Abstract
The aim of this meta-analysis was to determine the effect of pulmonary hypertension (PH) on survival in patients undergoing transcatheter aortic valve replacement (TAVR). The present study was conducted according to the guidelines of Preferred Reporting of Systematic Review and Meta-Analysis (PRISMA). We conducted a comprehensive search of electronic databases including PubMed/MEDLINE, Embase, Cochrane Library, and Web of Science from January 1, 2015, to March 10, 2024. Outcomes assessed in this meta-analysis included early and late all-cause mortality and cardiovascular mortality. Total 15 studies were integrated into the pooled analysis to assess the impact of PH on outcomes among patients undergoing TAVR, comprising a total sample size of 35,732 individuals. The pooled prevalence of PH stood at 52.57% (n=18,767). Predominantly, the studies were conducted in the United States (n=6), followed by Germany (n=3), with one study each from Japan, Italy, Switzerland, Brazil, Poland, and Australia. Pooled analysis showed that risk of short-term mortality was greater in patients with PH compared to patients without PH (risk ratio (RR): 1.46, 95% CI: 1.19 to 1.80). Risk of long-term mortality was greater in patients with PH (RR: 1.42, 95% CI: 1.29 to 1.55). Risk of cardiovascular mortality was also greater in patients with PH compared to patients without PH (RR: 1.66, 95% CI: 1.36 to 2.02). We advocate for further research to address gaps in understanding different types of PH and their impacts on mortality and cardiovascular outcomes.
Keywords: systematic review and meta analysis, cardiovascular mortality, all-cause mortality, transcatheter aortic valve replacement, pulmonary hypertension
Introduction and background
Pulmonary hypertension (PH) frequently occurs in patients diagnosed with severe aortic stenosis (AS), correlating with heightened mortality rates post-transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) [1,2]. Some studies indicate PH as an independent risk factor for cardiovascular and overall mortality subsequent to transcatheter aortic valve implantation (TAVI), alongside its well-established association with adverse outcomes following SAVR [3,4]. While TAVI emerges as a safe and efficacious treatment for AS, surpassing SAVR in attention, the heightened perioperative risk posed by PH necessitates thorough investigation into its balance of benefits and risks within this patient cohort [5]. Patients with PH undergoing TAVR may encounter elevated rates of post-procedure complications, prolonged hospital stays, increased mortality, and diminished functional outcomes compared to their counterparts without PH [6]. These findings underscore the importance of meticulous evaluation and management of PH in TAVR patients to optimize outcomes and enhance patient care. Furthermore, patients with PH may face an augmented risk of developing complications, such as sepsis, neurological issues like brain edema and hemorrhagic transformation, bleeding complications including gastrointestinal bleeding, and cardiovascular complications like congestive heart failure and atrial fibrillation with rapid ventricular response [7].
The evidence concerning short- and long-term mortality related to PH following TAVR remains inconclusive. Some studies suggest potential mortality benefits from ameliorating PH post-TAVR, yet a definitive conclusion is lacking. Moreover, the absence of a definitive PH threshold complicates the ability to predict which patients should refrain from TAVR or are at higher risk for post-TAVR mortality and morbidity. For instance, while PH (defined as pulmonary artery systolic pressure (PASP) ≥ 60 mmHg) is considered in the EuroSCORE criteria, it is not integrated into the Society of Thoracic Surgeons score [8]. The disparity in PH definitions employed across studies (e.g., elevated PASP or mean pulmonary artery pressure) further obfuscates the establishment of clear-cut values influencing mortality post-TAVR. Consequently, our objective in this study is to systematically review the literature and quantitatively synthesize data on the relationship between baseline PH and early as well as late cardiovascular and overall mortality.
