Abstract
Background
The 6-minute walk test (6MWT) represents a comprehensive functional assessment that is commonly used in patients with heart failure; however, data are lacking in patients with transthyretin cardiac amyloidosis (ATTR-CA).
Objectives
This study aimed to assess the prognostic importance of the 6MWT in patients with ATTR-CA.
Methods
A retrospective analysis of patients diagnosed with ATTR-CA at the National Amyloidosis Centre who underwent a baseline 6MWT between 2011 and 2023 identified 2,141 patients, of whom 1,118 had follow-up at 1 year.
Results
The median baseline 6MWT distance was 347 m (Q1-Q3: 250-428 m) and analysis by quartiles demonstrated an increased death rate with each distance reduction (deaths per 100 person-years: 6.3 vs 9.2 vs 13.6 vs 19.0; log-rank P < 0.001). A 6MWT distance of <350 m was associated with a 2.2-fold higher risk of mortality (HR: 2.15; 95% CI: 1.85-2.50; P < 0.001), with a similar increased risk across National Amyloidosis Centre disease stages (P for interaction = 0.761) and genotypes (P for interaction = 0.172). An absolute (reduction of >35 m) and relative worsening (reduction of >5%) of 6MWT at 1 year was associated with an increased risk of mortality (HR: 1.80; 95% CI: 1.51-2.15; P < 0.001 and HR: 1.89; 95% CI: 1.59-2.24; P < 0.001, respectively), which was similar across the aforementioned subgroups. When combined with established measures of disease progression (N-terminal pro–B-type natriuretic peptide progression and outpatient diuretic intensification), each incremental increase in progression markers was associated with an increased death rate (deaths per 100 person-years: 7.6 vs 13.9 vs 22.4 vs 32.9; log-rank P < 0.001).
Conclusions
The baseline 6MWT distance can refine risk stratification beyond traditional prognosticators. A worsening 6MWT distance can stratify disease progression and, when combined with established markers, identifies patients at the highest risk of mortality.
Key Words: 6-minute walk test, cardiac ATTR amyloidosis, prognosis
Central Illustration
Transthyretin cardiac amyloidosis (ATTR-CA) is a progressive and ultimately fatal cardiomyopathy, characterized by the deposition of misfolded transthyretin in the form of amyloid fibrils within the myocardial extracellular space, which disrupt cardiac structure and function.1,2 The sporadic, noninherited, wild type (wtATTR-CA) is a condition of older, predominantly male individuals, whereas the hereditary form (hATTR-CA) can present earlier in life with a varying clinical phenotype, often comprising both restrictive cardiomyopathy and polyneuropathy.3,4
The 6-minute walk test (6MWT) is a well-established measure of functional exercise capacity and has demonstrated utility in the stratification of prognosis and assessment of treatment response in patients with heart failure in both clinical practice and clinical trials.5,6
This metric has been widely used in clinical trials to assess the efficacy of ATTR-specific disease modifiers.7, 8, 9 Tafamidis and acoramidis, both highly specific transthyretin (TTR) stabilizers, reduce the composite of all-cause mortality and cardiovascular-related hospitalization in patients with ATTR-CA. The ATTR-ACT (Safety and Efficacy of Tafamidis in Patients With Transthyretin Cardiomyopathy) and ATTRibute-CM (Efficacy and Safety of AG10 in Subjects with Transthyretin Amyloid Cardiomyopathy) trials demonstrated that along with reduced all-cause mortality and cardiovascular-related hospitalization, treatment with tafamidis and acoramidis was associated with a slower rate of decline in 6MWT distance.7,8 More recently, the efficacy of the RNA interference agent patisiran was assessed in the APOLLO-B (A Study to Evaluate Patisiran in Participants With Transthyretin Amyloidosis With Cardiomyopathy) trial, where the primary endpoint was the change in 6MWT at 1 year, and treatment with patisiran was shown to slow the rate of decline in 6MWT distance.9
The only drug currently approved for the treatment of ATTR-CA is tafamidis.7 However, several novel compounds that inhibit TTR synthesis and subsequent amyloid formation are in advanced stages of development and are likely to become available soon in clinical practice.10 Worsening in functional capacity is one of the key elements in the assessment of disease progression and might highlight the need to switch to alternative agents with different mechanisms of action or consider combination therapy. Objective assessment of functional capacity with the 6MWT will therefore be crucial in guiding treatment decisions.
Despite widespread use of the 6MWT in clinical trials and the potential key role of the 6MWT in clinical practice, the prognostic importance of the 6MWT distance in patients with ATTR-CA as well as the significance of changes in 6MWT distance over time are yet to be characterized.
The aims of this study were to assess the prognostic importance of the baseline 6MWT distance and assess the prognostic importance of a change in 6MWT distance as a marker of disease progression in a large cohort of patients with ATTR-CA.
Methods
This is a retrospective observational cohort study of patients diagnosed with ATTR-CA at the National Amyloidosis Centre (NAC) between 2011 and 2023. The diagnosis of ATTR-CA was established on the basis of validated diagnostic criteria,11 and patients who underwent a baseline 6MWT in accordance with the American Thoracic Society guidelines were eligible for inclusion.12 All patients underwent genetic sequencing of the TTR gene and provided written consent for their data to be retrospectively analyzed and published, in line with the Declaration of Helsinki and approval from the Royal Free Hospital ethics committee (REC 21/PR/0620).
Statistical analysis
Statistical analysis was performed using Stata, release 17 (StataCorp). All continuous variables were tested for normality (Shapiro-Wilk test) and are presented as mean ± SD if the distribution is normal or median (Q1-Q3) otherwise, other than N-terminal pro–B-type natriuretic peptide (NT-proBNP), which was log-transformed for bivariate testing. The independent-samples Student’s t-test was used to compare means if the data were normally distributed in each group, or its nonparametric equivalent was used to compare the distributions of the 2 groups. One-way analysis of variance if the data were normally distributed in each group was used to compare means in more than 2 groups, or its nonparametric equivalent was used to compare the distributions of multiple groups. A significant result was followed by post hoc Bonferroni corrected pairwise comparisons to establish where differences lay. Categorical data are presented as absolute number and frequency (%) and compared using the chi-square test.
The relationships between the change in 6MWT, baseline characteristics, and other established markers of disease progression were assessed using linear regression, with the outcome variable being the 1-year 6MWT distance and explanatory variables being the baseline 6MWT distance and either NT-proBNP progression (defined as an increase of >700 ng/L and >30%) or outpatient diuretic intensification (ODI) (defined as any initiation or increment in the dosage of loop diuretic [furosemide equivalent]).13 To assess whether the decline in 6MWT changed according to the time period, the population was divided into a historical cohort (pre-2017) and a contemporary cohort (post-2017). The relationship was estimated by preforming a linear regression analysis, with the outcome variable being the 1-year 6MWT distance and the explanatory variables being the baseline 6MWT distance and the group being evaluated. The estimated regression coefficient represented the estimated difference in means at 1 year after adjustment for the baseline value. All variables with a P value of <0.10 in the univariable analysis were included in the multivariable analysis.
The optimal cutpoints for the baseline 6MWT distance and change in 6MWT distance at 1 year were established using time-dependent receiver operating characteristic curves, followed by the Youden method. The optimal cutpoint for the baseline 6MWT distance was 336 m (sensitivity: 56.5%, specificity: 64.0%), and a cutpoint of 350 m (sensitivity: 61.1%, specificity: 58.5%) was a good discriminator of survival by the log-rank test. The optimal cutpoint for the absolute change in 6MWT distance at 1 year was –35 m (sensitivity: 47.1%, specificity: 71.3%), and this was a good discriminator of survival by the log-rank test. The optimal cutpoint for a relative change in 6MWT distance was –5.5% (sensitivity: 57.6%, specificity: 62.2%), and cutpoint of a reduction of –5% (sensitivity: 58.3%, specificity: 61.3%) was a good discriminator of survival by the log-rank test. An absolute worsening of the 6MWT distance was defined as a reduction of >35 m, and a relative worsening was defined as a reduction of >5%, which exceeded the previously reported minimal clinically important difference in patients with chronic heart failure.14, 15, 16
Survival was evaluated using Cox proportional hazards regression analysis, providing estimated HRs with 95% CIs. The proportional hazards assumption was checked and confirmed using weighted Schoenfeld residuals. The initial survival analysis evaluated the association between baseline 6MWT distance and all-cause mortality. Significant results were followed by internal validation of the model, which was achieved by performing a bootstrapping procedure with 500 repeats, affording a comparison of the percentile and bias-corrected methods to ensure the results were unbiased, followed by a sensitivity analysis whereby patients were censored at the start date of disease-modifying therapy and clinical trials to account for their impact on survival. To assess the potential modification of effects across different baseline characteristics, additional models were created with an interaction term, and the equality of coefficients associated with the interaction term was explicitly tested. Multivariable Cox regression models were adjusted for known predictors of mortality, and collinearity was assessed using variance inflation factors with the threshold equal to 5.
