INTRODUCTION
Urticaria, also known as hives, is a common condition thought to affect up to 20% of individuals worldwide in their lifetime.1 This skin condition is characterized by the appearance of pruritic, erythematous papules or plaques with superficial swelling of the dermis. The major complaint is the symptom of pruritus. Angioedema, which involves a deeper swelling of dermal or mucosal tissues, may accompany urticaria. Urticaria can be classified by both time course of symptoms and the underlying etiology. Acute urticaria (AU) is defined as having a duration of urticaria for 6 weeks or less, whereas chronic urticaria (CU) is generally defined by the presence of urticaria on most days of the week for a period of greater than 6 weeks.1
CU is further classified by additional criteria. Approximately one-fifth of CU patients have a clear-cut physical or inducible trigger for their skin eruptions. This condition, termed chronic inducible urticaria (CIndU), is further classified according to the nature of the inciting stimulus (see the article by Altricher in this issue). In the remaining 80% of CU cases, no external allergen or causative disease is identified. This condition is termed chronic spontaneous urticaria (CSU), which was previously labeled as chronic idiopathic urticaria (CIU). In this review, the terms CSU and CIU are used synonymously. Approximately 30% CSU patients have concomitant CIndU.2 Up to 40% of patients with CSU report accompanying episodes of angioedema, whereas 10% or fewer have angioedema as their main manifestation.3,4
EPIDEMIOLOGY
Up to 1.5% of the United States and European populations are affected by CU.1 The overall prevalence is similar in other regions.1,5 Both children and adults can develop CU, but it is more common in adults, with women affected nearly twice as often as men.5 However, the male-to-female ratio varies in CSU based on the presence of angioedema, with the highest ratio observed in angioedema-only CSU (0.65) and lower in patients with wheals + angioedema or with wheals only (0.33 and 0.45, respectively).3 In children, the male-to-female ratio for CSU is 1:1.6 The average age of CSU patients suggests that the condition begins in the third to fifth decades of life5, however, a substantial portion of those affected are ≥65 years (4%−18.1%).7
PROGNOSIS AND RISK FACTORS
CU is a self-limited disorder in most cases, with an average duration of 2 to 5 years.1 In CSU patients, the rate of spontaneous remission at 1 year is approximately 30% to 50%.8,9 However, symptoms persist beyond 5 years in nearly one-fifth of patients.8,10 In a prospective study of 139 CU patients followed for 5 years, angioedema, severe disease, autologous serum skin test (ASST) positivity, and thyroid autoimmunity were associated with longer disease duration.10 However, other studies have not found an association between positive ASST and disease duration.9 The coexpression of CIndU in CSU patients is associated with a longer duration of CSU, higher CSU disease activity, and poor response to antihistamines.1
Thyroid and other Autoimmune Disorders
A higher incidence of thyroid disease in CU patients has been long recognized.11,12 The overall incidence of hypothyroidism (9.8%) and hyperthyroidism (2.6%) in CU is at least twice that of the general population.12 Reports also indicate a higher prevalence of antiperoxidase and antimicrosomal autoantibodies in CU subjects than in the general population13; however, thyroid function is normal in most CU patients with thyroid autoantibodies. Some view the higher frequency of thyroid autoimmunity in CSU as a common tendency to form autoantibodies rather than a causal link between the two diseases.11
An increased prevalence of thyroid disorders, Celiac disease, Sjögren’s syndrome, systemic lupus erythematosus (SLE), rheumatoid arthritis, and type 1 diabetes was observed in CU patients.12 A recent genome-wide association study (GWAS) of 1,230 CSU patients and 1,382 healthy controls revealed that CSU shares susceptibility single nucleotide polymorphisms with autoimmune diseases but not atopic diseases.14
Malignancy
There exist conflicting data on whether CSU patients are at increased risk for malignancies. In a Swedish study, 1155 patients with CSU were followed for an average of 8.2 years.15 The incidence of malignancy during the observation period was compared with the expected number of cancers from the Swedish Cancer Registry, yielding a relative risk of 0.88 (95% confidence interval [CI] 0.61–1.12). In another study, a cohort of 12,720 Taiwanese patients was identified as having CU, based on the ICD-9-CM code for urticaria along with use of an antihistamine for at least 6 months over a 2-year period. 16 In this study, the standardized incidence ratio of malignancy for patients with CU was 2.2 (95% CI 2.0–2.4). Rarely have malignancies been reported as a cause for CU.17
PATHOPHYSIOLOGY
Skin Pathology
The histologic pattern of urticarial lesions includes degranulated mast cells and perivascular infiltrates composed of lymphocytes, eosinophils, neutrophils, and basophils that have migrated to the skin lesion (Fig. 1).18,19 Upon activation, mast cells and basophils release histamine and other inflammatory mediators which are capable of causing local vasodilation, itch, and/or swelling of the skin. CSU skin biopsies lack leukocytoclasia or fibrinoid deposition such as seen in urticarial vasculitis.20,21 Complement deposition and immune deposits are also absent, distinguishing CSU from bullous pemphigoid and cutaneous lupus erythematosus.22,23 Given that pruritus is the major skin symptom and the overall benefits seen with antihistamine therapy 24, histamine is a central mediator in CU. Compared to healthy skin, blister fluid obtained from lesional and nonlesional CU skin showed greater histamine content and histamine-releasing activity based on in vitro testing with healthy basophils.25
Fig. 1.
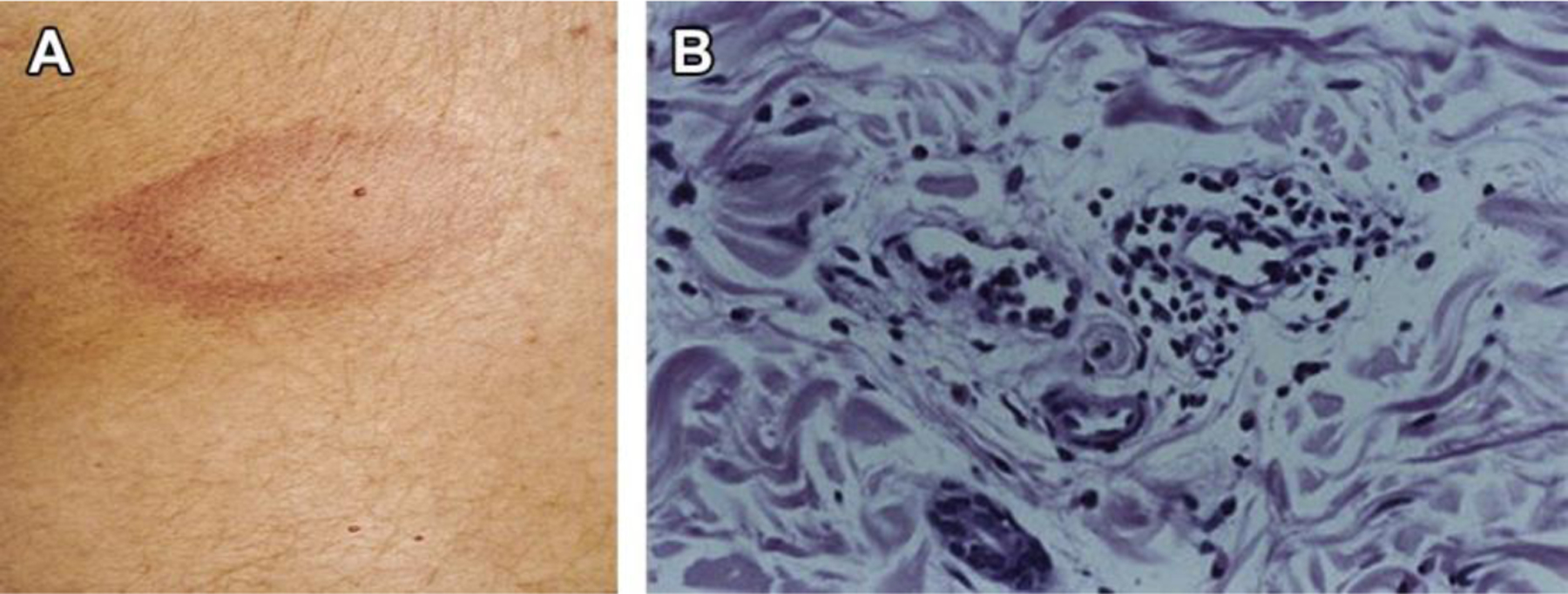
(A) Image of skin lesion. Superficial swellings of the dermis are called wheals. (B) Biopsy of of early spontaneous wheal shows perivascular lymphocytes and intraluminal and perivascular neutrophils, with perivascular and interstitial eosinophils. (From Grattan CE, Sabroe RA, Greaves MW. J Am Acad Dermatol 2002;46(5):645–60. Available at: http://dx.doi.org/10.1067/mjd.2002.122759; with permission).
The leukocyte infiltrate observed in CU skin biopsies are similar to allergen-induced late-phase skin reactions (LPRs). Although both feature mast-cell activation, skin LPRs express a Type 2 cytokine pattern (interleukin [IL]-4, IL-5), whereas both Type 2 cytokines (IL-4 and IL-5) and Type 1 cytokines (interferon-γ) are noted in CU lesions.18,26 Thymic stromal lymphopoietin (TSLP), IL-25, IL-33, IL-24, and IL-31 are increased in CSU lesions compared to healthy controls.26,27 Notably, IL-4 and IL-31 have been implicated in histamine-independent itch mechanisms.28 Little difference is found in the characteristics of skin biopsies of patients with and without serologic evidence for autoimmune urticaria.18,29
There is limited understanding of the roles of eosinophils, lymphocytes, and neutrophils in disease pathology.26 Eosinophil and basophil numbers are equivalent in CSU lesions.30 A predominance of neutrophils in the skin-lesion biopsy is termed neutrophilic urticaria, and can lead to alternative disease management.31 Other immune profiles described in CSU subjects are summarized in Table 1.
