ABSTRACT
Whether empirical therapy with carbapenems positively affects the outcomes of critically ill patients with bacterial infections remains unclear. This study aimed to investigate whether the use of carbapenems as the initial antimicrobial administration reduces mortality and whether the duration of carbapenem use affects the detection of multidrug-resistant (MDR) pathogens. This was a post hoc analysis of data acquired from Japanese participating sites from a multicenter, prospective observational study [Determinants of Antimicrobial Use and De-escalation in Critical Care (DIANA study)]. A total of 268 adult patients with clinically suspected or confirmed bacterial infections from 31 Japanese intensive care units (ICUs) were analyzed. The patients were divided into two groups: patients who were administered carbapenems as initial antimicrobials (initial carbapenem group, n = 99) and those who were not administered carbapenems (initial non-carbapenem group, n = 169). The primary outcomes were mortality at day 28 and detection of MDR pathogens. Multivariate logistic regression analysis revealed that mortality at day 28 did not differ between the two groups [18 (18%) vs 27 (16%), respectively; odds ratio: 1.25 (95% confidence interval (CI): 0.59–2.65), P = 0.564]. The subdistribution hazard ratio for detecting MDR pathogens on day 28 per additional day of carbapenem use is 1.08 (95% CI: 1.05–1.13, P < 0.001 using the Fine-Gray model with death regarded as a competing event). In conclusion, in-hospital mortality was similar between the groups, and a longer duration of carbapenem use as the initial antimicrobial therapy resulted in a higher risk of detection of new MDR pathogens.
IMPORTANCE
We found no statistical difference in mortality with the empirical use of carbapenems as initial antimicrobial therapy among critically ill patients with bacterial infections. Our study revealed a lower proportion of inappropriate initial antimicrobial administrations than those reported in previous studies. This result suggests the importance of appropriate risk assessment for the involvement of multidrug-resistant (MDR) pathogens and the selection of suitable antibiotics based on risk. To the best of our knowledge, this study is the first to demonstrate that a longer duration of carbapenem use as initial therapy is associated with a higher risk of subsequent detection of MDR pathogens. This finding underscores the importance of efforts to minimize the duration of carbapenem use as initial antimicrobial therapy when it is necessary.
KEYWORDS: initial empirical therapy, critically ill, infectious disease, carbapenem, multidrug resistance, intensive care unit
INTRODUCTION
Determining the appropriate antimicrobials to be used as the initial empirical therapy in critically ill patients with bacterial infections remains challenging, especially considering that the initial administration of antibiotics is time-sensitive in severely ill patients to achieve better outcomes (1, 2). Previous studies have revealed that inappropriate initial antimicrobial administration is associated with higher mortality (3–5); therefore, physicians tend to administer broad-spectrum antibiotics as initial therapy to avoid inappropriate administrations. The Determinants of Antimicrobial Use and De-escalation in Critical Care (DIANA) study, a multicenter international observational cohort study investigating critically ill adult patients receiving empirical antimicrobial therapy for suspected or confirmed bacterial infections in the intensive care unit (ICU), revealed that carbapenems are some of the most widely administered antimicrobials as initial therapy [389/1,495 (26%) patients were administered carbapenems as empirical antimicrobial therapy] (6). However, it remains unclear whether the empirical use of carbapenems improves mortality rates.
Questions regarding whether carbapenems as initial empirical therapy lead to improvement in mortality, have arisen for several reasons. First, various studies on the treatment of bacterial infections and risk assessment of involvement of multidrug-resistant (MDR) pathogens have been expanding globally (7–13). Several recent studies have identified the risk for MDR pathogens, helping us select narrow antibiotics more accurately in cases with a low risk for MDR pathogens (7–13). This can potentially counteract the benefits of using carbapenems empirically to avoid inappropriate therapy. Second, previous studies have reported that extended-spectrum beta-lactamase-producing Enterobacteriaceae, a major reason for the use of carbapenems as initial empirical therapy (14), are frequently susceptible to other antibiotics such as specific beta-lactam/beta-lactamase inhibitors (15, 16), although a few studies have indicated the possibility of the superiority of carbapenems over other antibiotics (17, 18). Several studies have implied that carbapenem-sparing regimens as empirical therapy for various infectious diseases show non-inferiority to carbapenems (15, 16, 19–24). However, the effect is unknown in an integrated cohort of critically ill patients with bacterial infection in the ICU setting.
