Abstract
Purpose:
Chronic musculoskeletal pain is common and debilitating among breast cancer survivors. The PEACE trial demonstrated that electro-acupuncture (EA) and battle field auricular acupuncture (BFAA) both reduced pain more than usual care (UC) in cancer survivors.
However, the comparative effectiveness between EA and BFAA among breast cancer survivors is unknown.
Methods:
EA and BFAA received ten weekly treatments. UC was offered ten EA treatments after week 12. The primary endpoint was change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12. We analyzed the subset of 165 (46%) trial participants with a breast cancer primary diagnosis. We conducted constrained linear mixed model analyses, which constrained all arms to a common pre-randomization baseline mean. Model-based mean estimates at weeks 12 and 24 were compared between arms using model contrasts.
Results:
Among 165 breast cancer survivors, common pre-randomization mean pain severity was 5.35 (95% Confidence Interval [CI]: 5.04, 5.66). At week 12, BPI pain severity score was 2.69 (2.26. 3.13) in EA, 3.60 (3.17, 4.02) in BFAA, and 5.06 (4.47, 5.65) in UC. EA reduced pain severity significantly more than BFAA at weeks 12 (−0.90 [−1.45, −0.36], p=0.001) and 24 (- 0.82, [−1.38, −0.27], p=0.004). EA and BFAA significantly improved both Patient-Reported Outcomes Measurement Information System (PROMIS)–Global Health physical health and mental health component scores at week 12 compared to UC. Mild toxicities were reported.
Conclusion:
EA was more effective than BFAA at reducing pain severity, but both similarly improved physical and mental health scores. Breast cancer survivors with chronic musculoskeletal pain may consider EA before BFAA.
Trial Registration:
ClinicalTrials.gov Identifier: NCT02979574, https://clinicaltrials.gov/ct2/show/NCT02979574
Keywords: pain, breast cancer, acupuncture, quality of life
INTRODUCTION
With early diagnosis and improved treatment, more than 3.8 million women in the United States live with a history of breast cancer.[1] Chronic musculoskeletal pain is common and debilitating among breast cancer survivors.[2] The majority of breast cancer patients has estrogen receptor positive tumors and will be treated with five to ten years of anti-estrogen agents, such as aromatase inhibitors (AIs), which have been associated with musculoskeletal pain in up to 47% of patients.[3] In addition, breast cancer survivors may also experience post-surgical pain, post- radiation discomfort that manifests as musculoskeletal pain, and post-chemotherapy arthralgia and pain associated with lymphedema or rotator cuff syndrome.[2]
Acupuncture is one of the most frequently used integrative medicine approaches among breast cancer survivors for symptom control. The efficacy of acupuncture in reducing AI-induced musculoskeletal pain among breast cancer survivors has been demonstrated in a recent phase III sham acupuncture and usual care-controlled trial.21 This trial showed that acupuncture significantly reduced AI-induced joint muscle pain by 1 point on a 0–10-point scale. There has also been research on the effect of acupuncture on reducing chronic pain, postoperative pain, and post-radiation pain.[4–7]
Electro-acupuncture (EA) is a technique of inserting acupuncture needles in specific points in the body with electronic stimulation connected between certain acupoints to obtain therapeutic effect. Licensed acupuncturists must administer EA,[8] and their licensing requires years of training. Battle field auricular acupuncture (BFAA) is a specific ear acupuncture protocol involving insertion of specific ear needles into up to five points in the ear on each side that was developed to treat chronic pain, it can be administered by clinicians without formal acupuncture training,[9] and has been widely implemented across the Veterans Health Administration nationwide. No large trials have been conducted to study the effect of BFAA in reducing musculoskeletal pain among cancer survivors.
Previously, we reported results from the Personalized Electro-acupuncture (EA) versus Battle Field Auricular Acupuncture (BFAA) Comparative Effectiveness (PEACE) trial, a three-arm, parallel, single center randomized trial investigating the effectiveness of EA and BFAA versus UC for chronic musculoskeletal pain in 360 cancer survivors[10]. We demonstrated that both acupuncture methods reduced pain more than usual care (UC) in all types of cancer survivors with mean pain reduction ranging from 1.6 to 1.9 points on a 0–10 numeric pain scale among all cancer types.[10] However, the comparative effectiveness between EA and BFAA among breast cancer survivors remains unknown.
Here, we report the results of subgroup analysis focusing on breast cancer survivors enrolled in the PEACE trial to help guide breast cancer survivors with chronic musculoskeletal pain about whether EA or BFAA would be more effective to reduce their pain with fewer side effects.
METHODS
Eligibility and Study Conduct
We have previously reported the details of the PEACE study.[11, 10] Briefly, eligible study participants were cancer survivors with no evidence of disease who were experiencing moderate to severe pain with the worst pain intensity in the past week ≥4 on a 0–10 numerical rating scale.
Participants were randomized with a 2:2:1 ratio to receive EA:BFAA:UC using randomly permuted blocks of random length stratified by accrual site and baseline opioid use. In this manuscript, we analyze the subset of trial participants with a primary diagnosis of breast cancer. This trial was conducted at Memorial Sloan Kettering Cancer Center, which includes a main campus in Manhattan, New York and five regional community-based clinical sites in New York and New Jersey.
Intervention
Patients in both EA and BFAA received ten weekly treatments over ten weeks. Patients in UC could receive ten EA treatments after week 12. Details of the EA and BFAA interventions have been described in the parent manuscript.[10] Briefly, in the EA arm, licensed acupuncturists with at least five years of experience placed acupuncture needles (SEIRIN) at four acupuncture points near the painful site and an additional four or more needles at distal points to address the coexisting symptoms. The local pain points were stimulated by a 2 Hz electric current with an E- STIMII device (Tens Plus Industrial Company).
In the BFAA arm, the same acupuncturists delivered auricular acupuncture following a standardized battlefield protocol.[12] ASP auricular acupuncture needles (Sedatelec) were inserted in up to five different auricular acupuncture points (cingulate gyrus, thalamus, omega 2, point zero, and shen men) in sequence depending on the patient’s response. The needles were left in place for three to four days and removed by the patients themselves.
