Abstract
This manuscript represents the official position of the Korean Society of Echocardiography on valvular heart diseases. This position paper focuses on the diagnosis and management of valvular heart diseases with referring to the guidelines recently published by the American College of Cardiology/American Heart Association and the European Society of Cardiology. The committee sought to reflect national data on the topic of valvular heart diseases published to date through a systematic literature search based on validity and relevance. In the part II of this article, we intend to present recommendations for diagnosis and treatment of mitral valve disease and tricuspid valve disease.
Keywords: Mitral stenosis, Mitral regurgitation, Tricuspid regurgitation
Background
This manuscript represents the official position of the Korean Society of Echocardiography (KSE) on valvular heart diseases. This position paper focuses on the diagnosis and management of valvular heart diseases with reference to the guidelines recently published by the American College of Cardiology/American Heart Association (ACC/AHA) [1] and the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) [2]. The committee sought to reflect national data on the topic of valvular heart diseases published to date through a systematic literature search based on validity and relevance. In part II of this article, we intend to present recommendations for diagnosis and treatment of mitral valve (MV) disease and tricuspid valve disease.
Mitral stenosis
Etiology
Rheumatic MS
The causes of mitral stenosis (MS) are mostly rheumatic or degenerative. Rheumatic fever is a poststreptococcal immune-mediated inflammatory disease, which results in a high degree of valve fibrosis and dysfunction through long-term inflammation, predominantly involving the MV, and sometimes causing multiple valve disease. According to the statistics of the global, regional, and national burden of rheumatic heart disease published in 2017 [3], the health-related burden of rheumatic heart disease has declined worldwide, but high rates of the disease persist in some underdeveloped regions in the world. In the 2015 statistics, Republic of Korea (hereinafter, Korea) had a very low prevalence [3]. Although there have been few new patients with rheumatic fever owing to the rapid improvement in socioeconomic status from the 1950s to the present, Korea still has a large number of aging patients with rheumatic MS because Korea is one of the developed countries that can systematically apply the latest imaging diagnosis and treatment for the disease [4]. Percutaneous mitral valvuloplasty (PMV) was started by Inoue et al. [5] in 1984 and has been established as an effective treatment for patients with symptomatic rheumatic MS for more than 30 years. Especially in Korea, some leading centers have accumulated extensive clinical experience on rheumatic MS and PMV [6–10].
Degenerative MS
There has been a gradual transition in the epidemiology of MS in the Western world, with rheumatic disease in rapid decline and an increasing recognition of degenerative mitral annular calcification (MAC)-related MS in the elderly [11]. A similar phenomenon is occurring in Korea as well [4]. MAC is a chronic degenerative process affecting the fibrous skeleton of the mitral ring [12, 13]. The main contributing factors for occurrence of MAC are age-related degeneration, elevated left ventricular afterload, atherosclerotic risk factors, and aberrant calcium-phosphate metabolism [14–18]. In the past, MAC was considered an incidental finding in the elderly population or in those with chronic kidney disease. However, growing evidence shows that MAC is associated with cardiovascular morbidity and mortality [19–22]. In addition, patients with hemodynamically significant MAC resulting in MS showed poor clinical outcomes and higher incidence of atrial fibrillation (AF) and stroke [15, 23]. Through several recent investigations [24, 25], the differences in clinical factors, echocardiographic characteristics and pathophysiology between rheumatic MS and MAC-related degenerative MS have been demonstrated as shown in Table 1.
Table 1.
Characteristics of rheumatic MS versus MAC-related degenerative MS
| Characteristic | Rheumatic MS | MAC-related degenerative MS |
|---|---|---|
| Anatomy | Funnel-shaped geometry | Tubular orifice geometry |
| Commissural fusion | Commissures spared | |
| Epidemiology | Younger population | Elderly, comorbid population |
| Echo assessment | MVA quantification validated | MVA quantification challenging |
| Greater MVA relative to mean gradient than rheumatic MS | ||
| Treatment | PMV if favorable valve morphology without contraindication | Poor valvuloplasty candidates |
| MV replacement | Transcatheter or surgical MV replacement |
MS Mitral stenosis, MAC Mitral annular calcification, MVA Mitral valve area, PMV Percutaneous mitral valvuloplasty
Stages
The stages of MS are defined by patient symptoms, valve anatomy, valve hemodynamics, and the consequences of valve obstruction on the left atrium (LA) and pulmonary circulation (Table 2).
Table 2.
Stages of mitral stenosis
| Characteristic | Stage | ||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Definition | At risk | Progressive | Asymptomatic severe | Symptomatic severe | |
| Severity | Normal to insignificant | Mild | Moderate | Severe | |
| Echocardiography morphology | |||||
| Leaflet | Mildly fibrotic or sclerotic, mild doming | Partial fibrothickening, sclerocalcified, doming | Severe fibrothickening, sclerocalcified, doming | ||
| Commissure | No fusion | Mild to moderate fusion | Severe fusion | ||
| Annulus | Mild calcification | Mild to moderate calcification | Severe calcification | ||
| Area (cm2) | - | > 2.5 | 1.6–2.5 | ≤ 1.5 (very severe, ≤ 1.0) | |
| MDPG (mmHg) | - | < 3 | 3–5 | ≥ 5 (very severe, ≥ 10) | |
| PHT (msec) | - | < 100 | 100–149 | ≥ 150 | |
| PASP (mmHg) | Normal | Normal | Normal at rest | Normal at rest or > 50 | > 50 |
| LA size | Normal | Normal or ↑ | ↑ | ↑ | |
| Symptom | None | None | None | DOE, EI | |
MDPG Mean diastolic pressure gradient, PHT Pressure half-time, PASP Pulmonary artery systolic pressure, LA Left atrium, DOE Dyspnea on exertion, EI exercise intolerance
The definition of “severe” MS is based on the severity of symptoms, as well as the severity at which intervention will improve symptoms. Thus, an MV area (MVA) ≤ 1.5 cm2 is considered severe, which typically corresponds to a transmitral mean diastolic pressure gradient (MDPG) greater than 5 mmHg at a normal heart rate. However, MDPG is highly dependent on transvalvular flow rate, diastolic filling period, and heart rate. Mitral pressure half-time also has limitations and is dependent upon left ventricular (LV) and LA compliance as well as stenosis severity. It is true that confusion has occurred in diagnosis and treatment criteria while correcting the cutoff value of the definition of severe MS to MVA of 1.5 cm2 from 1.0 cm2 in the AHA/ACC guideline in 2014 [26] and its focused update in 2017 [27].
According to the practice survey conducted by the KSE in 2022, 62.1% of institutions are still using the MVA cutoff of 1.0 cm2 for severe MS and 37.9% of institutions are complying with the cutoff of 1.5 cm2 [1, 2]. The reasons for sticking to the original criteria were diverse: lack of sufficient evidence for changes in cutoff values, worsening discrepancy between MVA and MDPG, the view that MVA of 1.5 cm2 as the tipping point for intervention was unreasonable, and inevitable confusion in patients on regular follow-up whose MS severity would be newly classified due to changes in cutoffs rather than progression of valve disease.
The KSE guideline committee has developed expert consensus on this issue through structured face-to-face meetings. In this position paper, MVA of 1.5 cm2 is determined as the cutoff for severe MS in rheumatic MS. In addition, the criterion for very severe MS is determined to be MVA ≤ 1.0 cm2. In cases of degenerative MS, that is, MAC-related MS, the role of MDPG is more emphasized, but the severity diagnostic criteria are equally recommended. As a diagnostic criterion for severe MS, the MVA standard refers to structural abnormalities that suggest the need for intervention. However, decision-making should be made based on the patient’s symptoms and hemodynamic consequences, such as pulmonary hypertension or atrial arrhythmia.
The cutoff values for MDPG correlating with MS severity were set to reduce the discrepancy between MVA and MDPG. When there is a discrepancy between MVA and MDPG, it is recommended to apply the biplane method or three-dimensional (3D) multiplanar reconstruction to obtain accurate measurement of MVA. In cases of degenerative MS due to MAC or functional MS due to other causes, it may be difficult to measure the MVA, so the clinical significance may have to be interpreted according to hemodynamic parameters.
Korean data
A recent nationwide hospital-based retrospective cohort study from the Korean Valve Survey collected clinical and echocardiographic data on valvular heart disease from 45 medical centers [28]. Among 4,089 patients, 244 patients showed MS and mean age of MS patients was 65.5 ± 10.9 years, which was significantly younger than those with mitral regurgitation (MR) or other valvular dysfunction. 74.6% of patients with MS had rheumatic etiology and 20.5% had degenerative causes.
Historically, abundant research on rheumatic MS has been reported in Korea [6–10, 29–32]. Kang et al. [29] compared the long-term outcomes of early preemptive PMV and a conventional treatment strategy to define the optimal timing of PMV in asymptomatic patients with moderate MS. At the time of conducting this study, the guidelines defined MVA 1.0 to 1.5 cm2 as moderate MS, so if interpreted using the current guidelines, this study was in fact about asymptomatic severe MS. In 244 asymptomatic patients with severe rheumatic MS (MVA, 1.0–1.5 cm2) who were potential candidates for early PMV, the risk of cardiovascular endpoint was significantly lower in the PMV group than in the conventional group after propensity matching. They concluded that the clinical benefits of early PMV may outweigh the risks associated with early intervention, but prospective randomized trials are required to confirm the efficacy of early PMV. Looking at the 20-year experience of a single-center study reported by Kim et al. [8] in 2018, the factors affecting the long-term outcome of PMV were echo score > 8 and post-PMV MVA of 1.76 cm2 or less. A recent report on 10-year trends in the incidence, treatment, and outcomes based on the Korean Health Insurance Review and Assessment Service (HIRA) database showed that the overall incidence of MS in Korea has decreased remarkably [30]. The data also demonstrated the increasing rate of anticoagulant use and similar incidence of systemic embolism, but increasing incidence of intracranial hemorrhage, suggesting a topic that should be considered in the treatment of MS in the future. To overcome the limitations of the previous retrospective study on early PMV in asymptomatic MS, a prospective randomized multicenter trial was conducted in Korea [31]. However, the effect of early PMV was not demonstrated in asymptomatic MS patients. In addition, a study on the trajectory profile of rheumatic MS was conducted in Korea to find factors that predict rapid progression [32]. In a total of 436 patients with severe MS with a valve area of 1.0 to 1.5 cm2, rapid progression of pulmonary artery systolic pressure determined prognosis, which was associated with a pulmonary artery systolic pressure of 40 mmHg on initial evaluation.
Recently, with an increase in the elderly population in Korea, research on degenerative MS and MAC is being actively conducted [15, 17, 21]. In particular, a study on the sex differences of risk factors in patients with MAC has been reported [21]. In hemodynamic comparisons between degenerative MS and rheumatic MS, degenerative MS presented with a greater MVA relative to MDPG than rheumatic MS [25]. Kim et al. [15] reported the clinical significance of morphological and structural characteristics of MAC. Morphological and functional features of MAC on echocardiogram were scored from 0 to 3 according to MAC mobility, presence of echo-dense mass with central echolucencies in the periannular region suggesting caseous necrosis, and functional stenosis in 460 subjects with MAC. They demonstrated that this MAC score was independently associated with stroke and had significant incremental value over demographic factors and comorbidities for predicting stroke. In the future, studies that more objectively assess MAC severity using cardiac computed tomography (CT) are expected to be conducted.
Diagnosis and follow-up
Rheumatic MS
Diagnosis
For patients with rheumatic MS, there is no doubt that transthoracic echocardiography (TTE) is a fundamentally important first-line imaging for evaluating structure and function in MS. In particular, it plays an important role in evaluating the severity of MS, determining feasibility of intervention, and classifying risk after intervention. The hallmark feature of rheumatic MS is commissural fusion, leading to the classic “fish mouth” appearance of the MV orifice, and thickening and restriction of the posterior leaflet. Notably, calcification in rheumatic MS primarily involves the leaflet tip, which is distinguishable from annular calcification found in degenerative MS. Among many echocardiographic parameters, MVA using 2D planimetry is the reference method of measuring MS severity, and severe MS is defined by an MVA ≤ 1.5 cm2 [1, 2]. Planimetry by 3D echocardiography may have an additional diagnostic value by permitting accurate identification of the MV orifice. Transmitral MDPG and pulmonary arterial pressures are useful parameters reflecting hemodynamic consequences of MS and providing prognostic information [33].
Table 3 shows the advantages, disadvantages, and indications of additional diagnostic tests in patients with MS. Stress testing is recommended in patients with no or equivocal symptoms or discordant symptoms with MS severity measured at rest. Exercise echocardiography has a role in these patients for the evaluation of changes in transmitral MDPG and pulmonary artery pressure. Dobutamine stress echocardiography is also a safe and feasible stress test; however, it has some limitations compared to exercise echocardiography [34]. The role of dobutamine stress echocardiography in these patients is primarily to assess contractile reserve and myocardial ischemia, which could contribute to symptoms or help identify underlying coronary artery disease. It can also provide information on the hemodynamic response to stress in patients who are unable to exercise or have limited exercise capacity. Nonetheless, exercise echocardiography is often considered superior to dobutamine stress echocardiography in the evaluation of MS, as dobutamine induces changes in loading conditions that are different from exercise-induced changes and thus does not simulate day-to-day physiologic stress as accurately. Cardiac catheterization is useful in patients with discordant grading of MS to further characterize hemodynamics and the cause of symptoms, as this test allows measurement of absolute pressures in the LA, LV, and pulmonary circulation at rest and even during exercise. Although TTE usually provides sufficient information for routine management of patients with rheumatic MS, transesophageal echocardiography (TEE) is superior to TTE in obtaining detailed information on mitral anatomy, particularly in assessing commissural zones, subvalvular apparatus, and other complex MV pathology. For this reason, TEE should be performed to determine suitability for PMV [35, 36], and also to exclude the presence of thrombus in LA or LA appendage before PMV.
Table 3.