The subsequent detection of MDR pathogens is another concern related to carbapenem use. A previous study revealed that the use of broad-spectrum antimicrobials, including carbapenems as initial empirical therapy for more than 72 h was associated with the detection of new MDR pathogens (25). However, no studies are focusing on carbapenems, which are broad-spectrum antimicrobials that may be considered to have the greatest impact on the emergence of MDR pathogens, except for a single-center study (26).
We hypothesized that the use of carbapenems as initial antimicrobial therapy would not improve outcomes and that the extended duration of the initial empirical therapy with carbapenems was a risk factor for the subsequent detection of MDR pathogens. This study was a post hoc analysis aimed at investigating whether the use of carbapenems as the initial administration can improve mortality on day 28 and whether the duration of use increases the subsequent detection of MDR pathogens.
RESULTS
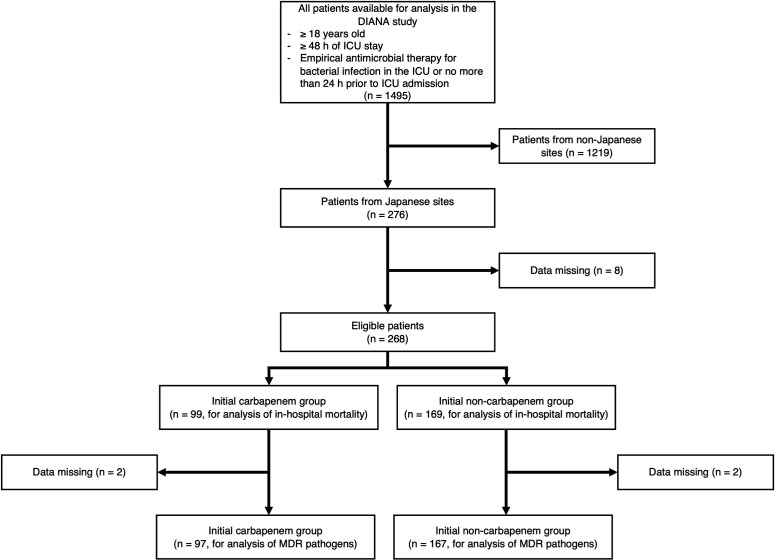
Figure 1 illustrates the patient flowchart of the study. Among the 1,495 patients registered in the DIANA study, 276 from 31 Japanese ICUs were eligible for inclusion in this study. Eight patients were excluded according to our preset criteria because of missing values required for the analyses. As a result, the remaining 268 patients were analyzed. Among them, 99 (37%) were administered carbapenems as initial empirical therapy, whereas 169 (63%) were administered other antibiotics. A list of initial antimicrobial therapy in the initial non-carbapenem group is shown in Table S1.
Fig 1.
Patient flowchart.
The baseline characteristics of the 268 patients are summarized in Table 1. There were no statistically significant differences between the two groups, except for antimicrobial exposure from hospitalization to study inclusion, the sources of infection, and bloodstream infection. In the initial carbapenem group, the median duration of carbapenem use as initial empirical therapy was 6 days (interquartile range: 3–9). Eighteen patients (18%) in the initial carbapenem group and 27 patients (16%) in the initial non-carbapenem group had died in the hospital by day 28. The odds ratio (OR) for the use of carbapenems for in-hospital mortality by day 28 was 1.25 (95% confidence interval (CI): 0.59–2.65) in the multivariate analysis (Table 2).
TABLE 1.
