Abstract
Aims
The lymphocyte adaptor protein (LNK) is a negative regulator of cytokine and growth factor signalling. The rs3184504 variant in SH2B3 reduces LNK function and is linked to cardiovascular, inflammatory, and haematologic disorders, including stroke. In mice, deletion of Lnk causes inflammation and oxidative stress. We hypothesized that Lnk−/− mice are susceptible to atrial fibrillation (AF) and that rs3184504 is associated with AF and AF-related stroke in humans. During inflammation, reactive lipid dicarbonyls are the major components of oxidative injury, and we further hypothesized that these mediators are critical drivers of the AF substrate in Lnk−/− mice.
Methods and results
Lnk−/− or wild-type (WT) mice were treated with vehicle or 2-hydroxybenzylamine (2-HOBA), a dicarbonyl scavenger, for 3 months. Compared with WT, Lnk−/− mice displayed increased AF duration that was prevented by 2-HOBA. In the Lnk−/− atria, action potentials were prolonged with reduced transient outward K+ current, increased late Na+ current, and reduced peak Na+ current, pro-arrhythmic effects that were inhibited by 2-HOBA. Mitochondrial dysfunction, especially for Complex I, was evident in Lnk−/− atria, while scavenging lipid dicarbonyls prevented this abnormality. Tumour necrosis factor-α (TNF-α) and interleukin-1 beta (IL-1β) were elevated in Lnk−/− plasma and atrial tissue, respectively, both of which caused electrical and bioenergetic remodelling in vitro. Inhibition of soluble TNF-α prevented electrical remodelling and AF susceptibility, while IL-1β inhibition improved mitochondrial respiration but had no effect on AF susceptibility. In a large database of genotyped patients, rs3184504 was associated with AF, as well as AF-related stroke.
Conclusion
These findings identify a novel role for LNK in the pathophysiology of AF in both experimental mice and humans. Moreover, reactive lipid dicarbonyls are critical to the inflammatory AF substrate in Lnk−/− mice and mediate the pro-arrhythmic effects of pro-inflammatory cytokines, primarily through electrical remodelling.
Keywords: Atrial fibrillation, Inflammation, Oxidative stress, Isolevuglandins, Pro-inflammatory cytokines
Graphical Abstract
Graphical Abstract.
Time of primary review: 25 days
See the editorial comment for this article ‘A mechanistic LNK between inflammation and atrial fibrillation?’, by J.A. Keefe et al., https://doi.org/10.1093/cvr/cvae083.
1. Introduction
Atrial fibrillation (AF), the most common sustained arrhythmia, is associated with an increased risk of stroke, heart failure, and death.1 Unfortunately, currently available therapies to prevent AF are inadequate1,2 due to an incomplete understanding of the mechanisms that promote the AF substrate.
Encoded by SH2B3, the lymphocyte adaptor protein (LNK) is a member of the Src homology 2-B family of adaptor proteins that regulate multiple intracellular signalling pathways.3 LNK is predominantly expressed in all haematopoietic cells and tissues, where it functions as a negative regulator of cytokine- and haematopoietic growth factor-mediated signalling and proliferation. LNK is also found in select non-haematopoietic cells, including the endothelium.3–5 In genome-wide association studies, a missense single-nucleotide polymorphism rs3184504 in SH2B3 has been linked to multiple cardiovascular, autoimmune, and haematologic diseases, including hypertension (HTN), myocardial infarction, stroke, Type I diabetes, rheumatoid arthritis, and inflammatory bowel disease, as well as leucocytosis, thrombocytosis, and myeloproliferative disorders.6–12 The prevalence of rs3184504 in individuals of European ancestry is 48–50%.5 In mice, deletion of Lnk causes systemic inflammation and oxidative stress due to the removal of a signalling ‘brake’ on haematopoietic cell proliferation, as well as renal and vascular inflammation and impaired vasorelaxation.13 These animals also display a propensity for HTN and aortic dissection during Angiotensin II infusion, although they are normotensive at baseline.14 Bone marrow transplantation experiments have shown that Lnk-deficient haematopoietic cells are responsible for inflammatory and hypertensive phenotypes, rather than non-haematopoietic cells, indicating that inflammation drives the pathophysiology present in these animals.13 In the setting of hypercholesterolaemia, Lnk−/− mice develop accelerated atherosclerosis and thrombosis.15 Recently, the rs3184504 polymorphism was modelled in mice, demonstrating a similar but weaker hypertensive phenotype, indicating that this variant causes loss of function.5 The role of LNK in AF is unknown.
Inflammation is linked to the pathophysiology of multiple AF risk factors, such as HTN, and in humans to AF itself. It is well recognized that cardiac surgery promotes systemic inflammation with up to 50% of patients developing post-operative AF.16 Pro-inflammatory cytokines are elevated in human AF,17 and experimental studies have implicated tumour necrosis factor-alpha (TNF-α),18 interleukin-1 beta (IL-1β),19 and interleukin-6 (IL-6)20 in detrimental pro-arrhythmic effects primarily in the ventricle. While the fundamental molecular mechanism(s) by which inflammation promotes AF susceptibility nonetheless remain unclear, one possible causative mechanism is inflammation-mediated oxidative stress due to excess reactive oxygen species (ROS). Inflammatory cells directly produce ROS, while inflammation-mediated cytokines, such as IL-1β, also increase ROS in the heart,19 and oxidative damage is present in the atria of humans with AF.21 Unfortunately, dietary antioxidants targeting ROS levels directly have proven ineffective to prevent AF in clinical trials.22,23 An alternative therapeutic strategy is to target pathologic mediators of ROS-related injury.24 A preferential substrate for ROS is polyunsaturated fatty acid, and lipid peroxidation generates multiple reactive lipid dicarbonyl products, exemplified by malondialdehyde (MDA) and isolevuglandins (IsoLGs), that adduct proteins, DNA, and other macromolecules to cause ROS-related disease (see Supplementary material online, Figure S1).25 In essence, lipid dicarbonyls serve as a biomarker for oxidative stress in cells and tissues. Among these compounds, IsoLGs are the most reactive, and they have been shown to participate in the pathogenesis of multiple inflammation-related diseases, including atherosclerosis,26 hypertension,27 and Alzheimer’s disease.28 In addition, IsoLGs were recently found to promote AF susceptibility in murine hypertension.24 In a murine model of atherosclerosis, both IsoLG and MDA adducts were similarly increased in atherosclerotic aortas,26 indicating the utility of IsoLGs as a representative marker.
Given the relationship among LNK, inflammation, and multiple AF risk factors, we hypothesized that mice deficient in Lnk are susceptible to AF. Due to the systemic inflammatory state of these mice, we further hypothesized a key role for oxidative stress, specifically lipid dicarbonyls, in the pathophysiology of AF susceptibility. Using this unique model, we identified for the first time that lipid dicarbonyls are critical drivers of inflammation-related AF that mediate the arrhythmogenic effects of pro-inflammatory cytokines. Moreover, we found that inhibition of soluble TNF-α, but not IL-1β, can prevent AF in these animals. Finally, we identified a novel association of rs3184504 with AF, as well as AF-related stroke, in humans.
2. Methods
Additional details regarding the methods used in this study are summarized in the supplementary material.
2.1. Animals
Mice with systemic deletion of Lnk (Lnk−/−) were generated as described29 with cardiac morphology assessed by echocardiography and routine histology. AF inducibility was performed using transoesophageal atrial pacing.30,31 Soluble TNF-α was inhibited by XPRO1595, a dominant-negative biologic, whereas IL-1β was inhibited using an anti-IL-1β antibody. Age-matched littermate controls were used, and treatments were randomly assigned.
2.2. Flow cytometry
Atrial immune cells were quantified by flow cytometry using antibodies specific for CD45, CD3, CD19, MHCII, CD11b, and NK1.1. IsoLG adducts were detected by intracellular staining with a fluorescently labelled anti-IsoLG lysyl antibody (D11 ScFv).32
2.3. Atrial superoxide and immunofluorescence
In frozen atrial sections, superoxide (O2•−) and IsoLG adducts were assessed using dihydroethidium and D11 ScFv, respectively. Co-immunostaining studies with IsoLGs were performed using D11 ScFv and antibodies directed against mouse NaV1.5 and Kv4.2.
2.4. mRNA and protein quantitation
Atrial mRNA expression (e.g. of collagen and ROS-related genes) was quantitated using real-time quantitative polymerase chain reaction (RT-qPCR), while representative ion channel protein subunits were assayed using western blot analysis.
2.5. Electrophysiology
To isolate atrial myocytes, hearts were excised and cannulated on a Langendorff apparatus followed by perfusion and digestion with collagenase. Single-cell action potentials and ionic currents were recorded using current clamp and voltage clamp techniques, respectively.
2.6. Ca2+ measurements
Previously published methods were used to quantitate Ca2+ sparks in permeabilized atrial myocytes and intracellular Ca2+ handling in intact cells.33
2.7. High-resolution respirometry
Atria were permeabilized and mechanically dissociated. Oxygen consumption was measured at baseline and following the addition of bioenergetic substrates.
