Abstract
The antiretroviral nucleoside analog 2′,3′-dideoxy-3′-thiacytidine (3TC) is a potent inhibitor of wild-type human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT). A methionine-to-valine or methionine-to-isoleucine substitution at residue 184 in the HIV-1 YMDD motif, which is located at the RT active site, leads to a high level of resistance to 3TC. We sought to determine whether 3TC can inhibit the replication of wild-type murine leukemia virus (MLV), which contains V223 at the YVDD active site motif of the MLV RT, and of the V223M, V223I, V223A, and V223S mutant RTs. Surprisingly, the wild type and all four of the V223 mutants of MLV RT were highly resistant to 3TC. These results indicate that determinants outside the YVDD motif of MLV RT confer a high level of resistance to 3TC. Therefore, structural differences among similar RTs might result in widely divergent sensitivities to antiretroviral nucleoside analogs.
Currently, 14 antiviral drugs have been approved for clinical use to combat human immunodeficiency virus type 1 (HIV-1) infections (11, 52). When used in combination, many of these drugs have been shown to prolong the life expectancy of infected individuals and slow the progression of AIDS (12, 14, 19, 20, 38, 40). Most of these drugs, both nucleoside and nonnucleoside inhibitors, have been designed to target reverse transcriptase (RT) (2, 10, 51). One of the compounds used in combination therapy is the nucleoside analog 2′,3′-dideoxy-3′-thiacytidine (3TC) (4, 19, 20, 35, 49). The triphosphate form of 3TC inhibits reverse transcription through chain termination of DNA polymerization, a function carried out by RT, and therefore has been observed to be a potent inhibitor of both HIV-1 and -2 replication (2, 9, 10). In addition to the clinical benefits (19, 20, 35, 44) associated with the use of this nucleoside analog, 3TC exhibits low toxicity even at millimolar concentrations (7, 9, 15, 34).
All of the drugs being used for therapy to combat HIV-1 infections eventually result in drug-resistant mutants, thereby allowing the progression of the disease. Resistance to 3TC is no exception and is characterized by a mutation at the methionine 184 position of the Tyr-Met-Asp-Asp (YMDD) motif found in HIV-1 RT (5, 16, 29, 46, 53). The YXDD motif, where X is a variable amino acid, is highly conserved among the many viral RNA polymerases as well as RNA-dependent DNA polymerases (41). For example, Rous sarcoma virus RT contains the YMDD motif, the RTs of retroelements such as 297 (Gypsy-like group) and Int 32 (Line-like group) contain both the YLDD and YADD sequences, and the poliovirus RNA polymerase contains the YGDD motif (41). Mutations in the YXDD motif can abolish enzymatic activity and alter the processivity and fidelity of RT (3, 6, 21, 25, 37). The prevalent mutation in HIV-1 RT associated with 3TC resistance is the M184V substitution (16, 46, 53), which confers a level of resistance 1,000 times greater than that displayed by the wild-type enzyme (16, 53). It has been observed that the M184I variant, which is resistant to 3TC but is less catalytically active, is selected first after initiation of 3TC treatment (5, 6, 29, 53) and then is replaced by the M184V variant after long-term treatment with 3TC.
Mutations in the motif analogous to the HIV-1 RT YMDD domain are also correlated with resistance to 3TC in other retroviruses. The YVDD, YIDD, and YTDD motifs are selected during 3TC treatment of cells infected with simian immunodeficiency virus or feline immunodeficiency virus (FIV) (8, 47). In addition, the YVDD and YIDD motifs arise during 3TC treatment of hepatitis B virus (HBV)-infected cells and patients (1). The selection for mutations in the YXDD motif in other retroviruses as well as in HBV has suggested that this determinant is widely associated with 3TC sensitivity.
Other mutations in HIV-1 and FIV RTs have been implicated in dual resistance to 3TC and other nucleoside analogs. Specifically, the E89G and G333E mutations in HIV-1 RT are correlated with dual resistance in tissue culture to 3TC and either phosphonoformic acid or 3′-azido-3′-deoxythymidine (AZT), respectively (27, 43). The K65R mutation in HIV-1 RT is associated with resistance to 3TC and 2′,3′-dideoxycytidine (ddC) (18). Finally, the P156S mutation in FIV RT appears to confer resistance to both AZT and 3TC (48).
Although HIV-1 and murine leukemia virus (MLV) RTs share only ∼25% amino acid sequence identity, the two proteins are structurally similar (17). Comparison of the finger and palm domains in HIV-1 and MLV RT crystal structures reveals similar tertiary structures (17, 23, 32). In addition, many of the sequence motifs present in HIV-1 RT, such as the YXDD motif, the deoxynucleoside triphosphate (dNTP) binding site, and the conserved Leu-Pro-Gln-Gly (LPQG) motif, are also present in MLV RT. Importantly, the antiretroviral nucleoside analogs AZT, ddC, 2′,3′-dideoxyinosine, and 2′,3′-didehydro-3′-deoxythimidine, which inhibit HIV-1 RT, also inhibit MLV RT (50). Therefore, since wild-type MLV RT contains the YVDD motif, it was expected and recently shown to be resistant to 3TC (42).
