Abstract
Background
Radiotherapy is essential in the treatment of prostate cancer. An alternative to conventional photon radiotherapy is the application of carbon ions, which provide a superior intratumoral dose distribution and less induced damage to adjacent healthy tissue. A common characteristic of prostate cancer cells is their dependence on androgens which is exploited therapeutically by androgen deprivation therapy in the advanced prostate cancer stage. Here, we aimed to analyze the transcriptomic response of prostate cancer cells to irradiation by photons in comparison to carbon ions, focusing on DNA damage, DNA repair and androgen receptor signaling.
Methods
Prostate cancer cell lines LNCaP (functional TP53 and androgen receptor signaling) and DU145 (dysfunctional TP53 and androgen receptor signaling) were irradiated by photons or carbon ions and the subsequent DNA damage was assessed by immuno-cytofluorescence. Furthermore, the cells were treated with an androgen-receptor agonist. The effects of irradiation and androgen treatment on the gene regulation and the transcriptome were investigated by RT-qPCR and RNA sequencing, followed by bioinformatic analysis.
Results
Following photon or carbon ion irradiation, both LNCaP and DU145 cells showed a dose-dependent amount of visible DNA damage that decreased over time, indicating occurring DNA repair. In terms of gene regulation, mRNAs involved in the TP53-dependent DNA damage response were significantly upregulated by photons and carbon ions in LNCaP but not in DU145 cells, which generally showed low levels of gene regulation after irradiation. Both LNCaP and DU145 cells responded to photons and carbon ions by downregulation of genes involved in DNA repair and cell cycle, partially resembling the transcriptome response to the applied androgen receptor agonist. Neither photons nor carbon ions significantly affected canonical androgen receptor-dependent gene regulation. Furthermore, certain genes that were specifically regulated by either photon or carbon ion irradiation were identified.
Conclusion
Photon and carbon ion irradiation showed a significant congruence in terms of induced signaling pathways and transcriptomic responses. These responses were strongly impacted by the TP53 status. Nevertheless, irradiation mode-dependent distinct gene regulations with undefined implication for radiotherapy outcome were revealed. Androgen receptor signaling and irradiations shared regulation of certain genes with respect to DNA-repair and cell-cycle.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13014-024-02480-z.
Keywords: Carbon ion irradiation, Photon irradiation, Prostate cancer, Androgen receptor, TP53
Introduction
Photon irradiation is an established technique for the treatment of various cancers including prostate cancer [1]. Carbon ion (12C-ion) irradiation by a particle accelerator is an innovative radiation technique in medicine with different physical properties allowing high precision targeting of the tumor region [2–4]. Particularly, the radiation energy of 12C-ions is directed as a Bragg-Peak to constrained predetermined tissue depths as opposed to the deposition of photon energy at broader tissue depths [5]. Thereby, 12C-ion irradiation can more precisely target the tumor volume while minimizing hazardous effects on healthy adjacent tissue. This may be superior for the irradiation of anatomically critical tumor entities such as those of the central nervous system, head-neck and prostate cancer [3, 6, 7].
Beside radiation physics, the genotoxic insults exerted by photon versus 12C-ion irradiation may have common and divergent characteristics in prostate cancer with impact on cellular responses of DNA-damage, DNA repair and gene regulation [8–10]. In addition, the responses may depend on the cancer cell specific context due to differences in the occurring genetic alterations as well as active signaling pathways that specifically drive tumor progression [11–13] potentially affecting the therapeutic outcome of irradiation.
In prostate cancer, irradiation therapy is a treatment option in all clinical stages of the disease [1]. One significant aspect of prostate cancer is the androgen receptor (AR) signaling pathway which is exploited as a drug target by pharmacologic androgen deprivation or antagonistic AR blockade therapy in certain tumor stages [14–16]. Critically, AR signaling has been shown to be interconnected with DNA-damage and with DNA-repair genes in prostate cancer cells and tumor models. In primary human prostate cancer samples, a link between AR signature and DNA repair genes was demonstrated. Combined AR intervention and irradiation resulted in decreased clonogenic survival of prostate cancer cells or tumor progression in mice models. Notably, the protocols of AR intervention included both antagonistic inhibition or agonistic activation of AR signaling in different doses [8–10]. Overall, these findings corroborate that intervention in AR signaling can favor the outcome of photon irradiation therapy. However, it remains unknown whether the gene regulatory effects are transferable to irradiation using 12C-ions.
Of further relevance is the prominent tumor suppressor TP53 pathway, which functions as a critical master of gene regulation [17–19]. In general, TP53 is crucial for therapeutic cancer interventions that target DNA integrity such as chemotherapeutics and irradiation. TP53 is activated by DNA damage such as DNA double strand breaks (DSB) and induces cell cycle arrest through a well-defined signaling cascade. TP53 is regulated by a feedback circuit involving MDM2 ubiquitination and proteasomal degradation. The cell cycle kinase inhibitor (CDKN1A) is a key target gene of TP53 that initiates the cell cycle arrest.
In prostate cancer, TP53 is dysfunctional in a considerable subset of cases in addition to AR mutations. Both TP53 and AR aberrations indicate a worse prognosis in metastatic androgen responsive prostate cancer [20].
Here, we aimed to explore common and selective effects of 12C-ion and photon irradiation with respect to DNA-damage and gene regulation, as reflected by alterations of mRNA levels in two human prostate cancer cell lines. We analyzed LNCaP cells with functional TP53 and AR, as well as DU145 cells with dysfunctional TP53 and AR. In particular, we focused on target genes related to DNA repair, cell cycle and DNA replication. Alongside, we monitored AR signaling that was induced as reference by dihydrotestosterone in LNCaP cells and examined correlations with the respective irradiation responses. These analyses were complemented by whole transcriptome analyses using RNA-seq technique. In essence, we identified common and unique changes in gene regulation induced by the different irradiation modes.
Materials and methods
Culturing of cell lines
The human prostate cancer cell lines LNCaP and DU145 were obtained and authenticated from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Germany (LNCaP, DSMZ-ACC 256; DU145, DSMZ-ACC 261). The shipment of LNCaP cells was in September 2017 and the latest authentication of DU145 cells in November 2018. Authentication was performed by DNA profiling using different and highly polymorphic short tandem repeat (STR) loci. The cell culture experiments were performed between 2018 and 2020. The experiments were performed with tested mycoplasma-free cells (Minerva Biolabs GmbH, Berlin, Germany). LNCaP cells have functional androgen receptor and TP53 signaling. DU145 cells are non-responsive to androgens and are non-functional for TP53 due to a missense mutation (cBioPortal v4.0.4: Cancer Cell Line Encyclopedia [21]). Cells were cultured in accordance with the recommended conditions in RPMI full growth medium supplemented with fetal bovine serum. LNCap and DU145 cells were treated by irradiations and LNCaP cells by addition of (5α, 17β)-17-hydroxy-androstane-3-one (di-hydro-testosterone; DHT) (20 nM) (Sigma-Aldrich). All treatments were performed in three independent triplicates with a calculated cell density of approximately 50% confluence at the time point of setup.
RNA and protein isolation
RNA was isolated by the RNeasy Mini Kit (QIAGEN, Hilden, Germany) procedure according to the manufacturer’s protocol. Protein was isolated with RIPA buffer (Cell signalling Technology Europe, Frankfurt a.M., Germany) supplemented with protease inhibitor cocktail (Sigma-Aldrich Chemie, GmbH, Munich, Germany). The isolation procedure was performed according to the manufacturer’s protocols.