Comparison of diagnostic tools for the evaluation of MS
| Test | Advantage | Disadvantage | Indication |
|---|---|---|---|
| Exercise SE | Physiological stress test | Limited in patients unable to exercise | Evaluation of exercise hemodynamics associated with MS |
| Minimal invasiveness | Objective assessment of symptoms and exercise capacity | ||
| Dobutamine SE | Relative ease of performance compared to exercise SE | Nonphysiological stress test | Assessment of contractile reserve and myocardial ischemia/viability |
| Minimal invasiveness | Risk of dobutamine-induced arrhythmias | ||
| Cardiac catheterization | Absolute pressure measurements | Risk of invasive complications: bleeding, infection, vascular damage | Possible confirmatory test for discordant or inconclusive results of MS severity grading |
| Hemodynamic assessment during exercise | Evaluation of hemodynamics responsible for exertional symptoms | ||
| TEE | Provision of high-resolution images | Semi-invasiveness | More thorough evaluation of valve morphology and function |
| Better visualization of mitral valve, LA, and LAA | Patient discomfort | Detection of thrombus in LA or LAA |
MS Mitral stenosis, SE Stress echocardiography, TEE Transesophageal echocardiography, LA Left atrium, LAA Left atrial appendage
-
(2)
Follow-up
As rheumatic MS is a slowly progressive disease, repeated TTE at intervals dictated by valve area is recommended for patients with asymptomatic MS. In patients with mild MS, although the rate of further narrowing is quite variable [37], the average rate of progression is a decrease in valve area of 0.1 cm2/yr [38]. However, it is important to note that progressive increases in right ventricular (RV) size and RV systolic pressure can be found, even in the absence of a decrease in MVA. Intervals of 2 to 3 years are appropriate in cases of moderate MS [1]. Close surveillance of disease progression with yearly TTE is recommended in asymptomatic patients with severe MS. Follow-up strategies for patients undergoing successful PMV are similar to those of asymptomatic patients and more frequent follow-up visits and evaluations are indicated if restenosis occurs even though patients remain asymptomatic.
Degenerative MS
Diagnosis
Defining the severity of degenerative MS is difficult and the lack of validation of the usual parameters used in rheumatic MS is still an issue. For instance, extensive calcification and irregular orifice of the MV hamper the accurate measurement of MVA, making it less reliable [39, 40]. Furthermore, abnormal LA and LV compliance, which are commonly present in patients with degenerative MS, cause a high transmitral MDPG even in the absence of significant MS [11]. Although MDPG is not useful for determining disease severity and threshold for intervention, this echocardiographic parameter may have prognostic value in patients with degenerative MS [23]. Echocardiography remains the initial imaging modality of choice for the assessment of MS when an intervention is planned, and cardiac CT is often necessary to evaluate the location and degree of calcification and to determine the feasibility of a planned intervention [41].
-
(2)
Follow-up
Data is scarce on the rate of progression in degenerative MS and the factors influencing progression rate. Evidence is lacking to support a recommendation for follow-up intervals in patients with degenerative MS, but follow-up may be performed similarly to rheumatic MS.
Medical therapy
Diuretics can improve symptoms by reducing LA pressure and pulmonary congestion. β-blockers, nondihydropyridine calcium channel blockers, and digoxin can also improve symptoms by prolonging diastole and consequently improving LV filling at rest and during exercise.
In patients with AF, anticoagulation with a vitamin K antagonist with a target international normalized ratio of 2 to 3 is recommended. Importantly, vitamin K antagonists should be preferred over direct oral anticoagulants in patients with moderate-to-severe rheumatic MS and AF. A recent study using the HIRA database suggests that the use of direct oral anticoagulants is promising in the prevention of thromboembolic events in patients with MS and AF [42], but there is no solid evidence to support this yet. In patients in sinus rhythm, an oral anticoagulant is recommended in the setting of prior systemic embolism or presence of a thrombus in the LA [43]. However, it is controversial whether long-term oral anticoagulation should be considered when there is only LA enlargement or spontaneous echocardiographic contrast on TEE [44, 45]. The benefit of rhythm control for AF should be considered based on severity of MS, LA size, and possibility of sinus conversion. In patients with significant MS, rhythm control strategies including cardioversion and ablation are not recommended before relieving MS because of the low probability of achieving durable restoration of sinus rhythm. However, cardioversion should be performed immediately after successful intervention in patients with AF of recent onset and moderately enlarged LA. Among antiarrhythmic medications, amiodarone is most effective in maintaining sinus rhythm after cardioversion of AF.
Timing of intervention
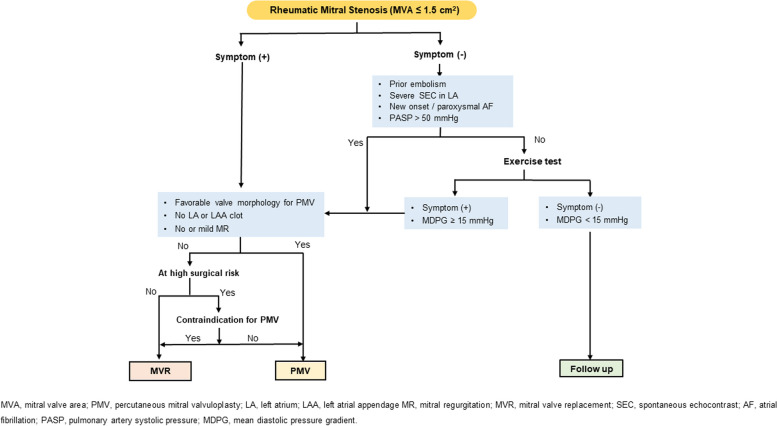
Determining the optimal timing for surgical or percutaneous interventions in patients with MS is important to avoid the risks of unnecessary early intervention and irreversible pulmonary hypertension and/or right heart failure due to delayed intervention. Although the natural history data of MS have a retrospective nature, are prone to selection bias, and are unclear regarding severity of stenosis, most of the data consistently show poor prognosis without intervention in symptomatic MS patients. The 10-year survival rate of symptomatic patients is 34% to 61% and the 20-year survival rate is 14% to 21%, which is very poor [46, 47]. In particular, the 5-year survival rate is 44%, the 10-year survival rate is 32%, and the 15-year survival rate is 19% in patients who were recommended but refused intervention [48]. The most obvious timing of intervention is when symptoms are present in patients with severe MS (MVA ≤ 1.5 cm2) (Fig. 1).
Fig. 1.
Treatment of rheumatic mitral stenosis. AF, atrial fibrillation; LA, left atrium; LAA, left atrial appendage; MDPG, mean diastolic pressure gradient; MR, mitral regurgitation; MVA, mitral valve area; MVR, mitral valve replacement; PASP, pulmonary artery systolic pressure; PMV, percutaneous mitral valvuloplasty; SEC, spontaneous echocontrast
The 20-year survival is excellent in asymptomatic patients with MS, but half of the patients experience sudden deterioration due to AF or systemic embolism [47–50]. Early PMV was expected to have a clinical benefit compared to conventional management in patients with asymptomatic severe MS, but randomized control trials did not show that early PMV reduces cardiovascular events, including PMV-related complications, cardiovascular mortality, cerebral infarction, and systemic thromboembolic events, when compared to conventional management [29, 31]. In asymptomatic MS patients with AF or a previous thromboembolic event, early PMV reduced the composite endpoint including cardiovascular mortality, cerebral infarction, and systemic embolic events compared to patients with conventional treatment only [2]. Intervention should be considered in asymptomatic MS patients with previous systemic embolism, severe spontaneous echo contrast in LA or new-onset or paroxysmal AF.
Pulmonary hypertension is common, with a prevalence of 15% to 81% in patients with severe MS [51–54]. Pulmonary hypertension in patients with MS develops through postcapillary and precapillary hypertension. In the early stages of MS, pulmonary hypertension is caused by increased LA pressure, but as the disease progresses, pulmonary hypertension is exacerbated by increased pulmonary vascular resistance. Pulmonary arterial hypertension in patients with MS has an adverse effect on long-term prognosis [55, 56]. When there is clinical evidence of pulmonary hypertension in MS patients, the prognosis is poor in patients treated only medically [1]. Patients with severe pulmonary hypertension or with high pulmonary vascular resistance prior to the intervention had a higher mortality rate and less improvement in pulmonary hypertension after the intervention [57, 58]. It is reasonable to perform intervention for MS when pulmonary hypertension develops.
In general, intervention is performed only in patients with severe MS, because the risk of intervention outweighs its benefit in patients with MVA > 1.5 cm2. However, patients with an MVA > 1.5 cm2 may have unexplained symptoms for other reasons. This is related to the potential limitations of MVA measured by 2D planimetry or pressure half-time. In this case, intervention can be considered if MDPG increases over 15 mmHg through exercise tests. Conversely, in cases of severe MS with ambiguous symptoms, intervention may be considered if symptoms develop through exercise testing.
Intervention timing for patients with degenerative MS is different from that for patients with rheumatic MS. Patients with degenerative MS have a high risk regardless of type of intervention because they have severe calcification, old age, and multiple comorbidities. Therefore, in patients with degenerative MS, intervention should be performed only in those with severe symptoms.
Choice of intervention
Effective treatment in patients with rheumatic MS is PMV or surgery. The choice of intervention is determined based on the clinical condition of the patient, the anatomical features of the valve, and the experience of the institution (Fig. 1).
PMV should be considered primarily for intervention except in cases where PMV is contraindicated or inappropriate. Unfavorable clinical characteristics for PMV include old age, prior commissurotomy, severe symptoms (New York Heart Association class IV), permanent AF, and severe pulmonary hypertension [37]. Echocardiography is used to confirm the absence of anatomical contraindications for PMV and to predict outcomes after PMV. In order to perform PMV, the following conditions must not be met: thrombus in LA, moderate or more MR, severe or bilateral commissural calcification, no commissural fusion, severe aortic valve disease or severe tricuspid stenosis and regurgitation, or requirement of concomitant coronary artery bypass surgery. The anatomical features of MV evaluated by echocardiography are related to the outcomes after PMV. Most investigators use Wilkins score or Cormier score to predict outcome after PMV [37, 59, 60]. Patients with Wilkins score greater than 8 showed suboptimal results [37]. Patients with valve morphology corresponding to Cormier groups 2 and 3 were 2.2 and 5.3 times, respectively, more likely to have inadequate results than those with valve morphology corresponding to group 1 [59]. PMV is also recommended if the patient has contraindications to surgery or is at high risk. In patients who underwent a successful PMV procedure, both the Inoue technique and the double balloon technique showed good long-term prognosis, regardless of the type of procedure [61]. A pre-PMV echocardiographic score > 8 and an immediate post-PMV MVA ≤ 1.76 cm2 are independent prognostic predictors of poor long-term prognosis, including MV reintervention, stroke, and cardiovascular death during a follow-up period greater than 10 years [8]. In a recent multicenter registry comparing the outcome of PMV and MV replacement, the treatment method did not affect cardiovascular mortality or heart failure hospitalization, but in very severe MS, early redo-intervention was found to increase in the PMV group [62].
In patients with symptomatic severe MS, PMV is recommended if the valve morphology is favorable for PMV, there is no clot in the LA or left atrial appendage, and significant MR is absent. However, if all the requirements for PMV are not met, MV replacement should be considered if the surgical risk is not high. In cases of high surgical risk, PMV may be considered in patients with resolved contraindications. For example, if thrombus in the LA or left atrial appendage disappears after anticoagulation, PMV can be done if other conditions are suitable for PMV. In patients requiring MV replacement, a mechanical valve is usually implanted because the durability of the prosthesis is better in mitral lesions and most patients require lifelong anticoagulant treatment due to AF.
In degenerative MS, unlike rheumatic MS, commissural fusion or leaflet involvement is rare, and calcification of the mitral annulus expands to the base of the leaflet, causing stenosis. Therefore, PMV or surgical commissurotomy has no role in patients with degenerative MS.
Mitral regurgitation
Etiology
MR is the most common valvular heart disease and is associated with excess mortality [63, 64]. The etiology of MR is divided mainly into primary MR and secondary MR. Primary MR is caused by primary anatomical and structural problems of the valve leaflets, chordae tendineae, and papillary muscles. The etiology of chronic primary MR includes the following: (1) MV prolapse (MVP) due to myxomatous change or fibroelastic deficiency; (2) degenerative leaflet thickening with calcification; (3) rheumatic change; and (4) congenital abnormality. MVP can occur in association with connective tissue diseases such as Marfan syndrome or Ehlers-Danlos syndrome [65]. In addition, the pathophysiology of primary MR due to MVP in patients with hypertrophic cardiomyopathy is associated with intrinsic MV abnormality and increased sheer stress [17, 65]. Papillary muscle rupture following myocardial infarction and destruction of MV leaflets due to bacterial endocarditis are the major causes of acute primary MR.
Secondary MR occurs by LV pathology, such as LV systolic dysfunction and enlarged LV size, resulting in impaired coaptation of MV due to the papillary muscle and chordae tendineae pulling the MV and forming MV tenting without problems in the MV apparatus [66]. Secondary MR can be caused by any cause of LV systolic dysfunction. It can occur not only from ischemic cardiomyopathy, but also from nonischemic cardiomyopathy, stress cardiomyopathy, and myocarditis. Of these, secondary MR caused by ischemic insult to the myocardium is called ischemic MR. Secondary MR can be caused by LA pathology, without an LV cause. LA enlargement and dilated MV annulus also result in impaired coaptation of MV, which is called atrial functional MR and is frequently observed in AF and heart failure with preserved ejection fraction [3].
Stages
The stages of MR are based on valve anatomy and valve hemodynamics, and the criteria for severe MR in primary MR and secondary MR are the same: vena contract width (VCW) ≥ 7 mm, effective regurgitant orifice area (EROA) ≥ 0.4 cm2, regurgitant volume ≥ 60 mL, and regurgitant fraction (RF) ≥ 50% [1]. Table 4 demonstrates the stages of chronic primary MR and Table 5 demonstrates the stages of secondary MR.
Table 4.