Review
Methodology
Search Strategy
The present study was conducted according to the guidelines of Preferred Reporting of Systematic Review and Meta-analysis (PRISMA). We conducted a comprehensive search of electronic databases including PubMed/MEDLINE, Embase, Cochrane Library, and Web of Science from January 1, 2015, to March 10, 2024. The search strategy combined keywords and MeSH terms related to ("pulmonary hypertension"[MeSH] OR "PH"[MeSH]) AND ("TAVR"[MeSH] OR "Transcatheter Aortic Valve Replacement"[MeSH]) AND ("death"[MeSH] OR "All-cause mortality"[MeSH] OR "cardiovascular mortality"[MeSH] OR "in-hospital mortality"[MeSH]). Boolean operators (AND, OR) were used to refine the search and ensure inclusivity. Additionally, we manually screened reference lists of relevant articles and reviews for additional studies. Search was performed by two authors independently and disagreements between two authors were resolved through consensus.
Study Selection
Two independent reviewers screened titles and abstracts of retrieved studies followed by full-text screening for eligibility based on predetermined inclusion and exclusion criteria. Studies were included if they: (1) involved adult patients (age ≥ 18 years) undergoing TAVR, (2) reported baseline PH status, (3) assessed cardiovascular and overall mortality outcomes, and (4) were written in English. Studies were excluded if they were case reports, reviews, or editorials. Any discrepancies in study selection were resolved through discussion or consultation with a third reviewer.
Data Extraction and Quality Assessment
Data extraction was performed independently by two reviewers using a standardized form. Extracted data included study characteristics (e.g., author name, year of publication, study region and sample size), patient demographics, definition of PH, follow-up duration, and outcome measures. Outcomes assessed in this meta-analysis included short term mortality (in-hospital and 30-day mortality), long-term mortality and cardiovascular related mortality. Quality assessment of included studies was performed using New-Castle Ottawa scale.
Data Analysis
In this meta-analysis, we employed random-effects models to conduct our analysis, allowing for the calculation of pooled effect estimates, specifically the risk ratio (RR), along with corresponding 95% CI, to evaluate the association between baseline PH and outcomes. We considered a p-value less than 0.05 as indicative of statistical significance. To assess heterogeneity among the included studies, we utilized the I2 statistic, where values exceeding 50% were deemed to indicate significant heterogeneity. All statistical analyses were carried out using RevMan software. Forest plots were used to present the pooled findings of included studies.
Results
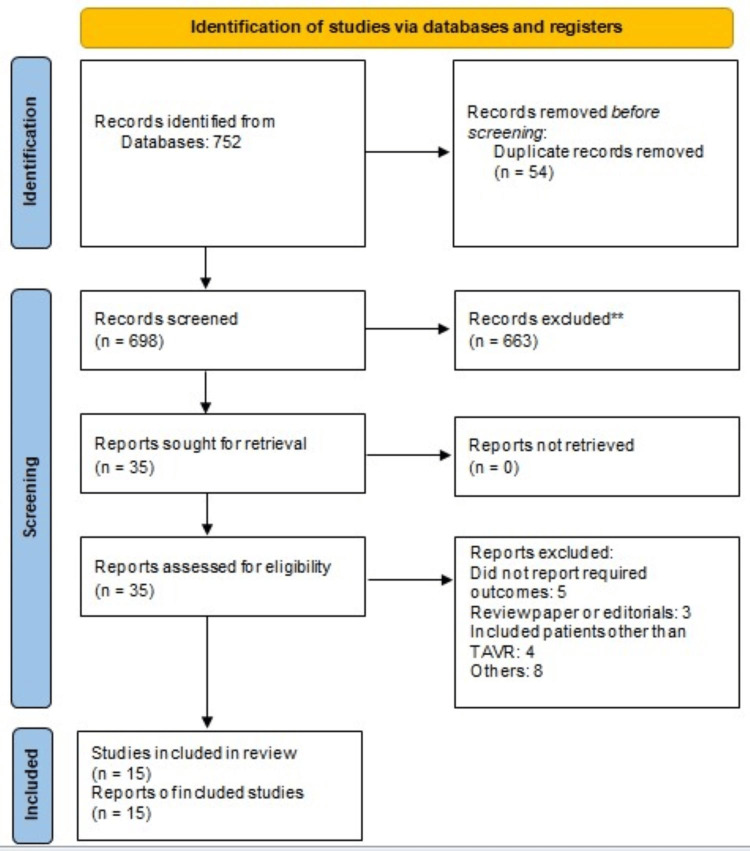
Our screening across major databases produced 752 results, which were then refined to 698 articles following the removal of duplicates. Screening occurred in two stages. Initially, abstracts and titles were scrutinized against predetermined inclusion and exclusion criteria. Subsequently, the full texts of eligible studies were retrieved, and a comprehensive evaluation was conducted to determine their suitability for inclusion in this meta-analysis. Ultimately, 15 studies were integrated into the pooled analysis to assess the impact of PH on outcomes among patients undergoing TAVR, comprising a total sample size of 35,732 individuals. Figure 1 illustrates the PRISMA flowchart, delineating the study selection process.
