Abstract
Purpose
Compared with older women diagnosed with breast cancer, younger women are more likely to die of breast cancer and more likely to suffer psychosocially in both the short-term and long term. The Young Women’s Breast Cancer Study (YWS) is a multisite prospective cohort study established to address gaps in our knowledge about this vulnerable and understudied population.
Participants
The YWS enrolled 1302 women newly diagnosed with stages 0–IV breast cancer at age 40 years or younger at 13 academic and community sites in North America between 2006 and 2016. Longitudinal patient-reported outcome data are complemented by clinical data abstraction and biospecimen collection at multiple timepoints.
Findings to date
Key findings related to fertility include that nearly 40% of participants were interested in pregnancy following diagnosis; of those who reported interest, 10% pursued fertility preservation. Overall, approximately 10% of YWS participants became pregnant in the first 5 years after diagnosis; follow-up is ongoing for pregnancies after 5 years. Studies focused on psychosocial outcomes have characterised quality of life, post-traumatic stress and fear of recurrence, with findings detailing the factors associated with the substantial psychosocial burden many young women face during and following active treatment. Multiple studies have leveraged YWS biospecimens, including whole-exome sequencing of tumour analyses that revealed that select somatic alterations occur at different frequencies in young (age≤35) versus older women with luminal A breast cancer, and a study that explored clonal hematopoiesis of indeterminate potential found it to be rare in young survivors.
Future plans
With a median follow-up of approximately 10 years, the cohort is just maturing for many relevant long-term outcomes and provides outstanding opportunities to further study and build collaborations to address gaps in our knowledge, with the ultimate objective to improve care and outcomes for young women with breast cancer.
Trial registration number
Keywords: ONCOLOGY, Breast tumours, Quality of Life
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Established in 2006, the Young Women’s Breast Cancer Study (YWS) is a multisite, prospective cohort of women with young-onset breast cancer.
The YWS was designed to conduct regular medical record review and collect biospecimens and serial patient-reported outcomes.
Eligibility criteria included: (1) being female; (2) a new diagnosis of stages 0–IV breast cancer; (3) age≤40 years at diagnosis; and (4) ability to understand written and spoken English.
Robust follow-up procedures optimise participant engagement and ensure accurate ascertainment of oncologic outcomes and vital status.
Recruitment was hospital based and may not be truly representative of the general population, potentially impacting generalisability.
Introduction
Greater than 14 000 women aged 40 years and younger are diagnosed with breast cancer annually in the USA alone, with thousands more diagnosed worldwide. Far less is known, however, about breast cancer in younger versus older women. Further, recent population-based data have demonstrated that the incidence of breast cancer is growing in this population.1 2 Breast cancer is the leading cause of cancer-related deaths in women under 40 years of age, and while improved over time, survival rates for young women with breast cancer remain lower compared with older women.3 4 The reasons for the poorer outcomes experienced by young women, as understood to date, are complex and multifactorial. Compared with older women, young women are more likely to present with symptoms and at a more advanced stage, due in part to diagnostic delays and lack of screening in this population.5 6 Breast cancers arising in young women tend to have more unfavourable pathologic features and aggressive subtypes, including greater proportions with luminal B, human epidermal growth factor receptor 2 (HER2)-positive or triple-negative disease.7 8 Young women are at high risk of non-adherence to risk-reducing adjuvant hormonal therapy.9–11 Compounding their disparate disease outcomes, young breast cancer survivors may be at greater risk of long-term or late morbidity, given the aggressive therapy that they usually receive and anticipated long life trajectory in survivorship, although there are limited data in this area.
Of particular significance are the variety of young age-related medical and psychosocial issues that this population faces as a result of their diagnosis and treatment, which contribute to a greater risk of psychosocial distress compared with older patients.12 These issues include hereditary predisposition and risks of future cancer, sexual dysfunction, infertility, premature menopause, body image concerns, role functioning including parenting, career and schooling disruption and the development of short-term and long-term comorbidities. Collectively, these problems may influence treatment decisions, as well as disease and psychosocial outcomes.
Purpose of the Young Women’s Breast Cancer Study (YWS)
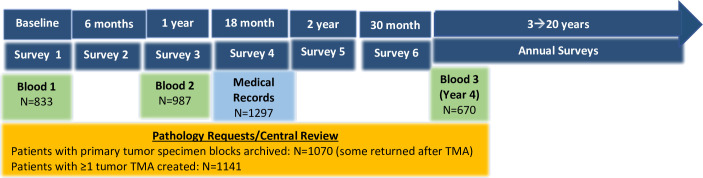
Young women are under-represented in large prospective cohorts as well as in randomised trials evaluating novel therapies and prognostic tools to guide breast cancer treatment decisions.13–15 Hesitancy to extrapolate data from studies of primarily older populations may result in young women being excluded or delayed in benefits from treatment improvements, or put at risk for overtreatment based solely on young age. Even in large prospective cohort studies and clinical trials inclusive of premenopausal women, as well as in population-based registries, there are rarely enough young women and/or adequate granularity of data to draw meaningful conclusions for this subpopulation. For example, the Nurses’ Health Studies have previously reported 374 incident cases age<40 years (vs 2533 cases in women age≥40),16 the Black Women’s Health Study had 529 cases of breast cancer diagnosed at age<45 years documented through 2013 (vs 1534 cases in women aged 45 years and older)17 and the Suppression of Ovarian Function Trial had 933 participants age<40 years at diagnosis,18 with limited details in follow-up for most. The YWS was designed to address gaps in knowledge regarding breast cancer in younger women, including unanswered questions pertaining to diagnosis and presentation, underlying disease biology, optimal treatment, survivorship, psychosocial issues and living with metastatic disease. The YWS is the first and one of the largest cohorts of women with young-onset breast cancer designed to conduct regular medical record review and collect biospecimens and patient-reported outcomes (PROs) (figure 1). The original study objectives were to (1) enrol a cohort of women age≤40 years newly diagnosed with breast cancer; (2) characterise the cohort at diagnosis and in follow-up regarding disease and psychosocial outcomes; and (3) archive tumour and blood specimens for future studies.