Stages of chronic primary mitral regurgitation
| Characteristic | Stage | |||||
|---|---|---|---|---|---|---|
| A | B | C1 | C2 | D | ||
| Definition | At risk | Progressive | Asymptomatic severe | Symptomatic severe | ||
| Severity | Normal to insignificant | Mild | Moderate | Severe | ||
| Echocardiography morphology | ||||||
| Leaflet | Mild thickening or restriction | Moderate to severe thickening or restriction | Severe thickening or restriction | |||
| Prolapse | Mild | Moderate to severe | Severe | |||
| Coaptation | Normal | Moderate to severe loss | Severe loss | |||
| VCW (mm) | < 3 | 3–7 | ≥ 7 | |||
| EROA (cm2) | < 0.2 | 0.2–0.3 | 0.3–0.4 | ≥ 0.4 | ||
| Regurgitant volume (mL) | < 30 | 30–44 | 45–59 | ≥ 60 | ||
| Regurgitant fraction (%) | < 30 | 20–39 | 40–49 | ≥ 50 | ||
| LVEF (%) | Normal | Normal | > 60 | ≤ 60 | - | |
| LVESD (mm) | Normal | Normal | < 40 | ≥ 40 | - | |
| LA size | Normal | Normal or ↑ | ↑ | |||
| LV size | Normal | Normal | ↑ | |||
| PV flow | Systolic dominance | Normal or systolic blunting | Systolic flow reversal | |||
| Symptom | None | None | None | DOE, EI | ||
VCW Vena contracta width, EROA Effective regurgitant orifice area by 2D-PISA method, Reg. Regurgitant, LVEF Left ventricular ejection fraction, LVESD Left ventricular end-systolic dimension, LA Left atrium, LV Left ventricle, PV Pulmonary vein, DOE Dyspnea on exertion, EI Exercise intolerance
Table 5.
Stages of secondary mitral regurgitation
| Characteristic | Stage | ||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Definition | At risk | Progressive | Asymptomatic severe | Symptomatic severe | |
| Severity | Normal to insignificant | Mild | Moderate | Severe | |
| Echocardiography morphology | |||||
| Tethering | Normal to insignificant | Mild to moderate | Severe | ||
| Coaptation | Normal | Limited | Severe loss | ||
| Annulus | Normal | Dilation | Dilation | ||
| VCW (mm) | < 3 | 3–7 | ≥ 7 | ||
| EROA (cm2) | - | 0.2–0.3 | 0.3–0.4 | ≥ 0.4 | |
| Regurgitant volume (mL) | - | 30–44 | 45–59 | ≥ 60 | |
| Regurgitant fraction (%) | - | 20–39 | 40–49 | ≥ 50 | |
| LA size | Normal or mildly ↑ | ↑ | ↑ | ||
| LV size | Normal or mildly ↑ | ↑ | ↑ | ||
| LV systolic function | Normal or ↓ | ↓ | ↓ | ||
| RWMA | Fixed or inducible | Presence | Presence | ||
| PV flow | Systolic dominance | Normal or systolic blunting | Systolic flow reversal | ||
| Symptom | None (or angina) | None (or angina) | None (or angina) | Angina, DOE, EI | |
VCW Vena contracta width, EROA Effective regurgitant orifice area by 2D-PISA method, LA Left atrium, LV Left ventricle, RWMA Regional wall motion abnormality, PV Pulmonary vein, DOE Dyspnea on exertion, EI Exercise intolerance
Korean data
A recent nationwide hospital-based retrospective cohort study from the Korean Valve Survey collected clinical and echocardiographic data on valvular heart disease from 45 medical centers [28]. It showed that MR (1,384 of 4,089, 33.8%) is the most common significant valvular heart disease. Primary MR (n = 795, 57%) showed a higher proportion than secondary MR (n = 598, 43%); degenerative change (44.3%) and MVP (33.7%) were the most common causes of primary MR. In secondary MR, nonischemic etiology is observed in two-thirds (63.0%) and ischemic etiology in one-third (29.3%). The mean LV ejection fraction (LVEF) of secondary MR was 38.9%, and proportion of LVEF less than 40% was 57.2%.
A recent study showed distinct phenogroups of patients with primary MR undergoing valve surgery [67]. In this study, 1,629 patients who underwent valve surgery with severe primary MR were divided into five groups (group 1, least comorbidities; group 2, men with LV enlargement; group 3, predominantly women with rheumatic MR; group 4, low-risk older patients; and group 5, high-risk older patients) and the patient's prognosis was evaluated. The elderly and high-risk patients (group 5) had the poorest prognosis, while the young and low-risk patients (group 1) had the best prognosis [67]. It was suggested that defining the patient group would help to predict the patient's prognosis and determine appropriate surgical timing according to phenotype. Other studies evaluated prognosis by measuring LA strain and LV global longitudinal strain in severe MR patients who underwent MV surgery [68, 69]. Preoperative LV global longitudinal strain predicted the postoperative outcome and preoperative LA strain was also an independent predictor of long-term prognosis after surgery [68, 69]. After the Korean Ministry of Food and Drug Safety approved transcatheter edge-to-edge repair (TEER) use for degenerative MR in 2019, the initial experiences with TEER in Korea show that postimplantation MR grade ≤ 2 could be achieved in 94% and 30-day mortality rate was 6%, which were acceptable for initial procedural outcome [70].
Mortality and morbidity of secondary MR are high in heart failure patients with LV systolic dysfunction. Due to the lack of evidence for pharmacological therapy, the PRIME study was conducted to confirm whether sacubitril/valsartan improves secondary MR [71]. A total of 118 patients with secondary MR from four centers in Korea were enrolled in this study. The sacubitril/valsartan group had a more significant decrease in ERO and LV end-diastolic volume index than the valsartan group. The PRIME study showed that angiotensin receptor-neprilysin inhibitors may be considered for optimal medical therapy in stable patients with heart failure and functional MR.
Diagnosis and follow-up
Primary MR
Evaluation of the pathologic change of MV apparatus (leaflets, chordae tendineae, papillary muscles, and annulus) and LV remodeling is crucial for grading and determining treatment strategy for primary MR. The presence of MR-related symptoms or arrhythmias such as AF should also be assessed to determine the optimal timing of MR intervention.
Diagnosis
TTE is a baseline imaging modality to diagnose and grade primary MR. Evaluation of pathoanatomic lesions of the MV causing MR is needed to identify a specific etiology and evaluate feasibility of surgical or transcatheter repair [72]. Parameters for evaluating MR severity include EROA, regurgitant volume, vena contracta, jet area by color Doppler, and transmitral jet velocity [73, 74]. Changes in LVEF, LV volume and pulmonary artery pressure should also be evaluated to guide the timing of MR intervention and predict long-term outcome. Although LVEF is a key factor in determining the timing of MR intervention, it is frequently load-dependent in patients with chronic MR. In this case, global longitudinal strain may be useful to reflect LV dysfunction more sensitively [75, 76]. When the MR is multijet or eccentric, 3D echocardiography may be helpful to accurately quantify regurgitant volume [77, 78]. In patients with primary MR, TEE is indicated for evaluation of severity and mechanism of MR when TTE provides inadequate image quality or discordant information. Especially, the en face view using 3D TEE can visualize the same inspection with a surgical view and therefore may be helpful for surgical planning. Cardiac magnetic resonance (CMR) provides a more accurate volumetric assessment of LV, LA, and LVEF than TTE or TEE. Thus, in cases where the echocardiographic image is too poor to measure chamber size, CMR may be indicated [75, 79, 80]. Although CMR is known as a gold standard for measuring regurgitant fraction in patients with MR, CMR is possible when the adequate quality of velocity-encoded imaging acquisition is ensured. Also, CMR is less useful for evaluating MV pathology. In asymptomatic patients with severe primary MR and nondilated cardiac chambers, patients who had low brain natriuretic peptide (BNP) levels showed more favorable outcomes than those with high BNP levels [81]. Therefore, checking BNP value can be useful for predicting the clinical course in asymptomatic patients with severe primary MR. Exercise echocardiography may identify the cardiac origin of dyspnea in patients with discordant symptoms and MR grade at rest [82, 83]. Changes in MR volume and pulmonary pressures during exercise is helpful in checking objective symptom and hemodynamic influence of MR [84, 85].
-
(2)
Follow-up
The clinical situation (symptom onset, chest x-ray, electrocardiogram) and echocardiography should be assessed periodically in patients with chronic MR. In patients with asymptomatic MR, serial echocardiography is required depending on the MR severity. Reassessment of MR severity, LA/LV size, LVEF, and pulmonary artery pressure every 3 to 5 years in patients with mild MR, every 1 to 2 years in patients with moderate MR, and every 6 to 12 months in patients with severe MR are recommended. Recently, the Asian Valve Registry demonstrated that LV mass index was independently associated with development of MR-related symptoms [86]. Therefore, when the occurrence of cardiac symptoms due to MR is not convincing, change in LV mass index may help the decision-making. Measurement of BNP levels, exercise echocardiography, electrocardiogram and CMR may be considered complementary diagnostic and risk stratification tools [86–89].
-
(3)
Medical therapy
In patients with acute MR, nitrates and diuretics are used to reduce filling pressures. Sodium nitroprusside reduces afterload and regurgitant fraction. However, there is no evidence to support the prophylactic use of vasodilators in chronic primary MR with preserved LVEF. In patients with overt heart failure, medical therapy is reasonable [90, 91].
-
(4)
Timing of intervention
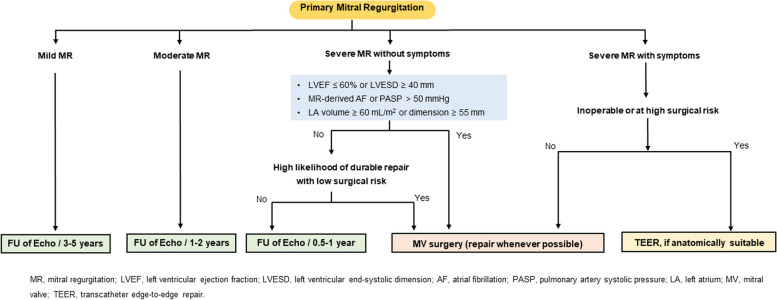
Patients with acute severe MR, caused by acute chordae rupture, papillary muscle rupture, or infective endocarditis, usually present with decompensated symptoms and signs of heart failure, therefore, urgent surgical treatment is recommended in acute severe MR. Indications for intervention in chronic primary MR are shown in Fig. 2. In symptomatic patients with severe primary MR, MV surgery is recommended irrespective of LV systolic function. The benefit of early surgery in asymptomatic patients with severe primary MR has been suggested in prospective, observational studies [92–94]. Especially in asymptomatic patients with severe primary MR and preserved LVEF over 50 years of age, the efficacy of early surgery on reducing cardiac mortality was much more significant than conservative management [94]. In this regard, imaging surveillance is useful to determine the ideal time for MV surgery before the development of LV systolic dysfunction. The presence of LVEF ≤ 60%, LV end-systolic dimension ≥ 40 mm, LA volume index ≥ 60 mL/m2 or diameter ≥ 55 mm, systolic pulmonary arterial pressure > 50 mmHg, and AF are considered triggers for intervention regardless of symptomatic status [95–99].
Fig. 2.
Treatment of primary mitral regurgitation (MR). AF, atrial fibrillation; FU, follow-up; LA, left atrium; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; MV, mitral valve; PASP, pulmonary artery systolic pressure; TEER, transcatheter edge-to-edge repair
-
(5)
Choice of intervention: surgical repair and replacement
When the surgical treatment is determined, MV repair should be considered in preference to replacement under multidisciplinary discussion by a heart team. Large prospective observational data have shown that MV repair is associated with lower operative mortality, better long-term survival, and fewer valve-related complications compared with MV replacement [100–102]. Excellent surgical and cardiac outcome of mitral repair was observed regardless of the type of annuloplasty ring in the prospective randomized data in Korea [103]. However, in patients with advanced rheumatic MR, durability of MV repair can be limited by thickened or calcified leaflets, or extensive subvalvular disease [104]. Therefore, durability and success of repair versus replacement in rheumatic MR may be assessed by a comprehensive valve center.
-
(6)
Choice of intervention: transcatheter MV repair
Studies of mitral TEER have demonstrated improvement of symptoms and a reduction in MR by 2 to 3 grades, leading to reverse remodeling of the LV in patients with symptomatic severe primary MR [105–107]. However, since surgical repair is the standard therapy in primary MR, TEER can only be considered in symptomatic patients with primary MR with high surgical risk and who are anatomically suitable for clipping. The 5-year follow-up data of TEER with MitraClip (Abbott) in patients with severe symptomatic primary MR are now available. Patients treated with percutaneous repair more commonly required surgery for residual MR during the first year after treatment [105]. However, between 1- and 5-year follow-ups, comparably low rates of surgery for MV dysfunction with either percutaneous or surgical therapy were reported [106, 107]. However, long-term follow-up data on the durability of TEER are needed. In addition, before determining TEER, a multidisciplinary discussion by the heart team regarding feasibility of MV anatomy for TEER and patient’s life expectancy should be considered.
Secondary MR
Diagnosis
Secondary MR is the condition of MR without significant pathologic problems of the leaflets [1, 2]. It results from LV dysfunction or remodeling by ischemic or nonischemic cardiomyopathies or atrial disease. Structurally, papillary muscular displacement with leaflet tethering and/or mitral annulus dilatation are the main pathologies [3, 108]. The most important diagnostic tool is TTE to identify the etiology and to assess severity of MR, LVEF, chamber sizes, and hemodynamic information including pulmonary arterial pressure [1, 2]. Coronary CT angiography or invasive coronary angiography is often required to evaluate the presence of coronary artery disease. Stress nuclear imaging, positron emission tomography (PET), CMR, or stress echocardiography can be considered to assess coronary artery disease as well as myocardial viability [109]. The utilization of TEE in secondary MR is focused on transcatheter valve interventions. It is indicated to determine suitability of transcatheter valve intervention or for guidance during the procedure [1, 2].
The definition of severe secondary MR is not different from primary MR. The recommended definition of severe secondary MR is EROA ≥ 0.4 cm2, regurgitant volume ≥ 60 mL, and regurgitant fraction ≥ 50% [1, 2]. Clinical evidence from previous studies indicates that secondary MR is associated with poorer outcomes compared to primary MR, even with smaller EROA [110, 111]. In addition, the discrepancy of estimated echocardiographic parameters may lead to difficulties in defining severe secondary MR due to following reasons: the total forward LV stroke volume is lower which can cause lower estimated regurgitant volume (< 60 mL); the crescent shape of the regurgitant orifice in secondary MR may lead to underestimation of the vena contracta width as well as EROA. Furthermore, multiple components other than the severity of MR can influence the prognosis of secondary MR, implying that the severity should not be determined only by the prognosis of the disease.