Western blot
Protein samples (40 µg) were analysed by sodium dodecyl sulphate polyacrylamide gel electrophoresis with subsequent electric transfer to a nitrocellulose membrane (Bio-Rad-System, Germany). The membranes were blocked in TRIS-buffered saline with 0.1% tween containing 5% dry milk and the primary antibodies were added and incubated at 4 °C for 24 h. The antibodies were as follows: H2A.X, rabbit mAb, #7631S, dilution 1:5000, Cell Signaling Technology; Phospho-Histone H2A.X (Ser139), rabbit mAb, #9718S, dilution 1:5000, Cell Signaling Technology; cytoplasmic β-actin, mouse #MAK6019 Linaris GmbH. Then, the respective secondary anti host antibodies coupled with horseradish peroxidase (Anti-Rabbit IgG: HRP, goat, #ZRH1158, Linaris; anti-Mouse IgG: HRP, goat, #31,430, dilution 1:5000, Thermo Scientific) were added for band detection with enhanced chemiluminescent luciferase kit (Thermo Scientific, Rockford, USA) by an imager system (Fluorchem IS-8900, Alpha Innotech, San Leandro, CA, USA).
cDNA synthesis and realtime RT-PCR
RNA (1 µg) was treated with DNAse I. cDNA synthesis was performed with random hexamer primers and M-MLV reverse transcriptase. The cDNA was submitted to SYBR green (ThermoScientific, UK) based RT-qPCR (IQ5, Biorad, Germany). Cycling conditions were: 95 °C, 7.5 m followed by 40 cycles (95 °C, 15 s; 58 °C, 30 s; 72 °C, 30 s). Melting curve analysis was performed by a temperature increment (0.5 °C, 10 s) from 60 up to 95 °C. Target mRNA levels are displayed as -ΔCt values (log 2-scale) normalized to TATA-binding protein (TBP)-mRNA as references. The primer sets (Biomers GmbH, Germany) were derived from sequence entries in GenBank and selected by Primer-Blast (NCBI National Center for Biotechnology Information). The corresponding sequences are listed (supplementary Table S). The RT-PCR amplicons were characterized by DNA length and melting temperature profile and contaminations were excluded by appropriate controls (data not shown).
Irradiations
For photon irradiation an X-RAD 320ix cabinet was used (X-ray unit (Precision X-Ray Inc, Denver, USA, 320 kV, 8 mA, filter: 0.5 mm Cu and 0.5 mm Al, dose rate 1 Gy/min).
12C-irradiation was performed at the Marburg Ion-Beam Therapy Centre (MIT). Cells were irradiated with a horizontal beam of 114.5–129.5 MeV/n 12C-ions and positioned in the middle of a spread-out Bragg peak (SOBP) of 10–20 mm. Fields were applied using active scanning with a square of 324 cm2.
Immuno-cytofluorescence of γ-H2AX/53BP1 DSB foci
For detection of DSB repair foci, co-staining with γ-H2AX and 53BP1 antibodies was performed. Asynchronous growing cells were seeded on glass cover slips 36 h prior to irradiation [22–24]. The cover slips were precoated by 0.01% Poly-L-Lysin-hydrobromide for improving cell adherence to the surface. Cells were fixed and stained at various time points (2–48 h) after irradiation using 4% para-formaldehyde/PBS for 10 min. Fixed cells were permeabilized with 0.2% Triton X-100, 1% BSA/PBS for 10 min, washed with 1% BSA/PBS and blocked in 3% BSA/PBS for 1 h. The primary antibody solution was incubated for 1.5 h at room temperature using the following antibodies: mouse monoclonal anti-phospho-S139-H2AX antibody (1:500, clone JBW301, Millipore, Darmstadt, Germany) and rabbit polyclonal 53BP1 antibody (1:500, Novus Biologicals, Wiesbaden, Germany). After washing three times with 0.1% Tween20/PBS for 10 min, the cells were incubated for 1.5 h with secondary anti- mouse Alexa-fluor594 (1:1000) and anti-rabbit Alexa-fluor488 (1:1000, both Invitrogen, Karlsruhe, Germany). Cells were again washed three times and mounted in ProLong Gold antifade reagent (Invitrogen, Karlsruhe, Germany) containing DAPI for staining of nuclei. Immunofluorescence analysis was conducted using the Leica DM5500 wide-field microscope. About twenty z-stacks (0.30 mm) per image were captured using an immersion objective with 63 × magnification and a numerical aperture of 1.25. To analyse the z-stacked images, they were deconvoluted and overlaid with the integrated LAS-AF software (Leica, Wetzlar, Germany), and foci were counted using FiJi software.
Statistical analysis
Correlation analyses was performed by non-parametric Spearman test and linear regression (Pearson correlation) analysis. The analyses were performed with MS Excel 2017 and Graphpad Prism 9.5.1.
RNAseq
For RNAseq, RNA quality was assessed using the Bioanalyzer RNA 6000 Nano Kit (Agilent). RNAseq libraries were prepared from total RNA with the QuantSeq 3′ mRNA-Seq Library Prep Kit FWD for Illumina (Lexogen) in combination with the UMI Second Strand Synthesis Module for QuantSeq FWD (Illumina, Read 1) (Lexogen) according to the manufacturer’s instructions. Quality of sequencing libraries was controlled on a Bioanalyzer 2100 using the Agilent High Sensitivity DNA Kit (Agilent). Pooled sequencing libraries were quantified and sequenced on the NextSeq550 platform (Illumina) with 75 bases single reads. The raw data has been submitted to EMBL Biostudies and can be accessed via the accession number E-MTAB-14099.
Data analyses and bioinformatics of RNA seq data
Unique molecular identifiers (UMI) were extracted from the sequenced reads and the first four nucleotides corresponding to the QuantSeq FWD-UMI 3′ spacer were removed. Trimmed reads were mapped to the Homo sapiens (revision 99, GRCh38) Ensembl reference genome, using STAR (version 2.6.1d) [25]. After alignment, UMIs were deduplicated using UMI-tools (version 1.1.1). UMI per gene were quantified and normalized to counts per million (CPM). Genes with CPM counts were below 1 in all samples were considered background noise and discarded. In addition, genes were restricted to protein-coding genes in the further analysis. Raw read counts of paired samples were used to assess differential gene expression via EdgeR (version 3.24.0). Obtained p-values were corrected via Benjamini–Hochberg correction. Genes with log2FC ≥ 1 as well as corrected p-values < 0.05 were considered differentially expressed. Principle component analysis was performed using scikit-learn. Gene set enrichment analysis [26] was performed using gene sets from Molecular Signatures Database (MSigDB: https://www.gsea-msigdb.org/gsea/msigdb/ index.jsp) and from [9] as listed. The conclusive parameters of GSEA comprise the enrichment score (ES) that can be positive or negative, the false discovery rate (FDR q-value) and the nominal p-value. ES-values with FDR < 0.25 and nominal p-value < 0.05 are considered as significant. The ES values are displayed in volcano-plots (ES vs − log FDR) (Fig. 4). For GSEA-graphs and parameters see supplementary information.
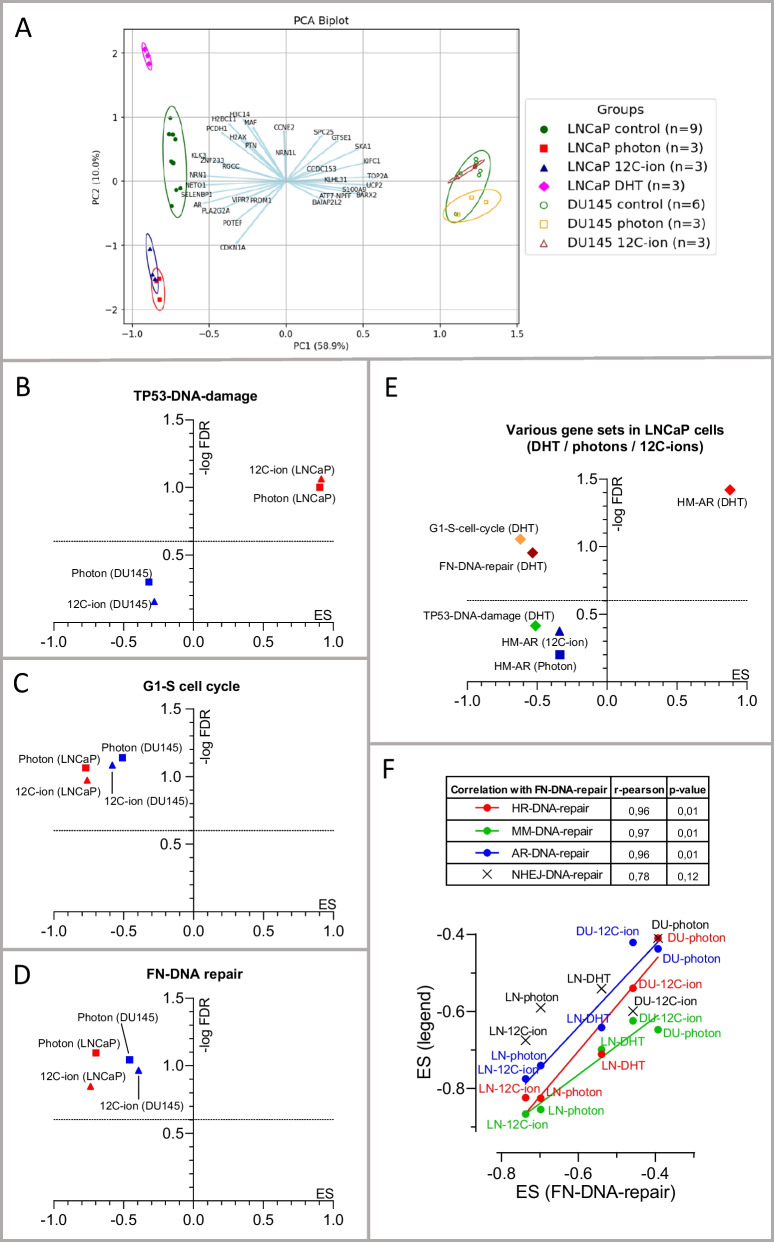
Fig. 4.
