Abstract
We demonstrate that human immunodeficiency virus type 1 (HIV-1)-specific CD8+ cytotoxic T lymphocytes (CTL) suppress HIV-1 replication in primary lymphocytes, monocytes, and dendritic cells individually. Viral inhibition is significantly diminished in lymphocyte-dendritic cell clusters, suggesting that these clusters in vivo could be sites where viral replication is more difficult to control by CTL.
Major histocompatibility complex (MHC) class I-restricted CD8+ cytotoxic T lymphocytes (CTL) provide an important protective immune response in many viral infections and are likely to play a crucial role in the control of human immunodeficiency virus (HIV-1) infection (reviewed in reference 40). Virus-specific CTL have been identified in HIV-1-exposed but uninfected individuals (23, 27, 30, 31) and therefore have been proposed to contribute to protective immunity from infection. Antiviral CTL activity in chronically infected persons has been correlated with clearance of viremia in acute infection (4, 21), delayed disease progression (17, 18, 29), and control of plasma viremia (24).
Our laboratory previously has shown that HIV-1-specific CTL clones can mediate potent antiviral effects in vitro, suppressing viral replication through direct cytolytic as well as soluble-factor-mediated mechanisms (35, 39). This activity is dependent on specific triggering of the T-cell receptor and is therefore MHC and antigen restricted. Although studies have documented that CTL suppress HIV-1 replication in immortalized (39) and primary (5, 39) CD4+ T lymphocytes, the antiviral activity of CTL in other cell types previously has not been examined systematically.
Besides lymphocytes, other CD4-expressing cells are thought to be infected in vivo. Monocytes and immature dendritic cells (14) support the replication of monocyte-tropic (M-tropic, R5) strains of HIV-1 in vitro. Monocytes/macrophages (M/M) have been proposed to be a reservoir of virus in vivo that may decay with slower kinetics than lymphocytes after the initiation of combination antiretroviral therapy (26). Dendritic cells (DC) are professional antigen-presenting cells that are also believed to play a crucial role in the pathogenesis of HIV-1 infection (reviewed in references 6, 16, and 19). In primary HIV-1 infection, immature DC in peripheral areas (e.g., mucosal surfaces) are hypothesized to transfer HIV-1 to CD4+ T cells in regional lymph nodes during the normal process of migration and antigen presentation (34). DC–T-cell clusters are known to be a site of vigorous viral replication (7, 28). These cells therefore may be responsible for the initial dissemination of virus to CD4+ T cells and other cellular reservoirs.
The capability of CTL to suppress viral replication in DC and monocytes would be an important prerequisite for CTL to prevent and control infection in vivo. In this study, we test the ability of MHC class I-restricted HIV-1-specific CTL clones to inhibit HIV-1 replication in primary CD4+ T lymphocytes, monocytes, monocyte-derived DC, and lymphocyte-DC clusters.
HIV-1 replication is suppressed in primary CD4+ lymphocytes by HIV-1-specific CTL clones.
Two HIV-1-seronegative individuals served as donors for the peripheral blood mononuclear cells (PBMC) used to generate the target cells used in these studies. The MHC haplotypes of these donors were determined by standard serologic methods at the tissue-typing laboratory of Massachusetts General Hospital, Boston, Mass. Donor A3 expressed MHC A3, A24, B48, and B51; donor B14 expressed MHC A23 (A9), B65 (a split of B14), and B44. Primary CD4+ lymphocytes (greater than 95% CD3- and CD4-expressing by flow cytometric analysis [data not shown]) were generated from freshly Ficoll gradient-purified PBMC and maintained as previously described (39) by using a CD3:8-bispecific monoclonal antibody (38). These cells were maintained in RPMI 1640 (Sigma) containing 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES, 100 U of penicillin per ml, and 10 μg of streptomycin per ml (R10) supplemented with 50 U of recombinant human interleukin-2 per ml (R10-50) and infected, 7 days after isolation or stimulation, with the monocyte-tropic HIV-1 strain JR-CSF (22) at a multiplicity of infection of 0.01 50% tissue culture infective dose per cell for 4 h at 37°C, followed by two washes. The acutely infected lymphocytes were cocultured with the HIV-1-specific CTL clones at a 1:1 ratio (5 × 105 each cell type) in a 24-well plate containing 2 ml of R10-50 per well. The CTL clones had been previously obtained from PBMC of HIV-1-infected individuals and characterized as described previously (37). The MHC A3-restricted CTL clone 11504/A7 (termed A3/Gag) was specific for an HIV-1 Gag p17 epitope (amino acids KIRLRPGGK). The MHC B14-restricted CTL clone 15160/D75 (termed B14/ENV) was specific for an HIV-1 gp41 epitope (ERYLK DQQL). The viral epitopes recognized by these clones were conserved in HIV-1 JR-CSF (sequence available through the Los Alamos National Laboratory HIV Database). At 2- to 4-day intervals, 1 ml of coculture supernatant was removed for quantitative HIV-1 p24 antigen capture enzyme-linked immunosorbent assay (NEN Life Sciences Products, Boston, Mass.) and replenished with R10-50.