In the UC arm, patients continued utilizing usual care including pain medications, physical therapy, and steroid injections as prescribed by their primary care providers and were offered ten acupuncture treatments after week 12.
Endpoints
The primary endpoint was the change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12. Change in BPI pain severity from baseline to week 24 was a secondary endpoint. The BPI has four questions related to pain severity, and the responses range from 0 (no pain) to 10 (pain as bad as you can imagine). The mean of these four responses (BPI pain severity score) was used as the primary outcome measure.[13] Other secondary endpoints included change from baseline to weeks 12 and 24 on scores from the Patient-Reported Outcomes Measurement Information System (PROMIS)–Global Health questionnaire, a ten-item validated instrument for quality of life.[14] The PROMIS instrument yields a physical health and a mental health component score, both scored on a T-score metric with 50 corresponding to the normative sample mean. To enhance the clinical interpretation of our results, we defined BPI pain response as a reduction of 30% or greater on the BPI pain severity score from baseline to 12 weeks, consistent with established definitions of analgesic response based on the BPI.22
Statistical Analysis
We analyzed each BPI and PROMIS outcome measure using separate constrained linear mixed models in which all arms were constrained to have a common pre-randomization baseline outcome measure mean.[15] Consistent with the statistical analysis plan for the full PEACE study, each model included the randomization stratification variables (accrual site and baseline opioid use), treatment arm, time (categorical), and the arm-by-time interaction as fixed effects and patient-specific random intercepts. All randomized patients with breast cancer as their primary diagnosis and with at least one outcome assessment were included in the models.
Model-based mean estimates at weeks 12 and 24 were compared between arms, with inferences based on model coefficients and contrasts of model-based means. We calculated the proportion of BPI pain severity responders at week 12 within each arm and compared the proportions using a Fisher’s chi-squared test. We used separate logistic regression models to evaluate whether surgery type or AI use was associated with BPI severity response, with each model containing the main effect for treatment arm and either surgery type or AI use. To assess the heterogeneity of pain treatment effect by these two variables, we added the arm-by-variable interactions to the respective main effect models.
RESULTS
Among the 360 cancer survivors, 165 had breast cancer as their primary cancer diagnosis. The baseline characteristics of these breast cancer survivors are listed in Table 1. In brief, mean (standard deviation [SD]) age was 60.3 (11.0) years, 35.8% were non-white, and mean time since cancer diagnosis was 5.4 (6.5) years. Patients had been experiencing pain for 5.6 (7.3) years, with baseline mean pain severity of 5.35 (95% CI: 5.04, 5.66) based on the constrained linear mixed model. 86.7% had a prior history of surgery, 43.0% chemotherapy, 64.8% radiotherapy, and 50.3% endocrine therapy. In addition, 50.3% of patients were on endocrine therapy, with 57.6% in the EA arm, 38.8% in the BFAA arm, and 59.4% in the UC arm. The common locations of pain were lower back (24.2%), knee/leg (23.6%), and shoulder/arm/elbow (13.9%). 107 (66.9%) patients were taking pain medication. Patients with breast cancer were balanced among three arms (Table 1).
Table 1:
Patient Demographics and Characteristics in Breast Cancer Subgroup
| Characteristic | N | Overall, N = 1651 | Electroacupuncture, N = 66 | Battle Field Auricular Acupuncture, N = 67 | Usual Care, N = 32 |
|---|---|---|---|---|---|
| Age, Mean+/−SD | 165 | 60.3+/−11.0 | 60.4+/−11.2 | 61.5+/−10.6 | 57.4+/−11.3 |
| Sex, n (%) | 165 | ||||
| Male | 1 (0.6) | 0 (0.0) | 1 (1.5) | 0 (0.0) | |
| Female | 164 (99.4) | 66 (100.0) | 66 (98.5) | 32 (100.0) | |
| Race, n (%) | 165 | ||||
| Non-white | 59 (35.8) | 28 (42.4) | 26 (38.8) | 5 (15.6) | |
| White | 106 (64.2) | 38 (57.6) | 41 (61.2) | 27 (84.4) | |
| Ethnicity, n (%) | 163 | ||||
| Hispanic | 19 (11.7) | 9 (13.8) | 8 (12.1) | 2 (6.2) | |
| Non-Hispanic | 144 (88.3) | 56 (86.2) | 58 (87.9) | 30 (93.8) | |
| Missing | 2 | 1 | 1 | 0 | |
| Breast Surgery, n (%) | 165 | 143 (86.7) | 57 (86.4) | 56 (83.6) | 30 (93.8) |
| Chemotherapy, n (%) | 165 | 71 (43.0) | 23 (34.8) | 32 (47.8) | 16 (50.0) |
| Radiation, n (%) | 165 | 107 (64.8) | 40 (60.6) | 45 (67.2) | 22 (68.8) |
| Reconstructive Surgery, n (%) | 165 | 43 (26.1) | 16 (24.2) | 17 (25.4) | 10 (31.2) |
| Endocrine therapy, n (%) | 165 | 83 (50.3) | 38 (57.6) | 26 (38.8) | 19 (59.4) |
| Years Since Cancer Diagnosis, Mean+/−SD | 165 | 5.4+/−6.5 | 5.3+/−6.5 | 5.4+/−6.6 | 5.4+/−6.5 |
| Duration of Pain Symptoms, years, Mean+/−SD | 165 | 5.6+/−7.3 | 5.8+/−7.3 | 5.2+/−7.1 | 6.0+/−8.2 |
| Taking Any Pain Medications, n (%) | 160 | 107 (66.9) | 43 (67.2) | 42 (64.6) | 22 (71.0) |
| Missing | 5 | 2 | 2 | 1 | |
| Location of Pain, n (%) | 165 | ||||
| Neck | 10 (6.1) | 2 (3.0) | 3 (4.5) | 5 (15.6) | |
| Upper Back | 9 (5.5) | 3 (4.5) | 4 (6.0) | 2 (6.2) | |
| Lower Back | 40 (24.2) | 21 (31.8) | 15 (22.4) | 4 (12.5) | |
| Shoulder/Arm/Elbow | 23 (13.9) | 9 (13.6) | 13 (19.4) | 1 (3.1) | |
| Wrist/Hand | 10 (6.1) | 5 (7.6) | 4 (6.0) | 1 (3.1) | |
| Hip/Thigh | 20 (12.1) | 8 (12.1) | 8 (11.9) | 4 (12.5) | |
| Knee/Leg | 39 (23.6) | 13 (19.7) | 16 (23.9) | 10 (31.2) | |
| Ankle/Feet | 14 (8.5) | 5 (7.6) | 4 (6.0) | 5 (15.6) |
Mean+/−SD; n (%)
Among the 165 breast cancer survivors, the baseline mean pain severity was 5.35 (95% confidence interval (CI): 5.04, 5.66). At week 12, the BPI pain severity score was 2.69 (2.26. 3.13) in EA, 3.60 (3.17, 4.02) in BFAA, and 5.06 (4.47, 5.65) in UC. The change in mean BPI severity score from baseline was −2.65 (−3.06, −2.25), −1.75 (−2.15, −1.35), and −0.29 (−0.86, 0.28) in EA, BFAA, and UC, respectively. At week 24, the mean BPI pain severity was 2.84 (2.40, 3.28) in EA and 3.67 (95% CI: 3.23, 4.10) in BFAA (Figure 1).