2.8. Transmission electron microscopy
Hearts were perfused with a cardioplegic buffer and fixed. Samples were post-fixed, embedded, and sectioned followed by image acquisition.
2.9. Quantification of cytokines
Pro-inflammatory cytokines were quantified in plasma and atrial tissue using a multiplex electrochemiluminescence immunoassay.34
2.10. Cell culture
O2•−, IsoLG adducts, cellular ATP, and action potential parameters were assessed in atrial HL-1 cells treated with TNF-α or IL-1β for 24 h or with acute exposure.
2.11. Human analyses
To test the hypothesis that the functional missense variant rs3184504 in SH2B3 is associated with human AF, association studies were performed in a large clinical population (>67 000) of European ancestry subjects with genotype and phenotype data.
2.12. Statistics
Results are presented as mean ± standard error of the mean (SEM) or median with inter-quartile ranges as appropriate. Data are analysed in GraphPad Prism (v8.2.1, Boston, MA) using Student’s t-test, one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test (MCT), Mann–Whitney U test, Kruskal–Wallis test with Dunn’s MCT, Fisher’s exact test, or two-way ANOVA for repeated measures as indicated in the figures. For isolated cardiomyocytes, data are analysed in RStudio using a previously validated hierarchical cluster analysis.35 The association between rs3184504 and AF in humans utilized logistic regression adjusted for age, sex, and 10 principal components.
2.13. Ethics statement
All animal procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and IACUC and conducted in accordance with the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services.
With regards to the human association study, the data utilized from BioVU was de-identified data. Therefore, upon review of this project the Vanderbilt Institutional Review Board deemed it exempt as non-human subject research in accordance with 45 Code of Federal Regulations 46.
3. Results
3.1. Cardiac morphology in Lnk−/− mice is normal
Given that mice deficient in Lnk could represent a novel model of AF susceptibility, initial studies were performed to characterize their cardiac structure. By echocardiography, there were no differences in ventricular function between wild-type (WT) and Lnk−/− mice (Figure 1A and B; Supplementary material online, Table S1). In a separate group of anaesthetized animals, left atrial (LA) diameter in Lnk−/− mice was quantitated and found to be similar to that of WT controls (see Supplementary material online, Figure S2). In addition, histochemical staining revealed no evidence of histologic abnormalities, increased fibrosis, or amyloid deposition in the Lnk−/− atria compared with WT controls (see Supplementary material online, Figure S3A and B). Atrial mRNA expression of Col1a1 and Col3a1, as well as heart and atrial weights, did not differ in the two groups (see Supplementary material online, Figure S3C–F). Finally, measurement of systolic blood pressure and glucose tolerance testing confirmed that these mice did not have overt HTN or diabetes (see Supplementary material online, Figure S4A and B). Thus, there was no evidence of any cardiovascular abnormalities in Lnk−/− mice that would predispose to AF.
Figure 1.
In the setting of structurally normal hearts, Lnk−/− mice display increased AF susceptibility that is prevented by the dicarbonyl scavenger 2-HOBA, while atrial inflammation is not affected. (A) Representative B- and M-mode images in WT and Lnk−/− mice with (B) quantification of ejection fraction (EF) and fractional shortening (FS; n = 9, 10). Summary data for (C) total AF duration and (D) sustained AF (≥15 s) incidence in WT littermate controls, and Lnk−/− mice treated with vehicle, 2-HOBA, or 4-HOBA (n = 19, 30, 18, 13). (E) Representative density plots and quantification of total atrial leucocytes by flow cytometry (CD45+; n = 26, 24, 14). Each data point represents the atria from 2 mice. Summary data for atrial (F) antigen-presenting cells (MHCII+/CD11b+; n = 22, 20, 9), (G) natural killer cells (NK1.1+; n = 26, 24, 14), (H) B cells (CD19+; n = 23, 24, 14), and (I) T cells (CD3+; n = 26, 24, 14). Statistical tests: (B) Student’s t-test; (C, E–G, I) Kruskal–Wallis followed by Dunn’s MCT; (D) Fisher’s exact test; and (H) one-way ANOVA with a Tukey’s post hoc test. EDD, end diastolic diameter; ESD, end systolic diameter; ns, not significant; SAX, short axis; SSC-A, side scatter area. *P < 0.05; **P < 0.01.
3.2. Lnk−/− mice demonstrate AF susceptibility that is suppressed by 2-HOBA
Lnk−/− mice display systemic inflammation,13 and we hypothesized that reactive lipid dicarbonyl products of inflammation-mediated oxidative stress promote AF in this setting. Indeed, compared with WT controls, Lnk−/− mice demonstrated a significant increase in total AF duration and the number of sustained AF episodes during transoesophageal atrial pacing (Figure 1C and D). Administration of a highly specific lipid dicarbonyl scavenger, 2-hydroxybenzylamine (2-HOBA; Supplementary material online, Figure S1), significantly reduced both parameters of AF susceptibility, whereas a structurally similar control compound with minimal scavenging activity, 4-hydroxybenzylamine (4-HOBA), had no effect (Figure 1C and D). Taken together, these findings implicate reactive lipid dicarbonyls as key mediators of the AF substrate in these mice.
3.3. 2-HOBA does not alter inflammation in Lnk−/− mouse atria
To investigate the specific role of atrial inflammation in Lnk−/− AF susceptibility, atrial leucocytes were assessed by flow cytometry (see Supplementary material online, Figure S5). In the Lnk−/− atria, there was a significant increase in total leucocytes (CD45+; Figure 1E) compared with WT controls. This elevation was characterized by increases in antigen-presenting cells (MHCII+/CD11b+) and natural killer cells (NK1.1+) but not B cells (CD19+) or T cells (CD3+; Figure 1F–I). Treatment with 2-HOBA had no effect on the number of total leucocytes present in the Lnk−/− atria nor any immune cell subtype (Figure 1E–I). Thus, Lnk deficiency increases atrial inflammation, but immune cell numbers are not affected by scavenging lipid dicarbonyls.
3.4. Lnk−/− atrial myocytes demonstrate increased oxidative stress and IsoLG adducts
Additional studies were undertaken to investigate atrial leucocytes and cardiomyocytes as potential sources of oxidative stress and reactive dicarbonyl mediators. O2•− was increased in both the LA and right atria (RA) of Lnk−/− mice compared with WT controls, as assessed by the fluorescent indicator dihydroethidium (Figure 2A). In addition, atrial expression of superoxide dismutase (SOD) 1 and glutathione peroxidase (Gpx) 1 was reduced (see Supplementary material online, Figure S6), indicating an impaired antioxidant response that would further promote ROS generation. Previous work has shown that IsoLG protein adducts develop not only in the heart but also in immune cells during hypertension, where they function as neoantigens.27 To investigate the location of IsoLG adducts within the Lnk−/− atria, we performed flow cytometry and immunofluorescence of atrial leucocytes and cardiomyocytes, respectively, using an anti-IsoLG lysyl antibody (D11 ScFv).36 Compared with WT, there was no difference in the percentage of total leucocytes containing IsoLG adducts (CD45+/D11+) in the Lnk−/− atria (Figure 2B). Moreover, the percentage of IsoLG-adducted cells was similar between WT and Lnk−/− mice for antigen-presenting cells (MHCII+/CD11b+/D11+), where pathophysiologic lipid dicarbonyls have been previously identified, as well as other atrial leucocyte subtypes (see Supplementary material online, Figure S7).27,37 In contrast, IsoLG-adducted proteins were increased throughout the left and right Lnk−/− atrial myocardium compared with WT controls, and they were reduced by treatment with 2-HOBA (Figure 2C). These results indicate that the Lnk−/− AF phenotype is characterized by increased lipid dicarbonyls within cardiomyocytes, but not immune cells, and this is abrogated by 2-HOBA.
Figure 2.
Superoxide and IsoLG-adducted proteins are increased in Lnk−/− atria. (A) Representative images of dihydroethidium (DHE) staining (n = 5–6; scale bars = 50 µm). (B) Quantification of IsoLG adducts in atrial leucocytes (CD45+) by flow cytometry using D11 ScFv (n = 14, 17, 12). Each data point represents atria from two mice. (C) Representative images of D11 ScFv immunostaining, demonstrating IsoLG-adducted proteins in the Lnk−/− atria (LA: n = 3, 3, 2; RA: n = 4, 4, 3; scale bars = 50 µm). Statistical test: Kruskal–Wallis with Dunn’s MCT post hoc. LA, left atria; ns, not significant; RA, right atria.
3.5. Inflammation promotes electrical remodelling in the Lnk−/− atria that is prevented by 2-HOBA
Recent evidence indicates that inflammation causes ventricular electrical remodelling, largely manifested by QT prolongation on the electrocardiogram (ECG) in humans.38 Moreover, previous work has shown that direct lipoxidative modification of cardiac ion channels can promote pro-arrhythmic effects.39 We hypothesized that the atrial inflammation present in Lnk−/− mice increases AF susceptibility through electrical remodelling. ECG analysis revealed that P-wave duration, as well as QRS and QT intervals, were significantly increased in Lnk-deficient mice compared with controls (Figure 3A–D; Supplementary material online, Table S2). Treatment with 2-HOBA prevented P-wave changes, but not those in the QRS or QT intervals. Compared with WT, action potential duration (at 90% repolarization; APD90) in Lnk−/− atrial myocytes was significantly prolonged, and this was completely prevented by treatment with 2-HOBA (Figure 3E and F). The maximal dV/dT of Phase 0 (Vmax) was also reduced in Lnk−/− atrial myocytes, but there was no effect by 2-HOBA (Figure 3G). There was no difference in resting membrane potential (RMP; Figure 3H) between control and Lnk−/− atrial myocytes.