Analysis of RNA seq data by Principal Component Analysis (PCA) and gene set enrichment analysis (GSEA) from samples of control, photon (6 Gy; 24 h) and 12C-ion (2 Gy; 24 h) irradiation or DHT treatment (20 nM; 24 h). A PCA Biplot of the mRNAs including all samples. The principal component 1 (PC1) covers 58.9% (x-axes) and PC2 covers 10% (y-axes) of the multidimensional data sets of the detected mRNA targets (n > 104). The samples of LNCaP or DU145 cells are grouped as control, photons, 12C-ions or DHT, as indicated by different colors and symbols (see legend). The n-number of controls is n = 9 for LNCaP and n = 6 DU145 since the respective matched control values (n = 3) were merged. The samples are encircled by a line representing the 95% confidence interval (95% CI) for each group. The loading vectors (light blue) represent several selected altered genes and indicate their contribution to PC1 or PC2. (B-E) Gene set enrichment analyses (GSEA) of enrichment score (ES) and false discovery rate (FDR) are displayed as volcano plots (ES vs. − log FDR). In GSEA, ES-values are considered as significant if FDR < 0.25 and p < 0.05. The dotted line at (− log FDR = 0.602) corresponds to FDR = 0.25, meaning that the ES values above this line have an FDR < 0.25. Here, these gene sets have nominal p-values < 0.01 (see supplementary information) and therefore the ES-values above the dotted line represent significant ES-values. The critical ES-values of the treatments are displayed with respect to different gene sets: B Amundson-DNA-damage-response-TP53 (TP53-DNA-damage) for photons and 12C-ions; C WP-G1-to S-cell cycle-control (G1-S-Cell cycle) photons and 12C-ions; D WP-DNA-repair pathway full network (FN-DNA-Repair) for photons and 12C-ions. In E the Hallmark Androgen Response (HM-AR) in DHT-treated LNCaP cells is compared with photon and 12C-ion irradiated LNCaP cells. In E, the gene sets shown in B–D are analyzed with respect to DHT treatment. In F, the ES values of FN-DNA-repair are correlated with those of other DNA-repair modes particularly, DNA-repair-homologue-recombination (HR-DNA-repair), non-homologue-end-joining (NHEJ-DNA-repair), mismatch repair-DNA-repair (MM-DNA-repair) and AR-targeted DNA-repair (AR-DNA-repair). The symbols refer to the DNA-repair mode (see inset table). Treatments and cells are indicated LNCaP (LN) and DU145 (DU)
Results
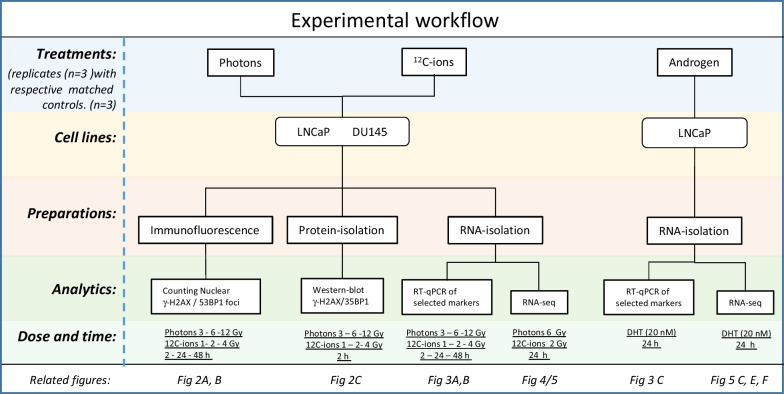
We analyzed cellular and molecular effects triggered by irradiation with photons or 12C-ions. We focused on DNA damage as well as selective and comprehensive gene regulation. For that, we analyzed the two cell lines LNCaP and DU145 that differ genetically with respect to TP53 and androgen signaling. LNCaP have functional TP53 and androgen receptor (AR) signaling whereas DU145 are dysfunctional for TP53 and AR signaling. AR signaling was induced in LNCaP cells by addition of dihydrotestosterone (DHT). A schematic diagram summarizes the experimental workflow (Fig. 1).
Fig. 1.
Schematic diagram of the experimental workflow
DNA damage evaluated by immuno-cytofluorescence of γ-H2AX and 53BP1
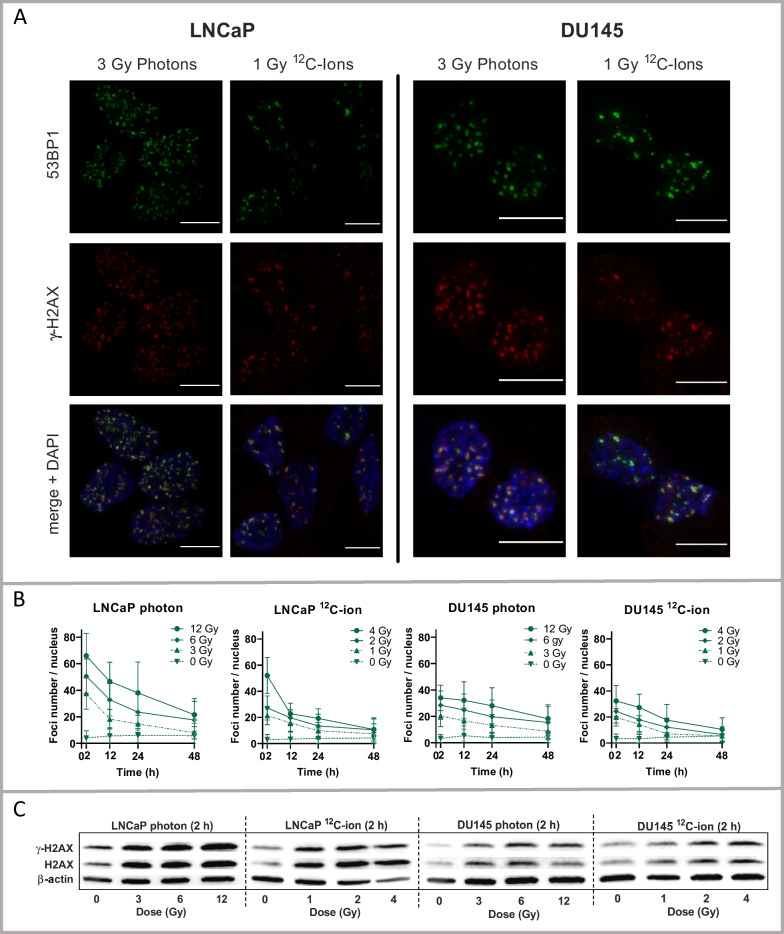
We measured DNA-damage caused by 12C-ion or photon irradiation by immunofluorescence for γ-H2AX and 53BP1 indicating the DNA-double-strand breaks (DSB) (Fig. 2A). The applied dose ranges for 12C-ions (0, 1, 2, 4 Gy) and photons (0, 3, 6, 12 Gy) were adjusted to take account of the relative biological effectiveness (RBE), which is higher for 12C-ions (approximately 3-times) than for photons [23, 27]. Using the number of DSB-foci as an indicator for DNA damage, the respective 12C-ion and photon dose curves strikingly overlapped for LNCaP and DU145 cells (Fig. 2B). Typically, the number of DSB-foci at 2 h after irradiation correlated with the irradiation dose. The numbers of DSB-foci per nucleus were higher in LNCaP than in DU145 cells. The decrease in DSB-foci over time reflected the process of DNA repair. Alongside, the formation of DSB-foci was paralleled by an increase of protein abundance of γ-H2AX and H2AX in cell lysates of LNCaP and DU145 cells (Fig. 2C) upon photons and 12C-ions.