Patient characteristics
| Variablea | Overall cohort (n = 268) | Initial carbapenem (n = 99) | Initial non-carbapenem (n = 169) |
|---|---|---|---|
| Age (yr)b | 73 (60–80) | 70 (62–80) | 74 (58–81) |
| Male sex, no. (%) | 151 (56) | 55 (56) | 96 (57) |
| APACHE II on ICU admissionb | 21 (15–26) | 21 (16–26) | 20 (15–27) |
| Abx exposure before inclusion, no. (%) | 123 (46) | 60 (61) | 63 (37) |
| Comorbidity | |||
| Chronic pulmonary disease, no. (%) | 28 (10) | 12 (12) | 16 (9) |
| Chronic hepatic disease, no. (%) | 15 (6) | 6 (6) | 9 (5) |
| Chronic renal disease, no. (%) | 32 (12) | 13 (13) | 19 (11) |
| Diabetes mellitus, no. (%) | 57 (21) | 25 (25) | 32 (19) |
| Cardiovascular disease, no. (%) | 61 (23) | 18 (18) | 43 (25) |
| Solid tumor, no. (%) | 43 (16) | 14 (14) | 29 (17) |
| Hematologic malignancy, no. (%) | 9 (3) | 6 (6) | 3 (2) |
| Cerebrovascular disease, no. (%) | 31 (12) | 14 (14) | 17 (10) |
| Healthcare exposurec, no. (%) | 115 (43) | 43 (43) | 72 (43) |
| Immunosuppression statusd, no. (%) | 37 (14) | 16 (16) | 21 (12) |
| Colonization with MDR pathogens prior to initiation of empirical antimicrobialse, no. (%) | 11 (4) | 6 (6) | 5 (3) |
| Source of infection | |||
| Abdominal, no. (%) | 66 (25) | 40 (40) | 26 (15) |
| Respiratory, no. (%) | 93 (35) | 17 (17) | 76 (45) |
| Urogenital, no. (%) | 24 (9) | 8 (8) | 16 (9) |
| Others, no. (%) | 93 (35) | 34 (34) | 59 (35) |
| Bloodstream infection, no. (%) | 68 (25) | 37 (37) | 31 (18) |
| Microbiologically documented infection, no. (%) | 137 (51) | 59 (60) | 78 (46) |
| Septic shock, no. (%) | 78 (29) | 35 (35) | 43 (25) |
APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; Abx, antibiotics; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; MDR, multidrug-resistant.
Data are presented as median (interquartile range).
Hospitalization for ≥2 days in the 12 months preceding study inclusion, antimicrobial exposure in the previous 3 months preceding study inclusion, resident in a nursing home or long-term care facility, receiving chronic hemodialysis or receiving invasive procedures (at home or in an outpatient clinic) in the previous 30 days preceding study inclusion.
Congenital immunodeficiency, neutropenia (absolute neutrophil count <1,000 neutrophils/μL), patient receiving corticosteroid treatment (prednisolone or equivalent >0.5 mg/kg/day for >3 months preceding study inclusion), solid organ transplant patient receiving immunosuppressive treatment, bone marrow transplant patient receiving immunosuppressive treatment, administration of chemotherapy within 1 year preceding study inclusion, administration of radiotherapy within 1 year preceding inclusion, patient with autoimmune disease receiving immunosuppressive treatment, or with HIV or AIDS.
Defined as the detection of MDR pathogens presumed to be present upon the ICU admission. This included those detected within 1 year prior to study inclusion, and those not present on ICU admission and detected before day 2 (day 0 was considered the start date of the empirical antimicrobial therapy).
TABLE 2.
Odds ratios and P values of mortality at day 28 and ICU mortality
| Outcomeb | Initial carbapenem (n = 99) | Initial non-carbapenem (n = 169) | Univariate | Multivariatea | ||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |||
| Mortality at day 28, no. (%) | 18 (18) | 27 (16) | 1.17 (0.61–2.25) | 0.735 | 1.25 (0.59–2.65) | 0.564 |
| ICU mortality, no. (%) | 11 (11) | 17 (10) | 1.12 (0.50–2.49) | 0.837 | 1.80 (0.71–4.56) | 0.216 |
APACHE II score on ICU admission, antimicrobial exposure between hospitalization and the day of inclusion, and source of infection were used for multivariate logistic regression analysis.
ICU, intensive care unit; CI, confidence interval; APACHE, Acute Physiology and Chronic Health Evaluation.
There was no significant difference regarding secondary outcomes between the two groups. Eleven patients (11%) in the initial carbapenem group and 17 patients (10%) in the initial non-carbapenem group died in the ICU. The odds ratio for ICU mortality by day 28 was 1.80 (0.71–4.56) in the multivariate analysis (Table 2). The inappropriate initial antimicrobial administration was 6 (6%) in the initial carbapenem group, and 7 (4%) in the initial non-carbapenem group (P = 0.559). The number of days spent in the ICU and hospital following the onset of infection under study [measured from inclusion (day 0) to day 28 and assessed in subgroups of ICU survivors (n = 240) and patients alive at day 28 (n = 223)] was not statistically different between the initial carbapenem and initial non-carbapenem groups [median (interquartile range) days in the ICU: 7 (4–16) vs 7 (4–18), P = 0.675; days in the hospital: 28 (20–28) vs 28 (19–28), P = 0.651]. Similarly, the length of stay (days) in the ICU and hospital on day 28 was not statistically different between the two groups {ICU [median (interquartile range)]: 7 (3–17) vs 7 (4–20), P = 0.262; hospital: 28 (22–30) vs 28 (21–30), P = 0.985}.