Multimodal imaging assessment is helpful in patients with secondary MR. Recent studies have demonstrated that CMR, global longitudinal strain, 3D echocardiography, and exercise echocardiography are additional useful tools to identify and prognosticate severe secondary MR [68, 79, 83, 112–116].
-
(2)
Follow-up
The follow-up interval for TTE is recommended as follows based on the known MR progression rate and impact on the LV and LA. It is recommended to reassess MR severity, LA/LV size, LVEF, and pulmonary artery pressure every 3 to 5 years for patients with mild MR, every 1 to 2 years for patients with moderate MR, and every 6 to 12 months for patients with severe MR. Since secondary MR is often dynamic according to hemodynamic changes, it is recommended to perform a repeat TTE in the case of treatment that will change the size or function of the LV and LA. In addition to routine periodic imaging, the onset of symptoms or a change in the physical examination should raise concern about the cardiac response to the MR, necessitating a repeat TTE.
-
(3)
Medical therapy
Chronic severe secondary MR with heart failure with reduced ejection fraction should receive four pillars of optimal guideline-directed medical therapy (GDMT), including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/angiotensin receptor-neprilysin inhibitors, β-blockers, aldosterone antagonists, and sodium glucose cotransporter-2 inhibitors, on top of adequate volume control with diuretics [90, 91]. The PRIME study showed that the reverse remodeling caused by angiotensin receptor-neprilysin inhibitors can reduce severity of secondary MR [71]. Other combined conditions that are related to heart failure need to be controlled according to the updated guidelines [90, 91]. Device therapy such as cardiac resynchronization therapy and optimal coronary artery revascularization are also helpful for reducing secondary MR.
-
(4)
Timing of intervention
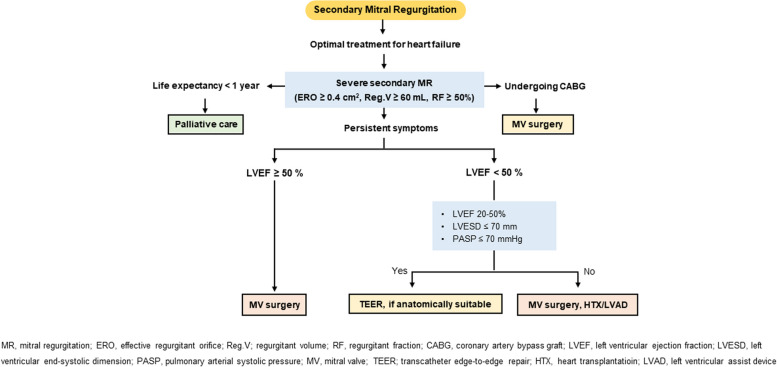
Figure 3 shows the decision-making process for secondary MR treatment. If symptoms persist after optimization of conventional heart failure therapy, further interventions should be evaluated before the deterioration of underlying LV disease [108, 117, 118]. The importance of decision-making by a multidisciplinary heart team consisting of a valve specialist, heart failure specialist, cardiac interventionist, and heart valve surgeon needs to be emphasized in this step [1, 2, 119]. When a patient has persistent symptoms despite the optimal treatment of heart failure, TEER or MV surgery is required according to the adequate indication [118, 120, 121]. For patients with severe comorbidities or life expectancy less than 1 year, palliative care could be considered.
Fig. 3.
Treatment of secondary mitral regurgitation (MR). CABG, coronary artery bypass graft; ERO, effective regurgitant orifice; HTX, heart transplantation; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; MV, mitral valve; PASP, pulmonary arterial systolic pressure; Reg.V; regurgitant volume; RF, regurgitant fraction; TEER; transcatheter edge-to-edge repair
-
(5)
Choice of intervention
When patients with severe secondary MR and LV systolic dysfunction (LVEF < 50%) have persistent symptoms despite the optimal GDMT of heart failure, TEER with MitraClip system is reasonable provided that the appropriate anatomy is ensured (20% ≤ LVEF ≤ 50%, LV end-systolic dimension ≤ 70 mm, pulmonary artery systolic pressure ≤ 70 mmHg) [119, 121–124]. Two previous randomized controlled trials (COAPT and MITRA-FR) evaluated the safety and efficacy of TEER in patients with symptomatic heart failure and severe secondary MR. Both studies proved that the procedure is safe and effectively reduces severity of MR [120–123]. However, only the COAPT trial showed benefit in primary endpoint of all-cause mortality or heart failure hospitalization owing to the greater severity of secondary MR and lesser extent of LV dilatation (disproportionate severe MR) [118, 121–123].
The surgical approach needs to be personalized according to each circumstance. In patients with severe secondary MR who require coronary artery bypass graft due to myocardial ischemia, concomitant MV surgery is reasonable [93, 125–127]. In selected patients without advanced LV remodeling, MV repair with an undersized complete rigid ring can be performed to restore valve competence, improve symptoms, and reverse LV remodeling [102]. Chordal sparing valve replacement may be considered in patients with echocardiographic predictors of repair failure [126, 128, 129]. Isolated MV surgery in severe secondary MR needs special consideration owing to high combined risk, high recurrence rate, and the absence of proven survival benefit. In cases of atrial MR, which is often associated with AF, ring annuloplasty with AF ablation can be beneficial [3].
In severe secondary MR, optimal GDMT according to heart failure status should be ensured. When symptoms persist despite appropriate management, TEER or surgical MV repair/replacement can be considered. A multidisciplinary heart team approach is especially crucial in secondary MR due to the disease’s complexity.
Tricuspid regurgitation
Etiology
Less than moderate tricuspid regurgitation (TR) is frequent with normal tricuspid valve (TV) structure. However, significant TR is not uncommon. In a community-based study, moderate and severe TR was 5% and 0.6%, respectively [130]. In an asymptomatic Korean population on health checkup, moderate and severe TR was observed in approximately 0.3% [131]. TR was more prevalent in the elderly (75 years or more) and in women [131]. There is growing concern about TR with the increase in the aging population. Significant TR is also associated with poor long-term outcomes [132].
The etiology of TR can be divided into primary TR, caused by abnormal TV leaflets, and secondary TR, caused by tricuspid annular dilatation and leaflet tethering in the presence of normal leaflets (Table 6) [1]. Primary causes of TR involve the tricuspid apparatus. They include rheumatic disease, infective endocarditis, myxomatous disease, carcinoid syndrome, cancer involvement, radiation, drugs, congenital diseases such as Ebstein anomaly, chest trauma, and trauma including iatrogenic damage. Recently, significant TR due to cardiac implantable electronic device leads or endomyocardial biopsies is also increasing and can be found in 20% to 30% of the patients who underwent those procedures [133–135].
Table 6.
Etiology of TR
| Primary TR | Secondary TR |
|---|---|
| Rheumatic | Pulmonary hypertension with RV remodeling |
| Infective endocarditis | Primary pulmonary arterial hypertension |
| Myxomatous disease | Secondary pulmonary hypertension (left-sided heart disease) |
| Carcinoid syndrome | Tricuspid annular dilation (associated with AF) |
| Cancer involvement | RV volume overload (shunt/high output) |
| Radiation or drugs | RV dysfunction (cardiomyopathies or ischemic insult) |
| Congenital disease (e.g., Ebstein anomaly) | |
| Chest trauma | |
| Iatrogenic (CIED, endomyocardial biopsy) |
TR Tricuspid regurgitation, RV Right ventricle, AF Atrial fibrillation, CIED Cardiac implantable electronic devices
Secondary TR is more common (≥ 90%) and is mainly related to the setting of RV remodeling. RV remodeling is frequently induced by pressure overload due to pulmonary hypertension either primary or secondary to left-sided heart disease, volume overload due to left–right shunting, RV dysfunction related to cardiomyopathies or ischemic insult. Furthermore, around 10% of patients with secondary TR have “isolated” TR without significant pulmonary hypertension (pulmonary artery systolic pressure < 50 mmHg), with normal LV systolic function (LVEF > 60%) and no left-sided valves disease, and with normal appearing TV leaflets; “isolated” TR is mainly attributable to AF and dilatation of the right atrial (RA) and tricuspid annulus [1, 136]. Secondary TR accompanying RV remodeling is called “ventricular TR” and when remodeling of RA and annulus due to AF is dominant, it is called “atrial TR”. Secondary TR can develop late after left-sided valve surgery, mostly related to AF [137–139].
Stages
The stages of TR are based on valve and annulus structure and valve hemodynamics, and the criteria for severe TR are VCW ≥ 7 mm, EROA ≥ 40 mm2, regurgitant volume ≥ 45 mL, and regurgitant fraction (RF) ≥ 50% [1]. Table 7 demonstrates the stages of TR. Severe TR is further classified into severe, massive, or torrential TR according to the degree of TR quantification [140, 141].
Table 7.
Stages of TR
| Characteristic | Stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| Definition | At risk | Progressive | Asymptomatic severe | Symptomatic severe | |||||
| Severity | Normal to trivial | Mild | Moderate | Severe | Massive | Torrential | |||
| Echocardiography morphology | |||||||||
| Leaflet | Normal | Mild to moderate thickening | Severe thickening, flail, perforation, retraction | ||||||
| Coaptation | Normal, CIED, AF | Limited, prolapse, lead impingement | Severe loss, prolapse | ||||||
| Annulus | Normal | Dilation | Dilation | ||||||
| VCW (mm) | - | < 3 | 3–6 | 7–13 | 14–20 | ≥ 21 | |||
| EROA (cm2) | - | < 0.2 | 0.2–0.4 | 0.4–0.6 | 0.6–0.8 | ≥ 0.8 | |||
| PISA radiusa (mm) | - | ≤ 5 | 6–8 | ≥ 9 | |||||
| Regurgitant volume (mL) | - | < 30 | 30–45 | 45–59 | 60–74 | ≥ 75 | |||
| Regurgitant fraction (%) | - | < 30 | 30–49 | ≥ 50 | |||||
| RA/RV size | - | Normal or ↑ | ↑ | ||||||
| Hepatic vein flow | - | Systolic dominance | Systolic blunting | Systolic flow reversal | |||||
| TR-CW Doppler jet | - | Faint/ parabolic | Dense/ parabolic | Dense, triangular | |||||
| Symptom | None | None | None | DOE, EI, right HF | |||||
TR Tricuspid regurgitation, CIED Cardiac implantable electronic devices, AF Atrial fibrillation, VCW Vena contracta width, EROA Effective regurgitant orifice area by 2D-PISA method, PISA Proximal isovelocity surface area, RA Right atrium, RV Right ventricle, CW Continuous wave, DOE Dyspnea on exertion, EI Exercise intolerance, HF Right heart failure
aRadius at Nyquist limit shift of 28 cm/sec
Korean data
A recent nationwide hospital-based retrospective cohort study from the Korean Valve Survey collected clinical and echocardiographic data on valvular heart disease from 45 medical centers [28]. Secondary TR accounted for 90% of TR in this valve survey. In Korean patients with moderate or more TR, half of the patients had AF or hypertension. Half of them had MR.
Korean researchers have conducted numerous studies on risk factors, imaging parameters, and prognosis of TR [142–154]. In a study of 299 patients who underwent successful PMV for MS, development of significant TR was associated with MV restenosis [142]. Age, female sex, rheumatic etiology, AF, and peak pressure gradient of TR at follow-up were the independent risk factors for the development of late significant TR after successful left-side valve surgery [138]. The degree of TR and some quantitative parameters of TR have been shown to predict adverse clinical outcomes. In a study of 429 patients who underwent TTE before and after pacemaker implantation, pacemaker-related TR was found in 9.8% and was associated with AF, history of open-heart surgery, and poorer cardiovascular outcomes [143]. In patients with severe isolated TR, VCW > 7 mm was a powerful independent predictor of adverse outcomes [144]. In another study that compared the VCW of 2D echocardiography with 3D echocardiography in functional TR, different VCW cutoff values were suggested because the cross-sectional shape of the VCW was ellipsoidal with a long anteroposterior direction [145]. In addition, it was demonstrated that VCW was determined by annular dilation and leaflet tenting in the corresponding directions. In a study with real-time 3D echocardiography, TR severity was determined by septal leaflet tethering, septal-lateral annular dilatation, and the severity of pulmonary hypertension [146].
In a total of 213 patients with moderate or severe TR, a cutoff value of 40 mm for tricuspid annulus was the best predictor for cardiovascular events [147]. In patients with severe isolated TR undergoing TV surgery, reduced RV global longitudinal strain [148], reduced peak atrial longitudinal strain [149], and preoperative RV free-wall longitudinal strain were associated with poor clinical outcomes [150]. In atrial functional TR, baseline RA enlargement, and RA area to RV end-systolic area ratio were strong risk factors for progression of atrial functional TR [151].
The role of CMR was also studied in TR. In 75 patients with severe functional TR, preoperative assessment of CMR-based RV ejection fraction provided independent and incremental prognostic information in patients undergoing corrective surgery for severe functional TR [152]. TR fraction by CMR was independently associated with adverse clinical outcomes in patients with heart failure with reduced ejection fraction [153].
Some imaging parameters were related to a better surgical approach. In 238 patients who underwent stand-alone TV surgery (repair, 132; replacement, 106) for severe TR, a trend favoring replacement was shown in patients with annular diameter > 44 mm compared to repair [154].
Diagnosis and follow-up
Diagnosis
TTE is most important to determine the etiology and severity of TR as well as for routine follow-up [1, 2]. CMR imaging can be used as an auxiliary means for determination of the severity of TR, as well as for volumetric assessment and tissue characterization of the RV. Invasive cardiac catheterization for measurement of the pulmonary artery pressures, pulmonary vascular resistance, right-sided pressures, and cardiac index can be useful when clinical and noninvasive data are inadequate.