Figure 3.
Inflammation promotes electrical remodelling in the atria of Lnk−/− mice, which is largely prevented by 2-HOBA. (A) Representative ECG recordings. Red lines denote P-wave duration and blue lines the QT interval. Quantification of (B) P-wave duration as well as (C) QRS and (D) QT intervals (n = 24, 44, 30). (E) Representative action potential recordings from isolated atrial myocytes (n = 24, 36, 18). Experimental data shown for (F) action potential duration at 90% repolarization (APD90), (G) the maximum phase 0 upstroke velocity (Vmax), and (H) RMP. Statistical tests: (B, C) Kruskal–Wallis followed by Dunn’s MCT; (D) one-way ANOVA with a Tukey’s MCT; and (F–H) hierarchical cluster analysis by Sikkel et al.35 ns, not significant. **P < 0.01; ***P < 0.001; ****P < 0.0001.
3.6. Modulation of ionic currents underlie Lnk−/− atrial action potential changes
To investigate the molecular basis for the action potential changes observed in Lnk−/− atrial myocytes, individual ionic currents were recorded in atrial cells. In Lnk-deficient mice, peak atrial Na+ current was reduced compared with WT controls, an effect that was prevented by 2-HOBA (Figure 4A–C). On the other hand, Lnk−/− atrial myocytes displayed an increase in late INa that was also abrogated by 2-HOBA (Figure 4D and E). There was no significant difference in the magnitude of L-type Ca2+ current between Lnk-deficient and WT atrial myocytes (Figure 4F–H). The dominant outward K+ current in these cells is the transient outward current ITo. This current was reduced in Lnk−/− atrial myocytes, with prevention by 2-HOBA (Figure 4I–K). The observed alterations in late INa and ITo are consistent with APD90 prolongation in Lnk−/− atrial myocytes, whereas the reduced INa peak is consistent with the increased P-wave duration. Taken together, these findings reveal that the inflammation and resultant oxidative stress present in Lnk−/− mice promote atrial electrical remodelling that is prevented by 2-HOBA, thus implicating lipid dicarbonyls as a causative mechanism of these effects.
Figure 4.
Alterations in INa and ITo are consistent with action potential changes in Lnk−/− atrial myocytes. (A) Representative families of Na+ currents (INa) are illustrated with the voltage clamp protocol shown in the inset. Experimental data for (B, C) peak and (D, E) late INa (n = 16, 16, 23). Late INa is shown as percentage of peak INa at −30 mV. (F) L-type Ca++ currents (ICa,L) are depicted along with the voltage clamp protocol used. (G, H) Summary data for ICa,L (n = 12, 23, 8). (I–K) Similar data are shown for ITo (n = 11, 13, 8). All statistical analyses (C, E, H, K) were performed using the hierarchical cluster analysis by Sikkel et al.35 ns, not significant. *P < 0.05; **P < 0.01. Data are shown as mean ± SEM for (B, G, J).
3.7. Mechanisms of ionic current modulation
Experiments were performed to investigate the mechanisms whereby inflammation-related lipid dicarbonyls modulate INa and ITo currents. Using western analysis, we found that expression of Nav1.5 was reduced in Lnk−/− atrial tissue but was unaffected by 2-HOBA, while expression of Kv4.2 was unchanged in Lnk-deficient atrium (see Supplementary material online, Figure S8). It was previously reported that IsoLGs can directly adduct to the Nav1.5 channel during exposure to the oxidant tert-butylhydroperoxide.39 Indeed, immunofluorescence experiments demonstrated evidence of co-staining for both Nav1.5 and Kv4.2 channels with IsoLG adducts at the plasma membrane, suggesting colocalization (see Supplementary material online, Figure S9). Thus, there is evidence supporting a role for direct channel modulation, as well as reduced channel expression in the case of Nav1.5, to account for the electrophysiologic remodelling in Lnk−/− mice.
3.8. Intracellular Ca2+ handling is comparable in WT and Lnk−/− atrial myocytes
In addition to ionic current remodelling, abnormal Ca2+ handling, specifically Ca2+ leak from hyperactive cardiac ryanodine receptors (RyR2), has also been implicated as a mechanism that promotes the AF substrate.40 Thus, studies were undertaken to determine whether this might also be the case for Lnk−/− mice. To examine RyR2 activity, we measured sarcoplasmic reticulum (SR) Ca2+ sparks in atrial myocytes isolated from WT and Lnk−/− mice. Atrial cells were permeabilized using saponin and bathed in an internal solution that promotes RyR2-mediated sparks.33 WT and Lnk−/− atrial cardiomyocytes had similar Ca2+ spark frequency and overall Ca2+ leak (see Supplementary material online, Figure S10). Since Ca2+ spark frequency is proportional to the SR Ca2+ load, we assessed SR Ca2+ content using the application of 10 mM caffeine at the end of the experiment. We did not observe any differences in SR Ca2+, suggesting that WT and Lnk−/− atrial myocytes have a similar Ca2+ load and no apparent differences in RyR2 activity. Similarly, in intact atrial cardiomyocytes, there were no differences between WT and Lnk−/− mice with respect to spontaneous Ca2+ release events, Ca2+ transient properties, or SR Ca2+ content (see Supplementary material online, Figure S11). Thus, there was no evidence that abnormal Ca2+ handling plays a role in AF susceptibility in these mice.
3.9. Lipid dicarbonyls promote mitochondrial dysfunction in Lnk−/− mouse atria
In the murine kidney, IsoLGs cause mitochondrial dysfunction by inactivating mitochondrial Complex I (CI).41 To determine whether lipid dicarbonyls can impair mitochondrial bioenergetics in the Lnk−/− mouse heart, we performed high-resolution respirometry using whole atrial tissue. Mitochondrial respiration was assessed at baseline and following addition of substrates for CI, fatty acid oxidation (FAO), and Complex II (CII), as well as adenosine diphosphate (ADP). Compared with WT, Lnk−/− atria displayed a significant loss of CI-mediated respiration and ADP-supported long chain FAO (Figure 5A). Notably, 2-HOBA increased basal as well as CI- and FAO-mediated respiration compared with vehicle-treated Lnk−/− mice (Figure 5B), indicating that lipid dicarbonyls drive mitochondrial abnormalities in the Lnk−/− atria.
Figure 5.
Inflammation promotes mitochondrial dysfunction and ultrastructural abnormalities in Lnk−/− atria. Comparison of atrial oxygen flux between (A) WT and Lnk−/− mice (n = 4, 4) and (B) vehicle- and 2-HOBA-treated Lnk−/− mice (n = 4, 4). (C) Representative transmission electron microscopy images highlighting atrial mitochondria [LA: n = 3, 3, 2; RA: n = 3, 3, 3; scale bars = 1 µm (2700×) and 400 nm (6500×)]. White arrows denote heterogenous mitochondrial damage, while red arrows identify dense atrial granules. Quantification of mitochondrial cristae in the (D) left (n = 379, 348, 263) and (E) right atria (n = 345, 374, 328). Statistical tests: (A, B) Student’s t-test; and (D, E) Kruskal–Wallis with Dunn’s MCT post hoc. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. CI, Complex I; CII, Complex II; FAO, fatty acid oxidation; LA, left atria; mt, mitochondria; OXPHOS, oxidative phosphorylation; RA, right atria.
Given this profound mitochondrial dysfunction, transmission electron microscopy was performed to investigate the cardiac ultrastructure. In Lnk−/− mice, heterogeneous mitochondrial damage was evident throughout both atria that was prevented by 2-HOBA (Figure 5C). Mitochondrial cristae abundance and integrity were blindly assessed using a previously validated scoring system [ranging from 0 (no sharply defined cristae) to 4 (regular and well-defined cristae)].42 There was evidence of significant cristae disruption with loss of normal structure in vehicle-treated but not 2-HOBA-treated Lnk−/− mice (Figure 5D and E). In addition, there was a noticeable increase in atrial dense granules containing natriuretic peptides43 in Lnk-deficient mice in both the absence and presence of 2-HOBA treatment. Thus, lipid dicarbonyls cause not only dysfunction but also structural damage in Lnk−/− atrial mitochondria.
3.10. TNF-α and IL-1β are elevated in Lnk−/− mice and promote electrical remodelling and mitochondrial dysfunction
Pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, are recognized to cause electrical and bioenergetic remodelling,19,38 with most effects reported in the ventricle. Given the similarity of these effects in Lnk−/− atria, we hypothesized that reactive lipid dicarbonyls are pro-arrhythmic mediators of these cytokines. Compared with WT, TNF-α was significantly increased in Lnk−/− plasma, while IL-6 and IL-1β were unchanged (Figure 6A–C). For Lnk−/− atrial tissue, IL-1β was elevated but not TNF-α or IL-6 (Figure 6D–F). Notably, 2-HOBA had no effect on cytokine levels in plasma or atria.