Table 1.
Immune profiles of CIU subjects relative to controls
| Immune Cell Type/Tissue | Profile |
|---|---|
| Lymphocytes | Altered signaling through the p21Ras pathway32 Increased frequency of IL-10+ peripheral T cells33 |
| Peripheral blood mononuclear cells (PBMC) | Increased stimulated production of TNF-a, IL-10, MIP-Iα, and RANTES32 Reduced IL-433 |
| Peripheral blood dendritic cells (pDCs) | Impaired TLR9 induced interferon-a production34 |
| Serum | Increased levels of TNF, IL-1β, IL-6, IL-13, IL-12p70, IL-10, and B-cell activating factor (BAFF); similar among those with CIU and CAU35–37 Increased IL-6, CRP in CU subjects with NSAID sensitivity after aspirin challenge38 |
| Skin | Increased IL-3, IL-4, IL-5, IL-17, IL-24, IL-25, IL-33, INF-γ, and TNF-α in CSU lesions compared to healthy skin26 Increased IL-3, IL-5, IL-25, IL-33, and TNF-α in CSU lesions compared to CSU nonlesional skin26 |
| Coagulation | Extrinsic coagulation pathway activated with increased levels of D-dimer and prothrombin fragments39 |
Abbreviations: CAU, chronic autoimmune urticaria; CIU, chronic idiopathic urticaria; CRP, C-reactive protein; CU, chronic urticaria; IL, interleukin; MIP, macrophage inflammatory protein; NSAID, nonsteroidal anti-inflammatory drug; RANTES, regulated on activation, normal T-cell expressed and secreted (chemokine ligand 5); TNF, tumor necrosis factor.
Pathogenesis
Although there are several theories regarding the pathogenesis of CU, none have been conclusively established.40
Autoimmune theory
A widely held theory is that 30% to 40% of CSU patients have an autoimmune disease, also referred to as CAU, more recently called type IIb autoimmune CSU.41 These patients have circulating immunoglobulin G (IgG) autoantibodies to either IgE or the high-affinity IgE receptor that are thought to be pathogenic by directly activating mast cells and basophils (Fig. 2).40 This theory arose from the recognition of increased incidence of thyroid autoimmunity in patients with CU (Fig. 3).12 Furthermore, increased clustering of human leukocyte antigen (HLA) class 2 DR antigen expression is noted in subjects with CSU and, in particular, those with evidence for serum autoimmune factors.42
Fig. 2.
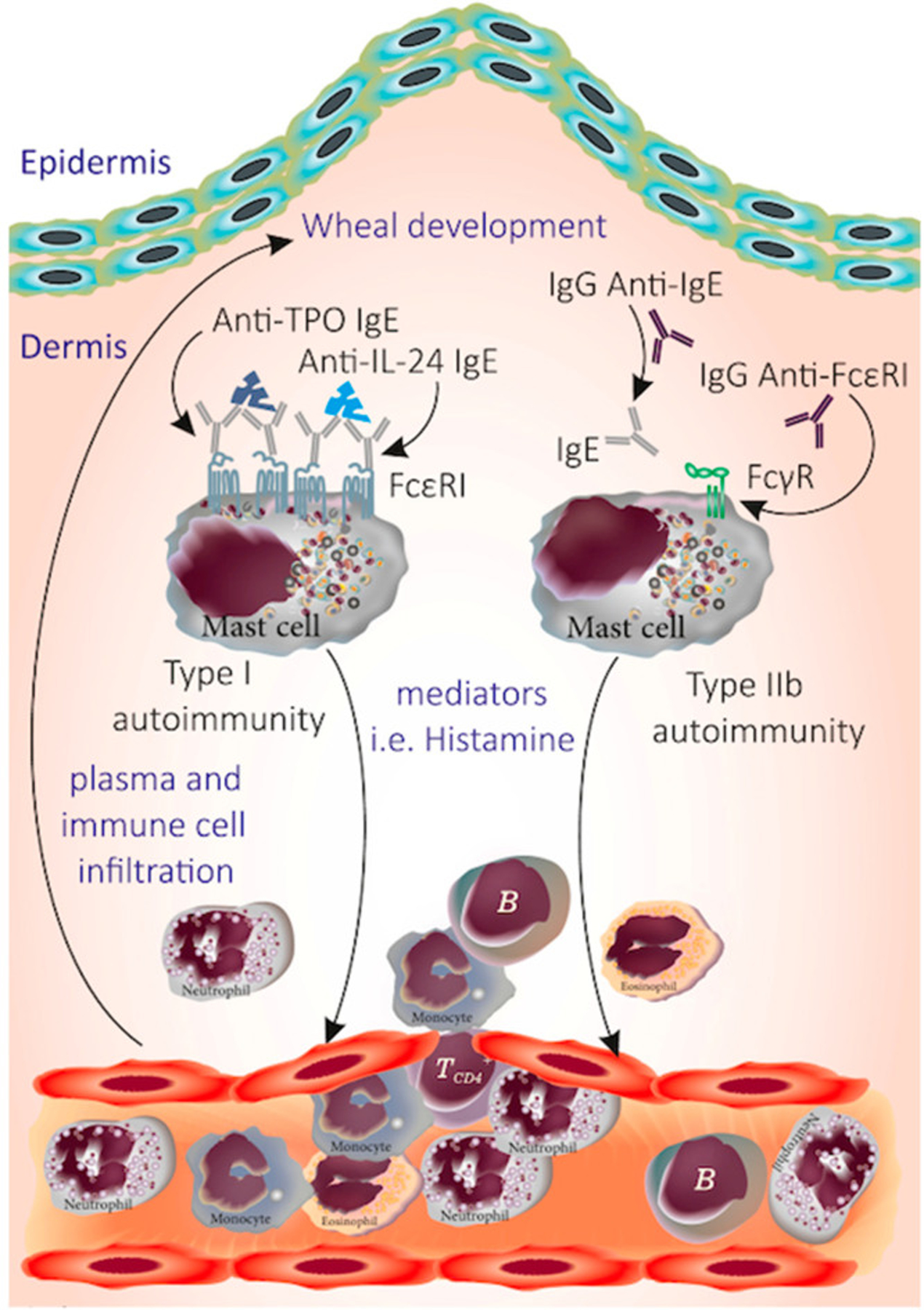
Proposed pathogenic antibodies in CSU. TPO, thyroperoxidase; Ig, immunoglobulin; MC, mast cell. (From Kocatürk et al. Viruses. 2023 Jul 20;15(7):1585. doi: 10.3390/v15071585. PMID: 37515272; PMCID: PMC10386070)
Fig. 3.
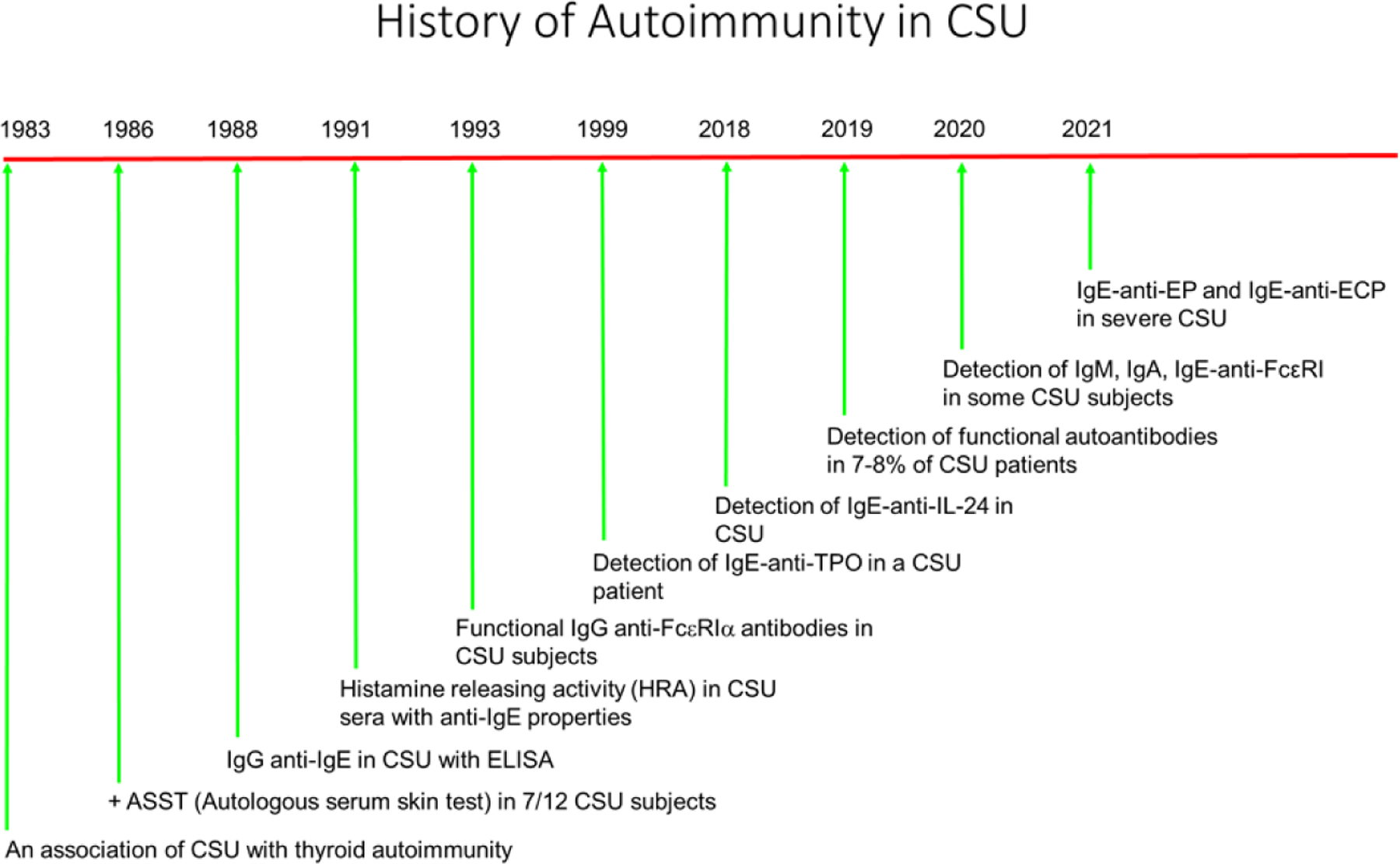
Timeline for autoantibody theory. (Data from Refs41,43,44,47–56)
The autoimmune theory dates to the 1980s when a serum factor that behaved like a cross-linking anti-IgE was detected in CU patients.43,44 Another report found that 7 of 12 (58%) CU patients developed wheal and flare reactions within 30 minutes at the site of intradermal injection of their own serum or ASST (Fig. 4).43 The skin pathology of an ASST reaction resembled that of an IgE-mediated LPR, supporting that mast-cell degranulation occurred in the ASST reaction via a presumed CSU disease-related factor.45 However, subsequent work reported positive ASST results in subjects with allergic respiratory disease and healthy controls, raising issues of specificity of this test for CU disease.46
Fig. 4.