Based on the similarities between the HIV-1 and MLV RTs, we hypothesized that the YMDD mutant of MLV RT would be sensitive to 3TC and the YIDD mutant would be resistant. To test this hypothesis, we generated viruses containing wild-type MLV RT as well as several mutations at position V223 within the YVDD motif and compared the titers of these viruses in several different target cells in the presence and absence of 3TC.
MLV mutants, target cells, and the ANGIE P cell line.
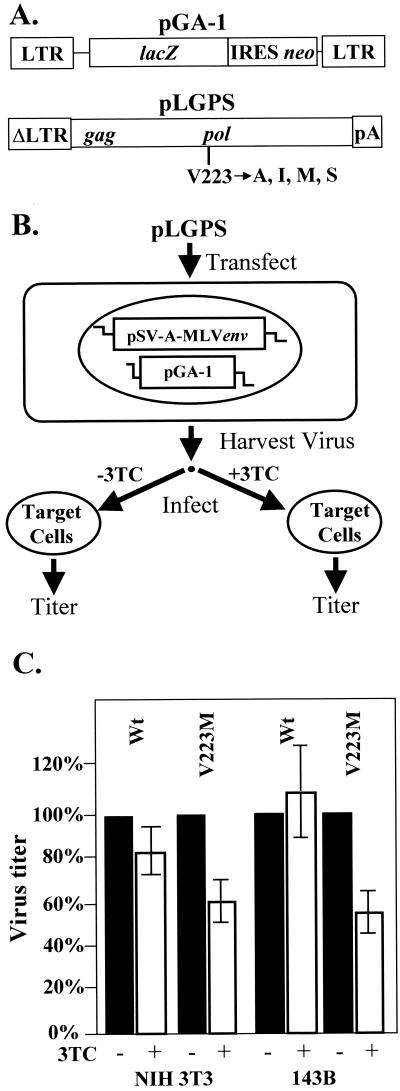
The construction of the V223A, V223I, V223M, and V223S mutants was described previously (21). The mutants were generated from the parent plasmid pLGPS (Fig. 1A), which expresses the MLV gag and pol genes from a truncated MLV long-terminal-repeat (ΔLTR) promoter (39).
FIG. 1.
Structures of MLV-based constructs and protocol used to determine sensitivity to 3TC. (A) Structures of the MLV-based vector pGA-1 and the gag-pol expression construct pLGPS. The pGA-1 vector contains both LTRs and all cis-acting elements of MLV and transcribes Escherichia coli lacZ and neo from the LTR promoter. The internal ribosomal entry site (IRES) (24) of encephalomyocarditis virus is used to express neo. The construct pLGPS expresses the MLV gag-pol gene from a truncated viral LTR. (B) Experimental protocol. ANGIE P, a D17-based cell line expressing pGA-1 and a vector carrying the amphotropic MLV env gene (pSV-A-MLVenv) was separately transfected with either the wild-type (V223) or mutated (V223A, V223I, V223M, and V223S) pLGPS constructs. Virus from pools of these transfected ANGIE P cells was harvested and used to infect target cells (NIH 3T3 or 143B cells) for 4 h in the presence and absence of 3TC (10 μM). The target cells were also treated with 3TC (10 μM) 4 h prior to infection and 24 h postinfection in the drug treatment groups. (C) Measurement of virus titer. The percent change in the virus titers of the wild-type and V223M mutant was measured in the absence and presence of 3TC (10 μM). Two to four independent experiments were performed for NIH 3T3 and 143B cells. Virus titers observed in the absence of 3TC treatment were defined as 100%. Error bars represent the standard error.
The targets of infection in this study included the murine fibroblast cell line NIH 3T3 and the human osteosarcoma cell line 143B (both obtained from the American Type Culture Collection). The ANGIE P cell line (Fig. 1B) is a D17-based (dog osteosarcoma) cell line expressing the construct pSV-A-MLVenv (obtained from the NIH AIDS Research and Reference Program) and pGA-1, an MLV-based retroviral vector (Fig. 1A) (21, 36). The expression construct pSV-A-MLVenv encodes the amphotropic MLV envelope gene, whereas pGA-1 expresses the bacterial β-galactosidase gene (lacZ) from the LTR promoter. In addition, pGA-1 contains the neomycin phosphotransferase gene (neo), which is utilized as a selectable marker during infection. All cells were maintained in Dulbecco's modified Eagle's medium (ICN Biochemicals) supplemented with penicillin (50 U/ml; Gibco), streptomycin (50 μg/ml; Gibco), and bovine calf serum (6% for ANGIE P and 143B cells and 10% for NIH 3T3 cells; HyClone Laboratories).
Protocol for determining sensitivity of MLV RT to 3TC.
The approach used to determine the sensitivity of MLV RT to 3TC is outlined in Fig. 1B. Briefly, either wild-type or mutated pLGPS along with pSVα3.6, a plasmid that confers resistance to ouabain (28), was cotransfected into the ANGIE P cell line. Transfections were carried out by the previously described dimethyl sulfoxide-Polybrene method (26), and the transfected cells were then selected for resistance to 10−7 M ouabain.