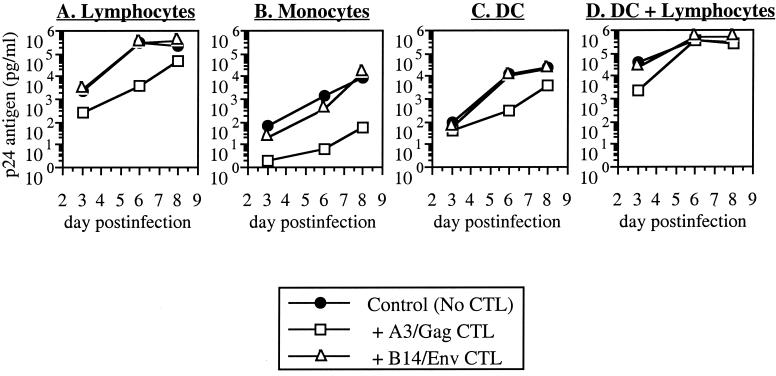
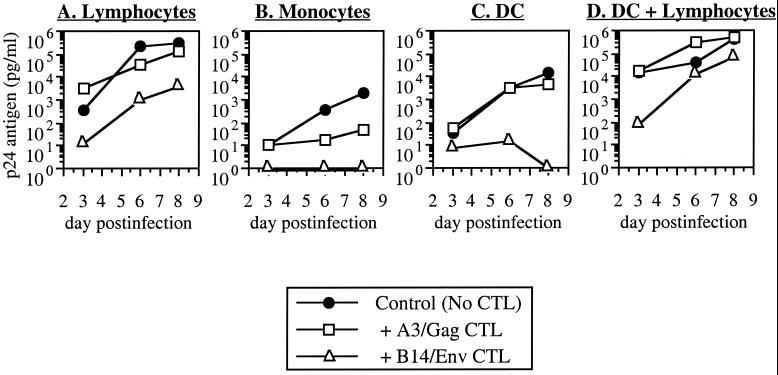
For lymphocytes from donor A3, the A3-restricted clone was inhibitory whereas the B14-restricted clone was not (Fig. 1A). Conversely, HIV-1 replication in lymphocytes from donor B14 was suppressed by the B14-restricted clone but not by the A3-restricted clone (Fig. 2A). In some experiments, nonspecific inhibition was observed with the MHC-mismatched CTL clone, but the matched CTL was always reproducibly more inhibitory (data not shown). Thus, in agreement with previous studies (5, 39), viral replication in lymphocytes was inhibited by CTL in an MHC-restricted fashion.
FIG. 1.
Inhibition of HIV-1 replication in lymphocytes, monocytes, DC, and DC-lymphocyte clusters from donor A3. Acutely infected primary CD4+ lymphocytes, monocytes, and DC from seronegative donor A3 (MHC A3+/B14−) were cocultured with HIV-1-specific CTL clones restricted by MHC A3 (A3/Gag) or B14 (B14/Env). These data are representative of two experiments.
FIG. 2.