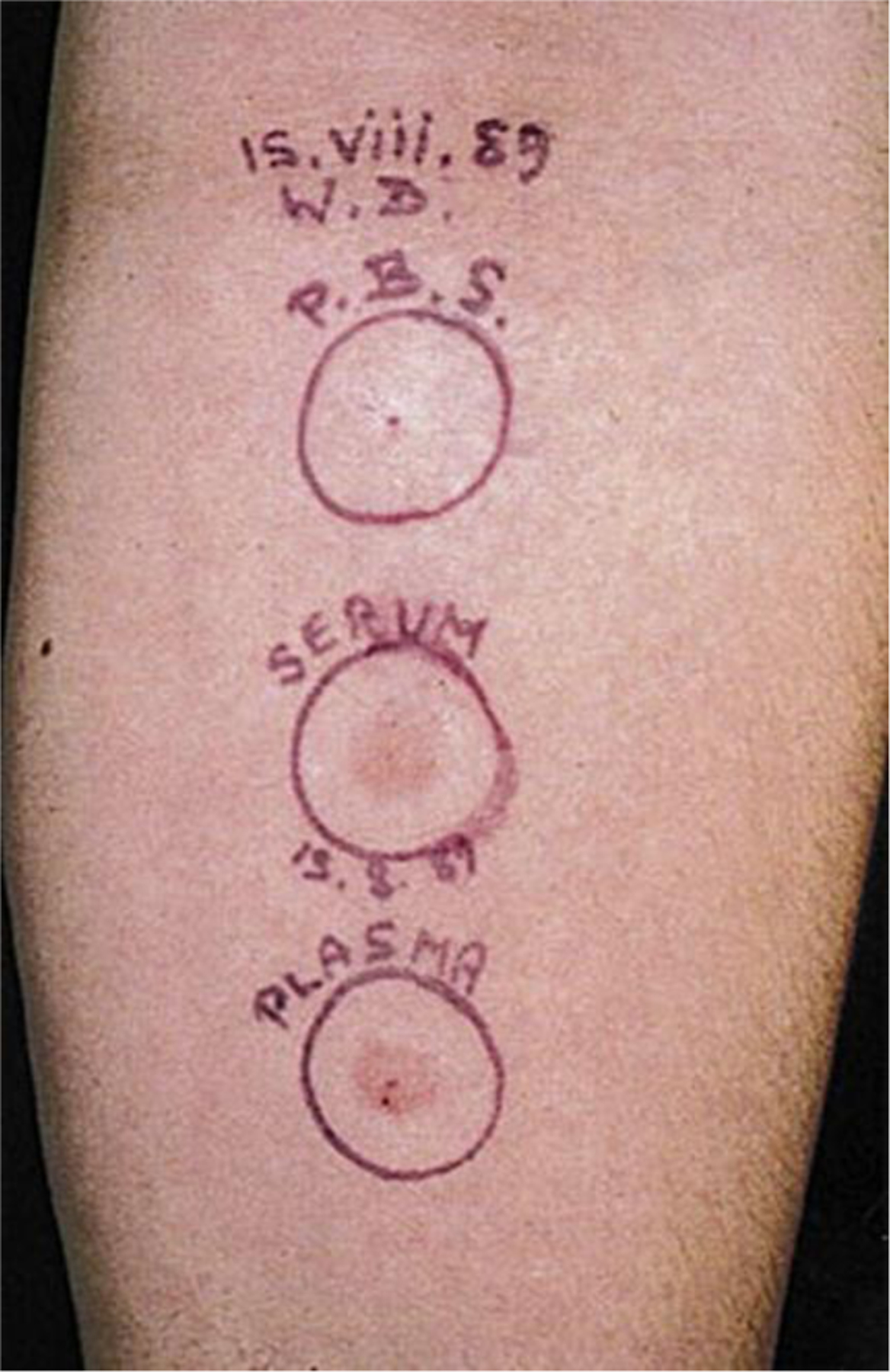
An autologous serum skin test (ASST) reaction. Phosphate-buffered saline (P.B.S.), saline solution negative control, serum, and plasma are injected in a volume of 0.05 mL and the reaction read at 30 minutes. Here, both serum and plasma have produced positive responses. (From Greaves M. Chronic urticaria. J Allergy Clin Immunol 2000;105(4):664–72; with permission).
In parallel with studies of ASST, a report emerged that CU serum contained IgG antibodies against the Fc region of IgE, and led to histamine release from the blood basophils of healthy subjects.44 This activity was termed serum histamine-releasing activity (HRA) (Fig. 5). A modification of the HRA assay examines CU serum for the ability to increase levels of basophil surface activation marker CD203c rather than histamine release.57 Initially, serum fractions responsible for HRA (>100 kDa) differed from fractions described as inducing an ASST (<20 kDa).44,58 Later, a single serum fraction (>100 kDa) was capable of inducing both a positive ASST and positive HRA.51 These findings culminated in a study identifying IgG anti-FcεRIα autoantibodies as the main serum factor responsible for HRA.59 It is unclear how some of these antibodies would be functional in vivo, given the overall high occupancy rate (>95%) of IgE receptors on basophils and mast cells.
Fig. 5.
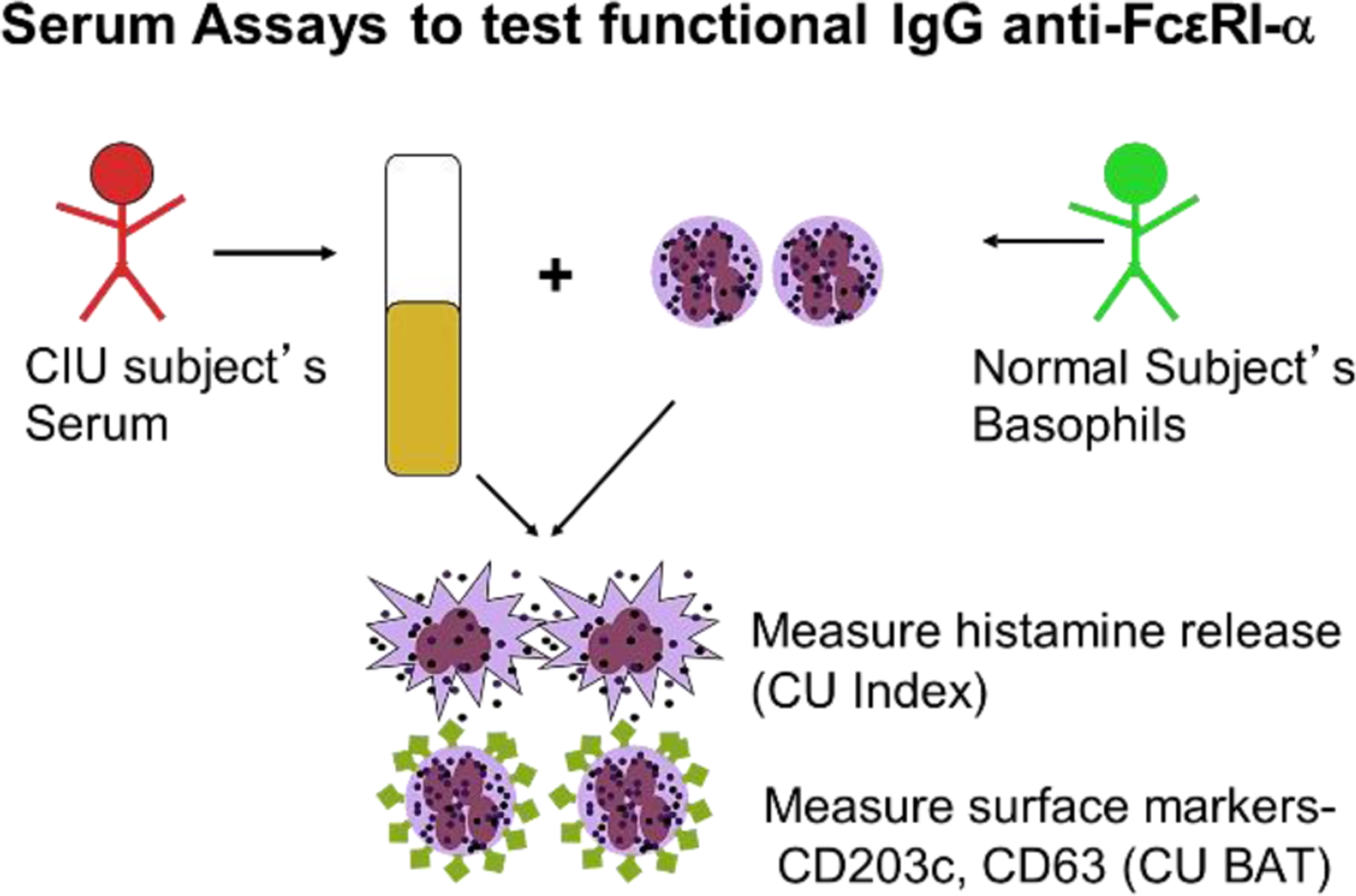
Basophil assays. Approach for measuring histamine release or activation markers. BAT, basophil activation test (Adapted from Brodell LA, Beck LA, Saini SS. Pathophysiology of chronic urticaria. Ann Allergy Asthma Immunol 2008;100(4):294; with permission)
Although early reports suggested that HRA activity and ASST reactions decreased in CU remission43, later reports noted persistence of ASST positivity in remission and even after IgG-depletion of the serum used in the ASST.60 In 163 CU subjects, a direct comparison of serum ASST results with those of HRA testing found ASST positivity in 60% of subjects, but only half of the ASST-positive sera evoked serum HRA from basophils.61 This highlighted that ASST reactivity reflected skin mast-cell–specific factors that differed from those shown to activate donor basophils (IgG anti-IgE, or anti-FcεRIα).62 IgG anti-FcεRIα HRA autoantibodies are estimated to occur in 30% to 40% of CU patients, whereas IgG anti-IgE autoantibodies occur in 10%.40 57
The exact nature of CU serum HRA factors, their role in disease, and the optimum test strategy for detecting such factors continues to be debated. Concerns with the assays are: the variability of the donor basophils for HRA assay; the confounding by other factors in serum that can trigger histamine release from normal basophils (IL-3, complement); and the occurrence of positive HRA in the serum of healthy subjects and those with SLE.63
Complement-fixing subclasses IgG1 and IgG3 were found in the serum of CU subjects by an enzyme-linked immunosorbent assay (ELISA).64 By contrast, non–complement-fixing subclasses IgG2 and IgG4 were detected in pemphigus vulgaris, dermatomyositis, SLE, and bullous pemphigoid at a similar frequency to CU but were nonfunctional in HRA assays. A later study failed to demonstrate a correlation between CU serum containing immunoreactive IgG antibodies to FcεRIα by Western blotting and serum positive for HRA.65 The lack of concordance between ASST, HRA, and ELISA/Western blotting remains a problem in identifying the best test to define CAU.62 Further, a relationship of antibody titers to CSU disease activity or remission has not been demonstrated.66
While older studies reported high rates of autoantibody presence, the percentage of CSU patients with functional serum autoantibodies to IgE and FcεRI was found to be approximately 7% using stringent assay criteria. These criteria included the following: 1) the patient’s sera induced histamine release in 2 different basophil donors; 2) histamine release was inhibited by SYK, BTK and PI3K inhibitors; 3) a known anti-IgE Ab could cross-desensitize serum-induced histamine release; 4) the sera did not induce histamine release from basophils with the non-releasing phenotype; and 5) either IgE- or FcεRIα-beads inhibited the ability of sera to induce histamine release.47 The PURIST Study found that 8% of CSU subjects met study criteria for autoimmunity which required a positive ASST, a positive basophil histamine release or basophil activation assay, and detection of IgG Ab against FcεRl and/or IgE with Western blot or ELISA.48 Using two very different approaches, the estimated prevalence of functional IgG autoantibodies in CSU has been reduced.
Recently, some have proposed that most cases of CSU can be divided into type I autoimmunity and type IIb autoimmunity.41 Type I autoimmunity, also referred to as autoallergic CSU, describes individuals who have detectable IgE against endogenous proteins such as TPO, EP, Tissue Factor, or IL-24.52–55 Type IIb autoimmunity describes those with IgG against FcεRI or IgE. Previously, these were described as 2 distinct subsets. However, a recent work found major overlap between these 2 groups with most patients with type IIb (based on triple positivity of the PURIST study) also having type I autoantibodies, but not vice versa.67 The significance of this autoimmune classification as a marker of severity or predictor of therapeutic response lacks sufficient evidence-based trials. There is also novel demonstration of IgE, IgM, and IgA autoantibodies to FcεRI, further complicating the definitions of type I and type II autoimmunity.55,56
Summary of Points Unresolved in the Autoimmune Theory
ASST lacks sensitivity and specificity for CSU46
ASST may indicate the presence of other vasoactive factors rather than IgG autoantibodies60
HRA testing depends on the basophil donor and is confounded by other serum active factors
HRA positives are seen in healthy subjects and SLE subjects63
Skin biopsies and clinical phenotype do not differ between CAU and CSU18,29