Fig. 2.
Analysis of DNA-damage in LNCaP and DU145 cells following photon and 12C-ion irradiation. A Immunofluorescence of γ-H2AX (red), 53BP1 staining (green) and DAPI-merge (weakly blue) for counterstaining of nuclei. The co-localized red and green foci indicate the DNA-double-strand-breaks (DSB) per nucleus. The white bar scale corresponds to 10 µm. The respective images of dose and time served for counting of DSB-foci. B Number of DSB-foci for photon and 12C-ion irradiation of LNCaP (left) and DU145 (right) time dependently for each dose of photon or 12C-ion irradiation (mean ± SD, n ≥ 95). C Western-blot analysis of γ-H2AX and H2AX, after (2 h) photon or 12C-ion irradiation, dose-dependent in LNCaP cells (left) and DU145 cells (right)
Regulation of mRNAs encoding genes for DNA damage, DNA repair, DNA replication and cell cycle after photon and 12C-ion irradiation in LNCaP and DU145 cells
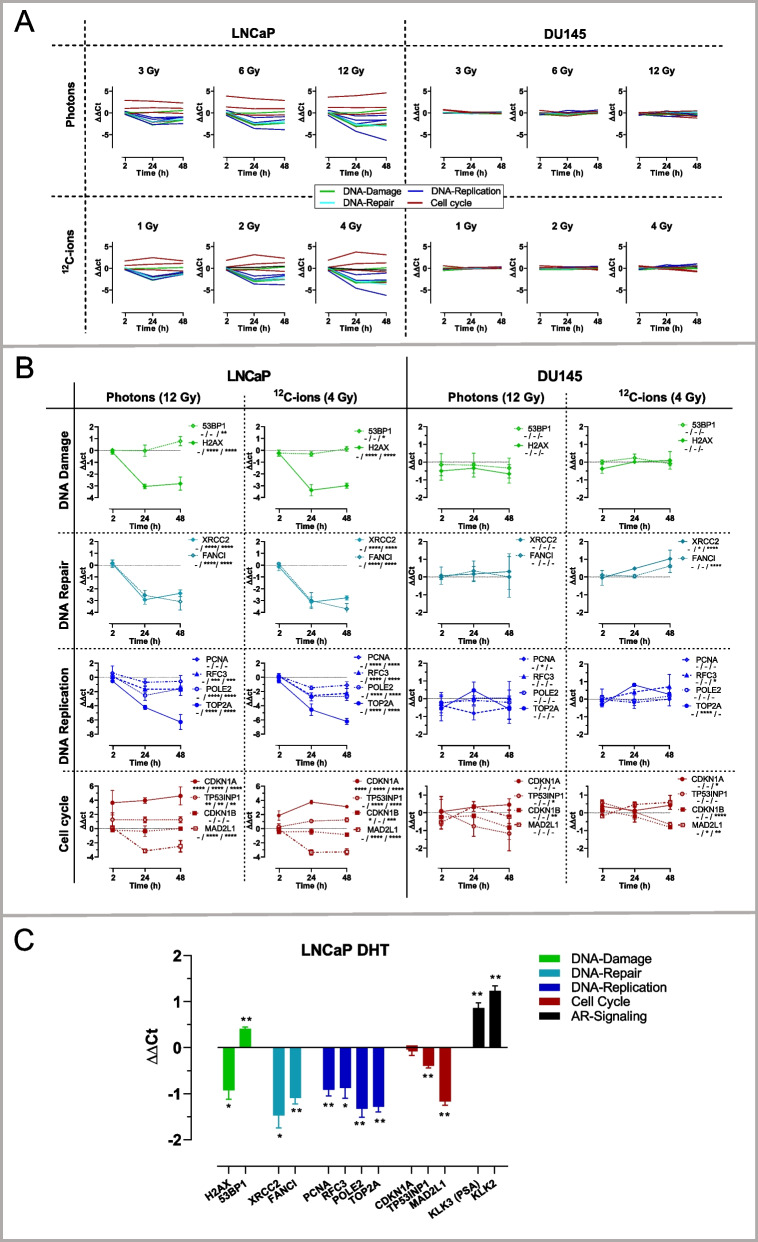
Next, we analyzed selected mRNAs of DNA-damage, DNA-repair, DNA replication and cell cycle in LNCaP and DU145 cells. An overview of the changes in mRNA levels upon photon and 12C-ion irradiation with all tested doses and time points is displayed (Fig. 3A). It reveals that the mRNA changes in LNCaP cells are strikingly stronger than in DU145 cells.
Fig. 3.
RT-qPCR analysis of selected mRNAs related to DNA-damage (green), DNA-repair (cyan), DNA replication (blue) and cell cycle (red) in LNCaP (left) and DU145 cells (right). A Overview of the changes of mRNA levels (ΔΔCt, mean, n = 3) upon photon and 12C-ion irradiations with all tested doses and time points. B Subsets of changes of mRNA levels (ΔΔCt) (mean ± SD, n = 3) in adjusted scales displayed for the irradiation modes with the maximum applied dose for photons (12 Gy) and for 12C-ions (4 Gy). Significant differences (2-way-ANOVA with multiple comparisons) are indicated for each time point (separated by slash) in the insets. C Changes of mRNA levels (ΔΔCt) after DHT treatment (20 nM; 24 h) of LNCaP cells for analysis of mRNAs related to DNA-damage (green), DNA-repair (cyan), DNA replication (blue), cell cycle (red) in addition to androgen receptor (AR) signaling (black). The changes of mRNA levels (ΔΔCt) are displayed (mean ± SD, n = 3). Significant differences between DHT and control were determined by unpaired Welch corrected t-test. Symbols of significances (*p < 0.05, **p < 0.01; ***p < 0.001; ****p < 0.0001)
We further divided the respective subsets of mRNAs and displayed their changes in adjusted scales for LNCaP and DU145 cells (Fig. 3B). Interestingly, in LNCaP cells, the initial increase in H2AX protein that was observed after 2 h (Fig. 3C) was counterbalanced by decreased H2AX-mRNA levels at later time points (24 h, 48 h) (Fig. 3B, 1st lane, left) whereas the 53BP1-mRNA levels were slightly upregulated (< twofold). In DU145 cells, both H2AX-mRNA and 53BP1-mRNA levels were unaffected by irradiation (Fig. 3B, 1st lane, right).
In addition to H2AX and 53BP1, we investigated additional mRNAs by RT-qPCR that are involved in DNA repair (XRCC2, FANCI) (Fig. 3B; 2nd lane), DNA replication (PCNA, RFC3, POLE2, TOP2A) (Fig. 3B; 3rd lane) and cell cycle control (CDKN1A, CDKN1B, TP53NIP1, MAD2L1) (Fig. 3B, 4th lane).
In LNCaP cells (Fig. 3B, left side), the mRNAs of DNA-repair- (XRCC2, FANCI) and DNA-regulator-genes (TOP2A, RFC3, POLE2; MAD2L1) except for PCNA were downregulated by both 12C-ion and photon irradiation. In particular, TOP2A mRNA was downregulated by almost 100-fold (ΔΔCt > 6). As a critical cell cycle inhibitor, CDKN1A-mRNA was upregulated by approximately 20-fold (ΔΔCt > 4) upon photon and ion irradiation.
In DU145 cells (Fig. 3B, right side), the alterations of mRNAs after irradiation with 12C-ions and photons were of considerably lower amplitude (< twofold; ΔΔCt < 1) than in LNCaP cells and are adequately displayed in a narrower scale.