Figure 1.
Overview of YWS data and biospecimen collection. YWS, Young Women’s Breast Cancer Study.
Cohort description
Overview
From 2006 to 2016, the YWS enrolled 1302 women from 13 North American academic and community sites (online supplemental table 1). There was high accrual of those approached for enrolment (1302/2186, 60% participate rate). 4 patients were determined to be ineligible, and 1 withdrew consent following enrolment leaving 1297 women who are followed. Reasons for non-participation are detailed in figure 2.
Figure 2.
YWS study flow diagram. YWS, Young Women’s Breast Cancer Study.
bmjopen-2023-081157supp001.pdf (94.7KB, pdf)
Enrolment procedures
At Massachusetts sites, potentially eligible participants were systematically identified by pathology review or by pathology or clinic list review by a research nurse on a monthly or bimonthly basis. The Dana-Farber Cancer Institute (DFCI) Survey and Data Management Core then reviewed the medical record to confirm eligibility. At sites outside of Massachusetts, study staff assisted with recruitment via systematic review of patient lists. Eligibility was confirmed prior to inviting. Inclusion criteria included: (1) being female; (2) a new diagnosis of stages 0–IV breast cancer; (3) age≤40 years at diagnosis; and (4) ability to understand written and spoken English to the extent necessary to complete the questionnaires. Participants enrolled a median of 4 (range: 0–29) months following diagnosis. Following receipt of written informed consent, participants were mailed a welcome letter, medical record release form, tumour specimen request form and the baseline survey. Current median follow-up of the cohort is 10 years (range: 0.4–16 years). Because calculation of follow-up is based on individual patient data, the lower end of this range (eg, 0.4 years) reflects the follow-up duration of patients who died soon after diagnosis and/or were lost to follow-up early in the study.
Participant characteristics
Table 1 includes a summary of YWS participant age at diagnosis, race and ethnicity. Baseline survey questions asked participants about their race and ethnicity. For baseline survey non-responders or instances where this information was not reported, race/ethnicity as determined from medical record at screening/enrolment was used. Regarding racial and ethnic background, approximately 85% of participants identified as White, 4% as Black or African American, and 7% as Asian; 4% idenified as Hispanic. The median age at diagnosis was 37 (range: 17–40) years. Key cancer information including stage, grade, subtype and BReast CAncer gene (BRCA) mutation status is detailed in table 2. Recognising that there have been changes in how we measure social determinants of health since the baseline survey was developed and to make these data more complete, we are sending YWS participants a supplemental survey including updated questions regarding race, ethnicity, gender and sexual identity.
Table 1.
Demographic characteristics
| N (%) | |
| Age at diagnosis (years) | |
| <25 | 25 (2) |
| 25–30 | 139 (11) |
| 31–35 | 353 (27) |
| 36–40 | 780 (60) |
| Race | |
| American Indian/Alaska Native | 6 (<1) |
| Asian | 88 (7) |
| Native Hawaiian/other Pacific Islander | 0 (0) |
| Black or African American | 48 (4) |
| White | 1101 (85) |
| More than one race | 16 (1) |
| Unknown or not reported | 38 (3) |
| Ethnicity | |
| Hispanic | 56 (4) |
| Non-Hispanic | 1045 (81) |
| Unknown or not reported | 196 (15) |
Table 2.
Disease/tumour characteristics and BRCA mutation status (n=1297)
| N (%) | |
| Stage | |
| 0 | 98 (8) |
| I | 413 (32) |
| II | 525 (41) |
| III | 197 (15) |
| IV | 64 (5) |
| Bilateral breast cancer | 21 (2) |
| Tumour grade | |
| 1 | 89 (7) |
| 2 | 445 (34) |
| 3 | 752 (58) |
| Missing/unknown | 11 (0.9) |
| ER status | |
| Positive | 945 (73) |
| Negative | 351 (27) |
| Missing/unknown | 1 (<1) |
| PR status | |
| Positive | 848 (65) |
| Negative | 441 (34) |
| Missing/unknown | 8 (<1) |
| HER2 status | |
| Positive | 360 (28) |
| Negative | 880 (68) |
| Missing/unknown/not performed* | 57 (4) |
| Subtype | |
| Luminal A-like | 395 (31) |
| Luminal B-like | 269 (21) |
| Luminal B/HER2 | 255 (20) |
| HER2-enriched (ER−, PR−, HER2+) | 105 (8) |
| Triple negative | 210 (16) |
| Missing/unknown | 63 (5) |
| BRCA mutation status | |
| BRCA1+ | 90 (7) |
| BRCA2+ | 54 (4) |
| Variant of unknown significance | 54 (4) |
| No mutation detected | 919 (71) |
| Not tested or unknown testing status | 180 (14) |
*Missing/unknown subtype includes cases of DCIS for which HER2 was not performed.