Figure 1.
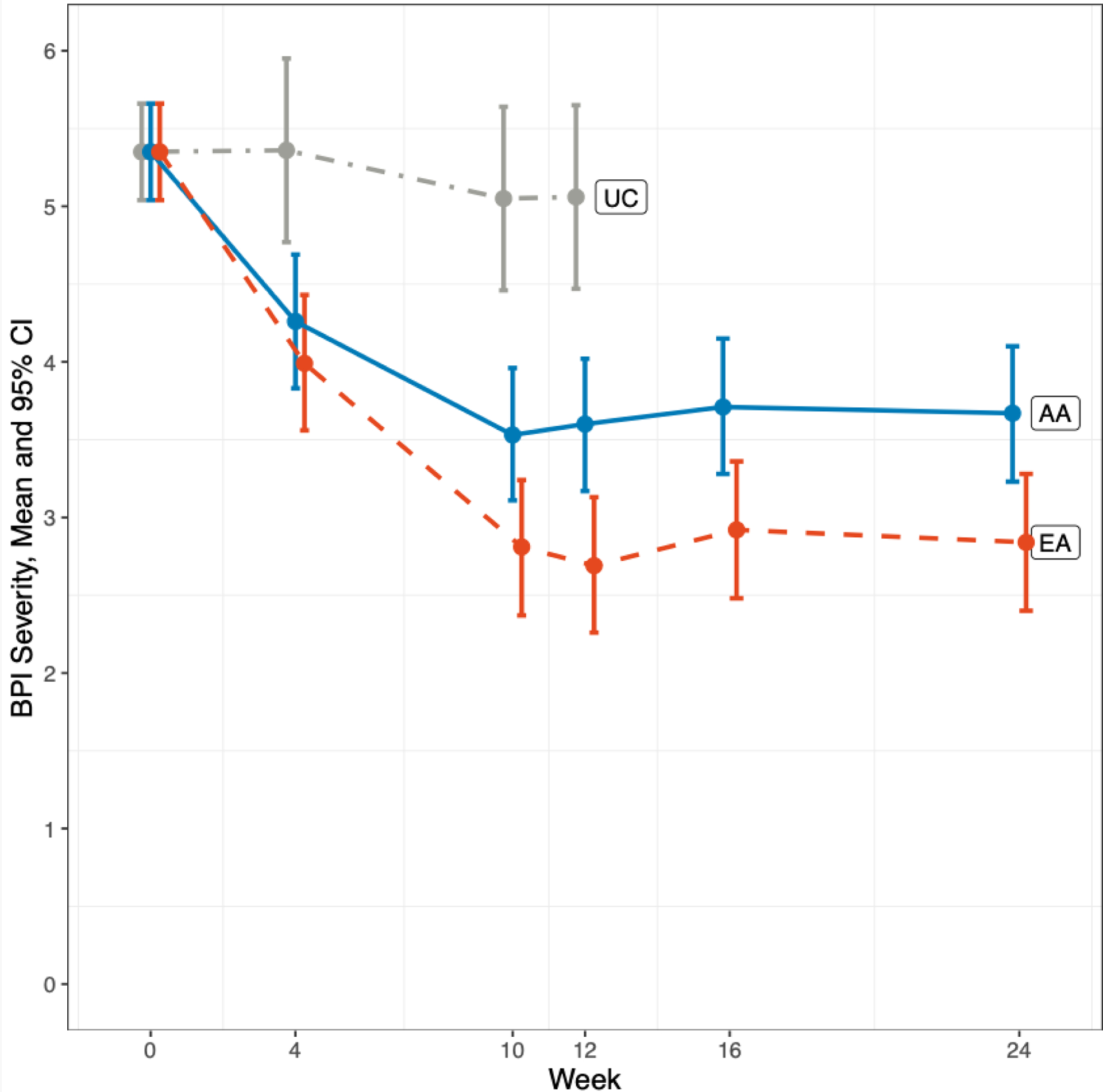
Brief Pain Inventory Severity Changes Over Time
This figure shows changes over time in the BPI pain severity score.
At week 12, EA reduced BPI pain severity by an average of 2.65 (95% CI: −3.06, −2.25) when compared to baseline on a 0–10 scale, which persisted at week 24 (2.51, 95% CI: −2.92, −2.10). In comparison, BFAA reduced pain severity by 1.75 (95% CI: −2.15, −1.35) at week 12, which also persisted at week 24 (−1.68, 95% CI: −2.09, −1.27). EA reduced pain severity significantly more than BFAA at both week 12, (−0.90, 95% CI: −1.45, −0.36, p=0.001) and week 24 (−0.82, 95% CI: −1.38, −0.27, p=0.004) (Table 2).