MDR pathogens were newly detected in 11 patients (11%) in the initial carbapenem group and 13 patients (8%) in the initial non-carbapenem group (P = 0.380). Two hundred and sixty-four patients were analyzed to evaluate the association between the detection of MDR pathogens and the duration of carbapenem use as the initial antimicrobial administration. Four patients were excluded owing to missing data regarding the detection of MDR pathogens. The association between the detection of MDR pathogens and the duration of use of carbapenems was statistically significant; subdistribution hazard ratios (sHRs) for detecting MDR pathogens according to the duration of carbapenem use were 1.08 (95% CI: 1.05–1.13) in the multivariate analysis (Table 3). A list of newly detected MDR pathogens is shown in Table S2.
TABLE 3.
Subdistribution hazard ratios and P values for detecting MDR pathogens according to the duration of carbapenem use as the initial antimicrobial administrationa
| Univariateb | Multivariatec | |||
|---|---|---|---|---|
| sHR (95% CI) for detection of MDR | P value | sHR (95% CI) for detection of MDR | P value | |
| Carbapenem use as initial antimicrobial administration (per additional day of carbapenem use) | 1.08 (1.05–1.11) | <0.001 | 1.08 (1.05–1.13) | <0.001 |
MDR, multidrug-resistant; sHR, subdistribution hazard ratio; CI, confidence interval; APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit.
Patients (n = 264) without missing data were analyzed.
APACHE II score on ICU admission, antimicrobial exposure between hospitalization and the day of inclusion, and source of infection were used for multivariate analysis.
The subgroup analysis of patients treated with monotherapy (n = 179 for mortality at day 28 and ICU mortality; n = 177 for the detection of new MDR pathogens) showed results consistent with our main analysis (Tables S3 and S4).
DISCUSSION
In this post hoc analysis of a prospective observational study of ICU patients with suspected or confirmed bacterial infections, we found no statistical difference in mortality by empirical use of carbapenems as initial antimicrobial therapy. We also demonstrated that a longer duration of carbapenem use resulted in a higher risk of emergence of new MDR pathogens.
One of the most common reasons for the use of carbapenems is to avoid inappropriate initial antimicrobial administration, which means that causative pathogens were in vitro not susceptible, considering that the treatment for patients with severe infections is time-sensitive (1, 2). However, the proportions of inappropriate initial antimicrobial administration in our study were 6% in the initial carbapenem group and 4% in the initial non-carbapenem group. These proportions were much lower than those reported in previous studies. One systematic review and meta-analysis reported that the overall proportion of inappropriate administration was 14.1%–78.9% (27). We assume that this discrepancy may be explained by recent improvements in the quality of treatment and those reflecting the guidelines for severe infections including those in Japan, such as enhanced clinical skills for estimating causative microorganisms and more precise risk assessment for MDR pathogens (7–13, 28), although further studies are required. The lack of significant difference in the mortality between those who were not administered carbapenems and those with carbapenem use in our study, which was similar to the recent study (29), may be associated with this low proportion of inappropriate initial antimicrobial administration in both groups.
We emphasize that our study does not imply that carbapenems are not required in actual clinical practice. For patients at high risk of MDR, we should not hesitate to use broad-spectrum antibiotics, including carbapenems (17, 18). A previous study reported that the unnecessary use of broad-spectrum antibiotics and inappropriate use of narrow-spectrum antibiotics were associated with poor patient outcomes (30). Although we did not evaluate whether the use of carbapenems as initial therapy in our study was necessary or too broad, we believe that our study highlights the importance of appropriate risk assessment for MDR pathogens and the selection of “appropriate” antibiotics according to risk. Further study is warranted from this perspective.