To determine the sensitivity of the MLV RTs to 3TC, we separately pooled and expanded more than 500 ouabain-resistant colonies for the wild type and V223 mutants. For each pLGPS construct, 5 × 106 ouabain-resistant cells were plated on 100-mm-diameter dishes and the medium was changed 24 h later. Virus was harvested after another 24 h and serially diluted. In the presence of Polybrene (50 μg/ml), the virus was used to infect either NIH 3T3 or 143B cells for 4 h. The target cells were plated at a density of 1 × 105 to 2 × 105 cells per 60-mm-diameter dish. In experiments conducted in the presence of the drug, the target cells were incubated with 10 μM 3TC 4 h prior to infection, 4 h during infection, and 24 h postinfection. The 3TC concentration used in this study was 15- to 4,000-fold higher than the mean 50% inhibitory concentration, ranging between 2.5 nM and 0.67 μM, which was previously shown to inhibit several different strains of HIV-1 (9). The infected cells were then subjected to selection with G418, an analog of neomycin (600 μg/ml for 143B and D17 cells and 1.2 mg/ml for NIH 3T3 cells), 24 h after infection. The effect of 3TC treatment on MLV replication (wild type and V223 mutants) was determined from the number of drug-resistant colonies obtained in the presence or absence of 3TC.
Comparison of viral titers in the presence or absence of 3TC.
Viral titers were determined by quantitation of G418-resistant NIH 3T3 and 143B cells after infection (the data are summarized in Table 1). ANGIE P cells transfected with wild-type pLGPS or the V223A, V223I, V223M, or V223S mutant were previously shown to produce infectious viral particles, and target cells infected with these viruses were expected to confer resistance to G418 (21). Two to four independent infections of NIH 3T3 and 143B cells, in the absence or presence of 3TC, were performed with virus containing either the wild-type pLGPS or one of the four V223 mutants.
TABLE 1.
The effect of 3TC treatment on MLV replication
| Target cells | MLV RTa | Expt | Titer (CFU/ml)
|
+3TC relative titer (mean ± SE)d | |
|---|---|---|---|---|---|
| −3TCb | +3TCc | ||||
| NIH 3T3 | WT | 1 | 3.7 × 104 | 2.6 × 104 | 0.70 |
| 2 | 2.7 × 104 | 1.5 × 104 | 0.56 | ||
| 3 | 7.2 × 104 | 9.2 × 104 | 1.28 | ||
| 4 | 1.3 × 105 | 9.5 × 104 | 0.73 | ||
| Avg | 0.82 ± 0.16 | ||||
| V223M | 1 | 2.9 × 102 | 1.4 × 102 | 0.48 | |
| 2 | 1.6 × 102 | 1.0 × 102 | 0.63 | ||
| 3 | 9.2 × 104 | 6.9 × 104 | 0.75 | ||
| 4 | 2.4 × 104 | 1.0 × 104 | 0.42 | ||
| Avg | 0.57 ± 0.07 | ||||
| V223I | 1 | 3.4 × 104 | 2.5 × 104 | 0.74 | |
| 2 | 2.6 × 104 | 3.3 × 104 | 1.27 | ||
| Avg | 1.01 ± 0.27 | ||||
| V223A | 1 | 1.3 × 102 | 1.9 × 102 | 1.46 | |
| 2 | 2.1 × 102 | 2.0 × 102 | 0.95 | ||
| Avg | 1.21 ± 0.26 | ||||
| V223S | 1 | 4.3 × 104 | 4.3 × 104 | 1.00 | |
| 2 | 8.3 × 103 | 1.2 × 104 | 1.45 | ||
| Avg | 1.23 ± 0.23 | ||||
| 143B | WT | 1 | 1.9 × 103 | 1.4 × 103 | 0.74 |
| 2 | 3.4 × 102 | 5.8 × 102 | 1.70 | ||
| 3 | 4.3 × 102 | 3.5 × 102 | 0.8 | ||
| 4 | 1.2 × 103 | 1.3 × 103 | 1.10 | ||
| Avg | 1.10 ± 0.23 | ||||
| V223M | 1 | 2.5 × 102 | 1.3 × 102 | 0.52 | |
| 2 | 2.0 × 102 | 8.3 × 101 | 0.42 | ||
| 3 | 1.3 × 101 | 1.1 × 101 | 0.85 | ||
| 4 | 1.0 × 101 | 0.5 × 101 | 0.50 | ||
| Avg | 0.57 ± 0.09 | ||||
MLV generated from transfection of pLGPS (wild type [WT] or V223 mutants of MLV RT) into the ANGIE P encapsidating cell line.
The virus titer for each experimental group was determined by serial dilutions. In experiments using NIH 3T3 target cells, four independent infections were performed with virus containing the wild-type or V223M mutant RT, whereas two independent infections were performed for the V223A, V223I, and V223S RTs. In experiments using 143B target cells, four independent infections were performed with virus containing the wild-type or V223M mutant RT.
The virus titer for each experimental group was determined by serial dilutions. Four independent infections were performed in the presence of 3TC (10 μM) as described in footnote b for virus containing either the wild-type or V223M RT. The 3TC treatment included incubation of the target cells 4 h prior to infection, 4 h during infection, and 24 h postinfection.
The +3TC relative titer was calculated as follows: titer obtained in the presence of 3TC ÷ titer obtained in the absence of 3TC.