Regulation of selected mRNAs by DHT
For evaluating AR-signaling, LNCaP cells were treated with DHT (Fig. 3C). The AR markers KLK3 (prostate specific antigen, PSA) and KLK2 were measured as known AR targets and displayed the typical DHT-dependent induction. In the DNA repair group, mRNAs for XRCC2, FANC1 and H2AX were significantly downregulated (twofold), while 53BP1 was slightly upregulated (1.5-fold) by DHT. Similarly, the DNA regulators PCNA, RFC3, POLE2 and TOP2A were downregulated (twofold). In the cell cycle group, CDKN1A was unaffected, whereas T53INP and MAD2L1 were downregulated by DHT.
In essence, certain genes from functional groups gave typical congruent responses in LNCaP cells to 12C-ion and photon irradiation. In particular, some DHT responses were common to the irradiation responses, most distinctly for DNA-damage and DNA repair genes and partially for DNA-regulators and cell cycle genes (e.g. TOP2A, MAD2L), with the exception of CDKN1A. In contrast to LNCaP cells, DU145 cells showed considerably lower changes in mRNA levels following irradiation.
Transcriptomic analysis by RNA-seq
Subsequently, we analyzed the transcriptomic mRNA response of LNCaP and DU145 cells by RNA-seq. Based on the previous results (Fig. 3A, B), the analysis was performed 24 h after irradiation with a dose of 6 Gy (photons) and 2 Gy (12C-ions). These intermediate irradiation doses as well as the intermediate time period exhibited significant gene regulatory effects and appeared suitable for comprehensive RNA-seq analysis. In particular, the higher selected dose range of photons versus 12C-ions is supported by literature [23, 27]. For analysis of AR response, LNCaP cells were incubated with 20 nM DHT. Each treatment included directly matched naïve control cells for analysis.
The obtained RNA seq data were evaluated using Principal Component Analysis (PCA) (Fig. 4A). PCA projected the huge multidimensional mRNA data (n > 104) of the treated and control samples to a 2-dimensional plane geometry with maximum possible differentiation for the first dimension 1 (X-axis: PC1 = 58.9%) and for the second dimension (Y-axis: PC2 = 10%) (Fig. 4A). The samples (dots) were labeled according to cell type and treatment group (control, photons, 12C-ions, or DHT). The groups of samples are encircled by respective lines indicating the 95% confidence interval (CI) of the treatment. In result, LNCaP cells (allocated on the left side) and DU145 cells (allocated on the right side) were separated. The LNCaP cells (filled shapes) could be separated into (i) control group (green circles) (ii) DHT group (red diamonds) and (iii) the overlapping irradiation modes 12C-ion (blue triangles) and photon (purple squares). Within DU145 cells (empty shapes), no separation was possible since the encircled 95% CI of control (green circles), photon (yellow squares) and 12C-ion (brown triangles) all overlapped. The loading vectors (light blue) refer to the most differentially expressed genes. Some of these have been already mentioned in Fig. 3 (CDKN1A, KLK3, TOP2A).
Gene set enrichment analysis of RNA-seq data
Furthermore, the RNA-seq data were analyzed using Gene set enrichment analysis (GSEA). GSEA statistically analyses gene sets that are preferentially altered as a whole in response to a trigger. Here, we focused on gene sets covering signaling pathways that are related to irradiation and AR signaling (listed in supplementary information). We tested gene sets related to Amundson-DNA-damage-response-TP53 (TP53-DNA-damage), WP-DNA-repair-pathway-full-network (FN-DNA-repair), WP-G1-to S-cell cycle-control (G1-S-cell cycle), and hallmark-androgen-response (HM-AR). Individual members of these pathways have been addressed previously (Fig. 3).
TP53-DNA-damage gene set
LNCaP cells displayed a significant positive enrichment score for the gene set TP53-DNA-damage after photon and 12C-ion-irradiation (Fig. 4B). In DU145 cells, which are dysfunctional for TP53, no significant ES for TP53-DNA-damage by photons or 12C-ions was determined (Fig. 4B).
G1-S-Cell-cycle gene set
LNCaP cells displayed significant negative ES for the gene set G1-S-cell cycle by photons and 12C-ions (Fig. 4C). In DU145 cells, this gene set also displayed significant negative ES, but to a lesser extent, when treated with photons and 12C-ions (Fig. 4C).
DNA-repair gene set
Next, we analyzed the gene set FN-DNA-repair. LNCaP cells showed significant negative ES after photon and 12C-ion-irradiation (Fig. 4D). In DU145 cells, the gene set FN-DNA-repair also displayed significant negative ES for both photons and 12C-ions (Fig. 4D).
DHT, photons and 12C-ions in relation to several gene set pathways
The androgen responsive LNCaP cells that had been treated with DHT were analyzed with respect to androgen receptor signaling using the gene set HM-AR (Fig. 4E). As expected, the gene set HM-AR exhibited a significant positive ES in DHT treated LNCaP. In comparison, the gene set HM-AR showed rather negative ES for photons and 12C-ions without significance. Alongside, TP53-DNA-damage, FN-DNA-repair and G1-S-cell cycle were analyzed for DHT treated LNCaP cells (Fig. 4E). TP53-DNA-damage was not significantly altered by DHT, whereas the ES values for FN-DNA-repair and G1-S-cell cycle control revealed significant negative enrichment by DHT. Complementarily, photons and 12C-ions both had negative ES-values for HM-AR but without significance (Fig. 4E). Furthermore, we compared FN-DNA-repair with subsets of DNA-repair modes including gene sets for kegg-homologues-recombination (HR-DNA-repair), kegg-non-homologues-end-joining (NHEJ-DNA-repair), kegg-mismatch-repair (MM-DNA-repair) and the subset of AR-targeted DNA-repair genes (AR-DNA-repair) [9]. The ES-values of FN-DNA-Repair significantly correlated with the listed DNA-repair modes except for NHEJ-DNA-repair (Fig. 4F).
Differentially expressed genes (DEG) after irradiation by photons or 12C-ions and DHT treatment
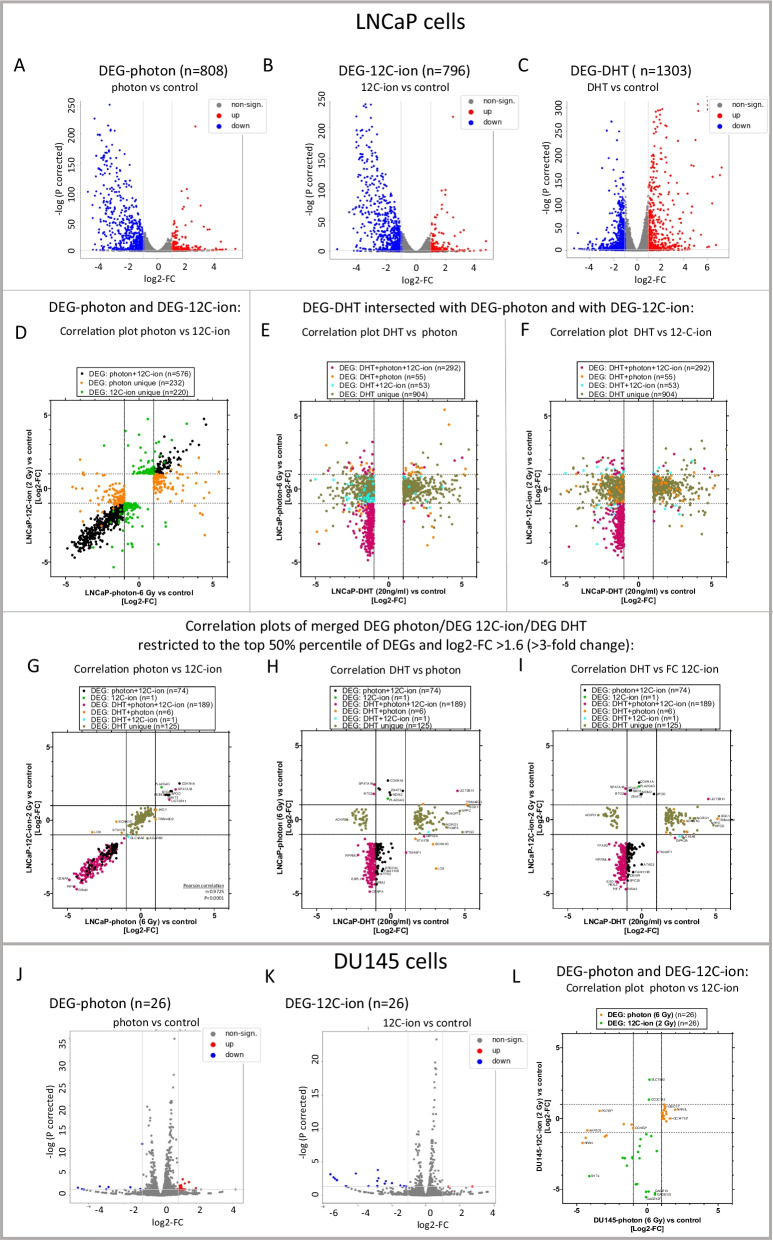
Next, DEG were determined from the RNA-seq data. DEG are defined as mRNAs that are altered with a log2FC ≥ 1 and corrected p-values < 0.05. DEG-photon and DEG-12C-ion were determined for LNCaP (Fig. 5A, B) and for DU145 cells (Fig. 5J, K). Furthermore, DEG-DHT were determined for LNCaP cells (Fig. 5C). The DEG are displayed in volcano plots.