Inhibition of HIV-1 replication in lymphocytes, monocytes, DC, and DC-lymphocyte clusters from donor B14. Acutely infected primary CD4+ lymphocytes, monocytes, and DC from seronegative donor B14 (MHC B14+/A3−) were cocultured with CTL clones as in Fig. 1. These data are representative of three experiments.
CTL inhibit HIV-1 replication in primary monocytes and DC.
To examine the ability of HIV-1-specific CTL clones to suppress viral replication in other HIV-1-permissive cell types, primary monocytes and monocyte-derived DC were generated from the same donors. Primary monocyte cultures (>95% by nonspecific esterase staining [data not shown]) were established by adherence for 2 h at 37°C of 5 × 106 freshly isolated PBMC per well in a polystyrene 24-well plate (Costar) containing macrophage serum-free medium (Life Technologies, Grand Island, N.Y.), followed by three washes to remove nonadherent cells and culture for 4 days in serum-free medium containing 1,000 U of recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF, Genzyme, Cambridge, Mass.) per ml. Monocyte-derived DC (>80% CD1a+ DR+ CD3− CD14− [data not shown]) were generated from freshly adherence-selected monocytes by culture for 7 days in R10 supplemented with recombinant human interleukin-4 (Genzyme) and rhGM-CSF at 1,000 U/ml each (3). After isolation and culture, the monocytes (assuming a yield of approximately 5 × 105 cells per well) and DC were incubated with JR-CSF at a multiplicity of infection of 0.1 and used for coculture assays with CTL as above.
Replication of HIV-1 in these cell types was less efficient than in lymphocytes despite a 10-fold higher initial input of virus (Fig. 1B and C and 2B and C). As with the lymphocytes, the MHC-matched CTL suppressed viral replication in either monocytes (Fig. 1B and 2B) or DC (Fig. 1C and 2C) whereas the mismatched CTL generally were not inhibitory. In this experiment, some inhibition in B14 monocytes was noted with the mismatched A3-restricted CTL clone, although the B14-restricted clone was completely inhibitory for detectable p24 antigen (Fig. 2B). Nonspecific inhibitory activity (of viral replication in MHC-mismatched target cells) was noted in some assays but was always weaker than inhibition by MHC-matched CTL clones (data not shown). Suppression of viral replication by CTL in these monocyte and DC cultures was therefore MHC restricted and comparable to that seen in lymphocytes.
HIV-1 replication in DC-lymphocyte clusters is less susceptible to inhibition by CTL.
DC-lymphocyte clusters have been modeled using an in vitro assay system to study the inhibition of HIV-1 by polyclonal CD8+ lymphocytes (1), and we therefore examined the ability of HIV-1-specific CTL clones to suppress viral replication in this system. DC were generated and infected as above, added to autologous uninfected CD4+ lymphocytes at a ratio of 1 × 105 DC to 5 × 105 lymphocytes per well, and cocultured with 5 × 105 CTL clones (Fig. 1D and 2D).
Infection under these conditions was marked by extremely vigorous viral replication, which exceeded that of monocytes and DC alone (Fig. 1B and C and 2B and C) and increased more rapidly than that of infected lymphocytes alone (Fig. 1A and 2A). MHC-matched CTL clones were mildly suppressive for HIV-1 replication by DC-lymphocyte clusters at the earliest time point, but inhibition was blunted or lost at later time points (Fig. 1D and 2D and data not shown). Mismatched CTL clones mediated no appreciable inhibition of replication. Statistical comparison (analysis of variance and Fisher's protected least significant difference analysis [StatView Reference; Abacus Concepts, Inc., Berkeley, Calif.]) of MHC-matched inhibition by CTL in multiple experiments revealed that inhibition in clusters was significantly lower than in lymphocytes, monocytes, or DC alone (Table 1) whereas the results for single-cell groups were not statistically different from each other.
TABLE 1.