Differential targeting of anti-FcεRI antibodies to occupied versus unoccupied receptors raises issues regarding functional role in disease in vivo
It is unclear why IgG anti-IgE leads to only skin-limited disease and not anaphylaxis
It is unclear why multiple isotypes are observed to the same antigen (e.g., FcεRI) in individuals and what the net consequences of these antibodies are in disease
Skin mast cells
Mast-cell degranulation is a central event in lesion development in urticaria18, and elevated histamine levels are noted in CU lesional skin blister fluid.25 There are inconsistent findings on the number of skin mast cells in CSU lesions relative to unaffected skin. Skin mast cells were reported as increased in both CSU skin lesions and nonlesional skin compared to healthy skin.19 Other studies find similar mast cells numbers in lesional and nonlesional CSU skin biopsies relative to the skin of healthy controls.68 Likewise, total serum tryptase levels, an indirect measure of total body mast cell numbers, is slightly elevated in CU subjects relative to healthy and atopic subjects, but well within the normal range.69 Although there are conflicting reports on whether histamine in increased in nonlesional CU skin25, culture-derived mast cells grown from peripheral blood CD34+cells of CU subjects showed elevated spontaneous histamine release when compared with mast cells grown from healthy donors.70
Decades ago, skin mast-cell hyperreleasability in active CU was shown by skin challenges with compound 48/80.71 It was discovered that compound 48/80 histamine release was mediated through the Mas-Related GPR family member X2 (MRGPRX2) receptor.72 MRGPRX2 is a G protein-coupled receptor which is activated by a diverse range of endogenous and foreign ligands. Studies utilizing a human mast cell line have revealed that several drugs, LL-37, and β-defensins bind to MRGPRX2, stimulating the release of histamine, TNF, PGD2, IP-10, RANTES, and IL-8.72 MRGPRX2 expression is higher on skin mast cells from subjects with severe CSU compared to healthy controls.68 Intradermal skin tests to the MRGPRX2 ligands atracurium and icatibant produced greater wheal size in CSU subjects compared to healthy controls.73 Other pathways that could activate mast cells remain to be established.
Basophils
Evidence that basophils are involved in the pathogenesis of CU has emerged over the past decades.49,74 In the 1970s, two groups identified that basophils of CSU subjects had reduced capacity to release histamine after IgE-receptor activation.75,76 Basophil histamine hyporeleasability was limited to the IgE pathway and not seen with stimulation by formyl-methionyl-leucyl-phenylalanine, bradykinin, or monocyte chemoattractant protein.77–79 Currently, there are 2 basophil functional phenotypes referred to as CSU responders (CSU-R) and CSU nonresponders (CSU-NR).80 CSU-R basophils show an IgE-based histamine degranulation profile similar to that of normal subjects, whereas basophils of CSU-NR subjects do not degranulate to ex vivo IgE-receptor activation and possess elevated levels of the IgE-receptor regulating inhibitory phosphatases, SH2-domain–containing inositol-5-phosphatase (SHIP)-1 and SHIP-2. These 2 functional phenotypes are stable in active CSU disease, are not related to the presence of autoimmune serum factors, and also reflect differences in some clinical features of subjects.69,81,82 Furthermore, basophil hyporeleasability improves in disease remission.66 While the mechanisms leading to these distinct basophil phenotypes remains unknown, these findings support that basophil responsiveness is relevant to the pathogenesis of urticaria.
Blood basopenia has been noted in CU subjects and linked to the presence of serum HRA.81 Skin biopsies have revealed basophils in both CSU lesional sites and nonlesional skin tissues, a finding not seen in healthy skin and other skin diseases.18,30 The degree of basopenia is correlated with disease severity, improves in CSU remission, and appears to reflect blood basophil recruitment to skin lesions.66 Phase III trials of monoclonal IgG anti-IgE therapy (omalizumab) resulted in a dose-dependent improvement in CSU clinical symptoms and a parallel increase in blood basophil numbers.82 In a mechanistic clinical study of omalizumab use in CSU, subjects having a baseline basophil functional phenotype of CSU-R demonstrated a more complete and rapid response than those with basopenia or CSU-NR status.83 While pathways for basophil recruitment in CSU have not been fully elucidated, mast cell-derived PGD2 may contribute to basophil trafficking.84
Eosinophils
In skin biopsies from CSU patients, eosinophils were increased in lesional and nonlesional skin compared with healthy controls.19 Eosinopenia was recently identified as a risk factor for more severe disease in CSU patients.85 Serum levels of eosinophil granule proteins, specifically eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP), were shown to directly correlate with CSU disease severity.86,87 Compared with healthy individuals, major basic protein (MBP) and ECP were increased in the skin and sera of CSU patients.88,89 MBP and eosinophil peroxidase (EP) were demonstrated to activate skin-derived cultured mast cells through MRGPRX2.68
Infections
Among infections implicated in CU are Helicobacter pylori, but the data are mixed.90,91 Although reports of CU induced by COVID-19 are infrequent, a recent case series described 14 cases of COVID-19-induced CSU cases where CSU urticaria appeared an average of 18 days following COVID-19.92,93 Acute urticaria can be observed in the early stages of hepatitis A, B, and C infection, but little evidence exists for hepatitis virus infection causing CU.94 Some studies have suggested that internal parasitic infections (helminths and protozoa) can cause CSU. Recently, a systematic review of 39 independent studies examined the prevalence of parasitic infections and found that two-thirds of studies reported infection rates of 10% or less.95 However, convincing evidence that parasitic infections cause CSU is lacking.
