Abstract
The infection of CD4-negative cells by variants of tissue culture-adapted human immunodeficiency virus type 1 (HIV-1) or HIV-2 strains has been shown to be mediated by the CXCR4 coreceptor. Here we show that two in vitro-established CD4−/CCR5−/CXCR4+ human pre-T-cell lines (A3 and A5) can be productively infected by wild-type laboratory-adapted T-cell-tropic HIV-1 and HIV-2 strains in a CD4-independent, CXCR4-dependent fashion. Despite the absence of CCR5 expression, A3 and A5 cells were susceptible to infection by the simian immunodeficiency viruses SIVmac239 and SIVmac316. Thus, at least in A3 and A5 cells, one or more of the chemokine receptors can efficiently support the entry of HIV and SIV isolates in the absence of CD4. These findings suggest that to infect cells of different compartments, HIV and SIV could have evolved in vivo to bypass CD4 and to interact directly with an alternative receptor.
The entry of human immunodeficiency virus type 1 (HIV-1) into target cells is initiated by the binding of the viral envelope (Env) protein gp120 to the CD4 receptor present on the cell surface and, subsequently, to a coreceptor belonging to the chemokine receptor family (1, 4, 7, 10, 37). HIV tropism, in fact, is strongly related to coreceptor usage. Studies with recombinant HIV-1 Env have indicated that differences in the sequence of the V3 loop of gp120 are sufficient to determine whether Env binds to CCR5 or CXCR4 (4, 7).
To enter target cells, simian immunodeficiency virus (SIV) isolates can use CCR5 but not CXCR4, CCR2b, CCR3, CCR1, or CCR4. Several orphan receptors, including gpr1, gpr15, and strl33, can also be exploited as entry cofactors by SIV, and specific changes in the V3 loop can influence the specific chemokine receptor used by different SIV variants (6, 10, 11, 14, 28, 29).
It is well established that CD4 is the main receptor for both HIV and SIV, and it has been proposed that a CD4-induced change in gp120 conformation is necessary for correct binding to the chemokine receptors. In fact, in the absence of CD4, the V3 loop is not correctly exposed to allow the direct binding of gp120 to chemokine receptors (2, 34, 37).
Recent studies have shown that some variants of HIV-1 and HIV-2 cultured in the laboratory can infect both lymphoid and nonlymphoid cells in the absence of CD4, indicating that these viruses can utilize coreceptors without a prior CD4 interaction (2, 9, 15, 20). In particular, HIV-1 and HIV-2 are able to infect CD4-negative cells, utilizing CXCR4 as a primary receptor (9, 12, 24, 26). Moreover, it has been demonstrated that while different SIV strains can infect CD4-negative cells by using CCR5, gpr15 supports the CD4-independent infection by viruses with SIVmac316 and SIVmac316BSS envelope glycoproteins but does not support a CD4-independent infection by viruses with the SIVmac239 envelope glycoprotein (28). By contrast, the utilization of gpr1, strl33, and CCR8 is strictly CD4 dependent (10, 18, 29).
In this study we examined the ability of two highly undifferentiated, in vitro-established CD4−/CCR5−/CXCR4+ human T-cell lines (A3 and A5) to support infection by wild-type laboratory-adapted T-cell-tropic HIV-1, HIV-2/ROD10, and SIV.
The A3 and A5 cell lines can be infected by HIV-1, HIV-2, or SIV in the absence of CD4.
A3 and A5 are highly undifferentiated T-cell lines derived from in vitro cloning of peripheral blood mononuclear cells of an asymptomatic HIV-1-seropositive subject (32). No HIV-1 sequence was ever detected in A3 and A5 cells by nested PCR of cell lysates using specific primers for the gag, pol, env, vpu, and nef regions (5, 32), by coculture with susceptible cells, or by determination of p24 production (data not shown). PCR analysis showed the absence of coinfecting viruses that could have been present in the HIV-1-infected patient, including human T-cell lymphotropic virus type 1 (HTLV-1) (36); cytomegalovirus; adenovirus; Epstein-Barr; herpesvirus types 1, 2, 7, 8, and 9 (25); JC virus; and BK virus (22) (data not shown).