Figure 1. PRISMA flowchart of study selection.
PRISMA: Preferred Reporting of Systematic Review and Meta-Analysis
Characteristics of Included Studies
The characteristics of the included studies are presented in Table 1. The pooled sample encompassed 35,732 patients. Among the included studies, the sample sizes varied from 136 to 25,969 patients undergoing TAVR. The pooled prevalence of PH stood at 52.57% (n= 18,767). Predominantly, the studies were conducted in the United States (n=6), followed by Germany (n=3), with one study each from Japan, Italy, Switzerland, Brazil, Poland, and Australia. The majority of the studies utilized mean pulmonary arterial pressure (mPAP) as a diagnostic criterion for PH. Table 2 presents quality assessment of included studies.
Table 1. Characteristics of included studies.
NS: Not specified; PH: Pulmonary hypertension; mPAP: Mean pulmonary arterial pressure; sPAP: Systolic pulmonary arterial pressure
| Author | Year | Study Design | Region | Criteria to assess PH | Groups | Sample Size | Follow-up | Age | Males | Previous MI | Diabetes | Afib |
| Ahmad et al [9] | 2023 | Retrospective | United States | mPAP≥25 mmHg | Normal | 150 | 30 days | NS | 87 | NS | 40 | 35 |
| PH | 324 | 165 | 155 | 116 | ||||||||
| Alushi et al [10] | 2019 | Retrospective | Germany | sPAP≥34 mmHg | Normal | 136 | 365 days | NS | NS | NS | NS | NS |
| PH | 481 | |||||||||||
| Barbash et al [11] | 2016 | Prospective | United States | sPAP> 50 mmHg | Normal | 172 | 365 days | 83 | 116 | 48 | 69 | 92 |
| PH | 243 | 84 | 80 | 23 | 55 | 73 | ||||||
| Keymel et al [12] | 2020 | Retrospective | Germany | mPAP≥25 mmHg | Normal | 52 | 30 days | 78 | 35 | 10 | 16 | NS |
| PH | 73 | 78.9 | 49 | 20 | 28 | |||||||
| Kleczynski et al [13] | 2017 | Prospective | Poland | NS | Normal | 83 | 365 days | 82 | 28 | 20 | 23 | 29 |
| PH | 65 | 82 | 28 | 28 | 25 | 23 | ||||||
| Lindman et al [14] | 2016 | Prospective | United States | mPAP≥35 mmHg | Normal | 785 | 365 days | 85 | 409 | 168 | 247 | NS |
| PH | 1395 | 83 | 741 | 382 | 570 | |||||||
| Masri et al [15] | 2018 | Retrospective | United States | mPAP≥25 mmHg | Normal | 134 | 700 days | 82.9 | 66 | 75 | 48 | 43 |
| PH | 102 | 82.8 | 50 | 33 | 38 | 64 | ||||||
| Mayr et al [16] | 2021 | Retrospective | Germany | NS | Normal | 359 | 30 days | 81 | 225 | NS | NS | NS |
| PH | 718 | 81 | 432 | |||||||||