Table 2.
Changes in Brief Pain Inventory Pain Severity From Baseline
| BPI Pain severity | UC | EA | BFAA | EA vs. BFAA | ||
|---|---|---|---|---|---|---|
| Change from baseline | Change from baseline | Difference from UC | Change from baseline | Difference from UC | Difference between EA and BFAA | |
|
Week 12 Mean
(95% CI) |
−0.29 (−0.86, 0.28) | −2.65* (−3.06, −2.25) | −2.37* (−3.05, −1.68) | −1.75* (−2.15, −1.35) | −1.46* (−2.14, −0.78) | −0.90* (−1.45, −0.36) |
|
Week 24 Mean
(95% CI) |
n/a | −2.51* (−2.92, −2.10) | n/a | −1.68 * (−2.09, −1.27) | n/a | −0.82 ** (−1.38, −0.27) |
p < 0.05
p, 0.01
p < 0.001
Abbreviations: BPI, Brief Pain Inventory; UC, usual care; EA, electro-acupuncture; BFAA, battle field auricular acupuncture; CI, confidence interval
Neither surgery type (mastectomy versus lumpectomy) (p=0.46) nor AI/Tamoxifen (AI vs. Tamoxifen vs. Neither) (p=0.66) was associated with BPI Severity response (defined as 30% improvement between baseline and Week 12). The n (%) with BPI response for the EA, BFAA, and UC arms, respectively, was 42 (68.9%), 37 (57.8%), and 5 (16.7%). P<0.001. There were no differences between the arms in the effect of AI/Tamoxifen on BPI response (arm*AI_Tamoxifen Interaction p=0.27). Similarly, there were no differences between the arms in the effect of surgery type on BPI response (arm*Surgery Interaction p=0.39).
Quality of Life
There were no differences between EA and BFAA in improvements in PROMIS physical health or mental health component scores, but both EA and BFAA significantly improved both PROMIS scores at week 12 compared to UC (Table 3 and Figures 2 and 3).
Table 3.
Changes in PROMIS Physical and Mental Health From Baseline
| UC | EA | BFAA | EA vs BFAA | |||
|---|---|---|---|---|---|---|
| Change from baseline | Change from baseline | Difference from UC | Change from baseline | Difference from UC | Difference between EA and BFAA | |
| PROMIS Physical Health | ||||||
|
Week 12 Mean
(95% CI) |
0.33 (−1.41, 2.08) | 5.21 (3.97, 6.44)*** | 4.87 (2.77, 6.97)*** | 4.36 (3.13, 5.59)*** | 4.03 (1.94, 6.12)*** | 0.84 (−0.85, 2.54) |
|
Week 24 Mean
(95% CI) |
n/a | 3.70 (2.46, 4.95)*** | n/a | 3.72 (2.46, 4.97)*** | n/a | −0.01 (−1.74, 1.71) |
| PROMIS Mental Health | ||||||
|
Week 12 Mean
(95% CI) |
−1.45 (−3.37, 0.47) | 2.12 (0.76, 3.47)** | 3.56 (1.25, 5.88)** | 2.96 (1.62, 4.31)*** | 4.41 (2.10, 6.72)*** | −0.85 (−2.72, 1.02) |
|
Week 24 Mean
(95% CI) |
n/a | 1.71 (0.35, 3.07)* | n/a | 1.80 (0.43, 3.18)* | n/a | −0.09 (−1.99, 1.80) |
p < 0.05
p, 0.01
p < 0.001
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; UC, usual care; EA, electro-acupuncture; BFAA, battle field auricular acupuncture; CI, confidence interval
Figure 2.
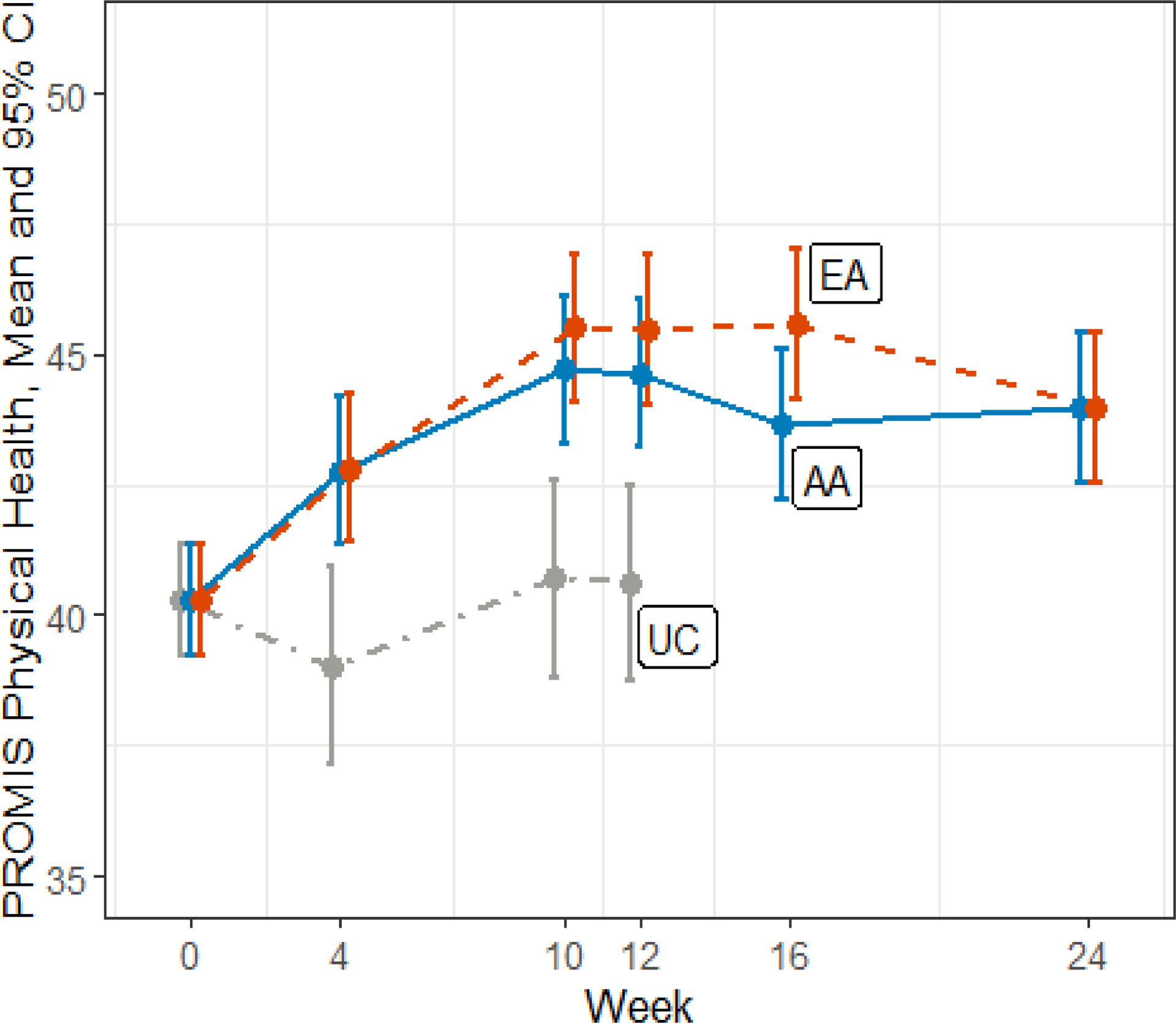
PROMIS Physical Health Changes Over Time
This figure shows changes over time in the PROMIS physical health score.