Fluorescence-activated cell sorter analysis of A3 and A5 cells using a CD4 panel of monoclonal antibodies that recognize different epitopes of the CD4 molecule (Q4120, D4056, L120, Q4116, RFT4, SK3, MT310, RPAT4, and OKT4a) showed no CD4 cell surface expression. Furthermore, intracellular staining with different CD4 monoclonal antibodies on permeabilized cells and radioimmunoprecipitation assays failed to show the presence of intracellular CD4 protein, despite the detection of CD4 mRNA by reverse transcriptase (RT) PCR in both the A3 and A5 cell lines (data not shown).
To assess whether these CD4− T-cell lines were susceptible to HIV-1 or SIV infection, A3 and A5 cells were infected with HIV-1/HXBc2, HIV-2/ROD10, SIVmac239, or SIVmac316 at a multiplicity of infection of 0.01 50% tissue culture infective dose/cell for 2 h at 37°C. Cells were then washed and cultured for an additional 2 weeks. Infection was detected by measuring the levels of viral p24 in the culture supernatants at different time points postinfection (p.i.). As shown in Fig. 1A, a productive replication of HIV-1, HIV-2, and SIV strains occurred rapidly and in the absence of syncytium formation. In addition, cell-free supernatants from these cultures infected Jurkat, C8166, SupT1, and CEMx174 cells, as shown by p24 detection in the cell supernatants and syncytium formation 24 h p.i. (data not shown).
FIG. 1.
Infection of the CD4-negative A3 and A5 cell lines by HIV-1, HIV-2, and SIVmac239. (A) Replication kinetics of HIV-1, HIV-2, and SIVmac239. (B) A3, A5, SupT1, and HSB-2 cells were preincubated in culture medium with 50 μg of either the anti-human CD4 monoclonal antibody Q4120 or the isotype control per ml at the indicated concentrations prior to the addition of cell-free HIV-1/HXBc2, HIV-2/ROD10, or SIVmac239 (multiplicity of infection of 0.01 50% tissue culture infective dose/cell) and maintained in culture with the monoclonal antibody for 4 days. On day 4, p24 core protein levels in the culture supernatants were determined. CD4-positive SupT1 and CD4-negative HSB-2 cells were included as controls. The experiment shown is representative of three independent experiments. (C) Infection of A3 and A5 cells by HIV-1 is independent of the CD4 molecule, as shown by the entry of wild-type and 368D/R gp120 mutant HXBc2 virus in both cell lines. Comparable amounts of cell lysates, after normalization to total protein content with the Micro BCA protein assay (Pierce), were used for the assays. CAT activity was calculated as the ratio of the amount of acetylated forms of chloramphenicol (upper spots) to that of the unacetylated form (bottom spots). SupT1 and HSB-2 cells were included as controls. The experiment shown is representative of three independent experiments.
Previous evidence suggested that laboratory-adapted T-cell-tropic viruses interact with the cell surface CD4 or soluble CD4, resulting in the enhanced infectivity of cultured cells even in the presence of a small amount of this receptor (16). To exclude the possibility of viral entry by the CD4 molecule, A3, A5, and SupT1 cells were incubated with 50 μg of an anti-human CD4 monoclonal antibody (Q4120) per ml or the same concentration of an isotype-matched control antibody prior to the addition of the virus to the cells. After 12 h, cells were washed and cultured in the presence of the antibodies. On day 4 p.i. the concentration of p24 in the culture supernatants was determined. As expected, the addition of a saturating concentration of anti-CD4 antibody inhibited the infection of SupT1 cells as measured by the release of p24, whereas it did not affect HIV-1, HIV-2, or SIV infection of A3 and A5 cells. No p24 was detected when HSB-2 cells were infected with different viruses (Fig. 1B). Similar results were obtained when cells were incubated with 100 μg of the anti-CD4 monoclonal antibody per ml.
Entry of HIV-1 into A3 and A5 cells is not affected by changes in the CD4-binding domain of gp120.
It has been previously shown that the mutation at residue 368 in the gp120 glycoprotein dramatically reduces the ability of this protein to form syncytia with target cells or to complement replication of an env-defective virus.
