Abstract
Cold injury is a major environmental stress affecting the growth and yield of crops. Brassinosteroids (BRs) and salicylic acid (SA) play important roles in plant cold tolerance. However, whether or how BR signaling interacts with the SA signaling pathway in response to cold stress is still unknown. Here, we identified an SA methyltransferase, TaSAMT1 that converts SA to methyl SA (MeSA) and confers freezing tolerance in wheat (Triticum aestivum). TaSAMT1 overexpression greatly enhanced wheat freezing tolerance, with plants accumulating more MeSA and less SA, whereas Tasamt1 knockout lines were sensitive to freezing stress and accumulated less MeSA and more SA. Spraying plants with MeSA conferred freezing tolerance to Tasamt1 mutants, but SA did not. We revealed that BRASSINAZOLE-RESISTANT 1 (TaBZR1) directly binds to the TaSAMT1 promoter and induces its transcription. Moreover, TaBZR1 interacts with the histone acetyltransferase TaHAG1, which potentiates TaSAMT1 expression via increased histone acetylation and modulates the SA pathway during freezing stress. Additionally, overexpression of TaBZR1 or TaHAG1 altered TaSAMT1 expression and improved freezing tolerance. Our results demonstrate a key regulatory node that connects the BR and SA pathways in the plant cold stress response. The regulatory factors or genes identified could be effective targets for the genetic improvement of freezing tolerance in crops.
Transcription factor TaBZR1 directly activates methyltransferase-encoding TaSAMT1-B under freezing stress by recruiting a histone acetyltransferase and increasing its histone H3 acetylation.
Introduction
Cold stress severely limits crop quality and productivity. As one of the most important staple crops worldwide, bread wheat (T. aestivum) provides approximately 20% of the daily dietary calories and protein (Shewry and Hey 2015; FAOSTAT 2020). As with other crops, climate-driven temperature extremes impair the vegetative and reproductive growth of wheat, resulting in lower yield (Thakur et al. 2010; Frederiks et al. 2015). Periods of cold are common in wheat-growing regions throughout the world, including Australia, China, Europe, and the United States (Holman et al. 2011; Trnka et al. 2014; Zheng et al. 2015; Crimp et al. 2016; Jiang et al. 2022). Therefore, understanding the molecular mechanisms underlying cold tolerance has become a high priority for research and wheat breeding programs.
Phytohormones influence diverse events associated with plant growth and tolerance under cold stress by dynamically modulating physiological, biochemical, and molecular responses through stress-responsive regulatory cascades (Eremina et al. 2016a; Raza et al. 2023). Abscisic acid (ABA), brassinosteroid (BR), ethylene, jasmonic acid (JA), and gibberellic acid (GA) signaling play important roles in regulating plant freezing tolerance via pathways that depend on or are independent of the C-REPEAT BINDING FACTOR (CBF) transcription factors (Hu et al. 2013; Venzhik et al. 2016; Li et al. 2017; Ye et al. 2019; Lantzouni et al. 2020; Yu et al. 2020; Huang et al. 2023).
In addition, molecular and physiological responses to cold stress are intricately linked to the regulation of phytohormones (Verma et al. 2016; Waadt et al. 2022). Low temperatures influence phytohormone pathways by activating or suppressing key molecular hubs in plant hormone biosynthesis, homeostasis, signaling, and downstream responses (Ding and Yang 2022). These regulatory steps typically depend on transcriptional, post-transcriptional, and posttranslational mechanisms, some of which have been directly connected to canonical CBF signaling (Ye et al. 2019; Jiang et al. 2020; Castroverde and Dina 2021). Moreover, crosstalk among plant hormones is important for the transcriptional activation of downstream genes essential for plant development and responses to stress. For example, JASMONATE-ZIM DOMAIN PROTEINs (JAZs) interact with ETHYLENE-INSENSITIVE3 (EIN3) and the related EIN3-LIKE1 (EIL1) to mediate JA, ethylene, and CBF signaling (Hu et al. 2013; Li et al. 2012; Shi et al. 2012, 2015). Likewise, regulation of DELLAs by PHYTOCHROME-INTERACTING FACTOR4 (PIF4)–BRASSINAZOLE-RESISTANT 1 (BZR1) modulates GA, BR, and CBF signaling pathways (Achard et al. 2008; Oh et al. 2012; Shi et al. 2015; Wang et al. 2020). However, the mechanisms underlying the crosstalk among different phytohormone signaling cascades remain to be investigated in the regulation of freezing tolerance.
Salicylic acid (SA), a small-molecule plant hormone that regulates plant development and plant responses to pathogen attack, also appears to be involved in cold tolerance (Siboza et al. 2014). Exogenous SA treatment mitigates freezing injury in the leaves of maize (Zea mays) and wheat plants grown under low-temperature conditions (Kang and Saltveit 2002; Taşgín et al. 2003). Treatment with SA also diminished cold injury symptoms in several horticultural crops (Ding et al. 2002; Wang et al. 2006; Sayyari et al. 2009). Notably, high concentrations of SA and the continuous application of SA impose severe growth damage and decreased cold tolerance capacity, suggesting that timely application of SA may enhance cold tolerance, whereas continuous SA application may lead to the opposite effect (Scott et al. 2004; Miura and Tada 2014; Nadarajah et al. 2021). Moreover, methyl SA (MeSA) enhances cold tolerance in various species, including Arabidopsis (Arabidopsis thaliana), tomato (Solanum lycopersicum), wheat, and several fruit trees (Sayyari et al. 2011; Zhang et al. 2011; Siboza et al. 2014; Habibi et al. 2019).