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; UC, usual care; EA, electro-acupuncture; AA, auricular acupuncture; CI, confidence interval
Figure 3.
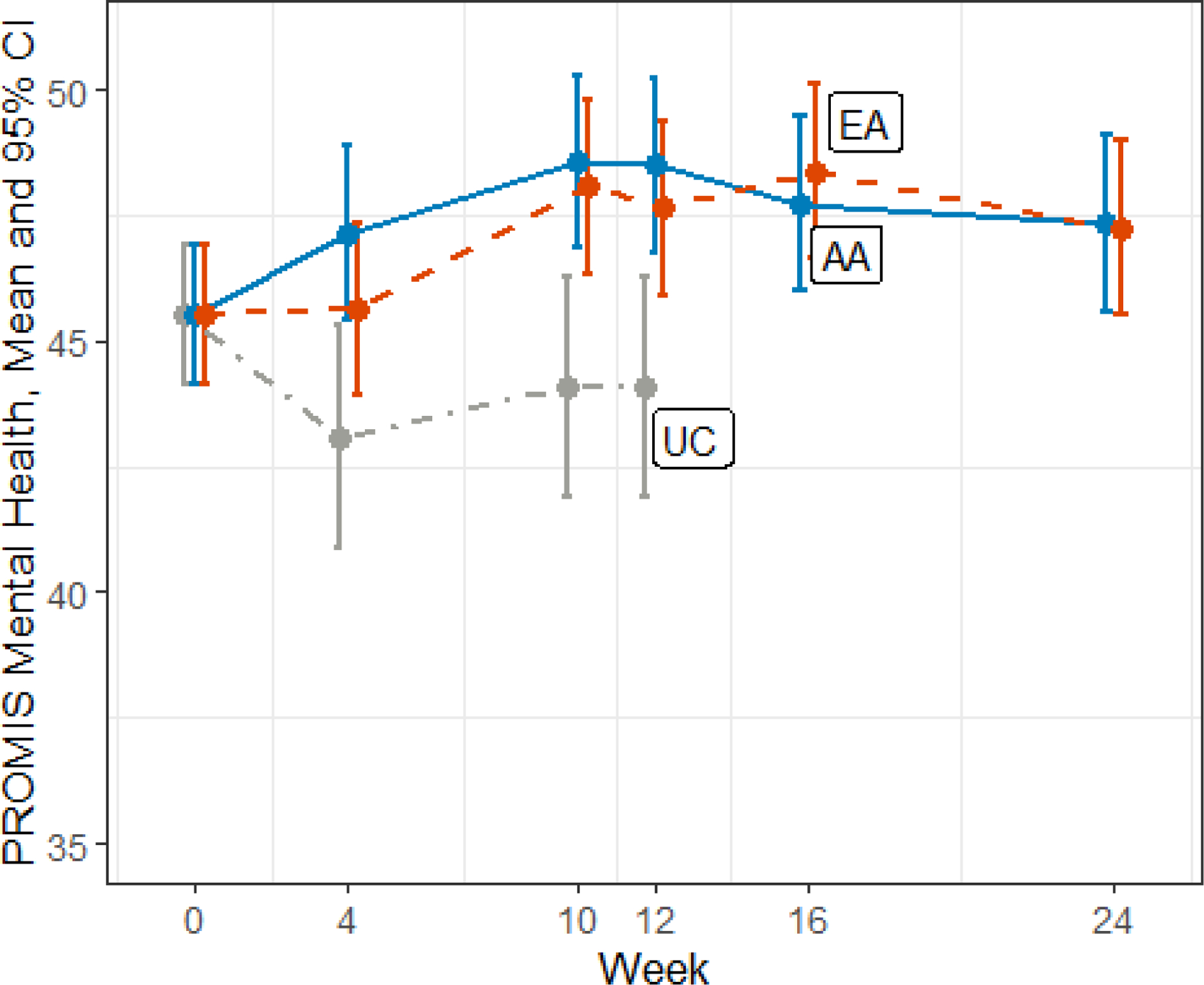
PROMIS Mental Health Changes Over Time
This figure shows changes over time in the PROMIS mental health score.
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; UC, usual care; EA, electro-acupuncture; AA, auricular acupuncture; CI, confidence interval
Mild study-related adverse events were reported among the 165 breast cancer survivors. Table 4 listed the toxicities in three arms. The BFAA arm generally had more adverse events than the EA arm, particularly pain at the needling site. Five (7.6%) EA patients and 11 (16.4%) BFAA patients did not complete treatment (p=0.12).