The likelihood ratio test was used to evaluate the contribution of adding the 6MWT distance to the NAC disease stage model. The Akaike information criterion and Harrell C-statistic were calculated to measure the discriminatory ability of each model. The C-statistics were compared by randomly dividing the dataset into 2 cohorts (1:1). The models were fitted to the first cohort, and the C-statistics were compared in the second cohort using Student’s t-test after creating jackknife standard errors.
Landmark survival analysis was carried out to assess the relationship between worsening of the 6MWT distance at the 1-year follow-up timepoint and all-cause mortality from the 1-year timepoint onward. Kaplan-Meier curves were constructed to view survival in different groups. Statistical significance was defined as P < 0.05.
Results
Baseline characteristics
The study population comprised 2,141 patients diagnosed with ATTR-CA, of whom 1,573 (73.5%) had wtATTR-CA, 292 (13.6%) had p.(V142I) hATTR-CA, and 276 (12.9%) had non-p.(V142I) hATTR-CA. The mean age of the population was 76.5 ± 8.4 years, and 1,885 (88.0%) were male. The median NT-proBNP level was 2,581 ng/L (Q1-Q3: 1,315-4,583 ng/L), the median estimated glomerular filtration rate (eGFR) was 60 mL/min/1.73 m2 (Q1-Q3: 47-73 mL/min/1.73 m2), and most of the population had NAC stage 1 (51.1%) or NAC stage 2 (34.2%) disease (Table 1). During follow-up, 259 patients were enrolled into clinical trials, and 212 were initiated on disease-modifying therapy (patisiran: n = 100; tafamidis: n = 97; inotersen: n = 10; vutrisiran: n = 5).
Table 1.
Baseline Characteristics for Patients Diagnosed With ATTR-CA (N = 2,141)
| Baseline characteristics | |
| Age, y | 76.5 ± 8.4 |
| Male | 1,885 (88.0) |
| Ethnicity | |
| White | 1,759 (82.2) |
| Afro-Caribbean | 353 (16.5) |
| Asian | 21 (1.0) |
| Other | 8 (0.4) |
| Genotypes | |
| wtATTR | 1,573 (73.5) |
| p.(V142I) hATTR | 292 (13.6) |
| Non-p.(V142I) hATTR | 276 (12.9) |
| Heart failure severity | |
| NYHA functional class | |
| I | 282 (13.2) |
| II | 1,368 (63.9) |
| III | 333 (15.6) |
| IV | 13 (0.6) |
| Missing | 145 |
| 6MWT distance, m | 347 (250-427) |
| 6MWT, % predicted | 71.2 ± 26.8 |
| Systolic blood pressure, mm Hg | 125.8 ± 17.3 |
| Diastolic blood pressure, mm Hg | 75.0 ± 10.2 |
| Heart rate, beats/min | 72.0 ± 12.8 |
| Blood biomarkers | |
| NAC stage | |
| 1 | 1,095 (51.1) |
| 2 | 732 (34.2) |
| 3 | 314 (14.7) |
| NT-proBNP, ng/L | 2,581 (1,315-4,581) |
| eGFR, mL/min/1.73 m2 | 60 (47-73) |
| Hemoglobin, g/L | 138 (127-148) |
| Serum total bilirubin, μmol/L | 13 (9-18) |
| Alanine transaminase, IU/L | 25 (19-33) |
| Alkaline phosphatase, IU/L | 90 (71-119) |
| GGT, IU/L | 67 (32-134) |
| Echocardiographic parameters | |
| IVSd, mm | 16.8 ± 2.5 |
| PWTd, mm | 16.3 ± 3.6 |
| LVEF, % | 49.0 ± 10.7 |
| Longitudinal strain, % | –11.2 ± 3.9 |
| E/e′ | 16.5 ± 7.2 |
Values are mean ± SD, n (%), n, or median (Q1-Q3).
ATTR-CA = transthyretin cardiac amyloidosis; eGFR = estimated glomerular filtration rate; GGT = gamma-glutamyl transferase; hATTR-CA = hereditary transthyretin cardiac amyloidosis; IVSd = interventricular septum in diastole; LVEF = left ventricular ejection fraction; NAC = National Amyloidosis Centre; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PWTd = posterior wall thickness in diastole; wtATTR-CA = wild-type transthyretin cardiac amyloidosis.
The median 6MWT distance was 347 m (Q1-Q3: 250-428 m), and the mean percent predicted distance corrected for age and height was 71.2% ± 27.8%. Analysis by 6MWT distance quartiles demonstrated that patients with a shorter 6MWT distance tended to be older, were more often female, were more often Afro-Caribbean, and more commonly had the p.(V142I) genotype. Patients with non-p.(V142I) hATTR-CA walked the farthest at their baseline assessment, followed by those with wtATTR-CA and p.(V142I) hATTR-CA (393 m [Q1-Q3: 293-468 m] vs 351 m [Q1-Q3: 255-426 m] vs 276 m [Q1-Q3: 184-388 m]; P < 0.001); however, after correction for age and height, patients with wtATTR-CA had the greatest percent predicted distance, followed by non-p.(V142I) hATTR-CA and p.(V142I) hATTR-CA (72.9% ± 26.3% vs 70.6% ± 26.3% vs 62.6% ± 27.9%; P < 0.001).
The severity of the cardiac phenotype also worsened with each reduction in 6MWT distance quartile, as evidenced by a greater proportion of patients being in NYHA functional class III/IV and a greater proportion having NAC stage 3 (severe) disease, driven by a significantly higher median NT-proBNP level and lower eGFR. Patients with a shorter 6MWT also had higher biomarkers suggestive of hepatic congestion (elevated alkaline phosphatase and gamma-glutamyl transferase levels). Assessment of echocardiographic parameters demonstrated patients with a shorter 6MWT had worse systolic and diastolic left ventricular function but similar increase in wall thickness (Table 2). Similar trends were also observed when patients were divided into quartiles based on their percent predicted 6MWT distance (Table 3).
Table 2.