Infection of NIH 3T3 cells with the wild-type virus harvested from a single pool produced titers that ranged from 2.7 × 104 to 1.3 × 105 CFU/ml in the absence of 3TC. Treatment of the target cells with 3TC had no significant effect on titers of the wild-type virus (82%, relative to the untreated control). Similarly, infection of NIH 3T3 cells with the V223M mutant produced titers that ranged from 1.6 × 102 to 9.2 × 104 CFU/ml in the absence of 3TC after virus was harvested from two different virus-producing pools. Treatment of the target cells with 3TC did not substantially affect the titers of the V223M mutant virus (57%, relative to the untreated control) compared to the inhibition of the luciferase-expressing HIV-1-based vector pNLuc (Fig. 2C). The twofold change observed in the titers in the absence or presence of 3TC is probably not biologically relevant due to the inherent variation that occurs during infections (21). We also assessed the sensitivity to 3TC of other V223 mutants (V223A, V223I, and V223S) in NIH 3T3 cells; the results were similar to those obtained with the wild-type and V223M mutant viruses. In summary, viral titers in the presence or absence of 3TC varied by only twofold, suggesting that 3TC did not substantially inhibit either the wild-type or V223 mutant RTs during infection of NIH 3T3 cells.
FIG. 2.
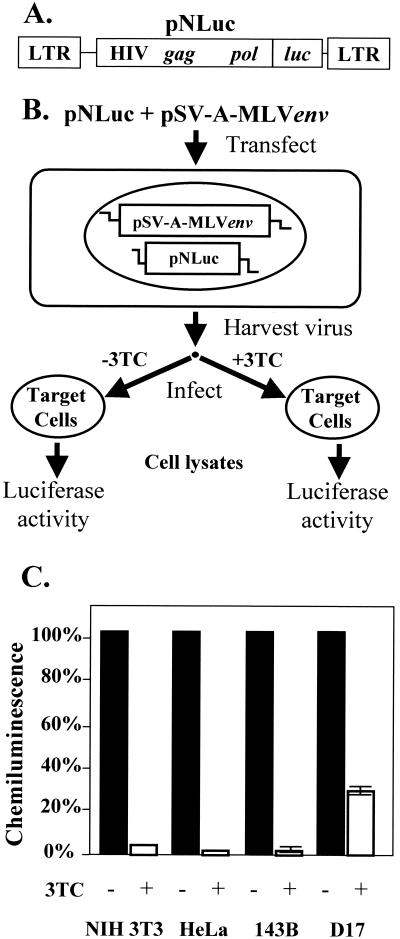
Structure of HIV-1-based constructs and activation of 3TC in NIH 3T3, HeLa, 143B, and D17 cell lines. (A) Structure of the HIV-1-based luciferase-expression vector pNLuc, which contains both LTRs, other cis-acting elements required for viral replication, and the gag-pol gene of HIV-1. pNLuc expresses the luciferase reporter gene (luc) from the LTR promoter. (B) Experimental protocol. The vectors pNLuc and pSV-A-MLVenv were cotransfected into 293T cells and pseudotyped virus was used to infect various target cells (NIH 3T3, HeLa, 143B, and D17) for 4 h in the presence or absence of 3TC (10 μM). The target cells were also treated with 3TC (10 μM) 4 h prior to infection and 24 h postinfection in the drug treatment groups. (C) Measurement of chemiluminescence. The percent chemiluminescence was measured in the absence and presence of 3TC (10 μM). At least two independent experiments were performed for NIH 3T3, HeLa, 143B, and D17 cells. Chemiluminescence measurements in the absence of 3TC treatment were defined as 100%. Error bars represent the standard error. The standard errors for both NIH 3T3 and HeLa are less than 0.3% (not shown).
To determine whether the lack of inhibition to MLV replication with 3TC treatment was specific to NIH 3T3 cells, we also tested infection of 143B cells and D17 cells. Infection of 143B cells with virus containing either the wild-type or V223M mutant RT produced results similar to those obtained with NIH 3T3 cells. Virus obtained from wild-type pLGPS produced viral titers that ranged from 3.4 × 102 to 1.9 × 103 CFU/ml in the absence of 3TC. Treatment of the target cells with 3TC had no significant effect on the titers of the wild-type virus (110%, relative to the untreated control). In addition, infection of 143B cells with virus containing the V223M mutant RT produced viral titers ranging from 1.0 × 101 to 2.5 × 102 CFU/ml in the absence of 3TC and 0.5 × 101 to 1.3 × 102 CFU/ml in the presence of 3TC (57%, relative to the untreated control). Similar results were obtained with D17 cells (data not shown).
Activation of 3TC in target cells.
The results obtained with viruses generated and harvested from ANGIE P cells that had been transfected with either the wild-type or V223 mutant constructs showed that all MLV RTs were resistant to 3TC (Table 1). There are two possible explanations for these results. First, structural differences between MLV RT and HIV-1 RT might account for the resistance of MLV RT to 3TC. Second, the uptake and/or phosphorylation of the drug by the target cells might be inefficient. To address these possibilities, we generated infectious HIV-1 particles and used them to infect the various target cells as previously described (30, 31) (Fig. 2A and B). Briefly, 293T (human embryonic kidney) cells were cotransfected with pNLuc and pSV-A-MLVenv. Pseudotyped virus stocks were harvested and used to infect NIH 3T3, HeLa, 143B, and D17 cells in the presence or absence of 10 μM 3TC. The target cells infected in the presence of drug were incubated with 3TC for 4 h prior to infection, 4 h during infection, and 24 h postinfection. Two days postinfection, the cells were lysed and the amount of luciferase activity present in the lysates was measured with a luminometer (Tropix) (Fig. 2B).