Figure 6.
Plasma TNF-α and atrial IL-1β are elevated and promote IsoLG formation with electrical remodelling and bioenergetic dysfunction. Concentrations of pro-inflammatory cytokines in the (A–C) plasma (n = 13, 16, 13) and (D–F) atria (normalized to total protein) of Lnk−/− mice (n = 10, 10, 10). Representative images demonstrating IsoLG adduct accumulation in atrial HL-1 cells following a 24 h exposure to (G) TNF-α or (H) IL-1β (n = 3 each; scale bars = 50 µm). Representative AP tracings from HL-1 cells treated with either (I) TNF-α or (K) IL-1β (n = 9–12) for 24 h. ATP production in atrial HL-1 cells following 24 h exposure to both (J) TNF-α (n = 5) and (L) IL-1β (n = 4). Statistical tests: (A–D) Kruskal–Wallis with Dunn’s MCT post hoc; (E, F) one-way ANOVA followed by a Tukey’s MCT; (J, L) Mann–Whitney U test. ns, not significant; *P < 0.05; **P < 0.01.
To investigate the role of these cytokines in the AF substrate, we exposed immortalized atrial myocytes (HL-1 cells) to TNF-α or IL-1β for 24 h. Both cytokines increased cardiomyocyte production of O2•− as well as IsoLG adducts (see Supplementary material online, Figure S12; Figure 6G and H). While previous work has shown that TNF-α and IL-1β alter the ventricular action potential,44 their effects on atrial myocytes remain unclear.45,46 After a 24 h incubation, both cytokines prolonged APD90, reduced Vmax, and lowered RMP in atrial HL-1 cells (Figure 6I and K; Supplementary material online, Table S3). With acute exposure, TNF-α and IL-1β had largely similar but smaller effects on the action potentials of isolated murine atrial myocytes (see Supplementary material online, Table S4). In addition, both cytokines suppressed ATP production in atrial HL-1 cells (Figure 6J and L). Taken together, these data demonstrate that IsoLGs are likely mediators of cytokine-induced electrical remodelling and mitochondrial dysfunction.
3.11. Soluble TNF-α, not IL-1β, promotes inflammation-mediated AF
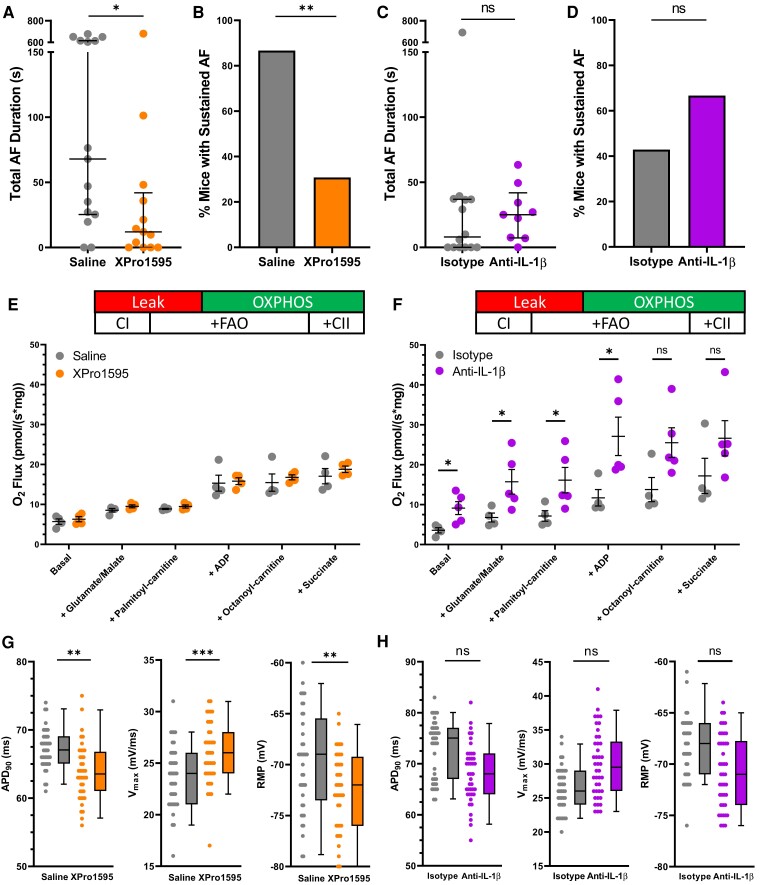
To determine the relative contribution of TNF-α and IL-1β to the AF substrate, we injected Lnk−/− mice with inhibitors of each cytokine. Compared with saline-treated controls, inhibition of soluble TNF-α with XPRO1595 significantly reduced total AF duration and episodes of sustained AF (Figure 7A and B). On the other hand, inhibition of IL-1β had no effect on AF susceptibility compared with isotype-injected controls (Figure 7C and D). With respect to bioenergetics, inhibition of soluble TNF-α had no effect on mitochondrial function whereas IL-1β inhibition increased basal respiration, CI activity, and FAO in the presence of ADP (Figure 7E and F). Interestingly, inhibition of soluble TNF-α shortened APD90, increased Vmax, and lowered RMP, while IL-1β inhibition had no effect on these parameters (Figure 7G and H). These data indicate that soluble TNF-α rather than IL-1β is a key driver of AF susceptibility in Lnk−/− mice due to ROS-mediated electrical remodelling.
Figure 7.
Inhibition of soluble TNF-α but not IL-1β prevents AF and electrical remodelling in Lnk−/− mouse atria. Summary data of total AF duration and sustained AF (≥15 s) incidence for Lnk−/− mice treated with (A, B) a soluble TNF-α inhibitor (XPRO1595; n = 15, 13) or (C, D) IL-1β inhibitor (anti-IL-1β antibody; n = 14, 9). Experimental data demonstrate the effect of (E) soluble TNF-α (n = 4, 4) and (F) IL-1β inhibition (n = 4, 5) on atrial oxygen flux. Action potential parameters in (G) XPRO1595- (n = 41, 40) and (H) anti-IL-1β antibody-treated (n = 40, 42) Lnk−/− mice. Statistical tests: (A, C) Mann–Whitney U test; (B, D) Fisher’s exact test; (E, F) Student’s t-test; (G, H) hierarchical cluster analysis by Sikkel et al.35 ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations are identical to Figures 3 and 5.
3.12. Association of rs3184504 with AF and AF-associated stroke in humans
In human analyses, we identified a statistically significant association between rs3184504 and both AF-associated phecodes ‘Atrial fibrillation and flutter’ [odds ratio: 1.06 (95% confidence interval, 1.01–1.12); P = 0.02] and ‘Atrial fibrillation’ [odds ratio: 1.05 (95% confidence interval, 1.00–1.10); P = 0.03]. There is overlap between these phecodes; thus, they are not independent signals.
In secondary analyses restricted to subjects with AF-associated phecodes, we observed an association between rs3184504 and ‘Cerebral ischaemia’ [odds ratio: 1.31 (95% confidence interval, 1.07–1.55); P = 0.03]. A review of ∼10% of cases showed this phecode to have a positive predictive value of >0.7 for ischaemic stroke by cardiologist expert adjudication.
4. Discussion
In this study, we found that Lnk−/− mice displayed AF susceptibility in the setting of electrical remodelling and mitochondrial dysfunction. Moreover, we identified reactive lipid dicarbonyls as critical drivers of an inflammatory AF substrate, as these abnormalities and arrhythmia vulnerability were prevented by 2-HOBA. Pro-arrhythmic remodelling coincided with the elevation of TNF-α and IL-1β in the plasma and atria of Lnk-deficient mice, respectively, and both cytokines altered atrial electrophysiology and bioenergetics in vitro. Interestingly, inhibition of soluble TNF-α prevented electrical remodelling and AF susceptibility in Lnk−/− mice, whereas IL-1β inhibition had no effect on these parameters. Taken together, these findings elucidated the arrhythmogenic mechanisms of the pro-inflammatory cytokine TNF-α in the atrium, and they identified lipid dicarbonyls as a novel target for inflammation-mediated AF. In addition, we identified for the first time a significant association between rs3184504, a loss-of-function LNK variant, and human AF as well as its thrombotic complications, demonstrating the relevance of this adaptor protein to the pathophysiology of clinical AF.