Detection of MDR pathogens, regardless of colonization or infection, is associated with poor patient outcomes (31–33). Although a previous study investigated the association between the duration of carbapenem use and subsequent infection with MDR pathogens (26), no study has investigated the association between the duration of carbapenem use as initial empirical therapy for critically ill patients with infections and the subsequent detection of MDR pathogens, regardless of colonization or infection. To the best of our knowledge, our study is the first to show that a longer duration of carbapenem use as initial therapy increases the rate of subsequent detection of MDR pathogens. Our findings suggest that care should be taken regarding the duration of carbapenem exposure when choosing initial empirical therapy and treatment should be de-escalated to more narrow-spectrum antibiotics as soon as possible if other antibiotics could be effective for the patient.
Our study had several limitations. First, because this was a post hoc analysis of a prospective observational study, a randomized controlled trial with sufficient participants is required to confirm the non-superiority of carbapenems as an initial empirical therapy in a well-selected patient population. Second, this study only included data obtained from participating hospitals in Japan. We need to confirm whether our findings are consistent with those of other countries, although our treatment strategy follows the guidelines for severe infections, including the Japanese national guidelines for sepsis, which are not significantly different from those of other countries (34). In addition, the proportion of resistance in Japan is not far from the majority of those reported worldwide (35). Third, we could not assess the effect of antibiotic duration on detecting new MDR pathogens because of multicollinearity. Therefore, as future studies, investigating the effects of overall exposure to antimicrobial agents in critically ill patients with bacterial infections would be beneficial.
In conclusion, in-hospital mortality was similar between critically ill patients who were administered carbapenems as initial empirical antimicrobials and those who were treated with other antibiotics, with a longer duration of carbapenem use resulting in a higher risk of new detection of MDR pathogens.
MATERIALS AND METHODS
Study design
This was a post hoc analysis of data obtained from Japanese participants of a prospective, multicenter international observational study (DIANA study), which analyzed 1,495 critically ill adult patients receiving empirical antimicrobial therapy for suspected or confirmed bacterial infections at 152 ICUs in 28 countries from October 2016 to May 2018 (6). Patients were included in the DIANA study if they were 18 years or older, admitted to an ICU with an anticipated need for at least 48 h of ICU support, and were treated with empirical antimicrobial therapy in the ICU, or no more than 24 h prior to ICU admission to treat a suspected or confirmed community-, healthcare-, hospital-, or ICU-acquired bacterial infection. Patients were excluded if they were already included in the DIANA study or had insufficient data on infection and/or microbiology. In this post hoc analysis, patients from 31 Japanese ICUs were included in the DIANA study. Patients with missing values for the variables required for analysis were excluded. The patients were divided into two groups: one group was administered carbapenems as initial empirical antimicrobial therapy (initial carbapenem group) and the other group without carbapenems (initial non-carbapenem group).
The study was conducted by the Declaration of Helsinki and was approved by the Institutional Review Board of Hiroshima University, which waived the requirement for informed consent to ensure participant anonymity, as stipulated in the Japanese government guidelines (approval no. E2021-2721).
Data set
In the original DIANA study, data on patients, infections (including information on MDR pathogens), antimicrobial treatment, and outcomes were collected from the day of study inclusion (day 0), defined as the start date of empirical antimicrobial therapy, to day 28 following initiation.
Definitions
Initial empirical antimicrobial administration was defined as the administration when the causative pathogen and susceptibility pattern were unidentified at the time of initiation of antimicrobial therapy. Day 0 was considered the start date of the empirical antimicrobial therapy. The detection of MDR pathogens was defined as detection between days 2 and 28 and absence before day 2, and the positive sample sites were categorized as follows: nose swab, throat swab, respiratory tract samples, urine samples, rectal swab/fecal samples, blood culture, perioperative samples, and others. Colonization with MDR pathogens before the initiation of empirical antimicrobials was defined as the detection of MDR pathogens presumed to be present upon ICU admission. It included those detected within 1 year prior to study inclusion, and those not present on ICU admission and detected before day 2. Multidrug resistance was defined as a pathogen producing extended-spectrum beta-lactamase or carbapenemase, Stenotrophomonas maltophilia, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus sp., or a pathogen resistant to three or more antimicrobial classes, according to the publication of Magiorakos et al. (36). Empirical therapy was considered inappropriate when a causative pathogen resistant to the initial agent(s) was present that leading to the addition or replacement of the empirical antimicrobial administration.