CLINICAL FEATURES
Urticarial lesions or hives are typically raised erythematous papules or plaques that are circumscribed (Fig. 6). In skin of color, urticarial lesions may be the same color as the surrounding skin or appear hyperpigmented (Fig. 7). These lesions can assume a variety of sizes, and may be flattened if a patient is using antihistamines. Any area of the body can be affected by lesions with a life span of less than 24 hours; therefore, photographs of skin lesions are useful to confirm the diagnosis. Lesions with a longer life span or with bruising may suggest a vasculitis. The severity of the pruritus can be disruptive of both sleep and daily activities (see the article by Stitt and Keller elsewhere in this issue).
Fig. 6.
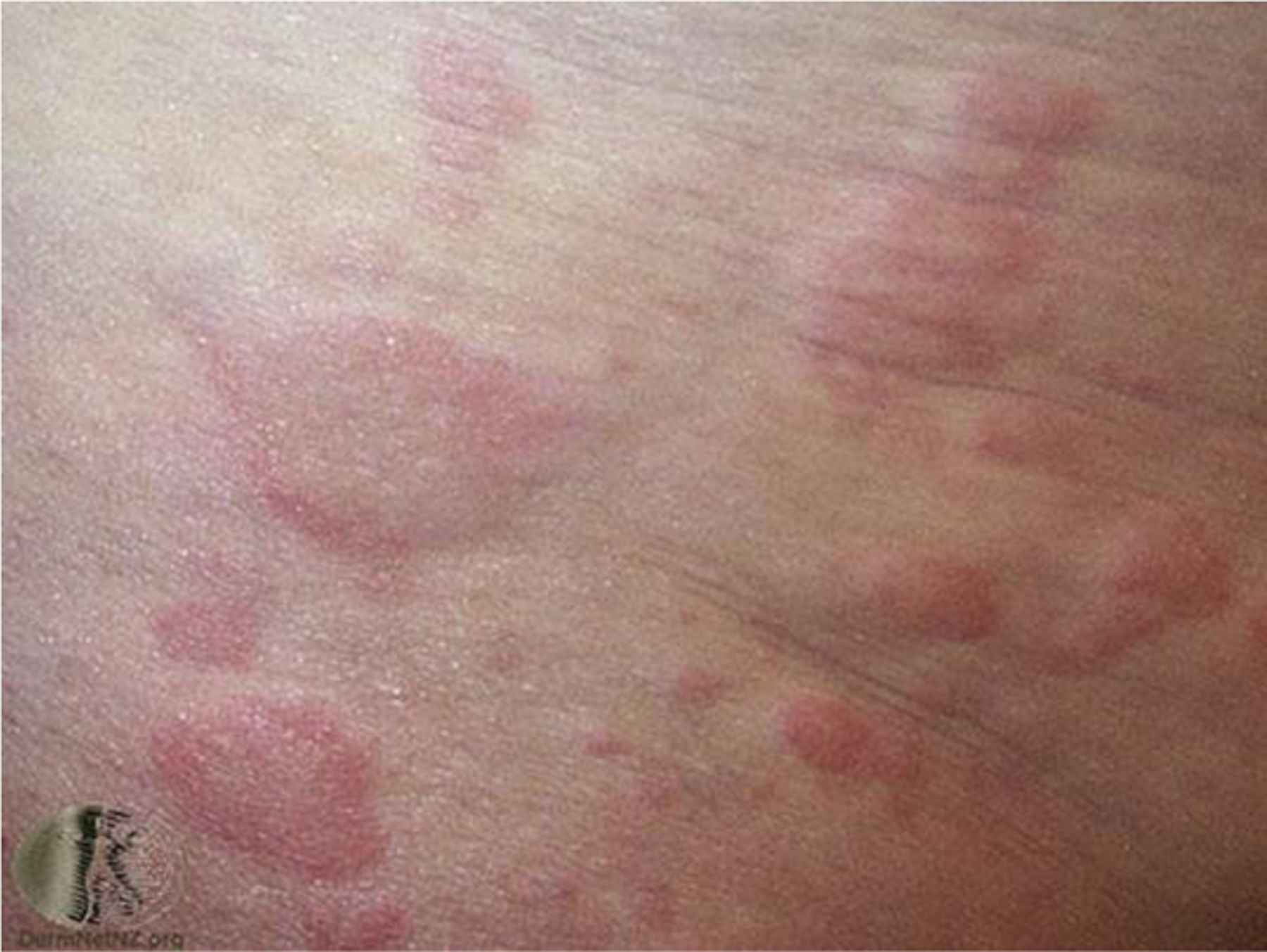
Urticaria. (Courtesy of New Zealand Dermatological Society Incorporated).
Fig. 7.
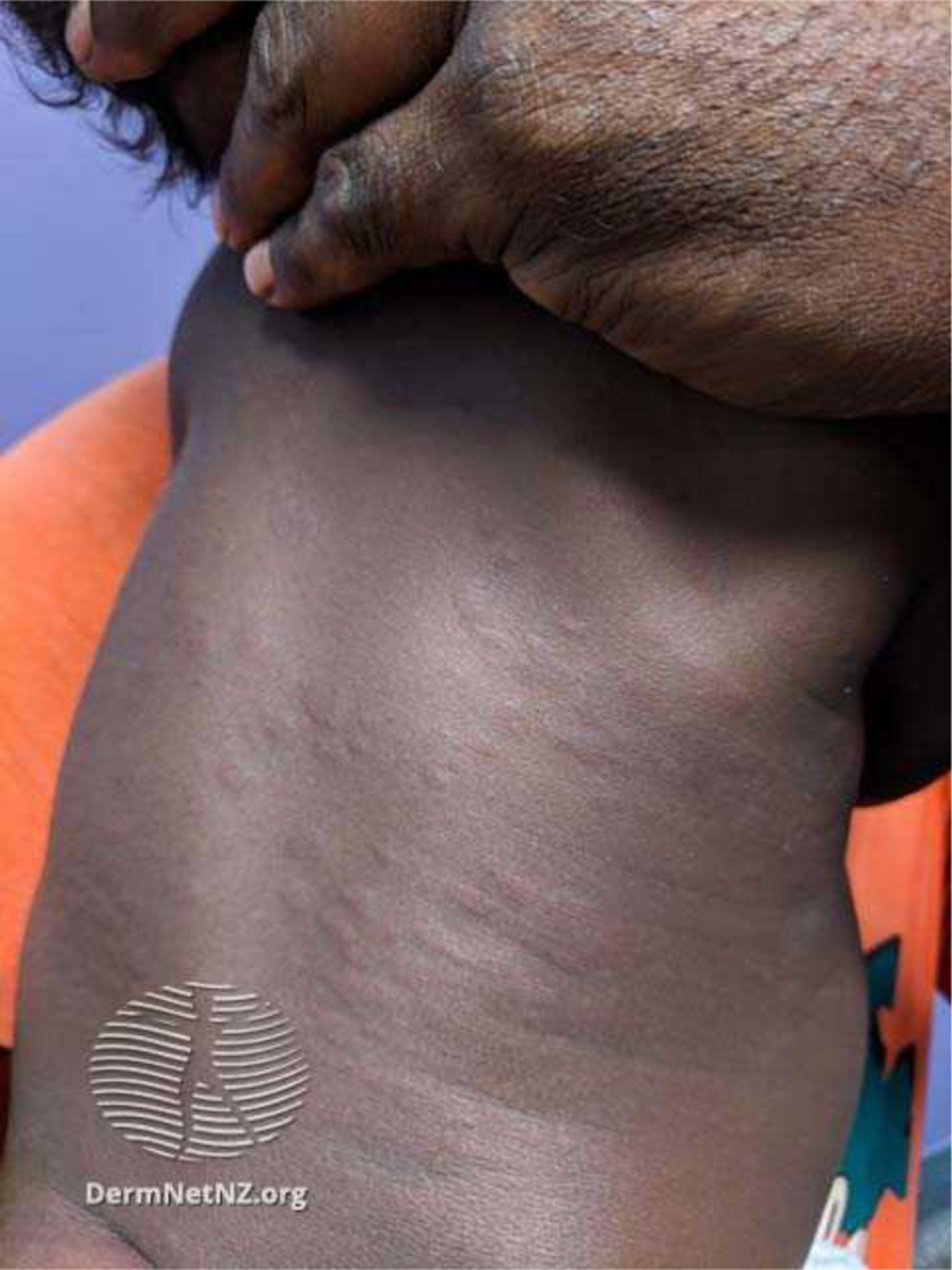
Urticaria in skin of color. (Courtesy of New Zealand Dermatological Society Incorporated).
Angioedema involves episodic submucosal or subcutaneous swelling that is asymmetric in distribution (Fig. 8). Numbness or tingling is common, rather than the pruritus described in urticaria. Body areas commonly involved include the lips, eyes, cheeks, and extremities.96 It is important to consider other causes of isolated angioedema such as drugs (angiotensin-converting enzyme inhibitor–induced lesions), hereditary angioedema, or acquired angioedema (see the relevant article by Zuraw elsewhere in this issue).
Fig. 8.