Table 4.
Summary of Adverse Events in Each Arm
| Adverse Events, n (%) | Overall N = 165 | Electro- acupuncture N = 66 | Battle Field Auricular Acupuncture N = 67 | Usual Care N = 32 |
|---|---|---|---|---|
| Pain/soreness at needling site, grade 1 | 14 (8.5) | 1 (1.5) | 13 (19.4) | 0 |
| Pain/soreness at needling site, grade 2 | 9 (5.5) | 0 (0) | 9 (13.4) | 0 |
| Bruising at needling site, grade 1 | 16 (9.7) | 14 (21.2) | 2 (3.0) | 0 |
| Bruising at needling site, grade 2 | 1 (0.6) | 0 (0) | 1 (1.5) | 0 |
| Nausea, grade 1 | 1 (0.6) | 0 (0) | 1 (1.5) | 0 |
| Dizziness, grade 1 | 4 (2.4) | 1 (1.5) | 3 (4.5) | 0 |
| Dizziness, grade 2 | 1 (0.6) | 0 (0) | 1 (1.5) | 0 |
| Bleeding at needling site, grade 1 | 7 4.2) | 4 (6.1) | 3 (4.5) | 0 |
| Bleeding at needling site, grade 2 | 1 (0.6) | 0 (0) | 1 (1.5) | 0 |
| Itchiness, grade 1 | 1 (0.6) | 1 (1.5) | 0 (0) | 0 |
| Spasm, grade 1 | 1 (0.6) | 1 (15) | 0 (0) | 0 |
DISCUSSION
Pain is a common symptom experienced by growing numbers of breast cancer survivors that negatively impacts their quality of life, functions, and potentially their adherence to hormonal treatment. Consistent with the parent PEACE study,[10] the subgroup analysis of the 165 breast cancer survivors showed that both EA and BFAA resulted in clinically meaningful and persistent pain reduction among breast cancer survivors when compared with usual care at weeks 12 and 24. The magnitude of such pain reduction is both statistically and clinically significant. EA was found to be more effective than BFAA at reducing pain severity with fewer side effects.
Our finding is consistent with existing literature that showed acupuncture significantly reduced AI-related musculoskeletal pain among breast cancer survivors with similar magnitude of pain reduction.[16] Additionally, both EA and BFAA were found to have long term treatment effects at week 24 despite stopping treatment at week 10. This long-term effect of acupuncture has been reported by a number of clinical trials. A multicenter randomized controlled trial comparing true acupuncture versus sham acupuncture versus usual care showed that true acupuncture was more effective in reducing AI-associated musculoskeletal pain when compared to sham acupuncture and usual care, not only at the end of intensive acupuncture treatment at week 6, but also at weeks 12, 24, and 52.[16, 17] This further suggests that both EA and BFAA may serve as durable treatments for cancer survivors with chronic pain. This is particularly important for breast cancer survivors, as medication treatment for pain often requires ongoing use. Long term use of non-steroidal anti-inflammatory drugs (NSAIDS) or acetaminophen (Tylenol) can cause gastrointestinal, hepatic, renal or cardiac toxicities. Together with these two trials results, it is reasonable to recommend acupuncture for breast cancer survivors with chronic musculoskeletal pain. Currently, one of the major barriers for cancer survivors to access acupuncture is the lack of insurance coverage.[18] Our trial, along with the large acupuncture AI-associated musculoskeletal pain trial,[16] provides strong evidence supporting the use of acupuncture for breast cancer survivors. These findings may help to advance the coverage of acupuncture by insurance companies.
The potential mechanism of EA for pain reduction involves stimulating neurohormonal pathways to release neuropeptides such as beta-endorphin secretion and increase pain threshold[19] and reducing systemic inflammation via decreasing pro-inflammatory cytokines.[20] A 2021 animal study showed that EA stimulation at rodent ST 36 acupuncture on the hind leg drove the vagal- adrenal anti-inflammatory axis through PROKR2 sensory neurons in the deep hind leg fascia and vagal efferent neurons in the dorsal motor nucleus of the vagus nerve.[21] The mechanism of auricular acupuncture is likely due to auriculovagal regulation which involves applying auricular acupuncture needles in the outer ear, causing auricular vagus nerve stimulation, which subsequently affects the nucleus tractus solitarii in the brain stem and reduces symptoms including pain, anxiety, and depression.[12, 22, 23]
EA differs from BFAA in multiple ways: For EA, an acupuncturist applies needles in both local pain points and distal points in the body to treat other underlying comorbidities, such as anxiety and insomnia. In BFAA, needles are only applied at ear points. EA uses electronic stimulation between acupoints on the body in the local pain area to increase stimulation; these needles stay in the body for about 25 minutes during each treatment. For BFAA, only special ear needles are inserted; they last for three to four days before falling off the ears. The shorter duration of EA treatment may be the reason EA has fewer toxicities when compared to BFAA, and, hence, a lower dropout rate. EA has more stimulation points than BFAA and has additional electric stimulation, which may be why it is more effective than BFAA in pain reduction in this population.
Both EA and BFAA improved quality of life in breast cancer survivors when compared to UC, whereas there was no significant difference between EA and BFAA. This suggests that the effect of EA-induced systemic nervous stimulation on quality of life is similar to that caused by BFAA- induced auriculo-vagal regulation. EA requires specialized training that may take up to four years to complete. BFAA requires much shorter training, a half day in our study, which makes it easier to disseminate. As such, for quality-of-life improvement in breast cancer survivors with musculoskeletal pain, BFAA is a reasonable alternative to EA in places where it is difficult to access experienced acupuncturists.
This subgroup analysis of the PEACE trial is limited by it being a single center trial, a lack of biomarkers to further explain the mechanisms, and a lack of sham control to tease out the placebo effect. This trial is also limited by using BFAA, which is not the typical auricular acupuncture as it uses up to five points in each ear without an option to personalize the point selection based on the patient’s symptoms. In addition, the trial was conducted in an academic center, which may limit its generalizability, though community-based network clinical sites were included. The strength of the parent PEACE study is its large sample size, novel design by comparing EA with BFAA and UC, and being the first large RCT comparing the effectiveness of EA versus BFAA among cancer survivors with chronic musculoskeletal pain.
