (4) |
| Other | 3 (21) | 4 (40) | 7 (29) |
| Unknown | 1 (7) | 2 (20) | 3 (12) |
| NTRK2 fusion gene partner, n (%) | |||
| KCTD16 | 1 (20) | 1 (33) | 2 (25) |
| Other | 3 (60) | 2 (67) | 5 (62) |
| Unknown | 1 (20) | 0 (0) | 1 (12) |
| NTRK3 fusion gene partner, n (%) | |||
| ETV6 | 8 (89) | 6 (46) | 14 (64) |
| EML4 | 0 (0) | 3 (23) | 3 (14) |
| EML3 | 0 (0) | 1 (8) | 1 (5) |
| Other | 1 (11) | 2 (15) | 3 (14) |
| Unknown | 0 (0) | 1 (8) | 1 (5) |
FISH = fluorescence in situ hybridization; NGS = next-generation sequencing; NTRK = neurotrophic tyrosine receptor kinase; RT-PCR = reverse transcription polymerase chain reaction; TRKi = tyrosine receptor kinase inhibitor.
The most common NTRK fusion type was NTRK1 at 45% (n = 25), followed by 40% NTRK3 (n = 22) and 15% NTRK2 (n = 8). The most common gene fusion partner was TPM3 at 29% (n = 7) for NTRK1 fusions, 25% KCTD16 (n = 2) for NTRK2, and 64% ETV6 (n = 14) for NTRK3.
The most frequent genetic alterations among patients with NTRK fusion–positive solid tumors were TP53 (24%), CDKN2A (15%), NTRK1 (15%), ARID1A (13%), and PTEN (13%) (Table 3).
TREATMENT PATTERNS
Prior to FDA approval of the first TRKi in 2018 (pre-TRKi period), pharmacologic treatment of NTRK fusion–positive solid tumors was primarily systemic chemotherapy in this cohort of patients (1L: 75% [n = 12], 2L: 60% [n = 6], and 3L: 60% [n = 3]) (Figure 2; Supplementary Table 4 (275.1KB, pdf) , available in online article). In the 1L setting, additional therapies used included other targeted therapies (19%, n = 3) and immunotherapy (6%, n = 1).
FIGURE 2.
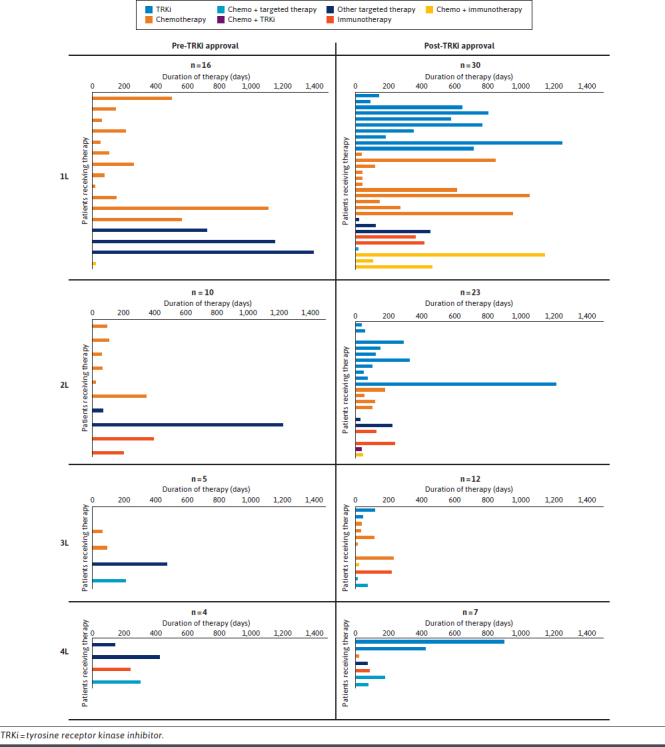
Treatment Patterns
Following TRKi approval (post-TRKi period), utilization of chemotherapy in the 1L setting was 33% (n = 10), 2L: 22% (n = 5), and 3L: 50% (n = 6) (Figure 2; Supplementary Table 4 (275.1KB, pdf) ). In the post-TRKi period, TRKi utilization was 30% in the 1L setting (n = 9), 2L: 48% (n = 11), and 3L: 17% (n = 2). Median duration of therapy (DoT) for TRKis was 610 days (IQR = 182-764) in the 1L setting, 103 days (47-292) in the 2L, and 79 days (44-114) in the 3L. Median DoT for all other therapies in the post-TRKi period was 207.5 (42-539) in the 1L setting, 101 (28-178) in the 2L, and 33 (12-112) in the 3L. TRKi utilization was also seen in the 4L (29%, n = 2), 5L (33%, n = 1), and 7L (100%, n = 1) settings. Additional 1L therapies included other targeted therapies (10%, n = 3), immunotherapy (10%, n = 3), chemotherapy + immunotherapy (10%, n = 3), and chemotherapy + other targeted therapy (3%, n = 1).