Baseline Characteristics for Patients Diagnosed With ATTR-CA Divided by the 6MWT Distance Quartile
| 6MWT Distance Quartiles |
P Value | ||||
|---|---|---|---|---|---|
| >427 m (n = 535) | 348-427 m (n = 535) | 250-347 m (n = 536) | <250 m (n = 535) | ||
| Baseline characteristics | |||||
| Age, y | 71.8 ± 9.2a,b,c | 76.0 ± 8.0d,e | 78.4 ± 7.0f | 79.6 ± 7.1 | <0.001 |
| Male | 503 (94.0)c | 481 (89.9)e | 481 (89.7)f | 420 (78.5) | <0.001 |
| Ethnicity | <0.001 | ||||
| White | 480 (89.7) | 459 (85.8)e | 431 (80.4)f | 389 (72.7) | |
| Afro-Caribbean | 50 (9.3) | 66 (12.3)e | 97 (18.1)f | 140 (26.2) | |
| Asian | 3 (0.6) | 7 (1.3) | 6 (1.1) | 5 (0.9) | |
| Other | 2 (0.4) | 3 (0.6) | 2 (0.4) | 1 (0.2) | |
| Genotypes | <0.001 | ||||
| wtATTR | 389 (72.7) | 412 (77.0)e | 408 (76.1)f | 364 (68.0) | |
| p.(V142I) hATTR | 43 (8.0)a,b,c | 52 (9.7) | 78 (14.6) | 119 (22.2) | |
| Non-p.(V142I) hATTR | 103 (19.3)a,b,c | 71 (13.3) | 50 (9.3) | 52 (9.7) | |
| Heart failure severity | |||||
| NYHA functional class | <0.001 | ||||
| I | 135 (25.2)a,b,c | 60 (11.2)e | 57 (10.6)f | 30 (5.6) | |
| II | 355 (62.6)c | 371 (69.3)e | 356 (66.4)f | 286 (53.5) | |
| III | 23 (4.3)c | 61 (11.4)e | 75 (14.0)f | 174 (32.5) | |
| IV | 0 (0.0)c | 1 (0.2)e | 2 (0.4) | 10 (1.9) | |
| Missing | 22 | 42 | 46 | 35 | |
| 6MWT, % predicted | 97.7 ± 15.5a,b,c | 83.5 ± 11.7d,e | 78.4 ± 7.0f | 35.6 ± 15.5 | <0.001 |
| Systolic blood pressure, mm Hg | 126.7 ± 16.2 | 126.9 ± 17.8 | 125.0 ± 17.5 | 124.7 ± 17.7 | 0.048 |
| Diastolic blood pressure, mm Hg | 77.0 ± 9.8b,c | 75.3 ± 10.4 | 74.2 ± 10.1 | 73.4 ± 10.0 | <0.001 |
| Heart rate, beats/min | 71.9 ± 13.3 | 70.8 ± 13.4e | 72.4 ± 12.1 | 72.9 ± 12.4 | 0.027 |
| Blood biomarkers | |||||
| NAC stage | <0.001 | ||||
| 1 | 374 (69.9)a,b,c | 307 (57.4)d,e | 237 (44.2)f | 177 (33.1) | |
| 2 | 127 (23.7)a,b,c | 176 (32.9)e | 219 (40.9) | 210 (39.9) | |
| 3 | 34 (6.4)b,c | 52 (9.7)e | 80 (14.9)f | 148 (27.7) | |
| NT-proBNP, ng/L | 1,725 (872-3,087)a,b,c | 2,384 (1,159-3,826)d,e | 3,554 (1,662-4,971)f | 3,594 (2,031-6,792) | <0.001 |
| eGFR, mL/min/1.73 m2 | 66 (55-80)a,b,c | 61 (51-74)d,e | 59 (46-71)f | 51 (40-66) | <0.001 |
| Hemoglobin, g/L | 143 (134-151)a,b,c | 139 (130-148)d,e | 136 (125-146)f | 131 (119-142) | <0.001 |
| Serum total bilirubin, μmol/L | 13 (9-18) | 13 (9-17) | 13 (9-20) | 13 (9-19) | 0.109 |
| Alanine transaminase, IU/L | 27 (21-35)a,b,c | 25 (19-32)e | 25 (19-33)f | 23 (18-31) | <0.001 |
| Alkaline phosphatase, IU/L | 83 (68-102)a,b,c | 88 (70-115)d,e | 96 (74-125) | 101 (76-139) | <0.001 |
| GGT, IU/L | 57 (29-113)a,b,c | 59 (30-115)e | 71 (35-139) | 82 (36-176) | <0.001 |
| Echocardiographic parameters | |||||
| IVSd, mm | 16.7 ± 2.7 | 16.8 ± 2.7 | 16.9 ± 2.5 | 16.8 ± 2.4 | 0.485 |
| PWTd, mm | 16.2 ± 2.8 | 16.3 ± 2.5 | 16.4 ± 2.7 | 16.3 ± 2.5 | 0.429 |
| LVEF, % | 50.5 ± 10.5b,c | 50.0 ± 10.5d,e | 48.2 ± 10.8 | 47.3 ± 10.7 | <0.001 |
| Longitudinal strain, % | –11.9 ± 4.0b,c | –11.8 ± 3.8d,e | –10.8 ± 3.8 | –10.5 ± 3.6 | <0.001 |
| E/e′ | 14.6 ± 6.8a,b,c | 16.5 ± 7.9e | 17.2 ± 6.7 | 17.6 ± 7.1 | <0.001 |
Values are mean ± SD, n (%), n, or median (Q1-Q3). P values for pairwise comparison.
Abbreviations as in Table 1.
P < 0.05 for >426 m vs 347-426 m.
P < 0.05 for >426 m vs 250-346 m.
P < 0.05 for >426 m vs <250 m.
P < 0.05 for 347-426 m vs 250-346 m.
P < 0.05 for 347-426 m vs <250 m.
P < 0.05 for 250-346 m vs <250 m.
Table 3.
Baseline Characteristics for Patients Diagnosed With ATTR-CA Divided by the Percent Predicted 6MWT Distance Quartile
| 6MWT Percent Predicted Quartiles |
P Value | ||||
|---|---|---|---|---|---|
| >89% (n = 546) | 75%-89% (n = 522) | 55%-74% (n = 546) | <55% (n = 527) | ||
| Baseline characteristics | |||||
| Age, y | 75.9 ± 7.9c | 75.6 ± 8.5e | 76.1 ± 9.1f | 78.3 ± 7.8 | <0.001 |
| Male | 491 (89.8)c | 468 (89.7)e | 496 (90.8)f | 430 (81.6) | <0.001 |
| Ethnicity | <0.001 | ||||
| White | 474 (86.8)c | 455 (87.2)e | 444 (81.3)f | 386 (73.2) | |
| Afro-Caribbean | 65 (11.9)b,c | 60 (11.5)d,e | 96 (17.6)f | 132 (25.0) | |
| Asian | 4 (0.7) | 6 (1.1) | 5 (0.9) | 6 (1.1) | |
| Other | 3 (0.5) | 1 (0.2) | 1 (0.2) | 3 (0.6) | |
| Genotypes | <0.001 | ||||
| wtATTR | 431 (78.9)c | 396 (75.9)e | 402 (73.6)f | 344 (65.3) | |
| p.(V142I) hATTR | 52 (9.5)c | 48 (9.2)e | 77 (14.2)f | 115 (21.8) | |
| Non-p.(V142I) hATTR | 63 (11.5) | 78 (14.9) | 67 (12.3) | 68 (12.9) | |
| Heart failure severity | |||||
| NYHA functional class | <0.001 | ||||
| I | 126 (23.1)a,b,c | 71 (13.6)d | 52 (9.5) | 33 (6.3) | |
| II | 372 (68.1)c | 343 (65.7)e | 371 (67.9)f | 282 (53.5) | |
| III | 32 (5.8)a,b,c | 56 (10.7)e | 78 (14.2)f | 167 (31.7) | |
| IV | 0 (0.0)c | 0 (0.0)e | 3 (0.5) | 10 (1.9) | |
| Missing | 16 | 52 | 42 | 35 | |
| 6MWT distance, m | 460 (414-515)a,b,c | 391 (351-432)d,e | 310 (276-349)f | 161 (92-211) | <0.001 |
| Systolic blood pressure, mm Hg | 128.3 ± 17.3b,c | 126.0 ± 17.1 | 125.2 ± 16.8 | 123.9 ± 17.8 | <0.001 |
| Diastolic blood pressure, mm Hg | 76.0 ± 9.9c | 75.4 ± 10.5e | 75.0 ± 10.2 | 73.5 ± 10.0 | 0.001 |
| Heart rate, beats/min | 70.1 ± 13.5b | 71.9 ± 12.7 | 72.9 ± 12.7 | 72.5 ± 12.4 | 0.011 |
| Blood biomarkers | |||||
| NAC stage | <0.001 | ||||
| 1 | 365 (66.8)a,b,c | 287 (55.0)e | 265 (48.5)f | 178 (33.8) | |
| 2 | 131 (24.0)a,b,c | 184 (35.2) | 210 (38.5) | 207 (39.3) | |
| 3 | 50 (9.2)c | 51 (9.8)e | 71 (13.0)f | 142 (26.9) | |
| NT-proBNP, ng/L | 1,785 (940-3,281)a,b,c | 2,476 (1,311-4,134)e | 2,764 (1,357-4,796)f | 3,577 (2,009-6,475) | <0.001 |
| eGFR, mL/min/1.73 m2 | 63 (53-78)b,c | 63 (50-72)e | 59 (48-74)f | 52 (40-66) | <0.001 |
| Hemoglobin, g/L | 142 (132-151)b,c | 140 (129-149)d,e | 137 (126-146)f | 132 (120-143) | <0.001 |
| Serum total bilirubin, μmol/L | 12 (9-17)b,c | 13 (9-18) | 13 (9-19) | 13 (9-19) | 0.005 |
| Alanine transaminase, IU/L | 26 (20-34)c | 25 (19-34)e | 25 (19-32) | 24 (18-31) | <0.001 |
| Alkaline phosphatase, IU/L | 83 (68-105)b,c | 89 (70-114)d,e | 93 (73-125) | 101 (76-140) | <0.001 |
| GGT, IU/L | 54 (28-105)b,c | 65 (32-118)e | 70 (32-143)f | 84 (38-185) | <0.001 |
| Echocardiographic parameters | |||||
| IVSd, mm | 16.6 ± 2.6 | 17.0 ± 2.7 | 16.8 ± 2.6 | 16.7 ± 2.4 | 0.035 |
| PWTd, mm | 16.1 ± 2.7 | 16.5 ± 2.6 | 16.4 ± 2.7 | 16.3 ± 2.5 | 0.048 |
| LVEF, % | 51.2 ± 10.2a,b,c | 49.0 ± 10.5e | 48.5 ± 11.0 | 47.1 ± 10.7 | <0.001 |
| Longitudinal strain, % | –12.0 ± 4.0b,c | –11.5 ± 3.8e | –11.0 ± 3.8 | –10.4 ± 3.6 | <0.001 |
| E/e′ | 15.3 ± 7.1a,b,c | 16.5 ± 6.6e | 17.0 ± 7.9 | 17.1 ± 7.0 | <0.001 |
Values are mean ± SD, n (%), n, or median (Q1-Q3). P values for pairwise comparison.