One unique aspect of our trial is that we report on the efficacy of BFAA, a specific ear acupuncture protocol involving insertion of ear needles into up to five points in the ear on each side that can be administered by clinicians without formal acupuncture training. However, it is difficulty to conclude that the BFAA administered by non-acupuncture clinicians is as effective as BFAA administered by licensed acupuncturists without additional studies. The regular cost of electroacupuncture is $75 to $150 per session in the United States, whereas BFAA could be offered at a lower cost and overall more cost effective due to the ability to leverage existing clinicians and the minimal space requirements needed. EA must be administered by trained acupuncturists, whereas BFAA could be administered by clinicians without formal acupuncture training, therefore improving its accessibility. Further, EA sessions usually last 30 to 45 minutes, whereas BFAA could take less time, 10 to 15 minutes.
On the other hand, our data shows that patients receiving EA had a marginally greater reduction in pain than those receiving BFAA. In addition to pain, EA has been shown to be an effective treatment for other symptoms related to cancer such as hot flashes, nausea, and insomnia. Many patients, especially in an oncology population, present with symptom clusters. For these patients, EA may be a more efficient tool to address their multiple complaints. Additionally, there was a higher incidence of drop-outs in the BFAA group, which was due in part to difficulty tolerating the procedure. While BFAA is easily taught to health care providers and thus more accessible, EA may be a better option for more sensitive patients, or those experiencing symptom clusters. As a result, if there is an option for patients to undergo either EA or BFAA, patients may benefit from EA. If no such option is available, BFAA is a great alternative for patients due to cost and accessibility issues.
In conclusion, EA was more effective than BFAA at reducing pain severity. Breast cancer survivors with chronic musculoskeletal pain may consider EA before BFAA for pain reduction. Both EA and BFAA are effective in improving quality of life when compared to UC.
Figure 4.
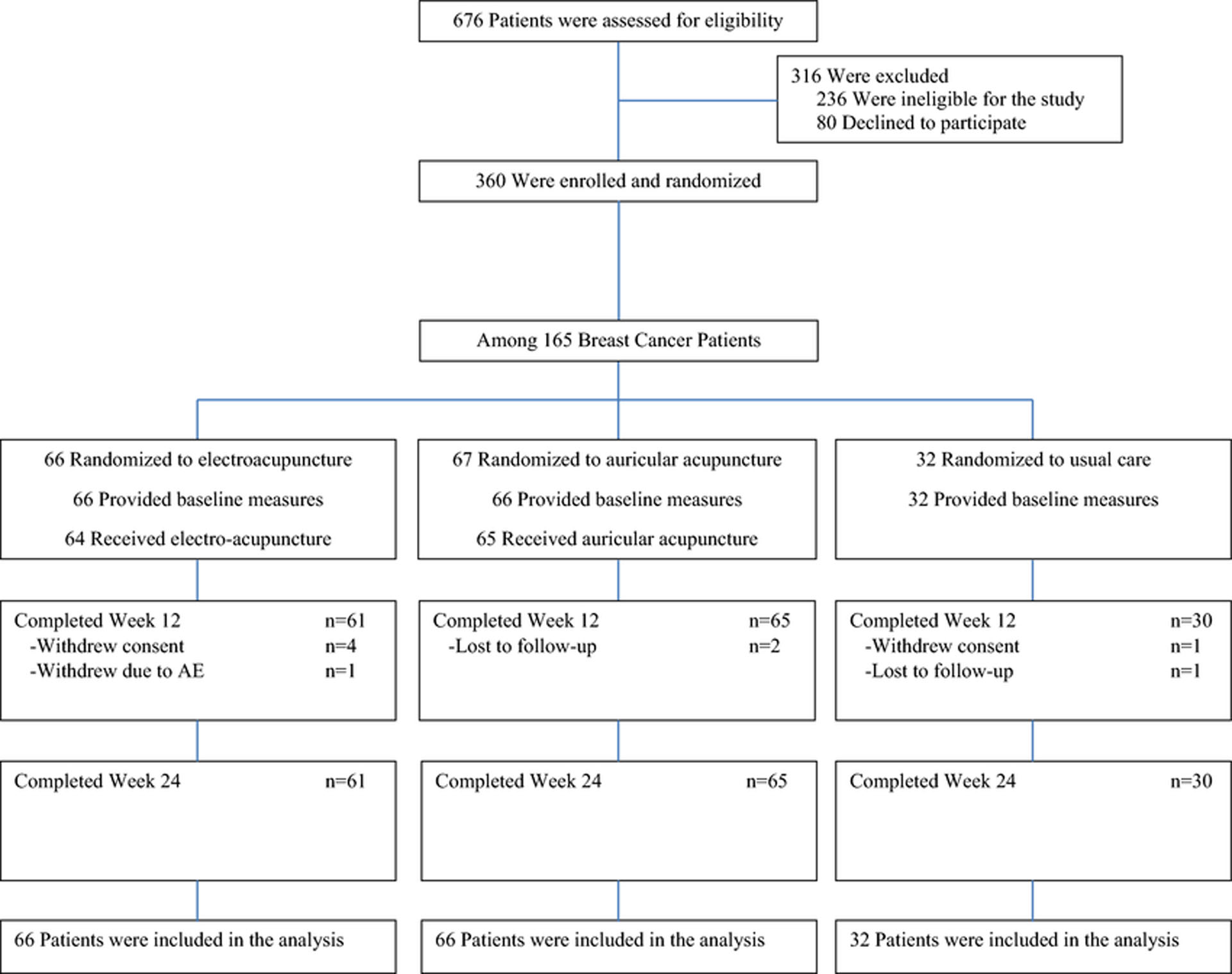
PEACE Trial Enrollment and Follow-Up Among Breast Cancer Patients
This figure is a consort diagram that shows PEACE trial enrollment and follow-up among breast cancer patients.