Fig. 5.
Analysis of differentially expressed genes (DEG) determined by RNA-seq. DEG of LNCaP cells for DEG-photon A, DEG-12C-ion B and DEG-DHT C and in DU145 cells for DEG-photon J and DEG-12C-ion K. DEG are defined as log2FC ≥ 1; corrected p < 0.05. The log2-FC in the heatmaps with the corresponding volcano plots indicate treatment versus control (n = 3). Upregulated DEG are red, downregulated DEG are blue and non-significantly altered mRNAs are grey. D Correlation graphs of log2-FC of DEG-photon and DEG-12C-ion in LNCaP cells. DEG-photon that intersected with DEG-12C-ion are black (n = 577). Unique DEG-photon are orange (n = 232) and unique DEG-12C-ion are green (n = 220). E, F Correlation graphs of DEG-DHT that intersected with DEG-photon or with DEG-12C-ion. The same samples are plotted once with (log2-FC-photon) on the y-axis E and once with (log2-FC-(12C-ion) on the y-axis F. The majority of DEG-DHT were unique (n = 904, olive). A prominent fraction of DEG-DHT intersected with DEG-12C-photon (orange; n = 55), with DEG 12C-ion (cyan; n = 53) or with both (red; n = 292). G–I Correlation graphs of merged DEG-photon, DEG-12C-ion and DEG-DHT that are restricted to those with high expression levels (top 50th percentile of all DEG) and strong fold changes (0.3 > FC > 3; adequately to (log2FC > 1.6) for at least one treatment. The samples are plotted with different XY-axes. G log2-FC of photon versus 12C-ion with Pearson correlation analysis (r = 0.9725, p < 0.0001). H log2-FC of DHT versus photon and I log2-FC of DHT versus 12C-ion. Respective unique and intersected DEG are assigned to different sample colors with the n-number in the legends. The names of strikingly altered DEG are indicated. L Correlation graphs of log2-FC of DEG-photon and DEG-12C-ion in DU145 cells. DEG-12C-ion (orange; n = 26) and DEG-12C-ion (green, n = 26) were all unique without intersection. Some DEG-photon in DU145 cells (n = 5) intersected with DEG-photon in LNCaP cells and are marked with an asterisk
DEG in LNCaP cells
Essentially, LNCaP cells (with functional TP53 and AR signaling) displayed considerable alterations of certain mRNA levels upon photon and 12C-ion irradiation. A higher proportion of DEG-photon (n = 809) and of DEG-12C-ion (n = 797) were downregulated (blue) than upregulated (red) (Fig. 5A, B). Concerning DEG-DHT (n = 1304), a higher proportion was upregulated (red) than downregulated (blue) (Fig. 5C). The individual names of DEG are listed (supplementary information).
Furthermore, correlation plots depicting the fold-change (FC) of DEG-photon, DEG-12C-ion and DEG-DHT were constructed. DEG-photon considerably overlapped with DEG-12C-ion (black; n = 577) (Fig. 5D). DEG-photon that were unique are labeled in orange (n = 232) and those that were unique for DEG-12C-ion are labeled in green (n = 220) (Fig. 5D). Moreover, we compared DEG-DHT with DEG-photon or DEG-12C-ion; once with log2-FC-photon on the Y-axis (Fig. 5E) and once with Log2-FC-12C-ion on the Y-axis (Fig. 5F). We found that the majority of DEG-DHT were unique (n = 904, olive). A prominent fraction of DEG-DHT intersected with DEG-12C-photon (orange; n = 55), with DEG 12C-ion (cyan; n = 53) or with both (purple; n = 292).
Next, we focused on those DEG that have high expression levels and are strongly changed upon treatments. To that end, we arbitrarily restricted the comparison to the DEG of the top 50th percentile with a minimum threefold change (log2FC > 1.6) under photon, 12C-ion or DHT treatment. These DEGs were then merged and displayed in three XY-graphs (Fig. 5G, H, I). Strikingly, a strong correlation between DEG-photon versus DEG-12C-ion became obvious in LNCaP cells (Pearson r = 0.9725; p < 0.0001) reflecting the particularly high degree of congruent regulation by 12C-ion and photon in the top level DEG. The extremely altered mRNAs are named (Fig. 5G). Alongside, we displayed the values of the same samples for log2-FC DHT (X-axes) versus log2-FC photon (Y-axes) (Fig. 5H) and for log2-FC DHT (X-axes) versus log2-FC 12C-ion (Y-axes) (Fig. 5I). It is noteworthy that the genes that are predominantly induced by DHT remain unaffected by photon- or 12C-ion-irradiation (e.g.: SGK1, NPPC, HPGD, NDRG1). On the other hand, those genes that are majorly induced by photons and 12C-ions remain unaffected by DHT (e.g. CDKN1A, ZMAT3, MDM2) (Fig. 5H, I).
DEG in DU145 cells
In DU145 cells (dysfunctional TP53), the number of DEG-photon (n = 26) and DEG-12C-ions (n = 26) (Fig. 5J, K) were significantly lower (1–2 magnitudes) than in LNCaP cells (Fig. 5A, B). In addition, the amplitude of the fold changes was considerably lower and the triplicate values of fold changes appear less conform. Furthermore, all DEG-photon and DEG-12C-ion were unique in DU145 cells without intersection. The DEG showing the most extreme fold changes are identified with labels. Some mRNAs (n = 5) of DEG-photon in DU145 cells intersected with DEG-photon in LNCaP cells and are indicated by an asterisk in the correlation graph (Fig. 5L). For DEG-12C-ion, no intersection was observed between DU145 and LNCaP cells.
Identification of putative 12C-ion and photon dependent pathways in LNCaP and DU145 cells
As a direct approach to identify putative 12C-ion and photon dependent pathways, GSEA was applied to 12C-ion irradiated versus photon irradiated samples. This allowed us to identify Notch signaling as significantly enriched in 12C-ion versus photon irradiated LNCaP cells. In DU145 cells, we identified the unfolded protein response, the reactive oxygen species pathway and oxidative phosphorylation as significantly enriched in 12C-ion versus photon irradiated samples (supplementary information).
Discussion
Photon irradiation is an established technique for the treatment of prostate cancer and 12C-ion irradiation represents an innovative radiation technique. The physical parameters of these irradiation modes are well defined and their performance in radiotherapy of patients with advantages and disadvantages have been documented [3, 4].
Here, we rather focussed on critical biological responses in prostate cancer cells. As an early response, we compared DNA-damage of the applied irradiation doses of photons versus 12C-ions. As a later manifesting response, we studied gene regulation related to DNA-damage, DNA-repair, cell cycle and AR-signaling that is relevant in prostate cancer progression. The key messages are briefly listed and subsequently discussed.
-
i.
The applied doses of photons and 12C-ions were suitable in terms of inducing adequate DNA damage and gene responses. Alongside, these conditions caused typical induction of TP53 DNA-damage pathway by both photons and 12C-ions in TP53 functional cells, but not in TP53 dysfunctional cells.
-
ii.