Outcome measurement
The primary outcomes were in-hospital mortality and detection of new MDR pathogens by day 28. Secondary outcomes were ICU mortality by day 28, inappropriate empirical antimicrobial administrations, and number of days in ICU and hospital following the onset of infection under study [measured from inclusion (day 0) to day 28, and assessed in subgroups of ICU survivors and patients alive at day 28, respectively]. Mortality was compared between the two groups. The risk of new detection of MDR pathogens was evaluated according to the duration (days) of use of carbapenems as the initial antimicrobial therapy; the duration was regarded as zero for the initial non-carbapenem group. Subgroup analyses were performed for patients treated with monotherapy.
Statistical analysis
A complete case analysis was conducted. Continuous variables were expressed as medians (interquartile range: 25–75), and categorical variables were expressed as proportions (%). Fisher’s exact test was used to compare categorical variables between the groups. The Mann–Whitney U-test was used to compare the continuous variables. In the multivariate logistic regression analysis, the adjustment factors were chosen beforehand according to clinical aspects: Acute Physiology and Chronic Health Evaluation II (APACHE II) scores on ICU admission, antimicrobial exposure between hospitalization and the day of inclusion, and the source of infection (abdominal, respiratory, urogenital, or others) (37–42). To determine the risk of detection of MDR pathogens according to the duration (days) of use of carbapenems as the initial antimicrobial therapy, the Fine-Gray model, with death as a competing event, was used. The adjustment factor was the same as that used in multivariate logistic regression analysis.
All reported P values were two-sided, and statistical significance was set at P < 0.05. All analyses were performed using R, version 4.2.2 (Vienna University of Economics and Business, Vienna, Austria) and JMP Pro 16 software (SAS Institute, Cary, NC, USA).
ACKNOWLEDGMENTS
We would like to thank Michihito Kyo and Shingo Ohki who advised us on the manuscript design and the study design. We also would like to thank Gaku Aoki, Reo Kawano, and Tomoyuki Akita who advised us on statistical analysis.
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
J.I., M.N., Y. Kida, and N.S. conceived the research idea and designed the study. L.D. B. and J. D. W. are the DIANA study principal investigators. They gathered and organized the patients’ data and supervised the writing of the manuscript. S.F. served as the Japanese national coordinator of the DIANA study. A.T., A. Kuriyama, A. Kobayashi, C.T., H. Hashi, H. Hashimoto, H.N., M.S., M.K., M.I., S.H., S. Katayama, S.F., S. Kameda, S.S., T. Komuro, T. Kawagishi., Y. Kida, Y.F., Y. Kawano, Y.H., and H.Y. gathered the patients’ data as local coordinators and contributed to the manuscript. J.I., M.N., Y. Kida., H.Y., and N.S. drafted the manuscript and took public responsibility for the contents of this paper. All authors read and approved the final manuscript.
Contributor Information
Junki Ishii, Email: ishii824@hiroshima-u.ac.jp.
Paschalis Vergidis, Mayo Foundation for Medical Education and Research, Rochester, Minnesota, USA.
DATA AVAILABILITY
Data are available upon reasonable request with the permission of participating facilities.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00342-24.
Tables S1-S4.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. 2017. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 376:2235–2244. doi: 10.1056/NEJMoa1703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. 2014. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 42:1749–1755. doi: 10.1097/CCM.0000000000000330 [DOI] [PubMed] [Google Scholar]
- 3. Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, Peters C, Ahsan M, Chateau D, Cooperative Antimicrobial Therapy of Septic Shock Database Research Group . 2009. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136:1237–1248. doi: 10.1378/chest.09-0087 [DOI] [PubMed] [Google Scholar]
- 4. Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155. doi: 10.1378/chest.118.1.146 [DOI] [PubMed] [Google Scholar]
