ABSTRACT
In the next decades, the increasing material and energetic demand to support population growth and higher standards of living will amplify the current pressures on ecosystems and will call for greater investments in infrastructures and modern technologies. A valid approach to overcome such future challenges is the employment of sustainable bio-based technologies that explore the metabolic richness of microorganisms. Collectively, the metabolic capabilities of Chloroflexota, spanning aerobic and anaerobic conditions, thermophilic adaptability, anoxygenic photosynthesis, and utilization of toxic compounds as electron acceptors, underscore the phylum’s resilience and ecological significance. These diverse metabolic strategies, driven by the interplay between temperature, oxygen availability, and energy metabolism, exemplify the complex adaptations that enabled Chloroflexota to colonize a wide range of ecological niches. In demonstrating the metabolic richness of the Chloroflexota phylum, specific members exemplify the diverse capabilities of these microorganisms: Chloroflexus aurantiacus showcases adaptability through its thermophilic and phototrophic growth, whereas members of the Anaerolineae class are known for their role in the degradation of complex organic compounds, contributing significantly to the carbon cycle in anaerobic environments, highlighting the phylum’s potential for biotechnological exploitation in varying environmental conditions. In this context, the metabolic diversity of Chloroflexota must be considered a promising asset for a large range of applications. Currently, this bacterial phylum is organized into eight classes possessing different metabolic strategies to survive and thrive in a wide variety of extreme environments. This review correlates the ecological role of Chloroflexota in such environments with the potential application of their metabolisms in biotechnological approaches.
KEYWORDS: filamentous anoxygenic phototroph, photoautotrophic bacteria, extremophiles, biotechnological applications, decontamination technologies, value-added substances production
INTRODUCTION
The utilization of microorganisms in biotechnological processes has been widely reported, and the search for new metabolisms can lead to the development of several innovative technologies focused on environmental decontamination, wastewater treatment, and production of energy and value-added substances (1–4). In all these applications, the adaptability and versatility of microorganisms make them valuable tools for addressing environmental and industrial challenges. In every microbial phylum, we can find interesting metabolisms, and in the specific case of the Chloroflexota phylum, it encompasses a wide spectrum of metabolic diversity, with some organisms exhibiting remarkable traits such as a bicycle-like mechanism for inorganic carbon fixation, others harnessing the power of halogens, and others performing denitrification, although often being involved in the cycling of several elements (5). This diversity, which arises from the natural adaptation of Chloroflexota to harsh environmental conditions, contains a unique array of metabolic processes and distinctive features that can be explored for biotechnological purposes.
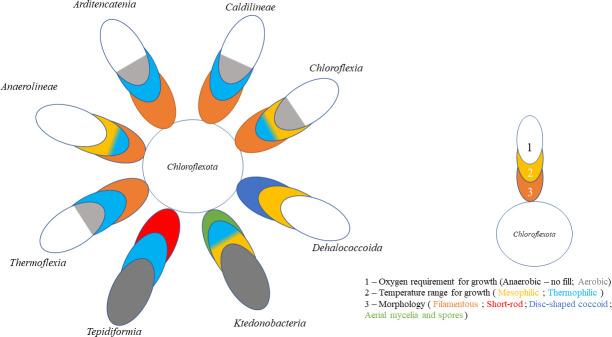
The Chloroflexota bacteria phylum, formerly known as green non-sulfur bacteria (GNSB), comprises extensively diverse microorganisms that can be found in several environments, both terrestrial and aquatic. This phylum nomenclature derives from the species Chloroflexus aurantiacus, first isolated and described by Pierson and Castenholtz (6) as a filamentous anoxygenic phototroph (FAP), a term currently used specifically for phototrophic members of the Chloroflexota. The constant discoveries of new microbial organisms and advances in phylogenetic analysis are leading to a continual redefinition and reorganization of this phylum. Currently, the Chloroflexota phylum is divided into eight classes: Anaerolineae, Ardenticatenia, Caldilineae, Chloroflexia, Dehalococcoidia, Ktedonobacteria, Tepidiformia, and Thermoflexia (Fig. 1). Interestingly, the entire phylum only has 18 families, with many of the classes owing their name to one single genus characteristic of the class.
Fig 1.
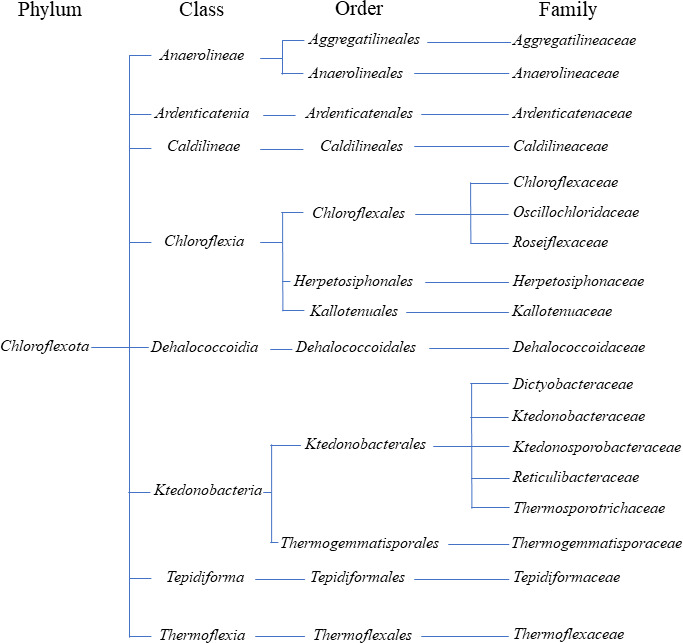
The Chloroflexota phylum is divided into eight classes of bacteria, each with its specific orders and families (https://lpsn.dsmz.de/phylum/chloroflexota, accessed 10 February 2024).
The uniqueness of Chloroflexota members is reviewed in this manuscript, analyzing its metabolic features and placing a great focus on the much-needed overview of the biotechnological potential of this bacterial phylum.
CHLOROFLEXOTA CLASSES AND CHARACTERISTICS
The Chloroflexota phylum is characterized according to the metabolism, phylogeny, cell shape, motility, ability to form multicellular aggregates, and spore formation capacity. There are several similar traits (morphology, environment, and growth conditions) between the constituents of this bacterial phylum, as shown in Fig. 2.
Fig 2.
The Chloroflexota phylum organized in terms of predominant morphology, oxygen requirements, and temperature range for growth (mesophiles: 20°C–45°C; thermophiles: 45°C–80°C).
The class Anaerolineae is divided into two orders, Aggregatilineales and Anaerolineales, each containing a single family. Its representatives are strictly anaerobic chemoorganotrophic organisms with a filamentous morphology. Organisms from this class have been isolated from diverse environments including anaerobic digesters, hot springs, and sub-seafloor sediments (7, 8).
The class Ardenticatenia was proposed based on a single species, Ardenticatena maritima, which was isolated from a costal hydrothermal field, and is a facultative aerobe that can reduce ferric ion and nitrate under anaerobic conditions. This chemoorganotrophic species forms thin multicellular filaments and can grow at temperatures of up to 75°C (9).
The class Caldilineae contains a single described order, Caldilineales, and family, Caldilineaceae, which consists of two genera, Caldilinea and Litorilinea. Species belonging to these genera are aerobic, facultative aerobic, or anaerobic chemoorganotrophic filamentous organisms found in hot springs and hot aquifers (7, 10, 11).
The class Chloroflexia contains the first known members of this phylum that possess phototrophic and/or chemoheterotrophic growth under mesophilic or moderately thermophilic conditions, presenting a filamentous growth morphology. Moreover, the phototrophic members of this class, usually referred to as FAP bacteria, belong to the Chloroflexales order, which can be divided into three families: Chloroflexaceae (photoheterotrophic but also with photoautotrophic abilities), Roseiflexaceae, and Oscillochloridaceae (both predominantly photoheterotrophic). The first two families are composed of thermophilic FAP bacteria isolated from terrestrial hot springs, whereas the latter contains photoheterotrophic mesophilic freshwater species (12, 13). Although phototrophy in Chloroflexota isolates is limited to Chloroflexia class, metagenomic studies indicate the existence of potentially phototrophic members also in other classes, which could be attributed to horizontal gene transfer of sequences for reaction center and bacteriochlorophyll synthesis proteins (14). The Chloroflexia class has two additional orders, Herpetosiphonales and Kallotenuales, containing non-photosynthetic species that rely on heterotrophic metabolism for growth (12, 15).
The class Dehalococcoidia is characterized by the disc-shaped coccoid form of its members, instead of the filamentous morphology attributed to other classes within the Chloroflexota phylum. All isolates from this class can grow in chemoorganotrophic conditions and perform dehalogenation of chlorinated and brominated alkanes under strict anaerobic conditions, which grants them great importance in the bioremediation field (16).
The class Ktedonobacteria contains heterotrophic bacteria capable of growing under microaerophilic conditions. They have been isolated from soil samples with the peculiarity of forming aerial mycelia and spores. This class is divided into two orders, Ktedonobacterales and Thermogemmatisporales. The order Ktedonobacterales contains all the mesophilic and some thermophilic representatives of this class, whereas the Thermogemmatisporales order encompasses only thermophilic species (17–22).
The class Tepidiformia is composed of one order, Tepidiformales, and one family, Tepidiformaceae, containing moderately thermophilic bacteria with a regular short rod morphology, being able to grow hetero- or auto-trophically in aerobic conditions (23).
The class Thermoflexia, proposed based on its type species Thermoflexus hugenholtzii, englobes filamentous thermophilic chemoheterotrophic microaerobes (optimally growing at 1% (vol/vol) O2 with an upper limit of 8% O2) being also facultatively anaerobic (24).
CHLOROFLEXOTA METABOLISMS AND ENVIRONMENTS
There are several metabolisms present within the Chloroflexota phylum, such as anoxygenic phototrophy, obligate anaerobic heterotrophy, organohalide respiration, and facultative or aerobic heterotrophy. For this reason, bacteria from the Chloroflexota phylum can be found in several environments (natural or industrial) with different properties and singularities (25–29). Their metabolic versatility allowed the adaptation of Chloroflexota to microbial mats, soils, aquatic environments, and other extreme environments as shown in Fig. 3.
Fig 3.
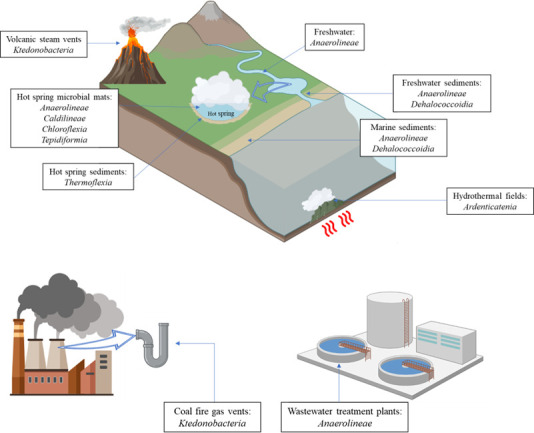
Natural and industrialized environments where Chloroflexota can be found. The pictures were designed with templates available at https://www.biorender.com/.
Anoxygenic phototrophy
The first metabolism to be described in this phylum was anoxygenic phototrophic growth (6), in which the filamentous phototrophs use light energy to generate chemical energy in the form of ATP. The isolated members from the Chloroflexota phylum exhibiting photoautotrophy are exclusively contained in the Chloroflexia class; however, most organisms belonging to this class demonstrated the ability to grow photoheterotrophically assimilating organic carbon compounds (30–33), or chemotrophically under aerobic dark conditions. Chloroflexia is commonly found in phototrophic microbial mat communities, specifically in neutral and alkaline spring waters with temperatures between 40°C and 70°C (6, 34–36).
