Abstract
This retrospective study investigates the efficacy of 2 treatment regimens, pregabalin alone versus pregabalin combined with ketamine, amitriptyline, and lidocaine cream, in reducing itch in patients with brachioradial pruritus at a tertiary care center. Electronic medical records of 64 brachioradial pruritus patients seen at the University of Miami Itch Center were analyzed. A significant reduction in itch scores was seen with both treatments, with no significant difference between the groups. A small number of patients experienced adverse effects, including drowsiness and weight gain with pregabalin and skin irritation with ketamine, amitriptyline, and lidocaine cream. Ultimately, our findings underscore the potential of utilizing combined therapy for difficult-to-treat brachioradial pruritus cases and implementing individualized approaches for managing neuropathic pruritus. Further controlled clinical trials are needed to establish optimal treatment protocols.
SIGNIFICANCE
This study on brachioradial pruritus presents valuable insights into effective treatments for a condition lacking standardized therapies. Additionally, treatment adverse effects and non-responder rates highlight the need for careful consideration and further research into optimal medication dosages and combinations. Our findings contribute to improving symptom management in brachioradial pruritus with both pregabalin alone and combined with topical treatment and the need to establish treatment guidelines for brachioradial pruritus patients.
Key words: brachioradial pruritus, neuropathic pruritus, pruritus, treatments
Brachioradial pruritus (BRP) is a rare form of neuropathic pruritus localized to the dorsolateral arms (1). Most therapeutic strategies have been reported in limited case reports (2–6) with no identified gold standard. We present here a large cohort of BRP patients examined at a tertiary center for itch reduction in response to 2 treatment regimens.
MATERIALS AND METHODS
In this study, approved by the University of Miami Institutional Review Board, electronic medical records of 64 patients seen at the University of Miami Itch Center from January 2017 to February 2023 were examined retrospectively. Itch Numerical Rating Scale (NRS) scores at the initial visit and 3-month follow-up were recorded. A paired samples t-test was conducted to compare the outcome measures of pregabalin at baseline and 3-month follow-up. An independent samples t-test was used to assess for significance between the 2 cohorts: oral pregabalin alone and oral pregabalin + compounded ketamine 10%, lidocaine 5%, and amitriptyline 5% in liposomal transdermal base (KAL cream).
RESULTS
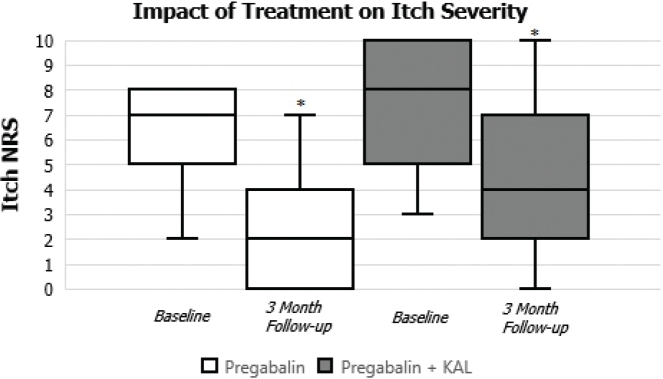
Of the 64 BRP patients, most were white (56 of the 64 or 88%) with a mean age of 64.09 ± 12.06 years. Some 45% (29) were men and 55% (35) were women with most patients being overweight or obese (36 of the 64 or 56%). The average daily dose of pregabalin prescribed for these patients was 300 mg daily; 28 patients were on oral pregabalin alone and 15 were prescribed KAL cream in addition to pregabalin. The other 21 patients were receiving various treatments other than pregabalin or in combination with compounded KAL cream and were not analyzed due to limited numbers. These other medications included gabapentin, KAL cream alone, Sarna lotion, hydrocortisone cream, triamcinolone ointment, capsaicin cream, pramoxine 1% cream, and desonide lotion. One patient did not have any treatment documented. In assessing the efficacy of pregabalin alone at baseline and at 3-month follow-up, the analysis revealed a significant reduction in mean itch scores between the 2 visits of 3.57 ± 1.251 (p < 0.0145, 2-tailed), 95% CI [0.85, 6.30], as shown in Fig. 1. This statistically significant difference suggests a meaningful impact of the treatment on the measured outcome. For the combined pregabalin and topical KAL cream group, the reduction in mean itch scores at 3-month follow-up from baseline was also significant at 3.00 ± 1.003 (p < 0.0057, 2-tailed), 95% CI [0.95, 5.05], as shown in Fig. 1. In comparing the 2 cohorts of patients receiving pregabalin (pregabalin alone vs pregabalin + KAL), however, no significant difference was noted.
Fig. 1.
Impact of pregabalin on brachioradial pruritus.