Baseline demographics and cancer characteristics among patients receiving 1L therapy stratified by pre- and post-TRKi approval are shown in Supplementary Tables 5 and 6 (275.1KB, pdf) , respectively. Of the patients receiving TRKis in the postapproval period (n = 10), the mean age was 49.8 (SD = 25) years and 20% were female, compared with 55.6 (20.3) years and 60% female among patients receiving other therapies (n = 20) (Supplementary Table 5 (275.1KB, pdf) ). TRKi-treated patients all had salivary gland (n = 4), thyroid (n = 3), lung (n = 2), or soft-tissue sarcoma (n = 1) cancers (Supplementary Table 6 (275.1KB, pdf) ).
The median time from cancer diagnosis to TRKi initiation was 332 days (IQR = 67-644). The median time from NTRK testing to TRKi initiation was 48 days (IQR = 9-207) (Figure 1).
At least 1 cycle of radiation therapy was received by 100% of patients (n = 27) in the non-TRKi cohort (Supplementary Table 2 (275.1KB, pdf) ). Among patients in the TRKi group (n = 28), 43% received at least 1 cycle of radiation.
Discussion
This retrospective cohort study conducted across several US academic cancer centers sheds light on the demographic, clinical, and genomic characteristics and treatment patterns of patients with NTRK fusion–positive solid tumors in the real-world practice setting.
This study observed real-world uptake of TRKis in the treatment of patients with NTRK fusion–positive solid tumors following the FDA approval of larotrectinib and entrectinib. Before TRKi approval, chemotherapy was the primary treatment modality for NTRK fusion–positive solid tumors, particularly in the 1L (75%) and 2L (60%) settings. After TRKi approval, utilization of chemotherapy in the 1L and 2L settings was 33% and 22%, and TRKi utilization was 30% and 48%, respectively. The uptake of TRKis in clinical practice likely reflects the limited treatment options available to patients prior to FDA approval of larotrectinib, as well as changes in provider practice given the favorable outcomes and improved adverse effect profile (compared with chemotherapy) of TRKis observed in the literature.16
Median DoT in the 1L setting was 610 days (IQR = 182-764) among the TRKi cohort and 207.5 days (IQR = 42-539) among the non-TRKi cohort. When converted to months, median DoT was 20.1 (IQR = 6-25.1) for the TRKi cohort and 6.8 (IQR = 1.4-17.7) for the non-TRKi cohort. Although this study took a descriptive approach and did not test for significance between the groups, this trend is in line with a study by Klink et al, which found a duration of 1L therapy of 16.8 months among TRKi-treated patients compared with 5.6 months for patients receiving other agents.13
This analysis also highlighted the time intervals between cancer diagnosis, NTRK testing, and TRKi initiation. The median time from cancer diagnosis to NTRK testing was 72 days for patients receiving TRKis; the time from diagnosis to TRKi initiation was 332 days (67-644). Updated National Comprehensive Cancer Network guidelines now recommend molecular biomarker testing (including NTRK testing) prior to 1L therapy for patients with locally advanced or metastatic disease.17,18 Of note, among the 32 patients diagnosed with cancer in the post-TRKi approval period, 5 experienced progressions to stage III or IV cancer between initial diagnosis and NTRK testing. Whether this delay in testing resulted in worsened outcomes is beyond the scope of this study, although previous literature has indicated that delays in time to treatment from diagnosis lead to worsened survival in patients with early-stage breast, lung, and colon cancers.19
The proportion of patients receiving NTRK testing prior to 1L therapy was 16% in the pre-TRKi period and 56% in the post-TRKi approval periods (overall: 35%). A one-time retrospective, multisite cohort study of 73 patients with NTRK fusion–positive solid tumors (diagnosed between January 1, 2016, and December 31, 2019) treated at primarily community cancer clinics found that 42.5% received NTRK testing prior to initiating 1L therapy, similar to the 35% detected in this study.20
STRENGTHS
The strengths of the study are the inclusion of cancer centers across multiple geographical regions in the United States including the West Coast, intermountain, Midwest, South, and Northeast. Further, previous real-world studies have focused on patients with NTRK fusion–positive solid tumors treated at community cancer clinics, whereas this study offers insight on patients treated within academic cancer centers.
LIMITATIONS
This study has several limitations. Data extraction was dependent on independent chart review conducted at each respective site. Although the CRF was standardized, variability in EHRs, databases, and data abstractor practices leads to the potential for misclassification errors in data. Further, not all EHRs contain the same level of detail, so some variables may be missing across the entire dataset. The relatively small sample size of this study was anticipated given the low prevalence of NTRK fusion–positive solid tumors. Nonetheless, it does pose significant limitations in the generalizability of our findings and results in increased sampling variability. Future research on larger cohorts is warranted to further elucidate the real-world treatment patterns and outcomes for patients with NTRK fusion–positive solid tumors.
Conclusions
This study provides valuable insights into the real-world management of NTRK fusion–positive solid tumors in academic medical centers in the United States. It demonstrates the evolving treatment landscape following the approval of TRKis. There is an opportunity to improve identification of NTRK fusions through broader testing earlier in care, which may lead to earlier initiation of TRKis.