| Miyamoto et al [17] | 2022 | Retrospective | Japan | sPAP>36 mmHg | Normal | 1027 | 700 days | 84.5 | 429 | NS | 263 | 229 |
| PH | 845 | 84.7 | 141 | 108 | 173 | |||||||
| Mujeeb et al [18] | 2021 | Retrospective | United States | NS | Normal | 12989 | 30 days | 80.8 | 6118 | NS | 4845 | 7118 |
| PH | 12980 | 80.9 | 6243 | 4893 | 7126 | |||||||
| Naing et al [19] | 2022 | Prospective | Australia | mPAP>20 mmHg | Normal | 320 | 365 days | NS | NS | NS | NS | NS |
| PH | 179 | |||||||||||
| O Sullivan et al [20] | 2015 | Prospective | Switzerland | mPAP≥25 mmHg | Normal | 108 | 365 days | 81.7 | 60 | 17 | 31 | 8 |
| PH | 325 | 82.5 | 136 | 48 | 95 | 52 | ||||||
| Souza et al [21] | 2015 | Prospective | Brazil | NS | Normal | 103 | 365 days | NS | NS | NS | NS | NS |
| PH | 33 | |||||||||||
| Sultan et al [22] | 2020 | Retrospective | United States | mPAP>25 mmHg | Normal | 201 | 1,825 days | 82.9 | 97 | 58 | 73 | 53 |
| PH | 360 | 82.1 | 189 | 146 | 161 | 146 | ||||||
| Testa et al [23] | 2016 | Retrospective | Italy | sPAP>40 mmHg | Normal | 346 | 365 days | 82 | 152 | 72 | 90 | 69 |
| PH | 644 | 78.5 | 300 | 133 | 184 | 163 |
Table 2. Quality assessment of included studies.
| Author | Selection | Comparability | Outcome or Exposure Assessment | Overall |
| Ahmad et al [9] | 3 | 2 | 3 | Good |
| Alushi et al [10] | 3 | 2 | 3 | Good |
| Barbash et al [11] | 3 | 2 | 2 | Good |
| Keymel et al [12] | 4 | 2 | 3 | Good |
| Kleczynski et al [13] | 4 | 1 | 3 | Good |
| Lindman et al [14] | 3 | 1 | 2 | Fair |
| Masri et al [15] | 3 | 2 | 2 | Good |
| Mayr et al [16] | 3 | 1 | 3 | Good |
| Miyamoto et al [17] | 3 | 2 | 2 | Good |
| Mujeeb et al [18] | 3 | 1 | 3 | Good |
| Naing et al [19] | 4 | 2 | 3 | Good |
| O Sullivan et al [20] | 3 | 2 | 3 | Good |
| Souza et al [21] | 3 | 2 | 2 | Good |
| Sultan et al [22] | 4 | 1 | 2 | Good |
| Testa et al [23] | 3 | 2 | 3 | Good |
Effect of PH on Short-Term Mortality
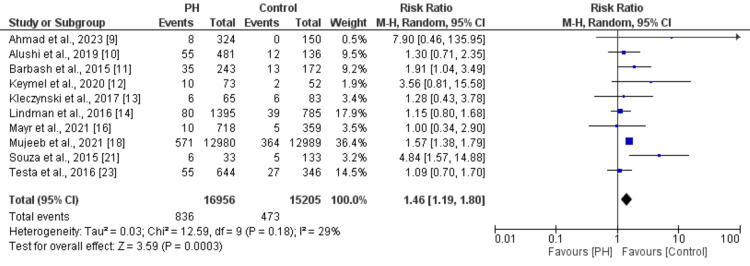
10 studies were included in the pooled analysis of estimating the effect of PH on short-term mortality in patients undergoing TAVR; the results are presented in Figure 2. Pooled analysis showed that risk of short-term mortality was 1.46 times greater in patients with PH compared to patients without PH (RR: 1.46, 95% CI: 1.19 to 1.80). No significant heterogeneity was reported among the study results (I2: 29%).
Figure 2. Effect of PH on short-term mortality [9-14,16,18,21,23].
