Abstract
Objective.
To compare minimally invasive (MILR) and open liver resections (OLR) for hepatocellular carcinoma (HCC) in patients with metabolic syndrome (MS).
Summary background data.
Liver resections for HCC on MS are associated with high perioperative morbidity and mortality. No data on the minimally invasive approach in this setting exist.
Methods.
A multicenter study involving 24 institutions was conducted. Propensity scores were calculated, and inverse probability weighting was used to weight comparisons. Short- and long-term outcomes were investigated.
Results.
996 patients were included, 580 in OLR and 416 in MILR. After weighing, groups were well matched. Blood loss was similar between groups (OLR 275.9±3.1 vs. MILR 226±4.0, p=0.146). There were no significant differences in 90-day morbidity (38.9% vs. 31.9% OLRs and MILRs, p=0.08) and mortality (2.4% vs. 2.2% OLRs and MILRs, p=0.84). MILRs were associated with lower rates of major complications (9.3% vs. 15.3%, p=0.015), post hepatectomy liver failure (0.6% vs. 4.3%, p=0.008) and bile leaks (2.2% vs. 6.4%, p=0.003); ascites was significantly lower at postoperative day 1 (2.7% vs. 8.1%, p=0.002) and day 3 (3.1% vs. 11.4%, p<0.001); hospital stay was significantly shorter (5.8±1.9 vs. 7.5±1.7, p<0.001). There was no significant difference in overall survival and disease-free survival.
Conclusions.
MILR for HCC on MS is associated with equivalent perioperative and oncological outcomes to OLRs. Fewer major complications, post hepatectomy liver failures, ascites and bile leaks can be obtained, with shorter hospital stay. The combination of lower short-term severe morbidity and equivalent oncologic outcomes favor MILR for MS when feasible.
Keywords: Hepatocellular carcinoma, metabolic syndrome, liver resection, non-alcoholic liver disease
INTRODUCTION
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the third leading cause of cancer-related deaths worldwide.1 Despite most HCCs developing in the setting of chronic viral hepatitis and/or alcohol-related liver disease, there has been a recent increase in the incidence of both cirrhosis and HCC related to metabolic syndrome (MS), a growing clinical entity in Western countries including hypertension, dyslipidaemia, obesity, and insulin resistance.2, 3 Indeed, metabolic syndrome leads to non-alcoholic fatty liver disease (NAFLD), a spectrum of hepatic parenchymal changes ranging from simple steatosis to significant fibrosis and cirrhosis.4, 5 NAFLD triggers carcinogenesis, which results in a 2–4 fold higher risk of developing HCC than the general population, and a yearly incidence as high as 2.6%.6
As surgery remains one of the few potentially curative options for HCC, an increasing number of patients with MS are considered for resection, especially in North America and Europe.7–9 Liver surgery for HCC patients with MS is associated with an up to three-fold risk of mortality and a two-fold risk of morbidity depending on the severity of patients’ comorbidities and parenchymal changes.8, 10–12 In this setting, strategies to decrease the impact of surgery on such complex patients are warranted. Minimally invasive liver resections (MILR) have gained widespread popularity due to the decreased morbidity, length of hospital stays, pain, and more rapid recovery compared to the open approach. Indeed, extensive clinical research, including randomized controlled trials, have disclosed the safety and the advantages of MILR for both benign and malignant diseases, with significant improvements reported over time.13, 14 Despite this, no data on MILRs for HCC in patients with MS have been described so far. These patients are likely to benefit from a mini-invasive approach but are frequently obese and have multiple comorbidities, which might limit the application of laparoscopy or robotics.
This study aimed to review a large Western experience of MILRs for HCC in patients with MS and to compare it to the open approach. Short-term and oncological outcomes were compared to assess surgical safety and oncological adequacy.
MATERIAL AND METHODS
Study population
Data from 24 institutions (12 European and 12 North American) with experience in the treatment of hepatobiliary malignancies were collected from November 1992 and May 2021. All centers involved in this study are experienced in minimally invasive liver resections, have an established minimally invasive program and are contributing to national registries. Based on volume of patients provided, centers were categorized in high volume (more than 70 patients), medium volume (between 30 and 70 patients), low volume (fewer than 30 patients). Patients with metabolic syndrome undergoing pure laparoscopic, robotic-assisted, or open liver resection for histologically proven hepatocellular carcinoma were included. Metabolic syndrome was defined by 3 out of 5 of the following criteria:15, 16 a) abdominal obesity (BMI>28.8 kg/m2 or waist circumference >102 cm in men and >88 cm in women);17 b) triglycerides >150 mg/dl; c) high-density lipoprotein cholesterol <40 mg/dl in men and <50 mg/dl in women); d) type 2 diabetes or glucose intolerance (fasting glucose >110 mg/dl); e) hypertension (blood pressure >130/85 mmHg). Patients with viral, alcoholic, autoimmune disease, hemochromatosis, and Wilson’s disease were excluded as well as cases of fibrolamellar or mixed hepatocellular-cholangiocellular histopathology. Patients with extrahepatic disease or requiring vascular and/or biliary reconstructions were also excluded.
The primary endpoint was the short-term outcome, focusing on 90-day postsurgical morbidity and mortality. Secondary endpoint was the long-term oncological outcome, including survival and disease-free survival.
Institutional Review Board (IRB) approval was obtained from the coordinating center (n° 16–801, approved December 7th, 2020). Data transfer agreement and IRB approval were included and requested by all participating institutions. Every case was discussed in a multidisciplinary setting according to the centers’ policies.