Lnk-deficient mice represent a unique model of AF susceptibility. Most notably, genetic deletion in animal models, the rs3184504 variant in humans, or the orthologous risk variant in mice causes the removal of a signalling ‘brake’ on haematopoietic cell proliferation, generating a state of systemic inflammation.5,13,47 This drives the production of pro-inflammatory cytokines, along with an oxidative stress state due to activation of the inflammatory cells themselves as well as cytokine-mediated ROS production. Consistent with this, we found that inflammation, ROS, and lipid dicarbonyls, a representative marker of oxidative stress, are all increased in the atria of these mice, without evidence of structural abnormalities or other factors that would promote remodelling and AF susceptibility. At baseline, Lnk−/− mice have evidence of renal and vascular inflammation/oxidative stress, but do not have overt hypertension or endothelial dysfunction.13 However, Lnk deficiency, or the risk variant of LNK, predisposes to the development of HTN and diabetes when challenged by a second hit.5 Prior work has shown that bone marrow transfer of Lnk−/− haematopoietic cells, as opposed to non-haematopoietic cells or tissue, into WT mice fully reproduced the pathophysiology, confirming a causative role for increased haematopoietic cells and inflammation in the phenotype.13 Moreover, we found that AF susceptibility in Lnk−/− mice is prevented by a specific dicarbonyl scavenger as well as cytokine inhibition, further supporting inflammation-mediated oxidative stress as the primary driver of AF in these mice. Several other experimental models have been used to investigate the pathogenesis of inflammation-mediated AF. In mice, cardiac-specific overexpression of TNF-α induced atrial arrhythmias,48 while activation of the NLRP3 (NACHT, LRR, and PYD domain containing protein 3) inflammasome with the generation of IL-1β and IL-18 increased AF susceptibility.49 However, the animals in both of those models had evidence of atrial enlargement and impaired ventricular function, which also enhanced AF susceptibility, thereby confounding the role of inflammation in the AF substrate. Recent studies also support a role for inflammatory signalling in AF susceptibility in the setting of obesity and diabetes.50–52
Our results provide direct evidence that pro-inflammatory cytokines mediate action potential remodelling in the atrium during systemic inflammation. Based on our data, the underlying mechanism for these effects is most likely direct channel modification by IsoLGs, with alterations in ionic currents that were abrogated by 2-HOBA, while the reduced channel expression observed of Nav1.5 was not affected by scavenging lipid dicarbonyls. In support of this, the oxidant tert-butylhydroperoxide caused a reduction in Nav1.5 current and leftward shift in channel availability, similar to our results in Lnk−/− atrial myocytes, and these effects were prevented by 2-HOBA.39 Analogous results were also obtained upon exposure to IsoLGs. Importantly, these changes were associated with direct lipoxidative modification of the Nav1.5 channel protein.39,53 In the human ventricle, inflammation lengthens cardiac repolarization, exemplified by QT prolongation with COVID-19 infections44 and rheumatoid arthritis,54 and consistent with our findings in Lnk−/− mice. To date, the effects of cytokines to modulate cardiac ion channels have largely been investigated in ventricular myocytes, while less is known about their effect on atrial action potentials. Multiple studies in ventricular cells have shown that TNF-α,55 IL-1β,56 and IL-657 cause APD prolongation, with a reduction in repolarizing K+ currents such as ITo and IKr, and occasionally increased ICa,L.38 Interestingly, TNF-α increased APD90 in rat ventricular myocytes by reducing ITo through an oxidative stress-dependent mechanism,58 with a similar ROS dependence for IL-1β-mediated prolongation of the ventricular action potential in a mouse model of diabetes-induced ventricular arrhythmias.19 In animal models of atrial arrhythmia susceptibility, the effects of inflammation on atrial repolarization have been controversial, with both APD shortening and prolongation reported.18,49,59 However, our results are consistent with findings in ventricular myocytes, with lengthening of atrial APD in Lnk−/− mice, as well as direct effects of TNF-α and IL-1β to prolong atrial repolarization.
Experimental evidence indicates that systemic inflammation also slows atrial20 and ventricular38 conduction. In human atrium, this pro-arrhythmic effect is primarily manifested by an increase in P-wave duration,20 a finding that we also observed in Lnk-deficient mice, along with reduced atrial myocyte Vmax and QRS prolongation. Based on our results and previous studies, these effects in the atrium likely result from at least two mechanisms. First, we found that cardiac Na+ current was reduced in Lnk−/− atrial myocytes compared with WT controls, with suppression of Vmax in atrial cells chronically exposed to TNF-α and IL-1β, along with reduced Nav1.5 expression. Second, prior work indicates that pro-inflammatory cytokines also alter expression and/or localization of Cx43 and Cx40 to slow conduction.20 Interestingly, inhibition of soluble TNF-α, but not IL-1β, prevented atrial electrical remodelling in Lnk−/− mice and reduced AF susceptibility, despite similar effects in vitro. Although TNF-α was not increased in the atria of Lnk−/− mice, it was increased in the circulation, reflecting the systemic inflammatory state in these mice. While Lnk is primarily present in haematopoietic cells, it should be recognized that the specific source of TNF-α in these mice remains unknown. Interestingly, the TNF-α inhibitor infliximab has been shown to reduce lipid peroxidation in a rat model of carbon tetrachloride (CCl4) toxicity.60 Of note, considerable experimental evidence has linked inflammation-mediated AF with abnormal Ca2+ handling, including aberrant RyR2 activity and spontaneous Ca2+ release events or sparks, as a pro-arrhythmic mechanism.50,61 However, we found no evidence of these abnormalities in Lnk−/− atrial cardiomyocytes.
In addition to electrical remodelling, atrial bioenergetics were altered in the Lnk−/− mouse atria resulting in mitochondrial dysfunction. While disturbances in mitochondrial function are strongly associated with AF in both animal models and humans,62–65 a mechanistic link connecting pro-inflammatory cytokines to mitochondrial dysfunction in the setting of AF has remained elusive.62 There is abundant evidence of oxidative stress with reduced mitochondrial respiration and structural abnormalities in the atria of patients with existing AF,66,67 as well as those who develop post-operative AF.68 Moreover, rapid pacing of human atrial tissue caused similar findings.66 Limited data indicate that pro-inflammatory cytokines can directly promote pro-arrhythmic mitochondrial dysfunction in the ventricle. In rat ventricular myocytes, TNF-α suppressed mitochondrial bioenergetics and DNA copy number through increased ROS production,69 while inhibition of IL-1β reduced SR Ca2+ leak and ventricular arrhythmias in diabetic mice.19 In atrial HL-1 cells, both TNF-α70 and IL-1β71 promoted mitochondrial abnormalities. Similarly, we found that these cytokines increased ROS production and suppressed ATP production in vitro, reflective of mitochondrial dysfunction. Interestingly, inhibition of IL-1β improved mitochondrial respiration with no effect on electrical remodelling yet failed to reduce AF susceptibility in Lnk−/− mice. On the other hand, soluble TNF-α inhibition did not alter mitochondrial function but prevented electrical remodelling and AF susceptibility. Thus, TNF-α-mediated electrical remodelling appears to be the predominant mechanism for inflammation-related AF susceptibility in Lnk−/− mice, as opposed to bioenergetic remodelling.
Importantly, our results demonstrate that in the setting of inflammation, ROS-derived lipid dicarbonyl products, exemplified by IsoLGs, are critical molecular mediators of pro-arrhythmic remodelling and AF susceptibility. While ROS are highly unstable, they generate lipid peroxidation products with longer half-lives and greater lipid solubility, with highly reactive IsoLGs being the primary mediators of oxidative injury. Administration of the dicarbonyl scavenger 2-HOBA to Lnk−/− mice prevented the development of AF susceptibility, as well as electrophysiologic and bioenergetic remodelling. Extensive work has revealed the selectivity of 2-HOBA for these lipid peroxidation products, without other known biologic effects. Previous studies have demonstrated pro-arrhythmic effects of IsoLGs on cardiac ion channels. As noted above, IsoLGs suppressed INa due to direct modification of the NaV1.5 channel protein,39 while IsoLGs have also been shown to reduce IKr current in AT-1 cells.72 These findings are consistent with our results, as peak INa was reduced, accompanied by a hyperpolarizing shift in the inactivation curve, in Lnk−/− atrial myocytes, and this was improved by 2-HOBA administration. IKr is not present in mouse atrial cells, but the reduction in ITo with inflammation was prevented by 2-HOBA. Interestingly, hydrogen peroxide has been shown to prolong APD90 in rat ventricular myocytes due to an increase in late INa,73 and we found that 2-HOBA prevented a similar effect in Lnk-deficient mice. In addition to electrophysiologic effects, oxidative stress has been shown to promote mitochondrial damage with loss of cristae in a mouse model of AF.74 Not surprisingly, IsoLGs caused mitochondrial dysfunction characterized by a reduction in mitochondrial CI activity,41,75 findings consistent with 2-HOBA-mediated improvement in mitochondrial CI-mediated respiration in the Lnk−/− atria.
A limitation of this study is that the co-immunostaining studies performed for IsoLG adducts and Kv4.2 do not prove that the channel protein is directly adducted. However, this has been previously shown for NaV1.5 as noted above. Given that lysine residues are the preferred targets for IsoLG adduction, it is highly likely that this also occurs for Kv4.2. To date, an association of additional LNK variants with AF has not been investigated in humans. These variants are most commonly associated with myeloproliferative neoplasms (MPNs) or enhanced erythrocytosis (e.g. polycythemia vera).76 Interestingly, patients with MPN demonstrated an increased incidence of atrial arrhythmias (primarily AF/flutter),77 but a correlation with LNK genotype has not been performed.