PH: Pulmonary hypertension
Effect of PH on Long-Term Mortality
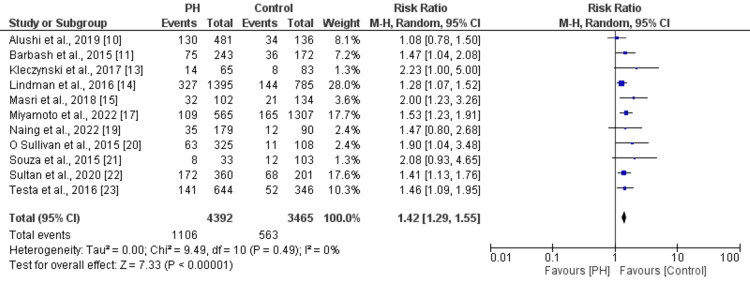
11 studies were included in the pooled analysis of estimating the effect of PH on long-term mortality in patients undergoing TAVR; the results are presented in Figure 3. Pooled analysis showed that risk of long-term mortality was 1.42 times greater in patients with PH compared to patients without PH (RR: 1.42, 95% CI: 1.29 to 1.55). No significant heterogeneity was reported among the study results (I2: 0%).
Figure 3. Effect of PH on long-term mortality in patients undergoing TAVR [10-15,17,19-23].
PH: Pulmonary hypertension
Effect of PH on Cardiovascular Mortality
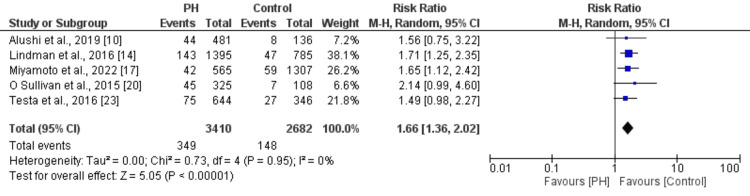
Five studies were included in the pooled analysis of estimating the effect of PH on cardiovascular mortality in patients undergoing TAVR; the results are presented in Figure 4. Pooled analysis showed that risk of cardiovascular mortality was 1.66 times greater in patients with PH compared to patients without PH (RR: 1.66, 95% CI: 1.36 to 2.02). No significant heterogeneity was reported among the study results (I2: 0%).
Figure 4. Effect of PH on cardiovascular mortality in patients undergoing TAVR [10,14,17,20,23].
PH: Pulmonary hypertension
Discussion
This meta-analysis, encompassing 15 studies published from 2015, aggregated individual findings concerning three distinct outcomes. Our analysis indicates a significant contrast in short-term mortality, long-term mortality, and cardiovascular mortality, with all these outcomes exhibiting notably higher risks following TAVR in patients with PH compared to those without PH at baseline.
Our findings align with previous studies underscoring the prognostic significance of baseline PH in patients with severe AS undergoing TAVI procedures. Tang et al. [23] conducted a comprehensive meta-analysis on this subject, echoing the impact of baseline PH on post-TAVI mortality. Despite numerous studies investigating the association between PH and post-TAVI outcomes, determining the optimal cutoff point for defining PH as a risk factor in the preoperative assessment of these patients remains unresolved.
Numerous studies have highlighted the association of baseline PH with delayed rather than immediate mortality and poorer clinical outcomes in patients undergoing TAVR [3,24]. Luçon et al found that baseline PH served as a predictive factor for one-year mortality post-TAVR, while Testa et al showed that baseline systolic pulmonary arterial pressure (sPAP) exceeding 60 mmHg independently predicted one-year mortality [2,24]. In line with these findings, our study revealed that baseline PH was linked to worse clinical outcomes at the two-year mark compared to patients without PH. Moreover, higher baseline sPAP levels correlated with increased occurrence of adverse clinical events. A meta-analysis from China and the US echoed the observations of the observational studies included in our analysis [25-26]. The randomized controlled trial by Lindman et al and a retrospective study recommended considering both objective PH findings and clinical factors in assessing mortality risk and guiding decisions regarding TAVR [14,27]. While Kleczynski et al demonstrated elevated all-cause mortality by stratifying PH based solely on tricuspid regurgitation velocity (TRV), they found no impact on quality of life in PH patients; however, further studies with larger cohorts are warranted to validate these findings [13].