Both laparoscopic and robotic-assisted procedures were included in the MILR group. Procedures converted to open were analysed in the MILR group following an intention-to-treat policy. Portal hypertension was defined as the radiological presence of significant splenomegaly, umbilical vein recanalization, and/or portosystemic shunts as well, as preoperative platelets count <100,000/mm3.18 Major liver resections were defined as the resection of 3 segments or more according to the Brisbane 2000 nomenclature and the “New World Terminology” of hepatectomies.19, 20 Liver segments II-III-Ivb-V and VI were defined as anterolateral while segments I-IVa-VII and VIII as posterosuperior.21, 22 Morbidity was graded according to the Clavien-Dindo classification and the Comprehensive Complication Index.23, 24 Complications of grade 3 or higher according to Clavien-Dindo classification were considered major morbidity. Postoperative ascites was characterized by a drainage output of more than 10 mL/kg/24h.25 Post hepatectomy liver failure (PHLF) and bile leakage were graded according to the International Study Group on Liver Surgery definition.26, 27
Statistical analysis
Variable’s distribution was assessed using Kolmogorov–Smirnov and Shapiro–Wilk tests. Quantitative data were expressed as mean and standard deviation (sd) or median and first and third quartile (Q1-Q3) as needed, for variable needing a logarithmic transformation, the geometric mean (GM) was reported to present data in the original unit measure. Categorical data were expressed as numbers and percentages (%). The chi-squared test was used to compare differences between categorical variables.
To account for the baseline imbalance of the two groups, propensity scores (PS) were calculated for each patient. The propensity score represented the predicted probability of receiving MILR and was determined using a multivariable logistic regression model using as the independent variables confounders related to both the exposure and the outcome selected a priori based on background knowledge. The model included the following variables: year of operation (2001–2015, 2016–2021), age, gender, BMI (<24.9, 25–29.9, ≥30), comorbidities, cirrhosis, the model for end-stage liver disease (MELD), previous HCC treatment (no treatment, locoregional, resection), the position of lesions (anterolateral, left lobe, posterosuperior, right lobe), number and size of lesions, previous surgery, type of hepatectomy (minor, major), and geographic area (Europe, North America). Inverse probability weight (IPW) was calculated for each patient and used to weight the analysis. Standardized differences (STD) calculated in unweighted and weighted samples were used to assess the balance of baseline covariates between the two groups. Stabilized weights were used to handle very small or very large propensity scores. An STD value ≤0.1 was considered a good balancing. Associations between the type of surgery and outcomes were investigated by weighted logistic, negative binomial, Poisson, and linear regression depending on the outcome variable. Long-term Overall survival (OS) and disease-free survival (DFS) were estimated using weighted Kaplan–Meier curves, and comparisons were performed with weighted Cox Regressions. OS was defined as the time interval from surgery to death for any cause or last follow-up while DFS as the interval from surgery to recurrence in the liver or elsewhere or the date of the last follow-up.
A p<0.05 was considered significant. Statistical analysis was performed with STATA version 16.1 (StataCorp, College Station, TX, USA).
RESULTS
A total of 1100 liver resections for HCC on metabolic syndrome were collected and reviewed. Because no minimally invasive procedures were performed before 2001, 13 patients operated on by open between 1992 and 2001 were excluded from the study. Furthermore, 91 patients were excluded because they had at least one missing value in the variables used to calculate the propensity scores. The final sample of the study consisted of 996 patients, 580 OLR (58.2%) and 416 (41.8%) MILR (Figure 1). Within the MILR group, 353 resections (85.0%) were performed by pure laparoscopy, 52 (12.5%) by hand-assisted laparoscopy, and 11 (3.0%) by robotics. Baseline characteristics before and after inverse probability weighting are depicted in Table 1. In the OLR group there were more patients with diabetes (59.5% vs. 52.6%, STD −0.14) and with a history of ischemic heart disease (22.2% vs. 15.9%, STD −0.16). In comparison, more patients with previous respiratory disease were operated on by minimally invasive approach (18% vs. 13.8%, STD 0.12). A greater proportion of cirrhotic patients were operated on by MILR (42.8% vs. 31.2%, STD 0.24). Tumors in the MILR group were smaller (40 mm (27–56) vs. 55 mm (36–85), STD −0.50) and more likely to be in the anterolateral segments of the liver (56.5% vs. 37.6%, STD 0.39). Minor hepatectomies were more common in MILR (80.3% vs. 60.9%, STD 0.44). while most of the major hepatectomies were performed by open (39.1% vs. 19.7%, STD −0.44). After inverse probability weighting, the above-mentioned differences were corrected, and the two groups were well balanced (Table 1). Furthermore, there were no significant differences in the two groups in additional baseline, intraoperative, and pathological characteristics (Supplementary table 1).
Figure 1.