BRCA, BReast CAncer gene; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Patient and public involvement
Since the study’s inception, we have invited patient volunteers and patient advocates to review survey content as well as assist with strategies to communicate results to YWS participants and other young patients. We routinely share lay summary results from YWS analyses directly through the DFCI Programme for Young Adults with Breast Cancer newsletter as well as intermittent webinars and patient forums. Newsletters and webinars are also archived on the programme’s public website (https://youngandstrong.dana-farber.org).
YWS data collection and follow-up
Surveys: Participants completed surveys at study baseline (median of 5 months post diagnosis), 6 months after enrolment and 1 year post diagnosis. Participants were then surveyed every 6 months through 3 years, and annually thereafter. Participants enrolled at the Toronto site (n=62) are sent abbreviated (‘short-form’) surveys that ask for sociodemographics, how their breast cancer presented, fertility and gynaecologic information and cancer endpoints. At the 10-year timepoint, Toronto participants are sent a full survey to complete. A smaller subset of participants (n=29) who enrolled at other sites chose to complete the short-form follow-up annual surveys that only ask about fertility, gynaecologic and cancer outcomes. For the initial 7 years of the YWS, all surveys were mailed to main study participants; participants completing short-form surveys were given the option of completing surveys on paper or online via Survey Monkey. Beginning at the 8-year survey timepoint, participants were offered the option to complete an electronic survey through REDCap. In March 2020, due to COVID-19 restrictions limiting the ability to mail materials, participants with a valid email address (98%) were sent surveys through REDCap. Collectively, these surveys have yielded an unparalleled resource of longitudinal PRO data related to menopausal status, fertility, anxiety, depression, quality of life (QOL), fear of recurrence, treatment side effects, employment, genetics and treatment decision-making (see table 3 for domains/timeline for the first 15 years of follow-up). We also periodically invite participant subsets to complete supplemental surveys to investigate salient issues in greater detail.19–24
Table 3.
Summary of primary survey domains and schedule, baseline through year 15
| Survey schedule | |||||||||||||||||||
| Month | Year | ||||||||||||||||||
| Baseline | 6 | 12 | 18 | 24 | 30 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Sociodemographics including insurance, finances | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Medical/family history | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Genetics | X | X | X | X | X | X | |||||||||||||
| Health-related activities/social history | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Fertility/gynaecologic | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Lactation assessment | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Contraception | X | X | |||||||||||||||||
| QOL/anxiety/depression | X | X | X | X | X | X | X | X | X | X | |||||||||
| Coping | X | X | X | ||||||||||||||||
| Fear of recurrence | X | X | X | X | X | X | X | X | X | ||||||||||
| Post-traumatic stress | X | ||||||||||||||||||
| Menopausal symptoms | X | X | X | X | X | X | X | X | X | X | |||||||||
| Social support | X | X | X | X | X | X | |||||||||||||
| Spirituality assessment | X | X | |||||||||||||||||
| Treatment decisions | X | X | |||||||||||||||||
| Medications list | X | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Tamoxifen adherence | X | ||||||||||||||||||
| Healthcare utilisation | X | X | |||||||||||||||||
QOL, quality of life.
Follow-up to optimise survey response rates: A research coordinator systematically contacts participants who do not complete their surveys with a phone call at 3 weeks after the initial survey is sent out; the survey is resent at 6 weeks if the participant has still not completed the survey at that timepoint. Additional calls and/or survey resends are then conducted monthly up to 6 months following the initial send out, as needed. Response rates (surveys received/surveys sent) for the majority of survey timepoints range from 86% to 91%. Response rates for 7-year and 10-year surveys are modestly lower (71% for 7-year survey and 65% for 10-year survey) due to our attempt to re-engage non-responding participants at those timepoints.
Medical records: Medical records through the first 18 months following diagnosis are available for 100% of participants. Records are also reviewed at additional timepoints (including at 10 years) to (1) confirm and collect specifics on cancer outcomes (recurrences, new primary breast cancers, other cancers) that are self-reported on surveys and (2) to update comorbidities including recurrences and new cancers and vital status among participants who do not regularly complete surveys. We have abstracted initial treatment information, including specific chemotherapy regimens started and received, surgery, radiation, genetic testing (panel type and results) and comorbidities using the Charlson framework25 26 from the medical record to validate/supplement what patients report on surveys, and to fill in missing data.