The severity of TR can be determined by a comprehensive consideration of multiple criteria, either semiquantitatively or quantitatively, as shown in Table 7. Mild TR usually does not have hemodynamic significance, while severe TR leads to RV and RA dilatation, remodeling, elevated systemic venous pressure, and eventually symptoms. While the current guidelines classify the severity of TR as mild, moderate, and severe [1, 2], there is increasingly a consensus that a spectrum of TR severity exists within the grade of “severe TR” which is inadequately reflected in the current grading scheme, especially with the advent of the transcatheter repair for TR. Thus, a new grading scheme increasing the grades to include massive and torrential TR has been proposed, using VCW and EROA [140, 141]. Due to the noncircular shape of the TR regurgitant orifice, measurement of the biplane VCW can be helpful [145]. The 3D regurgitant orifice area can be measured, but reported cutoffs have been diverging. The severity of TR can be dynamic and is affected by changes in preload and pulmonary artery pressure, and follow-up after optimal medical therapy should be done.
Besides the severity of TR, pulmonary artery pressures should be ascertained, either noninvasively with Doppler echocardiography or invasively with cardiac catheterization. Pulmonary artery systolic pressure can be estimated from the maximum TR velocity by continuous wave Doppler. However, the severity of pulmonary hypertension may be underestimated in patients with severe TR. In patients with suspected pulmonary hypertension of clinical significance or uncertain cause, invasive measurement of pulmonary artery pressures and pulmonary vascular resistance is essential for guiding treatment. The measurement of cardiac output using the thermodilution technique may be inaccurate with severe TR, and the Fick method using arterial and mixed venous oxygen content is recommended.
Also, annular dilatation is a risk factor for late TR in patients undergoing left-sided valve surgery. The tricuspid annulus should be measured in the RV-focused apical view at end-diastole, and it is reasonable for those with tricuspid annulus diameter > 40 mm (or 21 mm/m2) to receive concomitant TV repair at the time of left-sided valve surgery [1, 2]. RV function should be assessed, and normal RV systolic function is defined by several parameters including tricuspid annular plane systolic excursion > 16 mm, tricuspid annular systolic velocity > 10 cm/sec, and RV end-systolic area < 20 cm2 or fractional area change > 35% [1, 2]. RV strain and 3D echocardiographic measurements of the RV volumes can be considered to overcome the limitations of these conventional RV function indices [148, 150, 151]. CMR can provide more accurate volumetric measurements of the RV and assessment of RV function and tissue characterization [152, 155, 156].
Follow-up
The severity of TR and its consequences should be reassessed every 3 to 5 years in patients with mild TR, every 1 to 2 years in patients with moderate TR, and every 6 to 12 months in patients with severe TR. Similar to MR, TR is often dynamic according to volume status, changes in left-side chambers, and pulmonary artery systolic pressure. Therefore, it is recommended to perform a repeat TTE in case of significant changes in volumes and hemodynamic status.
Medical therapy
Severe TR can lead to signs and symptoms of right-sided heart failure, such as peripheral edema, ascites, fatigue, and indigestion. Lifestyle changes such as a low-salt diet and support stockings can be helpful. Medical therapies for severe TR itself are limited, and the mainstay is diuretics [1, 2]. The appropriate use of diuretics is important to decrease volume overload in severe TR. Loop diuretics are integral in treatment, but use can be limited due to worsening low cardiac output in the presence of RV dysfunction. Adding aldosterone antagonists can be beneficial, especially as hepatic congestion from right-sided heart failure can promote activation of the renin–angiotensin–aldosterone system.
In cases of severe secondary TR, therapies to treat the primary cause can be useful and may mitigate TR [1, 2]. Pulmonary vasodilators for selected patients with pulmonary arterial hypertension can reduce pulmonary vascular resistance and resulting RV pressure overload. GDMT for patients with heart failure with reduced LVEF is effective for reducing RV volume and pressure overload and alleviating secondary TR. Rhythm control for AF may be helpful for preventing TR development related to tricuspid annular dilatation [139].
Timing of intervention
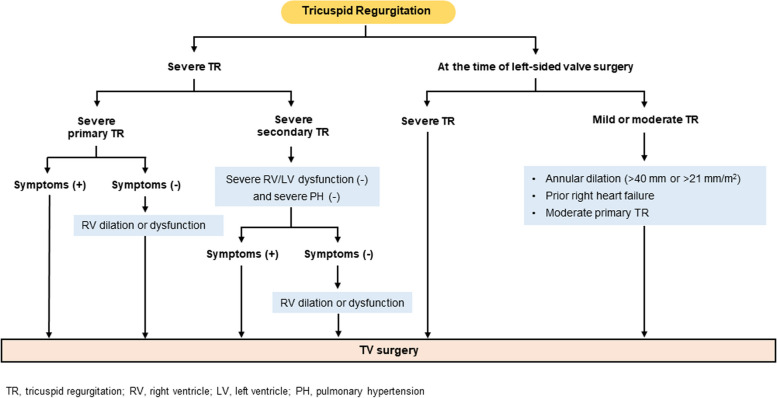
Trivial or mild TR in patients with normal valves is commonly observed in routine echocardiography examinations and is of no clinical consequence. However, significant TR is associated with poor survival [157, 158]. Severe TR should be intervened before development of irreversible end-organ damage, such as advanced RV dysfunction, hepatic failure, and renal failure, which also increase surgical risk and affect postsurgical outcomes [159, 160]. Recommendations for intervention in TR are shown in Fig. 4. The latest ACC/AHA guideline [1] and ESC/EACTS guidelines [2] on the management of valvular heart disease mostly agree on the timing of intervention for TR, although the ESC/EACTS guidelines recommend earlier TV surgery for asymptomatic patients with severe TR [2].
Fig. 4.
Treatment of tricuspid regurgitation (TR). LV, left ventricle; PH, pulmonary hypertension; RV, right ventricle; TV, tricuspid valve
In patients undergoing left-sided valve surgery, TV surgery should be performed liberally, especially considering the risk of late TR development [137–139]. In patients undergoing left-sided valve surgery, TV surgery is recommended in those with severe primary or secondary TR, regardless of symptoms [1, 2]. In these patients, TV surgery is also reasonable for those with mild or moderate TR and tricuspid annular dilatation (> 40 mm or 21 mm/m2), prior symptoms of right-sided heart failure, or moderate primary TR [1, 2].
In patients with symptomatic severe primary TR, TV surgery is recommended before the onset of severe RV dysfunction [1, 2]. In patients with asymptomatic severe primary TR with acceptable surgical risk, surgery should be considered when progressive RV dilatation or dysfunction is observed [1, 2]. However, exact thresholds have not been established. In otherwise healthy patients without other comorbidities and in the absence of RV dysfunction or pulmonary hypertension, the surgical risk associated with isolated TV surgery is low (< 1%–2% operative mortality) [1].
In patients with severe secondary TR, the benefit of isolated TV surgery is less well-established [161], and operative mortality is non-negligible, at around 10% [159, 162, 163]. However, with careful patient selection in the modern era, TV surgery can be performed with lower mortality than traditionally reported (< 4%–5%) [1, 164]. A prerequisite for consideration of TV surgery in severe secondary TR is the absence of severe RV and LV dysfunction and severe pulmonary hypertension [1, 2]: these patients should be treated medically due to the risk of postinterventional RV failure. Both the ACC/AHA guideline [1] and the ESC/EACTS guidelines [2] consider TV surgery to be reasonable in patients with symptomatic severe secondary TR. However, in patients with asymptomatic severe secondary TR, only the European guideline states that TV surgery is reasonable in those with RV dilatation [2]. In patients with previous left-sided valve surgery and symptomatic severe TR, isolated TV surgery is also reasonable and should not be delayed to improve the poor postoperative prognosis and high mortality rates related to reoperation and late referral [2].
Choice of intervention
In TV surgery, valve repair, namely annuloplasty with prosthetic rings, is preferable to valve replacement whenever possible, and valve replacement should only be considered when there is severe TV leaflet tethering and annular dilatation or leaflet abnormality precluding valve repair [1, 2]. Observational data have shown that TV repair is associated with lower surgical risk than TV replacement, but there may be a selection bias, as TV replacement would be done in patients with a severely dilated annulus or abnormal leaflets [165, 166]. In the case of TV replacement, the selection of bioprosthetic or mechanical valve should be done considering the risk of lifelong anticoagulation and the risk of reoperation [167–169]. A recent prospective randomized trial of tricuspid TEER for severe TR showed that tricuspid TEER was safe for patients with severe TR, reduced the severity of TR, and was associated with an improvement of quality of life [170]. In Korea, clinical application of tricuspid TEER to high-risk patients in surgery is expected to begin in the near future.
Conclusions
In the absence of guidelines tailored to the Korean population, clinical treatment has faced several limitations in dealing with MR, MS, and TR. This position paper on valvular heart disease complies research results in Korea and combines foreign guidelines to provide guidance on diagnosis and treatment of MS, MR, and TR. Based on this position paper, further interest and research on clinical unmet needs are warranted in the future.
Acknowledgements
This position paper reflects data from the Korean Valve Survey conducted with the participation of 45 institutions in Korea and the contents of the Korean valve practical survey supported by the Korean Society of Echocardiography.
List of participating centers and principal investigators of the Korean Valve Survey
Kye Hun Kim, MD, PhD, Chonnam National University Hospital; Wook-Jin Chung, MD, PhD, Gachon University Gil Medical Center; Chan Seok Park, The Catholic University of Korea, Bucheon St. Mary’s Hospital; Hyo-Suk Ahn, MD, PhD; The Catholic University of Korea, Uijeongbu St. Mary’s Hospital; Woo-Baek Chung, MD, PhD, The Catholic University of Korea, Seoul St. Mary’s Hospital; Eun Joo Cho, MD, PhD, The Catholic University of Korea, Yeouido St. Mary’s Hospital; Jung Sun Cho, MD, PhD, The Catholic University of Korea, Daejeon St. Mary’s Hospital; Dong Ryeol Ryu, MD, PhD, Kangwon National University School of Medicine and Kangwon National University Hospital; Dong Heon Yang, MD, PhD, Kyungpook National University Hospital; Jeong Rang Park, MD, PhD, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital; Woo-Shik Kim, MD, PhD, Kyung Hee University Healthcare System; Il Suk Sohn, MD, PhD, Kyung Hee University Hospital at Gangdong; In-Cheol Kim, MD, PhD, Hyungseop Kim, MD, PhD, Keimyung University Dongsan Medical Center; Jin Oh Na, MD, PhD, Korean University Guro Hospital; Seong-Mi Park, MD, PhD, Korea University Anam Hospital; Sun Ho Hwang, MD, Gwangju Veterans Hospital; Ji-Yong Choi, MD, PhD, Daegu Catholic University Medical Center; Tae-Ho Park, MD, PhD, Dong-A University Medical Center; Yong Hyun Park, MD, PhD, Pusan National University Yangsan Hospital; Jung Hyun Choi, MD, Pusan National University Hospital; Hack-Lyoung Kim, MD, PhD, Seoul Metropolitan Government-Seoul National University Boramae Medical Center; Jun-Bean Park, MD, PhD, Seoul National University Hospital; Eun Kyoung Kim, MD, PhD, Samsung Medical Center; Hye Sun Seo, MD, PhD, Soonchunhyang University Bucheon Hospital; Jin-Sun Park, MD, PhD, Ajou University School of Medicine; Jung-Woo Son, MD, Wonju Severance Christian Hospital, Yonsei University College of Medicine; Chi Young Shim, MD, PhD, Severance Cardiovascular Hospital, Yonsei University College of Medicine; Eui-Young Choi, MD, PhD, Gangnam Severance Hospital, Yonsei University College of Medicine; Jang-Won Son, MD, PhD, Yeungnam University Hospital; Shin-Jae Kim, MD, PhD, Ulsan University Hospital; Sahmin Lee, MD, PhD, Asan Medical Center; Sang Jae Rhee, MD, PhD, Wonkwang University Hospital; In-Jeong Cho, MD, PhD, Ewha Womans University Seoul Hospital; Young Sup Byun, MD, Inje University Sanggye Paik Hospital; Jeong-Sook Seo, MD, PhD, Inje University Busan Paik Hospital; Sung-Hee Shin, MD, PhD, Inha University Hospital; Se-Jung Yoon, MD, PhD, National Health Insurance Service Ilsan Hospital; Sun Hwa Lee, MD, PhD, Jeonbuk National University Hospital; Jong Wook Beom, MD, Jeju National University Hospital; Byung Joo Sun, MD, PhD; Chungnam National University Hospital and Chungnam National University School of Medicine; Ju-Hee Lee, MD, PhD, Dae-Hwan Bae, MD, Chungbuk National University Hospital, Chungbuk National University College of Medicine; Sung-Ai Kim, MD, Hallym Sacred Heart Hospital and Hallym University College of Medicine; Dae Gyun Park, MD, PhD, Hallym University Kangdong Sacred Heart Hospital; Min-Kyung Kang, MD, PhD, Hallym University Kangnam Sacred Heart Hospital; Kyung-Soon Hong, MD, PhD, Hallym University Chuncheon Sacred Heart Hospital; Ran Heo, MD, PhD, Hanyang University Medical Center, Hanyang University College of Medicine.
Abbreviations
- ACC/AHA
American College of Cardiology/American Heart Association
- AF
Atrial fibrillation
- BNP
Brain natriuretic peptide
- CMR
Cardiac magnetic resonance
- CT
Computed tomography
- D
Dimensional
- ERO
Effective regurgitant orifice
- EROA
Effective regurgitant orifice area
- ESC/EACTS
European Society of Cardiology/European Association for Cardio-Thoracic Surgery
- GDMT
Guideline-directed medical therapy
- HIRA
Korean Health Insurance Review and Assessment Service
- KSE
Korean Society of Echocardiography
- LA
Left atrium
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- MAC
Mitral annular calcification
- MDPG
Mean diastolic pressure gradient
- MR
Mitral regurgitation
- MS
Mitral stenosis
- MV
Mitral valve
- MVA
Mitral valve area
- MVP
Mitral valve prolapse
- PMV
Percutaneous mitral valvuloplasty
- RA
Right atrial
- RV
Right ventricular
- TEE
Transesophageal echocardiography
- TEER
Transcatheter edge-to-edge repair
- TR
Tricuspid regurgitation
- TTE
Transthoracic echocardiography
- TV
Tricuspid valve
- VCW
Vena contract width
Authors’ contributions
Conceptualization: JHP, KHK, DHK, JWH, HK; Data curation: JWS, HYK; Formal analysis: HK; Funding acquisition: DHK; Investigation: CYS, EKK, DHC, JBP, JSS, ICK, Sang-Hyun Lee, RH, HJL, SL, BJS, SJY, Sun Hwa Lee, HYK, HMK, KHK, HK; Project administration: KHK, DHK, HK; Resources: JWH; Supervision: Sun Hwa Lee, JHP, GRH, HOJ, YJK, KHK, JWH, HK; Visualization: Sang-Hyun Lee, HJL, SJY; Writing–original draft: CYS, EKK, DHC, JBP, JSS, JWS, ICK, Sang-Hyun Lee, RH, HJL, SL, BJS, SJY, Sun Hwa Lee, HYK, HMK; Writing–review & editing: CYS, EKK, RH, BJS, GRH, HOJ, YJK, HK. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are included in this manuscript. Additional data used to support the findings of this review are also available in this published article.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyungseop Kim, Email: khyungseop@dsmc.or.kr.