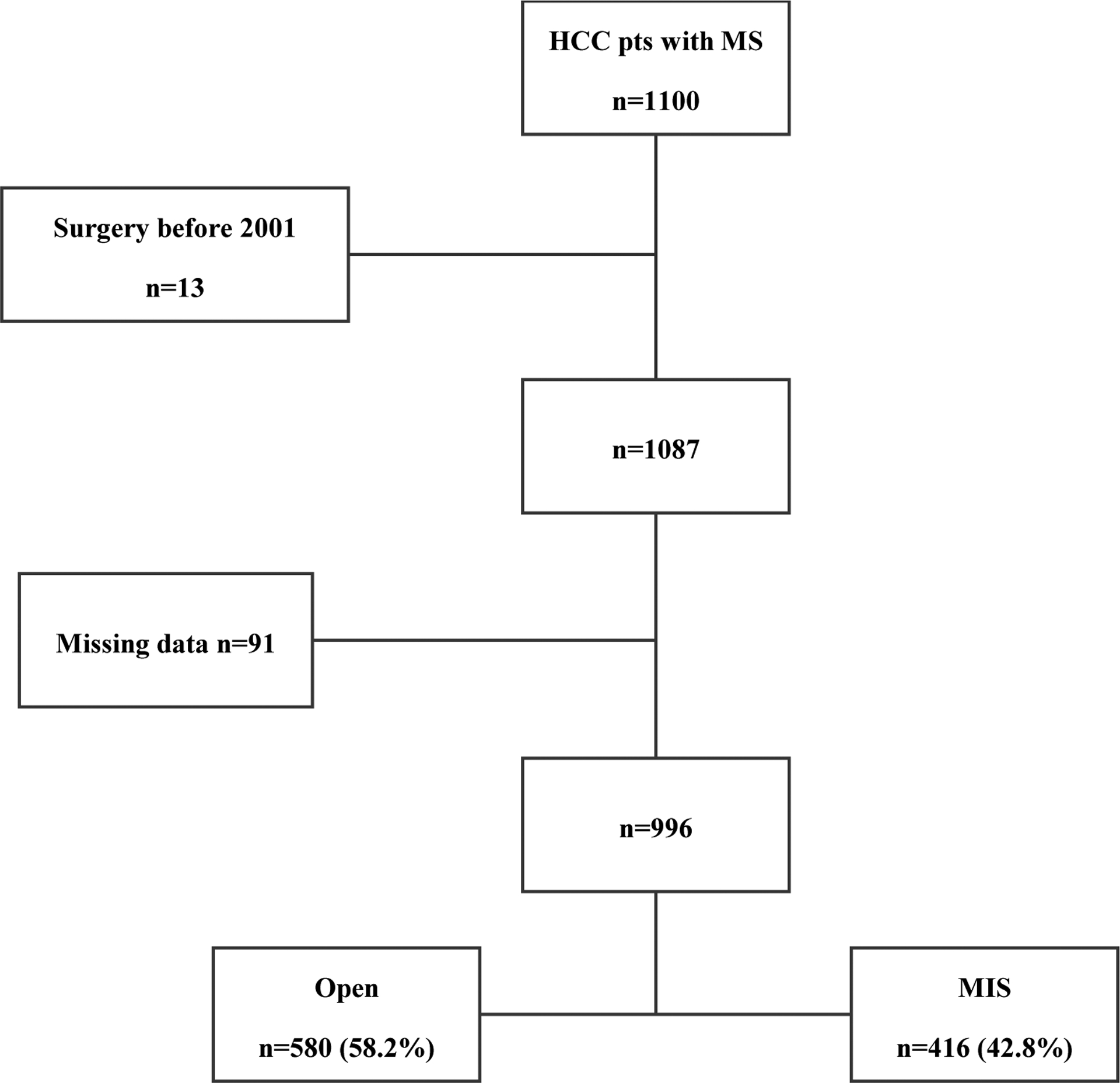
Flow chart of the patients included in the study.
HCC, hepatocellular carcinoma; MS, metabolic syndrome; MIS, minimally invasive surgery
Table 1.
Baseline variables used for propensity score in unweighted and weighted populations.
| Unweighted population | Weighted population | |||||
|---|---|---|---|---|---|---|
| Open (n=580) | Minimally Invasive (n=416) | Unweighted STD | Open (n=580) | Minimally Invasive (n=416) | Weighted STD | |
| Age (years) | 68.6 (±9.2) | 68.9 (±9.0) | 0.04 | 68.7 (9.3) | 69.2 (8.9) | 0.06 |
| Male gender | 422 (72.8%) | 293 (70.4%) | −0.05 | 72.1% | 73.1% | 0.02 |
| Geographic Area | ||||||
| Europe | 319 (55.0%) | 219 (52.6%) | −0.05 | 56.7% | 58.5% | 0.04 |
| North America | 261 (45.0%) | 197 (47.4%) | 0.05 | 43.3% | 41.5% | −0.04 |
| Centers’ volume | ||||||
| High | 275 (47.4%) | 208 (50.0%) | 0.05 | 45.6% | 47.1% | 0.03 |
| Medium | 250 (43.1%) | 167 (40.1%) | −0.06 | 45.0% | 42.5% | −0.05 |
| Low | 55 (9.5%) | 41 (9.9%) | 0.01 | 9.4% | 10.4% | 0.03 |
| Body mass index | ||||||
| <24.9 kg/m 2 | 113 (19.5%) | 80 (19.2%) | 0.00 | 19.5% | 20.1% | 0.01 |
| 25–29.9 kg/m 2 | 204 (35.2%) | 158 (38.0%) | 0.06 | 34.8% | 36.8% | 0.01 |
| ≥ 30 kg/m2 | 263 (45.3%) | 178 (42.8%) | −0.05 | 45.7% | 43.1% | −0.05 |
| Hypertension | 462 (79.7%) | 314 (75.5%) | −0.01 | 78.1% | 77.9% | 0.00 |
| Diabetes | 345 (59.5%) | 219 (52.6%) | −0.14 | 57.9% | 57.7% | 0.00 |
| Ischemic heart disease | 129 (22.2%) | 66 (15.9%) | −0.16 | 19.7% | 19.4% | −0.01 |
| Congestive cardiac failure | 27 (4.7%) | 24 (5.8%) | 0.05 | 5.1% | 4.7% | −0.02 |
| Respiratory disease | 80 (13.8%) | 75 (18.0%) | 0.12 | 15.1% | 16.9% | 0.05 |
| Dyslipidemia | 308 (53.1%) | 225 (54.1%) | 0.02 | 52.8% | 51.6% | −0.02 |
| Previous surgery | 99 (17.1%) | 74 (17.8%) | 0.02 | 17.9% | 19.6% | 0.04 |
| Cirrhosis | 181 (31.2%) | 178 (42.8%) | 0.24 | 34.4% | 34.5% | 0.00 |
| MELD score | 8 (6.4–9) | 8 (6–9.4) | 0.12 | 8 (6.4–9.1) | 7 (6–9) | −0.01 |
| Previous HCC treatment | ||||||
| Locoregional | 73 (12.6%) | 42 (10.1%) | −0.08 | 86.7% | 85.3% | 0.05 |
| Resection | 14 (2.4%) | 9 (2.16%) | −0.02 | 11.2% | 12.7% | −0.01 |
| Year of surgery | ||||||
| 2001–2015 | 415 (71.6%) | 153 (36.8%) | −0.74 | 57.3% | 55.9% | −0.03 |
| 2015–2021 | 165 (28.5%) | 263 (63.2%) | 0.74 | 42.7% | 44.1% | 0.03 |
| Number of lesions | 1 (1–1) | 1 (1–1) | −0.12 | 1 (1–1) | 1 (1–1) | −0.03 |
| Size of lesions (mm) | 55 (36–85) | 40 (27–56) | −0.50 | 50 (32–76) | 48 (32–75) | 0.04 |
| Position of lesions | ||||||
| Anterolateral | 218 (37.6%) | 235 (56.5%) | 0.39 | 44.9% | 46.7% | 0.04 |
| Left lobe | 55 (9.5%) | 30 (7.2%) | −0.08 | 8.6% | 9.0% | 0.01 |
| Posterosuperior | 163 (28.1%) | 111 (26.7%) | −0.03 | 27.6% | 25.1% | −0.06 |
| Right lobe | 144 (24.8%) | 40 (9.6%) | −0.41 | 18.9% | 19.2% | 0.01 |
| Type of hepatectomy | ||||||
| Minor | 353 (60.9%) | 334 (80.3%) | 0.44 | 68.5% | 68.1% | −0.01 |
| Major | 227 (39.1%) | 82 (19.7%) | −0.44 | 31.5% | 31.9% | 0.01 |
STD, Standardized differences. MELD, Model for End Stage Liver Disease. HCC, hepatocellular carcinoma