Abbreviations as in Table 1.
P < 0.05 for >89% vs 75%-89%.
P < 0.05 for >89% vs 55%-74%.
P < 0.05 for >89% vs <55%.
P < 0.05 for 75%-89% vs 55%-74%.
P < 0.05 for 75%-89% vs <55%.
P < 0.05 for 55%-74% vs <55%.
Worsening 6-MWT at 1 year
At 1 year, 1,118 patients had a repeat 6MWT. The median absolute reduction in 6MWT distance was 17 m, and the mean relative percent reduction was 4.5%. The median absolute reduction was greater in patients with p.(V142I) hATTR and those with non-p.(V142I) hATTR than in patients with wtATTR-CA (33 m vs 33 m vs 13 m; P < 0.001), and a similar trend was observed when comparing the mean relative percent reduction across genotypes (9.3% vs 7.9% vs 3.2%; P < 0.001).
At 1 year, 365 (32.7%) patients experienced NT-proBNP progression (defined as an increase of >700 ng/L and >30%). Patients who experienced NT-proBNP progression had a greater decline in 6MWT distance than those with a stable NT-proBNP, whereby the difference in the mean 6MWT distance (adjusted for baseline 6MWT distance) between the 2 groups was –37.8 m (95% CI: –48.3 to –27.4 m; P < 0.001). This relationship was maintained when NT-proBNP was assessed as a continuous variable (Supplemental Appendix). At 1 year, 303 (27.1%) patients experienced ODI. Patients who experienced ODI also had a greater decline in 6MWT distance than those with a stable diuretic dosage, with an adjusted mean difference of –24.5 m (95% CI: –35.7 to –13.3 m; P < 0.001). A similar trend was observed in the assessment of the change in percent predicted 6MWT distance (NT-proBNP progression: –8.4%; 95% CI: –10.6% to –6.1%; P < 0.001; ODI: –5.1%; 95% CI: –7.5% to –2.7%; P < 0.001). To assess whether the magnitude of 6MWT decline changed over time, patients were divided into historical (pre-2017: n = 604) and contemporary cohorts (post-2017: n = 514). Patients in the historical cohort had a more advanced cardiac phenotype at diagnosis (Supplemental Table 1) and experienced a greater decline in the absolute 6MWT distance than those in the contemporary cohort (median reduction: 23 m vs 11 m; P = 0.004), with an adjusted mean difference of –17.1 m (95% CI: –27.2 to –7.0 m; P = 0.001). A similar trend was observed in the assessment of the percent predicted 6MWT distance (–4.5%; 95% CI: –2.3% to –6.7%; P < 0.001). These variables alongside hATTR-CA and log NT-proBNP remained independently associated with a decline in the absolute 6MWT distance and percent predicted 6MWT distance in a multivariable linear regression model (Supplemental Table 2).
Worsening of the 6MWT distance was also assessed as an absolute worsening (reduction of >35 m) and a relative worsening (reduction of >5%). At 1 year, 429 (38.4%) patients experienced an absolute worsening of the 6MWT, and 548 (49.0%) experienced a relative worsening of the 6MWT. Patients who experienced worsening more commonly had the p.(V142I) or non-p.(V142I) genotype than wtATTR-CA, a lower eGFR level, and a lower hemoglobin level (Table 4). Patients in the historical cohort more commonly experienced an absolute (258 [42.7%] vs 171 [33.3%]; P = 0.001) and relative worsening (320 [53.0%] vs 228 [44.4%]; P = 0.004) of the 6MWT distance.
Table 4.
Baseline Characteristics for Patients Who Experienced an Absolute and Relative Reduction in the 6MWT Distance Compared to Those Who Did Not
| Absolute Reduction in 6MWT Distance |
Relative Reduction in 6MWT Distance |
|||||
|---|---|---|---|---|---|---|
| Stable Distance (n = 689; 61.6%) | Reduction >35 m (n = 429; = 38.4%) | P Value | Stable Distance (n = 570; 51.0%) | Reduction >5% (n = 548; 49.0%) | P Value | |
| Baseline characteristics | ||||||
| Age, y | 75.5 ± 8.5 | 74.7 ± 8.2 | 0.038 | 75.0 ± 8.8 | 75.3 ± 8.1 | 0.957 |
| Male | 621 (90.1) | 375 (87.4) | 0.156 | 513 (90.0) | 483 (88.1) | 0.318 |
| Ethnicity | 0.010 | 0.005 | ||||
| White | 574 (83.3) | 83 (19.3) | 482 (84.6)a | 432 (78.8) | ||
| Afro-Caribbean | 106 (15.4) | 83 (19.3) | 80 (14.0)a | 109 (19.9) | ||
| Asian | 9 (1.3) | 2 (0.5) | 8 (1.4) | 3 (0.5) | ||
| Other | 0 (0.0)a | 4 (0.9) | 8 (1.4)a | 3 (0.5) | ||
| Genotypes | <0.001 | 0.001 | ||||
| wtATTR | 530 (76.9)a | 285 (66.4) | 443 (77.7)a | 372 (67.9) | ||
| p.(V142I) hATTR | 77 (11.2)a | 68 (15.9) | 60 (10.5)a | 85 (15.5) | ||
| Non-p.(V142I) hATTR | 82 (11.9)a | 76 (17.7) | 67 (11.8)a | 91 (16.6) | ||
| Heart failure severity | ||||||
| NYHA functional class | 0.039 | 0.252 | ||||
| I | 101 (14.7) | 54 (12.6) | 87 (15.3) | 68 (12.4) | ||
| II | 444 (64.4) | 307 (71.6)a | 374 (65.6) | 377 (68.8) | ||
| III | 125 (18.1) | 61 (14.2) | 97 (17.0) | 89 (16.2) | ||
| IV | 5 (0.7) | 0 (0.0) | 1 (0.2) | 4 (0.7) | ||
| Missing | 14 | 7 | 11 | 10 | ||
| Baseline 6MWT distance, m | 365 (264-438) | 385 (301-448) | 0.005 | 372 (276-450) | 368 (286-434) | 0.842 |
| Baseline percent predicted 6MWT, % | 72.1 ± 25.6 | 78.0 ± 22.2 | 0.004 | 73.3 ± 25.7 | 75.4 ± 23.3 | 0.630 |
| Systolic blood pressure, mm Hg | 126.5 ± 17.0 | 124.5 ± 17.1 | 0.036 | 126.3 ± 16.8 | 125.0 ± 17.3 | 0.144 |
| Diastolic blood pressure, mm Hg | 75.0 ± 10.0 | 73.9 ± 10.5 | 0.019 | 75.1 ± 10.0 | 74.0 ± 10.4 | 0.029 |
| Heart rate, beats/min | 71.7 ± 12.8 | 72.8 ± 14.1 | 0.420 | 71.6 ± 12.9 | 72.7 ± 13.6 | 0.267 |
| Blood biomarkers | ||||||
| NAC stage | 0.220 | 0.109 | ||||
| 1 | 404 (58.6) | 229 (53.4) | 339 (59.5) | 294 (53.6) | ||
| 2 | 215 (31.2) | 149 (34.7) | 177 (31.1) | 187 (34.1) | ||
| 3 | 70 (10.2) | 51 (11.9) | 54 (9.5) | 67 (12.2) | ||
| NT-proBNP, ng/L | 2,313 (1,115-4,030) | 2,436 (1,281-4,224) | 0.135 | 2,286 (1,073-3,958) | 2,461 (1,281-4,263) | 0.018 |
| eGFR, mL/min/1.73 m2 | 62 (52-77) | 60 (48-74) | 0.009 | 63 (52-77) | 59 (48-74) | 0.002 |
| Hemoglobin, g/L | 140 (130-149) | 137 (126-148) | 0.006 | 141 (130-149) | 137 (127-148) | 0.005 |
| Serum total bilirubin, μmol/L | 12 (9-18) | 13 (9-18) | 0.393 | 12 (9-17) | 13 (9-18) | 0.577 |
| Alanine transaminase, IU/L | 25 (19-33) | 25 (19-33) | 0.865 | 25 (19-34) | 25 (19-32) | 0.544 |
| Alkaline phosphatase, IU/L | 87 (69-114) | 87 (68 -115) | 0.967 | 87 (68-114) | 87 (70-115) | 0.517 |
| GGT, IU/L | 63 (30-129) | 70 (33-134) | 0.327 | 62 (31-129) | 70 (32-133) | 0.223 |
| Echocardiographic parameters | ||||||
| IVSd, mm | 16.6 ± 2.5 | 16.8 ± 2.5 | 0.111 | 16.6 ± 2.5 | 16.8 ± 2.5 | 0.112 |
| PWTd, mm | 16.2 ± 2.6 | 16.5 ± 2.6 | 0.074 | 16.1 ± 2.6 | 16.4 ± 2.6 | 0.074 |
| LVEF, % | 49.3 ± 10.6 | 49.5 ± 10.7 | 0.702 | 49.3 ± 10.7 | 49.5 ± 10.5 | 0.628 |
| Longitudinal strain, % | –11.3 ± 3.7 | –11.6 ± 3.6 | 0.181 | –11.3 ± 3.7 | –11.5 ± 3.6 | 0.495 |
| E/e′ | 15.8 ± 7.5 | 16.2 ± 6.5 | 0.171 | 15.6 ± 7.5 | 16.4 ± 6.7 | 0.010 |
Values are mean ± SD, n (%), n, or median (Q1-Q3).