Infection of NIH 3T3, 143B, and HeLa cells with the pNLuc-derived virus was decreased at least 30-fold by 3TC treatment (Fig. 2C). The D17 cells exhibited a fourfold decrease in pNLuc expression relative to D17 cells not treated with 3TC. Thus, in the same cell lines in which MLV infectivity was not substantially affected, HIV-1 infectivity was significantly reduced.
The infection of NIH 3T3 cells was decreased 33-fold (3% of wild type, average of two experiments) and infection of 143B cells was decreased 100-fold (1% of wild type, average of two experiments) by 3TC treatment. Under the same conditions, infection of both NIH 3T3 and 143B cells by the wild-type MLV RT was not significantly decreased. Therefore, the wild-type MLV RT was 33- to 100-fold less sensitive to 3TC than the HIV-1 RT. Similarly, infection of both NIH 3T3 and 143B cells was reduced to 57% by 3TC treatment. Even though the significance of the less-than-twofold reduction of the virus titer is doubtful, the results clearly indicated that the YMDD mutant of MLV RT was at least 20-fold less sensitive to 3TC in NIH 3T3 cells and 57-fold less sensitive to 3TC in 143B cells than the HIV-1 RT. While it is possible that the YMDD mutant of MLV RT may display some sensitivity to 3TC when very high concentrations of 3TC are used, it is clear from the data presented here that both the wild-type and the YMDD mutant of MLV RT exhibit a marked resistance to 3TC relative to the wild-type HIV-1 RT.
These results also indicate that the lack of an effect of 3TC on MLV infectivity was not due to problems associated with the uptake, phosphorylation, or other mechanisms that may interfere with the inhibitory activity of the nucleoside analog in these target cells. The less efficient inhibition of pNLuc expression in D17 cells than in the other target cells (Fig. 2C) could have been caused by either reduced uptake or phosphorylation of 3TC in D17 cells. Alternatively, this cell line might actively export the nucleoside analog, thus reducing its efficacy. Regardless of the mechanism, HIV-1 infecting different cell types in vivo might display divergent susceptibilities to 3TC or other RT inhibitors. Thus, the data obtained with the D17 cell line might have implications for drug therapy in HIV-1-infected patients.
Mechanism of 3TC resistance in MLV RT.
3TC resistance arises in both retroviral (HIV-1, FIV, and simian immunodeficiency virus) and nonretroviral (HBV) polymerases with catalytic sites containing the YMDD motif (1, 8, 16, 29, 46, 47, 53). This resistance usually results from a substitution of methionine to threonine, isoleucine, or valine. Based on these observations, we expected that the wild-type MLV RT containing the YVDD motif would be resistant (42) and the V223M mutant would be sensitive to 3TC. It was therefore surprising that both the wild type and the V223M mutant were highly resistant to 3TC.
The mechanism by which the M184V mutant of HIV-1 RT confers resistance to 3TC is unclear. The methionine-to-isoleucine substitution at position 184 in HIV-1 RT results in a repositioning of the template-primer complex, and this rearrangement might result in a misalignment of the 3TC triphosphate with the template, resulting in a decrease in the turnover rate (45). Molecular modeling of the wild type and the M184I mutant of HIV-1 RT has also suggested that steric hindrance between the β-l-oxathiolane ring of 3TC triphosphate and the β-branched amino acids (valine, isoleucine, and threonine) at position 184 interferes with 3TC binding (23, 45). It is important to note that the proposed steric hindrance model does not preclude 3TC binding to RT in a mode that is unfavorable to its incorporation. In this regard, Feng and Anderson (13) reported that 3TC triphosphate binds to the M184V mutant with a much higher Kd value (5.2 μM) relative to the wild-type HIV-1 RT (0.24 μM). Similarly, Wilson et al. (54) suggested that β-l-2′,3′-dideoxy-5-fluoro-3′-thiacytidine, a nucleoside analog that is structurally similar to 3TC, binds with a higher affinity to the wild-type HIV-1 RT than the M184V mutant. However, Krebs and coworkers did not find a substantial difference in Kd values for 3TC binding to the wild type and M184V mutants (33). Recent evidence indicates that 3TC can bind to the M184V and M184I mutants of HIV-1 RT, which results in a conformational change in the enzyme that affects the nature of RNase H cleavages (H.-Q. Gao, P. L. Boyer, S. G. Sarafianos, E. Arnold, and S. Hughes, personal communication).
