Abstract
We assessed the diagnostic yield of urine GeneXpert MTB/RIF Ultra and factors associated with a positive test among adult patients suspected to have extrapulmonary tuberculosis. Urine Ultra was positive in 14% of participants with definite or probable tuberculosis. Hospitalization, disseminated tuberculosis, and human immunodeficiency virus infection were associated with a positive result.
Keywords: diagnostic yield, tuberculosis, urine Xpert MTB/RIF Ultra, extrapulmonary tuberculosis, human immunodeficiency virus
Microbiological confirmation of tuberculosis (TB) and resistance determination are crucial for accurate diagnosis and treatment. However, globally microbiological confirmation was achieved only in 63% of people diagnosed with pulmonary tuberculosis (PTB) in 2022 [1], and in not more than 24% of those diagnosed with extrapulmonary tuberculosis (EPTB) [2].
Xpert MTB/RIF Ultra (Cepheid Inc, Sunnyvale, California) (Ultra), a nucleic acid amplification test with an improved lower limit of detection (defined as the smallest number of colony-forming units (CFUs) per sample that can be consistently distinguished from negative samples with 95% confidence) of 15.6 CFU/mL of sputum compared to 112.6 CFU/mL for conventional Xpert MTB/RIF (Xpert) [3], was endorsed by the World Health Organization (WHO) in 2017 for the diagnosis of TB. Studies have shown it to have a sensitivity ranging from 75% in pleural fluid to 89.4% in cerebrospinal fluid, with specificities of 87% and 91.2%, respectively, compared to culture. In lymph node aspirates, the sensitivity was 70% with a specificity of 100% against a composite reference standard [4].
Small studies have shown that urine Ultra could be useful in the diagnosis of TB affecting sites other than the urogenital system, with a specificity of 98.1% for PTB and 98% for tuberculous lymphadenitis, but a low sensitivity of 17.2% and 18%, respectively [5]. Despite the comparatively low sensitivity [5], modeling studies have suggested the ease of acquiring a sample might positively impact test performance irrespective of diagnostic accuracy [6]. The diagnostic yield of urine Ultra in addition to other microbiological tests is unknown.
We assessed the diagnostic yield of urine Ultra in addition to other microbiological tests in patients with suspected EPTB, and determined factors associated with urine Ultra positivity.
METHODS
We conducted a secondary analysis of data from a large randomized 2-center trial assessing the use of ultrasound on the management of adults (aged >15 years) suspected to have EPTB. Participants were recruited in the rural St Francis Regional Referral Hospital and the urban Mwananyamala Regional Referral Hospital in Tanzania, from September 2018 to December 2020. At trial enrollment, sputum and urine samples were collected. Additionally, punctures and biopsies were done as clinically indicated. Ultra was done in all samples except sputum, which was tested by conventional Xpert MTB/RIF. All samples except urine were cultured [7, 8].
For this analysis, we included participants with definite or probable TB. Definite TB was defined as the presence of positive microbiology, histology, cytology, culture, or an adenosine deaminase level ≥40 U/mL in pleural fluid, or ≥35 U/mL in ascites and pericardial fluid. Probable TB was determined by an end-point review committee based on clinical, radiological, sonographical, and follow-up information [7]. Disseminated TB was defined as having either a miliary pattern on chest X-ray, having 2 or more microbiologically proven sites other than urine, having probable EPTB and PTB, or having a microbiologically proven and a probable second TB site other than urine.
We expressed categorical variables as frequencies and percentages, and continuous variables as medians and interquartile ranges (IQRs). The χ2 test and Fisher exact test were used to assess the differences between categorical variables. Continuous variables were compared by the classical 2-sample t test or Wilcoxon rank-sum test. Logistic regression models were used to determine factors associated with urine Ultra positivity with variables for the final estimation model (body mass index [BMI], human immunodeficiency virus [HIV] status, hemoglobin level, disseminated TB, and hospitalization) selected a priori based on clinical importance or prior reported association with urine Ultra and/or lipoarabinomannan (LAM) positivity [6, 9]. Participants with missing data were excluded from the respective analyses. We used Stata software version 16 for Windows (StataCorp, College Station, Texas).