To perform their main metabolism, the phototrophic bacteria rely on light-harvesting organelles known as chlorosomes, oval structures attached to the inner surface of the cytoplasmic membrane, consisting of paracrystalline aggregates of bacteriochlorophylls that are surrounded by a galactolipid non-unit membrane (37). Regarding the antenna pigments, there are some slight differences within the order Chloroflexales: for example, members of the Chloroflexus and Oscillochloris genera possess bacteriochlorophyll c, whereas Chloronema is rich in bacteriochlorophylls c and d (31, 32). The presence of bacteriochlorophylls c or d gives cells their green color, and carotenoids (β- and γ-carotene, and derivatives) are also present as part of the light-harvesting cellular apparatus. Roseiflexus castenholzii and Heliothrix oregonensis (from the family Roseiflexaceae) contain solely bacteriochlorophyll a, which makes them chlorosome-free. These cells usually present a red/orange color because of their oxo-γ-carotene and glucoside-rich nature. Moreover, the carotenoid β-carotene (which is present in Chloroflexus, Oscillochloris, and Chloronema) is missing in the red-colored genera Roseiflexus and Heliothrix (38–40).
In terms of inorganic carbon fixation, C. aurantiacus can use the 3-hydropropionate bi-cycle, in which bicarbonate fixation is proceeded by the carboxylation of acetyl-CoA and propionyl-CoA yielding pyruvate as the net product; glyoxylate, an intermediate of the bi-cycle, can also be assimilated into cell material (41–44). Energy-wise, inorganic carbon fixation via the 3-hydroxypropionate bi-cycle requires 7 ATP, 5 NAD(P)H, and 3 HCO3− to produce one pyruvate. Comparatively, alternative carbon fixation pathways found in other phototrophs can produce pyruvate through the reverse tricarboxylic cycle, which requires, equivalently, 3 CO2, 2 ATP, and 5 NAD(P)H, whereas the Calvin-Benson-Bassham cycle inputs 3 CO2, 7 ATP, and 5 NAD(P)H to produce one pyruvate molecule (45, 46). However, the 3-hydroxypropionate bi-cycle allows the consumption of bicarbonate, which can provide a competitive advantage to Chloroflexus in alkaline environments or under low carbon dioxide availability (47). To support inorganic carbon fixation, different compounds can be used as electron sources, such as sulfide, thiosulfate, small organic molecules, or molecular hydrogen (45, 48–50).
Given the ability to use a wide range of electron donors, members of the Chloroflexia class (specifically members of the Chloroflexales order) have also been observed in marine and hypersaline microbial mats (51, 52), which points to the adaptability of such organisms to different environments. Indeed, the presence of Chloroflexales members has also been reported in extreme soil environments such as sediments from the Arctic (53). Furthermore, Chloroflexia has been isolated from volcanic vents (54), and Chloroflexus islandicus has been isolated from a geyser in Iceland (30). Ecologically, Chloroflexia serves as both primary and secondary producers (55–57), performing mixotrophic growth using CO2 and simple organic molecules as carbon sources.
Anaerobic heterotrophy
Strict anaerobic chemoheterotrophic growth has been reported in the classes Anaerolineae and Dehalococcoidia. The strict anaerobic metabolisms of Anaerolineae are characterized by a fermentative metabolism, with some isolates performing sugar fermentation to produce acetate, lactate, succinate, propionate, and hydrogen and this class has shown to be present in methanogenic sludge systems (58), wastewaters with recalcitrant compounds (59), and sugar-fed microbial fuel cells systems (60, 61). Additionally, members of the Anaerolineae class have been reported to contribute to the transformation of cellulose and hemi-cellulose to smaller carbon molecules such as lactate, formate, and acetate, even in adverse conditions, such as uranium-rich sediments, which was possible, given the presence of genes related to uranium tolerance (62). Members of the Anaerolineae classes can also be found in marine environments contributing to the re-cycling of dissolved organic matter and degrading carbohydrates (8, 63, 64).
Bacteria from the Dehalococcoidia class can perform anaerobic organohalide respiration, being repeatedly found in marine sediments at different worldwide locations, often with high relative abundances (65, 66). In fact, Krzmarzick et al. (29) investigated the role of Dehalococcoidia on chlorine cycle by establishing a correlation between their concentration and the concentration of organochlorine compounds, stating the pivotal role of these bacteria in the biogeochemical chlorine cycle (29). Additionally, a sulfur-oxidizing/reducing ability was reported by different authors, which could imply a role of Dehalococcoidia in the sulfur cycle of marine shallow surfaces (67).
Overall, members of the Anaerolineae and Dehalococcoidia contribute to the fermentation of sugars and fixation of carbon dioxide, participating in carbon cycling and constituting around 5%–25% of the bacterial communities detected in freshwater sediments from lakes and rivers (68, 69).
Facultative or aerobic heterotrophy
Aerobic chemoheterotrophic metabolism can be found in the Ardenticatenia, Ktedonobacteria, Tepidiformia, and Thermoflexia classes. A. maritima (sole species of the Ardenticatenia class) is an aerobe that can also use ferric iron as an electron acceptor and tolerates high NaCl concentrations and temperatures, which can explain its abundance in iron-rich coastal hydrothermal fields (9). Members of Ktedonobacteria can grow in mesophilic or thermophilic conditions and have been reported to be dominant in coal-fire gas vents at 58°C, able to oxidize hydrogen and carbon monoxide for its metabolism (70). Additionally, Ktedonobacteria members have been found in steam vents from volcanoes (54). Thermoflexia members have been reported to have optimal growth at 72.5°C–75°C in microaerophilic conditions (1% vol/vol of O2), conditions usually found in hot springs sediments, where these bacteria may be found (24).
Within the aerobic organisms of the Chloroflexota, Tepidiformia class members, here represented by its single species Tepidiforma bonchosmolovskayae, are aerobic bacteria that can grow chemoorganoheterotrophically using different carbohydrates or volatile fatty acids and chemolithoautotrophically using FeCO3 as the electron donor, being usually found in hot springs (23).
Chloroflexota members exhibit several metabolisms, and despite being taxonomically divided into only eight classes, these bacteria can be found in extremely diverse environments adapted to different conditions, playing a role in the cycle of several elements such as carbon, sulfur, and halogens (71). The adaptability of Chloroflexota to different organic matter inputs allows their survival in adverse environments, a feature undoubtedly important for their application in the biotechnological industry.
BIOTECHNOLOGICAL RELEVANCE
The metabolic versatility of Chloroflexota and its natural occurrence in different habitats make this a very interesting group of bacteria to be used in several biotechnological applications, which can range from the production of chemical compounds to the degradation of contaminants, among many others (Fig. 4).
Fig 4.
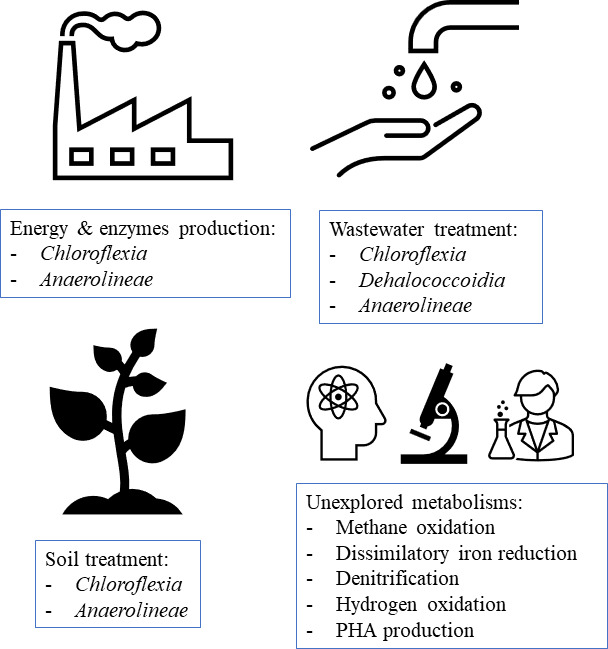
Applications of Chloroflexota members in different biotechnological areas.
In fact, Chloroflexota bacteria can be found as sole contributors to certain applications or as an integrative of mixed microbial culture-based solutions. Regardless, this review aims to discuss Chloroflexota role in each technology, providing insights about the advantages of the utilization of these bacteria and prospecting the unexplored metabolisms that can be applied to the development of new biotechnological approaches.
Production of enzymes and energy
Energy production is fundamental for human activities, powering essentially every aspect of daily life. Transportation, communications, and industrial production are paramount examples of the current energy demands. Complementarily, enzymes are widely applied in various industries (food and beverages, nutrition, textiles, cleaning products, and health and drugs sector) (72–74), ensuring product quality and stability while increasing production efficiency. The development of new approaches to produce enzymes and energy is imperative to decrease the environmental footprint of these activities, namely through the reduction of waste generation as well as water, energy, and raw materials demand.
Within Chloroflexota, the genus Chloroflexus has been a great source of enzymes. Shin et al. cloned and expressed in Escherichia coli the homodimer enzyme α-L-rhamnosidase (200 kDa MW) from C. aurantiacus and purified it as a soluble enzyme to use in the transformation of rutin (the bioflavonoid vitamin P) into isoquercitrin (flavonoid) (75). The obtained product has several important properties acting as an antioxidant, anti-inflammatory, anti-carcinogenic, antidiabetic, and anti-allergic agent (76), with the results displaying a natural ability from C. aurantiacus to effectively produce isoquercitrin. In fact, the purified α-L-rhamnosidase displayed the highest substrate-specific activity when compared with other isoquercitrin-producing enzymes. Moreover, isoquercitrin productivity from C. aurantiacus-derived α-L-rhamnosidase was almost two times higher than commercial α-L-rhamnosidase. Interestingly, this enzyme is also widely used in the industrial field for debittering citrus fruit juices, enhancement of wine aromas, and drug precursor production (77).
C. aurantiacus metabolic versatility was also investigated in the search for thermophilic alcohol dehydrogenases (78). These enzymes catalyze the regio- and stereo-selective reduction in aldehydes or ketones to primary or secondary alcohols, a process applied in several industrial-scale processes (79). Loderer et al. expressed this enzyme gene from C. aurantiacus in E.coli and reported its optimal temperature activity (70°C) and possible substrates, a relevant result given the industries’ demand for a larger diversity of well-characterized enzymes (78). The expressed enzyme showed high tolerance to ethylenediamine tetraacetic acid (EDTA), compared with other alcohol dehydrogenases, reflecting a stronger binding of the catalytic zinc ion, attributed to a more robust enzyme fold derived from a thermophilic host. Overall, the unique properties of the Chloroflexota enzyme (high-temperature activity, substrate versatility, and high tolerance to EDTA) broaden the enzyme’s applicability in various industrial processes, such as in the synthesis of fine chemicals, pharmaceuticals, or biofuels, benefiting from high-temperature stability, substrate flexibility, and robustness in the presence of chelating agents.
Chloroflexia bacteria are indeed a unique reservoir of new biocatalytic activities and the production of ene-reductase enzymes by Chloroflexus aggregans was investigated (80). These enzymes catalyze the asymmetric hydrogenation of alkenes and have collected great interest from academia and industries. Robescu et al. reported that the ene-reductases produced by C. aggregans were robust biocatalysts with high thermostability, presenting acceptable solvent tolerance and a wide range of optimal pH, which can be important for bigger-scale applications of this enzyme.
Chloroflexi bacterium, unclassified Chloroflexota, has been used to obtain ω-transaminases (ω-TA) highly reactive to aromatic amino donors/receptors at pH 8.5, 40°C, and showing affinity to cyclic substrates such as 1-Boc-3-piperidone (81). The enzymatic properties of this Chloroflexota-derived enzyme displayed good thermal stability, organic solvent tolerance, and broad substrate specificity. The ω-TA enzymes produce chiral amines with applications in the medical and fine chemical industries, such as the oral antihyperglycemic drug, sitagliptin. Therefore, the results of this study showed that Chloroflexota is a valuable source of catalysts for the asymmetric synthesis of these chiral amines from the corresponding aldehydes or ketones.
There are several examples of enzyme production with Chloroflexota bacteria, such as α-L-rhamnosidase, alcohol dehydrogenase, ene-reductase, and ω-transaminases. However, the reported studies have been using strains from the Chloroflexia class, which means that given the metabolic diversity presented by Chloroflexota members, there is still a tremendous amount of unexplored potential for enzyme production within this bacterial phylum, and further understanding of metabolic pathways and enzyme functions could help overcome this bottleneck.