Ascertainment of cancer outcomes and vital status: Recurrences, new primary breast cancers and new (non-breast) cancers are assessed on each survey. In addition to site(s) of recurrence or new cancer, participants are asked to report the date of the recurrence. Any self-report of a recurrence or new cancer, including site and date (either radiologic or pathologic confirmation) is confirmed by medical record review. Because this process relies on survey responses to ascertain outcomes, for those participants who are no longer responding to surveys, we request and review updated medical records for these patients every 4 years. For participants treated at DFCI, Brigham and Women’s Hospital, Massachusetts General Hospital or an affiliate network site, the National Death Index is queried periodically to obtain vital status information. Following the 2018–2019 update, we were able to ascertain oncologic outcomes and vital status for 321 patients who were survey non-responders. As of September 2023, among the entire cohort, we have documented 181 participants with distant metastatic recurrences, 96 participants with locoregional recurrences, 22 participants with new primary breast cancers and 49 participants with new non-breast primary cancers, and 186 deaths.
Tissue specimen collection/storage: Pathology reports, H&E-stained slides and a representative paraffin block of tumour were requested from the institution where the patient had her surgery; the patient’s signed release for the specimens and reports were sent to the pathology department. A YWS study pathologist reviewed each pathology report and an H&E-stained section of the tumour block to annotate tumour details.8 27 Slides from the core needle biopsy were requested as well as from the surgery in neoadjuvant therapy cases. Hormone and HER2 receptor expression, and lymph node status, were extracted from the pathology reports. Of 1297 participants followed, 1278 (99%) consented to providing a tumour specimen for review. Primary tumour pathology has been centrally reviewed on 97% of patients (1242/1278), with blocks (n=1120) and cores (n=21) for the tissue microarray from tumour specimens collected on 1141 patients (88%). Currently, there are 1370 specimens from 1242 patients available in the tissue microarray blocks (patients who received neoadjuvant therapy may have more than one block).
Blood collection/storage: Blood is collected at three timepoints (two 10 mL tubes of whole blood at each timepoint), each with a several month ‘window’ to maximise our ability to collect blood while minimising burden: baseline (enrolment up to 9 months post diagnosis); 1 year (9 months to 2 years post diagnosis) and 4 year (3.5–5 years post diagnosis). Overall, 94% (1224/1297) of participants consented to blood collection. Of these, there is at least one sample available from 92% of participants, with 73% of participants providing samples at two or more timepoints (online supplemental table 2). Women were asked to go to their treating institution or a local laboratory with the materials provided in the blood specimen kit, unless drawn at DFCI where kits are provided on site by staff. Specimens were processed at the DFCI Breast Cancer CORE Blood Repository, where whole blood (four aliquots/2 mL each) and plasma (two aliquots/2 mL each) were isolated and banked for future studies.
Findings to date
Since its inception, YWS investigations have characterised more fully the experience of breast cancer in young women, creating a platform from which to study molecular and biologic issues, as well as the health and psychosocial repercussions of a breast cancer diagnosis and treatment at a young age. As the YWS has matured, we have expanded our investigations to include collaborations with basic and translational scientists enabling us to address new questions, advancing knowledge of the biology of the disease as well as to improve clinical care and outcomes. In addition, research findings from the YWS have informed international guidelines and clinical practice.28 29
Fertility issues and pregnancy
In 2014, we published findings from the baseline survey regarding fertility concerns and fertility preservation strategies used.30 Since then, additional publications from the YWS have described breast cancer diagnosed during pregnancy,31 pregnancy after breast cancer,32 treatment-related amenorrhoea,33 fertility concerns and endocrine therapy decision-making,34 and fertility concerns in germline genetic carriers.35 Key findings included the observation that nearly 40% of participants were interested in pregnancy after breast cancer; of those interested, 10% pursued fertility preservation strategies.30 Another study documented that approximately 20% of women become amenorrhoeic in survivorship after standard chemotherapy, with variability by chemotherapy type and age.33 A third study evaluated postdiagnosis pregnancies, observing that 10% became pregnant in the first 5 years after breast cancer.32 Fertility outcomes remain a primary outcome of interest in continued follow-up of the cohort and the rich and detailed fertility and gynaecologic data collected at each timepoint will allow for novel and detailed investigations regarding impact of pregnancy on cancer endpoints, and menopausal and related comorbidity outcomes. Findings from this work have been referenced in international guidelines28 29 and informed an international trial that evaluated the safety of pregnancy after breast cancer.36 We are also participating in an international, multisite, retrospective cohort study (PI: Dr Matteo Lambertini, University of Genoa, IT) inclusive of >1400 patients with BRCA+ breast cancer from 30 sites across North America, Europe, Latin America and Israel, contributing clinical, genetic and PRO data on 125 YWS participants with BRCA mutations. To date, there have been two major publications reporting analyses of pregnancy outcomes as well as clinical outcomes from this collaboration.37 38
Systemic treatment and disease recurrence and response
In 2017, we conducted a study examining the prognostic value of genomic expression prediction assays in the YWS. Prior research that led to the routine incorporation of such tools into clinical care had included relatively few women<40 years old, resulting in hesitation among providers to use them for young women. We demonstrated that Recurrence Score (RS) was prognostic and appeared to be predictive of chemotherapy benefit or lack thereof in patients with node-negative disease.39 These data have informed recent guidelines and clinical practice supporting the use of RS in young patients to influence chemotherapy decisions.28 We subsequently demonstrated the association of RS with pathologic complete response after neoadjuvant chemotherapy for hormone receptor-positive disease40; results from this study are providing preliminary data for planned correlative work in a preoperative clinical trial in the Alliance for Clinical Trials in Oncology.