The Korean Valve Survey Investigators:
Wook-Jin Chung, Chan Seok Park, Hyo-Suk Ahn, Woo-Baek Chung, Eun Joo Cho, Jung Sun Cho, Dong Ryeol Ryu, Dong Heon Yang, Jeong Rang Park, Woo-Shik Kim, Il Suk Sohn, Jin Oh Na, Seong-Mi Park, Sun Ho Hwang, Ji-Yong Choi, Tae-Ho Park, Yong Hyun Park, Jung Hyun Choi, Hack-Lyoung Kim, Hye Sun Seo, Jin-Sun Park, Eui-Young Choi, Jang-Won Son, Shin-Jae Kim, Sang Jae Rhee, In-Jeong Cho, Young Sup Byun, Sung-Hee Shin, Sun Hwa Lee, Jong Wook Beom, Ju-Hee Lee, Dae-Hwan Bae, Sung-Ai Kim, Dae Gyun Park, Min-Kyung Kang, and Kyung-Soon Hong
References
- 1.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 3.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 4.Jang SY, Ju EY, Seo SR, Choi JY, Park SJ, Kim DK, et al. Changes in the etiology of valvular heart disease in the rapidly aging Korean population. Int J Cardiol. 2014;174:355–359. doi: 10.1016/j.ijcard.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. doi: 10.1016/S0022-5223(19)37390-8. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Lee WK, Shim WH, Cho SY, Tahk SJ, Kim SS. Percutaneous mitral valvuloplasty using the double balloon technique: immediate results and determinant factors of increasing mitral regurgitation. Korean J Intern Med. 1991;6:51–57. doi: 10.3904/kjim.1991.6.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JS, Joung B, Kang SM, Ha JW, Ko YG, Choi D, et al. Comparison of long-term(over 10 years) outcome of percutaneous mitral balloon valvuloplotomy between moderate and severe mitral stenosis. Korean Circ J. 2006;36:208–213. doi: 10.4070/kcj.2006.36.3.208. [DOI] [Google Scholar]
- 8.Kim D, Chung H, Nam JH, Park DH, Shim CY, Kim JS, et al. Predictors of long-term outcomes of percutaneous mitral valvuloplasty in patients with rheumatic mitral stenosis. Yonsei Med J. 2018;59:273–278. doi: 10.3349/ymj.2018.59.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JK, Song JM, Kang DH, Yun SC, Park DW, Lee SW, et al. Restenosis and adverse clinical events after successful percutaneous mitral valvuloplasty: immediate post-procedural mitral valve area as an important prognosticator. Eur Heart J. 2009;30:1254–1262. doi: 10.1093/eurheartj/ehp096. [DOI] [PubMed] [Google Scholar]
- 10.Kang DH, Park SW, Song JK, Kim HS, Hong MK, Kim JJ, et al. Long-term clinical and echocardiographic outcome of percutaneous mitral valvuloplasty: randomized comparison of Inoue and double-balloon techniques. J Am Coll Cardiol. 2000;35:169–175. doi: 10.1016/S0735-1097(99)00502-1. [DOI] [PubMed] [Google Scholar]
- 11.Reddy YN, Murgo JP, Nishimura RA. Complexity of defining severe "stenosis" from mitral annular calcification. Circulation. 2019;140:523–525. doi: 10.1161/CIRCULATIONAHA.119.040095. [DOI] [PubMed] [Google Scholar]
- 12.Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol. 2015;66:1934–1941. doi: 10.1016/j.jacc.2015.08.872. [DOI] [PubMed] [Google Scholar]
- 13.Elmariah S, Budoff MJ, Delaney JA, Hamirani Y, Eng J, Fuster V, et al. Risk factors associated with the incidence and progression of mitral annulus calcification: the multi-ethnic study of atherosclerosis. Am Heart J. 2013;166:904–912. doi: 10.1016/j.ahj.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanjanauthai S, Nasir K, Katz R, Rivera JJ, Takasu J, Blumenthal RS, et al. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2010;213:558–562. doi: 10.1016/j.atherosclerosis.2010.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Shim CY, Hong GR, Jeong H, Ha JW. Morphological and functional characteristics of mitral annular calcification and their relationship to stroke. PLoS One. 2020;15:e0227753. doi: 10.1371/journal.pone.0227753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patlolla SH, Schaff HV, Nishimura RA, Geske JB, Lahr BD, Lee AT, et al. Mitral annular calcification in obstructive hypertrophic cardiomyopathy: prevalence and outcomes. Ann Thorac Surg. 2022;114:1679–1687. doi: 10.1016/j.athoracsur.2021.09.077. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Seo J, Cho I, Hong GR, Ha JW, Shim CY. Prognostic implication of mitral valve disease and its progression in east asian patients with hypertrophic cardiomyopathy. J Am Heart Assoc. 2023;12:e024792. doi: 10.1161/JAHA.121.024792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Shim CY, Kim YJ, Chang BC. Complicated infective endocarditis on mitral annular calcification with left ventricular pseudoaneurysm. Eur J Cardiothorac Surg. 2018;53:886. doi: 10.1093/ejcts/ezx434. [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Vasan RS, Parise H, Levy D, O'Donnell CJ, D'Agostino RB, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.CIR.0000058168.26163.BC. [DOI] [PubMed] [Google Scholar]
- 20.Kato N, Guerrero M, Padang R, Amadio JM, Eleid MF, Scott CG, et al. Prevalence and natural history of mitral annulus calcification and related valve dysfunction. Mayo Clin Proc. 2022;97:1094–1107. doi: 10.1016/j.mayocp.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Seo J, Jeong H, Cho I, Hong GR, Ha JW, Shim CY. Sex differences in mitral annular calcification and the clinical implications. Front Cardiovasc Med. 2021;8:736040. doi: 10.3389/fcvm.2021.736040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pressman GS, Rodriguez-Ziccardi M, Gartman CH, Obasare E, Melendres E, Arguello V, et al. Mitral annular calcification as a possible nidus for endocarditis: a descriptive series with bacteriological differences noted. J Am Soc Echocardiogr. 2017;30:572–578. doi: 10.1016/j.echo.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Bertrand PB, Churchill TW, Yucel E, Namasivayam M, Bernard S, Nagata Y, et al. Prognostic importance of the transmitral pressure gradient in mitral annular calcification with associated mitral valve dysfunction. Eur Heart J. 2020;41:4321–4328. doi: 10.1093/eurheartj/ehaa819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churchill TW, Yucel E, Deferm S, Levine RA, Hung J, Bertrand PB. Mitral valve dysfunction in patients with annular calcification: JACC review topic of the week. J Am Coll Cardiol. 2022;80:739–751. doi: 10.1016/j.jacc.2022.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pressman GS, Ranjan R, Park DH, Shim CY, Hong GR. Degenerative mitral stenosis versus rheumatic mitral stenosis. Am J Cardiol. 2020;125:1536–1542. doi: 10.1016/j.amjcard.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Choi YJ, Son JW, Kim EK, Kim IC, Kim HY, Seo JS, et al. Epidemiologic profile of patients with valvular heart disease in korea: a nationwide hospital-based registry study. J Cardiovasc Imaging. 2023;31:51–61. doi: 10.4250/jcvi.2022.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang DH, Lee CH, Kim DH, Yun SC, Song JM, Lee CW, et al. Early percutaneous mitral commissurotomy vs. conventional management in asymptomatic moderate mitral stenosis. Eur Heart J. 2012;33:1511–7. doi: 10.1093/eurheartj/ehr495. [DOI] [PubMed] [Google Scholar]
- 30.Kim JY, Kim SH, Myong JP, Choi Y, Hwang YM, Kim TS, et al. Ten-year trends in the incidence, treatment and outcomes of patients with mitral stenosis in Korea. Heart. 2020;106:746–750. doi: 10.1136/heartjnl-2019-315883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang DH, Park SJ, Lee SA, Lee S, Kim DH, Park DW, et al. Early percutaneous mitral commissurotomy or conventional management for asymptomatic mitral stenosis: a randomised clinical trial. Heart. 2021;107:1980–1986. doi: 10.1136/heartjnl-2021-319857. [DOI] [PubMed] [Google Scholar]
- 32.Ko KY, Cho I, Kim S, Seong Y, Kim DY, Seo JW, et al. Identification of distinct subgroups in moderately severe rheumatic mitral stenosis using data-driven phenotyping of longitudinal hemodynamic progression. J Am Heart Assoc. 2022;11:e026375. doi: 10.1161/JAHA.121.026375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Reis G, Motta MS, Barbosa MM, Esteves WA, Souza SF, Bocchi EA. Dobutamine stress echocardiography for noninvasive assessment and risk stratification of patients with rheumatic mitral stenosis. J Am Coll Cardiol. 2004;43:393–401. doi: 10.1016/j.jacc.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Bouleti C, Iung B, Laouénan C, Himbert D, Brochet E, Messika-Zeitoun D, et al. Late results of percutaneous mitral commissurotomy up to 20 years: development and validation of a risk score predicting late functional results from a series of 912 patients. Circulation. 2012;125:2119–2127. doi: 10.1161/CIRCULATIONAHA.111.055905. [DOI] [PubMed] [Google Scholar]
- 36.Nunes MC, Tan TC, Elmariah S, do Lago R, Margey R, Cruz-Gonzalez I, et al. The echo score revisited: impact of incorporating commissural morphology and leaflet displacement to the prediction of outcome for patients undergoing percutaneous mitral valvuloplasty. Circulation. 2014;129:886–95. doi: 10.1161/CIRCULATIONAHA.113.001252. [DOI] [PubMed] [Google Scholar]
- 37.Abascal VM, Wilkins GT, O'Shea JP, Choong CY, Palacios IF, Thomas JD, et al. Prediction of successful outcome in 130 patients undergoing percutaneous balloon mitral valvotomy. Circulation. 1990;82:448–456. doi: 10.1161/01.CIR.82.2.448. [DOI] [PubMed] [Google Scholar]
- 38.Sagie A, Freitas N, Padial LR, Leavitt M, Morris E, Weyman AE, et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol. 1996;28:472–479. doi: 10.1016/S0735-1097(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 39.Movva R, Murthy K, Romero-Corral A, Seetha Rammohan HR, Fumo P, Pressman GS. Calcification of the mitral valve and annulus: systematic evaluation of effects on valve anatomy and function. J Am Soc Echocardiogr. 2013;26:1135–1142. doi: 10.1016/j.echo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Chu JW, Levine RA, Chua S, Poh KK, Morris E, Hua L, et al. Assessing mitral valve area and orifice geometry in calcific mitral stenosis: a new solution by real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:1006–1009. doi: 10.1016/j.echo.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urena M, Himbert D, Brochet E, Carrasco JL, Iung B, Nataf P, et al. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves: a step-by-step approach. JACC Cardiovasc Interv. 2017;10:1905–1919. doi: 10.1016/j.jcin.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 42.Kim JY, Kim SH, Myong JP, Kim YR, Kim TS, Kim JH, et al. Outcomes of direct oral anticoagulants in patients with mitral stenosis. J Am Coll Cardiol. 2019;73:1123–1131. doi: 10.1016/j.jacc.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 43.Omran H, Rang B, Schmidt H, Illien S, Schimpf R, Maccarter D, et al. Incidence of left atrial thrombi in patients in sinus rhythm and with a recent neurologic deficit. Am Heart J. 2000;140:658–662. doi: 10.1067/mhj.2000.109213. [DOI] [PubMed] [Google Scholar]
- 44.Manjunath CN, Srinivasa KH, Panneerselvam A, Prabhavathi B, Ravindranath KS, Rangan K, et al. Incidence and predictors of left atrial thrombus in patients with rheumatic mitral stenosis and sinus rhythm: a transesophageal echocardiographic study. Echocardiography. 2011;28:457–460. doi: 10.1111/j.1540-8175.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamoorthy KM. Incidence and predictors of left atrial thrombus in patients with rheumatic mitral stenosis and sinus rhythm: a transesophageal echocardiographic study. Echocardiography. 2012;29:876–877. doi: 10.1111/j.1540-8175.2012.01702.x. [DOI] [PubMed] [Google Scholar]
- 46.Olesen KH. The natural history of 271 patients with mitral stenosis under medical treatment. Br Heart J. 1962;24:349–357. doi: 10.1136/hrt.24.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowe JC, Bland EF, Sprague HB, White PD. The course of mitral stenosis without surgery: ten- and twenty-year perspectives. Ann Intern Med. 1960;52:741–749. doi: 10.7326/0003-4819-52-4-741. [DOI] [PubMed] [Google Scholar]
- 48.Horstkotte D, Niehues R, Strauer BE. Pathomorphological aspects, aetiology and natural history of acquired mitral valve stenosis. Eur Heart J. 1991;12 Suppl B:55–60. doi: 10.1093/eurheartj/12.suppl_B.55. [DOI] [PubMed] [Google Scholar]
- 49.Chiang CW, Lo SK, Ko YS, Cheng NJ, Lin PJ, Chang CH. Predictors of systemic embolism in patients with mitral stenosis A prospective study. Ann Intern Med. 1998;128:885–889. doi: 10.7326/0003-4819-128-11-199806010-00001. [DOI] [PubMed] [Google Scholar]
- 50.Diker E, Aydogdu S, Ozdemir M, Kural T, Polat K, Cehreli S, et al. Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease. Am J Cardiol. 1996;77:96–98. doi: 10.1016/S0002-9149(97)89145-X. [DOI] [PubMed] [Google Scholar]
- 51.Pourafkari L, Ghaffari S, Ahmadi M, Tajlil A, Aslanabadi N, Nader ND. Pulmonary hypertension in rheumatic mitral stenosis revisited. Herz. 2017;42:746–751. doi: 10.1007/s00059-016-4509-2. [DOI] [PubMed] [Google Scholar]
- 52.Fawzy ME, Osman A, Nambiar V, Nowayhed O, El DA, Badr A, et al. Immediate and long-term results of mitral balloon valvuloplasty in patients with severe pulmonary hypertension. J Heart Valve Dis. 2008;17:485–491. [PubMed] [Google Scholar]
- 53.Fawzy ME, Hassan W, Stefadouros M, Moursi M, El Shaer F, Chaudhary MA. Prevalence and fate of severe pulmonary hypertension in 559 consecutive patients with severe rheumatic mitral stenosis undergoing mitral balloon valvotomy. J Heart Valve Dis. 2004;13:942–947. [PubMed] [Google Scholar]