Funding Statement
This study was funded by Bayer Pharmaceuticals. Bayer provided input into the initial study concept and design but had no influence over the study execution and the decision to publish.
REFERENCES
- 1.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5(1):25-34. doi:10.1158/2159-8290.CD-14-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1(2):e000023. doi:10.1136/esmoopen-2015-000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khotskaya YB, Holla VR, Farago AF, Mills Shaw KR, Meric-Bernstam F, Hong DS. Targeting TRK family proteins in cancer. Pharmacol Ther. 2017;173:58-66. doi:10.1016/j.pharmthera.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 4.Robbins HL, Hague A. The PI3K/Akt pathway in tumors of endocrine tissues. Front Endocrinol (Lausanne). 2016;6:188. doi:10.3389/fendo.2015.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamura R, Boichard A, Kato S, Sicklick JK, Bazhenova L, Kurzrock R. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: Implications for NTRK-targeted therapeutics. JCO Precis Oncol. 2018;2018(2):1-20. doi:10.1200/PO.18.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange AM, Lo HW. Inhibiting TRK proteins in clinical cancer therapy. Cancers (Basel). 2018;10(4):105. doi:10.3390/cancers10040105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons C, Deyell RJ, MacNeill AJ, et al. Canadian consensus on TRK-inhibitor therapy for NTRK fusion-positive sarcoma. Int J Cancer. 2021;149(9):1691-704. doi:10.1002/ijc.33723 [DOI] [PubMed] [Google Scholar]
- 8.Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531-40. doi:10.1016/S1470-2045(19)30856-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitrakvi. Package insert. Bayer Healthcare Pharmaceuticals; 2018. Accessed September 11, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211710s000lbl.pdf [Google Scholar]
- 10.Demetri GD, De Braud F, Drilon A, et al. Updated integrated analysis of the efficacy and safety of entrectinib in patients with NTRK fusion-positive solid tumors. Clin Cancer Res. 2022;28(7):1302-12. doi:10.1158/1078-0432.CCR-21-3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou KI, Vashistha V, Guo A, Ahmed S, Kelley MJ. Real-world experience with neurotrophic tyrosine receptor kinase fusion-positive tumors and tropomyosin receptor kinase inhibitors in veterans. JCO Precis Oncol. 2023;7(7):e2200692. doi:10.1200/PO.22.00692 [DOI] [PubMed] [Google Scholar]
- 12.Hibar DP, Demetri GD, Peters S, et al. Real-world survival outcomes in patients with locally advanced or metastatic NTRK fusion-positive solid tumors receiving standard-of-care therapies other than targeted TRK inhibitors. PLoS One. 2022;17(8):e0270571. doi:10.1371/journal.pone.0270571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klink AJ, Kavati A, Gassama A, Kozlek T, Gajra A, Antoine R. Treatment patterns of real-world patients with TRK fusion cancer treated by US community oncologists. Target Oncol. 2022;17(5):549-61. doi:10.1007/s11523-022-00909-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen EY, Goldman DA, Hechtman JF, et al. TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin Cancer Res. 2020;26(7):1624-32. doi:10.1158/1078-0432.CCR-19-3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JC, Ashok A, Liu C, Kang H. Real-world experience of NTRK fusion-positive thyroid cancer. JCO Precis Oncol. 2022;6(6):e2100442. doi:10.1200/PO.21.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bokemeyer C, Paracha N, Lassen U, et al. Survival outcomes of patients with tropomyosin receptor kinase fusion-positive cancer receiving larotrectinib versus standard of care: A matching-adjusted indirect comparison using real-world data. JCO Precis Oncol. 2023;7(7):e2200436. doi:10.1200/PO.22.00436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Non–small cell lung cancer: Recommendations for biomarker testing and treatment. J Natl Compr Canc Netw. 2021;19(5.5):610-3. doi:10.6004/jnccn.2021.5020 [Google Scholar]
- 18.Valderrabano P, Eszlinger M, Stewardson P, Paschke R. Clinical value of molecular markers as diagnostic and prognostic tools to guide treatment of thyroid cancer. Clin Endocrinol (Oxf). 2023;98(6):753-62. doi:10.1111/cen.14882 [DOI] [PubMed] [Google Scholar]
- 19.Ossowski S, Neeman E, Borden C, et al. Improving time to molecular testing results in patients with newly diagnosed, metastatic non-small-cell lung cancer. JCO Oncol Pract. 2022;18(11):e1874-84. doi:10.1200/OP.22.00260 [DOI] [PubMed] [Google Scholar]
- 20.Klink AJ, Kavati A, Gassama AT, Kozlek T, Gajra A, Antoine R. Timing of NTRK gene fusion testing and treatment modifications following TRK fusion status among US oncologists treating TRK fusion cancer. Target Oncol. 2022;17(3):321-8. doi:10.1007/s11523-022-00887-w [DOI] [PMC free article] [PubMed] [Google Scholar]