During surgery, blood loss weighted geometric mean was 275.9 ml (±3.1) in OLR and 226.9 ml (±4.0) in MILR (p=0.146) with 12.2% and 13.1% of patients receiving intraoperative blood transfusions in OLR and MILR respectively (p=0.785). There were no significant differences in terms of any 90 day morbidity (38.9% vs. 31.9% OLRs and MILRs, p=0.08) and mortality (2.4% vs. 2.2% OLRs and MILRs, p=0.84) between groups (Table 2). However, MILRs were associated with lower rates of major complications (9.3% vs. 15.3%, p=0.015), post hepatectomy liver failure (0.6% vs. 4.3%, p=0.008) and bile leaks (2.2% vs. 6.4%, p=0.003). Furthermore, ascites was significantly lower at postoperative day 1 (2.7% vs. 8.1%, p=0.002) and day 3 (3.1% vs. 11.4%, p<0.001). No further differences in specific complications were observed (Supplementary table 2). Finally, hospital stay was significantly shorter in MILR (5.8±1.9 days vs. 7.5±1.7 days, p<0.001). Oncologic outcomes were similar between both approaches. There was no difference in margin positivity rates between OLR and MILR (7.6% and 11.6% respectively; p=0.179). After a median follow-up of 33 (15–59) months for OLR and 31 (16–64) months for MILR, there were also no significant differences in overall survival between groups (HR=0.90, 95% CI 0.69–1.19, p=0.461). Five years overall survival rate was 58.2% in OLR and 60.8% in MILR (Figure 2). Overall, 460 patients (46.1%) recurred after resection, 297 in open and 163 in the minimally invasive group. There were no significant differences in disease-free survival between groups (HR=0.84 95% CI 0.66–1.06 p=0.138). Five years disease-free survival rate was 39.3% in OLR and 47.8% in MILR (Figure 3).
Table 2.
Regression analysis for postoperative outcomes after inverse probability weighting.
| Open n=580 |
Minimally invasive n=416 |
Minimally invasive vs. Open | 95% confidence interval | p | ||
|---|---|---|---|---|---|---|
| 90 day mortality | 2.4% | 2.2% | OR | 0.92 | 0.38–2.22 | 0.845* |
| 90 day morbidity (any) | 38.9% | 31.9% | OR | 0.73 | 0.52–1.04 | 0.081* |
| Number of complications | 0.68 (±1.1) | 0.55 (±1.0) | Beta | −0.22 | −0.57–0.13 | 0.218- |
| Major morbidity | 15.3% | 9.3% | OR | 0.57 | 0.36–0.90 | 0.015 * |
| CCI | 31.3 (±22.7) | 31.9 (±22.2) | Beta | 0.63 | −4.73–5.99 | 0.817# |
| Postoperative ascites | 11.2% | 7.2% | OR | 0.61 | 0.28–1.34 | 0.22* |
| Post hepatectomy liver failure | 4.3% | 0.6% | OR | 0.14 | 0.03–0.60 | 0.008 * |
| Bile leak | 6.4% | 2.2% | OR | 0.33 | 0.15–0.69 | 0.003 * |
| Postoperative day 1 ascites | 8.1% | 2.7% | OR | 0.31 | 0.15–0.65 | 0.002 * |
| Postoperative day 3 ascites | 11.4% | 3.1% | OR | 0.25 | 0.12–0.51 | <0.001 * |
| Postoperative day 5 ascites | 13.7% | 7.2% | OR | 0.51 | 0.17–1.57 | 0.244* |
| Hospital stay (days) | 7.5 (±1.7) | 5.8 (±1.9) | Beta | −0.11 | −0.15–0.7 | <0.001 + |
| Readmission within 30 days | 8.4% | 8.6% | OR | 1.02 | 0.54–1.93 | 0.956* |
| R1 resection | 7.6% | 11.3% | OR | 1.55 | 0.82–2.95 | 0.179* |
| Margin width (mm) | 3.6 (±7.5) | 3.1 (±9.6) | Beta | −0.06 | −0.24–0.12 | 0.497+ |
Continuous data were expressed as mean±standard deviation or median (25th-75th percentile).
calculated from weighted logistic regression.
calculate from weighted negative binomial regression.
calculated from weighted poisson regression.
calculated from weighted linear regression.
calculated from weighted linear regression on log10 values.