Abbreviations as in Table 1.
P values for pairwise comparison < 0.05.
Survival
Baseline 6MWT and risk stratification
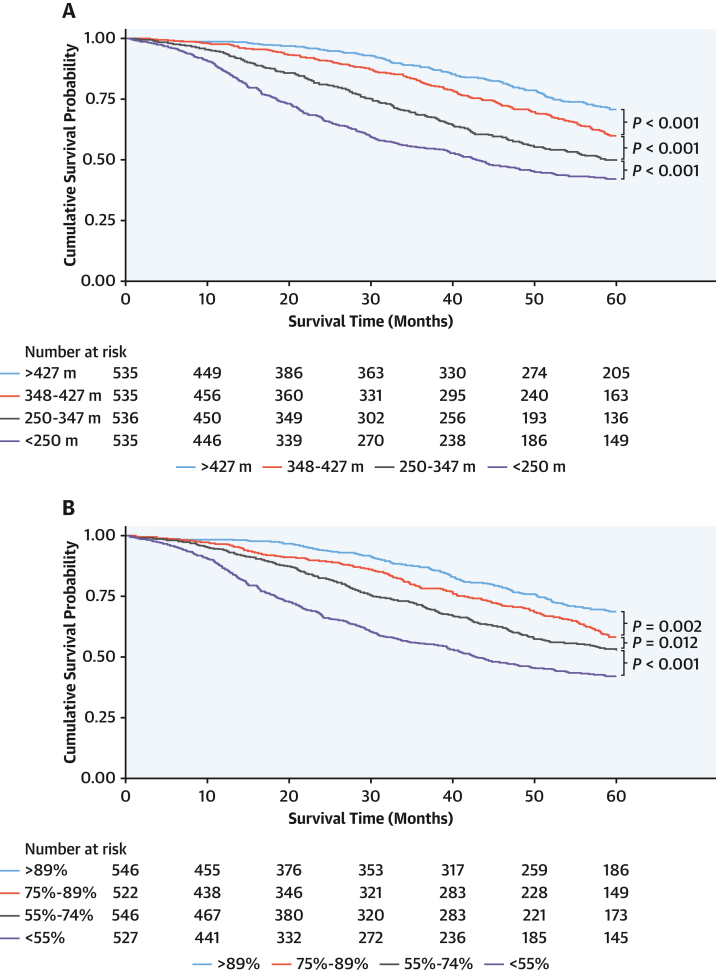
At a median follow-up of 43.1 months (Q1-Q3: 14.2-60.0 months), analysis by baseline 6MWT distance quartiles demonstrated a significant increase in death rate with each reduction in 6MWT distance. Compared with patients who walked >427 m, in whom the death rate was 6.3 deaths per 100 person-years (py) (95% CI: 5.3-7.6 deaths per 100 py), the death rate was 9.2 deaths per 100 py (95% CI: 7.8-10.7 deaths per 100 py) in patients who walked 347 to 426 m, 13.6 deaths per 100 py (95% CI: 11.9-15.6 deaths per 100 py) in patients who walked 250 to 346 m, and 19.0 deaths per 100 py (95% CI: 16.9-21.3 deaths per 100 py) in patients who walked <250 m. Analysis by percent predicted 6MWT distance demonstrated a similar incremental increase in mortality (Figure 1).
Figure 1.
Baseline 6-Minute Walk Test Quartiles and Survival
Kaplan-Meier curves demonstrating the effect of (A) 6-minute walk test distance quartile and (B) percent predicted 6-minute walk test quartile on survival.
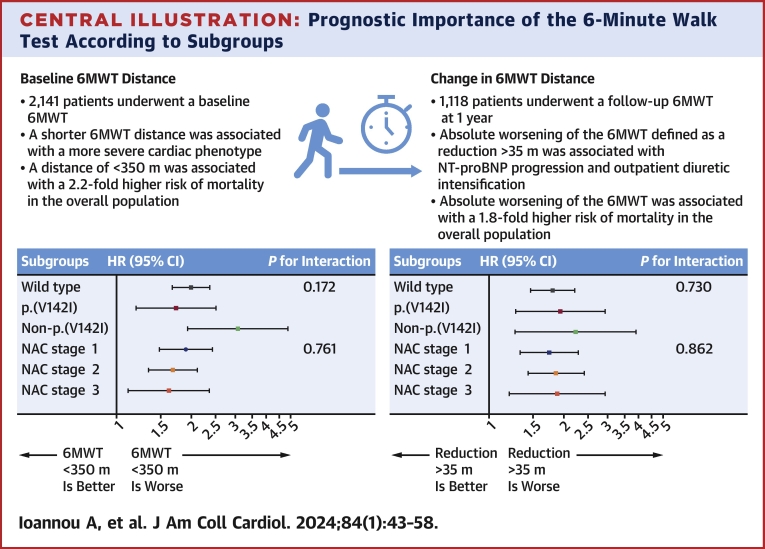
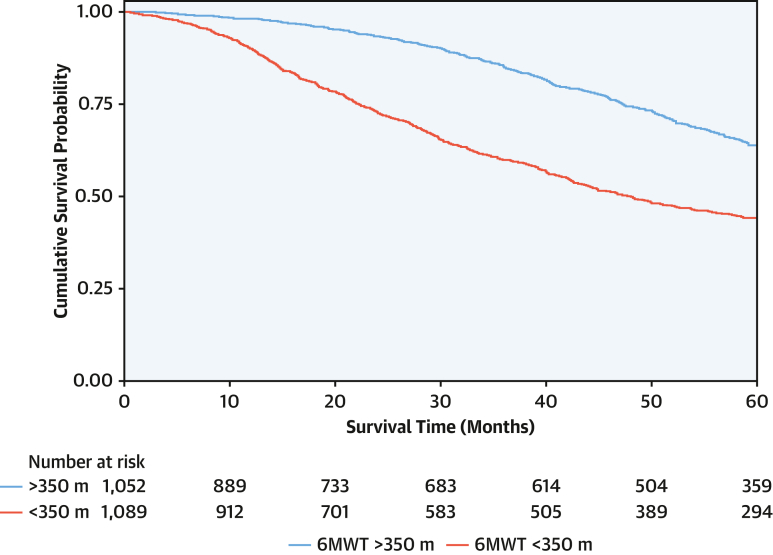
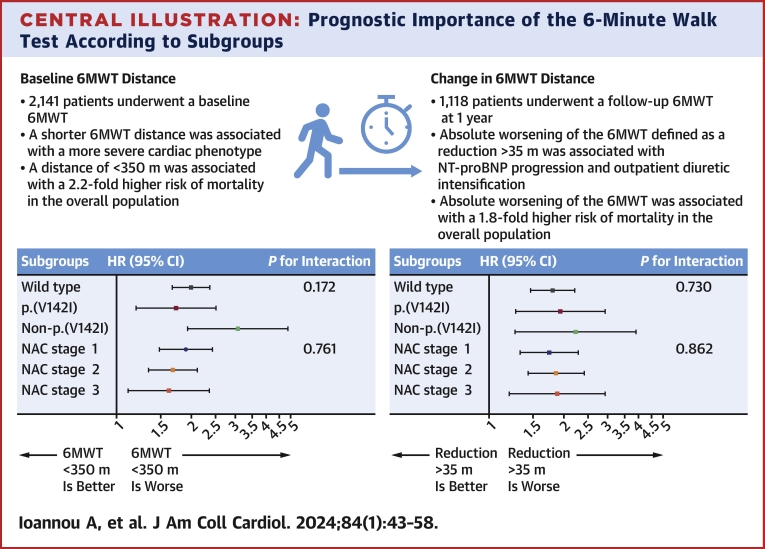
In the overall population, the 6MWT distance was associated with survival (HR: 0.98 for every 10-m increase; 95% CI: 0.97-0.99 for every 10-m increase; P < 0.001). Patients with a baseline 6MWT distance of <350 m had a higher death rate (16.1 deaths per 100 py; 95% CI: 14.7-17.5 deaths per 100 py) than those with a 6MWT of >350 m (7.7 deaths per 100 py; 95% CI: 6.8-8.6 deaths per 100 py). A baseline 6MWT distance of <350 m was associated with a 2.2-fold higher risk of mortality (HR: 2.15; 95% CI: 1.85-2.50; P < 0.001), and the bootstrapped results indicated that the coefficients remained constant across the resamples, suggesting robustness in the association between a 6MWT distance of <350 m and mortality (Figure 2). The increased risk associated with a 6MWT distance of <350 m was consistent across the spectrum of NAC disease stages (NAC stage 1 disease: HR: 1.90; 95% CI: 1.48-2.46; P < 0.001; NAC stage 2 disease: HR: 1.68; 95% CI: 1.34-2.11; P < 0.001; NAC stage 3 disease: HR: 1.62; 95% CI: 1.11-2.36; P = 0.012; P for interaction = 0.761) and the 3 genotypes (wild type: HR: 1.99; 95% CI: 1.67-2.37; P < 0.001; p.[V142I]: HR: 1.73; 95% CI: 1.20-2.50; P < 0.001; non-p.[V142I]: HR: 3.07; 95% CI: 1.93-4.88; P < 0.001; P for interaction = 0.172). The increased risk remained present regardless of background heart failure therapy and disease-modifying therapies (Supplemental Table 3).