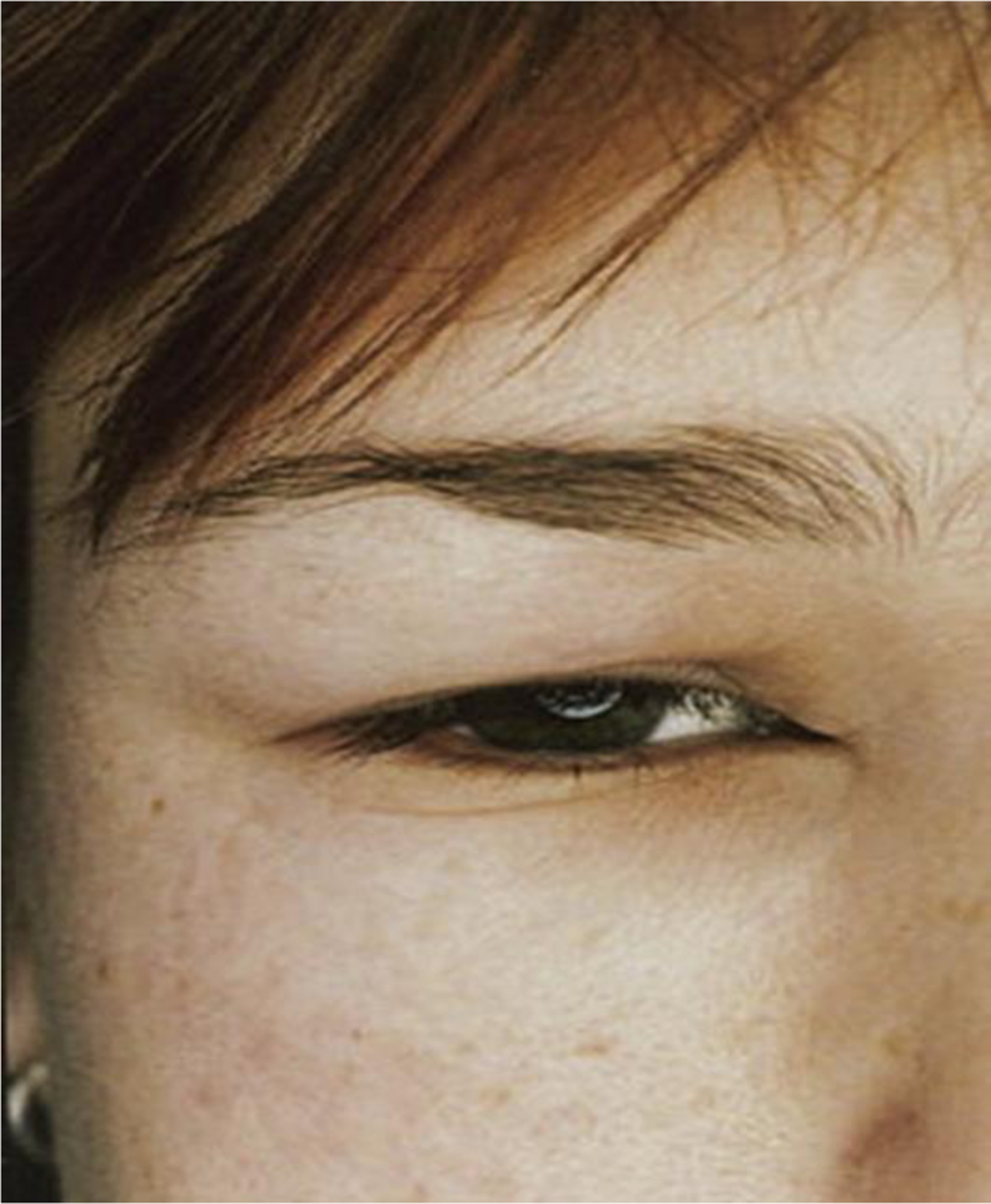
Angioedema. Deeper swelling of the skin and submucosa called angioedema are less well demarcated and do not necessarily show any difference in color from unaffected skin. (From Grattan CE, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol 2002;46(5):645–60; with permission).
DIFFERENTIAL DIAGNOSIS AND OTHER CONSIDERATIONS
Numerous disorders must be considered in the evaluation of patients with CSU. Acute urticaria is often associated with viral and bacterial infections, especially in children. Stool studies to exclude parasites as a cause of urticaria are only relevant in individuals who have recently traveled to endemic areas. It is clear that food and drug anaphylaxis often demonstrate skin symptoms within 2 hours of ingestion, and is rarely determined to be the cause of CU. One exception is delayed anaphylaxis to mammalian meat, also known as alpha gal syndrome.97
Among factors that are thought to contribute to disease exacerbations of urticarial are nonsteroidal anti-inflammatory medications (NSAIDs), alcohol ingestion, and stress.98 NSAID-induced urticaria and angioedema (NIUA) presents with urticaria and/or angioedema within 1–6 hours of taking aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without a history of CSU.99 NSAID-exacerbated CSU, also referred to as NSAID-exacerbated cutaneous disease or aspirin-intolerant CSU, occurs in roughly one-third of CSU patients and resolves in some with remission of CSU.100
FUTURE CONSIDERATIONS AND SUMMARY
Further studies are clearly needed to advance the understanding of pathogenic factors that trigger skin symptoms in both acute and chronic urticarial disease. The diverse nature of inciting factors and systemic diseases that are associated with urticaria suggest that multiple pathways lead to mast-cell activation and blood leukocyte migration. In addition, better biomarkers to predict the course of CSU disease and assist in the selection of therapeutics are needed.
Clinics Care Points
Mast cell degranulation is a central event in CSU disease expression.
Histamine is a principal mediator of itch and wheals in CSU.
Basopenia and eosinopenia may reflect more severe symptoms.
NSAIDs can flare symptoms in in one-third of CU cases.
CU disease is self-limited to a few years in most cases.
KEY POINTS.
Acute urticaria occurs in up to 20% of the population, and may be associated with an infection, medication, or food allergy. It is generally self-limited.
Chronic urticaria affects up to 1% of the population; in most cases, lesions occur spontaneously without an identifiable external cause.
There are multiple theories of pathogenesis for chronic spontaneous urticaria, none of which are clearly established. One such theory is that autoantibodies mediate disease in subset of individuals.
Abnormalities in skin mast cells and blood basophils have been described in chronic urticaria.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
ETO: Grant Support: National Institutes of Health (K23AI139394), Robert Meyerhoff Professorship. Advisory Board/Consultant: Novartis, Regeneron/ Sanofi.
SSS: Grant Support: Novartis, Sanofi, Regeneron, National Institutes of Health, Allakos, Escient. Advisory Board/Consultant: Allakos, Granular Therapeutics, Novartis, Aquestive, Regeneron, Escient, Evommune, Innate, Celltrion, Sanofi.
REFERENCES
- 1.Kolkhir P, Gimenez-Arnau AM, Kulthanan K, Peter J, Metz M, Maurer M. Urticaria. Nat Rev Dis Primers. 2022;8(1):61-z. doi: 10.1038/s41572-022-00389-z. [DOI] [PubMed] [Google Scholar]
- 2.Ornek Ozdemir S, Kuteyla Can P, Degirmentepe EN, Cure K, Singer R, Kocaturk E . A comparative analysis of chronic inducible urticaria in 423 patients: Clinical and laboratory features and comorbid conditions. J Eur Acad Dermatol Venereol. 2023. doi: 10.1111/jdv.19637. [DOI] [PubMed] [Google Scholar]
- 3.Buttgereit T, Vera C, Aulenbacher F, et al. Patients with chronic spontaneous urticaria who have wheals, angioedema, or both, differ demographically, clinically, and in response to treatment-results from CURE. J Allergy Clin Immunol Pract. 2023;11(11):3515–3525.e4. doi: 10.1016/j.jaip.2023.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Sabate-Bresco M, Rodriguez-Garijo N, Azofra J, et al. A comparative study of sex distribution, autoimmunity, blood, and inflammatory parameters in chronic spontaneous urticaria with angioedema and chronic histaminergic angioedema. J Allergy Clin Immunol Pract. 2021;9(6):2284–2292. doi: 10.1016/j.jaip.2021.03.038. [DOI] [PubMed] [Google Scholar]
- 5.Gaig P, Olona M, Munoz Lejarazu D, et al. Epidemiology of urticaria in spain. J Investig Allergol Clin Immunol. 2004;14(3):214–220. [PubMed] [Google Scholar]
- 6.Ozceker D, Can PK, Terzi O, et al. Differences between adult and pediatric chronic spontaneous urticaria from a cohort of 751 patients: Clinical features, associated conditions and indicators of treatment response. Pediatr Allergy Immunol. 2023;34(2):e13925. doi: 10.1111/pai.13925. [DOI] [PubMed] [Google Scholar]
- 7.Longhurst HJ, Goncalo M, Godse K, Ensina LF. Managing chronic urticaria and recurrent angioedema differently with advancing age. J Allergy Clin Immunol Pract. 2021;9(6):2186–2194. doi: 10.1016/j.jaip.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol. 1969;81(8):588–597. [DOI] [PubMed] [Google Scholar]
- 9.Kulthanan K, Jiamton S, Thumpimukvatana N, Pinkaew S. Chronic idiopathic urticaria: Prevalence and clinical course. J Dermatol. 2007;34(5):294–301. doi: 10.1111/j.1346-8138.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- 10.Toubi E, Kessel A, Avshovich N, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: A prospective study of 139 patients. Allergy. 2004;59(8):869–873. [DOI] [PubMed] [Google Scholar]
- 11.Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5(5):408–412. [DOI] [PubMed] [Google Scholar]
- 12.Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: Associations found in a large population study. J Allergy Clin Immunol. 2012;129(5):1307–1313. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi Y, Fann T, Kaplan AP. Antithyroid antibodies in chronic urticaria and angioedema. J Allergy Clin Immunol. 2003;112(1):218. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Qiu L, Wu J, et al. GWAS of chronic spontaneous urticaria reveals genetic overlap with autoimmune diseases, not atopic diseases. J Invest Dermatol. 2023;143(1):67–77.e15. doi: 10.1016/j.jid.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Lindelof B, Sigurgeirsson B, Wahlgren CF, Eklund G. Chronic urticaria and cancer: An epidemiological study of 1155 patients. Br J Dermatol. 1990;123(4):453–456. doi: 10.1111/j.1365-2133.1990.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Wu C, Shen J, Chen T, Chang Y. Cancer risk in patients with chronic urticaria: A population-based cohort study. Arch Dermatol. 2012;148(1):103–108. doi: 10.1001/archdermatol.2011.682. [DOI] [PubMed] [Google Scholar]
- 17.Larenas-Linnemann D, Saini SS, Azamar-Jacome AA, Maurer M. Chronic urticaria can be caused by cancer and resolves with its cure. Allergy. 2018;73(7):1562–1566. doi: 10.1111/all.13434. [DOI] [PubMed] [Google Scholar]
- 18.Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: Comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109(4):694–700. [DOI] [PubMed] [Google Scholar]
- 19.Kay AB, Ying S, Ardelean E, et al. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br J Dermatol. 2014;171(3):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas N, Hermes B, Henz BM. Adhesion molecules and cellular infiltrate: Histology of urticaria. J Investig Dermatol Symp Proc. 2001;6(2):137–138. doi: 10.1046/j.0022-202x.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 21.Puhl V, Bonnekoh H, Scheffel J, et al. A novel histopathological scoring system to distinguish urticarial vasculitis from chronic spontaneous urticaria. Clin Transl Allergy. 2021;11(2):e12031. doi: 10.1002/clt2.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okon LG, Werth VP. Cutaneous lupus erythematosus: Diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27(3):391–404. doi: 10.1016/j.berh.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamata A, Kurihara Y, Funakoshi T, et al. Basement membrane zone IgE deposition is associated with bullous pemphigoid disease severity and treatment results. Br J Dermatol. 2020;182(5):1221–1227. doi: 10.1111/bjd.18364. [DOI] [PubMed] [Google Scholar]