An important finding of this study is the significant association between combined PH and heightened mortality risk compared to the absence of PH. However, there was no notable difference observed between precapillary PH, isolated PH, and the absence of PH in terms of all-cause mortality [20,22]. It's worth noting that only two of the included studies directly compared mortality risk across different types of PH. Consequently, we advocate for further research to delve into and compare mortality and cardiovascular risks among the various types of PH. Furthermore, baseline PH (defined as mPAP ≥ 25 mmHg) is recognized as one of the risk factors incorporated in the pulmonary, bleeding, osler, sex, and skeletal complications score (PBOSS) for predicting TAVI outcomes in chronic obstructive pulmonary disorder (COPD) patients, alongside parameters such as BMI (below 21 kg/m2), oxygen dependency, and covering less than 200 m in the six-minute walk test. The adverse outcomes observed in patients with baseline PH underscore the necessity for continued investigation and the development of innovative therapies aimed at reducing PASP alongside TAVI for AS [28,29]. Indeed, considering the well-established link between post-TAVI PASP levels and subsequent survival, the imperative for supplementary medical interventions targeting PASP reduction warrants attention in forthcoming studies [30].
Currently, the only established scoring systems that take into account pre-TAVI PH values are the euroSCORE, which incorporates PASP assessed via echocardiography at 60 mmHg and the PBOSS score, designed specifically for risk assessment in COPD patients. Although most studies analyzed in our meta-analysis utilized the mPAP score, they applied different cutoff values. It is crucial for future research efforts to focus on determining mortality rates across different cutoff points to fully understand the implications of PH.
This meta-analysis was conducted using real-world studies, and therefore, our findings should be understood within the framework of observational research and its inherent limitations. Additionally, several of the included observational studies were retrospective in nature, which heightens the potential for bias. There was also a lack of consistency among the studies regarding the definition and diagnostic method of PH, which could lead to underestimation or overestimation of the study results. Furthermore, only two studies directly compared the risk of outcomes among different types of PH. Consequently, future research endeavors should prioritize the comparison of outcomes across various types of PH.
Conclusions
In conclusion, our meta-analysis of 15 studies on PH and outcomes post-TAVR reveals significant associations with short-term mortality, long-term mortality, and cardiovascular mortality. Despite variations in study designs and definitions of PH, our findings underscore the heightened risks faced by patients with PH undergoing TAVR. We advocate for further research to address gaps in understanding different types of PH and their impacts on mortality and cardiovascular outcomes. Moreover, the need for standardized diagnostic criteria and cutoff points for PH assessment in TAVR patients is evident, emphasizing the importance of ongoing investigation in this area.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Acquisition, analysis, or interpretation of data: Sujith K. Palleti, Samuel K. Dadzie, Merid Moqattash, Sulafa Khalil, Sajog Kansakar, Praveen Kumar Komminni, Mandeep Kaur
Drafting of the manuscript: Sujith K. Palleti, Godfrey Tabowei, Samuel K. Dadzie, Merid Moqattash, Sulafa Khalil, Sajog Kansakar, Praveen Kumar Komminni, Mandeep Kaur
Concept and design: Godfrey Tabowei, Sulafa Khalil
Critical review of the manuscript for important intellectual content: Godfrey Tabowei
References
- 1.Impact of pulmonary hypertension hemodynamic status on long-term outcome after transcatheter aortic valve replacement. Schewel J, Schmidt T, Kuck KH, Frerker C, Schewel D. JACC Cardiovasc Interv. 2019;12:2155–2168. doi: 10.1016/j.jcin.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: study from the FRANCE 2 Registry. Luçon A, Oger E, Bedossa M, et al. Circ Cardiovasc Interv. 2014;7:240–247. doi: 10.1161/CIRCINTERVENTIONS.113.000482. [DOI] [PubMed] [Google Scholar]