Another relevant application is the utilization of Chloroflexota metabolic pathways to produce energy precursors, namely hydrogen or biogas. Both can be used as renewable sources of energy (82), and the development of new production approaches can contribute to the implementation of completely circular and bio-based solutions.
Production of hydrogen by Chloroflexota has been studied through the utilization of catalytic systems based on whole cells or organelles of photosynthetic bacteria specialized in the conversion of light energy into H2 (83). For this reason, Gogotov et al. studied the hydrogenases of several photosynthetic bacteria (including C. aurantiacus), proving the involvement of Ni (from the enzyme Ni-Fe active center) on the activation of molecular hydrogen and reporting a high denaturing factor resistance from these enzymes, which can be attributed to their thermophilic origin. Moreover, hydrogenase from C. aurantiacus was able to reversibly activate H2 at a high rate at more anaerobic conditions, in contrast with other studied hydrogenases that exhibited low activity under similar conditions. The ability to activate hydrogen efficiently at low redox potentials could be an evolutionary adaptation of C. aurantiacus to its natural habitat. In fact, hot springs often present anaerobic and reduced environments, and the microbial life in these locations has evolved mechanisms to optimally harness available resources, like hydrogen, for survival and growth. This feature can also be linked to potential biotechnological applications. For instance, enzymes that are active at low redox potentials and high temperatures could be advantageous in industrial processes involving hydrogen gas, such as in biohydrogen production or in enzymatic fuel cells.
Hydrogen production in photosynthetic microbial mats has been further expanded by biogeochemical and molecular studies reporting H2 production mainly under dark and anoxic conditions (84). The authors stated the importance of incorporating carbon during sunlight availability for dark production of H2 and concluded about the inexistence of competition between nitrogen fixation and H2 production. Chloroflexales participated in this process by being involved in the carbon capture and producing reduction equivalents for the dark production of H2, which states the importance of Chloroflexota bacteria in a mixed microbial culture approach specialized in coupling carbon capture with energy production (84).
Recent research reported the presence of Anaerolineae in the upgrading of antibiotic fermentation residue (AFR) to biogas (85). The study showed that the addition of Fe3O4 acted as a biostimulator for Anaerolineae activity, which enhanced methane production by as high as 48%. The contribution of Anaerolineae for the valorization of this protein-rich biosolid is another proof of the potential role of Chloroflexota bacteria in residue management strategies and energy production.
Biodegradation technologies
Environmentally, microorganisms are the most important agents for the breakdown of organic pollutants or biodegradation, given their ability to use different harmful substances as carbon and energy sources (86). There are several examples of Chloroflexota microbial organisms’ contribution to biodegradation and decontamination technologies.
In the case of phenol-polluted environments, Chloroflexota can be used for their bioremediation. Phenolic compound pollution can be associated with wastewater discharges from several industries (87), with its removal being considered a priority by several countries and entities. Huang et al. assessed and stated the optimal conditions for phenol degradation (1.5 M of NaCl and 350 mg/L of phenol) using a mixed bacterial culture containing Chloroflexus sp. from a saline environment (88). Moreover, the authors studied the metabolic pathways related to phenol biodegradation and reported the importance of phenol hydroxylase, catechol 1,2-dioxygenase, and catechol 2,3-dioxygenase for this process. Furthermore, the dependence of this bacteria on ectoine and hydroxyectoine presence was established, contributing to improving bioremediation strategies in phenol-contaminated saline environments. Sanchéz-González et al. also stated the involvement of Chloroflexales in the degradation of phenol, highlighting the extremophile’s participation on the degradation process and the microbial community strategies to survive under severe environmental conditions (89). Overall, Chloroflexales bacteria can be considered effective phenolic degraders, and given their ability to thrive in anaerobic conditions, their utilization might be advantageous when treating certain types of industrial effluents. Moreover, their ability to establish synergies with other species can lead to more efficient degradation processes compared with microbes working in isolation. In comparison, genera like Pseudomonas, Acinetobacter, and Sphingomonas are more versatile and efficient in aerobic conditions and are widely used in industrial bioremediation processes (90, 91). Fungi offer a different mechanism through extracellular enzymatic degradation and are particularly effective against more complex pollutants (92). However, Chloroflexales might have a unique niche in phenol degradation, especially in anaerobic and extreme environments.
Zhang et al. studied the interactions of bacterial populations along sediment pollution gradients in shallow eutrophic lakes (93). The authors reported that Chloroflexales were among the dominant taxa at severe pollution concentrations, possibly contributing to photosynthesis and pollutant degradation, which further demonstrates the adaptability of Chloroflexota bacteria to adverse conditions and their importance in bioremediation technologies.
The organohalide respiration of Dehalococcoides presents great biotechnological interest in bioremediation applications, and Zanaroli et al. described the dechlorination capacity of members of this class, which was able to dechlorinate more than 75% of polychlorinated biphenyls (PCBs) in just 30 weeks (94). This extensive removal of pollutants is remarkable, given that PCBs are persistent organic pollutants and, due to bioaccumulation, are responsible for negative health effects on humans (95). In addition to being the first dechlorinator identified in marine sediments, the displayed dechlorination activity and specificity were more comprehensive than other bacteria described in the literature. Moreover, the activities of dechlorination took place under biogeochemical circumstances that closely mirror those found naturally in marine environments. This aspect is particularly important when considering the development of customized approaches for encouraging the in situ dechlorination of aged PCBs.
Padilla-Crespo et al. studied the environmental distribution of the genetic sequence encoding for the reductive dehalogenase, which catalyzes the dichloro elimination of 1,2-dichloropropane (a carcinogenic compound formerly used as industrial solvent) to propene (96). The authors reported gene sequences from different continents sharing high sequence identities (>98%), indicating that this enzyme is highly conserved or was recently disseminated. Moreover, Dehalococcoides mccarty, from the Dehalococcoidia class, appeared to be the major microbial contributor for this bioremediation process.
Organohalide-respiring Dehalococcoidia has been described, and its importance in bioremediation technologies has been stated before (16, 97). In fact, Dehalococcoidia presence has also been observed in industrialized estuaries sediments after extreme weather conditions (98). In this study, after Hurricane Harvey in 2017, the presence of several xenobiotic and polychlorinated compounds degrading microorganisms was correlated with sediment properties and contaminant concentrations of the estuary water.
Chloroflexota can also contribute to the biodegradation of polycyclic aromatic hydrocarbons (PAHs) and mutagenic/carcinogenic toxic compounds produced by incomplete combustion of fossil fuels. Specifically, in constructed wetlands, the low dissolved oxygen concentrations decrease the activity of PAHs-degrading microorganisms, such as Pseudomonas aeruginosa and Pseudomonas putida, which require aerobic conditions to perform aromatic hydrocarbon degradation (99). Therefore, the development of anaerobic approaches is important to enhance PAH removal for polluted sites with low available oxygen. Hence, Lu et al. reported the important role of Anaerolineae bacterium in the degradation of PAHs in an iron-enhanced anaerobic process, in which the metal presence functioned as an electron conduit to promote interspecies electron transfer between iron-reducing bacteria and Anaerolineae (100).
Wastewater treatment technologies
The consistently high abundance of Chloroflexota bacteria in wastewater treatment systems illustrates their ecological role in nutrient transformation processes. These bacteria are often identified in nutrient (phosphorous and nitrogen) removal systems acting as anaerobic chemoorganotrophs with sugar fermentation abilities, being present in floccular biomass and/or in bulking-related representatives (101, 102). The understanding of Chloroflexota distribution and physiology is determinant to establish correlations between their ecology and operational issues in full-scale plants. Table 1 summarizes processes for wastewater treatment processes involving Chloroflexota bacteria.
TABLE 1.
Wastewater treatment processes where the involvement of Chloroflexota bacteria was reported
| Water type | Pollutant removal | Process type | Bacteria | Reference |
|---|---|---|---|---|
| Synthetic wastewater | 97% of COD 97% of nitrogen |
Submerged membrane bioreactors | Chloroflexia, Anaerolineae | (103) |
| Municipal wastewater | 90% of DOC 92% of phosphorus 38% of nitrogen |
Submerged membrane bioreactors | Unspecified Chloroflexota | (104) |
| Mainstream wastewater | 90% of nitrogen | Anammox at low temperature | Chloroflexales | (105) |
| Aniline wastewater | 80% of aniline 100% of nitrogen |
Electro-enhanced sequencing batch reactor | Anaerolineae | (106) |
| Domestic saline wastewater | 93% of COD | Membrane-aerated biofilm reactor | Anaerolineae | (107) |
| Saline wastewater | 51.8% of COD | Constructed wetlands | Anaerolineae, Dehalococcoides | (108) |
| Thermal hydrolysis and anaerobic digestion wastewater | 12% of COD 0.58 ± 0.06 g N/(L⋅d) |
Partial nitritation-anammox | Anaerolineae | (109) |
| p-Fluoronitrobenzene (p-FNB) wastewater |
100% of p-FNB | Bioelectrochemical degradation | Anaerolineae | (110) |
| Quinoline wastewater | 83.5% of quinoline | Anaerobic degradation | Anaerolineae | (111) |
| Effluents of wastewater treatment plants | 83% of nitrogen 43% of COD |
Constructed wetlands |
Anaerolineae Other Chloroflexota |
(112) |
As can be observed, these bacteria are present in several treatment processes, contributing to pollutant removal in a wide range of wastewater types, often as dissolved organic matter decomposers.
Specifically, Chloroflexota members have been detected in membrane bioreactors acting as soluble microbial product decomposers (103), this feature being previously demonstrated by the work of Miura et al., who correlated the carbohydrates degradation ability of the system with the concentration of Chloroflexota and stated an imperative Chloroflexota cell concentration above 10% to avoid membrane fouling (104), suggesting the ecological significance of Chloroflexota members in the reduction of membrane fouling in membrane bioreactors.
Chloroflexota bacteria were also found to be important in specific treatments of mainstream wastewaters. Lv et al. reported an abundance of Chloroflexales genera in flocculent sludge establishing symbiotic microbial interactions with anaerobic ammonia-oxidizing bacteria, which favored the annamox process in mainstream wastewaters at low temperatures, maintaining a nitrogen removal efficiency above 90% (105). Therefore, the coexistence of anaerobic ammonia-oxidizing bacteria and Chloroflexales appears to be an effective solution to overcome the challenges of anammox processes at low temperatures.
Other reports displayed the importance of Anaerolineae in the effectiveness of aniline and nitrogen removal (106), treatment of saline wastewater (107, 108), treatment of waste streams rich in ammonium and low on organic compounds (109), biodegradation of p-fluoronitrobenzene (110), complete anaerobic mineralization of quinoline (111), and denitrification processes (112).
Soil treatment technologies
Chloroflexota bacteria have been reported in numerous approaches for soil treatment, contributing as producers of biodegradable organic matter and nutrients, and degrading recalcitrant molecules. They have been found in metal-contaminated soils of abandoned mines, and their concentration varied with the application of different phytostabilization techniques (113). In this study, among other reported bacteria, Chloroflexota organisms belonged to the Chloroflexales order and could perform anaerobic photoheterotrophy and, in dark aerobic conditions, chemoheterotrophy. Moreover, Chloroflexota members can present heavy metal resistance (114), which could imply their involvement in the decontamination of soils, especially in the reduction of heavy metal bioavailability and in the production of biodegradable organic matter.
Studies on the improvement of soil fertility also reported the relevance of Chloroflexota. Huang et al. successfully applied biochar to alleviate salt stress in a rice plantation field, to prevent crop production inhibition (115). The study reported the abundance of Chloroflexota (Anaerolineaceae family members) in the untreated soil, which decreased after the treatment with biochar mainly due to changes in the soil pH, suggesting that the regulation of the bacterial community is a key factor in achieving a satisfactory soil decontamination. Despite decreasing in concentration, Chloroflexota bacteria were still important in the transformation of inorganic carbon into organic matter and production of nutrients such as phosphorous and nitrogen, implying a central role in the symbiotic relationships established between soil bacteria, fungi, and plants. Indeed, in a study by Chen et al., Chloroflexota was demonstrated to be beneficial for soil treatment, acting as organic matter and nutrient producers and contributing to the decrease in N-loss bacterial activities. The authors studied a maize rhizosphere for 3 years and concluded that the presence of bacteria from the unclassified group Chloroflexia KD4-96 in soils contributed not only to plant growth but also to grain production (116).