Figure 2.
Baseline 6-Minute Walk Test Distance and Survival
Kaplan-Meier curve demonstrating the association between a 6-minute walk test (6MWT) distance of <350 m and survival.
In a multivariable analysis adjusted for covariates age, NAC disease stage, and genotype, a 6MWT distance of <350 m remained independently associated with mortality (HR: 1.54; 95% CI: 1.31-1.81; P < 0.001) (Supplemental Table 4). The likelihood ratio test demonstrated that the addition of a 6MWT distance of <350 m to the NAC disease stage significantly improved the goodness of fit of the model (chi-square = 53.30; P < 0.001) and significantly improved the discriminatory ability of the classification compared to the NAC disease stage alone (Akaike information criterion: 10,756 vs 10,781; Harrell C-statistic: 0.66; [95% CI: 0.63-0.68] vs 0.63 [95% CI: 0.60-0.66]; P < 0.001).
Change in 6MWT and disease progression
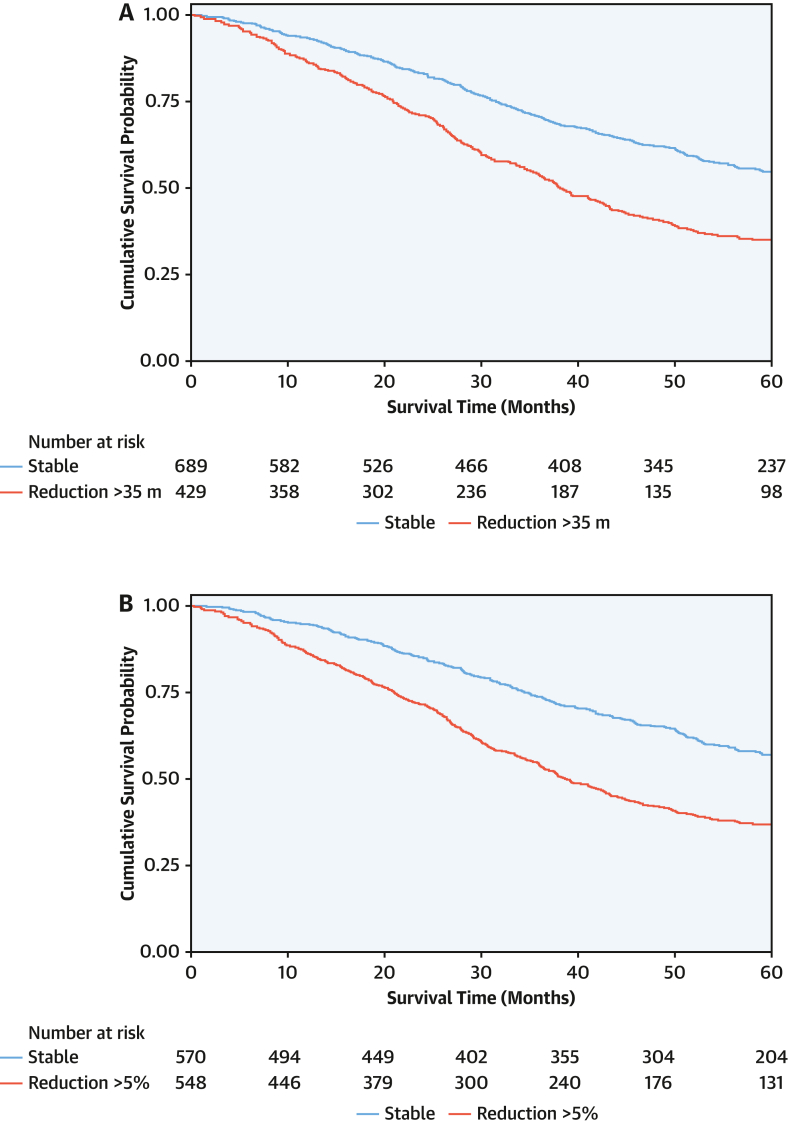
At 1 year, patients with an absolute reduction in the 6MWT distance of >35 m had a higher mortality rate (20.3 deaths per 100 py; 95% CI: 18.0-23.1 deaths per 100 py) than those with a stable 6MWT distance (11.5 deaths per 100 py; 95% CI: 10.3-13.1 deaths per 100 py), and a reduction of >35 m was associated with a 1.8-fold higher risk of mortality (HR: 1.80; 95% CI: 1.51-2.15; P < 0.001). Patients with a relative reduction in the 6MWT distance of >5% had a higher mortality rate (19.6 deaths per 100 py; 95% CI: 17.6-22.0 deaths per 100 py) than those with a stable 6MWT distance (10.7 deaths per 100 py; 95% CI: 9.4-12.2 deaths per 100 py), and a reduction of >5% was associated with a 1.9-fold higher risk of mortality (HR: 1.89; 95% CI: 1.59-2.24; P < 0.001). The bootstrapped results for both an absolute reduction of >35 m and relative reduction of >5% indicate that the coefficients remained constant across the resamples, suggesting robustness in the association between the change in 6MWT distance and mortality. In the overall population, the model that classified 6MWT worsening as a relative reduction of >5% had a lower Akaike information criterion (6,818 vs 6,825) and marginally higher Harrell C-statistic (0.59 vs 0.57) than the model that classified 6MWT worsening as an absolute reduction of >35 m (Figure 3).
Figure 3.
Change in 6-Minute Walk Test Distance and Survival
Landmark Kaplan-Meier curves demonstrating the association between (A) an absolute reduction in the 6-minute walk test distance of >35 m and (B) a relative reduction in 6-minute walk test distance of >5% at the 1-year timepoint and subsequent survival.
In 2 separate multivariable analyses adjusted for the covariates age, NAC disease stage, genotype, and 6MWT distance of <350 m, both an absolute reduction of >35 m (HR: 1.87; 95% CI: 1.57-2.23; P < 0.001) and relative reduction of >5% (HR: 1.91; 95% CI: 1.60-2.28; P < 0.001) remained independently associated with mortality (Supplemental Table 5). Furthermore, the increased risk of mortality associated with both an absolute reduction of >35 m and a relative reduction of >5% was consistent across the spectrum of NAC disease stages (P for interaction = 0.862 and 0.323, respectively) and the 3 genotypes (P for interaction = 0.730 and 0.945, respectively) (Central Illustration). Similarly, the increased risk of mortality was consistent across patients who walked >350 m and <350 m at baseline (P for interaction = 0.978 and 0.722, respectively) (Supplemental Table 6).
Central Illustration.
Prognostic Importance of the 6-Minute Walk Test According to Subgroups
The risk of mortality associated with the baseline and change in 6-minute walk test (6MWT) according to subgroups. The first P value for interaction is the interaction between genotypes, and the second is the interaction between disease stages. NAC = National Amyloidosis Centre.