- 3.Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Tamburino C, Capodanno D, Ramondo A, et al. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 4.Pulmonary artery hypertension in severe aortic stenosis: incidence and mechanism. Silver K, Aurigemma G, Krendel S, Barry N, Ockene I, Alpert J. Am Heart J. 1993;125:146–150. doi: 10.1016/0002-8703(93)90067-j. [DOI] [PubMed] [Google Scholar]
- 5.Outcomes of preprocedural pulmonary hypertension on all-cause and cardiac mortality in patients undergoing transcatheter aortic valve implantation: a systematic review. Desai A, Desai DM, Jamil A, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.34300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Incidence and risk factors for long-term persistence of diastolic dysfunction after aortic valve replacement for aortic stenosis compared with aortic regurgitation. Iliuță L, Andronesi AG, Scafa-Udriște A, Rădulescu B, Moldovan H, Furtunescu FL, Panaitescu E. J Cardiovasc Dev Dis. 2023;10 doi: 10.3390/jcdd10030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulmonary hypertension: a contemporary review. Johnson S, Sommer N, Cox-Flaherty K, Weissmann N, Ventetuolo CE, Maron BA. Am J Respir Crit Care Med. 2023;208:528–548. doi: 10.1164/rccm.202302-0327SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The relative performance characteristics of the logistic European System for Cardiac Operative Risk Evaluation score and the Society of Thoracic Surgeons score in the Placement of Aortic Transcatheter Valves trial. Beohar N, Whisenant B, Kirtane AJ, et al. J Thorac Cardiovasc Surg. 2014;148:2830–2837. doi: 10.1016/j.jtcvs.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Association between pulmonary hypertension and its effect on 30-day mortality, readmission, and cost after transcatheter aortic valve replacement: a multicenter study. Ahmad M, Del Cid Fratti J, Henien M, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.40976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulmonary hypertension in patients with severe aortic stenosis: prognostic impact after transcatheter aortic valve replacement: pulmonary hypertension in patients undergoing TAVR. Alushi B, Beckhoff F, Leistner D, et al. JACC Cardiovasc Imaging. 2019;12:591–601. doi: 10.1016/j.jcmg.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Prevalence and impact of pulmonary hypertension on patients with aortic stenosis who underwent transcatheter aortic valve replacement. Barbash IM, Escarcega RO, Minha S, et al. Am J Cardiol. 2015;115:1435–1442. doi: 10.1016/j.amjcard.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Patients with severe aortic stenosis and coexisting pulmonary hypertension treated by transapical transcatheter aortic valve replacement-Is there a need for increased attention? Keymel S, Papadopoulos G, Minol JP, et al. Catheter Cardiovasc Interv. 2020;95:1001–1008. doi: 10.1002/ccd.28358. [DOI] [PubMed] [Google Scholar]
- 13.Prognostic value of tricuspid regurgitation velocity and probability of pulmonary hypertension in patients undergoing transcatheter aortic valve implantation. Kleczynski P, Dziewierz A, Wiktorowicz A, et al. Int J Cardiovasc Imaging. 2017;33:1931–1938. doi: 10.1007/s10554-017-1210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risk stratification in patients with pulmonary hypertension undergoing transcatheter aortic valve replacement. Lindman BR, Zajarias A, Maniar HS, et al. Heart. 2015;101:1656–1664. doi: 10.1136/heartjnl-2015-308001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Outcomes of persistent pulmonary hypertension following transcatheter aortic valve replacement. Masri A, Abdelkarim I, Sharbaugh MS, et al. Heart. 2018;104:821–827. doi: 10.1136/heartjnl-2017-311978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TAVI for patients with pulmonary arterial hypertension-impact of the anesthesia regime on the short-term outcome. Mayr P, Rheude TR, Pellegrini C, et al. Eur Heart J. 2021;42:0. [Google Scholar]