Psychosocial implications of breast cancer in young women
A large focus of the YWS has been to study the early psychological and social repercussions of breast cancer in young women to identify areas for tailored support and management strategies. We have characterised sexual functioning and body image issues, the impact of a diagnosis on employment, post-traumatic stress, fear of recurrence, anxiety and depression in women with de novo stage IV disease, as well as the impact of the diagnosis on the partners of YWS participants.41–48 Recent studies have found that more extensive surgery and radiation are associated with poorer QOL21 49 as well as increased arm morbidity.50 Collectively, our findings indicate that young women experience a substantial psychosocial burden during and following the completion of active treatment, and this burden is associated with certain patient, disease and treatment variables which can be targeted for intervention.
Biological differences in breast cancer by age
Collaborative efforts have yielded important findings to date including a comparison of whole-exome sequencing profiles of the youngest 100 participants (≤35 years at diagnosis) to older women from The Cancer Genome Atlas.51 52 This analysis revealed that somatic alterations in three genes (PIK3CA, GATA3 and ARID1A) occur at different frequencies in young versus older women with luminal A breast cancer.52 Additional investigation of these genes could delineate biological susceptibilities for young patients. Several additional studies have used or pooled tissue and/or blood specimens including a genome wide association study characterising single nucleotide polymorphisms in young patients,53 a study of RasGAP genes in luminal B tumours,54 a study of somatic mutations in patients with metastatic triple negative breast cancer,55 and the development of a test to identify minimal residual disease in early-stage breast cancer.51 Most recently, we used novel DNA sequencing methods to evaluate the prevalence and mutation spectrum of clonal hematopoiesis in young women and its association with patient and treatment characteristics and outcomes.56 Reassuringly, clonal hematopoiesis of indeterminate potential was rare, with a prevalence of <3% among the 878 women in the analytic cohort.56 The YWS has also provided clinical data detailing cancer outcomes of nulliparous versus recently parous women, for an analysis supporting the novel preclinical finding that weaning-induced liver involution establishes a prometastatic microenvironment, a potential explanation for more poor cancer outcomes of recently parous women.57 Several analyses to understand the biologic underpinnings of early onset breast cancer are underway.
Collaboration
The YWS is uniquely positioned for investigations of age-related tumour and host biology as well as studies evaluating the impact of hereditary predisposition, postdiagnosis pregnancy, premature menopause, psychosocial, lifestyle and care delivery on cancer endpoints and comorbidities. The YWS research team includes medical, surgical and radiation oncologists, epidemiologists, pathologists and biostatisticians, and our collaborations have grown to include experts in cancer genomics, biology, tumour microenvironment and behavioural health. While data are not publicly available, investigators interested in accessing YWS-generated data and/or biospecimens should contact the principal investigator (AHP) and submit a request to the Dana-Farber Harvard Cancer Center breast users committee (https://www.dfhcc.harvard.edu/research/research-programs/clinical-based-programs/breast-cancer/program-resources/dfhcc-breast-users-committee/).
Future directions
The YWS has proven to be a rich scientific resource that has resulted in impactful research that has advanced our understanding of breast cancer in young women, generated new lines of investigation and informed clinical guidelines. Breast cancer is the leading cause of cancer-related deaths in young women, and while improved over time, survival rates for young women with breast cancer remain lower compared with older women.3 4 Thus, identifying risk factors for these poorer outcomes in younger women is critical. With the cohort maturing, we expect the number of recurrent disease events expected to increase by ~50% over the next 5 years and beyond, allowing for further evaluation of predictors of oncologic outcomes, including breast cancer free survival and overall survival. With relatively large numbers of young patients treated with contemporary regimens and well-annotated treatment details, we will be well-positioned to evaluate the impact of postdiagnosis pregnancies on recurrence, new primary cancer or death, assessment of risk factors for second cancers by breast cancer phenotype and germline pathogenic variant status, as well as risk factors for late recurrences among women diagnosed at a young age. Studies of the role of ctDNA on predicting late recurrence and exploring tumour and host characteristics that may be conducive to recurrence/disease resistance to therapy in young women (eg, tumour immune microenvironment differences by recent parity status at diagnosis) have great potential.