- 5. Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863. doi: 10.1128/AAC.00627-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Bus L, Depuydt P, Steen J, Dhaese S, De Smet K, Tabah A, Akova M, Cotta MO, De Pascale G, Dimopoulos G, Fujitani S, Garnacho-Montero J, Leone M, Lipman J, Ostermann M, Paiva J-A, Schouten J, Sjövall F, Timsit J-F, Roberts JA, Zahar J-R, Zand F, Zirpe K, De Waele JJ, DIANA study group . 2020. Antimicrobial de-escalation in the critically ill patient and assessment of clinical cure: the DIANA study. Intensive Care Med 46:1404–1417. doi: 10.1007/s00134-020-06111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, et al. 2021. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Critical Care Medicine 49:e1063–e1143. doi: 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 8. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552. doi: 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 9. IDSA Sepsis Task Force . 2018. Infectious diseases society of America (IDSA) POSITION STATEMENT: why IDSA did not endorse the surviving sepsis campaign guidelines. Clin Infect Dis 66:1631–1635. doi: 10.1093/cid/cix997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, Wilson APR. 2018. Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British society for antimicrobial chemotherapy/healthcare infection society/British infection association joint working party. J Antimicrob Chemother 73:iii2–iii78. doi: 10.1093/jac/dky027 [DOI] [PubMed] [Google Scholar]
- 11. Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, Pulcini C, Garnacho-Montero J, Seme K, Tumbarello M, Lindemann PC, Gandra S, Yu Y, Bassetti M, Mouton JW, Tacconelli E, Rodríguez-Baño J. 2022. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect 28:521–547. doi: 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 12. Seah VXF, Ong RYL, Lim ASY, Chong CY, Tan NWH, Thoon KC. 2017. Impact of a carbapenem antimicrobial stewardship program on patient outcomes. Antimicrob Agents Chemother 61:e00736-17. doi: 10.1128/AAC.00736-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells DA, Johnson AJ, Lukas JG, Mason D, Cleveland KO, Bissell A, Hobbs ALV. 2022. Criteria restricting inappropriate meropenem empiricism (CRIME): a quasi-experimental carbapenem restriction pilot at a large academic medical centre. Int J Antimicrob Agents 60:106661. doi: 10.1016/j.ijantimicag.2022.106661 [DOI] [PubMed] [Google Scholar]
- 14. America IDSo . 2022. IDSA guidance on the treatment of antimicrobial-resistant gram-negative infections, version 1.0. Available from: https://wwwidsocietyorg/practice-guideline/amr-guidance/
- 15. Palacios-Baena ZR, Gutiérrez-Gutiérrez B, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, et al. 2017. Empiric therapy with carbapenem-sparing regimens for bloodstream infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: results from the INCREMENT cohort. Clin Infect Dis 65:1615–1623. doi: 10.1093/cid/cix606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, et al. 2016. A multinational, preregistered cohort study of beta-lactam/beta-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4159–4169. doi: 10.1128/AAC.00365-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ofer-Friedman H, Shefler C, Sharma S, Tirosh A, Tal-Jasper R, Kandipalli D, Sharma S, Bathina P, Kaplansky T, Maskit M, Azouri T, Lazarovitch T, Zaidenstein R, Kaye KS, Marchaim D. 2015. Carbapenems versus piperacillin-tazobactam for bloodstream infections of nonurinary source caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Infect Control Hosp Epidemiol 36:981–985. doi: 10.1017/ice.2015.101 [DOI] [PubMed] [Google Scholar]
- 18. Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership Group . 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á, Extended-Spectrum Beta-Lactamases–Red Española de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group . 2012. β-lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174. doi: 10.1093/cid/cir790 [DOI] [PubMed] [Google Scholar]
- 20. Kang CI, Park SY, Chung DR, Peck KR, Song JH. 2012. Piperacillin-tazobactam as an initial empirical therapy of bacteremia caused by extended-spectrum beta-Lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Infect 64:533–534. doi: 10.1016/j.jinf.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 21. Ng TM, Khong WX, Harris PNA, De PP, Chow A, Tambyah PA, Lye DC. 2016. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLoS One 11:e0153696. doi: 10.1371/journal.pone.0153696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gudiol C, Royo-Cebrecos C, Abdala E, Akova M, Álvarez R, Maestro-de la Calle G, Cano A, Cervera C, Clemente WT, Martín-Dávila P, et al. 2017. Efficacy of beta-lactam/beta-lactamase inhibitor combinations for the treatment of bloodstream infection due to extended-spectrum-beta-lactamase-producing Enterobacteriaceae in hematological patients with neutropenia. Antimicrob Agents Chemother 61:e00164-17. doi: 10.1128/AAC.00164-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ko JH, Lee NR, Joo EJ, Moon SY, Choi JK, Park DA, Peck KR. 2018. Appropriate non-carbapenems are not inferior to carbapenems as initial empirical therapy for bacteremia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: a propensity score weighted multicenter cohort study. Eur J Clin Microbiol Infect Dis 37:305–311. doi: 10.1007/s10096-017-3133-2 [DOI] [PubMed] [Google Scholar]