Comparison of HIV-1 inhibition in different cell types by MHC-matched CTLa
| Sample | % Suppression of viral replication inb:
|
|||
|---|---|---|---|---|
| Lymphocytes | Monocytes | DC | Clusters | |
| 1 | 58.8 | 98.7 | 83.7 | 39.4 |
| 2 | 64.6 | 100.0 | 96.2 | −48.0 |
| 3 | 100.0 | 98.2 | 88.5 | 98.8 |
| 4 | 98.7 | 99.6 | 97.2 | 13.8 |
| 5 | 99.6 | 99.7 | 99.5 | 69.7 |
| Mean | 84.32 | 98.24 | 93.02 | 34.74 |
Inhibition of replication in different cell types was performed as described in the text, using cells from donor A3 (two experiments) and donor B14 (three experiments). Data from Fig. 1 and 2 are included as samples 4 and 5, respectively.
Suppression of viral replication compared to controls (no CTL) was calculated at the day 5 or 6 time point, yielding mean inhibition by CTL in each cell type. P = 0.019, 0.004, and 0.008 for comparisons of lymphocytes, monocytes, and DC, respectively, with DC-lymphocyte clusters. Differences between the individual cell types were not statistically significant (P = 0.445 to 0.749).
Suppression of HIV-1 replication in DC-lymphocyte clusters is dependent on the ratio of added DC.
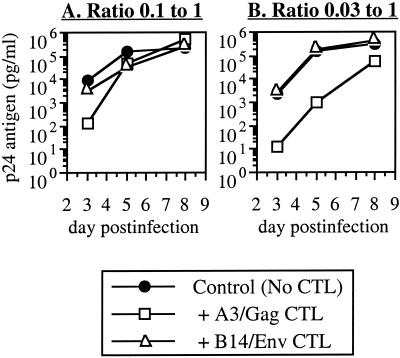
Because this DC-to-T-cell ratio could be supraphysiologic, we also examined whether CTL might be more effective at lower ratios of added DC (Fig. 3). Infected DC were added to autologous uninfected lymphocytes at ratios of 0.1:1 or 0.03:1, followed by coculture with the CTL clones (keeping the number of lymphocytes and CTL constant at 5 × 105 per well). Again, at the 0.1:1 ratio, replication was suppressed by the MHC-matched CTL clone only at the earliest time point and this was followed by loss of inhibition (Fig. 3A). In contrast, at the 0.03:1 ratio, the matched CTL clone was clearly more suppressive and inhibition was sustained at later time points as well (Fig. 3B). Thus, inhibition of viral replication in lymphocytes was less efficient in the presence of DC in a dose-dependent manner.
FIG. 3.
Effect of DC-to-lymphocyte ratio on inhibition by CTL. Acutely infected DC from donor A3 were added to autologous uninfected CD4+ lymphocytes at the indicated ratios, followed by coculture with A3/Gag CTL.
Most of the work investigating direct antiviral activity of HIV-1-specific CTL has been limited to the use of lymphocytes as target cells for investigating the antiviral activities of CTL clones (5, 39). The ability of CTL to act on other cell types permissive for HIV-1 replication has not been well defined previously. One potential explanation for the failure of CTL to clear HIV-1 infection in vivo could be the inability of CTL to control viral replication in a particular cell type. In the present study, we examined the ability of CTL to suppress HIV-1 replication in primary monocytes, DC, and DC-lymphocyte clusters.
M/M have been shown to be a reservoir of viral replication in vivo (8). It has been suggested that the kinetics of HIV-1 production by these cells might differ from that for lymphocytes in vivo; Perelson et al. suggested that productively infected lymphocytes have a half-life of less than 2 days whereas infected M/M have a half life of approximately 2 weeks, and their contribution to viremia may be approximately 1% (26). The tissue viral burden may be much higher, as demonstrated by examination of gastrointestinal macrophages (32) or macrophages at sites of microbial infection (36). Furthermore, M/M are the primary infected cell type in the central nervous system (11, 13, 20). In this study we found that M/M are susceptible to the antiviral effects of CTL, and M/M therefore do not represent an intrinsically resistant reservoir for viral replication.