RESULTS
We included 398 participants with definite (n = 209) or probable (n = 189) TB (Supplementary Figure 1). Median age was 35 (IQR, 27–44) years; the majority were male (n = 232 [58%]) and HIV-negative (n = 248 [62%]; Table 1). Urine was obtained in 99% and sputum was available in 59% of the participants. Participants with a positive urine Ultra (n = 55 [14%]) differed from those with a negative urine Ultra in being more likely to be HIV-positive (56% vs 34%, P = .002), to be hospitalized (35% vs 13%, P < .001), and to have a lower median BMI (18.94 [IQR, 16.23–20.06] kg/m2 vs 19.53 [IQR, 17.49–22.07] kg/m2; P = .032), a lower estimated glomerular filtration rate (78.96 [IQR, 60.09–107.18] mL/min/1.73 m2 vs 94.97 [IQR, 75.09–120.72] mL/min/1.73 m2; P = .008), and a lower hemoglobin level (9.70 [IQR, 7.40–11.70] g/dL vs 10.85 [IQR, 9.05–12.30] g/dL; P = .008; Supplementary Table 1).
Table 1.
Patients' Baseline Characteristics by Type of Tuberculosis
| Characteristic | Total Participants | Definite Tuberculosis | Probable Tuberculosis |
|---|---|---|---|
| (n = 398) | (n = 209) | (n = 189) | |
| Age, y, median (IQR) | 35 (27–44) | 34 (26–44) | 36 (28–45) |
| Sex, male, No. (%) | 232 (58) | 125 (60) | 107 (57) |
| BMI, kg/m2, median (IQR) | 19 (17–22) | 19 (17–21) | 20 (18–23) |
| HIV status, negative, No. (%) | 248 (62) | 121 (58) | 127 (67) |
| CD4 counta among PWH, cells/μL, median (IQR) | 122 (45–234) | 76 (37–187) | 226 (119–314) |
| History of previous EPTB or PTB, No. (%) | |||
| Yes | 30 (8) | 9 (4) | 21 (11) |
| No | 364 (91) | 199 (95) | 165 (87) |
| Unknown | 4 (1) | 1 (0) | 3 (2) |
| Hospitalization (yes), No. (%) | 66 (17) | 45 (22) | 21 (11) |
| WHO screening questions, No. (%) | |||
| Any cough | 320 (80) | 169 (81) | 151 (80) |
| Fever | 336 (84) | 178 (85) | 158 (84) |
| Night sweats | 210 (53) | 118 (56) | 92 (49) |
| Weight loss | 376 (94) | 200 (96) | 176 (93) |
| Laboratory results, median (IQR) | |||
| eGFR, mL/min/1.73 m2 | 94 (72–117) | 89 (67–112) | 98 (78–124) |
| Hemoglobin, g/dL | 11 (9–12) | 10 (8–12) | 11 (10–13) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; IQR, interquartile range; PTB pulmonary tuberculosis, PWH, people with human immunodeficiency virus; WHO, World Health Organization.
aOf 150 PWH included in the study, only 61 had a CD4 count result.
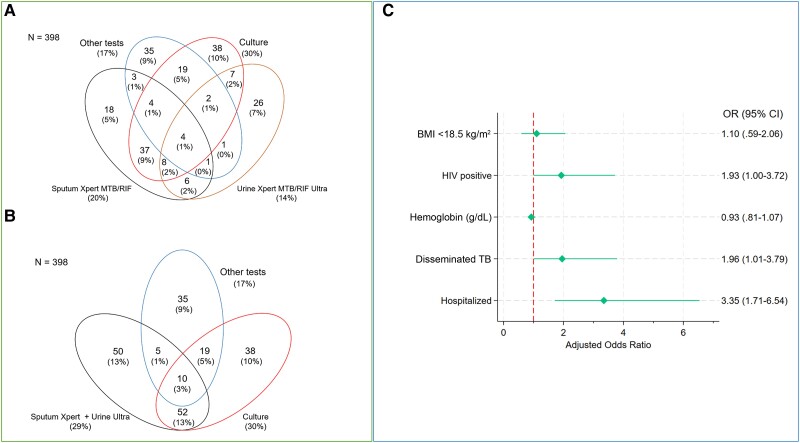
Among participants with definite or probable TB, sputum culture was the most frequent positive test (n = 86 [22%]; Supplementary Tables 2 and 3). Sputum Xpert had a yield of 20% (n = 79) and urine Ultra of 14% (n = 55) (Figure 1A). Urine Ultra was the only positive test in 26 (7%) participants. The addition of urine Ultra to sputum Xpert testing resulted in the detection of 36 more cases, with a combined yield of 29% (n = 117; Figure 1B). In participants with definite EPTB, urine Ultra had a yield of 26% and was the only positive test in 12% of the participants (Supplementary Figure 2). Further details on the yield of urine Ultra in relation to other diagnostic tests can be found in Supplementary Table 4.