- 24.Guillen-Aguinaga S, Jauregui Presa I, Aguinaga-Ontoso E, Guillen-Grima F, Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: A systematic review and meta-analysis. Br J Dermatol. 2016;175(6):1153–1165. doi: 10.1111/bjd.14768. [DOI] [PubMed] [Google Scholar]
- 25.Claveau J, Lavoie A, Brunet C, Bedard PM, Hebert J. Chronic idiopathic urticaria: Possible contribution of histamine-releasing factor to pathogenesis. J Allergy Clin Immunol. 1993;92(1 Pt 1):132–137. doi: 10.1016/0091-6749(93)90047-j. [DOI] [PubMed] [Google Scholar]
- 26.Gimenez-Arnau AM, DeMontojoye L, Asero R, et al. The pathogenesis of chronic spontaneous urticaria: The role of infiltrating cells. J Allergy Clin Immunol Pract. 2021;9(6):2195–2208. doi: 10.1016/j.jaip.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Kay AB, Clark P, Maurer M, Ying S. Elevations in Th2-initiating cytokines (IL-33, IL-25, TSLP) in lesional skin from chronic spontaneous (“idiopathic”) urticaria. Br J Dermatol. 2014. [DOI] [PubMed] [Google Scholar]
- 28.Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228.e13. doi: 10.1016/j.cell.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabroe RA, Poon E, Orchard GE, et al. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: Comparison of patients with and without anti-FcepsilonRI or anti-IgE autoantibodies. J Allergy Clin Immunol. 1999;103(3 Pt 1):484–493. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66(8):1107–1113. [DOI] [PubMed] [Google Scholar]
- 31.Kulthanan K, Chularojanamontri L, Tuchinda P, Buranaporn P, Karoopongse E. Unveiling the role of neutrophils in chronic spontaneous urticaria: Beyond mast cells. Asian Pac J Allergy Immunol. 2023;41(3):179–185. doi: 10.12932/AP-180623-1638. [DOI] [PubMed] [Google Scholar]
- 32.Piconi S, Trabattoni D, Iemoli E, et al. Immune profiles of patients with chronic idiopathic urticaria. Int Arch Allergy Immunol. 2002;128(1):59–66. doi: 10.1159/000058004. [DOI] [PubMed] [Google Scholar]
- 33.Confino-Cohen R, Goldberg A, Aharoni D, et al. Low stimulated IL-4 secretion in PBMC from patients with chronic idiopathic urticaria. Cytokine. 2004;27(2–3):74–80. [DOI] [PubMed] [Google Scholar]
- 34.Futata E, Azor M, Dos Santos J, et al. Impaired IFN-alpha secretion by plasmacytoid dendritic cells induced by TLR9 activation in chronic idiopathic urticaria. Br J Dermatol. 2011;164(6):1271–1279. doi: 10.1111/j.1365-2133.2010.10198.x. [DOI] [PubMed] [Google Scholar]
- 35.Dos Santos JC, Azor MH, Nojima VY, et al. Increased circulating pro-inflammatory cytokines and imbalanced regulatory T-cell cytokines production in chronic idiopathic urticaria. Int Immunopharmacol. 2008;8(10):1433–1440. doi: 10.1016/j.intimp.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Caproni M, Volpi W, Giomi B, et al. Chronic idiopathic and chronic autoimmune urticaria: Clinical and immunopathological features of 68 subjects. Acta Derm Venereol. 2004;84(4):288–290. doi: 10.1080/00015550410026939. [DOI] [PubMed] [Google Scholar]
- 37.Kessel A, Yaacoby-Bianu K, Vadasz Z, Peri R, Halazs K, Toubi E. Elevated serum B-cell activating factor in patients with chronic urticaria. Hum Immunol. 2012;73(6):620–622. doi: 10.1016/j.humimm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Kasperska-Zajac A, Grzanka A, Czecior E, Misiolek M, Rogala B, Machura E. Acute phase inflammatory markers in patients with non-steroidal anti-inflammatory drugs (NSAIDs)-induced acute urticaria/angioedema and after aspirin challenge. J Eur Acad Dermatol Venereol. 2013;27(8):1048–1052. doi: 10.1111/j.1468-3083.2012.04486.x. [DOI] [PubMed] [Google Scholar]
- 39.Asero R, Tedeschi A, Coppola R, et al. Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119(3):705–710. doi: 10.1016/j.jaci.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39(6):777–787. doi: 10.1111/j.1365-2222.2009.03256.x. [DOI] [PubMed] [Google Scholar]
- 41.Kolkhir P, Munoz M, Asero R, et al. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol. 2022;149(6):1819–1831. doi: 10.1016/j.jaci.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell BF, O’Neill CM, Francis DM, et al. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br J Dermatol. 1999;140(5):853–858. [DOI] [PubMed] [Google Scholar]
- 43.Grattan CE, Wallington TB, Warin RP, Kennedy CT, Bradfield JW. A serological mediator in chronic idiopathic urticaria--a clinical, immunological and histological evaluation. Br J Dermatol. 1986;114(5):583–590. [DOI] [PubMed] [Google Scholar]
- 44.Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90(2):213–217. [DOI] [PubMed] [Google Scholar]
- 45.Grattan CE, Boon AP, Eady RA, Winkelmann RK. The pathology of the autologous serum skin test response in chronic urticaria resembles IgE-mediated late-phase reactions. Int Arch Allergy Appl Immunol. 1990;93(2–3):198–204. doi: 10.1159/000235301. [DOI] [PubMed] [Google Scholar]
- 46.Guttman-Yassky E, Bergman R, Maor C, Mamorsky M, Pollack S, Shahar E. The autologous serum skin test in a cohort of chronic idiopathic urticaria patients compared to respiratory allergy patients and healthy individuals. J Eur Acad Dermatol Venereol. 2007;21(1):35–39. [DOI] [PubMed] [Google Scholar]
- 47.MacGlashan D Autoantibodies to IgE and FcepsilonRI and the natural variability of spleen tyrosine kinase expression in basophils. J Allergy Clin Immunol. 2019;143(3):1100–1107.e11. doi: 10.1016/j.jaci.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoepke N, Asero R, Ellrich A, et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: Results of the PURIST study. Allergy. 2019;74(12):2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 49.Saini SS. Urticaria and basophils. Allergol Int. 2023;72(3):369–374. doi: 10.1016/j.alit.2023.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119(8):636–640. [PubMed] [Google Scholar]
- 51.Grattan CE, Francis DM, Hide M, Greaves MW. Detection of circulating histamine releasing autoantibodies with functional properties of anti-IgE in chronic urticaria. Clin Exp Allergy. 1991;21(6):695–704. [DOI] [PubMed] [Google Scholar]
- 52.Bar-Sela S, Reshef T, Mekori YA. IgE antithyroid microsomal antibodies in a patient with chronic urticaria. J Allergy Clin Immunol. 1999;103(6):1216–1217. doi: 10.1016/s0091-6749(99)70204-6. [DOI] [PubMed] [Google Scholar]
- 53.Schmetzer O, Lakin E, Topal FA, et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;142(3):876–882. doi: 10.1016/j.jaci.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez J, Sanchez A, Munera M, et al. Presence of IgE autoantibodies against eosinophil peroxidase and eosinophil cationic protein in severe chronic spontaneous urticaria and atopic dermatitis. Allergy Asthma Immunol Res. 2021;13(5):746–761. doi: 10.4168/aair.2021.13.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asero R, Marzano AV, Ferrucci S, Lorini M, Carbonelli V, Cugno M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin Exp Immunol. 2020;200(3):242–249. doi: 10.1111/cei.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altrichter S, Zampeli V, Ellrich A, Zhang K, Church MK, Maurer M. IgM and IgA in addition to IgG autoantibodies against FcvarepsilonRIalpha are frequent and associated with disease markers of chronic spontaneous urticaria. Allergy. 2020;75(12):3208–3215. doi: 10.1111/all.14412. [DOI] [PubMed] [Google Scholar]
- 57.Yasnowsky KM, Dreskin SC, Efaw B, et al. Chronic urticaria sera increase basophil CD203c expression. J Allergy Clin Immunol. 2006;117(6):1430–1434. [DOI] [PubMed] [Google Scholar]
- 58.Grattan CE, Hamon CG, Cowan MA, Leeming RJ. Preliminary identification of a low molecular weight serological mediator in chronic idiopathic urticaria. Br J Dermatol. 1988;119(2):179–183. doi: 10.1111/j.1365-2133.1988.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 59.Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328(22):1599–1604. [DOI] [PubMed] [Google Scholar]
- 60.Fagiolo U, Kricek F, Ruf C, Peserico A, Amadori A, Cancian M. Effects of complement inactivation and IgG depletion on skin reactivity to autologous serum in chronic idiopathic urticaria. J Allergy Clin Immunol. 2000;106(3):567–572. doi: 10.1067/mai.2000.108913. [DOI] [PubMed] [Google Scholar]
- 61.Niimi N, Francis DM, Kermani F, et al. Dermal mast cell activation by autoantibodies against the high affinity IgE receptor in chronic urticaria. J Invest Dermatol. 1996;106(5):1001–1006. [DOI] [PubMed] [Google Scholar]
- 62.Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154(5):813–819. [DOI] [PubMed] [Google Scholar]