The fact that both the YMDD and YVDD motifs are highly resistant to 3TC strongly suggests that other structural determinants of MLV RT may interfere with the nature of 3TC binding through steric hindrance. The previous observation that MLV RT is sensitive to ddC (50) suggests that the steric hindrance involves the β-l-oxathiolane ring of 3TC. In accordance with the relatively low (∼25%) amino acid sequence identity between the MLV and HIV-1 RTs (17), structural alterations at or near the active site not related to the YVDD motif of MLV RT may lead to steric hindrance and prevent 3TC binding. A comparison of distances between residues of the YXDD motif and dNTP binding pocket of HIV-1 and MLV RTs reveals substantial differences (17, 22). It should be noted that the MLV RT crystal structure lacks the thumb, connection, and RNase H domain. Therefore, the observed differences in the relative spacing of residues in the YXDD motif and the dNTP binding site may be the result of the partial MLV RT fragment being folded differently than in the intact enzyme. Nevertheless, a comparison of the crystal structures suggests structural differences that might contribute to the divergent sensitivities to 3TC. Distances in MLV RT, specifically between the residues of the YVDD motif and K103, which is equivalent to K65 of HIV-1 RT (E. K. Halvas, E. S. Svarovskaia, and V. K. Pathak, unpublished data), are more than 2 Å longer than the distances in HIV-1 RT. Interestingly, the K65 residue of HIV-1 RT is associated with dual resistance to 3TC and ddC (18). Additionally, amino acid differences between the MLV and HIV-1 RTs around the dNTP binding pocket may also provide the steric hindrance needed to confer 3TC resistance. Specifically, the MLV RT possesses a phenylalanine at position 155, which is equivalent to the tyrosine 115 in HIV-1 RT. The Y115 of HIV-1 RT has been shown to interact with the deoxyribose ring of a dNTP substrate (23). Therefore, substitution of phenylalanine for the tyrosine in the MLV RT dNTP binding site may alter the nature of 3TC binding.
It is also conceivable that the structural differences between MLV and HIV-1 RTs near the active site or dNTP binding site alter the affinity or nature of binding of 3TC triphosphate relative to dCTP by a mechanism not involving steric hindrance. The nature of the structural differences that confer high-level resistance to 3TC despite the presence of a YMDD motif appears to be unique to MLV RT, since several other retroviral RTs as well as HBV polymerase display sensitivity to 3TC when the YMDD motif is present (1, 8, 47). Understanding the nature of the structural differences that lead to 3TC resistance in MLV RT might provide insights into the general mechanisms by which retroviral RTs acquire resistance to nucleotide analogs.
Acknowledgments
We especially thank Wei-Shau Hu for critical reading of the manuscript and valuable intellectual input and discussions throughout the project. We also especially thank Stephen H. Hughes for communicating unpublished results, intellectual input, and critical reading of the manuscript. We also thank Benjamin Beasley, Sara Cheslock, Que Dang, Krista Delviks, Carey Hwang, Timur Kabdulov, Terence Rhodes, Yegor Voronin, and Wen Hui Zhang for critical reading of the manuscript and discussion of results. Finally, we extend our thanks to Ann Arthur for her editorial expertise and revisions.
This work was supported in part by Public Health Service grant CA58875 from the National Institutes of Health, HIV Drug Resistance Program, National Cancer Institute, and Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Allen M I, Deslauriers M, Andrews C W, Tipples G A, Walters K A, Tyrrell D L, Brown N, Condreay L D. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology. 1998;27:1670–1677. doi: 10.1002/hep.510270628. [DOI] [PubMed] [Google Scholar]
- 2.Arts E J, Wainberg M A. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1996;40:527–540. doi: 10.1128/aac.40.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhanashvili M, Avidan O, Hizi A. Mutational studies of human immunodeficiency virus type 1 reverse transcriptase: the involvement of residues 183 and 184 in the fidelity of DNA synthesis. FEBS Lett. 1996;391:257–262. doi: 10.1016/0014-5793(96)00747-8. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini J, Pelemans H, Karlsson A, De Clerc Q E, Kleim J P. Concomitant combination therapy for HIV infection preferable over sequential therapy with 3TC and non-nucleoside reverse transcriptase inhibitors. Proc Natl Acad Sci USA. 1996;93:13152–13157. doi: 10.1073/pnas.93.23.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher C A B, Cammack N, Schipper P, Schuurman R, Rouse P, Wainberg M A, Cameron J M. High-level resistance to (−) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993;37:2231–2234. doi: 10.1128/aac.37.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer P L, Hughes S H. Analysis of mutations at position 184 in reverse transcriptase of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1995;39:1624–1628. doi: 10.1128/aac.39.7.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C N, Doong S L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C H, Cheng Y C, Liotta D, Schinazi R. Deoxycytidine deaminase-resistant stereoisomer is the active form of (+/−)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. . (Erratum, 267:24148.) [PubMed] [Google Scholar]