Chloroflexota has also been demonstrated to participate in composting processes, specifically in the treatment of textile wastes (117). In this study, the authors mixed several textile waste concentrations with paper waste for composting and reported the presence of Chloroflexota bacteria participating in recalcitrant molecular degradation for mixtures containing 40%–60% of textile wastes.
Anaerolineae carbon fixation metabolism, via Arnon-Buchanan cycle (118), was also demonstrated to be important during the reduction of contaminants bioavailability by natural processes in anthropized freshwater sediments with high phosphorous concentrations and alkaline pH (119). The carbon fixation activity of Anaerolineae in surface sediments creates a flux of carbon that aids in the degradation of xenobiotic compounds by bacteria residing in the deeper, non-surface sediments. This dynamic illustrates a noteworthy symbiotic relationship, where the metabolic processes of surface-dwelling Anaerolineae enhance the capacity of deeper sediment bacteria to recover contaminated sites.
Hereabove, a variety of Chloroflexota metabolisms (carbon fixation, nutrient production, switch between anaerobic photoheterotrophy, and, in dark aerobic conditions, chemoheterotrophy) were highlighted, being mostly found in microbial communities for the treatment of soils and sediments. Their wide presence can be attributed to their resistance to adverse environmental conditions, and their role as primary producers may potentiate the activity of other microorganisms, establishing symbiotic relationships in these applications. However, it can be observed that most of the described applications that make use of Chloroflexota mainly report bacteria from the Chloroflexia, Anaerolineae, and Dehalococcoides classes (Table 2). Therefore, the discovery of new metabolic pathways and functions can act as a driving force for the development of biobased technologies containing Chloroflexota bacteria as the main players or as important contributors in mixed microbial systems.
TABLE 2.
Reported applications for bacteria belonging to the Chloroflexota phyluma
| Bacterial class | Application | Metabolism | Reference |
|---|---|---|---|
| Chloroflexia | H2 production | Anaerobic photoheterotrophic growth | (84) |
| Anaerolineae | Biogas production | Carbohydrate hydrolysis and proteolysis | (85) |
| Chloroflexia | Phenolic compound removal | Meta-cleavage pathway | (88, 89) |
| Dehalococcoides | PCB treatment | Dehalogenation | (94, 96) |
| Anaerolineae | Biodegradation of PAHs | Anaerobic heterotrophic growth | (100) |
|
Anaerolineae
Chloroflexia |
Avoidance of membrane fouling in SMBR | Anaerobic heterotrophic growth | (103, 104) |
|
Anaerolineae
Chloroflexia |
N-removal, biodegradation of pollutants | Anaerobic (photo)-heterotrophic growth | (105, 106, 110) |
| Chloroflexia | Reduction of heavy metal availability and production of biodegradable organic matter | Anaerobic photoheterotrophic and chemoheterotrophic growth | (114) |
|
Anaerolineae
Chloroflexia |
Soil treatment | Anaerobic photoautotrophic and heterotrophic growth | (115, 116) |
| Anaerolineae | Reduction of contaminant bioavailability | Carbon fixation (Arnon-Buchanan cycle) | (119) |
PCBs, polychlorinated byiphenyls; PAHs, polycyclic aromatic hydrocarbons; SMBR, submerged membrane bioreactor.
PROSPECTIVE BIOTECHNOLOGICAL APPLICATIONS
Chloroflexota metabolic diversity continues to expand as scientific studies report previously unknown microbial processes. For instance, (120) described a new candidate of the Chloroflexota phylum (Candidatus Chlorolinea photoalkanotrophicum) with the ability to both perform phototrophy and oxidize methane and/or other small alkanes (120), which not only extends the known metabolic diversity of Chloroflexota but also offers exciting possibilities for biotechnological applications, especially in the areas of environmental remediation (biodegradation of alkanes), renewable energy (conversion of methane into biofuels), and carbon cycle management (carbon sequestration or transformation). In fact, the metagenomic-assembled genome of this species showed the ability of this species to perform several metabolisms, namely phototrophy, aerobic respiration, reduction of nitrites, carbon monoxide oxidation, and oxidation of carbon from methane and/or propane, and potentially fixate carbon using the pathway composed of hybridized components of the serine cycle and the 3-hydroxypropionate bi-cycle. These findings contribute to demonstrating the evolution and incorporation of new pathways into Chloroflexota promoted by horizontal gene transfer occurrences in natural habitats (120, 121).
The work of Kawaichi et al. described, for the first time, another interesting metabolism for a representative of the Chloroflexota phylum that could be biotechnologically viable (9). In this study, an isolate (belonging to the Ardenticatenia class) from a hydrothermal field with high iron concentrations was shown to perform dissimilatory iron reduction. Presenting a versatile metabolism with the ability to grow using oxygen, ferric iron, and nitrate as electron acceptors, this isolate also grows at different temperatures (30°C–75°C) and salt concentrations (0%–6% NaCl). This metabolic trait can play an important role in the bioremediation of subsurface environments contaminated with organic or metal contaminants.
Regarding the discovery of interesting metabolic pathways for Chloroflexota bacteria, there are also studies reporting the existence of newly found aerobic respiration and partial denitrification for Anaerolineae (122). In fact, despite most described Anaerolineae being classified as strict anaerobes, Levilinea saccharolytica displayed a branched aerobic respiration pathway, containing a NADH dehydrogenase, a succinate dehydrogenase, a heme-copper oxygen reductase, and a bd oxidase. Moreover, two nitrite reduction pathways were reported containing different nitrite reductases, able to reduce nitrite into nitric oxide or into ammonia. The presence of previously unknown pathways in Anaerolineae species suggests a wider physiological diversity than previously recognized for this Chloroflexota class, offering new opportunities for biotechnological applications: the aerobic and denitrification capabilities of these bacteria could play a role in converting nitrite into less toxic forms, contributing to the health of the soil microbiome, improving soil quality, and potentially enhancing crop growth. Moreover, the presence of enzymes like NADH dehydrogenase, succinate dehydrogenase, and oxygen reductases indicates their potential role in more efficient breakdown of organic matter in wastewater, leading to more effective and possibly faster treatment methods.
A. maritima, from the Ardenticatenia class, also presents a wide range of physiologies, which include aerobic respiration (containing enzymes from complex I, II, III, and three oxygen reductases), iron reduction, and a complete denitrification pathway composed of nitrate reductase, nitrite reductase, nitric oxide reductase, and nitrous oxide reductase (123). These findings could enhance the role of Chloroflexota bacteria in nitrogen removal technologies in wastewater treatment plants and, to a broader extent, the coupling of nitrogen, sulfur, and carbon cycles to be used in multipurpose bioreactors, which is of utmost technological relevance (124).
Hydrothermal systems, including terrestrial hot springs, contain diverse geochemical conditions that promote the discovery of novel metabolisms. Among the isolated bacteria from the different redox environments existing in an intertidal, anoxic, iron-, and hydrogen-rich hot spring that mixes with the oxygenated atmosphere and sulfate-rich seawater, there was a Chloroflexota member that not only presented the ability to fixate carbon, via Calvin cycle, but also had genes encoding for a hydrogenase, suggesting a lithoautotrophic capacity to oxidize hydrogen (125). This ability could be important in the utilization of these bacteria in waste-free biotechnological processes to directly convert electrical energy and inorganic substances into amino acids and other biologically active substances, contributing to sustainable bioproduction, where the goal is to minimize waste and maximize efficiency. Overall, the thermophilic nature of these Chloroflexota bacteria, thriving in hydrothermal systems, suggests that they possess enzymes and proteins that are stable and active at high temperatures. This thermophilic property can be particularly advantageous in industrial processes that operate at elevated temperatures, providing more robust and efficient systems, as in the production of bioenergy or in biocatalysis processes.
Chloroflexota from the Ktenodobacteria class (Thermogemmatispora sp. T81) has been demonstrated to persist using sub-atmospheric levels of H2 and CO (126). The authors reported that group 1 h [NiFe]-hydrogenases and type I carbon monoxide dehydrogenases were encoded in most of the studied reference genomes within the Ktedonobacteriales. Additionally, a meta-transcriptome study revealed that homologs of the group 1 f[NiFe]-hydrogenase of Roseiflexus species are highly expressed in geothermal microbial mats at night (55), possibly indicating atmospheric H2 oxidation within the photosynthetic Chloroflexota strains. These findings could also indicate a possible application of Chloroflexota bacteria in the treatment of syngas and industrial off-gas streams, often rich in hydrogen and carbon monoxide. In fact, the ability of C. aurantiacus to use hydrogen or sulfide for photoautotrophic growth (127, 128), combined with its capacity for polyhydroxyalkanoates (PHA) accumulation (129) and pigment production (130), could be explored as a circular process focused on carbon dioxide mitigation coupled with the production of value-added substances. PHA are natural polyesters with thermoplastic properties that are internally accumulated by bacteria as carbon and energy reserves, being considered interesting candidates for substituting traditional plastics (131). Recently, purple phototrophic bacteria have been described as suitable PHA-accumulating organisms (132), and its upscale challenges have been reviewed (133). There are several examples of phototrophic production of PHA using purple bacteria or cyanobacteria (134–138), and these bacteria have been reviewed as suitable phototrophic factories to couple resource recovery with the production of value-added substances (139). However, the PHA production capacity of phototrophic organisms of the Chloroflexota phylum remains mostly unexplored. In fact, phototrophy has been demonstrated to be present in seven bacterial phyla, and the development of omics methodologies can contribute to valuable metabolic, ecological, and physiological insights regarding photosynthesis and carbon fixation (5). A potential Chloroflexota member to develop such studies is C. aurantiacus, a phototrophic species that can use the 3-hydroxypropionate bi-cycle pathway for autotrophic carbon fixation, in which some intermediates of the pathway (such as acetyl-coA and propionyl-coA) are precursors for PHA accumulation (140). Studying PHA production in Chloroflexota, specifically C. aurantiacus, is crucial due to its unique metabolic pathways which may offer more efficient or varied PHA synthesis compared with well-studied purple bacteria. The photoautotrophic growth capabilities of C. aurantiacus, using hydrogen or sulfide, presents opportunities for sustainable, energy-efficient bioplastic production. In fact, exploring Chloroflexota’s PHA production can broaden the understanding of bioplastic synthesis and applications, complementing and extending the current knowledge derived from studies on purple bacteria. Furthermore, Cloroflexus potential to use CO2 fixation toward PHA production could contribute to new carbon capture technologies focused on bioplastic production, hence advancing new Chloroflexota-based bioprocesses for photoautotrophic biodegradable polymers production.
CONCLUSION
The Chloroflexota phylum encompasses several biotechnologically interesting bacteria that can be frequently found in extreme environments and naturally adapted to unfavorable conditions due to the unique characteristics of its members. The resilience of these organisms can be attributed to their metabolic diversity, responding to site-specific requirements. In fact, Chloroflexota is present in several biotechnological approaches, including water treatment, pollutant biodegradation, and energy production. Due to the metabolic diversity and adaptability of the members belonging to this bacterial phylum, several interesting Chloroflexota enzymes have been isolated and studied, often reported as thermostable and highly efficient. However, the available literature reveals that several Chloroflexota microorganisms remain unexplored, which means that several bacterial functions and possibly interesting metabolisms are being overlooked. Therefore, further research is required to unlock the full potential of these microorganisms, especially within their photoautotrophic members, which could be useful for the development of CO2-negative technologies. The understanding of Chloroflexota role in natural cycles, under specific conditions, is fundamental for the development of new technologies based on these microorganisms.
ACKNOWLEDGMENTS
This work was supported by national funds from FCT - Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences - UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy - i4HB. A. Freches also acknowledges the financial support of FCT - Fundação para a Ciência e a Tecnologia through the doctoral grant SFRH/BD/145555/2019.
Contributor Information
Joana Costa Fradinho, Email: j.fradinho@fct.unl.pt.
Haruyuki Atomi, Kyoto University, Kyoto, Japan.