Our results demonstrate that deletion of murine Lnk increases AF susceptibility coincident with electrical remodelling and mitochondrial dysfunction but normal cardiac function, representing a novel model of atrial arrhythmia vulnerability. In the setting of systemic inflammation and oxidative stress, reactive lipid dicarbonyls are generated that mediate the arrhythmogenic effects of pro-inflammatory cytokines in Lnk−/− mice. Moreover, soluble TNF-α-mediated electrical remodelling is the dominant mechanism promoting the inflammatory AF substrate rather than IL-1β-induced mitochondrial dysfunction. Given that a common loss-of-function LNK variant is associated with clinical AF, these results identify lipid dicarbonyls and soluble TNF-α as novel potential targets for the prevention of AF in humans.
Translational perspectives.
Our findings identify a novel role for deficiency/reduced function of the lymphocyte adaptor protein in murine and human atrial fibrillation (AF) through enhanced inflammation. Our results also demonstrate that cytokine-mediated oxidative stress plays an essential pathophysiologic role, and they support the concept of pre-emptively scavenging reactive downstream lipid dicarbonyl mediators, rather than targeting the generation of reactive oxidative species per se, as a potential therapeutic approach to prevent AF in this setting. Phase 2 clinical trials have recently been initiated with the best-studied dicarbonyl scavenger 2-hydroxybenzylamine to examine its efficacy to prevent AF.
Supplementary Material
Acknowledgements
The authors thank INmune Bio for providing XPRO1595.
Contributor Information
Matthew B Murphy, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Zhenjiang Yang, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Tuerdi Subati, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Eric Farber-Eger, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA.
Kyungsoo Kim, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Daniel J Blackwell, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Matthew R Fleming, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA.
Joshua M Stark, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Joseph C Van Amburg, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Kaylen K Woodall, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Justin P Van Beusecum, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Vineet Agrawal, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA.
Charles D Smart, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Ashley Pitzer, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
James B Atkinson, Department of Pathology, Microbiology, and Immunology, Vanderbilt University School of Medicine, 1161 21 Avenue South, Nashville, TN 37232, USA.
Agnes B Fogo, Department of Pathology, Microbiology, and Immunology, Vanderbilt University School of Medicine, 1161 21 Avenue South, Nashville, TN 37232, USA.
Julie A Bastarache, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA.
Annet Kirabo, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Quinn S Wells, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA; Department of Biomedical Informatics, Vanderbilt University School of Medicine, 2525 West End Avenue, Nashville, TN 37203, USA.
Meena S Madhur, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Joey V Barnett, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Katherine T Murray, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, 559 PRB, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University School of Medicine, 2220 Pierce Avenue, Nashville, TN 37232, USA.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
M.B.M. performed transoesophageal atrial pacing, histological analysis, RT-qPCR, cell culture, immunofluorescence, superoxide, flow cytometry, high-resolution respirometry, transmission electron microscopy, cytokine quantification, and animal experiments. Z.Y. performed electrophysiology experiments. T.S. performed experiments involving echocardiography, transmission electron microscopy, co-immunostaining, and animals. J.M.S. and J.C.V.A. performed experiments involving animals. K.K.W. isolated atrial cells from mice. V.A. performed high-resolution respirometry experiments. J.P.V.B., C.D.S., A.P., A.K., and M.S.M. performed flow cytometry experiments. K.K. and D.J.B. performed the calcium handling experiments. M.R.F. performed the western analysis experiments. J.B.A. and A.B.F. performed histological analysis. J.A.B. quantified cytokines in mice. E.F.-E. and Q.S.W. performed and analysed human association studies. M.B.M., J.V.B., and K.T.M. conceived the project, but all authors contributed to the conceptual design and formulated the experiments. M.B.M. and K.T.M. wrote the original draft of the manuscript, and all authors made substantive suggestions and approved the final version.
Funding
This work was supported by grants from the National Institutes of Health (grant numbers HL096844 and HL133127 to K.T.M., HL147818, HL155041, and HL144941 to A.K., DK56942 to A.B.F., and HL150783 to J.A.B.); the American Heart Association (grant numbers 18SFRN34230125 to K.T.M., 903918 to M.B.M., and 23CDA1042141 to M.R.F.); and the National Center for Advancing Translational Sciences of the National Institute of Health (grant number 5UL1 TR002243-08). Confocal and transmission electron microscopy were performed through the Vanderbilt Cell Imaging Shared Resource and supported by the National Institutes of Health (grant numbers S10OD021630, CA68485, DK20593, DK58404, DK59637, and EY08126). Histological analysis was performed through the Vanderbilt Translational Pathology Shared Resource and supported by the National Institutes of Health (grant number P30CA068485).
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:E199–E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoemaker MB, Hemnes AR, Robbins IM, Langberg JJ, Ellis CR, Aznaurov SG, Fredi JL, Slosky DA, Roden DM, Murray KT, Piana RN, Mendes LA, Whalen SP. Left atrial hypertension after repeated catheter ablations for atrial fibrillation. J Am Coll Cardiol 2011;57:1918–1919. [DOI] [PubMed] [Google Scholar]
- 3. Devalliere J, Charreau B. The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem Pharmacol 2011;82:1391–1402. [DOI] [PubMed] [Google Scholar]
- 4. BL D, Madhur MS. Linking inflammation and hypertension via LNK/SH2B3. Curr Opin Nephrol Hypertens 2016;25:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alexander MR, Hank S, Dale BL, Himmel L, Zhong X, Smart CD, Fehrenbach DJ, Chen YH, Prabakaran N, Tirado B, Centrella M, Ao MF, Du LP, Shyr Y, Levy D, Madhur MS. A single nucleotide polymorphism in SH2B3/LNK promotes hypertension development and renal damage. Circ Res 2022;131:731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MCY, Baum L, So WY, Wong KS, Chan JCN, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RCW, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448:353–357. [DOI] [PubMed] [Google Scholar]
- 7. The International Consortium for Blood Pressure Genome-Wide Association Studies . Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann TJ, Ehret GB, Nandakumar P, Ranatunga D, Schaefer C, Kwok PY, Iribarren C, Chakravarti A, Risch N. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat Gen 2017;49:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marquez A, Kerick M, Zhernakova A, Gutierrez-Achury J, Chen WM, Onengut-Gumuscu S, Gonzalez-Alvaro I, Rodriguez-Rodriguez L, Rios-Fernandez R, Gonzalez-Gay MA, Mayes MD, Raychaudhuri S, Rich SS, Wijmenga C, Martin J; Coeliac Disease Immunochip Consortium, Rheumatoid Arthritis Consortium International for Immunochip (RACI), International Scleroderma Group, Type 1 Diabetes Genetics Consortium. Meta-analysis of immunochip data of four autoimmune diseases reveals novel single-disease and cross-phenotype associations. Genome Med 2018;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vuckovic D, Bao EL, Akbari P, Lareau CA, Mousas A, Jiang T, Chen MH, Raffield LM, Tardaguila M, Huffman JE, Ritchie SC, Megy K, Ponstingl H, Penkett CJ, Albers PK, Wigdor EM, Sakaue S, Moscati A, Manansala R, Lo KS, Qian HJ, Akiyama M, Bartz TM, Ben-Shlomo Y, Beswick A, Bork-Jensen J, Bottinger EP, Brody JA, van Rooij FJA, Chitrala KN, Wilson PWF, Choquet H, Danesh J, Di Angelantonio E, Dimou N, Ding JZ, Elliott P, Esko T, Evans MK, Felix SB, Floyd JS, Broer L, Grarup N, Guo MH, Guo Q, Greinacher A, Haessler J, Hansen T, Howson JMM, Huang W, Jorgenson E, Kacprowski T, Kahonen M, Kamatani Y, Kanai M, Karthikeyan S, Koskeridis F, Lange LA, Lehtimaki T, Linneberg A, Liu YM, Lyytikainen LP, Manichaikul A, Matsuda K, Mohlke KL, Mononen N, Murakami Y, Nadkarni GN, Nikus K, Pankratz N, Pedersen O, Preuss M, Psaty BM, Raitakari OT, Rich SS, Rodriguez BAT, Rosen JD, Rotter JI, Schubert P, Spracklen CN, Surendran P, Tang H, Tardif