- 8.Cherry E, Slater M, Salomon H, Rud E, Wainberg M A. Mutations at codon 184 in simian immunodeficiency virus reverse transcriptase confer resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1997;41:2763–2765. doi: 10.1128/aac.41.12.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates J A V, Cammack N, Jenkinson H J, Jowett A J, Jowett M I, Pearson B A, Penn C R, Rouse P L, Viner K C, Cameron J M. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffin J M, Hughes S H, Varmus H E. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 11.Cohen J. The daunting challenge of keeping HIV suppressed. Science. 1997;277:32–33. doi: 10.1126/science.277.5322.32. [DOI] [PubMed] [Google Scholar]
- 12.Cooley T P, Kunches L M, Saunders C A, Ritter J K, Perkins C J, McLaren C, McCaffrey R P, Liebman H A. Once-daily administration of 2′,3′-dideoxyinosine (ddI) in patients with the acquired immunodeficiency syndrome or AIDS-related complex. Results of a Phase I trial. N Engl J Med. 1990;322:1340–1345. doi: 10.1056/NEJM199005103221902. [DOI] [PubMed] [Google Scholar]
- 13.Feng J Y, Anderson K S. Mechanistic studies examining the efficiency and fidelity of DNA synthesis by the 3TC-resistant mutant (184V) of HIV-1 reverse transcriptase. Biochemistry. 1999;38:9440–9448. doi: 10.1021/bi990709m. [DOI] [PubMed] [Google Scholar]
- 14.Fischl M A, Richman D D, Grieco M H, Gottlieb M S, Volberding P A, Laskin O L, Leedom J M, Groopman J E, Mildvan D, Schooley R T, Jackson G G, Durack D T, King D The AZT Collaborative Working Group. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 15.Furman P A, Davis M, Liotta D C, Paff M, Frick L W, Nelson D J, Dornsife R E, Wurster J A, Wilson L J, Fyfe J A, Tuttle J V, Miller W H, Condreay L, Averett D R, Schinazi R F, Painter G R. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Q, Gu Z, Parniak M A, Cameron J, Cammack N, Boucher C, Wainberg M A. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1993;37:1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgiadis M M, Jessen S M, Ogata C M, Telesnitsky A, Goff S P, Hendrickson W A. Mechanistic implications from the structure of a catalytic fragment of Moloney murine leukemia virus reverse transcriptase. Structure. 1995;3:879–892. doi: 10.1016/S0969-2126(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 18.Gu Z, Gao Q, Fang H, Salomon H, Parniak M A, Goldberg E, Cameron J, Wainberg M A. Identification of a mutation at codon 65 in the IKKK motif of reverse transcriptase that encodes human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1994;38:275–281. doi: 10.1128/aac.38.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Jonas L, Meibohm A, Holder D, Schleif W A, Condra J H, Emini E A, Isaacs R, Chodakewitz J A, Richman D D. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. JAMA. 1998;280:35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 21.Halvas E K, Svarovskaia E S, Pathak V K. Development of an in vivo assay to identify structural determinants in murine leukemia virus reverse transcriptase important for fidelity. J Virol. 2000;74:312–319. [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiou Y, Ding J, Das K, Clark A D, Jr, Hughes S H, Arnold E. Structure of unliganded HIV-1 reverse transcriptase at 2.7 A resolution: implications of conformational changes for polymerization and inhibition mechanisms. Structure. 1996;4:853–860. doi: 10.1016/s0969-2126(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Chopra R, Verdine G L, Harrison S C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 24.Jang S K, Kräusslich H-G, Nicklin M J H, Duke G M, Palmenberg A C, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushik N, Singh K, Alluru I, Modak M J. Tyrosine 222, a member of the YXDD motif of MuLV RT, is catalytically essential and is a major component of the fidelity center. Biochemistry. 1999;38:2617–2627. doi: 10.1021/bi9824285. [DOI] [PubMed] [Google Scholar]
- 26.Kawai S, Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984;4:1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemp S D, Shi C, Bloor S, Harrigan P R, Mellors J W, Larder B A. A novel polymorphism at codon 333 of human immunodeficiency virus type 1 reverse transcriptase can facilitate dual resistance to zidovudine and l-2′,3′-dideoxy-3′-thiacytidine. J Virol. 1998;72:5093–5098. doi: 10.1128/jvi.72.6.5093-5098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent R B, Emanuel J R, Ben Neriah Y, Levenson R, Housman D E. Ouabain resistance conferred by expression of the cDNA for a murine Na+, K+-ATPase alpha subunit. Science. 1987;237:901–903. doi: 10.1126/science.3039660. [DOI] [PubMed] [Google Scholar]
- 29.Keulen W, Back N K T, van Wijk A, Boucher C A B, Berkhout B. Initial appearance of the 184Ile variant in lamivudine-treated patients is caused by the mutational bias of human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1997;71:3346–3350. doi: 10.1128/jvi.71.4.3346-3350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiernan R E, Freed E O. Cleavage of the murine leukemia virus transmembrane Env protein by human immunodeficiency virus type 1 protease: transdominant inhibition by matrix mutations. J Virol. 1998;72:9621–9627. doi: 10.1128/jvi.72.12.9621-9627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiernan R E, Ono A, Englund G, Freed E O. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–4126. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohlstaedt L A, Wang J, Friedman J M, Rice P A, Steitz T A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 33.Krebs R, Immendorfer U, Thrall S H, Wohrl B M, Goody R S. Single-step kinetics of HIV-1 reverse transcriptase mutants responsible for virus resistance to nucleoside inhibitors zidovudine and 3-TC. Biochemistry. 1997;36:10292–10300. doi: 10.1021/bi970512z. [DOI] [PubMed] [Google Scholar]