CCI, Comprehensive complication index. GM, Geometric mean.
Figure 2.
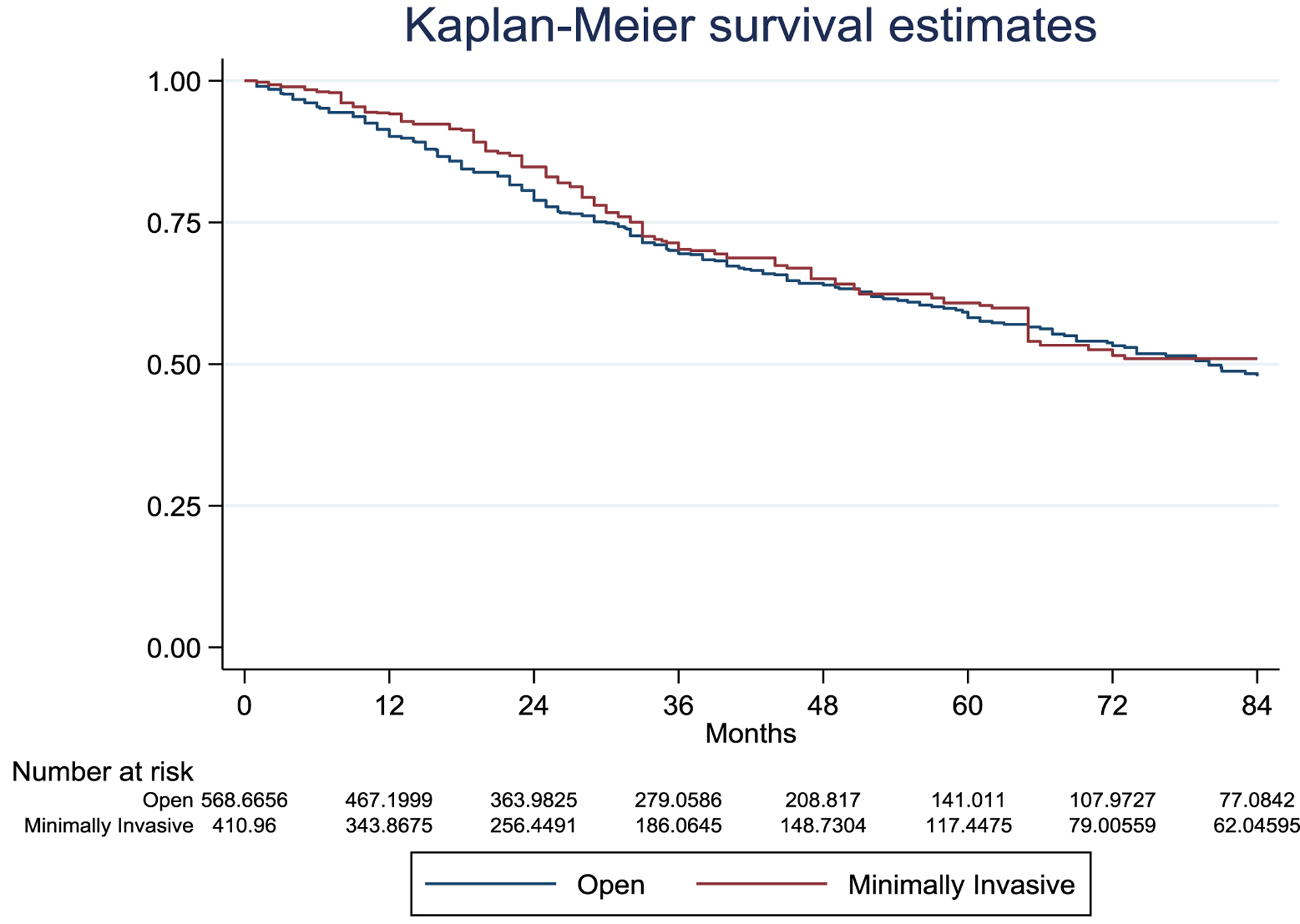
Inverse probability weighted Kaplan-Meier curves of overall survival according to treatment assignment.
Overall survival HR=0.90, 95% CI 0.69–1.19, p=0.461. Number of patients represent the number in the synthetic pseudo-population generated by the inverse probability treatment weighting
Figure 3.