- 17.Impact of periprocedural pulmonary hypertension on outcomes after transcatheter aortic valve replacement. Miyamoto J, Ohno Y, Kamioka N, et al. J Am Coll Cardiol. 2022;80:1601–1613. doi: 10.1016/j.jacc.2022.08.757. [DOI] [PubMed] [Google Scholar]
- 18.Burden of pulmonary hypertension in transcatheter aortic valve implantation. Mujeeb FA, Mostafa MR, Alqahtani F, Trabulsi AM, Chundrigar M, Rawasia WF. Cardiovasc Revasc Med. 2021;29:93–94. doi: 10.1016/j.carrev.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Impact of pulmonary hypertension in patients undergoing transcatheter aortic valve implantation: the experience from a centre of excellence. Naing P, Murdoch D, Burstow D, et al. Heart Lung Circ. 2022;31:207–208. [Google Scholar]
- 20.Effect of pulmonary hypertension hemodynamic presentation on clinical outcomes in patients with severe symptomatic aortic valve stenosis undergoing transcatheter aortic valve implantation: insights from the new proposed pulmonary hypertension classification. O'Sullivan CJ, Wenaweser P, Ceylan O, et al. Circ Cardiovasc Interv. 2015;8:0. doi: 10.1161/CIRCINTERVENTIONS.114.002358. [DOI] [PubMed] [Google Scholar]
- 21.Transcatheter aortic valve implantation and morbidity and mortality-related factors: a 5-year experience in Brazil. Souza AL, Salgado CG, Mourilhe-Rocha R, et al. Arq Bras Cardiol. 2016;106:519–527. doi: 10.5935/abc.20160072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Impact of combined pre and postcapillary pulmonary hypertension on survival after transcatheter aortic valve implantation. Sultan I, Fukui M, Bianco V, et al. Am J Cardiol. 2020;131:60–66. doi: 10.1016/j.amjcard.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 23.Meta-analysis of outcomes and evolution of pulmonary hypertension before and after transcatheter aortic valve implantation. Tang M, Liu X, Lin C, et al. Am J Cardiol. 2017;119:91–99. doi: 10.1016/j.amjcard.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Persistence of severe pulmonary hypertension after transcatheter aortic valve replacement: incidence and prognostic impact. Testa L, Latib A, De Marco F, et al. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003563. [DOI] [PubMed] [Google Scholar]
- 25.Methodological remarks in the meta-analysis on the impact of baseline pulmonary hypertension on post transcatheter aortic valve implantation outcomes. Kokkinidis DG, Oikonomou EK, Papanastasiou CA, Theochari CA, Giannakoulas G. Am J Cardiol. 2017;120:513–514. doi: 10.1016/j.amjcard.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 26.The predictive value of baseline pulmonary hypertension in early and long term cardiac and all-cause mortality after transcatheter aortic valve implantation for patients with severe aortic valve stenosis: a systematic review and meta-analysis. Kokkinidis DG, Papanastasiou CA, Jonnalagadda AK, et al. Cardiovasc Revasc Med. 2018;19:859–867. doi: 10.1016/j.carrev.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Echocardiographic pulmonary hypertension probability is associated with clinical outcomes after transcatheter aortic valve implantation. Nijenhuis VJ, Huitema MP, Vorselaars VM, et al. Int J Cardiol. 2016;225:218–225. doi: 10.1016/j.ijcard.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Outcomes of patients with chronic lung disease and severe aortic stenosis treated with transcatheter versus surgical aortic valve replacement or standard therapy: insights from the PARTNER trial (placement of AoRTic TraNscathetER Valve) Dvir D, Waksman R, Barbash IM, et al. J Am Coll Cardiol. 2014;63:269–279. doi: 10.1016/j.jacc.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 29.The role and clinical implications of diastolic dysfunction in aortic stenosis. Kampaktsis PN, Kokkinidis DG, Wong SC, Vavuranakis M, Skubas NJ, Devereux RB. Heart. 2017;103:1481–1487. doi: 10.1136/heartjnl-2017-311506. [DOI] [PubMed] [Google Scholar]
- 30.Decrease of pulmonary hypertension impacts on prognosis after transcatheter aortic valve replacement. Sinning JM, Hammerstingl C, Chin D, et al. https://eurointervention.pcronline.com/article/decrease-of-pulmonary-hypertension-impacts-on-prognosis-after-transcatheter-aortic-valve-replacement. EuroIntervention. 2014;9:1042–1049. doi: 10.4244/EIJV9I9A177. [DOI] [PubMed] [Google Scholar]