JC, Ghanbari M, Volker U, Volzke H, Watkins NA, Weiss S, Cai N, Kundu K, Watt SB, Walter K, Zonderman AB, Cho K, Li Y, Loos RJF, Knight JC, Georges M, Stegle O, Evangelou E, Okada Y, Roberts DJ, Inouye M, Johnson AD, Auer PL, Astle WJ, Reiner AP, Butterworth AS, Ouwehand WH, Lettre G, Sankaran VG, Soranzo N; VA Million Veteran Program . The polygenic and monogenic basis of blood traits and diseases. Cell 2020; 182:1214–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saevarsdottir S, Stefansdottir L, Sulem P, Thorleifsson G, Ferkingstad E, Rutsdottir G, Glintborg B, Westerlind H, Grondal G, Loft IC, Sorensen SB, Lie BA, Brink M, Arlestig L, Arnthorsson AO, Baecklund E, Banasik K, Bank S, Bjorkman LI, Ellingsen T, Erikstrup C, Frei O, Gjertsson I, Gudbjartsson DF, Gudjonsson SA, Halldorsson GH, Hendricks O, Hillert J, Hogdall E, Jacobsen S, Jensen DV, Jonsson H, Kastbom A, Kockum I, Kristensen S, Kristjansdottir H, Larsen MH, Linauskas A, Hauge EM, Loft AG, Ludviksson BR, Lund SH, Markusson T, Masson G, Melsted P, Moore KHS, Munk H, Nielsen KR, Norddahl GL, Oddsson A, Olafsdottir TA, Olason PI, Olsson T, Ostrowski SR, Horslev-Petersen K, Rognvaldsson S, Sanner H, Silberberg GN, Stefansson H, Sorensen E, Sorensen IJ, Turesson C, Bergman T, Alfredsson L, Kvien TK, Brunak S, Steinsson K, Andersen V, Andreassen OA, Rantapaa-Dahlqvist S, Hetland ML, Klareskog L, Askling J, Padyukov L, Pedersen OB, Thorsteinsdottir U, Jonsdottir I, Stefansson K; Members of the DBDS Genomic Consortium, The Danish RA Genetics Working Group, The Swedish Rheumatology Quality Register Biobank Study Group (SRQb) . Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset. Ann Rheum Dis 2022;81:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 2015;125:1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laroumanie F, Korneva A, Bersi MR, Alexander MR, Xiao L, Zhong X, Van Beusecum JP, Chen Y, Saleh MA, McMaster WG. LNK deficiency promotes acute aortic dissection and rupture. JCI Insight 2018;3:e122558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang W, Tang Y, Wang Y, Tascau L, Balcerek J, Tong W, Levine RL, Welch C, Tall AR, Wang N. LNK/SH2B3 loss of function promotes atherosclerosis and thrombosis. Circ Res 2016;119:E91–E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace 2012;14:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harada M, Wagoner DR V, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J 2015;79:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, Wang QL, Farman GP, Yang F, Yang W, Dorian D, Simpson JA, Tuomi JM, Jones DL, Nanthakumar K, Cox B, Wehrens XHT, Dorian P, Backx PH. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNF alpha. Nat Commun 2015;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Zhao Y, Xie A, Kim TY, Terentyeva R, Liu M, Shi GB, Feng F, Choi BR, Terentyev D, Hamilton S, Dudley SC. Interleukin-1 beta, oxidative stress, and abnormal calcium handling mediate diabetic arrhythmic risk. JACC-Basic Transl Sci 2021;6:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lazzerini PE, Laghi-Pasini F, Acampa M, Srivastava U, Bertolozzi I, Giabbani B, Finizola F, Vanni F, Dokollari A, Natale M, Cevenini G, Selvi E, Migliacci N, Maccherini M, Boutjdir M, Capecchi PL. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6 mediated changes in connexin expression. J Am Heart Assoc 2019;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mihm MJ, Yu FS, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation 2001;104:174–180. [DOI] [PubMed] [Google Scholar]
- 22. Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace 2011;13:308–328. [DOI] [PubMed] [Google Scholar]
- 23. Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European society of cardiology guidelines. Part II: secondary prevention. Europace 2011;13:610–625. [DOI] [PubMed] [Google Scholar]
- 24. Prinsen JK, Kannankeril PJ, Sidorova TN, Yermalitskaya LV, Boutaud O, Zagol-Ikapitte I, Barnett JV, Murphy MB, Subati T, Stark JM, Christopher IL, Jafarian-Kerman SR, Saleh MA, Norlander AE, Loperena R, Atkinson JB, Fogo AB, Luther JM, Amarnath V, Davies SS, Kirabo A, Madhur MS, Harrison DG, Murray KT. Highly reactive isolevuglandins promote atrial fibrillation caused by hypertension. JACC-Basic Transl Sci 2020;5:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. May-Zhang LS, Kirabo A, Huang JS, Linton MF, Davies SS, Murray KT. Scavenging reactive lipids to prevent oxidative injury. Annu Rev Pharmacol Toxicol 2021;61:291–308. [DOI] [PubMed] [Google Scholar]
- 26. Tao H, Huang JS, Yancey PG, Yermalitsky V, Blakemore JL, Zhang YM, Ding L, Zagol-Ikapitte I, Ye F, Amarnath V, Boutaud O, Oates JA, Roberts LJ, Davies SS, Linton MF. Scavenging of reactive dicarbonyls with 2-hydroxybenzylamine reduces atherosclerosis in hypercholesterolemic Ldlr( −/−) mice. Nat Commun 2020;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirabo A, Fontana V, de Faria APC, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno LH, Madhur MS, Roberts J, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 2014;124:4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, Olson SJ, Montine TJ, Amarnath V, Roberts LJ. Treatment with a γ-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J Alzheimers Dis 2011;27:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takaki S, Sauer K, Iritani BM, Chien S, Ebihara Y, Tsuji K, Takatsu K, Perlmutter RM. Control of B cell production by the adaptor protein Lnk: definition of a conserved family of signal-modulating proteins. Immunity 2000;13:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy M, Kim K, Kannankeril P, Murray K. Optimization of transesophageal atrial pacing to assess atrial fibrillation susceptibility in mice. J Vis Exp 2022;184:e64168. [DOI] [PubMed] [Google Scholar]
- 31. Murphy MB, Kim K, Kannankeril PJ, Subati T, Van Amburg JC, Barnett JV, Murray KT. Optimizing transesophageal atrial pacing in mice to detect atrial fibrillation. Am J Physiol Heart Circ Physiol 2022;322:H36–H43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Beusecum JP, Barbaro NR, McDowell Z, Aden LA, Xiao L, Pandey AK, Itani HA, Himmel LE, Harrison DG, Kirabo A. High salt activates CD11c(+) antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 2019;74:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim K, Blackwell DJ, Yuen SL, Thorpe MP, Johnston JN, Cornea RL, Knollmann BC. The selective RyR2 inhibitor ent-verticilide suppresses atrial fibrillation susceptibility caused by Pitx2 deficiency. J Mol Cell Cardiol 2023;180:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bastarache JA, Koyama T, Wickersham NE, Ware LB. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. J Immunol Methods 2014;408:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sikkel MB, Francis DP, Howard J, Gordon F, Rowlands C, Peters NS, Lyon AR, Harding SE, MacLeod KT. Hierarchical statistical techniques are necessary to draw reliable conclusions from analysis of isolated cardiomyocyte studies. Cardiovasc Res 2017;113:1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davies SS, Talati M, Wang XH, Mernaugh RL, Amarnath V, Fessel J, Meyrick BO, Sheller J, Roberts LJ. Localization of isoketal adducts in vivo using a single-chain antibody. Free Radic Biol Med 2004;36:1163–1174. [DOI] [PubMed] [Google Scholar]
- 37. Ngwenyama N, Kirabo A, Aronovitz M, Velazquez F, Carrillo-Salinas F, Salvador AM, Nevers T, Amarnath V, Tai A, Blanton RM, Harrison DG, Alcaide P. Isolevuglandin-modified cardiac proteins drive CD4+ T-cell activation in the heart and promote cardiac dysfunction. Circulation 2021;143:1242–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lazzerini P, Capecchi P, Laghi-Pasini F. Long QT syndrome: an emerging role for inflammation and immunity. Front Cardiovsc Med 2015;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakajima T, Davies SS, Matafonova E, Potet F, Amarnath V, Tallman KA, Serwa RA, Porter NA, Balser JR, Kupershmidt S, Roberts LJ. Selective γ-ketoaldehyde scavengers protect Nav1.5 from oxidant-induced inactivation. J Mol Cell Cardiol 2010;48:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dobrev D, Wehrens XHT. Calcium-mediated cellular triggered activity in atrial fibrillation. J Physiol 2017;595:4001–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mayorov V, Uchakin P, Amarnath V, Panov AV, Bridges CC, Uzhachenko R, Zackert B, Moore CS, Davies S, Dikalova A, Dikalov S. Targeting of reactive isolevuglandins in mitochondrial dysfunction and inflammation. Redox Biol 2019;26:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lam J, Katti P, Biete M, Mungai M, AshShareef S, Neikirk K, Lopez EG, Vue Z, Christensen TA, Beasley HK, Rodman TA, Murray SA, Salisbury JL, Glancy B, Shao JQ, Pereira RO, Abel ED, Hinton A. A universal approach to analyzing transmission electron microscopy with ImageJ. Cells 2021;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Donnell PJ, Driscoll WJ, Back N, Muth E, Mueller GP. Peptidylglycine-alpha-amidating monooxygenase and pro-atrial natriuretic peptide constitute the major membrane-associated proteins of rat atrial secretory granules. J Mol Cell Cardiol 2003;35:915–922. [DOI] [PubMed] [Google Scholar]