Figure 1.
A, Venn diagram showing diagnostic yield of the different tests among participants with definite or probable tuberculosis (TB). B, Venn diagram showing the combined diagnostic yield of sputum Xpert and urine Ultra testing among participants with definite or probable TB. “Other tests” comprise Xpert MTB/RIF Ultra in ascites, pleural fluid, cerebrospinal fluid, abscess and lymph node fluid, acid-fast bacilli staining, histology, cytology, and adenosine deaminase (Supplementary Table 3). C, Forest plot presenting results of the multivariate logistic regression analysis for factors associated with urine Xpert TB/RIF Ultra positivity. The final multivariable model comprises covariates with clinical importance or prior reported association with urine Ultra and/or lipoarabinomannan positivity [6, 9]. Seven participants were excluded from the final logistic regression model (6 had missing urine Ultra results and 1 had no hemoglobin results), lowering the denominator to 391 participants. Abbreviations: BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
Hospitalization (adjusted odds ratio [aOR], 3.35 [95% confidence interval {CI}, 1.71–6.54]; P < .001), disseminated TB (aOR, 1.96 [95% CI, 1.01–3.79]; P = .047), and HIV (aOR, 1.93 [95% CI, 1.00–3.72]; P = .050) were associated with urine Ultra positivity in the final multivariable model (Figure 1C and Supplementary Table 5).
DISCUSSION
In this analysis on the diagnostic yield of urine Ultra in participants with suspected EPTB, urine was obtained in almost all of the participants while only 59% could provide sputum. Urine Ultra was the only microbiological confirmatory test in 26 participants, with a yield of 7% for those with definite or probable TB.
To the best of our knowledge, this is the first analysis on the diagnostic yield of urine Ultra in adults with suspected EPTB. A cross-sectional study in patients with PTB found urine Ultra to be more sensitive in people with HIV with advanced disease, but led to the identification of just 2 additional cases not detected by sputum Xpert [10]. A cohort study on HIV-infected adults with suspected tuberculous meningitis showed a urine Ultra positivity rate of 41% [9], which is consistent with our finding of participants with disseminated infection being more likely to have a positive urine Ultra.
Participants with a positive urine Ultra were more likely to be HIV-infected, severely ill, and hospitalized. These characteristics are similar to those for whom WHO recommends urine LAM testing [11], suggesting urine Ultra as an alternative or additional test to urine LAM.
Our results are in line with a prospective cohort study from South Africa showing a yield of 64% of the conventional Xpert in urine within 24 hours of admission of PWH with a high number of advanced HIV disease. Similarly, 99.5% of participants were able to provide urine, while only 37% provided a sputum sample [12]. A cross-sectional study assessed urine Xpert yield in newly diagnosed PWH and found an increase in the diagnostic yield of TB by 58% compared to sputum Xpert testing alone [13]. In contrast to these studies, we decided to indicate the yield primarily for definite and probable TB, as this better reflects clinical realities [1, 2]. Furthermore, patients report to prefer urine over sputum testing for reasons including ease and discreetness of acquiring a sample [14].
Limitations of the study include, first, that we did not compare urine Ultra with WHO-recommended urine LAM due to unavailability of LAM at the time of the trial. Second, the initial trial only included participants suspected to have EPTB, affecting the generalizability to patients with PTB; however, 7% of our study participants had PTB, and 30% had both EPTB and PTB. Third, only 61 PWH had a CD4 cell count available. We were therefore unable to assess the association of CD4 count and urine Ultra positivity, which would be important information in people with HIV. Last, our definition of probable TB included ultrasound findings, which is not routinely done in other settings.
CONCLUSIONS
Given the ease of acquiring a urine sample, the added yield, and the additional potential information on rifampicin resistance, our study supports the integration of urine Ultra testing in the diagnostics of EPTB. This is particularly important for people with HIV, severely ill patients, and those who are hospitalized.
Supplementary Material
Contributor Information
Robert Ndege, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania; Department of Medicine, Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland; Internal Medicine Department, St Francis Regional Referral Hospital, Ifakara, Tanzania.
Martin Rohacek, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania; Department of Medicine, Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland; Internal Medicine Department, St Francis Regional Referral Hospital, Ifakara, Tanzania.