To confirm that the infection of A3 and A5 cells by laboratory-adapted HIV isolates was independent from the CD4 molecule, we used a defective HIV-1 genome whose ability to infect is dependent on the ability of envelope glycoproteins to support in trans virus transmission either in a cell-free fashion or in a combined cell-free and cell-to-cell manner. Since the defective HIV is capable of only one cycle of replication, the env complementation assay allowed us to quantitatively measure the efficiency at which different envelope glycoproteins can mediate early phases of virus infection (4). To produce recombinant HIV-1 virions, 293 cells were cotransfected with the pHXBH10ΔenvCAT plasmid, containing an HIV-1 provirus with a deletion in the env gene and the chloramphenicol acetyltransferase (CAT) gene replacing the nef gene, and the pSVIII plasmid, encoding wild-type or mutant envelope glycoproteins. To assess whether infection of A3 and A5 cells was independent of the CD4 molecule, a mutant with a change at residue 368 of the prototypical HXBc2 gp120 glycoprotein was also used.
It has been shown previously that mutation of D to R at position 368 (368D/R mutation) reduces the CD4-binding ability of HIV-1 glycoproteins as well their ability to complement virus entry (31). Recombinant viruses were harvested 72 h after transfection of 293 cells, normalized to 25,000 cpm of RT activity, and incubated with A3, A5, SupT1, or HSB-2 cells. CAT activity was then measured in the target cells 60 h after infection, providing an assessment of the abilities of the cells to support the entry of HIV-1 variants containing different envelope glycoproteins. Comparable amounts of cell lysates, after normalization to total protein content with the Micro BCA protein assay (Pierce), were used for CAT assays. CAT activity was calculated as the ratio of the amount of acetylated forms of chloramphenicol to that of the unacetylated form. Figure 1C shows that the entry of 368D/R mutant virus into A3 and A5 cells was as efficient as that of the wild-type virus. By contrast, the entry of 368D/R mutant virus into SupT1 cells was less successful than that of the wild-type virus. No entry of wild-type or mutant virus was detected in HSB-2 cells. These results indicated that the efficiency of infection of A3 and A5 cells by HIV-1 is not affected by the absence of the CD4 molecule on the cell membrane.
Coreceptor use by HIV and SIV strains for entry into A3 and A5 cells.
HIV entry is dependent not only on the surface expression of the CD4 molecule but also on the expression of chemokine coreceptors, such as CXCR4 and CCR5. To determine the surface expression levels of these chemokine receptors, the A3 and A5 cell lines were analyzed by flow cytometry using monoclonal antibodies specific for human CXCR4 (12G5), CCR5 (2D7), CXCR1 (5A12), and CXCR2 (6C6). CXCR4 was expressed at high levels in both clones, whereas no expression of CCR5, CXCR1, or CXCR2 was detected (data not shown). In agreement with these results, RT-PCR analysis revealed that A3 and A5 cells expressed high levels of CXCR4 but did not express CCR5. In addition, both cell lines expressed detectable levels of CCR8, gpr1, strl33, gpr15, ebi1, and ebi2 mRNA compared with controls. mRNAs coding for CCR2b, CCR3, apj, rdc, dez, and gpr4 were not expressed (data not shown).
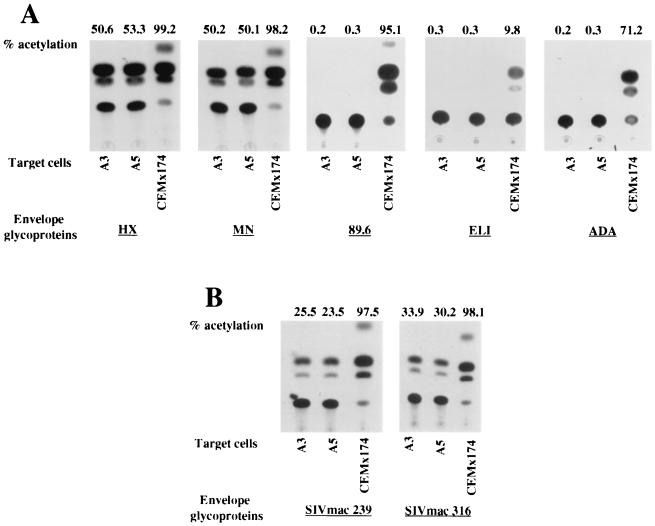
Expression of chemokine receptor mRNAs does not necessarily establish the expression of the proteins on the cell surface, which is essential for viral entry. Therefore, we evaluated the possibility that the previously tested chemokine receptors could still support the entry of HIV-1 and SIV into A3 and A5 cells. As mentioned above, pseudotyped CAT-expressing recombinant HIV-1 allows the quantitation of the efficiency of viral entry into susceptible cells. CAT-expressing recombinant HIV-1 bearing the envelope glycoproteins derived from laboratory-adapted T-cell-tropic (HXBc2 and MN), macrophage-adapted (ADA), T-cell-tropic primary (ELI), or dualtropic (89.6) HIV-1 isolates or from SIVmac239 or SIVmac316 were therefore used to infect A3 and A5 cells. Figure 2A shows that the recombinant viruses containing the HXBc2 and MN glycoproteins were able to infect A3 and A5 cells with comparable efficiencies. In contrast, viruses containing ADA, ELI, and 89.6 envelope glycoproteins were unable to infect A3 and A5 cells.