- 63.Cho CB, Stutes SA, Altrich ML, Ardoin SP, Phillips G, Ogbogu PU. Autoantibodies in chronic idiopathic urticaria and nonurticarial systemic autoimmune disorders. Ann Allergy Asthma Immunol. 2013;110(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. identification of a structure-function relationship. J Clin Invest. 1998;101(1):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kikuchi Y, Kaplan AP. Mechanisms of autoimmune activation of basophils in chronic urticaria. J Allergy Clin Immunol. 2001;107(6):1056–1062. [DOI] [PubMed] [Google Scholar]
- 66.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128(8):1956–1963. [DOI] [PubMed] [Google Scholar]
- 67.Xiang Y, Kolkhir P, Scheffel J, et al. Most patients with autoimmune chronic spontaneous urticaria also have autoallergic urticaria, but not ViceVersa. J Allergy Clin Immunol Pract. 2023;11(8):2417–2425.e1. doi: 10.1016/j.jaip.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Fujisawa D, Kashiwakura J, Kita H, et al. Expression of mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134(3):622–633.e9. [DOI] [PubMed] [Google Scholar]
- 69.Doong JC, Chichester K, Oliver ET, Schwartz LB, Saini SS. Chronic idiopathic urticaria: Systemic complaints and their relationship with disease and immune measures. J Allergy Clin Immunol Pract. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saini SS, Paterniti M, Vasagar K, Gibbons SP, Sterba PM, Vonakis BM. Cultured peripheral blood mast cells from chronic idiopathic urticaria patients spontaneously degranulate upon IgE sensitization: Relationship to expression of syk and SHIP-2. Clin Immunol. 2009;132(3):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacques P, Lavoie A, Bedard PM, Brunet C, Hebert J. Chronic idiopathic urticaria: Profiles of skin mast cell histamine release during active disease and remission. J Allergy Clin Immunol. 1992;89(6):1139–1143. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z, Babina M. MRGPRX2 signals its importance in cutaneous mast cell biology: Does MRGPRX2 connect mast cells and atopic dermatitis? Exp Dermatol. 2020;29(11):1104–1111. doi: 10.1111/exd.14182. [DOI] [PubMed] [Google Scholar]
- 73.Shtessel M, Limjunyawong N, Oliver ET, et al. MRGPRX2 activation causes increased skin reactivity in patients with chronic spontaneous urticaria. J Invest Dermatol. 2021;141(3):678–681.e2. doi: 10.1016/j.jid.2020.06.030. [DOI] [PubMed] [Google Scholar]
- 74.Saini SS. Basophil responsiveness in chronic urticaria. Curr Allergy Asthma Rep. 2009;9(4):286–290. doi: 10.1007/s11882-009-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greaves MW, Plummer VM, McLaughlan P, Stanworth DR. Serum and cell bound IgE in chronic urticaria. Clin Allergy. 1974;4(3):265–271. [DOI] [PubMed] [Google Scholar]
- 76.Kern F, Lichtenstein LM. Defective histamine release in chronic urticaria. J Clin Invest. 1976;57(5):1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luquin E, Kaplan AP, Ferrer M. Increased responsiveness of basophils of patients with chronic urticaria to sera but hypo-responsiveness to other stimuli. Clin Exp Allergy. 2005;35(4):456–460. [DOI] [PubMed] [Google Scholar]
- 78.Rauber MM, Pickert J, Holiangu L, Mobs C, Pfutzner W. Functional and phenotypic analysis of basophils allows determining distinct subtypes in patients with chronic urticaria. Allergy. 2017;72(12):1904–1911. doi: 10.1111/all.13215. [DOI] [PubMed] [Google Scholar]
- 79.Matsubara D, Yanase Y, Ishii K, et al. Basophils activation of patients with chronic spontaneous urticaria in response to C5a despite failure to respond to IgE-mediated stimuli. Front Immunol. 2022;13:994823. doi: 10.3389/fimmu.2022.994823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vonakis BM, Vasagar K, Gibbons SP,Jr, et al. Basophil FcepsilonRI histamine release parallels expression of src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. J Allergy Clin Immunol. 2007;119(2):441–448. [DOI] [PubMed] [Google Scholar]
- 81.Grattan CE, Walpole D, Francis DM, et al. Flow cytometric analysis of basophil numbers in chronic urticaria: Basopenia is related to serum histamine releasing activity. Clin Exp Allergy. 1997;27(12):1417–1424. doi: 10.1046/j.1365-2222.1997.1630972.x. [DOI] [PubMed] [Google Scholar]
- 82.Saini SS, Omachi TA, Trzaskoma B, et al. Effect of omalizumab on blood basophil counts in patients with chronic idiopathic/spontaneous urticaria. J Invest Dermatol. 2017;137(4):958–961. doi: 10.1016/j.jid.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 83.Johal KJ, Chichester KL, Oliver ET, et al. The efficacy of omalizumab treatment in chronic spontaneous urticaria is associated with basophil phenotypes. J Allergy Clin Immunol. 2021;147(6):2271–2280.e8. doi: 10.1016/j.jaci.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oliver ET, Sterba PM, Devine K, Vonakis BM, Saini SS. Altered expression of chemoattractant receptor-homologous molecule expressed on T2 cells on blood basophils and eosinophils in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015. [DOI] [PubMed] [Google Scholar]
- 85.Kolkhir P, Church MK, Altrichter S, et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2020;8(1):318–325.e5. doi: 10.1016/j.jaip.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 86.Saleh AA, Al-Obaidi AM, Behiry EG, Hamed AM. Serum levels of eosinophil-derived neurotoxin in patients with chronic urticaria. J Clin Aesthet Dermatol. 2020;13(9):21–23. [PMC free article] [PubMed] [Google Scholar]
- 87.Lorenzo GD, Mansueto P, Melluso M, et al. Blood eosinophils and serum eosinophil cationic protein in patients with acute and chronic urticaria. Mediators Inflamm. 1996;5(2):113–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peters MS, Schroeter AL, Kephart GM, Gleich GJ. Localization of eosinophil granule major basic protein in chronic urticaria. J Invest Dermatol. 1983;81(1):39–43. doi: 10.1111/1523-1747.ep12538380. [DOI] [PubMed] [Google Scholar]
- 89.Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol. 2009;148(2):170–174. doi: 10.1159/000155748. [DOI] [PubMed] [Google Scholar]
- 90.Baskan EB, Turker T, Gulten M, Tunali S. Lack of correlation between helicobacter pylori infection and autologous serum skin test in chronic idiopathic urticaria. Int J Dermatol. 2005;44(12):993–995. doi: 10.1111/j.1365-4632.2005.02280.x. [DOI] [PubMed] [Google Scholar]
- 91.Di Campli C, Gasbarrini A, Nucera E, et al. Beneficial effects of helicobacter pylori eradication on idiopathic chronic urticaria. Dig Dis Sci. 1998;43(6):1226–1229. doi: 10.1023/a:23109. [DOI] [PubMed] [Google Scholar]
- 92.Kocaturk E, Munoz M, Elieh-Ali-Komi D, et al. How infection and vaccination are linked to acute and chronic urticaria: A special focus on COVID-19. Viruses. 2023;15(7):1585. doi: 10.3390/v15071585. doi: 10.3390/v15071585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oztas Kara R, Demir S, Sarac E, et al. Features of chronic spontaneous urticaria induced by COVID-19. Int Arch Allergy Immunol. 2023;184(8):792–796. doi: 10.1159/000530610. [DOI] [PubMed] [Google Scholar]
- 94.Cribier B. Urticaria and hepatitis. Clin Rev Allergy Immunol. 2006;30(1):25–29. [DOI] [PubMed] [Google Scholar]
- 95.Kolkhir P, Balakirski G, Merk HF, Olisova O, Maurer M. Chronic spontaneous urticaria and internal parasites--a systematic review. Allergy. 2016;71(3):308–322. doi: 10.1111/all.12818. [DOI] [PubMed] [Google Scholar]
- 96.Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: A large clinical survey. CMAJ. 2006;175(9):1065–1070. doi: 10.1503/cmaj.060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Platts-Mills TAE, Commins SP, Biedermann T, et al. On the cause and consequences of IgE to galactose-alpha-1,3-galactose: A report from the national institute of allergy and infectious diseases workshop on understanding IgE-mediated mammalian meat allergy. J Allergy Clin Immunol. 2020;145(4):1061–1071. doi: 10.1016/j.jaci.2020.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kozel MM, Bossuyt PM, Mekkes JR, Bos JD. Laboratory tests and identified diagnoses in patients with physical and chronic urticaria and angioedema: A systematic review. J Am Acad Dermatol. 2003;48(3):409–416. [DOI] [PubMed] [Google Scholar]
- 99.Asero R, Bavbek S, Blanca M, et al. Clinical management of patients with a history of urticaria/angioedema induced by multiple NSAIDs: An expert panel review. Int Arch Allergy Immunol. 2013;160(2):126–133. doi: 10.1159/000342424. [DOI] [PubMed] [Google Scholar]
- 100.Grattan CE. Aspirin sensitivity and urticaria. Clin Exp Dermatol. 2003;28(2):123–127. [DOI] [PubMed] [Google Scholar]