REFERENCES
- 1. Kuleshova T, Rao A, Bhadra S, Garlapati VK, Sharma S, Kaushik A, Goswami P, Sreekirshnan TR, Sevda S. 2022. Plant microbial fuel cells as an innovative, versatile agro-technology for green energy generation combined with wastewater treatment and food production. Bio Bioener 167:106629. doi: 10.1016/j.biombioe.2022.106629 [DOI] [Google Scholar]
- 2. Sodhi KK, Mishra LC, Singh CK, Kumar M. 2022. Perspective on the heavy metal pollution and recent remediation strategies. Curr Res Microb Sci 3:100166. doi: 10.1016/j.crmicr.2022.100166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. George DM, Vincent AS, Mackey HR. 2020. An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable resource recovery. Biotechnol Rep (Amst) 28:e00563. doi: 10.1016/j.btre.2020.e00563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaashikaa PR, Senthil Kumar P, Varjani SJ, Saravanan A. 2019. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J CO2 Util 33:131–147. doi: 10.1016/j.jcou.2019.05.017 [DOI] [Google Scholar]
- 5. Thiel V, Tank M, Bryant DA. 2018. Diversity of chlorophototrophic bacteria revealed in the omics era. Annu Rev Plant Biol 69:21–49. doi: 10.1146/annurev-arplant-042817-040500 [DOI] [PubMed] [Google Scholar]
- 6. Pierson BK, Castenholz RW. 1974. A Phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol 100:5–24. doi: 10.1007/BF00446302 [DOI] [PubMed] [Google Scholar]
- 7. Yamada T, Sekiguchi Y, Hanada S, Imachi H, Ohashi A, Harada H, Kamagata Y. 2006. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int J Syst Evol Microbiol 56:1331–1340. doi: 10.1099/ijs.0.64169-0 [DOI] [PubMed] [Google Scholar]
- 8. Nakahara N, Nobu MK, Takaki Y, Miyazaki M, Tasumi E, Sakai S, Ogawara M, Yoshida N, Tamaki H, Yamanaka Y, Katayama A, Yamaguchi T, Takai K, Imachi H. 2019. Aggregatilinea Lenta gen. nov., sp. nov., a slow-growing, facultatively anaerobic bacterium isolated from subseafloor sediment, and proposal of the new order Aggregatilineales ord. nov. within the class Anaerolineae of the phylum Chloroflexi. Int J Syst Evol Microbiol 69:1185–1194. doi: 10.1099/ijsem.0.003291 [DOI] [PubMed] [Google Scholar]
- 9. Kawaichi S, Ito N, Kamikawa R, Sugawara T, Yoshida T, Sako Y. 2013. “Ardenticatena maritima gen. nov., sp. nov., a ferric iron- and nitrate-reducing bacterium of the phylum ‘Chloroflexi’ isolated from an iron-rich coastal hydrothermal field, and description of Ardenticatenia classis nov”. Int J Syst Evol Microbiol 63:2992–3002. doi: 10.1099/ijs.0.046532-0 [DOI] [PubMed] [Google Scholar]
- 10. Kale V, Björnsdóttir SH, Friðjónsson ÓH, Pétursdóttir SK, Ómarsdóttir S, Hreggviðsson GÓ. 2013. Litorilinea aerophila gen. nov., sp. nov., an aerobic member of the class Caldilineae, phylum Chloroflexi, isolated from an Intertidal hot spring. Int J Syst Evol Microbiol 63:1149–1154. doi: 10.1099/ijs.0.044115-0 [DOI] [PubMed] [Google Scholar]
- 11. Sekiguchi Y, Yamada T, Hanada S, Ohashi A, Harada H, Kamagata Y. 2003. Caldilinea aerophila gen. nov., sp. nov., novel filamentous thermophiles that represent a previously uncultured lineage of the domain bacteria at the subphylum level. Int J Syst Evol Microbiol 53:1843–1851. doi: 10.1099/ijs.0.02699-0 [DOI] [PubMed] [Google Scholar]
- 12. Gupta RS, Chander P, George S. 2013. Phylogenetic framework and molecular signatures for the class Chloroflexi and its different clades; proposal for division of the class Chloroflexi class. nov. into the suborder Chloroflexineae subord. nov., consisting of the emended family Oscillochloridaceae and the family Chloroflexaceae fam. nov., and the suborder Roseiflexineae subord. nov., containing the family Roseiflexaceae fam. nov. Antonie Van Leeuwenhoek 103:99–119. doi: 10.1007/s10482-012-9790-3 [DOI] [PubMed] [Google Scholar]
- 13. Keppen OI, Tourova TP, Kuznetsov BB, Ivanovsky RN, Gorlenko VM. 2000. Proposal of Oscillochloridaceae fam. nov. on the basis of a phylogenetic analysis of the filamentous anoxygenic phototrophic bacteria, and emended description of Oscillochloris and Oscillochloris trichoides in comparison with further new isolates. Int J Syst Evol Microbiol 50 Pt 4:1529–1537. doi: 10.1099/00207713-50-4-1529 [DOI] [PubMed] [Google Scholar]
- 14. Ward LM, Hemp J, Shih PM, McGlynn SE, Fischer WW. 2018. Evolution of phototrophy in the Chloroflexi phylum driven by horizontal gene transfer. Front Microbiol 9:260. doi: 10.3389/fmicb.2018.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole JK, Gieler BA, Heisler DL, Palisoc MM, Williams AJ, Dohnalkova AC, Ming H, Yu TT, Dodsworth JA, Li W-J, Hedlund BP. 2013. Kallotenue papyrolyticum gen. nov., sp. nov., a cellulolytic and filamentous thermophile that represents a novel lineage (Kallotenuales ord. nov., Kallotenuaceae fam. nov.) within the class Chloroflexia. Int J Syst Evol Microbiol 63:4675–4682. doi: 10.1099/ijs.0.053348-0 [DOI] [PubMed] [Google Scholar]
- 16. Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, Müller JA, Fullerton H, Zinder SH, Spormann AM. 2013. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol 63:625–635. doi: 10.1099/ijs.0.034926-0 [DOI] [PubMed] [Google Scholar]
- 17. Wang C-M, Zheng Y, Sakai Y, Toyoda A, Minakuchi Y, Abe K, Yokota A, Yabe S. 2019. Tengunoibacter tsumagoiensis gen. nov., sp. nov., Dictyobacter kobayashii sp. nov., Dictyobacter alpinus sp. nov., and description of Dictyobacteraceae fam. nov. within the order Ktedonobacterales isolated from Tengu-no-mugimeshi, a soil- like granular mass of micro-organisms, and emended descriptions of the genera Ktedonobacter and Dictyobacter. Int J Syst Evol Microbiol 69:1910–1918. doi: 10.1099/ijsem.0.003396 [DOI] [PubMed] [Google Scholar]
- 18. Cavaletti L, Monciardini P, Bamonte R, Schumann P, Rohde M, Sosio M, Donadio S. 2006. New lineage of filamentous, spore-forming, Gram-positive bacteria from soil. Appl Environ Microbiol 72:4360–4369. doi: 10.1128/AEM.00132-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan B, Guo X, Liu M, Huang Y. 2020. Ktedonosporobacter rubrisoli gen. nov., sp. nov., a novel representative of the class Ktedonobacteria, isolated from red soil, and proposal of Ktedonosporobacteraceae fam. nov. Int J Syst Evol Microbiol 70:1015–1025. doi: 10.1099/ijsem.0.003864 [DOI] [PubMed] [Google Scholar]
- 20. Yabe S, Zheng Y, Wang C, Sakai Y, Abe K, Yokota A, Donadio S, Cavaletti L, Monciardini P. 2021. Reticulibacter mediterranei gen. nov., sp. nov., within the new family Reticulibacteraceae fam. nov., and Ktedonospora formicarum gen. nov., sp. nov., Ktedonobacter robiniae sp. nov., Dictyobacter formicarum sp. nov. and Dictyobacter arantiisoli sp. nov., belonging to the class Ktedonobacteria. Int J Syst Evol Microbiol 71:1–24. doi: 10.1099/ijsem.0.004883 [DOI] [PubMed] [Google Scholar]
- 21. Yabe S, Aiba Y, Sakai Y, Hazaka M, Yokota A. 2010. Thermosporothrix hazakensis gen. nov., sp. nov., isolated from compost, description of Thermosporotrichaceae fam. nov. within the class Ktedonobacteria cavaletti et al. 2007 and emended description of the class Ktedonobacteria. Int J Syst Evol Microbiol 60:1794–1801. doi: 10.1099/ijs.0.018069-0 [DOI] [PubMed] [Google Scholar]
- 22. Yabe S, Aiba Y, Sakai Y, Hazaka M, Yokota A. 2011. Thermogemmatispora onikobensis gen. nov., sp. nov. and Thermogemmatispora foliorum sp. nov., isolated from fallen leaves on geothermal soils, and description of Thermogemmatisporaceae fam. nov. and Thermogemmatisporales ord. nov. within the class Ktedonobacteria. Int J Syst Evol Microbiol 61:903–910. doi: 10.1099/ijs.0.024877-0 [DOI] [PubMed] [Google Scholar]
- 23. Kochetkova TV, Zayulina KS, Zhigarkov VS, Minaev NV, Chichkov BN, Novikov AA, Toshchakov SV, Elcheninov AG, Kublanov IV. 2020. Tepidiforma bonchosmolovskayae gen. nov., sp. nov., a moderately thermophilic Chloroflexi bacterium from a Chukotka hot spring (Arctic, Russia), representing a novel class, tepidiformia, which includes the previously uncultivated lineage olb14. Int J Syst Evol Microbiol 70:1192–1202. doi: 10.1099/ijsem.0.003902 [DOI] [PubMed] [Google Scholar]
- 24. Dodsworth JA, Gevorkian J, Despujos F, Cole JK, Murugapiran SK, Ming H, Li W-J, Zhang G, Dohnalkova A, Hedlund BP. 2014. Thermoflexus hugenholtzii gen. nov., sp. nov., a thermophilic, microaerophilic, filamentous bacterium representing a novel class in the Chloroflexi, thermoflexia classis nov., and description of Thermoflexaceae fam. nov. and Thermoflexales ord. nov. Int J Syst Evol Microbiol 64:2119–2127. doi: 10.1099/ijs.0.055855-0 [DOI] [PubMed] [Google Scholar]
- 25. Mehrshad M, Salcher MM, Okazaki Y, Nakano SI, Šimek K, Andrei AS, Ghai R. 2018. Hidden in plain sight - highly abundant and diverse planktonic freshwater Chloroflexi. Microbiome 6:176. doi: 10.1186/s40168-018-0563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamada T, Sekiguchi Y, Imachi H, Kamagata Y, Ohashi A, Harada H. 2005. Diversity, localization, and physiological properties of filamentous microbes belonging to Chloroflexi subphylum I in mesophilic and thermophilic methanogenic sludge granules. Appl Environ Microbiol 71:7493–7503. doi: 10.1128/AEM.71.11.7493-7503.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanada S. 2003. Filamentous anoxygenic phototrophs in hot springs. Microb Environ 18:51–61. doi: 10.1264/jsme2.18.51 [DOI] [Google Scholar]
- 28. Gorlenko VM, Bryantseva IA, Samylina OS, Ashikhmin AA, Sinetova MA, Kostrikina NA, Kozyaeva VV. 2020. Filamentous anoxygenic phototrophic bacteria in microbial communities of the Kulunda Steppe soda lakes (Altai Krai, Russia). Microbiology 89:697–707. doi: 10.1134/S0026261720060053 [DOI] [Google Scholar]
- 29. Krzmarzick MJ, Crary BB, Harding JJ, Oyerinde OO, Leri AC, Myneni SCB, Novak PJ. 2012. Natural niche for organohalide-respiring Chloroflexi. Appl Environ Microbiol 78:393–401. doi: 10.1128/AEM.06510-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaisin VA, Kalashnikov AM, Grouzdev DS, Sukhacheva MV, Kuznetsov BB, Gorlenko VM. 2017. Chloroflexus islandicus sp. nov., a thermophilic filamentous anoxygenic phototrophic bacterium from a geyser. Int J Syst Evol Microbiol 67:1381–1386. doi: 10.1099/ijsem.0.001820 [DOI] [PubMed] [Google Scholar]
- 31. Hanada S, Takaichi S, Matsuura K, Nakamura K. 2002. Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium that lacks chlorosomes. Int J Syst Evol Microbiol 52:187–193. doi: 10.1099/00207713-52-1-187 [DOI] [PubMed] [Google Scholar]