FIG. 2.
CAT activity in the A3 and A5 cell lines after incubation with recombinant viruses containing the HXBc2, MN, 89.6, ELI, or ADA envelope glycoprotein or with SIVmac239 or SIVmac316 envelope glycoprotein. CEMx174 and SupT1 cells were used as controls.
These results might reflect the chemokine receptor expression pattern observed in A3 and A5 cells. Thus, a CD4-independent infection of A3 and A5 cells by the T-cell-line-adapted virus could occur mainly via CXCR4, whereas it is possible that primary strains need a prior interaction with a specific coreceptor in order to infect target cells.
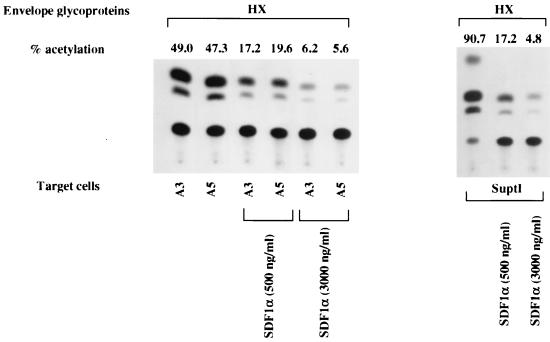
An efficient infection of these cells by recombinant viruses containing SIVmac239 and SIVmac316 envelope glycoproteins was also observed (Fig. 2B). Since A3 and A5 cells do not express CCR5 intracellularly or at the cell surface level, other coreceptors, including strl33, gpr1, gpr15, and CCR8, could be involved in the CD4-independent entry of SIV into these cells. These results indicated that laboratory-adapted T-cell-tropic viruses (HXBc2 and MN) might enter CD4-negative cell lines using CXCR4. In order to test this hypothesis, the inhibition of CXCR4-mediated infection of recombinant HIV-1 by CXCR4 natural ligand SDF-1 was performed (3). A3, A5, and SupT1 cells were incubated with equivalent amounts of RT activity (25,000 cpm) of recombinant HIV-1-CAT pseudotyped with HXBc2 envelope glycoprotein in the presence of different concentrations of SDF-1, as already described (3). As expected, the entry of laboratory-adapted T-cell-tropic viruses into A3, A5, and SupT1 cells was inhibited by SDF-1 in a dose-dependent fashion, as shown by the results of the CAT assays (Fig. 3). Moreover, the replication of HIV-1/HXBc2 and HIV-2/ROD10 could be inhibited by blocking CXCR4 on A3 and A5 cells with saturating levels of SDF-1 (data not shown). This result indicated that the entry of these viruses into A3 and A5 cells could be principally mediated by the CXCR4 molecule. In contrast, a saturating concentration of the 12G5 monoclonal antibody failed to block infection by laboratory-adapted T-cell-tropic HIV-1/HXBc2 or HIV-2/ROD10 as shown by the determination of p24 levels in A3 and A5 cell supernatants (data not shown). According to a previous report (30), this suggests that CXCR4 might be differently exposed on the membranes of different cell lines (i.e., A3 and A5 cells) or that different virus strains could bind different epitopes on the CXCR4 molecule.
FIG. 3.
CAT activity in A3 and A5 cells after a preincubation of the cells with SDF-1 at the indicated concentrations, followed by infection with recombinant viruses. The experiment shown is representative of three independent experiments.
To rule out the possibility that the entry of HIV-1 into A3 and A5 cells was mediated by different forms of CXCR4 exposed on the cellular surface, the nucleotide sequence of CXCR4 expressed by A3 and A5 cells was analyzed by sequencing. No mutations were found. This finding suggested that the cellular phenotype responsible for viral entry in the absence of CD4 was independent of mutations in the CXCR4 sequence (data not shown). These results indicated that laboratory-adapted T-cell viruses use CXCR4 as a receptor to enter A3 and A5 cells independently of CD4.