The gene sets of DNA-repair and G1-S-cell-cycle were downregulated by photon- and 12C-ion-irradiation in both TP53-functional and TP53-dysfunctional cells.
-
iii.
AR-signaling was typically induced by DHT and this was paralleled by downregulation of the gene sets of DNA-repair and G1-S-cell-cycle. Photons and 12C-ions did not cause significant changes in canonical AR-signaling gene sets.
-
iv.
The identified uniquely regulated genes by photons and 12C-ions may serve as biological markers for the physical differences between photon and 12C-ion irradiation. In addition, GSEA-based analysis directly comparing 12C-ion and photon irradiation suggests some differentially regulated signaling pathways.
Dose ranges of photons and 12C-ions in relation to biological responses
The selected dose ranges of photons (3–6–12 Gy) and 12C-ions (1–2–4 Gy) caused comparable amounts of DSB-foci in LNCaP and DU145 cells, as well as an increased level of cellular H2AX protein within the cells which is in-line with an observation made for photons [28].
With respect to gene regulation of LNCaP cells, the selected mRNAs displayed a plateau of changes in the applied dose ranges for photons (6–12 Gy) and 12C-ions (2–4 Gy). In addition, the time frame (2–24–48 h) covered the critical range due to its asymptotic dynamics (Fig. 3A, B). Additionally, correlation analysis of RNA-seq data confirmed almost equal changes of the intersected mRNAs (DEG) upon exposure to photons and 12C-ions in LNCaP cells (r = 0.9725, p < 0.0001 in Fig. 5G). Thus, the dose ranges align with the data for RBE demonstrated to be lower for photons than for 12C-ions [23, 27]. Apparently, (i) the early response to irradiation indicated by DSB, (ii) the later response manifested at the level of gene regulation and (iii) the final response manifested by cell death (RBE) are roughly related.
DNA damage and TP53 status
The greater amount of DNA damage observed in LNCaP compared to DU145 cells following exposure to photons and 12C-ions is consistent with previous findings for photons [29]. Speculatively, DNA repair may be initiated more rapidly in DU145 cells than in LNCaP cells, so that the repair process is already evident at the first recorded time period of 2 h post-irradiation [30]. Another explanation may be related to the TP53 status [31]. Naturally, the TP53-DNA damage signaling was strikingly responsive towards photons and 12C-ions in LNCaP (functional TP53), but not in DU145 cells (dysfunctional TP53) (Fig. 3B, Fig. 4B). Of relevance, TP53 was suggested to break up DNA condensation by disassembling protective tight protein/DNA interactions, resulting in higher resistance to DNA-damage in TP53 dysfunctional cells [31, 32].
DNA damage upon photon and 12C-ion irradiation and DNA-repair mechanisms
Several studies suggested that DNA-damage caused by 12C-ions and photons differ with respect to the specific mechanisms of DNA-repair such as HR-DNA-repair and NHEJ-DNA repair. However, these studies are not consistent [23, 33–35]. Generally, our study revealed negative enrichment scores for various gene subsets of DNA-repair upon photons and 12C-ions with differences summarized in the correlation analysis (Fig. 4F). As a limitation of our study, the connection between the regulation of certain DNA-repair genes and functional DNA-repair remains elusive and would require biochemical data of the active DNA-repair mechanisms for explanation. The observed downregulation of DNA-repair genes upon photon irradiation in prostate cancer cells has been described elsewhere [36]. As a potential mechanism for downregulation in a distinct context, it was demonstrated that irradiation induced miR-711, which subsequently inhibited several DNA-repair genes [37]. Based on our data, we hypothesize that the regulation of specific targets for DNA repair and damage may diverge between protein and mRNA as exemplified for the increased expression of H2AX protein alongside the decreased level of H2AX-mRNA (Figs. 2B, C). The diverging levels of the related protein and mRNA markers may reflect active regulatory adaptation to the disturbed condition.
Regulation of AR-signaling by DHT in relation to photons and 12C-ions
The HM-AR gene set pathway was not significantly affected by photons or 12C-ions in LNCaP cells. The DHT-treated LNCaP group served as a control for HM-AR gene set induction. Similarly, the DNA-damage-TP53 pathway was not significantly affected by DHT. However, analogous to the irradiations, DHT did suppress DNA-repair and G1-S cell cycle gene sets. Specifically, the subsets of the DNA-repair pathways AR-DNA-repair, HR and MMR were significantly suppressed by DHT. Upon initial review, our findings differ from a previously conducted study that demonstrated AR-dependent induction of DNA-repair gene sets [9]. The aforementioned study utilized the AR agonist R1881 at a concentration of 1 nM, which resulted in the induction of the AR-DNA-repair gene set [9]. Methodologically different, we used the cellular agonist DHT at supraphysiological dose (20 nM) and importantly the AR-DNA-repair gene set in addition to other DNA-repair gene sets were significantly downregulated by DHT. Our results are quite robust and the experimental performance was corroborated by the typical induction of the AR pathway through DHT (Fig. 4E). A related study showed that employing supra-physiological doses of DHT (up to 100 nM) caused DNA-damage [10]. Another study demonstrated that exposure of LNCaP cells to high dose of R1881 (100 nM) blocked LNCaP proliferation, an effect that could be attributed to cell cycle dependent proteasomal degradation of AR [38, 39]. These fundamental observations may be associated with the results of a clinical trial [40, 41] where bipolar androgen therapy with alternating supraphysiological R1881 doses versus castrate-serum androgen levels revealed a benefit for patients with prostate cancer. Overall, our data indicate that using supra-physiological doses of DHT, as opposed to the lower androgen doses found in literature [9], results in the downregulation of DNA-repair genes. In this regard, DHT has similar effects on DNA repair genes as photon and 12C-ion irradiation.
Limitations of the study with respect to 12C-ion and photon dependent signaling pathways
The limitations of the study relate to the general meaning of differentially expressed genes reflecting responses towards photon and 12C-ion irradiation in only two genetically different prostate cancer cell lines. An interesting finding is that these two cell lines considerably differ in their transcriptomic response towards the irradiation modes between each other.
Furthermore, GSEA-based analysis was used to directly compare 12C-ion and photon irradiation and this approach identified differences in the magnitude of certain gene responses (supplementary information). In particular, responses related to Notch signaling, unfolded protein response, reactive-oxygen-species pathway and oxidative phosphorylation differed significantly between the irradiation modes in a cell type-specific manner. Notch signaling is altered in several types of cancer and promotes epithelial-mesenchymal transition and angiogenesis [42]. The unfolded protein response of the endoplasmic reticulum maintains a healthy proteome and its disturbance is relevant in the pathogenesis of cancer [43]. The reactive oxygen species pathway is frequently disrupted in cancer cells and is affected by irradiation [44]. Oxidative phosphorylation along with glycolysis are altered in cancer cells as well [45]. The cause and exact biological significance of the differently altered signaling pathways between the irradiation modes is currently still unknown. It is noteworthy that the observed differential regulation of these pathways in direct comparison of the irradiation modes would need to be examined in more detail in a dose-dependent manner for confirmation.
Conclusion
In conclusion, this study demonstrated similar gene regulatory responses to photon and 12C-ion irradiation with respect to DNA-damage, DNA-repair and cell cycle, with specificity to the utilized cell type. Androgen receptor-signaling and irradiations shared basic responses with respect to gene sets of DNA-repair and G1-S-cell-cycle. Alongside, distinctive mRNA alterations elicited through photons versus 12C-ions are suggested.
Supplementary Information
Acknowledgements
We thank Susanne Lingelbach and Stefanie Preising for excellent medical technical assistance.
Abbreviations
- AR
Androgen receptor
- 53BP1
DSB marker protein
- 12C-ions
Carbon ions
- CDKN1A
Cell cycle kinase inhibitor
- CI
Confidence interval
- DEG
Differentially expressed gene
- DHT
(5α, 17β)-17-Hydroxy-androstane-3-one (di-hydro-testosterone)
- DSB
DNA double strand break
- ES
Enrichment score
- FC
Fold change
- FDR
False discovery rate
- GSEA
Gene set enrichment analysis
- H2AX
DSB marker protein
- PCA
Principal component analysis
Author contributions
JH, LMM, and AH contributed to study conception. JH, FSBS, ML, and AH contributed to funding acquisition. LMM, DKT, TP, KR, AN, and JH performed experiments and data analyses. UT, FE organized and managed the irradiation experiments. MM performed bioinformatics analyses. TS, RH, REC and AH supervised. JH and LMM wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a grant from the Marburg Ion Beam Therapy Center (MIT) Research, Philipps University Marburg.