As a survivorship cohort, evaluating long-term, late effects of breast cancer treatment and survivorship is a central objective of the YWS. A critical charge of cancer survivorship research is identifying potentially modifiable factors that predict these risks. Young breast cancer survivors may be at high risk of long-term or late morbidity given the aggressive therapy they usually receive and anticipated long life trajectory after the cancer. Additionally, they may have hereditary, behavioural and comorbidity predispositions to disease. The concerns unique to or accentuated by their stage of life at diagnosis also appear to contribute to the increased risk of psychological distress seen in this age group both at diagnosis and in long-term follow-up.12 With a median follow-up of 10 years, we are now well poised to study late and long-term morbidity, including premature menopause (age<45 years), which in non-cancer populations is associated with substantial multimorbidity including increased risk of depression, hyperlipidemia, heart disease, asthma, chronic obstructive pulmonary disease, arthritis and osteoporosis.58 On each survey, YWS participants are asked about their menstrual status, including date of last menstrual period, reasons for periods stopping, as well as questions about gynaecologic procedures providing granular data to inform analyses. Given the current age of the cohort, the next several years are a critical time to conduct in-depth assessment of the menopausal transition among women who remained premenopausal following treatment, including timing, risk factors for premature menopause and the impact of early menopause on QOL and comorbidities in this population. This data resource will also facilitate investigations ranging from healthcare utilisation assessments, changes in lifestyle factors over time, and trajectories of QOL, to evaluations of the association of specific co-morbidities (eg, heart disease, diabetes) with premature menopause, weight gain, body composition, metabolic biomarkers, inflammatory mediators, hormone levels and biomarkers of accelerated ageing.
Strengths and limitations
With a repository of biospecimens, clinical data and serial PRO collection, the YWS is a robust platform from which to study molecular and biologic issues, as well as the health and psychosocial repercussions of a breast cancer diagnosis and treatment in young women. One of the core strengths of the YWS is high participant engagement, which has facilitated our ability to follow participants for both medical and psychosocial outcomes in extended follow-up. Additionally, we have leveraged the collection of blood specimens at three timepoints—baseline, 1 year and 4 years after diagnosis—for novel investigations that are complemented with clinical data that have been systematically abstracted from the medical record. However, as the YWS is an observational cohort study, establishing causality versus association can be challenging and there is a risk of unmeasured or unaccounted for confounders in analyses. As the main objective of the YWS was to establish a survivorship cohort that followed young women diagnosed with breast cancer, women older than 40 and women without a history of cancer were not enrolled; thus, our study is not designed to enable cross-age comparisons or comparisons with non-cancer, age-matched ‘controls.’ Additionally, while the YWS is a multisite study that included both academic and community sites, women enrolled in the YWS are predominantly white, non-Hispanic and highly educated, which may impact generalizability of findings to young breast cancer survivors from more diverse racial, ethnic and socioeconomic backgrounds. Acknowledging this limitation, more recent studies conducted by our team, including intervention trials informed by YWS findings, have focused efforts to improve outreach to patients from historically under-represented racial and ethnic groups.
Supplementary Material
Acknowledgments
We thank all participants for their past and continued contributions to the YWS study. We also thank Ms. Kate Bifolck for editorial and submission assistance; she is a full-time employee of Dana-Farber Cancer Institute.
Footnotes
Contributors: AHP, EPW, RMT and SG conceptualised and developed the cohort and methodology. LC provided oversight for pathology review. MEM, CS and GJK have supported data and biospecimen collection and management. SG and YZ have provided statistical support. KR, LS, JP, SC, VFB and EW have served as site investigators. AHP, KR and PDP provided oversight for medical record/clinical data review. AHP and SMR provided general oversight and management of the study. AHP provided funding for the study. AHP is the guarantor of the study. All authors read and approved the final manuscript.
Funding: This study was supported by Susan G. Komen (SAC100008, Partridge) and Breast Cancer Research Foundation (BCRF-21-124, Partridge)
Competing interests: SMR reports grant funding from Conquer Cancer/Pfizer. VFB reports research funding (paid to institution) from SeaGen, AstraZeneca, Gilead and Olema; consultation for SeaGen, AstraZeneca, Lilly, Gilead and Olema; and stock/founder of PEARL Scientific. AHP reports royalties from Wolters Kluwer (paid to self) for authorship of UpToDate. All remaining authors have no disclosures to report.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Cohort description section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Requests to access YWS data/biospecimens should be made to the principal investigator, AHP (ann_partridge@dfci.harvard.edu).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The YWS is approved by the institutional review board at Dana-Farber Harvard Cancer Center (IRB# 06-169) and other participating sites. Participants gave informed consent to participate in the study before taking part.