It has been suggested that DC may be the first cells to encounter HIV-1 after host exposure at mucosal sites during primary infection, and these cells could be responsible for the transport of virus to regional lymph nodes and subsequent transfer to CD4+ T lymphocytes (6, 16, 19, 33). These cells therefore are proposed by some investigators to play a crucial role in the establishment of HIV-1 primary infection, mediating transmission of virus from the periphery to systemic lymphoid tissues. DC of the immature phenotype found in the periphery can be productively infected by R5 HIV-1 in vitro (14). Our finding that virus-specific CTL can suppress HIV-1 replication in DC suggests that CTL are potentially effective against infected DC at peripheral sites. Recent data obtained with a murine system suggest that mucosal antiviral CTL are required for protective immunity after mucosal exposure to HIV-1 (2), consistent with this hypothesis. If infection of DC is indeed a prerequisite for primary HIV-1 infection, the generation of virus-specific CTL at potential sites of exposure may be an important consideration in strategies to induce protective immunity, with the goal of preventing the initial spread of infection to regional lymph nodes.
As professional antigen-presenting cells, DC are potent activators of CD4+ T lymphocytes (9, 25). Activation of CD4+ lymphocytes by infected DC leads to vigorous viral replication; DC-lymphocyte clusters are known to be sites of explosive HIV-1 replication (7, 28), presumably due to the induction of cellular transcription factors (15). Although the contribution to the viral burden in vivo of infected DC themselves is controversial (6), it has been demonstrated that active viral replication occurs in DC-lymphocyte clusters in lymphoid tissues (10). We found that CTL-mediated suppression of HIV-1 replication in DC-lymphocyte clusters appeared markedly less efficient than in lymphocytes alone. The mechanism is unclear. Accelerated or increased viral replication could simply overwhelm CTL. Alternatively, the interaction of DC with the CD4+ lymphocytes could indirectly affect the function of CTL. The recent identification of a DC surface molecule, DC-SIGN, which apparently can trap and mediate the transfer of HIV-1 from DC to lymphocytes (12) further raises the possibility that DC-SIGN could play a role in the transfer of HIV-1 from uninfected DC (and therefore unrecognized by CTL) to activated lymphocytes. Further study is required to clarify these issues. Our in vitro findings, however, suggest a potential mechanism whereby DC-lymphocyte clusters in lymphoid tissues could be sites of relative resistance to the antiviral activities of CTL.
In summary, we have found that HIV-1-specific CTL clones are capable of suppressing viral replication in primary CD4+ lymphocytes, monocytes, and DC. CTL are thus potentially capable of controlling viral replication in these individual cell types in vivo. Inhibition in DC-lymphocyte clusters may be less efficient than in either cell type alone. Because productively infected DC have been suggested to spread infection to regional lymphatic tissues as an early event in primary infection with HIV-1, the induction of peripheral antiviral CTL responses may be important in the generation of protective immunity.
Acknowledgments
This work was supported by Public Health Service grant RO1 AI043202.
We thank Maurice Gately and Hoffman LaRoche for the generous contribution of interleukin-2 used for these studies.
REFERENCES
- 1.Barker T D, Weissman D, Daucher J A, Roche K M, Fauci A S. Identification of multiple and distinct CD8+ T cell suppressor activities. J Immunol. 1996;156:4476–4483. [PubMed] [Google Scholar]
- 2.Belyakov I M, Ahlers J D, Brandwein B Y, Earl P, Kelsall B L, Moss B, Strober W, Berzofsky J A. The importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J Clin Investig. 1998;102:2072–2081. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buseyne F, Fevrier M, Garcia S, Gougeon M L, Riviere Y. Dual function of a human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte clone: inhibition of HIV replication by noncytolytic mechanisms and lysis of HIV-infected CD4+ cells. Virology. 1996;225:248–253. doi: 10.1006/viro.1996.0597. [DOI] [PubMed] [Google Scholar]
- 6.Cameron P, Pope M, Granelli-Piperno A, Steinman R M. Dendritic cells and the replication of HIV-1. J Leukoc Biol. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- 7.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. . (Erratum, 257:1848.) [DOI] [PubMed] [Google Scholar]
- 8.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y H, Brookmeyer R, Zeiger M A, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 9.Flechner E R, Freundenthal P S, Kaplan P S, Steinman R M. Antigen-specific T lymphocytes cluster with dendritic cells in the human primary mixed-lymphocyte reaction. Cell Immunol. 1988;111:183–195. doi: 10.1016/0008-8749(88)90062-7. [DOI] [PubMed] [Google Scholar]