- 32. Gorlenko VM, Bryantseva IA, Kalashnikov AM, Gaisin VA, Sukhacheva MV, Gruzdev DS, Kuznetsov BB. 2014. “Candidatus ‘Chloroploca asiatica’ gen. nov., sp. nov., a new mesophilic filamentous anoxygenic phototrophic bacterium. Microbiology 83:838–848. doi: 10.1134/S0026261714060083 [DOI] [Google Scholar]
- 33. Gaisin VA, Burganskaya EI, Grouzdev DS, Ashikhmin AA, Kostrikina NA, Bryantseva IA, Koziaeva VV, Gorlenko VM. 2019. Candidatus viridilinea mediisalina”, a novel phototrophic Chloroflexi bacterium from a Siberian soda Lake. FEMS Microbiol Lett 366:fnz043. doi: 10.1093/femsle/fnz043 [DOI] [PubMed] [Google Scholar]
- 34. Gaisin VA, Burganskaya EI, Grouzdev DS, Osipova NS, Ashikhmin AA, Sinetova MA, Krutkina MS, Bryantseva IA, Sukhacheva MV, Kochetkova TV, Koziaeva VV, Kalashnikov AM, Gorlenko VM. 2019. Candidatus oscillochloris fontis’: a novel mesophilic phototrophic Chloroflexota bacterium belonging to the ubiquitous Oscillochloris genus. FEMS Microbiol Lett 366:fnz097. doi: 10.1093/femsle/fnz097 [DOI] [PubMed] [Google Scholar]
- 35. Bennett AC, Murugapiran SK, Hamilton TL. 2020. Temperature impacts community structure and function of phototrophic Chloroflexi and cyanobacteria in two alkaline hot springs in Yellowstone National Park. Environ Microbiol Rep 12:503–513. doi: 10.1111/1758-2229.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaisin VA, Grouzdev DS, Namsaraev ZB, Sukhacheva MV, Gorlenko VM, Kuznetsov BB. 2016. Biogeography of thermophilic phototrophic bacteria belonging to Roseiflexus genus. FEMS Microbiol Ecol 92:fiw012. doi: 10.1093/femsec/fiw012 [DOI] [PubMed] [Google Scholar]
- 37. Sprague SG, Staehelin LA, DiBartolomeis MJ, Fuller RC. 1981. Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus. J Bacteriol 147:1021–1031. doi: 10.1128/jb.147.3.1021-1031.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frigaard N-U, Gomez A, Chew M, Maresca JA, Bryant DA. 2006. Bacteriochlorophyll biosynthesis in green bacteria, p 201–221. In Grimm B, Porra RJ, Rudiger W, Scheer H (ed), Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. Springer. [Google Scholar]
- 39. Bryant DA, Liu Z, Li T, Zhao F, Costas AMG, Klatt CG, Ward DM, Frigaard N-U, Overmann J. 2012. Comparative and functional genomics of anoxygenic green bacteria from the taxa Chlorobi, Chloroflexi, and Acidobacteria, p 47–102. In Burnap RI, Vermaas WFJ (ed), Functional genomics and evolution of photosynthetic systems, advances in photosynthesis and respiration. Springer. [Google Scholar]
- 40. Takaichi S, Maoka T, Yamada M, Matsuura K, Haikawa Y, Hanada S. 2001. Absence of carotenes and presence of a tertiary methoxy group in a carotenoid from a thermophilic filamentous photosynthetic bacterium Roseiflexus castenholzii . Plant Cell Physiol 42:1355–1362. doi: 10.1093/pcp/pce172 [DOI] [PubMed] [Google Scholar]
- 41. Garritano AN, Song W, Thomas T. 2022. Carbon fixation pathways across the bacterial and archaeal tree of life. PNAS Nexus 1:pgac226. doi: 10.1093/pnasnexus/pgac226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sirevag R, Castenholz R. 1979. Aspects of carbon metabolism in Chloroflexus. Arch Microbiol 120:151–153. doi: 10.1007/BF00409101 [DOI] [Google Scholar]
- 43. van der Meer MT, Schouten S, de Leeuw JW, Ward DM. 2000. Autotrophy of green non-sulphur bacteria in hot spring microbial mats: biological explanations for isotopically heavy organic carbon in the geological record. Environ Microbiol 2:428–435. doi: 10.1046/j.1462-2920.2000.00124.x [DOI] [PubMed] [Google Scholar]
- 44. Liang B, Zhao Y, Yang J. 2020. Recent advances in developing artificial autotrophic microorganism for reinforcing CO2 fixation. Front Microbiol 11:592631. doi: 10.3389/fmicb.2020.592631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zarzycki J, Fuchs G. 2011. Coassimilation of organic substrates via the autotrophic 3-hydroxypropionate bi-cycle in Chloroflexus aurantiacus. Appl Environ Microbiol 77:6181–6188. doi: 10.1128/AEM.00705-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hügler M, Sievert SM. 2011. Beyond the calvin cycle: autotrophic carbon fixation in the ocean. Annu Rev Mar Sci 3:261–289. doi: 10.1146/annurev-marine-120709-142712 [DOI] [PubMed] [Google Scholar]
- 47. Zarzycki J, Brecht V, Müller M, Fuchs G, Bryant DA. 2009. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc Natl Acad Sci U S A 106:21317–21322. doi: 10.1073/pnas.0908356106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Overmann J. 2008. Green nonsulfur bacteria, p 1–10. In Encyclopedia of life sciences. Wiley. [Google Scholar]
- 49. Frigaard NU, Dahl C. 2008. Sulfur metabolism in phototrophic sulfur bacteria. Adv Microb Physiol 54:103–200. [DOI] [PubMed] [Google Scholar]
- 50. Camacho A. 2009. Sulfur bacteria, p 261–278. In Protists, bacteria and fungi: planktonic and attached. Elsevier. [Google Scholar]
- 51. Klappenbach JA, Pierson BK. 2004. “Phylogenetic and physiological characterization of a filamentous anoxygenic photoautotrophic bacterium “Candidatus chlorothrix halophila” gen. nov., sp. nov., recovered from hypersaline microbial mats”. Arch Microbiol 181:17–25. doi: 10.1007/s00203-003-0615-7 [DOI] [PubMed] [Google Scholar]
- 52. Burganskaya EI, Bryantseva IA, Krutkina MS, Grouzdev DS, Gorlenko VM. 2019. Bacterial communities of the microbial mats of chokrak sulfide springs. Arch Microbiol 201:795–805. doi: 10.1007/s00203-019-01648-6 [DOI] [PubMed] [Google Scholar]
- 53. Gaisin VA, Grouzdev DS, Krutkina MS, Ashikhmin AA, Sinetova MA, Osipova NS, Koziaeva VV, Gorlenko VM. 2020. Candidatus Oscillochloris kuznetsovii’ a novel mesophilic filamentous anoxygenic phototrophic chloroflexales bacterium from arctic coastal environments. FEMS Microbiol Lett 367:fnaa158. doi: 10.1093/femsle/fnaa158 [DOI] [PubMed] [Google Scholar]
- 54. Brito EMS, Romero-Núñez VM, Caretta CA, Bertin P, Valerdi-Negreros JC, Guyoneaud R, Goñi-Urriza M. 2019. The bacterial diversity on steam vents from Paricutín and Sapichu volcanoes. Extremophiles 23:249–263. doi: 10.1007/s00792-019-01078-8 [DOI] [PubMed] [Google Scholar]
- 55. Klatt CG, Liu Z, Ludwig M, Kühl M, Jensen SI, Bryant DA, Ward DM. 2013. Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring. ISME J 7:1775–1789. doi: 10.1038/ismej.2013.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kawai S, Martinez JN, Lichtenberg M, Trampe E, Kühl M, Tank M, Haruta S, Nishihara A, Hanada S, Thiel V. 2021. In-situ metatranscriptomic analyses reveal the metabolic flexibility of the thermophilic anoxygenic photosynthetic bacterium Chloroflexus aggregans in a hot spring cyanobacteria-dominated microbial mat. Microorganisms 9:1–22. doi: 10.3390/microorganisms9030652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van der Meer MTJ, Schouten S, Sinninghe Damsté JS, de Leeuw JW, Ward DM. 2003. Compound-specific isotopic fractionation patterns suggest different carbon metabolisms among chloroflexus-like bacteria in hot-spring microbial mats. Appl Environ Microbiol 69:6000–6006. doi: 10.1128/AEM.69.10.6000-6006.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun L, Toyonaga M, Ohashi A, Matsuura N, Tourlousse DM, Meng XY, Tamaki H, Hanada S, Cruz R, Yamaguchi T, Sekiguchi Y. 2016. Isolation and characterization of Flexilinea flocculi gen. nov., sp. nov., a filamentous, anaerobic bacterium belonging to the class anaerolineae in the phylum Chloroflexi. Int J Syst Evol Microbiol 66:988–996. doi: 10.1099/ijsem.0.000822 [DOI] [PubMed] [Google Scholar]
- 59. Cao S, Yan W, Yu L, Zhang L, Lay W, Zhou Y. 2021. Challenges of THP-AD centrate treatment using partial nitritation-anammox (PN/A) – inhibition, biomass washout, low alkalinity, recalcitrant and more. Water Res 203:117555. doi: 10.1016/j.watres.2021.117555 [DOI] [PubMed] [Google Scholar]
- 60. Park Y, Cho H, Yu J, Min B, Kim HS, Kim BG, Lee T. 2017. Response of microbial community structure to pre-acclimation strategies in microbial fuel cells for domestic wastewater treatment. Bioresour Technol 233:176–183. doi: 10.1016/j.biortech.2017.02.101 [DOI] [PubMed] [Google Scholar]
- 61. Zhang B, Tian C, Liu Y, Hao L, Liu Y, Feng C, Liu Y, Wang Z. 2015. Simultaneous microbial and electrochemical reductions of vanadium (V) with bioelectricity generation in microbial fuel cells. Bioresour Technol 179:91–97. doi: 10.1016/j.biortech.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 62. Sutcliffe B, Chariton AA, Harford AJ, Hose GC, Stephenson S, Greenfield P, Midgley DJ, Paulsen IT. 2018. Insights from the Genomes of microbes thriving in uranium-enriched sediments. Microb Ecol 75:970–984. doi: 10.1007/s00248-017-1102-z [DOI] [PubMed] [Google Scholar]
- 63. Bayer K, Jahn MT, Slaby BM, Moitinho-Silva L, Hentschel U. 2018. Marine sponges as Chloroflexi hot spots: genomic insights and high-resolution visualization of an abundant and diverse symbiotic clade. mSystems 3:117555. doi: 10.1128/mSystems.00150-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Q, Liu W, Yuan X, Wang R, Liu L, Li H, Zhao C, Kong Q. 2022. Characteristics of bacterial and archaeal communities in microbial-enhanced constructed wetlands under NaCl stress. Clean Soil Air Wat 50:2100152. doi: 10.1002/clen.202100152 [DOI] [Google Scholar]
- 65. Yang Y, Zhang Y, Cápiro NL, Yan J. 2020. Genomic characteristics distinguish geographically distributed Dehalococcoidia. Front Microbiol 11:546063. doi: 10.3389/fmicb.2020.546063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vuillemin A, Kerrigan Z, D’Hondt S, Orsi WD. 2020. Exploring the abundance, metabolic potential and gene expression of subseafloor Chloroflexi in million-year-old oxic and anoxic abyssal clay. FEMS Microbiol Ecol 96:fiaa223. doi: 10.1093/femsec/fiaa223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wasmund K, Cooper M, Schreiber L, Lloyd KG, Baker BJ, Petersen DG, Jørgensen BB, Stepanauskas R, Reinhardt R, Schramm A, Loy A, Adrian L. 2016. Single-cell genome and group-specific dsrAB sequencing implicate marine members of the class Dehalococcoidia (phylum Chloroflexi) in sulfur cycling. mBio 7:e00266-16. doi: 10.1128/mBio.00266-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Biderre-Petit C, Dugat-Bony E, Mege M, Parisot N, Adrian L, Moné A, Denonfoux J, Peyretaillade E, Debroas D, Boucher D, Peyret P. 2016. Distribution of Dehalococcoidia in the anaerobic deep water of a remote meromictic crater lake and detection of Dehalococcoidia-derived reductive dehalogenase homologous genes. PLoS One 11:e0145558. doi: 10.1371/journal.pone.0145558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gich F, Garcia-Gil J, Overmann J. 2001. Previously unknown and phylogenetically diverse members of the green nonsulfur bacteria are indigenous to freshwater lakes. Archives Microb 177:1–10. doi: 10.1007/s00203-001-0354-6 [DOI] [PubMed] [Google Scholar]