- 54.Yang B, DeBenedictus C, Watt T, Farley S, Salita A, Hornsby W, et al. The impact of concomitant pulmonary hypertension on early and late outcomes following surgery for mitral stenosis. J Thorac Cardiovasc Surg. 2016;152:394–400. doi: 10.1016/j.jtcvs.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 55.Ward C, Hancock BW. Extreme pulmonary hypertension caused by mitral valve disease. Natural history and results of surgery. Br Heart J. 1975;37:74–8. doi: 10.1136/hrt.37.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walston A, Peter RH, Morris JJ, Kong Y, Behar VS. Clinical implications of pulmonary hypertension in mitral stenosis. Am J Cardiol. 1973;32:650–655. doi: 10.1016/S0002-9149(73)80058-X. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro PA, al Zaibag M, Abdullah M. Pulmonary artery pressure and pulmonary vascular resistance before and after mitral balloon valvotomy in 100 patients with severe mitral valve stenosis. Am Heart J. 1993;125:1110–4. doi: 10.1016/0002-8703(93)90121-O. [DOI] [PubMed] [Google Scholar]
- 58.Emanuel R, Ross K. Pulmonary hypertension in rheumatic heart disease. Prog Cardiovasc Dis. 1967;9:401–413. doi: 10.1016/S0033-0620(67)80015-X. [DOI] [PubMed] [Google Scholar]
- 59.Iung B, Cormier B, Ducimetière P, Porte JM, Nallet O, Michel PL, et al. Immediate results of percutaneous mitral commissurotomy. A predictive model on a series of 1514 patients. Circulation. 1996;94:2124–30. doi: 10.1161/01.CIR.94.9.2124. [DOI] [PubMed] [Google Scholar]
- 60.Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S, Kang DH, Kim DH, Song JM, Song JK, Park SW, et al. Late outcome of percutaneous mitral commissurotomy: Randomized comparison of Inoue versus double-balloon technique. Am Heart J. 2017;194:1–8. doi: 10.1016/j.ahj.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Kim DY, Cho I, Kim K, Gwak SY, Ha KE, Lee HJ, et al. Outcomes of severe mitral stenosis with the revised severity criteria: mitral valve replacement vs percutaneous mitral valvuloplasty. Can J Cardiol. 2023;S0828-282X(23):01659–8. doi: 10.1016/j.cjca.2023.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–864. doi: 10.1038/s41569-021-00570-z. [DOI] [PubMed] [Google Scholar]
- 64.d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuttard J, Birks J, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129:2158–2170. doi: 10.1161/CIRCULATIONAHA.113.006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Gara PT, Mack MJ. Secondary mitral regurgitation. N Engl J Med. 2020;383:1458–1467. doi: 10.1056/NEJMcp1903331. [DOI] [PubMed] [Google Scholar]
- 67.Kwak S, Lee SA, Lim J, Yang S, Choi HM, Hwang IC, et al. Long-term outcomes in distinct phenogroups of patients with primary mitral regurgitation undergoing valve surgery. Heart. 2023;109:305–313. doi: 10.1136/heartjnl-2022-321305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HM, Cho GY, Hwang IC, Choi HM, Park JB, Yoon YE, et al. Myocardial strain in prediction of outcomes after surgery for severe mitral regurgitation. JACC Cardiovasc Imaging. 2018;11:1235–1244. doi: 10.1016/j.jcmg.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Oh JK, Yoon YH, Roh JH, Kim M, Sun BJ, Jung SH, et al. Prognostic impact of left atrial strain after mitral valve repair surgery in patients with severe mitral regurgitation. Korean Circ J. 2022;52:205–217. doi: 10.4070/kcj.2021.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong SJ, Kim JS, Hong GR. Recent evidence and initial experiences of transcatheter edge-to-edge repair of the mitral valve in South Korea. J Chest Surg. 2021;54:165–171. doi: 10.5090/jcs.21.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. doi: 10.1161/CIRCULATIONAHA.118.037077. [DOI] [PubMed] [Google Scholar]
- 72.Gavazzoni M, Taramasso M, Zuber M, Russo G, Pozzoli A, Miura M, et al. Conceiving MitraClip as a tool: percutaneous edge-to-edge repair in complex mitral valve anatomies. Eur Heart J Cardiovasc Imaging. 2020;21:1059–1067. doi: 10.1093/ehjci/jeaa062. [DOI] [PubMed] [Google Scholar]
- 73.Bargiggia GS, Tronconi L, Sahn DJ, Recusani F, Raisaro A, De Servi S, et al. A new method for quantitation of mitral regurgitation based on color flow Doppler imaging of flow convergence proximal to regurgitant orifice. Circulation. 1991;84:1481–1489. doi: 10.1161/01.CIR.84.4.1481. [DOI] [PubMed] [Google Scholar]
- 74.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 75.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 77.Cawley PJ, Hamilton-Craig C, Owens DS, Krieger EV, Strugnell WE, Mitsumori L, et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging. 2013;6:48–57. doi: 10.1161/CIRCIMAGING.112.975623. [DOI] [PubMed] [Google Scholar]
- 78.Penicka M, Vecera J, Mirica DC, Kotrc M, Kockova R, Van Camp G. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with organic mitral regurgitation: comparison with Doppler echocardiography-derived integrative approach. Circulation. 2018;137:1349–1360. doi: 10.1161/CIRCULATIONAHA.117.029332. [DOI] [PubMed] [Google Scholar]
- 79.Garg P, Swift AJ, Zhong L, Carlhäll CJ, Ebbers T, Westenberg J, et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol. 2020;17:298–312. doi: 10.1038/s41569-019-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myerson SG, d'Arcy J, Christiansen JP, Dobson LE, Mohiaddin R, Francis JM, et al. Determination of clinical outcome in mitral regurgitation with cardiovascular magnetic resonance quantification. Circulation. 2016;133:2287–2296. doi: 10.1161/CIRCULATIONAHA.115.017888. [DOI] [PubMed] [Google Scholar]
- 81.Pizarro R, Bazzino OO, Oberti PF, Falconi M, Achilli F, Arias A, et al. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol. 2009;54:1099–1106. doi: 10.1016/j.jacc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Bakkestrøm R, Banke A, Christensen NL, Pecini R, Irmukhamedov A, Andersen M, et al. Hemodynamic characteristics in significant symptomatic and asymptomatic primary mitral valve regurgitation at rest and during exercise. Circ Cardiovasc Imaging. 2018;11:e007171. doi: 10.1161/CIRCIMAGING.117.007171. [DOI] [PubMed] [Google Scholar]
- 83.Utsunomiya H, Hidaka T, Susawa H, Izumi K, Harada Y, Kinoshita M, et al. Exercise-stress echocardiography and effort intolerance in asymptomatic/minimally symptomatic patients with degenerative mitral regurgitation combined invasive-noninvasive hemodynamic monitoring. Circ Cardiovasc Imaging. 2018;11:e007282. doi: 10.1161/CIRCIMAGING.117.007282. [DOI] [PubMed] [Google Scholar]
- 84.Magne J, Lancellotti P, Pierard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol. 2010;56:300–309. doi: 10.1016/j.jacc.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 85.Magne J, Lancellotti P, Pierard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation. 2010;122:33–41. doi: 10.1161/CIRCULATIONAHA.110.938241. [DOI] [PubMed] [Google Scholar]
- 86.Amano M, Izumi C, Kim YJ, Park SJ, Park SW, Tanaka H, et al. Changes of echocardiographic parameters in primary mitral regurgitation and determinants of symptom: an assessment from the Asian Valve Registry data. Heart Vessels. 2020;35:555–563. doi: 10.1007/s00380-019-01514-x. [DOI] [PubMed] [Google Scholar]
- 87.Detaint D, Messika-Zeitoun D, Avierinos JF, Scott C, Chen H, Burnett JC, et al. B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome. Circulation. 2005;111:2391–2397. doi: 10.1161/01.CIR.0000164269.80908.9D. [DOI] [PubMed] [Google Scholar]
- 88.Sutton TM, Stewart RA, Gerber IL, West TM, Richards AM, Yandle TG, et al. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. 2003;41:2280–2287. doi: 10.1016/S0735-1097(03)00486-8. [DOI] [PubMed] [Google Scholar]
- 89.Clavel MA, Tribouilloy C, Vanoverschelde JL, Pizarro R, Suri RM, Szymanski C, et al. Association of B-type natriuretic peptide with survival in patients with degenerative mitral regurgitation. J Am Coll Cardiol. 2016;68:1297–1307. doi: 10.1016/j.jacc.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 90.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 91.Youn JC, Kim D, Cho JY, Cho DH, Park SM, Jung MH, et al. Korean Society of Heart Failure guidelines for the management of heart failure: treatment. Int J Heart Fail. 2023;5:66–81. doi: 10.36628/ijhf.2023.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119:797–804. doi: 10.1161/CIRCULATIONAHA.108.802314. [DOI] [PubMed] [Google Scholar]
- 93.Kang DH, Heo R, Lee S, Baek S, Kim DH, Song JM, et al. Initial surgery versus conservative management of symptomatic severe mitral regurgitation in the elderly. Heart. 2018;104:849–854. doi: 10.1136/heartjnl-2017-311759. [DOI] [PubMed] [Google Scholar]
- 94.Kang DH, Park SJ, Sun BJ, Cho EJ, Kim DH, Yun SC, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol. 2014;63:2398–2407. doi: 10.1016/j.jacc.2014.02.577. [DOI] [PubMed] [Google Scholar]
- 95.Grigioni F, Clavel MA, Vanoverschelde JL, Tribouilloy C, Pizarro R, Huebner M, et al. The MIDA Mortality Risk Score: development and external validation of a prognostic model for early and late death in degenerative mitral regurgitation. Eur Heart J. 2018;39:1281–1291. doi: 10.1093/eurheartj/ehx465. [DOI] [PubMed] [Google Scholar]
- 96.Tribouilloy C, Grigioni F, Avierinos JF, Barbieri A, Rusinaru D, Szymanski C, et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol. 2009;54:1961–1968. doi: 10.1016/j.jacc.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 97.Essayagh B, Antoine C, Benfari G, Messika-Zeitoun D, Michelena H, Le Tourneau T, et al. Prognostic implications of left atrial enlargement in degenerative mitral regurgitation. J Am Coll Cardiol. 2019;74:858–870. doi: 10.1016/j.jacc.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 98.Rusinaru D, Tribouilloy C, Grigioni F, Avierinos JF, Suri RM, Barbieri A, et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging. 2011;4:473–481. doi: 10.1161/CIRCIMAGING.110.961011. [DOI] [PubMed] [Google Scholar]
- 99.Grigioni F, Benfari G, Vanoverschelde JL, Tribouilloy C, Avierinos JF, Bursi F, et al. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J Am Coll Cardiol. 2019;73:264–274. doi: 10.1016/j.jacc.2018.10.067. [DOI] [PubMed] [Google Scholar]
- 100.Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. 2017;135:410–422. doi: 10.1161/CIRCULATIONAHA.116.023340. [DOI] [PubMed] [Google Scholar]
- 101.David TE, David CM, Tsang W, Lafreniere-Roula M, Manlhiot C. Long-term results of mitral valve repair for regurgitation due to leaflet prolapse. J Am Coll Cardiol. 2019;74:1044–1053. doi: 10.1016/j.jacc.2019.06.052. [DOI] [PubMed] [Google Scholar]
- 102.David TE, Armstrong S, McCrindle BW, Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127:1485–1492. doi: 10.1161/CIRCULATIONAHA.112.000699. [DOI] [PubMed] [Google Scholar]
- 103.Chang BC, Youn YN, Ha JW, Lim SH, Hong YS, Chung N. Long-term clinical results of mitral valvuloplasty using flexible and rigid rings: a prospective and randomized study. J Thorac Cardiovasc Surg. 2007;133:995–1003. doi: 10.1016/j.jtcvs.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 104.Dillon J, Yakub MA, Kong PK, Ramli MF, Jaffar N, Gaffar IF. Comparative long-term results of mitral valve repair in adults with chronic rheumatic disease and degenerative disease: is repair for "burnt-out" rheumatic disease still inferior to repair for degenerative disease in the current era? J Thorac Cardiovasc Surg. 2015;149:771–779. doi: 10.1016/j.jtcvs.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 105.Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 106.Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–2854. doi: 10.1016/j.jacc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 107.Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DR, Stebbins A, et al. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT Registry report. J Am Coll Cardiol. 2017;70:2315–2327. doi: 10.1016/j.jacc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 108.Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol. 2015;65:1231–1248. doi: 10.1016/j.jacc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 109.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Writing Committee Members et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary. A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2021;78:2218–61. doi: 10.1016/j.jacc.2021.07.052. [DOI] [PubMed] [Google Scholar]
- 110.Benfari G, Antoine C, Essayagh B, Batista R, Maalouf J, Rossi A, et al. Functional mitral regurgitation outcome and grading in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2021;14:2303–2315. doi: 10.1016/j.jcmg.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 111.Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure A. quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675–80. doi: 10.1136/hrt.2011.225789. [DOI] [PubMed] [Google Scholar]
- 112.Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65:1078–1088. doi: 10.1016/j.jacc.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 113.Bartko PE, Arfsten H, Heitzinger G, Pavo N, Toma A, Strunk G, et al. A unifying concept for the quantitative assessment of secondary mitral regurgitation. J Am Coll Cardiol. 2019;73:2506–2517. doi: 10.1016/j.jacc.2019.02.075. [DOI] [PubMed] [Google Scholar]
- 114.Cavalcante JL, Kusunose K, Obuchowski NA, Jellis C, Griffin BP, Flamm SD, et al. Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2020;13:1489–1501. doi: 10.1016/j.jcmg.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 115.Kamperidis V, Marsan NA, Delgado V, Bax JJ. Left ventricular systolic function assessment in secondary mitral regurgitation: left ventricular ejection fraction vs. speckle tracking global longitudinal strain. Eur Heart J. 2016;37:811–6. doi: 10.1093/eurheartj/ehv680. [DOI] [PubMed] [Google Scholar]