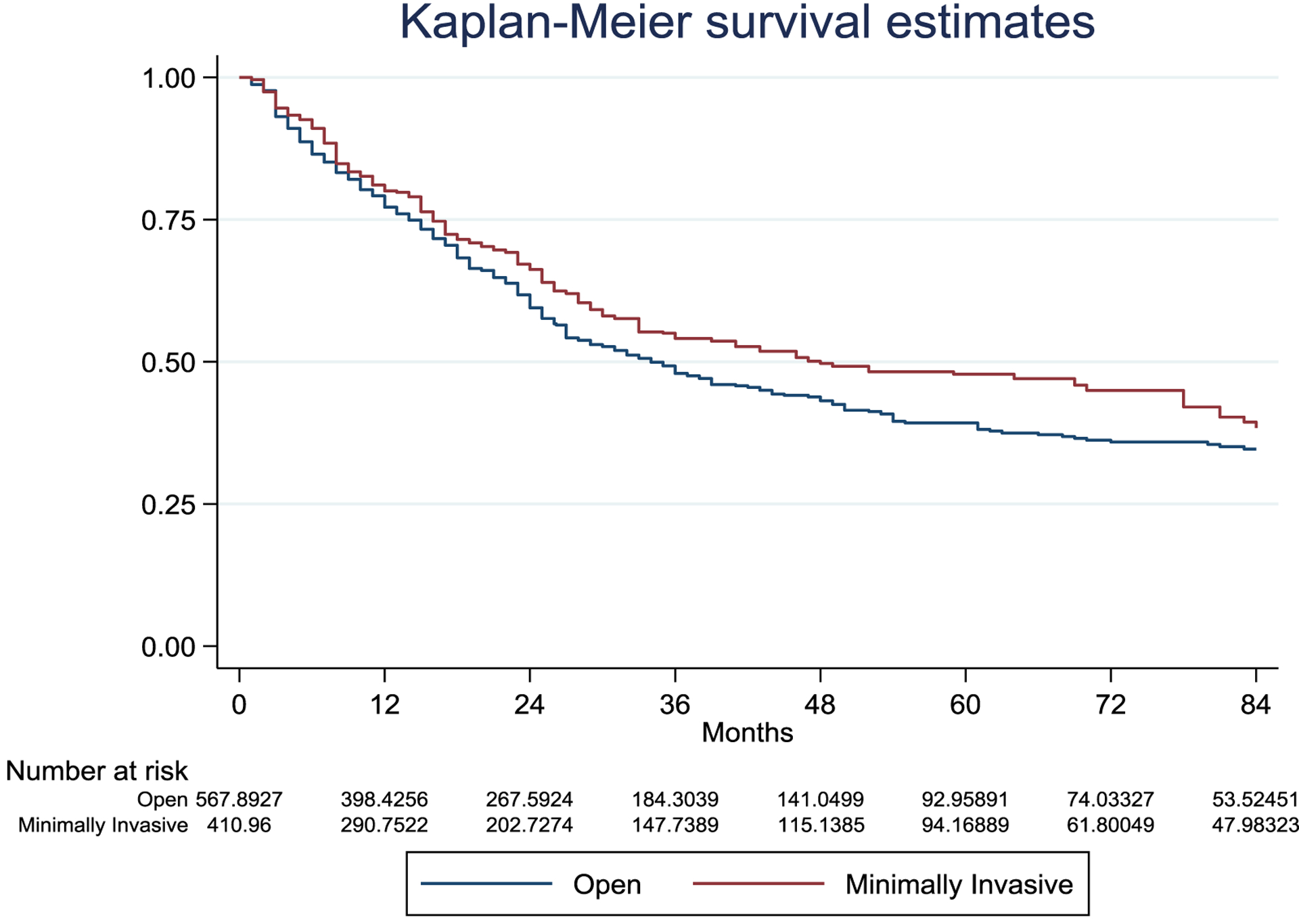
Inverse probability weighted Kaplan-Meier curves of disease-free survival according to treatment assignment.
Disease free survival HR=0.84 95% CI 0.66–1.06 p=0.138. Number of patients represent the number in the synthetic pseudo-population generated by the inverse probability treatment weighting
DISCUSSION
In this study, we have shown that compared to OLR, MILR for patients with HCC and MS is associated with lower rates of major complications, post hepatectomy liver failure, ascites, bile leaks, and shorter hospital stay. Furthermore, MILR was associated with similar long-term oncologic outcomes to OLR.
Because of the high calorie-low fiber diet and the scarce physical activity, a growing number of patients with metabolic syndrome and NAFLD have been diagnosed with HCC and are increasingly being considered for surgery, especially in the West. Liver resections in these patients bear high rates of perioperative morbidity and mortality, and strategies to decrease the risks of surgery are warranted.28, 29 Minimally invasive approaches are safely performed in many hepatobiliary centers worldwide. Indeed, two International consensus conferences, one European guideline, and one dedicated society (the International laparoscopic liver society, ILLS) facilitated the safe implementation and standardization of the technique over the years.13, 30–32 Given this global success, MILRs have been successfully adopted under challenging scenarios such as unfavorable locations, advanced cirrhosis, liver transplantation, and resection for recurrent tumors, challenging the limits of the technique and expanding the commonly accepted indications.21, 33–36 In general, patients with HCC and MS represent a complex clinical scenario, and minimally invasive liver resections in this setting could be at high risk of perioperative complications. Indeed, these patients are frequently obese, have multiple comorbidities, different degrees of portal hypertension, and underlying parenchymal changes (i.e., steatosis, fibrosis, cirrhosis). In our study, most patients were overweight (BMI ≥25 kg/m2) or obese (BMI ≥30 kg/m2) and had preexisting cardiovascular and/or respiratory conditions. Interestingly, before matching, a greater proportion of cirrhotic patients were operated on in the MILR group. Morise et al. and Cipriani et al. have previously demonstrated that minimally invasive approach for HCC allows expanding the indications to patients with advanced cirrhosis given the reduced surgical stress and the lower chances of postoperative decompensation.37, 38 In this setting, it seems reasonable that surgeons might have preferred a minimally invasive approach in cirrhotic patients, to potentially decrease the rates of postoperative morbidity, thereby explaining this imbalance before matching in our study. Despite this, MILRs remain technically more challenging, and easier resections are usually attempted relative to those performed using an open approach. Indeed, more resections in the anterolateral segments were performed by MILRs while major hepatectomies and lesions located posteriorly or superiorly in the so-called unfavorable segments were more commonly operated on by open techniques. After IPW, the above-mentioned imbalances were corrected, and groups were homogenous. Although the overall morbidity was similar, MILR was associated with fewer major complications. In our opinion, this is an important result as patients who develop high-grade complications require invasive intervention, significantly impacting hospital stay, costs, quality of life, and potentially delaying oncological treatments. In addition, complications have been associated with worse long-term oncologic outcomes.39 Reducing the rates of major complications translates into reduced hospital costs and better patient outcomes, both in the short- and long-term. Patients’ decompensation following surgery for HCC is not uncommon and depends on the degree of portal hypertension and preoperative liver conditions. Indeed, adaptations of the portosystemic circulation induced by the altered intrahepatic pressures lead to a fragile equilibrium that can be potentially dismantled with surgery, eventually leading to decompensation. In this study, we have shown that MILR allows respecting this equilibrium, decreasing the rates of post hepatectomy liver failure and the production of ascites. Surgical stress is significantly reduced, portosystemic shunts are respected, extensive liver mobilizations and manipulations are avoided, the abdominal content is not exposed to the air, and less fluids are required, limiting electrolyte imbalances.40 The rates of bile leaks were also reduced in MILR. This may be related to the magnified vision provided by laparoscopic and robotic surgery platforms. This may assist in identifying small structures, allowing the surgeon to be more selective during parenchymal transection. Nowadays, this is further enhanced by technologies such as high definition, 4K, 3D, near-infrared light, and indocyanine green dye. Accurate parenchymal transection and biliostasis allow for opportunities to reduce postoperative bile leaks, bilomas, and eventually major morbidity and invasive procedures.