- 70. Kadnikov VV, Mardanov AV, Beletsky AV, Grigoriev MA, Karnachuk OV, Ravin NV. 2021. Thermophilic Chloroflexi dominate in the microbial community associated with coal-fire gas vents in the Kuznetsk coal Basin, Russia. Microorganisms 9:948. doi: 10.3390/microorganisms9050948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu R, Wei X, Song W, Wang L, Cao J, Wu J, Thomas T, Jin T, Wang Z, Wei W, Wei Y, Zhai H, Yao C, Shen Z, Du J, Fang J. 2022. Novel Chloroflexi Genomes from the deepest ocean reveal metabolic strategies for the adaptation to deep-sea habitats. Microbiome 10:75. doi: 10.1186/s40168-022-01263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Raveendran S, Parameswaran B, Ummalyma SB, Abraham A, Mathew AK, Madhavan A, Rebello S, Pandey A. 2018. Applications of microbial enzymes in food industry. Food Technol Biotechnol 56:16–30. doi: 10.17113/ftb.56.01.18.5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meghwanshi GK, Kaur N, Verma S, Dabi NK, Vashishtha A, Charan PD, Purohit P, Bhandari HS, Bhojak N, Kumar R. 2020. Enzymes for pharmaceutical and therapeutic applications. Biotechnol Appl Biochem 67:586–601. doi: 10.1002/bab.1919 [DOI] [PubMed] [Google Scholar]
- 74. Singh R, Kumar M, Mittal A, Mehta PK. 2016. Microbial enzymes: industrial progress in 21st century. 3 Biotech 6:174. doi: 10.1007/s13205-016-0485-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shin K-C, Seo M-J, Oh D-K, Choi M-N, Kim D-W, Kim Y-S, Park C-S. 2019. Cloning and characterization of a -L-rhamnosidase from Chloroflexus aurantiacus and its application in the production of isoquercitrin from rutin. Biotechnol Lett 41:419–426. doi: 10.1007/s10529-019-02648-8 [DOI] [PubMed] [Google Scholar]
- 76. Makino T, Kanemaru M, Okuyama S, Shimizu R, Tanaka H, Mizukami H. 2013. Anti-allergic effects of enzymatically modified Isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-Oligoglucosyl rutin, and quercetin, when administered orally to mice. J Nat Med 67:881–886. doi: 10.1007/s11418-013-0760-5 [DOI] [PubMed] [Google Scholar]
- 77. Vila-Real H, Alfaia AJ, Bronze MR, Calado ART, Ribeiro MHL. 2011. Enzymatic synthesis of the flavone glucosides, prunin and isoquercetin, and the aglycones, naringenin and quercetin, with selective α-L-rhamnosidase and β-D-glucosidase activities of naringinase. Enzyme Res 2011:692618. doi: 10.4061/2011/692618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Loderer C, Wagner D, Morgenstern F, Spieß A, Ansorge-Schumacher MB. 2018. Discovery of a novel thermostable Zn2+-dependent alcohol dehydrogenase from Chloroflexus aurantiacus through conserved domains mining. J Appl Microbiol 124:480–490. doi: 10.1111/jam.13664 [DOI] [PubMed] [Google Scholar]
- 79. Huisman GW, Liang J, Krebber A. 2010. Practical chiral alcohol manufacture using ketoreductases. Curr Opin Chem Biol 14:122–129. doi: 10.1016/j.cbpa.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 80. Robescu MS, Niero M, Loprete G, Cendron L, Bergantino E. 2021. A new thermophilic ene‐reductase from the filamentous anoxygenic phototrophic bacterium Chloroflexus aggregans. Microorganisms 9:953. doi: 10.3390/microorganisms9050953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang C, Tang K, Dai Y, Jia H, Li Y, Gao Z, Wu B. 2021. Identification, characterization, and site-specific mutagenesis of a thermostable ω-transaminase from Chloroflexi bacterium. ACS Omega 6:17058–17070. doi: 10.1021/acsomega.1c02164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Torzillo G, Scoma A, Faraloni C, Giannelli L. 2015. Advances in the biotechnology of hydrogen production with the microalga Chlamydomonas reinhardtii. Crit Rev Biotechnol 35:485–496. doi: 10.3109/07388551.2014.900734 [DOI] [PubMed] [Google Scholar]
- 83. Gogotov IN, Zorin NA, Serebriakova LT. 1991. Hydrogen production by model systems including hydrogenases from phototrophic bacteria. Intern J Hydrogen Ene 16:393–396. doi: 10.1016/0360-3199(91)90137-8 [DOI] [Google Scholar]
- 84. Burow LC, Woebken D, Bebout BM, McMurdie PJ, Singer SW, Pett-Ridge J, Prufert-Bebout L, Spormann AM, Weber PK, Hoehler TM. 2012. Hydrogen production in photosynthetic microbial mats in the Elkhorn slough estuary, Monterey Bay. ISME J 6:863–874. doi: 10.1038/ismej.2011.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cai C, Li L, Hua Y, Liu H, Dai X. 2021. Ferroferric oxide promotes metabolism in anaerolineae other than microbial syntrophy in anaerobic methanogenesis of antibiotic fermentation residue. Sci Total Environ 758:143601. doi: 10.1016/j.scitotenv.2020.143601 [DOI] [PubMed] [Google Scholar]
- 86. Bala S, Garg D, Thirumalesh BV, Sharma M, Sridhar K, Inbaraj BS, Tripathi M. 2022. Recent strategies for bioremediation of emerging pollutants: a review for a green and sustainable environment. Toxics 10:484. doi: 10.3390/toxics10080484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arutchelvan V, Kanakasabai V, Elangovan R, Nagarajan S, Muralikrishnan V. 2006. Kinetics of high strength phenol degradation using Bacillus brevis. J Hazard Mater 129:216–222. doi: 10.1016/j.jhazmat.2005.08.040 [DOI] [PubMed] [Google Scholar]
- 88. Huang Z, Wang P, Li H, Lin K, Lu Z, Guo X, Liu Y. 2014. Community analysis and metabolic pathway of halophilic bacteria for phenol degradation in saline environment. Intern Bio Biod 94:115–120. doi: 10.1016/j.ibiod.2014.07.003 [DOI] [Google Scholar]
- 89. Sánchez-González M, Álvarez-Uribe H, Rivera-Solís R, González-Burgos A, Escalante-Réndiz D, Rojas-Herrera R. 2018. Analysis of a phenol-adapted microbial community: degradation capacity, taxonomy and metabolic description. J Appl Microbiol 126:771–779. [DOI] [PubMed] [Google Scholar]
- 90. Li Q, Huang Y, Wen D, Fu R, Feng L. 2020. Application of alkyl polyglycosides for enhanced bioremediation of petroleum hydrocarbon-contaminated soil using Sphingomonas changbaiensis and Pseudomonas stutzeri . Sci Total Envir 719:137456. doi: 10.1016/j.scitotenv.2020.137456 [DOI] [PubMed] [Google Scholar]
- 91. Méndez V, Fuentes S, Morgante V, Hernández M, González M, Moore E, Seeger M. 2017. Novel hydrocarbonoclastic metal-tolerant Acinetobacter and Pseudomonas strains from Aconcagua river oil-polluted soil. J Soil Sci Plant Nutr 17:1074–1087. doi: 10.4067/S0718-95162017000400017 [DOI] [Google Scholar]
- 92. Silva A, Delerue-Matos C, Figueiredo SA, Freitas OM. 2019. The use of algae and fungi for removal of pharmaceuticals by bioremediation and Biosorption processes: a review. Water 11:1555. doi: 10.3390/w11081555 [DOI] [Google Scholar]
- 93. Zhang H, Yang L, Li Y, Wang C, Zhang W, Wang L, Niu L. 2022. Pollution gradients shape the co-occurrence networks and interactions of sedimentary bacterial communities in Taihu lake, a shallow eutrophic lake. J Environ Man 305:114380. doi: 10.1016/j.jenvman.2021.114380 [DOI] [PubMed] [Google Scholar]
- 94. Zanaroli G, Balloi A, Negroni A, Borruso L, Daffonchio D, Fava F. 2012. A Chloroflexi bacterium dechlorinates polychlorinated biphenyls in marine sediments under in situ -like biogeochemical conditions. J Hazard Mater 209–210:449–457. doi: 10.1016/j.jhazmat.2012.01.042 [DOI] [PubMed] [Google Scholar]
- 95. Carpenter DO. 2006. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health 21:1–23. doi: 10.1515/reveh.2006.21.1.1 [DOI] [PubMed] [Google Scholar]
- 96. Padilla-Crespo E, Yan J, Swift C, Wagner DD, Chourey K, Hettich RL, Ritalahti KM, Löffler FE. 2014. Identification and environmental distribution of dcpA, which encodes the reductive dehalogenase catalyzing the dichloroelimination of 1,2-dichloropropane to propene in organohalide-respiring Chloroflexi. Appl Environ Microbiol 80:808–818. doi: 10.1128/AEM.02927-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yan J, Rash BA, Rainey FA, Moe WM. 2009. “Detection and quantification of Dehalogenimonas and “Dehalococcoides” populations via PCR-based protocols targeting 16S rRNA genes”. Appl Environ Microbiol 75:7560–7564. doi: 10.1128/AEM.01938-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Govindarajan A, Crum M, Adolacion J, Kiaghadi A, Acuña-Gonzalez E, Rifai HS, Willson RC. 2022. Sediment and their bacterial communities in an industrialized estuary after hurricane harvey. Mar Pollut Bull 175:113359. doi: 10.1016/j.marpolbul.2022.113359 [DOI] [PubMed] [Google Scholar]
- 99. Sakshi Haritash AK. 2020. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch Microbiol 202:2033–2058. doi: 10.1007/s00203-020-01929-5 [DOI] [PubMed] [Google Scholar]
- 100. Lu J, Guo Z, Pan Y, Li M, Chen X, He M, Wu H, Zhang J. 2022. Simultaneously enhanced removal of PAHs and nitrogen driven by Fe2+/Fe3+ Cycle in constructed Wetland through automatic tidal operation. Water Res 215:118232. doi: 10.1016/j.watres.2022.118232 [DOI] [PubMed] [Google Scholar]
- 101. Kiersztyn B, Chróst R, Miłobędzka A. 2023. Homogenisation and dilution in metabolic evaluation of activated sludge rich in Chloroflexi. Int J Environ Sci Technol 20:3295–3308. doi: 10.1007/s13762-022-04191-y [DOI] [Google Scholar]
- 102. Speirs LBM, Rice DTF, Petrovski S, Seviour RJ. 2019. The phylogeny, biodiversity, and ecology of the Chloroflexi in activated sludge. Front Microbiol 10:2015. doi: 10.3389/fmicb.2019.02015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xiao X, You S, Guo H, Ma F, Zhang J, Zhang R, Bao X. 2021. Mycelial pellets for alleviation of membrane fouling in membrane bioreactor. J Membrane Sci 635:119545. doi: 10.1016/j.memsci.2021.119545 [DOI] [Google Scholar]
- 104. Miura Y, Watanabe Y, Okabe S. 2007. Significance of Chloroflexi in performance of submerged membrane bioreactors (MBR) treating municipal wastewater. Environ Sci Technol 41:7787–7794. doi: 10.1021/es071263x [DOI] [PubMed] [Google Scholar]
- 105. Lv Y, Pan J, Huo T, Li J, Liu S. 2020. Enhance the treatment of low strength wastewater at low temperature with the coexistence system of AnAOB and heterotrophic bacteria: performance and bacterial community. Sci Total Environ 714:136799. doi: 10.1016/j.scitotenv.2020.136799 [DOI] [PubMed] [Google Scholar]