- 24. Son SK, Lee NR, Ko JH, Choi JK, Moon SY, Joo EJ, Peck KR, Park DA. 2018. Clinical effectiveness of carbapenems versus alternative antibiotics for treating ESBL-producing Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 73:2631–2642. doi: 10.1093/jac/dky168 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida H, Motohashi T, De Bus L, De Waele J, Takaba A, Kuriyama A, Kobayashi A, Tanaka C, Hashi H, Hashimoto H, et al. 2022. Use of broad-spectrum antimicrobials for more than 72 H and the detection of multidrug-resistant bacteria in Japanese intensive care units: a multicenter retrospective cohort study. Antimicrob Resist Infect Control 11:119. doi: 10.1186/s13756-022-01146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donaldson AD, Razak L, Liang LJ, Fisher DA, Tambyah PA. 2009. Carbapenems and subsequent multiresistant bloodstream infection: does treatment duration matter?. Int J Antimicrob Agents 34:246–251. doi: 10.1016/j.ijantimicag.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 27. Marquet K, Liesenborgs A, Bergs J, Vleugels A, Claes N. 2015. Incidence and outcome of inappropriate in-hospital Empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care 19:63. doi: 10.1186/s13054-015-0795-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Society of America and the society for healthcare epidemiology of America. Clin Infect Dis 62:e51–e77. doi: 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umemura Y, Yamakawa K, Tanaka Y, Yoshimura J, Ogura H, Fujimi S. 2023. Efficacy of carbapenems compared with noncarbapenem broad-spectrum beta-lactam antibiotics as initial antibiotic therapy against sepsis: a nationwide observational study. Crit Care Med 51:1210–1221. doi: 10.1097/CCM.0000000000005932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhee C, Kadri SS, Dekker JP, Danner RL, Chen HC, Fram D, Zhang F, Wang R, Klompas M, Program CPE. 2020. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open 3:e202899. doi: 10.1001/jamanetworkopen.2020.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cosgrove SE. 2006. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 42 Suppl 2:S82–9. doi: 10.1086/499406 [DOI] [PubMed] [Google Scholar]
- 32. Gandra S, Tseng KK, Arora A, Bhowmik B, Robinson ML, Panigrahi B, Laxminarayan R, Klein EY. 2019. The mortality burden of multidrug-resistant pathogens in India: a retrospective, observational study. Clin Infect Dis 69:563–570. doi: 10.1093/cid/ciy955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallaher JR, Banda W, Lachiewicz AM, Krysiak R, Cairns BA, Charles AG. 2018. Colonization with multidrug-resistant Enterobacteriaceae is associated with increased mortality following burn injury in sub-Saharan Africa. World J Surg 42:3089–3096. doi: 10.1007/s00268-018-4633-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egi M, Ogura H, Yatabe T, Atagi K, Inoue S, Iba T, Kakihana Y, Kawasaki T, Kushimoto S, Kuroda Y, et al. 2021. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). J Intensive Care 9:53. doi: 10.1186/s40560-021-00555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. OECD . 2018. Stemming the superbug tide – just A few dollars more. OECD health policy studies [Google Scholar]
- 36. Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 37. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. [PubMed] [Google Scholar]
- 38. Huang H, Chen B, Liu G, Ran J, Lian X, Huang X, Wang N, Huang Z. 2018. A multi-center study on the risk factors of infection caused by multi-drug resistant Acinetobacter baumannii . BMC Infect Dis 18:11. doi: 10.1186/s12879-017-2932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rello J, Ausina V, Ricart M, Castella J, Prats G. 1993. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest 104:1230–1235. doi: 10.1378/chest.104.4.1230 [DOI] [PubMed] [Google Scholar]
- 40. Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. 2010. Accuracy of American thoracic society/infectious diseases society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect 16:902–908. doi: 10.1111/j.1469-0691.2009.03027.x [DOI] [PubMed] [Google Scholar]
- 41. Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall J-R, Payen D, Sepsis Occurrence in Acutely Ill Patients Investigators . 2006. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a [DOI] [PubMed] [Google Scholar]
- 42. Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. 2014. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311:1308–1316. doi: 10.1001/jama.2014.2637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4.
Data Availability Statement
Data are available upon reasonable request with the permission of participating facilities.