Data availability
All the data supporting the findings of this study are available within the paper. The raw data of RNA-seq has been submitted to EMBL Biostudies and can be accessed via the accession number E-MTAB-14099.
Declarations
Ethical approval and consent to participate
Not applicable, since the cell lines were used from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Germany.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jörg Hänze and Lilly M. Mengen have contributed equally to this work and share first authorship.
References
- 1.Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Li X, Yao L, et al. Clinical efficacy and safety of proton and carbon ion radiotherapy for prostate cancer: A systematic review and meta-analysis. Front Oncol. 2021;11:709530. doi: 10.3389/fonc.2021.709530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byun HK, Han MC, Yang K, et al. Physical and biological characteristics of particle therapy for oncologists. Cancer Res Treat. 2021;53(3):611–620. doi: 10.4143/crt.2021.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(8):953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 5.Malouff TD, Mahajan A, Krishnan S, et al. Carbon ion therapy: A modern review of an emerging technology. Front Oncol. 2020;10:82. doi: 10.3389/fonc.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen AD, Nikoghosyan AV, Poulakis M, et al. Combined intensity-modulated radiotherapy plus raster-scanned carbon ion boost for advanced adenoid cystic carcinoma of the head and neck results in superior locoregional control and overall survival. Cancer. 2015;121(17):3001–3009. doi: 10.1002/cncr.29443. [DOI] [PubMed] [Google Scholar]
- 7.Durante M, Debus J, Loeffler JS. Physics and biomedical challenges of cancer therapy with accelerated heavy ions. Nat Rev Phys. 2021;3(12):777–790. doi: 10.1038/s42254-021-00368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, Ma T, Den RB, Dicker AP, Feng FY, Knudsen KE. (2013) A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254–71. doi: 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3(11):1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedayati M, Haffner MC, Coulter JB, et al. Androgen deprivation followed by acute androgen stimulation selectively sensitizes AR-positive prostate cancer cells to ionizing radiation. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22(13):3310–3319. doi: 10.1158/1078-0432.CCR-15-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abeshouse A, Ahn J, Akbani R, Ally A, Amin S, Andry CD, Annala M, Aprikian A, Armenia J, Arora A, Auman JT. The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–25. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow LJ, Shen MM. SnapShot: prostate cancer. Cancer Cell. 2013;24(3):400.e1. doi: 10.1016/j.ccr.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Robinson D, van Allen EM, Wu Y-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi J, Afridi A, Vatsia S, et al. The molecular biology of prostate cancer: current understanding and clinical implications. Prostate Cancer Prostatic Dis. 2018;21(1):22–36. doi: 10.1038/s41391-017-0023-8. [DOI] [PubMed] [Google Scholar]
- 15.Bennett NC, Gardiner RA, Hooper JD, et al. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42(6):813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer. 2010;17(4):R305–R315. doi: 10.1677/ERC-10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer. 2020;20(8):471–480. doi: 10.1038/s41568-020-0262-1. [DOI] [PubMed] [Google Scholar]
- 18.Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26(9):1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 19.Fischer M. Census and evaluation of p53 target genes. Oncogene. 2017;36(28):3943–3956. doi: 10.1038/onc.2016.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stopsack KH, Nandakumar S, Wibmer AG, et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(13):3230–3238. doi: 10.1158/1078-0432.CCR-20-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghandi M, Huang FW, Jané-Valbuena J, et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature. 2019;569(7757):503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziemann F, Seltzsam S, Dreffke K, et al. Roscovitine strongly enhances the effect of olaparib on radiosensitivity for HPV neg. but not for HPV pos. HNSCC cell lines. Oncotarget. 2017;8(62):105170–105183. doi: 10.18632/oncotarget.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerch S, Berthold S, Ziemann F, et al. HPV-positive HNSCC cell lines show strongly enhanced radiosensitivity after photon but not after carbon ion irradiation. Radiother Oncol J Eur Soc Therapeut Radiol Oncol. 2020;151:134–140. doi: 10.1016/j.radonc.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 24.Ding D, Zhang Y, Wang J, et al. γ-H2AX/53BP1/pKAP-1 foci and their linear tracks induced by in vitro exposure to radon and its progeny in human peripheral blood lymphocytes. Sci Rep. 2016;6:38295. doi: 10.1038/srep38295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinform (Oxf, Engl) 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shioyama Y, Tsuji H, Suefuji H, et al. Particle radiotherapy for prostate cancer. Int J Urol Off J Jpn Urol Assoc. 2015;22(1):33–39. doi: 10.1111/iju.12640. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Capetillo O, Chen H-T, Celeste A, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4(12):993–997. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 29.van Oorschot B, Hovingh SE, Rodermond H, et al. Decay of γ-H2AX foci correlates with potentially lethal damage repair in prostate cancer cells. Oncol Rep. 2013;29(6):2175–2180. doi: 10.3892/or.2013.2364. [DOI] [PubMed] [Google Scholar]
- 30.Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 31.Böhnke A, Westphal F, Schmidt A, et al. Role of p53 mutations, protein function and DNA damage for the radiosensitivity of human tumour cells. Int J Radiat Biol. 2004;80(1):53–63. doi: 10.1080/09553000310001642902. [DOI] [PubMed] [Google Scholar]
- 32.El-Awady RA, Dikomey E, Dahm-Daphi J. Radiosensitivity of human tumour cells is correlated with the induction but not with the repair of DNA double-strand breaks. Br J Cancer. 2003;89(3):593–601. doi: 10.1038/sj.bjc.6601133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Si J, Gan L, et al. Harnessing the targeting potential of differential radiobiological effects of photon versus particle radiation for cancer treatment. J Cell Physiol. 2021;236(3):1695–1711. doi: 10.1002/jcp.29960. [DOI] [PubMed] [Google Scholar]
- 34.Zafar F, Seidler SB, Kronenberg A, et al. Homologous recombination contributes to the repair of DNA double-strand breaks induced by high-energy iron ions. Radiat Res. 2010;173(1):27–39. doi: 10.1667/RR1910.1. [DOI] [PubMed] [Google Scholar]
- 35.Taleei R. Modelling DSB repair kinetics for DNA damage induced by proton and carbon ions. Radiat Prot Dosimetry. 2019;183(1–2):75–78. doi: 10.1093/rpd/ncy304. [DOI] [PubMed] [Google Scholar]
- 36.Simone CB, John-Aryankalayil M, Palayoor ST, et al. mRNA expression profiles for prostate cancer following fractionated irradiation are influenced by p53 status. Translat Oncol. 2013;6(5):573–585. doi: 10.1593/tlo.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabirzhanov B, Makarevich O, Barrett JP, et al. Irradiation-induced upregulation of miR-711 inhibits DNA repair and promotes neurodegeneration pathways. Int J Mol Sci. 2020 doi: 10.3390/ijms21155239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vander Griend DJ, Litvinov IV, Isaacs JT. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell cycle (Georget Tex) 2007;6(6):647–651. doi: 10.4161/cc.6.6.4028. [DOI] [PubMed] [Google Scholar]
- 39.Litvinov IV, Vander Griend DJ, Antony L, et al. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci USA. 2006;103(41):15085–15090. doi: 10.1073/pnas.0603057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isaacs JT, D’Antonio JM, Chen S, et al. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012;72(14):1491–1505. doi: 10.1002/pros.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci Transl Med. 2015;7(269):269ra2. doi: 10.1126/scitranslmed.3010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Q, Xue C, Zeng Y, et al. Notch signaling pathway in cancer: from mechanistic insights to targeted therapies. Signal Transduct Target Ther. 2024;9:128. doi: 10.1038/s41392-024-01828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer. 2022;22:280–297. doi: 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- 45.Ashton TM, McKenna WG, Kunz-Schughart LA, et al. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24:2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available within the paper. The raw data of RNA-seq has been submitted to EMBL Biostudies and can be accessed via the accession number E-MTAB-14099.