- 106. Feng J, Zhang Q, Tan B, Li M, Peng H, He J, Zhang Y, Su J. 2022. Microbial community and metabolic characteristics evaluation in start-up stage of electro-enhanced SBR for aniline wastewater treatment. J Water Pro Eng 45:102489. doi: 10.1016/j.jwpe.2021.102489 [DOI] [Google Scholar]
- 107. Tian H, Liu J, Feng T, Li H, Wu X, Li B. 2017. Assessing the performance and microbial structure of biofilms adhering on aerated membranes for domestic saline sewage treatment. RSC Adv 7:27198–27205. doi: 10.1039/C7RA03755D [DOI] [Google Scholar]
- 108. Wang Q, Cao Z, Liu Q, Zhang J, Hu Y, Zhang J, Xu W, Kong Q, Yuan X, Chen QF. 2019. Enhancement of COD removal in constructed wetlands treating saline wastewater: intertidal Wetland sediment as a novel inoculation. J Environ Manage 249:109398. doi: 10.1016/j.jenvman.2019.109398 [DOI] [PubMed] [Google Scholar]
- 109. Wang S, Yu H, Su Q, Zuo J. 2021. Exploring the role of heterotrophs in partial nitritation-anammox process treating thermal hydrolysis process - anaerobic digestion reject water. Bio Tech 341:125762. doi: 10.1016/j.biortech.2021.125762 [DOI] [PubMed] [Google Scholar]
- 110. Feng H, Wang Y, Zhang X, Shen D, Li N, Chen W, Huang B, Liang Y, Zhou Y. 2017. Degradation of p-fluoronitrobenzene in biological and bioelectrochemical systems: differences in kinetics, pathways, and microbial community evolutions. Chem Eng J 314:232–239. doi: 10.1016/j.cej.2016.12.097 [DOI] [Google Scholar]
- 111. Wang Y, Tian H, Huang F, Long W, Zhang Q, Wang J, Zhu Y, Wu X, Chen G, Zhao L, Bakken LR, Frostegård Å, Zhang X. 2017. Time-resolved analysis of a denitrifying bacterial community revealed a core microbiome responsible for the anaerobic degradation of quinoline. Sci Rep 7:14778. doi: 10.1038/s41598-017-15122-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang Y, Ji Z, Pei Y. 2021. Nutrient removal and microbial community structure in an artificial-natural coupled wetland system. Process Saf Environmental Prot 147:1160–1170. doi: 10.1016/j.psep.2021.01.036 [DOI] [Google Scholar]
- 113. Garaiyurrebaso O, Garbisu C, Blanco F, Lanzén A, Martín I, Epelde L, Becerril JM, Jechalke S, Smalla K, Grohmann E, Alkorta I. 2017. Long-term effects of aided phytostabilisation on microbial communities of metal-contaminated mine soil. FEMS Microbiol Ecol 93:fiw252. doi: 10.1093/femsec/fiw252 [DOI] [PubMed] [Google Scholar]
- 114. Rastogi G, Barua S, Sani RK, Peyton BM. 2011. Investigation of microbial populations in the extremely metal-contaminated Coeur d’Alene river sediments. Microb Ecol 62:1–13. doi: 10.1007/s00248-011-9810-2 [DOI] [PubMed] [Google Scholar]
- 115. Huang J, Zhu C, Kong Y, Cao X, Zhu L, Zhang Y, Ning Y, Tian W, Zhang H, Yu Y, Zhang J. 2022. Biochar application alleviated rice salt stress via modifying soil properties and regulating soil bacterial abundance and community structure. Agronomy 12:409. doi: 10.3390/agronomy12020409 [DOI] [Google Scholar]
- 116. Chen L, Li K, Shang J, Wu Y, Chen T, Wanyan Y, Wang E, Tian C, Chen W, Chen W, Mi G, Sui X. 2021. Plant growth–promoting bacteria improve maize growth through reshaping the rhizobacterial community in low-nitrogen and low-phosphorus soil. Biol Fertil Soils 57:1075–1088. doi: 10.1007/s00374-021-01598-6 [DOI] [Google Scholar]
- 117. Biyada S, Merzouki M, Dėmčėnko T, Vasiliauskienė D, Marčiulaitienė E, Vasarevičius S, Urbonavičius J. 2022. The effect of feedstock concentration on the microbial community dynamics during textile waste composting. Front Ecol Evol 10:813488. doi: 10.3389/fevo.2022.813488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Banda DM, Pereira JH, Liu AK, Orr DJ, Hammel M, He C, Parry MAJ, Carmo-Silva E, Adams PD, Banfield JF, Shih PM. 2020. Novel bacterial clade reveals origin of form I Rubisco. Nat. Plants 6:1158–1166. doi: 10.1038/s41477-020-00762-4 [DOI] [PubMed] [Google Scholar]
- 119. Madueño L, Starevich VA, Agnello AC, Coppotelli BM, Laprida C, Vidal NC, Di Marco P, Oneto ME, Del Panno MT, Morelli IS. 2021. Assessment of biological contribution to natural recovery of anthropized freshwater sediments from Argentina: autochthonous microbiome structure and functional prediction. Front Microbiol 12:601705. doi: 10.3389/fmicb.2021.601705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ward LM, Shih PM, Hemp J, Kakegawa T, Fischer WW, McGlynn SE. 2019. Genomic evidence for phototrophic oxidation of small alkanes in a member of the Chloroflexi Phylum. bioRxiv. doi: 10.1101/531582 [DOI]
- 121. Ward LM, Shih PM, Hemp J, Kakegawa T, Fischer WW, McGlynn SE. 2019. Phototrophic methane oxidation in a member of the Chloroflexi phylum. bioRxiv. 531582. [Google Scholar]
- 122. Hemp J, Ward LM, Pace LA, Fischer WW. 2015. Draft genome sequence of Levilinea saccharolytica KIBI-1, a member of the Chloroflexi class Anaerolineae. Genome Announc 3:24–25. doi: 10.1128/genomeA.01357-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hemp J, Ward LM, Pace LA, Fischer WW. 2015. Draft genome sequence of Ardenticatena maritima 110S, a thermophilic nitrate- and iron-reducing member of the Chloroflexi class Ardenticatenia. Genome Announc 3:110–111. doi: 10.1128/genomeA.01347-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. González-Blanco G, Casas-Reyes A, Velasco-Garduño O, Ruiz-Gómez ML, Aguirre-Garrido JF, Beristain-Cardoso R. 2021. simultaneous nitrification/denitrification and desulfurization of wastewater polluted with ammonium, COD and sulfide: effectiveness of a new up-flow vertical hybrid reactor. 3 Biotech 11:123. doi: 10.1007/s13205-021-02671-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ward LM, Idei A, Nakagawa M, Ueno Y, Fischer WW, McGlynn SE. 2019. Geochemical and metagenomic characterization of jinata onsen, a proterozoic- analog hot spring, reveals novel microbial diversity including iron-tolerant phototrophs and thermophilic lithotrophs. Microbes Environ 34:278–292. doi: 10.1264/jsme2.ME19017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Islam ZF, Cordero PRF, Feng J, Chen Y-J, Bay SK, Jirapanjawat T, Gleadow RM, Carere CR, Stott MB, Chiri E, Greening C. 2019. Two Chloroflexi classes independently evolved the ability to persist on atmospheric hydrogen and carbon monoxide. ISME J 13:1801–1813. doi: 10.1038/s41396-019-0393-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kanno N, Haruta S, Hanada S. 2019. Sulfide-dependent photoautotrophy in the filamentous anoxygenic phototrophic bacterium, Chloroflexus aggregans. Microbes Environ 34:304–309. doi: 10.1264/jsme2.ME19008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kawai S, Nishihara A, Matsuura K, Haruta S. 2019. Hydrogen-dependent autotrophic growth in phototrophic and chemolithotrophic cultures of thermophilic bacteria, Chloroflexus aggregans and Chloroflexus aurantiacus, isolated from Nakabusa hot springs. FEMS Microbiol Lett 366:fnz122. doi: 10.1093/femsle/fnz122 [DOI] [PubMed] [Google Scholar]
- 129. Rahman A, Susmi TF, Yasmin F, Karim ME, Hossain MU. 2020. Functional annotation of an ecologically important protein from Chloroflexus aurantiacus involved in polyhydroxyalkanoates (PHA) biosynthetic pathway. SN Appl Sci 2:1810. doi: 10.1007/s42452-020-03598-x [DOI] [Google Scholar]
- 130. Izaki K, Haruta S. 2020. Aerobic production of bacteriochlorophylls in the filamentous anoxygenic photosynthetic bacterium, Chloroflexus aurantiacus in the light. Microbes Environ 35:ME20015. doi: 10.1264/jsme2.ME20015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Almeida J.R., Serrano E, Fernandez M, Fradinho JC, Oehmen A, Reis MAM. 2021. Polyhydroxyalkanoates production from fermented domestic wastewater using phototrophic mixed cultures. Wat Res 197:117101. doi: 10.1016/j.watres.2021.117101 [DOI] [PubMed] [Google Scholar]
- 132. Almeida Juliana R., Fradinho JC, Carvalho G, Oehmen A, Reis MAM. 2022. Dynamics of microbial communities in phototrophic polyhydroxyalkanoate accumulating cultures. Microorganisms 10:351. doi: 10.3390/microorganisms10020351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fradinho J, Allegue LD, Ventura M, Melero JA, Reis MAM, Puyol D. 2021. Up-scale challenges on biopolymer production from waste streams by purple phototrophic bacteria mixed cultures: a critical review. Bioresour Technol 327:124820. doi: 10.1016/j.biortech.2021.124820 [DOI] [PubMed] [Google Scholar]
- 134. Afreen R, Tyagi S, Singh GP, Singh M. 2021. Challenges and perspectives of polyhydroxyalkanoate production from microalgae/cyanobacteria and bacteria as microbial factories: an assessment of hybrid biological system. Front Bioeng Biotechnol 9:624885. doi: 10.3389/fbioe.2021.624885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ángeles R, Arnaiz E, Gutiérrez J, Muñoz R, Lebrero R. 2021. Biogas-based production of glycogen by Nostoc muscorum: assessing the potential of transforming co2 into value added products. Chemosphere 275:129885. doi: 10.1016/j.chemosphere.2021.129885 [DOI] [PubMed] [Google Scholar]
- 136. Higuchi-Takeuchi M, Morisaki K, Toyooka K, Numata K. 2016. Synthesis of high-molecular-weight polyhydroxyalkanoates by marine photosynthetic purple bacteria. PLoS One 11:e0160981. doi: 10.1371/journal.pone.0160981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Higuchi-Takeuchi M, Morisaki K, Numata K. 2016. A screening method for the isolation of polyhydroxyalkanoate-producing purple non-sulfur photosynthetic bacteria from natural seawater. Front Microbiol 7:1509. doi: 10.3389/fmicb.2016.01509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Arias DM, Fradinho JC, Uggetti E, García J, Oehmen A, Reis MAM. 2018. Polymer accumulation in mixed cyanobacterial cultures selected under the feast and famine strategy. Algal Res 33:99–108. doi: 10.1016/j.algal.2018.04.027 [DOI] [Google Scholar]
- 139. Fradinho JC, Carvalho VCF, Reis MAM. 2021. New Phototrophic factories for resource recovery, p 413–438. In Moura JJG, Moura I, Maia LB (ed), Enzymes for solving humankind’s problems: Natural and artificial systems in health, agriculture, environment and energy. Springer. [Google Scholar]
- 140. Shih PM, Ward LM, Fischer WW. 2017. Evolution of the 3-hydroxypropionate bicycle and recent transfer of anoxygenic photosynthesis into the Chloroflexi. Proc Natl Acad Sci U S A 114:10749–10754. doi: 10.1073/pnas.1710798114 [DOI] [PMC free article] [PubMed] [Google Scholar]