In this report we demonstrate that two in vitro-established CD4−/CCR5−/CXCR4+ pre-T-cell lines (A3 and A5) can be infected by wild-type laboratory-adapted T-cells-tropic HIV-1, HIV-2/ROD10, and SIV isolates in the absence of CD4.
Previous reports showed that gp120 binds to CXCR4 on the cell surface without a prior CD4 interaction and that some HIV-1 and HIV-2 mutants that are cultured in the laboratory can infect CD4-negative cells (2, 12, 13, 35). Our data show that if one uses a replication-competent HXBc2 virus, A3 and A5 cells are fully permissive for HIV-1 infection as well as HIV-2 infection. In addition, although SIV can infect CD4− cells through CCR5 (29), we found that the A3 and A5 cell lines can be infected by SIVmac239 and by macrophage-tropic SIVmac316 even in the absence of CCR5 expression at the cell surface level.
Inhibition experiments using saturating doses of a specific anti-human CD4 monoclonal antibody (Q4120) demonstrated that the entry of HIV-1/HXBc2, HIV-2/ROD10, SIVmac239, and SIVmac316 into A3 and A5 cells was independent of the CD4 molecule, making it unlikely that low levels of CD4 contributed to the susceptibility of these cell clones to HIV-1, HIV-2, or SIV infection. When A3 and A5 cells were infected with laboratory-adapted T-cell-tropic viruses in the presence of an anti-CXCR4 monoclonal antibody (12G5), no inhibitory effects on the infection were observed. These data are consistent with previous reports that showed cell type and virus strain dependency for this monoclonal antibody (2).
On the other hand, SDF-1, the natural ligand for CXCR4, inhibited infection by the fully competent laboratory-adapted T-cell-tropic HXBc2 virus as well as by HIV-2 or recombinant viruses pseudotyped with the envelope glycoproteins from laboratory-adapted HXBc2 or MN. This occurred in a concentration-dependent manner, showing that CXCR4 functions as the primary receptor and no longer needs the CD4 molecule.
These results strongly suggest that CXCR4 could probably interact directly with the viral envelope glycoprotein, and unlike CD4, it could be necessary and sufficient for viral entry. Therefore, at least in this case, viral entry does not require binding of the envelope glycoprotein directly to the primary receptor CD4 in order to promote a conformational change for exposure of a binding site to chemokine receptors. Thus, in certain cases the chemokine receptors could represent the primary receptor, and the use of CD4 as a receptor might have evolved subsequently.
Since CXCR4 expressed by A3 and A5 cells does not show any difference in nucleotide sequence or amino acids compared to those of wild-type human CXCR4, there exists the possibility that CXCR4 may be modified in a cell-type-specific manner, resulting in the susceptibility of these cells to HIV-1 infection. Alternatively, CD4-independent envelope glycoproteins could interact with an unknown cell surface component(s) that may be required for CXCR4-mediated viral entry. For example, the importance of heparan sulfate proteoglycans on the cell surface in the attachment of HIV-1 to target cells has been shown (34). In addition, although A3 and A5 cells do not express the CCR5 and CD4 molecules on the cell surface, they are permissive to a recombinant virus pseudotyped with the envelope glycoproteins of SIVmac239 and SIVmac316. This suggests that SIV may use gpr1, gpr15, strl33, or other chemokine receptors in the absence of CD4 or that other still-unknown molecules may be required as cofactors and tropism determinants for some HIV and SIV isolates.
Conversely, no infection of A3 and A5 cells with macrophage-tropic (ADA) as well as ELI and dualtropic (89.6) pseudotyped viruses was detected. We can hypothesize that, as opposed to SIV strains, the ADA strain is unable to use gpr15 or alternative coreceptors on A3 and A5 cells in the absence of CD4 or that SIV may use an unknown molecule(s) as a coreceptor.
CXCR4 in association with CD4 is an efficient receptor for HIV-1 isolates 89.6 and ELI (4). Since A3 and A5 cells are resistant to infection by 89.6 and ELI, it is possible that different viruses interact with different regions of CXCR4 or that primary strains need a conformational change induced in their envelope glycoproteins by CD4 to expose a binding domain for the coreceptors (27). The precise molecular ratio between CD4 and chemokine receptors that is necessary for a productive infection at the single-cell level is still unknown. It has been shown that cells expressing a large amount of CD4 on the membrane require only a trace amount of CCR5 for maximal infection by macrophage-tropic strains. In contrast, cells with low surface expression of CD4 are more dependent on coreceptor expression levels (16, 21, 23).