References
- 1. Ellington TD, Miller JW, Henley SJ, et al. Trends in breast cancer incidence, by race, ethnicity, and age among women aged >/=20 years - United States, 1999-2018. MMWR Morb Mortal Wkly Rep 2022;71:43–7. 10.15585/mmwr.mm7102a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller KD, Fidler-Benaoudia M, Keegan TH, et al. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin 2020;70:443–59. 10.3322/caac.21637 [DOI] [PubMed] [Google Scholar]
- 3. Guo F, Kuo Y-F, Shih YCT, et al. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer 2018;124:3500–9. 10.1002/cncr.31638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim HJ, Kim S, Freedman RA, et al. The impact of young age at diagnosis (age <40 years) on prognosis varies by breast cancer subtype: A U.S. SEER database analysis. Breast 2022;61:77–83. 10.1016/j.breast.2021.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Partridge AH, Hughes ME, Ottesen RA, et al. The effect of age on delay in diagnosis and stage of breast cancer. Oncologist 2012;17:775–82. 10.1634/theoncologist.2011-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zabicki K, Colbert JA, Dominguez FJ, et al. Breast cancer diagnosis in women < or = 40 versus 50 to 60 years: increasing size and stage disparity compared with older women over time. Ann Surg Oncol 2006;13:1072–7. 10.1245/ASO.2006.03.055 [DOI] [PubMed] [Google Scholar]
- 7. Azim HA, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res 2014;16:427. 10.1186/s13058-014-0427-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzmán-Arocho YD, Rosenberg SM, Garber JE, et al. Clinicopathological features and BRCA1 and BRCA2 Mutation status in a prospective cohort of young women with breast cancer. Br J Cancer 2022;126:302–9. 10.1038/s41416-021-01597-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011;126:529–37. 10.1007/s10549-010-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 2003;21:602–6. 10.1200/JCO.2003.07.071 [DOI] [PubMed] [Google Scholar]
- 11. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 2010;28:4120–8. 10.1200/JCO.2009.25.9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howard-Anderson J, Ganz PA, Bower JE, et al. Fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:386–405. 10.1093/jnci/djr541 [DOI] [PubMed] [Google Scholar]
- 13. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29. 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 14. Kalinsky K, Barlow WE, Gralow JR, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 2021;385:2336–47. 10.1056/NEJMoa2108873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14. 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warner ET, Colditz GA, Palmer JR, et al. Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: are there differences before and after age 40? Breast Cancer Res Treat 2013;142:165–75. 10.1007/s10549-013-2721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertrand KA, Bethea TN, Adams-Campbell LL, et al. Differential patterns of risk factors for early-onset breast cancer by ER status in African American women. Cancer Epidemiol Biomarkers Prev 2017;26:270–7. 10.1158/1055-9965.EPI-16-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 2018;379:122–37. 10.1056/NEJMoa1803164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borstelmann NA, Gray TF, Gelber S, et al. Psychosocial issues and quality of life of parenting partners of young women with breast cancer. Support Care Cancer 2022;30:4265–74. 10.1007/s00520-022-06852-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borstelmann NA, Rosenberg S, Gelber S, et al. Partners of young breast cancer survivors: a cross-sectional evaluation of Psychosocial concerns, coping, and mental health. J Psychosoc Oncol 2020;38:670–86. 10.1080/07347332.2020.1823546 [DOI] [PubMed] [Google Scholar]
- 21. Dominici L, Hu J, Zheng Y, et al. Association of local therapy with quality-of-life outcomes in young women with breast cancer. JAMA Surg 2021;156:e213758. 10.1001/jamasurg.2021.3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med 2013;159:373–81. 10.7326/0003-4819-159-6-201309170-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker HE, Rosenberg SM, Stanton AL, et al. Perceptions, attributions, and emotions toward endocrine therapy in young women with breast cancer. J Adolesc Young Adult Oncol 2016;5:16–23. 10.1089/jayao.2015.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sella T, Fell GG, Miller PG, et al. Patient perspectives on testing for clonal hematopoiesis of indeterminate potential. Blood Adv 2022;6:6151–61. 10.1182/bloodadvances.2022008376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 26. Sangha O, Stucki G, Liang MH, et al. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–63. 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 27. Collins LC, Marotti JD, Gelber S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat 2012;131:1061–6. 10.1007/s10549-011-1872-9 [DOI] [PubMed] [Google Scholar]
- 28. Paluch-Shimon S, Cardoso F, Partridge AH, et al. ESO-ESMO 4TH international consensus guidelines for breast cancer in young women (BCY4). Ann Oncol 2020;31:674–96. 10.1016/j.annonc.2020.03.284 [DOI] [PubMed] [Google Scholar]
- 29. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:1994–2001. 10.1200/JCO.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 30. Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol 2014;32:1151–6. 10.1200/JCO.2013.52.8877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee GE, Rosenberg SM, Mayer EL, et al. Contemporary management of breast cancer during pregnancy and subsequent Lactation in a multicenter cohort of young women with breast cancer. Breast J 2019;25:1104–10. 10.1111/tbj.13431 [DOI] [PubMed] [Google Scholar]
- 32. Poorvu PD, Gelber SI, Zheng Y, et al. Pregnancy after breast cancer: results from a prospective cohort of young women with breast cancer. Cancer 2021;127:1021–8. 10.1002/cncr.33342 [DOI] [PubMed] [Google Scholar]