- 44. Lazzerini PE, Laghi-Pasini F, Boutjdir M, Capecchi PL. Inflammatory cytokines and cardiac arrhythmias: the lesson from COVID-19. Nat Rev Immunol 2022;22:270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitrokhin VM, Mladenov MI, Kamkin AG. IL-1 provokes electrical abnormalities in rat atrial myocardium. Int Immunopharmacol 2015;28:780–784. [DOI] [PubMed] [Google Scholar]
- 46. Abramochkin DV, Kuzmin VS, Mitrochin VM, Kalugin L, Dvorzhak A, Makarenko EY, Schubert R, Kamkin A. TNF-α provokes electrical abnormalities in rat atrial myocardium via a NO-dependent mechanism. Pflugers Arch 2013;465:1741–1752. [DOI] [PubMed] [Google Scholar]
- 47. Dou HJ, Kotini A, Liu WL, Fidler T, Endo-Umeda K, Sun XL, Olszewska M, Xiao T, Abramowicz S, Yalcinkaya M, Hardaway B, Tsimikas S, Que XC, Bick A, Emdin C, Natarajan P, Papapetrou EP, Witztum JL, Wang N, Tall AR. Oxidized phospholipids promote NETosis and arterial thrombosis in LNK(SH2B3) deficiency. Circulation 2021;144:1940–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saba S, Janczewski AM, Baker LC, Shusterman V, Gursoy EC, Feldman AM, Salama G, McTiernan CF, London B. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol 2005;289:H1456–H1467. [DOI] [PubMed] [Google Scholar]
- 49. Yao CX, Veleva T, Scott L, Cao SY, Li LG, Chen G, Jeyabal P, Pan XL, Alsina KM, Abu-Taha I, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Muller FU, El-Armouche A, Eissa T, Beeton C, Nattel S, Wehrens XHT, Dobrev D, Li N. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 2018;138:2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scott L, Fender AC, Saljic A, Li LG, Chen XH, Wang XL, Linz D, Lang JL, Hohl M, Twomey D, Pham TT, Diaz-Lankenau R, Chelu MG, Kamler M, Entman ML, Taffet GE, Sanders P, Dobrev D, Li N. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc Res 2021;117:1746–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fender AC, Kleeschulte S, Stolte S, Leineweber K, Kamler M, Bode J, Li N, Dobrev D. Thrombin receptor PAR4 drives canonical NLRP3 inflammasome signaling in the heart. Basic Res Cardiol 2020;115:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu XH, Liu Y, Tu DM, Liu XJ, Niu SL, Suo Y, Liu T, Li GP, Liu CL. Role of NLRP3-inflammasome/caspase-1/galectin-3 pathway on atrial remodeling in diabetic rabbits. J Cardiovasc Transl Res 2020;13:731–740. [DOI] [PubMed] [Google Scholar]
- 53. Mont S, Davies SS, Roberts Second LJ, Mernaugh RL, McDonald WH, Segal BH, Zackert W, Kropski JA, Blackwell TS, Sekhar KR, Galligan JJ, Massion PP, Marnett LJ, Travis EL, Freeman ML. Accumulation of isolevuglandin-modified protein in normal and fibrotic lung. Sci Rep 2016;6:24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lazzerini PE, Capecchi PL, Acampa M, Galeazzi M, Laghi-Pasini F. Arrhythmic risk in rheumatoid arthritis: the driving role of systemic inflammation. Autoimmun Rev 2014;13:936–944. [DOI] [PubMed] [Google Scholar]
- 55. Petkova-Kirova PS, Gursoy E, Mehdi H, McTiernan CF, London B, Salama G. Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-α. Am J Physiol Heart Circ Physiol 2006;290:H2098–H2107. [DOI] [PubMed] [Google Scholar]
- 56. Li YH, Rozanski GJ. Effects of human recombinant interleukin-1 on electrical properties of Guinea pig ventricular cells. Cardiovasc Res 1993;27:525–530. [DOI] [PubMed] [Google Scholar]
- 57. Hagiwara Y, Miyoshi S, Fukuda K, Nishiyama N, Ikegami Y, Tanimoto K, Murata M, Takahashi E, Shimoda K, Hirano T, Mitamura H, Ogawa S. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent I-CaL, ca2+ (i) transient, and APD increase in cardiomyocytes. J Mol Cell Cardiol 2007;43:710–716. [DOI] [PubMed] [Google Scholar]
- 58. Fernandez-Velasco M, Ruiz-Hurtado G, Hurtado O, Moro MA, Delgado C. TNF-α downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am J Physiol Heart Circ Physiol 2007;293:H238–H245. [DOI] [PubMed] [Google Scholar]
- 59. Dobrev D, Heijman J, Hiram R, Li N, Nattel S. Inflammatory signalling in atrial cardiomyocytes: a novel unifying principle in atrial fibrillation pathophysiology. Nat Rev Cardiol 2023;20:145–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ozdemir A, Tumkaya L, Kalcan S, Uyan M, Karakaya A, Demiral G, Celik Samanci T, Mercantepe T, Cumhur Cüre M, Cüre E. The effects of TNF-α inhibitors on carbon tetrachloride-induced nephrotoxicity. Clin Exp Hypertens 2022;44:291–296. [DOI] [PubMed] [Google Scholar]
- 61. Heijman J, Muna AP, Veleva T, Molina CE, Sutanto H, Tekook M, Wang Q, Abu-Taha IH, Gorka M, Künzel S, El-Armouche A, Reichenspurner H, Kamler M, Nikolaev V, Ravens U, Li N, Nattel S, Wehrens XHT, Dobrev D. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res 2020;127:1036–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pool L, Wijdeveld L, de Groot NMS, Brundel B. The role of mitochondrial dysfunction in atrial fibrillation: translation to druggable target and biomarker discovery. Int J Mol Sci 2021;22:8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mason FE, Pronto JRD, Alhussini K, Maack C, Voigt N. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res Cardiol 2020;115:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang KC, Bonini MG, Dudley SC. Mitochondria and arrhythmias. Free Radic Biol Med 2014;71:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Muszynski P, Bonda TA. Mitochondrial dysfunction in atrial fibrillation-mechanisms and pharmacological interventions. J Clin Med 2021;10:2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bukowska A, Schild L, Keilhoff G, Hirte D, Neumann M, Gardemann A, Neumann KH, Rohl FW, Huth C, Goette A, Lendeckel U. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp Biol Med 2008;233:558–574. [DOI] [PubMed] [Google Scholar]
- 67. Emelyanova L, Ashary Z, Cosic M, Negmadjanov U, Ross G, Rizvi F, Olet S, Kress D, Sra J, Tajik AJ, Holmuhamedov EL, Shi Y, Jahangir A. Selective downregulation of mitochondrial electron transport chain activity and increased oxidative stress in human atrial fibrillation. Am J Physiol Heart Circ Physiol 2016;311:H54–H63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Montaigne D, Marechal X, Lefebvre P, Modine T, Fayad G, Dehondt H, Hurt C, Coisne A, Koussa M, Remy-Jouet I, Zerimech F, Boulanger E, Lacroix D, Staels B, Neviere R. Mitochondrial dysfunction as an arrhythmogenic substrate. J Am Coll Cardiol 2013;62:1466–1473. [DOI] [PubMed] [Google Scholar]
- 69. Suematsu N, Tsutsui H, Wen J, Kang DC, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-α-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003;107:1418–1423. [DOI] [PubMed] [Google Scholar]
- 70. Shanmugam G, Narasimhan M, Sakthivel R, Kumar RR, Davidson C, Palaniappan S, Claycomb WW, Hoidal JR, Darley-Usmar VM, Rajasekaran NS. A biphasic effect of TNF-α in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox Biol 2016;9:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang LL, Tian JL, Diao SJ, Zhang GW, Xiao MC, Chang D. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1 beta-induced metabolic disturbance and mitochondrial dysfunction. Chem Biol Interact 2020;332:7. [DOI] [PubMed] [Google Scholar]
- 72. Brame CJ, Boutaud O, Davies SS, Yang T, Oates JA, Roden D, Roberts LJ. Modification of proteins by isoketal-containing oxidized phospholipids. J Biol Chem 2004;279:13447–13451. [DOI] [PubMed] [Google Scholar]
- 73. Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol 1997;500:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xie WJ, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX, Marks AR. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep 2015;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dikalova A, Mayorov V, Xiao L, Panov A, Amarnath V, Zagol-Ikapitte I, Vergeade A, Ao MF, Yermalitsky V, Nazarewicz RR, Boutaud O, Lopez MG, Billings FT, Davies S, Roberts LJ, Harrison DG, Dikalov S. Mitochondrial isolevuglandins contribute to vascular oxidative stress and mitochondria-targeted scavenger of isolevuglandins reduces mitochondrial dysfunction and hypertension. Hypertension 2020;76:1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maslah N, Cassinat B, Verger E, Kiladjian JJ, Velazquez L. The role of LNK/SH2B3 genetic alterations in myeloproliferative neoplasms and other hematological disorders. Leukemia 2017;31:1661–1670. [DOI] [PubMed] [Google Scholar]
- 77. Mahé K, Delluc A, Chauveau A, Castellant P, Mottier D, Dalbies F, Berthou C, Guillerm G, Lippert E, Ianotto JC. Incidence and impact of atrial arrhythmias on thrombotic events in MPNs. Ann Hematol 2018;97:101–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.