MILRs are associated with safe oncological outcomes for the treatment of both primary and secondary liver malignancies.41, 42 This has been confirmed in prospective randomized control trials for CRLM and HCC.43, 44 The results of MILRs for HCC in patients with MS have never been investigated. Authors suggest that survivals in this setting are better than those in viral or alcoholic diseases, probably because fewer patients present with cirrhosis and impaired liver function.29, 45 Indeed, NAFLD triggers carcinogenesis, and many patients with MS develop HCC in the absence of cirrhosis. In the present study, we confirm the good survival results of this subset of patients, even when treated by minimally invasive technique. Indeed, 61% of patients were alive, and 48% were disease-free at five years. Importantly, these results were comparable to OLR.
This study has some limitations. The retrospective design might have introduced selection bias. Indeed, this is a cohort of surgical candidates while patients with advanced disease, major comorbidities, or advanced cirrhosis were likely to be excluded. Intention-to-treat studies investigating unselected patients are warranted as they could be informative of the process of selection for both open and minimally invasive surgery. The MILR group of our study was composed of patients undergoing pure laparoscopic, hand-assisted, and robotic liver resections. Despite these being all minimally invasive procedures and having overall comparable short and long-term outcomes, minor differences should be considered when interpreting results. Hand-assisted procedures are used at the beginning of the learning curve as a gradual shift from open to laparoscopy and still represent the preferred approach in a few centers. On the contrary, the experience in robotic surgery is growing rapidly, with exciting results compared to laparoscopy.46 Comparisons with laparoscopy in the setting of MS are warranted and could be of great interest. A recent definition of metabolic-associated fatty liver disease (MAFLD) was proposed:47, 48 unfortunately, collection of data was ongoing when this new definition was proposed. MAFLD should be investigated and validated to standardize terminology among the literature. The findings of reduced ascites and biliary fistula with MILR of the present study should be interpreted with caution. Indeed, we don’t know the exact number of procedures in each group in which drainage was left in place. This can significantly vary between centers and type of resections. Even though inverse probability weighting aims at reducing differences among groups, selection bias at single variable level is not easy to avoid. Pathological differences in T stage and microvascular invasion could confound results, especially survivals, and this should be considered when interpreting results. Finally, this is a Western cohort of patients, and results should not be generalized to Eastern populations in which metabolic syndrome may have a similar prognostic significance but different incidence, risk factors, and pathogenesis.
CONCLUSIONS
Minimally invasive liver resections for hepatocellular carcinoma in patients with metabolic syndrome are associated with comparable overall morbidity,and long-term oncologic outcomes to the open approach. In contrast, minimally invasive liver resections are associated with a significant reduction in major complications, post hepatectomy liver failure, ascites, bile leaks, and shorter hospital stay. The combination of a favorable morbidity profile and equivalent oncologic outcomes favor a minimally invasive surgical approach when feasible for patients with metabolic syndrome. Selection of patients should take into account baseline characteristics, the difficulty of the resection, and surgeon’s experience.