Acknowledgments and Funding:
This work was supported by the Department of Defense office of the Congressionally Directed Medical Research Programs through the Peer Reviewed Medical Research Program Clinical Trial Award (W81XWH-15-1-0245). This work was also supported in part by a National Institutes of Health/National Cancer Institute (NIH/NCI) Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center. Dr. Bao is supported in part by NCI R37 CA248563. Dr. Mao is supported in part by NCI 1 R01 CA240417. Dr. Gillespie is supported in part by NCI K08 CA252640.
Competing Interests:
Jun J. Mao reports research funding provided to Memorial Sloan Kettering Cancer Center from Tibet Cheezheng Tibetan Medicine Company, Ltd. outside the submitted work. Ting Bao reports a consultation role in Eisai Inc.
Footnotes
Ethics Approval:
The Institutional Review Board at Memorial Sloan Kettering Cancer Center approved the trial protocol, and the study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. The trial registration records are available at ClinicalTrials.gov, identifier: NCT02979574, https://clinicaltrials.gov/ct2/show/NCT02979574.
Consent to Participate: Informed consent was obtained from all individual participants included in the study.
Data Availability:
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.American Cancer Society. About Breast Cancer: Key Statistics for Breast Cancer. In: Breast Cancer. American Cancer Society. 2022. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html. Accessed 08/08/2022 2022. [Google Scholar]
- 2.Bao T, Seidman A, Li Q, Seluzicki C, Blinder V, Meghani SH et al. Living with chronic pain: perceptions of breast cancer survivors. Breast Cancer Res Treat. 2018;169(1):133–40. doi: 10.1007/s10549-018-4670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25(25):3877–83. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 4.Bauml J, Basal C, Mao JJ. Treatment of post-mastectomy pain syndrome with acupuncture: a case report. Acupunct Med. 2014;32(2):183–5. doi: 10.1136/acupmed-2013-010459. [DOI] [PubMed] [Google Scholar]
- 5.Djaali W, Simadibrata CL, Nareswari I. Acupuncture Therapy in Post-Radiation Head-and- Neck Cancer with Dysgeusia. Med Acupunct. 2020;32(3):157–62. doi: 10.1089/acu.2020.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickers AJ, Vertosick EA, Lewith G, MacPherson H, Foster NE, Sherman KJ et al. Acupuncture for Chronic Pain: Update of an Individual Patient Data Meta-Analysis. J Pain. 2018;19(5):455–74. doi: 10.1016/j.jpain.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Wen J, Hong J. The Effects of Auricular Therapy for Cancer Pain: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2020;2020:1618767. doi: 10.1155/2020/1618767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan AY, Faggert S. Distribution of licensed acupuncturists and educational institutions in the United States in early 2015. J Integr Med. 2018;16(1):1–5. doi: 10.1016/j.joim.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Niemtzow R, Baxter J, Gallagher RM, Pock A, Calabria K, Drake D et al. Building Capacity for Complementary and Integrative Medicine Through a Large, Cross-Agency, Acupuncture Training Program: Lessons Learned from a Military Health System and Veterans Health Administration Joint Initiative Project. Mil Med. 2018;183(11–12):e486–e93. doi: 10.1093/milmed/usy028. [DOI] [PubMed] [Google Scholar]
- 10.Mao JJ, Liou KT, Baser RE, Bao T, Panageas KS, Romero SAD et al. Effectiveness of Electroacupuncture or Auricular Acupuncture vs Usual Care for Chronic Musculoskeletal Pain Among Cancer Survivors: The PEACE Randomized Clinical Trial. JAMA Oncol. 2021;7(5):720–7. doi: 10.1001/jamaoncol.2021.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou KT, Baser R, Romero SAD, Green J, Li QS, Orlow I et al. Personalized electro- acupuncture versus auricular-acupuncture comparative effectiveness (PEACE): A protocol of a randomized controlled trial for chronic musculoskeletal pain in cancer survivors. Medicine (Baltimore). 2020;99(21):e20085. doi: 10.1097/MD.0000000000020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemtzow RC. Battlefield Acupuncture: My Story. Med Acupunct. 2018;30(2):57–8. doi: 10.1089/acu.2018.29077.rcn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 14.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–80. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med. 2009;28(20):2509–30. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, Unger JM, Greenlee H, Capodice JL, Lew DL, Darke AK et al. Effect of Acupuncture vs Sham Acupuncture or Waitlist Control on Joint Pain Related to Aromatase Inhibitors Among Women With Early-Stage Breast Cancer: A Randomized Clinical Trial. Jama. 2018;320(2):167–76. doi: 10.1001/jama.2018.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershman D, Unger JM, Greenlee H, Capodice J, Lew DL, Darke AK et al. Long-term results from a randomized blinded sham- and waitlist-controlled trial of acupuncture for joint symptoms related to aromatase inhibitors in early stage breast cancer (S1200). J Clin Oncol. 2021:12018. [Google Scholar]
- 18.Sedhom R, Gupta A, Wang L, Paller C, Bao T. Payer Coverage of Integrative Medicine Interventions for Symptom Control in Patients With Cancer. JCO Oncol Pract. 2021;17(10):587–90. doi: 10.1200/OP.21.00361. [DOI] [PubMed] [Google Scholar]
- 19.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 20.Jeong HJ, Hong SH, Nam YC, Yang HS, Lyu YS, Baek SH et al. The effect of acupuncture on proinflammatory cytokine production in patients with chronic headache: a preliminary report. Am J Chin Med. 2003;31(6):945–54. doi: 10.1142/S0192415X03001661. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598(7882):641–5. doi: 10.1038/s41586-021-04001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemtzow RC. Implementing Battlefield Acupuncture Through a Large Medical System: Overcoming Barriers. Med Acupunct. 2020;32(6):377–80. doi: 10.1089/acu.2020.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He W, Wang X, Shi H, Shang H, Li L, Jing X et al. Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med. 2012;2012:786839. doi: 10.1155/2012/786839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