Farida Bani, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania; Internal Medicine Department, St Francis Regional Referral Hospital, Ifakara, Tanzania.
Omary Ngome, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania.
James Okuma, Department of Medicine, Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland.
Mohamed Sasamalo, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania.
Dorcas Mnzava, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania.
Klaus Reither, Department of Medicine, Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland.
Fiona Vanobberghen, Department of Medicine, Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland.
Jerry Hella, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania.
Daniel H Paris, Department of Medicine, Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland.
Maja Weisser, Biomedical Research and Clinical Trials Department, Ifakara Health Institute, Ifakara, Tanzania; Department of Medicine, Swiss Tropical and Public Health Institute, Allschwil, Switzerland; University of Basel, Basel, Switzerland; Division of Infectious Diseases and Hospital Epidemiology, University Hospital, Basel, Basel, Switzerland.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. R. N., M. R., M. W., K. R., D.H.P, and F. V. contributed to the conceptualization of the study. R. N., M. W., J. O., and F. V. contributed to data analysis. R. N. wrote the first draft of the manuscript. F. B., O. N., M. S., D. M., D.H.P, and J. H. contributed in the study design and reviewed the manuscript. All authors read and approved the final version of the manuscript.
Patient consent. The main trial was registered under the Pan African Clinical Trials registry (PACTR201712002829221) and obtained appropriate ethical approvals. Written informed consent was sought from patients before recruitment [7]. No additional consent was required for this analysis. Permission to publish was obtained from the Director General of the National Institute for Medical Research.
Financial support. The authors did not receive any financial support for this study. The main trial was funded by the Rudolf Geigy Foundation and the Gottfried and Julia Bangerter-Rhyner Foundation, Basel, Switzerland.
References
- 1. World Health Organization . Global tuberculosis report 2023. Geneva, Switzerland: World Health Organization; 2023. [Google Scholar]
- 2. Zurcher K, Ballif M, Kiertiburanakul S, et al. Diagnosis and clinical outcomes of extrapulmonary tuberculosis in antiretroviral therapy programmes in low- and middle-income countries: a multicohort study. J Int AIDS Soc 2019; 22:e25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chakravorty S, Simmons AM, Rowneki M, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 2017; 8:e00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kohli M, Schiller I, Dendukuri N, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2021; 1:CD012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rindi L. Rapid molecular diagnosis of extra-pulmonary tuberculosis by Xpert/RIF Ultra. Front Microbiol 2022; 13:817661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broger T, Koeppel L, Huerga H, et al. Diagnostic yield of urine lipoarabinomannan and sputum tuberculosis tests in people living with HIV: a systematic review and meta-analysis of individual participant data. Lancet Global Health 2023; 11:e903–16. [DOI] [PubMed] [Google Scholar]
- 7. Ndege R, Ngome O, Bani F, et al. Ultrasound in managing extrapulmonary tuberculosis: a randomized controlled two-center study. BMC Infect Dis 2020; 20:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ndege R, Ngome O, Vanobberghen F, et al. Ultrasononography in managing extrapulmonary tuberculosis: a randomized, controlled, parallel, superiority, open-label trial. Clin Infect Dis 2023; 76:1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cresswell FV, Ellis J, Kagimu E, et al. Standardized urine-based tuberculosis (TB) screening with TB-lipoarabinomannan and Xpert MTB/RIF Ultra in Ugandan adults with advanced human immunodeficiency virus disease and suspected meningitis. Open Forum Infect Dis 2020; 7:ofaa100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andama A, Jaganath D, Crowder R, et al. Accuracy and incremental yield of urine Xpert MTB/RIF Ultra versus determine TB-LAM for diagnosis of pulmonary tuberculosis. Diagn Microbiol Infect Dis 2020; 96:114892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV: policy update 2019. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 12. Lawn SD, Kerkhoff AD, Burton R, et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC Med 2015; 13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutschke A, Steiniche D, Jespersen S, et al. Xpert MTB/RIF on urine samples to increase diagnosis of TB in people living with HIV in Guinea-Bissau. Int J Infect Dis 2022; 124(Suppl 1):S63–8. [DOI] [PubMed] [Google Scholar]
- 14. Lissouba P, Rücker SCM, Otieno LA, et al. Experiences and perceptions of urine sampling for tuberculosis testing among HIV patients: a multisite qualitative descriptive study. BMJ Open 2023; 13:e058805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.