- 116.Bertrand PB, Schwammenthal E, Levine RA, Vandervoort PM. Exercise dynamics in secondary mitral regurgitation: pathophysiology and therapeutic implications. Circulation. 2017;135:297–314. doi: 10.1161/CIRCULATIONAHA.116.025260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goliasch G, Bartko PE, Pavo N, Neuhold S, Wurm R, Mascherbauer J, et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J. 2018;39:39–46. doi: 10.1093/eurheartj/ehx402. [DOI] [PubMed] [Google Scholar]
- 118.Praz F, Grasso C, Taramasso M, Baumbach A, Piazza N, Tamburino C, et al. Mitral regurgitation in heart failure: time for a rethink. Eur Heart J. 2019;40:2189–2193. doi: 10.1093/eurheartj/ehz222. [DOI] [PubMed] [Google Scholar]
- 119.Coats AJS, Anker SD, Baumbach A, Alfieri O, von Bardeleben RS, Bauersachs J, et al. The management of secondary mitral regurgitation in patients with heart failure: a joint position statement from the Heart Failure Association (HFA), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), and European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur Heart J. 2021;42:1254–1269. doi: 10.1093/eurheartj/ehab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 121.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 122.Iung B, Messika-Zeitoun D, Boutitie F, Trochu JN, Armoiry X, Maucort-Boulch D, et al. Characteristics and outcome of COAPT-eligible patients in the MITRA-FR trial. Circulation. 2020;142:2482–2484. doi: 10.1161/CIRCULATIONAHA.120.049743. [DOI] [PubMed] [Google Scholar]
- 123.Pibarot P, Delgado V, Bax JJ. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. 2019;20:620–4. doi: 10.1093/ehjci/jez073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adamo M, Fiorelli F, Melica B, D'Ortona R, Lupi L, Giannini C, et al. COAPT-like profile predicts long-term outcomes in patients with secondary mitral regurgitation undergoing MitraClip implantation. JACC Cardiovasc Interv. 2021;14:15–25. doi: 10.1016/j.jcin.2020.09.050. [DOI] [PubMed] [Google Scholar]
- 125.Mihaljevic T, Lam BK, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 126.Acker MA, Jessup M, Bolling SF, Oh J, Starling RC, Mann DL, et al. Mitral valve repair in heart failure: five-year follow-up from the mitral valve replacement stratum of the Acorn randomized trial. J Thorac Cardiovasc Surg. 2011;142:569–574. doi: 10.1016/j.jtcvs.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 127.Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125:2639–2648. doi: 10.1161/CIRCULATIONAHA.111.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harmel EK, Reichenspurner H, Girdauskas E. Subannular reconstruction in secondary mitral regurgitation: a meta-analysis. Heart. 2018;104:1783–1790. doi: 10.1136/heartjnl-2017-312277. [DOI] [PubMed] [Google Scholar]
- 130.Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. 2019;12:433–442. doi: 10.1016/j.jcmg.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 131.Kim MS, Cho SJ, Park SJ, Cho SW, Choi SH, Kim HS, et al. Frequency and clinical associating factors of valvular heart disease in asymptomatic Korean adults. Sci Rep. 2019;9:16741. doi: 10.1038/s41598-019-53277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JW, Song JM, Park JP, Lee JW, Kang DH, Song JK. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J. 2010;74:375–380. doi: 10.1253/circj.CJ-09-0679. [DOI] [PubMed] [Google Scholar]
- 133.Kim JB, Spevack DM, Tunick PA, Bullinga JR, Kronzon I, Chinitz LA, et al. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: an observational study. J Am Soc Echocardiogr. 2008;21:284–287. doi: 10.1016/j.echo.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 134.Höke U, Auger D, Thijssen J, Wolterbeek R, van der Velde ET, Holman ER, et al. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart. 2014;100:960–968. doi: 10.1136/heartjnl-2013-304673. [DOI] [PubMed] [Google Scholar]
- 135.Anvardeen K, Rao R, Hazra S, Hay K, Dai H, Stoyanov N, et al. Prevalence and significance of tricuspid regurgitation post-endocardial lead placement. JACC Cardiovasc Imaging. 2019;12:562–564. doi: 10.1016/j.jcmg.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 136.Fender EA, Zack CJ, Nishimura RA. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart. 2018;104:798–806. doi: 10.1136/heartjnl-2017-311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kwak JJ, Kim YJ, Kim MK, Kim HK, Park JS, Kim KH, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. 2008;155:732–737. doi: 10.1016/j.ahj.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 138.Song H, Kim MJ, Chung CH, Choo SJ, Song MG, Song JM, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart. 2009;95:931–936. doi: 10.1136/hrt.2008.152793. [DOI] [PubMed] [Google Scholar]
- 139.Kim HK, Kim YJ, Kim KI, Jo SH, Kim KB, Ahn H, et al. Impact of the maze operation combined with left-sided valve surgery on the change in tricuspid regurgitation over time. Circulation. 2005;112(9 Suppl):I14–I19. doi: 10.1161/CIRCULATIONAHA.104.524496. [DOI] [PubMed] [Google Scholar]
- 140.Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. 2017;18:1342–1343. doi: 10.1093/ehjci/jex139. [DOI] [PubMed] [Google Scholar]
- 141.Hahn RT, Badano LP, Bartko PE, Muraru D, Maisano F, Zamorano JL, et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. Eur Heart J Cardiovasc Imaging. 2022;23:913–929. doi: 10.1093/ehjci/jeac009. [DOI] [PubMed] [Google Scholar]
- 142.Lee SP, Kim HK, Kim KH, Kim JH, Park HE, Kim YJ, et al. Prevalence of significant tricuspid regurgitation in patients with successful percutaneous mitral valvuloplasty for mitral stenosis: results from 12 years' follow-up of one centre prospective registry. Heart. 2013;99:91–97. doi: 10.1136/heartjnl-2012-302602. [DOI] [PubMed] [Google Scholar]
- 143.Seo J, Kim DY, Cho I, Hong GR, Ha JW, Shim CY. Prevalence, predictors, and prognosis of tricuspid regurgitation following permanent pacemaker implantation. PLoS One. 2020;15:e0235230. doi: 10.1371/journal.pone.0235230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang WI, Shim CY, Kang MK, Chang HJ, Chung N, Cho SY, et al. Vena contracta width as a predictor of adverse outcomes in patients with severe isolated tricuspid regurgitation. J Am Soc Echocardiogr. 2011;24:1013–1019. doi: 10.1016/j.echo.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 145.Song JM, Jang MK, Choi YS, Kim YJ, Min SY, Kim DH, et al. The vena contracta in functional tricuspid regurgitation: a real-time three-dimensional color Doppler echocardiography study. J Am Soc Echocardiogr. 2011;24:663–670. doi: 10.1016/j.echo.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 146.Park YH, Song JM, Lee EY, Kim YJ, Kang DH, Song JK. Geometric and hemodynamic determinants of functional tricuspid regurgitation: a real-time three-dimensional echocardiography study. Int J Cardiol. 2008;124:160–165. doi: 10.1016/j.ijcard.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 147.Kim H, Kim IC, Yoon HJ, Park HS, Cho YK, Nam CW, et al. Prognostic usefulness of tricuspid annular diameter for cardiovascular events in patients with tricuspid regurgitation of moderate to severe degree. Am J Cardiol. 2018;121:1343–1350. doi: 10.1016/j.amjcard.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 148.Kim DY, Seo J, Cho I, Lee SH, Lee S, Hong GR, et al. Prognostic implications of biventricular global longitudinal strain in patients with severe isolated tricuspid regurgitation. Front Cardiovasc Med. 2022;9:908062. doi: 10.3389/fcvm.2022.908062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seo JH, Park I, Park SJ, Jeong DS, Bak M, Kim J, et al. Association of longitudinal left atrial strain with mortality after tricuspid valve surgery. ESC Heart Fail. 2022;9:3868–3875. doi: 10.1002/ehf2.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim M, Lee HJ, Park JB, Kim J, Lee SP, Kim YJ, et al. Preoperative right ventricular free-wall longitudinal strain as a prognosticator in isolated surgery for severe functional tricuspid regurgitation. J Am Heart Assoc. 2021;10:e019856. doi: 10.1161/JAHA.120.019856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kwak S, Lim J, Yang S, Rhee TM, Choi YJ, Lee HJ, et al. Atrial functional tricuspid regurgitation: importance of atrial fibrillation and right atrial remodeling and prognostic significance. JACC Cardiovasc Imaging. 2023;16:575–587. doi: 10.1016/j.jcmg.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 152.Park JB, Kim HK, Jung JH, Klem I, Yoon YE, Lee SP, et al. Prognostic value of cardiac MR imaging for preoperative assessment of patients with severe functional tricuspid regurgitation. Radiology. 2016;280:723–734. doi: 10.1148/radiol.2016151556. [DOI] [PubMed] [Google Scholar]
- 153.Seo J, Hong YJ, Batbayar U, Kim DY, Cho I, Kim YJ, et al. Prognostic value of functional tricuspid regurgitation quantified by cardiac magnetic resonance in heart failure. Eur Heart J Cardiovasc Imaging. 2023;24:742–750. doi: 10.1093/ehjci/jeac224. [DOI] [PubMed] [Google Scholar]
- 154.Park SJ, Oh JK, Kim SO, Lee SA, Kim HJ, Lee S, et al. Determinants of clinical outcomes of surgery for isolated severe tricuspid regurgitation. Heart. 2021;107:403–410. doi: 10.1136/heartjnl-2020-317715. [DOI] [PubMed] [Google Scholar]
- 155.Park JB, Lee SP, Lee JH, Yoon YE, Park EA, Kim HK, et al. Quantification of right ventricular volume and function using single-beat three-dimensional echocardiography: a validation study with cardiac magnetic resonance. J Am Soc Echocardiogr. 2016;29:392–401. doi: 10.1016/j.echo.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 156.Kim HK, Kim YJ, Park EA, Bae JS, Lee W, Kim KH, et al. Assessment of haemodynamic effects of surgical correction for severe functional tricuspid regurgitation: cardiac magnetic resonance imaging study. Eur Heart J. 2010;31:1520–1528. doi: 10.1093/eurheartj/ehq063. [DOI] [PubMed] [Google Scholar]
- 157.Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185–1194. doi: 10.1016/j.jcmg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 158.Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv-Baran T, Richert E, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21:157–165. doi: 10.1093/ehjci/jez216. [DOI] [PubMed] [Google Scholar]
- 159.Kim YJ, Kwon DA, Kim HK, Park JS, Hahn S, Kim KH, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009;120:1672–1678. doi: 10.1161/CIRCULATIONAHA.109.849448. [DOI] [PubMed] [Google Scholar]
- 160.Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Surgical outcomes of severe tricuspid regurgitation: predictors of adverse clinical outcomes. Heart. 2013;99:181–187. doi: 10.1136/heartjnl-2012-302856. [DOI] [PubMed] [Google Scholar]
- 161.Axtell AL, Bhambhani V, Moonsamy P, Healy EW, Picard MH, Sundt TM, et al. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:715–725. doi: 10.1016/j.jacc.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zack CJ, Fender EA, Chandrashekar P, Reddy YN, Bennett CE, Stulak JM, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. 2017;70:2953–2960. doi: 10.1016/j.jacc.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 163.Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–1049. doi: 10.1016/j.jtcvs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 164.Hamandi M, Smith RL, Ryan WH, Grayburn PA, Vasudevan A, George TJ, et al. Outcomes of isolated tricuspid valve surgery have improved in the modern era. Ann Thorac Surg. 2019;108:11–15. doi: 10.1016/j.athoracsur.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 165.Dhoble A, Zhao Y, Vejpongsa P, Loghin C, Smalling RW, Estrera A, et al. National 10-year trends and outcomes of isolated and concomitant tricuspid valve surgery. J Cardiovasc Surg (Torino) 2019;60:119–127. doi: 10.23736/S0021-9509.18.10468-X. [DOI] [PubMed] [Google Scholar]
- 166.Hwang HY, Kim KH, Kim KB, Ahn H. Treatment for severe functional tricuspid regurgitation: annuloplasty versus valve replacement. Eur J Cardiothorac Surg. 2014;46:e21–e27. doi: 10.1093/ejcts/ezu224. [DOI] [PubMed] [Google Scholar]
- 167.Chang BC, Lim SH, Yi G, Hong YS, Lee S, Yoo KJ, et al. Long-term clinical results of tricuspid valve replacement. Ann Thorac Surg. 2006;81:1317–1323. doi: 10.1016/j.athoracsur.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 168.Cho WC, Park CB, Kim JB, Jung SH, Chung CH, Choo SJ, et al. Mechanical valve replacement versus bioprosthetic valve replacement in the tricuspid valve position. J Card Surg. 2013;28:212–217. doi: 10.1111/jocs.12093. [DOI] [PubMed] [Google Scholar]
- 169.Negm S, Arafat AA, Elatafy EE, Fawzy HF. Mechanical versus bioprosthetic valve replacement in the tricuspid valve position: a systematic review and meta-analysis. Heart Lung Circ. 2021;30:362–371. doi: 10.1016/j.hlc.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 170.Sorajja P, Whisenant B, Hamid N, Naik H, Makkar R, Tadros P, et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. 2023;388:1833–1842. doi: 10.1056/NEJMoa2300525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are included in this manuscript. Additional data used to support the findings of this review are also available in this published article.