It has been recently demonstrated that HIV Env is able to interact in a CD4-independent manner with CXCR4 (2, 13, 37), although Mondor et al. (21) reported that the determinant in the interaction between the envelope glycoproteins of different HIV-1 viruses and CXCR4 is the level of CD4 expression. Results obtained from these studies using soluble forms of the envelope glycoprotein are not fully representative of an in vivo infection that utilizes infectious virus. A3 and A5 are the first CD4-negative T-cell lines permissive of infection by wild-type HIV-1 laboratory-adapted and SIV isolates.
Since the A3 and A5 cell lines appear to resemble immature T cells blocked at the earliest intrathymic T-cell differentiation stage, they may represent a useful model to study putative cellular receptors that might be present during the early phase of differentiation of T cells. Furthermore, studies of A3 and A5 cells may also provide new insights into the usage of the CXCR4 molecule by different HIV strains. It remains to be shown whether the CD4-independent, CXCR4-dependent phenotype exhibited by laboratory-adapted viruses and the CD4-independent phenotype exhibited by SIV isolates have implications for pathogenesis in vivo.
Acknowledgments
We thank J. Sodroski, R. Desrosiers, H. Choe, M. Farzan, P. Lusso, and the Medical Research Council (MRC) for reagents, A. Amadori and D. M. R. Negri for critical reading of the manuscript, M. Tripaldi for technical assistance, and A. Lippa and F. M. Regini for editorial assistance.
This work was supported by grants from the AIDS Project of the Ministry of Health, Rome, Italy.
REFERENCES
- 1.Alkhatib G, Combardiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1957. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Bandres J C, Wang Q F, O'Leary J, Baleaux F, Amara A, Hoxie J A, Zolla-Pazner S, Gorny M K. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. doi: 10.1128/jvi.72.3.2500-2504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleul C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for lester/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 4.Choe H, Farzan M, Sun Y, Sullivan N, Barret R, Ponath P D, Wu L, Mackay C R, La Rosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 5.Delassus S, Cheynier R, Wain-Hobson S. Evolution of human immunodeficiency virus type 1 nef and long terminal repeat sequences over 4 years in vivo and in vitro. J Virol. 1991;65:225–231. doi: 10.1128/jvi.65.1.225-231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng H, Unutmaz D, Kewalramani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;338:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 7.Deng H, Liu R, Ellemeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 8.Dragic T, Litwin V, Alloway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR5. Nature. 1996;381:666–671. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 9.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleaux F, Briand P, Hazan U. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edinger A L, Hoffman T L, Sharron M, Lee B, O'Dowd B, Doms R W. Use of gpr1, gpr15, and strl33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology. 1998;249:367–373. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- 11.Edinger A L, Hoffman T L, Sharron M, Lee B, Yi Y, Choe W, Kolson D L, Mitrovic B, Zhou Y, Faulds D, Collman R G, Hesselgesser J, Horuk R, Doms R W. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–7940. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 13.Hesselgesser J, Halks-Miller M, Del Vecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff F, Pöhlmann S, Hamacher M, Means R E, Kraus T, Überla K, Di Marzio P. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolchinsky P, Mirzabekov T, Farzan M, Kiprilov E, Cayabyab M, Mooney L J, Choe H, Sodroski J. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J Virol. 1999;73:8120–8126. doi: 10.1128/jvi.73.10.8120-8126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littman D R. Chemokine receptors: keys to AIDS pathogenesis? Cell. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 18.Martin K A, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard N P. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 19.McKnight Á, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Missè D, Cerutti M, Schmidt I, Jansen A, Devauchelle G, Jansen F, Veas F. Dissociation of the CD4 and CXCR4 binding properties of human immunodeficiency virus type 1 gp120 by deletion of the first putative alpha-helical conserved structure. J Virol. 1998;72:7280–7288. doi: 10.1128/jvi.72.9.7280-7288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondor L, Moulard M, Ugolini S, Klasse P J, Hoxie J, Amara A, Delaunay T, Wyatt R, Sodroski J, Sattentau Q. Interaction among HIV gp120, CD4, and CXCR4: dependence on CD4 expression level, gp120 viral origin, conservation of the gp120 COOH and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. Virology. 1998;248:394–405. doi: 10.1006/viro.1998.9282. [DOI] [PubMed] [Google Scholar]