- 10.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 11.Gabuzda D H, Hirsch M S. Neurologic manifestations of infection with human immunodeficiency virus. Clinical features and pathogenesis. Ann Intern Med. 1987;107:383–391. doi: 10.7326/0003-4819-107-2-383. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek T B H, Kwon D S, Torensma R, van Vliet S J, van Duijnhoven G C F, Middel J, Cornelissen I L M H A, Nottet H S L M, KewalRamani V N, Littman D R, Figdor C G, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Nature. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 13.Giulian D, Vaca K, Noonan C. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1597. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 14.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman R M. Immature dendritic cells selectively replicate macrophagetropic (m-tropic) human immunodeficiency virus type 1 while mature cells efficiently transmit both m- and t-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granelli-Piperno A, Pope M, Inaba K, Steinman R M. Coexpression of NF-kappa B/Rel and Sp1 transcription factors in human immunodeficiency virus 1-induced, dendritic cell-T-cell syncytia. Proc Natl Acad Sci USA. 1995;92:10944–10948. doi: 10.1073/pnas.92.24.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grouard G, Clark E A. Role of dendritic and follicular dendritic cells in HIV infection and pathogenesis. Curr Opin Immunol. 1997;9:563–567. doi: 10.1016/s0952-7915(97)80111-2. [DOI] [PubMed] [Google Scholar]
- 17.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 18.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1356–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight S C. Bone-marrow-derived dendritic cells and the pathogenesis of AIDS. AIDS. 1996;10:807–817. doi: 10.1097/00002030-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Koenig S, Gendelman H E, Orenstein J M, Dal Canto J M, Pezeshkpour G H, Yungbluth M, Janotta F, Askamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 21.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 23.Langlade-Demoyen P, Ngo-Giang-Huong N, Ferchal F, Oksenhendler E. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contacts of HIV-infected patients. J Clin Investig. 1993;93:1293–1297. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 25.Pancholi P, Steinman R M, Bhardwaj N. Dendritic cells efficiently immunoselect mycobacterial-reactive T cells in human blood, including clonable antigen-reactive precursors. Immunology. 1992;76:217–224. [PMC free article] [PubMed] [Google Scholar]
- 26.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 27.Pinto L A, Sullivan J, Berzofsky J A, Clerici M, Kessler H A, Landay A L, Shearer G M. Env-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J Clin Investig. 1995;96:867–876. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pope M, Betjes M G, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 29.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowland-Jones S, Dong T, Fowke K R, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Ngugi E, Bwayo J, MacDonald K S, McMichael A J, Plummer F A. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Investig. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowland-Jones S, Sutton J, Ariyoski K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 32.Smith P D, Meng G, Li L. Infection of gastrointestinal tract macrophages by human immunodeficiency virus type 1. J Leukoc Biol. 1997;62:72–77. doi: 10.1002/jlb.62.1.72. [DOI] [PubMed] [Google Scholar]
- 33.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahl-Hennig C, Steinman R M, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 35.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternack M S, Luster A D. Beta chemokines are released from HIV-1-specific cytolytic T cell granules complexed to proteoglycans. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 36.Wahl S M, Orenstein J M. Immune stimulation and HIV-1 replication. J Leukoc Biol. 1997;62:67–71. doi: 10.1002/jlb.62.1.67. [DOI] [PubMed] [Google Scholar]
- 37.Walker B D, Flexner C, Birch L K, Fisher L, Paradis T J, Aldovini A, Young R, Moss B, Schooley R T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong J T, Colvin R B. Bi-specific monoclonal antibodies: selective binding and complement fixation to cells that express two different surface antigens. J Immunol. 1987;139:1369–1374. [PubMed] [Google Scholar]
- 39.Yang O O, Kalams S A, Trocha A, Cao H, Luster A, Johnson R P, Walker B D. Suppression of HIV-1 replication by CD8+ cells: Evidence for HLA class I restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang O O, Walker B D. CD8+ cells in human immunodeficiency virus type 1 pathogenesis: cytolytic and non-cytolytic inhibition of viral replication. Adv Immunol. 1997;66:273–311. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]