Patients with an itch NRS score reduction of less than 3 points between the two visits were considered non-responders. For patients receiving pregabalin alone, 2 were non-responders, both of whom were on 50 mg twice daily. For the pregabalin + KAL cream group, 6 patients were non-responders: 4 on 75 mg pregabalin 2 times/day + KAL, 1 on 50 mg pregabalin daily + KAL, and 1 on 75 mg pregabalin 4 times/day + KAL. These findings emphasize the need for higher doses of GABAergic drugs (2). Conversely, patients with an itch NRS score reduction to 0 or 1 point at follow-up were considered to have fully responded to treatment. For the pregabalin-only group, 3 patients had full response. In the pregabalin + KAL group, 7 patients had full response. Regarding adverse effects, 6 patients reported drowsiness and 2 could not tolerate higher pregabalin doses. Additionally, 2 patients reported weight gain of up to 2 kg, and 1 patient reported leg edema. These are consistent with known side effects of GABAergic drugs. Amongst patients using KAL, 2 reported skin irritation and 4 stopped using the cream altogether due to no perceived improvement in itch.
Only 5 patients were found to be on KAL cream alone and were excluded from the analysis; 3 of these 5 patients did not have itch NRS scores recorded during their dermatology visits. One patient showed no reduction in itch scores from baseline to 3-month follow-up. Another patient showed a significant reduction in itch scores at the 3-month follow-up visit from baseline (itch score was 3 at baseline and 0 at follow-up).
DISCUSSION
Pregabalin is a γ-aminobutyric acid analog that acts by antagonizing the alpha-2-delta subunit of voltage-gated calcium channels and reduces the central neural release of excitatory molecules such as substance P and other neuropeptides involved in pruritus (4). The mechanism of action for its reduction in chronic pruritus is thought to involve increasing the threshold for neuronal depolarization by itch (7). Comparably, the combination of amitriptyline, ketamine, and lidocaine has been reported to be effective for neuropathic itch (8) by preventing neuronal excitation through synergistic effects on the synapse and axon. It is possible that the beneficial effect in BRP seen in our patients for both treatments is at least partly due to an inhibitory effect on central sensitization processes of itch chronification. These mechanisms likely overlap with those generating chronic pain. Moreover, most of our patients in the pregabalin + KAL group started with higher baseline itch NRS scores, signifying difficult-to-treat pruritus that may have previously failed improvement with other therapies. KAL cream may thus be used as an adjunct to oral medications for localized itch relief in these patients. In fact, a prior retrospective study we performed analyzing the efficacy and tolerability of topical ketamine–amitriptyline–lidocaine (TKAL) reported significant improvement in average itch scores before and after its application for BRP patients but with a limited number of patients (8).
Our study was limited by its retrospective nature and associated drawbacks including lack of control over original patient data collection, patient recall bias, and possible concomitant use of other systemic medications. Controlled clinical trials are needed to verify the efficacy and optimal dosing for BRP treatments and help establish clinical guidelines for treatment escalation decisions.
ACKNOWLEDGEMENTS
IRB approval status
This study was reviewed and approved by University of Miami IRB; approval #20230362.
Conflict of interest disclosures
GY: Consultant and Board Member Sanofi, Regeneron, Pfizer, Galderma, Novartis, Eli Lilly, Abbvie, Kiniksa, Trevi, Pierre Gabre, LEo, Escient. Celldex, Bellus, Research support Pfizer, Sanofi Regeneron, Leo, Eli Lilly, Kiniksa, Novartis, Escient, Bellus, Galderma, Celldex.
REFERENCES
- 1.Kavanagh KJ, Mattei PL, Lawrence R, Burnette C. Brachioradial pruritus: an etiologic review and treatment summary. Cutis 2023; 112: 84–87. [DOI] [PubMed] [Google Scholar]
- 2.Kwatra SG, Elmariah S, Chisolm S, Mollanazar N, Fassett M, Cole EF, et al. United States expert panel consensus on uniform nomenclature and diagnosis for neuropathic pruritus. Itch 2024; 9: e0073. [Google Scholar]
- 3.Baka P, Birklein F. Neuropathischer Pruritus – evidenzbasierte Behandlungsempfehlungen. Nervenarzt 2023; 94: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sreekantaswamy SA, Mollanazar N, Butler DC. Gabapentinoids for pruritus in older adults: a narrative review. Dermatol Ther (Heidelb) 2021; 11: 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atis G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther 2017; 30. [DOI] [PubMed] [Google Scholar]
- 6.Zakaria A, Amerson E. Treatment modalities in brachioradial pruritis: a systematic review. Dermatol Online J 2022; 28. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda KM, Sharma D, Schonfeld AR, Kwatra SG. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol 2016; 75: 619–625 e616. [DOI] [PubMed] [Google Scholar]
- 8.Lee HG, Grossman SK, Valdes-Rodriguez R, Berenato F, Korbutov J, Chan YH, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol 2017; 76: 760–761. [DOI] [PubMed] [Google Scholar]