- 22.Pietropaolo V, Di Taranto C, Degener A M, Jin L, Sinibaldi L, Baiocchini A, Melis M, Orsi N. Transplacental transmission of human polyomavirus BK. J Med Virol. 1998;56:372–376. doi: 10.1002/(sici)1096-9071(199812)56:4<372::aid-jmv14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potempa S, Picard L, Reeves J D, Wilkinson D, Weiss R A, Talbot S J. CD4-independent infection by human immunodeficiency virus type 2 strain ROD/B: the role of the N-terminal domain of CXCR-4 in fusion and entry. J Virol. 1997;71:4419–4424. doi: 10.1128/jvi.71.6.4419-4424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozo F, Tenorio A. Detection and typing of lymphotropic herpesviruses by multiplex polymerase chain reaction. J Virol Methods. 1999;79:9–19. doi: 10.1016/s0166-0934(98)00164-5. [DOI] [PubMed] [Google Scholar]
- 26.Reeves J D, Schulz T F. The CD4-independent tropism of human immunodeficiency virus type 2 involves several regions of the envelope protein and correlates with a reduced activation threshold for envelope-mediated fusion. J Virol. 1997;71:1453–1465. doi: 10.1128/jvi.71.2.1453-1465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves J D, Hibbitts S, Simmons G, McKnight Á, Azevedo-Pereira J M, Moniz-Pereira J, Clapham P R. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J Virol. 1999;73:7795–7804. doi: 10.1128/jvi.73.9.7795-7804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenten D, Marcon L, Karlsson G B, Parolin C, Kodama T, Gerard N, Sodroski J. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J Virol. 1999;73:5373–5380. doi: 10.1128/jvi.73.7.5373-5380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strizki J M, Turner J D, Collman R G, Hoxie J, González-Scarano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with the dual-tropic human immunodeficiency virus type 1 isolate HIV-189.6 but not the T-tropic isolate HIV-1HxB. J Virol. 1997;71:5678–5683. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thali M, Olshevsky U, Furman C, Gabuzda D, Li J, Sodroski J. Effects of changes in gp120-CD4 binding affinity on human immunodeficiency virus type 1 envelope glycoprotein function and soluble CD4 sensitivity. J Virol. 1991;65:5007–5012. doi: 10.1128/jvi.65.9.5007-5012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Titti F, Borsetti A, Federico M, Testa U, Meccia U, Samoggia E, Peschle P, Verani P, Rossi G B. Extrachromosomal human immunodeficiency virus type 1 DNA forms in fresh peripheral blood lymphocytes and in two interleukin-2-independent T cell lines derived from peripheral blood lymphocytes of a seropositive subject. J Gen Virol. 1993;73:3087–3097. doi: 10.1099/0022-1317-73-12-3087. [DOI] [PubMed] [Google Scholar]
- 33.Ugolini S, Mondor I, Sattentau Q J. HIV-1 attachment: another look. Trends Microbiol. 1999;7:144–149. doi: 10.1016/s0966-842x(99)01474-2. [DOI] [PubMed] [Google Scholar]
- 34.Ugolini S, Moulard M, Mondor I, Barois N, Demandolx D, Hoxie J, Brelot A, Alizon M, Davoust J, Sattentau Q. HIV-1 gp120 induces an association between CD4 and the chemokine receptor CXCR4. J Immunol. 1997;159:3000–3008. [PubMed] [Google Scholar]
- 35.Valenzuela A, Blanco J, Krust B, Franco R, Hovanessian A G. Neutralizing antibodies against the V3 loop of human immunodeficiency virus type 1 gp120 block the CD4-dependent and -independent binding of virus to cells. J Virol. 1997;71:8289–8298. doi: 10.1128/jvi.71.11.8289-8298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandamme A M, Van Laethem K, Liu H F, Van Brussel M, Delaporte E, de Castro Costa C M, Fleisher C, Taylor G, Bertazzoni U, Desmyter J, Goubau P. Use of a generic polymerase chain reaction assay detecting human T lymphotropic virus (HTLV) types I, II and divergent simian strains in the evaluation of individuals with indeterminate HTLV serology. J Med Virol. 1997;52:1–7. [PubMed] [Google Scholar]
- 37.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]