- 33. Poorvu PD, Hu J, Zheng Y, et al. Treatment-related amenorrhea in a modern, prospective cohort study of young women with breast cancer. NPJ Breast Cancer 2021;7:99. 10.1038/s41523-021-00307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sella T, Poorvu PD, Ruddy KJ, et al. Impact of fertility concerns on endocrine therapy decisions in young breast cancer survivors. Cancer 2021;127:2888–94. 10.1002/cncr.33596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewinsohn R, Zheng Y, Rosenberg SM, et al. Fertility preferences and practices among young women with breast cancer: germline genetic carriers versus noncarriers. Clin Breast Cancer 2023;23:317–23. 10.1016/j.clbc.2022.12.012 [DOI] [PubMed] [Google Scholar]
- 36. Partridge AH, Niman SM, Ruggeri M, et al. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med 2023;388:1645–56. 10.1056/NEJMoa2212856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lambertini M, Ameye L, Hamy A-S, et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J Clin Oncol 2020;38:3012–23. 10.1200/JCO.19.02399 [DOI] [PubMed] [Google Scholar]
- 38. Lambertini M, Ceppi M, Hamy A-S, et al. Clinical behavior and outcomes of breast cancer in young women with germline BRCA pathogenic variants. NPJ Breast Cancer 2021;7:16. 10.1038/s41523-021-00224-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poorvu PD, Gelber SI, Rosenberg SM, et al. Prognostic impact of the 21-gene recurrence score assay among young women with node-negative and node-positive ER-positive/Her2-negative breast cancer. J Clin Oncol 2020;38:725–33. 10.1200/JCO.19.01959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sella T, Gelber SI, Poorvu PD, et al. Response to neoadjuvant chemotherapy and the 21-gene breast recurrence score test in young women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat 2021;186:157–65. 10.1007/s10549-020-05989-5 [DOI] [PubMed] [Google Scholar]
- 41. Borstelmann NA, Rosenberg SM, Ruddy KJ, et al. Partner support and anxiety in young women with breast cancer. Psychooncology 2015;24:1679–85. 10.1002/pon.3780 [DOI] [PubMed] [Google Scholar]
- 42. Rosenberg SM, Vaz-Luis I, Gong J, et al. Employment trends in young women following a breast cancer diagnosis. Breast Cancer Res Treat 2019;177:207–14. 10.1007/s10549-019-05293-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vazquez D, Rosenberg S, Gelber S, et al. Posttraumatic stress in breast cancer survivors diagnosed at a young age. Psychooncology 2020;29:1312–20. 10.1002/pon.5438 [DOI] [PubMed] [Google Scholar]
- 44. Park EM, Gelber S, Rosenberg SM, et al. Anxiety and depression in young women with metastatic breast cancer: a cross-sectional study. Psychosomatics 2018;59:251–8. 10.1016/j.psym.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Hippel C, Rosenberg SM, Austin SB, et al. Identifying distinct trajectories of change in young breast cancer survivors' sexual functioning. Psycho-Oncology 2019;28:1033–40. 10.1002/pon.5047 [DOI] [PubMed] [Google Scholar]
- 46. Rosenberg SM, Tamimi RM, Gelber S, et al. Treatment-related amenorrhea and sexual functioning in young breast cancer survivors. Cancer 2014;120:2264–71. 10.1002/cncr.28738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg SM, Tamimi RM, Gelber S, et al. Body image in recently diagnosed young women with early breast cancer. Psychooncology 2013;22:1849–55. 10.1002/pon.3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schapira L, Zheng Y, Gelber SI, et al. Trajectories of fear of cancer recurrence in young breast cancer survivors. Cancer 2022;128:335–43. 10.1002/cncr.33921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosenberg SM, Dominici LS, Gelber S, et al. Association of breast cancer surgery with quality of life and Psychosocial well-being in young breast cancer survivors. JAMA Surg 2020;155:1035–42. 10.1001/jamasurg.2020.3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuijer A, Dominici LS, Rosenberg SM, et al. Arm morbidity after local therapy for young breast cancer patients. Ann Surg Oncol 2021;28:6071–82. 10.1245/s10434-021-09947-3 [DOI] [PubMed] [Google Scholar]
- 51. Parsons HA, Rhoades J, Reed SC, et al. Sensitive detection of minimal residual disease in patients treated for early-stage breast cancer. Clin Cancer Res 2020;26:2556–64. 10.1158/1078-0432.CCR-19-3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waks AG, Kim D, Jain E, et al. Somatic and germline genomic alterations in very young women with breast cancer. Clin Cancer Res 2022;28:2339–48. 10.1158/1078-0432.CCR-21-2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rath M, Li Q, Li H, et al. Evaluation of significant genome-wide association studies risk - SNPs in young breast cancer patients. PLoS One 2019;14:e0216997. 10.1371/journal.pone.0216997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olsen SN, Wronski A, Castaño Z, et al. Loss of rasgap tumor suppressors underlies the aggressive nature of luminal B breast cancers. Cancer Discov 2017;7:202–17. 10.1158/2159-8290.CD-16-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stover DG, Parsons HA, Ha G, et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol 2018;36:543–53. 10.1200/JCO.2017.76.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gibson CJ, Fell G, Sella T, et al. Clonal Hematopoiesis in young women treated for breast cancer. Clin Cancer Res 2023;29:2551–8. 10.1158/1078-0432.CCR-23-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goddard ET, Hill RC, Nemkov T, et al. The rodent liver undergoes weaning-induced involution and supports breast cancer metastasis. Cancer Discov 2017;7:177–87. 10.1158/2159-8290.CD-16-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc 2016;91:1577–89. 10.1016/j.mayocp.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081157supp001.pdf (94.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. Requests to access YWS data/biospecimens should be made to the principal investigator, AHP (ann_partridge@dfci.harvard.edu).