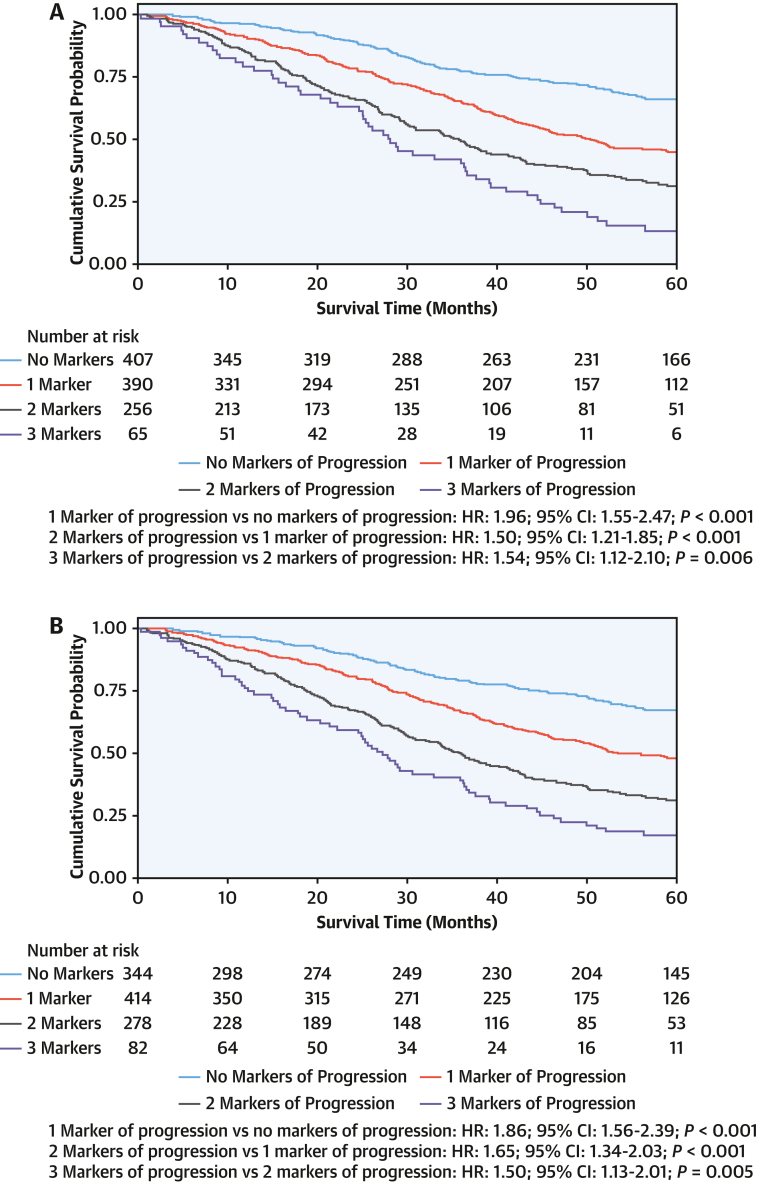
In a multivariable model with the covariates age, NAC disease stage, and baseline 6MWT distance of <350 m, NT-proBNP progression (HR: 1.65; 95% CI: 1.38-1.97; P < 0.001), ODI (HR: 1.58; 95% CI: 1.32-1.89; P < 0.001), and absolute reduction in 6MWT of >35 m (HR: 1.70; 95% CI: 1.43-2.03; P < 0.001) remained independently associated with an increased risk of mortality. Compared with patients who did not have any markers of disease progression at 1 year, in whom the death rate was 7.6 deaths per 100 py (95% CI: 6.2-9.3), the death rate was 13.9 deaths per 100 py (95% CI: 12.1-16.1 deaths per 100 py) in patients who had 1 marker of disease progression, 22.4 deaths per 100 py (95% CI: 19.4-26.1 deaths per 100 py) in patients with 2 markers of disease progression, and 32.9 deaths per 100 py (95% CI: 25.8-42.0 deaths per 100 py) in patients with 3 markers of disease progression (Figure 4).
Figure 4.
Markers of Progression and Survival
Landmark Kaplan-Meier curves demonstrating the association between markers of disease progression at the 1-year timepoint and subsequent survival. (A) The 6-minute walk test worsening as an absolute reduction (>35 m). (B) The 6-minute walk test worsening as a relative reduction (>5%).
The same variables remained independently associated with an increased risk of mortality after adjustment for a relative reduction in the 6MWT distance of >5% (NT-proBNP progression: HR: 1.67; 95% CI: 1.40-1.99; P < 0.001; ODI: HR: 1.55; 95% CI: 1.30-1.86; P < 0.001; 6MWT reduction of >5%: HR: 1.73; 95% CI: 1.45-2.07; P < 0.001), and similarly, each additional marker of progression was associated with an incremental increased risk of mortality (Supplemental Table 7). The increased risk associated with an incremental increase in markers of disease progression at 1 year was similar across the spectrum of NAC disease stages (P for interaction = 0.307) and the 3 genotypes (P for interaction = 0.708).
Discussion
This study is the first, to our knowledge, to comprehensively evaluate the importance of the 6MWT in a large population of patients with ATTR-CA, and it demonstrated the following: 1) 6MWT distance was significantly reduced in patients with ATTR-CA, with the degree of reduction being more severe in older, female patients with the p.(V142I) genotype and those with an advanced disease stage; 2) the 6MWT distance at baseline was independently associated with mortality; 3) absolute (>35 m) and relative (>5%) reductions in the 6MWT distance at 1 year were independently associated with mortality; and 4) combining worsening 6MWT with established measures of disease progression enables further refinement of risk and could be highly relevant both in clinical practice to stratify disease progression and as an endpoint in clinical trials.
The 6MWT represents a simple and easily performed yet comprehensive, standardized functional assessment that accurately reflects the severity of cardiac disease and can risk stratify patients beyond the conventional biomarkers (NT-proBNP and eGFR) used in the NAC staging system.17 A baseline distance of <350 m demonstrates remarkable capacity for identifying patients at an increased risk of mortality, with the associated risk being similar across the spectrum of NAC disease stages and the 3 different genotypes. Regardless of the cardiac disease severity or clinical phenotype, the 6MWT can be used alongside conventional markers to refine risk stratification during the baseline assessment and identify high-risk patients who may benefit from a more intensive treatment strategy and more frequent follow-up.
The 6MWT is the architype functional test that reflects each individual patient’s capability to conduct activities of daily living, and it is an objective measure that quantifies exercise capacity in a way that is important to patients. Therefore, longitudinal changes in the 6MWT distance have been widely used to demonstrate the efficacy of ATTR-specific disease-modifying therapies. However, the change in 6MWT distance in response to treatment has varied significantly among different agents.7, 8, 9 In the present study, the historical cohort (pre-2017) experienced a more rapid decline than the contemporary cohort (post-2017). This difference is likely to reflect patients in the contemporary cohort being diagnosed earlier in the course of their disease, with a milder cardiac phenotype, and therefore experiencing a slower decline.1 The impact of disease severity at baseline on the trajectory of 6MWT distance decline has been mirrored across different clinical trials. By comparing the trajectories of 6MWT distance decline in placebo patients across clinical trials, patients in more recent trials (ATTRibute-CM) had slower rates of disease progression compared with those in older trials (ATTR-ACT), supporting the hypothesis that the rate of 6MWT distance decline is significantly influenced by the disease stage at baseline.7,8 In clinical trials, the treatment effect on the rate of 6MWT distance decline will depend on the degree of deterioration in placebo patients, making simple comparisons of treatment effect across different trials based on changes in 6MWT challenging. This further emphasizes the need to define a minimal clinically important difference in the 6MWT at an individual level to ensure that novel therapies are appropriately assessed in the changing landscape of the clinical phenotype of patients with ATTR-CA.
In the current study, which represents the largest analysis of functional exercise testing in patients with ATTR-CA to our knowledge, an absolute reduction of >35 m or a relative reduction of >5% at 1 year was independently associated with an increased risk of mortality, even after adjustment for baseline characteristics and other established measures of disease progression. Furthermore, both absolute and relative worsening of the 6MWT distance were consistently associated with a similar increased risk of mortality across the spectrum of NAC disease stages and the 3 different genotypes. Patients with both p.(V142I) and non-p.(V142I) hATTR-CA experienced a more rapid decline in 6MWT distance than those with wtATTR-CA, and the presence of a hereditary genotype remained independently associated with the decline in 6MWT distance after adjustment for baseline characteristics and established markers of disease progression. It is possible that these differences are explained by intrinsic differences in disease biology between the different genotypes. The p.(V142I) genotype is associated with a more aggressive and rapidly progressive cardiomyopathy, whereas the non-p.(V142I) genotype is most commonly associated with a mixed phenotype, comprising a cardiomyopathy and peripheral polyneuropathy.18 The progression of cardiac disease invariably negatively influences exercise performance, and neuropathic involvement impairs balance, mobility, coordination, and postural stability, the combination of which are all assessed during the 6MWT. This illustrates the utility of the 6MWT as a comprehensive measure of functional capacity and its importance in monitoring the complex and multifaceted ATTR-CA disease process. The novel definitions of 6MWT worsening identified in this study could easily be applied in clinical practice to guide optimization of treatment strategies and as an endpoint in clinical trials. The use of 6MWT worsening as a surrogate marker of disease progression in clinical trials would enable the measurement of a metric that is clinically meaningful to both patients and clinicians alike while also potentially reducing the number of patients and length of follow-up required to evaluate the efficacy of novel agents.