- 34.Kukhanova M, Liu S H, Mozzherin D, Lin T S, Chu C K, Cheng Y C. l- and d-enantiomers of 2′,3′-dideoxycytidine 5′-triphosphate analogs as substrates for human DNA polymerases. Implications for the mechanism of toxicity. J Biol Chem. 1995;270:23055–23059. doi: 10.1074/jbc.270.39.23055. [DOI] [PubMed] [Google Scholar]
- 35.Lafeuillade A, Poggi C, Tamalet C, Profizi N, Tourres C, Costes O. Effects of a combination of zidovudine, didanosine, and lamivudine on primary human immunodeficiency virus type 1 infection. J Infect Dis. 1997;175:1051–1055. doi: 10.1086/516442. [DOI] [PubMed] [Google Scholar]
- 36.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larder B A, Purifoy D J, Powell K L, Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. Nature. 1987;327:716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- 38.Merigan T C, Skowron G, Bozzette S A, Richman D, Uttamchandani R, Fischl M, Schooley R, Hirsch M, Soo W, Pettinelli C, Schaumburg H The ddC Study Group of the AIDS Clinical Trials Group. Circulating p24 antigen levels and responses to dideoxycytidine in human immunodeficiency virus (HIV) infections. A phase I and II study. Ann Intern Med. 1989;110:189–194. doi: 10.7326/0003-4819-110-3-189. [DOI] [PubMed] [Google Scholar]
- 39.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray H W, Squires K E, Weiss W, Sledz S, Sacks H S, Hassett J, Cross A, Anderson R E, Dunkle L M. Stavudine in patients with AIDS and AIDS-related complex: AIDS clinical trials group 089. J Infect Dis. 1995;171(Suppl. 2):S123–S130. doi: 10.1093/infdis/171.supplement_2.s123. [DOI] [PubMed] [Google Scholar]
- 41.Poch O, Sauvaget I, Delarue M, Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989;8:3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell S K, Artlip M, Kaloss M, Brazinski S, Lyons R, McGarrity G J, Otto E. Efficacy of antiretroviral agents against murine replication-competent retrovirus infection in human cells. J Virol. 1999;73:8813–8816. doi: 10.1128/jvi.73.10.8813-8816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quan Y, Gu Z, Li X, Liang C, Parniak M A, Wainberg M A. Endogenous reverse transcriptase assays reveal synergy between combinations of the M184V and other drug resistance-conferring mutations in interactions with nucleoside analog triphosphates. J Mol Biol. 1998;277:237–247. doi: 10.1006/jmbi.1997.1592. [DOI] [PubMed] [Google Scholar]
- 44.Rinaldo C R, Jr, Liebmann J M, Huang X L, Fan Z, Al-Shboul Q, McMahon D K, Day R D, Riddler S A, Mellors J W. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;179:329–336. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]
- 45.Sarafianos S G, Das K, Clark A D, Jr, Ding J, Boyer P L, Hughes S H, Arnold E. Lamivudine (3TC) resistance in HIV-1 reverse transcriptase involves steric hindrance with beta-branched amino acids. Proc Natl Acad Sci USA. 1999;96:10027–10032. doi: 10.1073/pnas.96.18.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schinazi R F, Lloyd R M, Jr, Nguyen M-H, Cannon D L, McMillan A, Ilksoy N, Chu C K, Liotta D C, Bazmi H Z, Mellors J W. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37:875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith R A, Remington K M, Lloyd R M, Jr, Schinazi R F, North T W. A novel Met-to-Thr mutation in the YMDD motif of reverse transcriptase from feline immunodeficiency virus confers resistance to oxathiolane nucleosides. J Virol. 1997;71:2357–2362. doi: 10.1128/jvi.71.3.2357-2362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith R A, Remington K M, Preston B D, Schinazi R F, North T W. A novel point mutation at position 156 of reverse transcriptase from feline immunodeficiency virus confers resistance to the combination of (−)-β-2′,3′-dideoxy-3′-thiacytidine and 3′-azido-3′-deoxythymidine. J Virol. 1998;72:2335–2340. doi: 10.1128/jvi.72.3.2335-2340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solder B, Wintergerst U, Notheis G, Eberle J, Gurtler L, Belohradsky B H. Effect of antiretroviral combination therapy (zidovudine/didanosine or zidovudine/lamivudine) on quantitative plasma human immunodeficiency virus–ribonucleic acid in children and adolescents infected with human immunodeficiency virus. J Pediatr. 1997;130:293–299. doi: 10.1016/s0022-3476(97)70358-5. [DOI] [PubMed] [Google Scholar]
- 50.Strair R K, Nelson C J, Mellors J W. Use of recombinant retroviruses to characterize the activity of antiretroviral compounds. J Virol. 1991;65:6339–6342. doi: 10.1128/jvi.65.11.6339-6342.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tantillo C, Ding J, Jacobo-Molina A, Nanni R G, Boyer P L, Hughes S H, Pauwels R, Andries K, Janssen P A, Arnold E. Locations of anti-AIDS drug binding sites and resistance mutations in the three-dimensional structure of HIV-1 reverse transcriptase. Implications for mechanisms of drug inhibition and resistance. J Mol Biol. 1994;243:369–387. doi: 10.1006/jmbi.1994.1665. [DOI] [PubMed] [Google Scholar]
- 52.Temesgen Z, Wright A J. Antiretrovirals. Mayo Clin Proc. 1999;74:1284–1301. doi: 10.4065/74.12.1284. [DOI] [PubMed] [Google Scholar]
- 53.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson J E, Aulabaugh A, Caligan B, McPherson S, Wakefield J K, Jablonski S, Morrow C D, Reardon J E, Furman P A. Human immunodeficiency virus type-1 reverse transcriptase. Contribution of Met-184 to binding of nucleoside 5′-triphosphate. J Biol Chem. 1996;271:13656–13662. doi: 10.1074/jbc.271.23.13656. [DOI] [PubMed] [Google Scholar]