Supplementary Material
Footnotes
No conflict of interest
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017; 24(3):1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer 2009; 115(24):5651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turati F, Talamini R, Pelucchi C, et al. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer 2013; 108(1):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 2011; 54(2):463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010; 51(5):1820–32. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal S, Daruwala C. Metabolic syndrome and hepatic resection: improving outcome. HPB (Oxford) 2011; 13(12):846–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg 2013; 100(1):113–21. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018; 69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 10.de Meijer VE, Kalish BT, Puder M, et al. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg 2010; 97(9):1331–9. [DOI] [PubMed] [Google Scholar]
- 11.Koh YX, Tan HJ, Liew YX, et al. Liver Resection for Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma. J Am Coll Surg 2019; 229(5):467–478 e1. [DOI] [PubMed] [Google Scholar]
- 12.Wakai T, Shirai Y, Sakata J, et al. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg 2011; 15(8):1450–8. [DOI] [PubMed] [Google Scholar]
- 13.Berardi G, Van Cleven S, Fretland AA, et al. Evolution of Laparoscopic Liver Surgery from Innovation to Implementation to Mastery: Perioperative and Oncologic Outcomes of 2,238 Patients from 4 European Specialized Centers. J Am Coll Surg 2017; 225(5):639–649. [DOI] [PubMed] [Google Scholar]
- 14.Fretland AA, Dagenborg VJ, Bjornelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018; 267(2):199–207. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 16.Eckel RH, Alberti KG, Grundy SM, et al. The metabolic syndrome. Lancet 2010; 375(9710):181–3. [DOI] [PubMed] [Google Scholar]
- 17.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010; 51(6):1972–8. [DOI] [PubMed] [Google Scholar]
- 18.Santambrogio R, Kluger MD, Costa M, et al. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 2013; 15(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005; 12(5):351–5. [DOI] [PubMed] [Google Scholar]
- 20.Nagino M, DeMatteo R, Lang H, et al. Proposal of a New Comprehensive Notation for Hepatectomy: The “New World” Terminology. Ann Surg 2021; 274(1):1–3. [DOI] [PubMed] [Google Scholar]
- 21.Berardi G, Aghayan D, Fretland AA, et al. Multicentre analysis of the learning curve for laparoscopic liver resection of the posterosuperior segments. Br J Surg 2019; 106(11):1512–1522. [DOI] [PubMed] [Google Scholar]
- 22.Kazaryan AM, Rosok BI, Marangos IP, et al. Comparative evaluation of laparoscopic liver resection for posterosuperior and anterolateral segments. Surg Endosc 2011; 25(12):3881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013; 258(1):1–7. [DOI] [PubMed] [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg 2009; 144(1):46–51. [DOI] [PubMed] [Google Scholar]
- 26.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011; 149(5):680–8. [DOI] [PubMed] [Google Scholar]
- 27.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011; 149(5):713–24. [DOI] [PubMed] [Google Scholar]
- 28.Paro A, Tsilimigras DI, Dalmacy D, et al. Impact of Metabolic Syndrome on Postoperative Outcomes Among Medicare Beneficiaries Undergoing Hepatectomy. J Gastrointest Surg 2021; 25(10):2545–2552. [DOI] [PubMed] [Google Scholar]
- 29.Vigano L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter matched analysis with HCV-related HCC. J Hepatol 2015; 63(1):93–101. [DOI] [PubMed] [Google Scholar]
- 30.Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018; 268(1):11–18. [DOI] [PubMed] [Google Scholar]
- 31.Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009; 250(5):825–30. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015; 261(4):619–29. [DOI] [PubMed] [Google Scholar]
- 33.Colasanti M, Berardi G, Mariano G, et al. Laparoscopic Left Hepatectomy for Hepatocellular Carcinoma Recurrence Following Liver Transplantation. Ann Surg Oncol 2022. [DOI] [PubMed] [Google Scholar]
- 34.Morise Z, Aldrighetti L, Belli G, et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: a multicentre propensity score-based study. Br J Surg 2020; 107(7):889–895. [DOI] [PubMed] [Google Scholar]
- 35.Suh KS, Hong SK, Lee S, et al. Pure laparoscopic living donor liver transplantation: Dreams come true. Am J Transplant 2022; 22(1):260–265. [DOI] [PubMed] [Google Scholar]
- 36.Troisi RI, Berardi G, Morise Z, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg 2021; 108(2):196–204. [DOI] [PubMed] [Google Scholar]
- 37.Cipriani F, Fantini C, Ratti F, et al. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc 2018; 32(2):617–626. [DOI] [PubMed] [Google Scholar]
- 38.Morise Z, Ciria R, Cherqui D, et al. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci 2015; 22(5):342–52. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda A, Matsumoto S, Seya T, et al. Does postoperative complication have a negative impact on long-term outcomes following hepatic resection for colorectal liver metastasis?: a meta-analysis. Ann Surg Oncol 2013; 20(8):2485–92. [DOI] [PubMed] [Google Scholar]
- 40.Kabir T, Tan ZZ, Syn NL, et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg 2021; 109(1):21–29. [DOI] [PubMed] [Google Scholar]
- 41.Ciria R, Gomez-Luque I, Ocana S, et al. A Systematic Review and Meta-Analysis Comparing the Short- and Long-Term Outcomes for Laparoscopic and Open Liver Resections for Hepatocellular Carcinoma: Updated Results from the European Guidelines Meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann Surg Oncol 2019; 26(1):252–263. [DOI] [PubMed] [Google Scholar]
- 42.Ciria R, Ocana S, Gomez-Luque I, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc 2020; 34(1):349–360. [DOI] [PubMed] [Google Scholar]
- 43.Aghayan DL, Kazaryan AM, Dagenborg VJ, et al. Long-Term Oncologic Outcomes After Laparoscopic Versus Open Resection for Colorectal Liver Metastases : A Randomized Trial. Ann Intern Med 2021; 174(2):175–182. [DOI] [PubMed] [Google Scholar]
- 44.El-Gendi A, El-Shafei M, El-Gendi S, et al. Laparoscopic Versus Open Hepatic Resection for Solitary Hepatocellular Carcinoma Less Than 5 cm in Cirrhotic Patients: A Randomized Controlled Study. J Laparoendosc Adv Surg Tech A 2018; 28(3):302–310. [DOI] [PubMed] [Google Scholar]
- 45.Chin KM, Prieto M, Cheong CK, et al. Outcomes after curative therapy for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: a meta-analysis and review of current literature. HPB (Oxford) 2021; 23(8):1164–1174. [DOI] [PubMed] [Google Scholar]
- 46.Ciria R, Berardi G, Alconchel F, et al. The impact of robotics in liver surgery: A worldwide systematic review and short-term outcomes meta-analysis on 2,728 cases. J Hepatobiliary Pancreat Sci 2020. [DOI] [PubMed] [Google Scholar]
- 47.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol 2020; 73(1):202–209. [DOI] [PubMed] [Google Scholar]
- 48.Eslam M, Sanyal AJ, George J, et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020; 158(7):1999–2014 e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