Despite the 6MWT representing an important tool in the assessment of ATTR-specific disease-modifying therapies, many heart failure therapies shown to improve outcomes in the general heart failure population have failed to improve the 6MWT distance. This discrepancy is likely multifactorial in nature. Trials of heart failure therapies tend to reassess the 6MWT distance between 4 and 6 months,19,20 whereas in trials of ATTR-specific therapies, reassessment took place between 12 and 30 months.7, 8, 9 The magnitude of change in 6MWT distance is likely to be amplified as the follow-up duration extends, and therefore longer follow-up may be required to detect meaningful differences. This observation may also reflect inherent differences in disease biology, with ATTR-CA representing a well-defined, severe form of heart failure with a known pathophysiology for which highly-specific treatments are available.21 The severity of ATTR-CA is evident in accelerated 6MWT distance decline, and treatments targeting ATTR-specific disease pathways have consistently demonstrated clinically meaningful and significant changes in the 6MWT distance across all 3 large, randomized, placebo-controlled trials of disease-modifying therapy.7, 8, 9 This observation emphasizes important distinctions between different heart failure etiologies and underscores the necessity for tailored functional tests to evaluate the efficacy of disease-specific therapies.
Although the decline in 6MWT distance was associated with both NT-proBNP progression and ODI, all 3 markers of disease progression remained independently associated with mortality, indicating that each marker is capturing a slightly different aspect of the underlying disease process. NT-proBNP elevation reflects a combination of pathophysiologic biochemical processes occurring in response to cardiac amyloid infiltration, such as neurohormonal activation, impaired renal filtration, and fluid status.17 ODI acts as a surrogate marker of worsening heart failure symptoms secondary to fluid accumulation,13 and the 6MWT is a comprehensive functional assessment that provides a measure of cardiovascular robustness while also accounting for the extent of neuropathic involvement.5,6 The combination of these variables provides incremental information to each individual variable and allows further refinement of the rate of disease progression and risk of mortality.
Considering the likely availability of various novel therapeutic agents in the near future, the ability to reliably detect patients experiencing disease progression is of paramount importance.10 The presence of all 3 markers of disease progression was associated with a >4-fold higher risk of mortality and could signify the need to either switch to alternative agents with different mechanisms of actions or consider intensifying treatment with combination therapy. These markers are widely available, simple to measure, and inexpensive. Their universal applicability across the spectrum of NAC disease stages and different genotypes should support their widespread adoption to identify patients experiencing disease progression.
Study limitations
There is a possible selection bias, in that only patients who were able to complete a baseline 6MWT assessment were eligible for inclusion. There is also an invariable survival bias in the assessment of 6MWT worsening, and therefore it may be that the extent of differences is underestimated, and rapid disease progression may have resulted in death before the follow-up assessment.
Conclusions
In this large cohort of patients with ATTR-CA, the baseline 6MWT distance refined risk stratification and reliably identified patients at a higher risk of mortality regardless of their disease stage or genotype. Worsening of the 6MWT at 1 year was common and independently associated with an increased risk of mortality, even after adjustment for established markers of disease progression. The combination of 3 markers of progression (NT-proBNP progression, ODI, and 6MWT worsening) enabled further refinement of risk and facilitated the detection of patients experiencing rapid disease progression who are at the highest risk of mortality.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: The 6MWT distance at baseline is associated with an increased mortality in patients with ATTR-CA, across genotypes and disease stages.
TRANSLATIONAL OUTLOOK: Further research is needed to assess the incremental value of the 6MWT beyond other biomarkers to improve risk stratification and guide treatment in patients with ATTR-CA.
Funding Support and Author Disclosures
Dr Wechalekar has received consulting income from Alexia, AstraZeneca, Janssen, Attralus, and Prothena. Dr Solomon has received research grants from Alexion, Alnylam, AstraZeneca, Bellerophon, Bayer, BMS, Cytokinetics, Eidos, Gossamer, GSK, Ionis, Lilly, MyoKardia, National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI; and has consulted for Abbott, Action, Akros, Alexion, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, and Valo. Dr Gilmore has received consulting income from Ionis, Eidos, Intellia, Alnylam, and Pfizer. Dr Fontana is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/21/33447); and has received consulting income from Intellia, Novo Nordisk, Pfizer, Eidos, Prothena, Akcea, Alnylam, Caleum, Alexion, Janssen, Ionis, and AstraZeneca. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Listen to this manuscript's audio summary by Editor Emeritus Dr Valentin Fuster onwww.jacc.org/journal/jacc.
James L. Januzzi, MD, served as Guest Associate Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and an expanded Results section, please see the online version of this paper.
Appendix
References
- 1.Ioannou A., Patel R.K., Razvi Y., et al. Impact of earlier diagnosis in cardiac ATTR amyloidosis over the course of 20 years. Circulation. 2022;146:1657–1670. doi: 10.1161/CIRCULATIONAHA.122.060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ioannou A., Patel R.K., Razvi Y., et al. Multi-imaging characterization of cardiac phenotype in different types of amyloidosis. J Am Coll Cardiol Img. 2022;16:464–477. doi: 10.1016/j.jcmg.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Porcari A., Razvi Y., Masi A., et al. Prevalence, characteristics and outcomes of older patients with hereditary versus wild-type transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2023;25:515–524. doi: 10.1002/ejhf.2776. [DOI] [PubMed] [Google Scholar]
- 4.Patel R.K., Ioannou A., Razvi Y., et al. Sex differences among patients with transthyretin amyloid cardiomyopathy—from diagnosis to prognosis. Eur J Heart Fail. 2022;24:2355–2363. doi: 10.1002/ejhf.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bittner V., Weiner D.H., Yusuf S., et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 6.Opasich C., Pinna G.D., Mazza A., et al. Six-minute walking performance in patients with moderate-to-severe heart failure; is it a useful indicator in clinical practice? Eur Heart J. 2001;22:488–496. doi: 10.1053/euhj.2000.2310. [DOI] [PubMed] [Google Scholar]
- 7.Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 8.Gillmore J.D., Judge D.P., Cappelli F., et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390:132–142. doi: 10.1056/NEJMoa2305434. [DOI] [PubMed] [Google Scholar]
- 9.Maurer M.S., Kale P., Fontana M., et al. Patisiran treatment in patients with transthyretin cardiac amyloidosis. N Engl J Med. 2023;389:1553–1565. doi: 10.1056/NEJMoa2300757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannou A., Fontana M., Gillmore J.D. RNA targeting and gene editing strategies for transthyretin amyloidosis. BioDrugs. 2023;37:127–142. doi: 10.1007/s40259-023-00577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Pavia P., Bengel F., Brito D., et al. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2021;23:895–905. doi: 10.1002/ejhf.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crapo R.O., Casaburi R., Coates A.L., et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Ioannou A., Cappelli F., Emdin M., et al. Stratifying disease progression in patients with cardiac ATTR amyloidosis. J Am Coll Cardiol. 2024;83(14):1276–1291. doi: 10.1016/j.jacc.2023.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M.S., Anker S.D., Friede T., et al. Minimal clinically important differences in 6-minute walk test in patients with HFrEF and iron deficiency. J Card Fail. 2023;29:760–770. doi: 10.1016/j.cardfail.2022.10.423. [DOI] [PubMed] [Google Scholar]
- 15.Mathai S.C., Puhan M.A., Lam D., Wise R.A. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:428–433. doi: 10.1164/rccm.201203-0480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohannon R.W., Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23:377–381. doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 17.Gillmore J.D., Damy T., Fontana M., et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 18.Razvi Y., Ioannou A., Patel R.K., et al. Deep phenotyping of p.(V142I)-associated variant ATTR amyloid cardiomyopathy: distinct from wild-type ATTR amyloidosis? Eur J Heart Fail. 2023;26(2):383–393. doi: 10.1002/ejhf.3088. [DOI] [PubMed] [Google Scholar]
- 19.Mcmurray J.J.V., Docherty K.F., de Boer R.A., et al. Effect of dapagliflozin versus placebo on symptoms and 6-minute walk distance in patients with heart failure: the DETERMINE randomized clinical trials. Circulation. 2023;149(11):825–838. doi: 10.1161/CIRCULATIONAHA.123.065061. [DOI] [PubMed] [Google Scholar]
- 20.Pieske B., Wachter R., Shah S.J., et al. Effect of sacubitril/valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. 2021;326:1919–1929. doi: 10.1001/jama.2021.18463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannou A., Massa P., Patel R.K., et al. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur Heart J. 2023;44:2893–2907. doi: 10.1093/eurheartj/ehad347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.