SA was initially postulated to be a mobile signal in defense signaling pathways and required for systemic acquired resistance (SAR) (Gaffney et al. 1993; Durrant and Dong 2004), despite grafting studies suggesting that SA maybe not be a translocated mobile signal during SAR (Vernooij et al. 1994). Recent reports have shown that translocation of SA from primary infected tissue to distal tissues likely occurs via the apoplast and is regulated by leaf transpiration (Lim et al. 2016, 2020). MeSA is a volatile form of SA but can be also transported (Attaran et al. 2009). Intriguingly, MeSA also acts as a critical mobile signal and is assembled via the phloem to stimulate SAR (Park et al. 2007; Klessig et al. 2018). SA is converted to MeSA by the activity of carboxyl methyltransferase (SAMT) enzymes (Dudareva et al. 1998). In Arabidopsis, this enzyme is BSMT1 (also named S-ADENOSYL-L-METHIONINE-DEPENDENT METHYLTRANSFERASE1 [SAMT1]), which uses SA as substrate and forms MeSA (Chen et al. 2003; Liu et al. 2010). MeSA was also proposed as a signal molecule in plant responses to abiotic stress, as it improved plant tolerance against chilling injury (Seydpour and Sayyari 2016; Ren et al. 2020; Gondor et al. 2022). However, the function and the regulatory mechanisms of SAMT expression during the cold stress response are still largely unknown.
BR signaling also regulates plant cold tolerance (Li et al. 2017; Fang et al. 2019). After BR perception at the plasma membrane by the receptors BR INSENSITIVE 1 (BRI1) and brassinosteroid-associated kinase 1 (BAK1, also named SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE 3 [SERK3]), a well-established cascade relays BR signals to members of the BRI1-EMS-SUPPRESSOR 1 (BES1) and BZR1 family of transcription factors, which control BR-regulated gene expression (Nolan et al. 2020). BRs promote plant cold tolerance via governing different cold-responsive transcriptional cascades through translational and post-translational modifications of key regulators (Eremina et al. 2016b). For example, active BR signaling leads to the accumulation of the active unphosphorylated form of BZR1, modulating plant freezing tolerance through CBF-dependent and -independent pathways that positively regulate cold stress responses (Li et al. 2017).
BRs integrate multiple phytohormone and environmental inputs, eventually leading to the activation or repression of BR-regulated genes. Extensive research in recent years has helped characterize the key points of interaction between BRs and other phytohormones or the signaling components regulating stress responses in plants (Keuskamp et al. 2011; Oh et al. 2012; Bernardo-García et al. 2014). However, whether or how the BR signaling pathway interacts synergistically with the SA signaling pathway to establish or modulate plant cold tolerance remains unknown.
Regulation of gene expression in response to cold stress often employs posttranslational histone modifications (To et al. 2011; Yuan et al. 2013; Kim et al. 2015; Ding et al. 2019). Furthermore, several studies have indicated that epigenetic modifications also have critical roles in coordinating environmental and BR signals (Yu et al. 2008; Sui et al. 2012; Zhang et al. 2014). However, a direct demonstration of a function for epigenetic regulators in establishing crosstalk with the BR and SA signaling pathways to confer cold tolerance is still elusive.
In this study, we report that the methyltransferase TaSAMT1 plays a pivotal role in freezing tolerance by promoting SA conversion to MeSA in wheat. Moreover, we demonstrate that TaBZR1, a master regulator of the BR signaling pathway, interacts with histone acetyltransferase of the GNAT family 1 (TaHAG1) to potentiate TaSAMT1 transcription by increasing the levels of histone acetylation under cold stress, thus playing key roles in the freezing tolerance of wheat. These findings provide an in-depth understanding of cold stress tolerance in wheat and reveal a key regulatory module integrating the BR and SA signaling pathways, providing effective targets for breeding cold-tolerant wheat varieties.
Results
TaSAMT1 is a salicylate carboxyl methyltransferase in wheat
In a previous whole-transcriptome analysis of cold stress performed in wheat, the transcript levels of TraesCS3B02G445100 increased upon a cold treatment of 4°C compared to 22°C (Supplementary Fig. S1) and were induced by exposure to low temperatures (Li et al. 2015). Sequence analysis of the encoded protein revealed that this gene encodes a salicylate carboxyl methyltransferase, a putative ortholog of SAMTs from barley (Hordeum vulgare), purple false brome (Brachypodium distachyon), and rice (Oryza sativa); we thus named TraesCS3B02G445100 TaSAMT1 (Supplementary Fig. S2). A BLAST search against the genomes of the wheat varieties Chinese Spring, Fielder, and others identified three TaSAMT1 homoeologs in hexaploid wheat, designated as TaSAMT1-A, TaSAMT1-B, and TaSAMT1-D, according to their respective subgenomes (Walkowiak et al. 2020; Sato et al. 2021).
Intriguingly, the three TaSAMT1s encoded by these homoeologs showed substantial variation in sequence, with TaSAMT1-A consisting of a truncated peptide of only 130 amino acids (aa), TaSAMT1-B (encoded by TraesCS3B02G445100) being a 356-aa protein with a complete carboxyl methyltransferase domain, and TaSAMT1-D comprising 240 aa and lacking the N terminus present in the other two TaSAMT1s (Supplementary Fig. S3). In addition, a protein structure prediction analysis suggested that only TaSAMT1-B harbors a complete catalytic domain (Supplementary Fig. S3), prompting us to choose TaSAMT1-B for analysis of its role in cold responses. TaSAMT1-B clustered together with HvSAMT1, BdSAMT1, and OsSAMT1, with which it showed 90.45%, 63.1%, and 54.1% amino acid identity, respectively (Supplementary Fig. S2). We detected a complete SAM-dependent carboxyl methyltransferase domain in TaSAMT1-B and SAMT1s from the three other grasses, suggesting that TaSAMT1-B may play a conserved role in methylation (Supplementary Fig. S2).
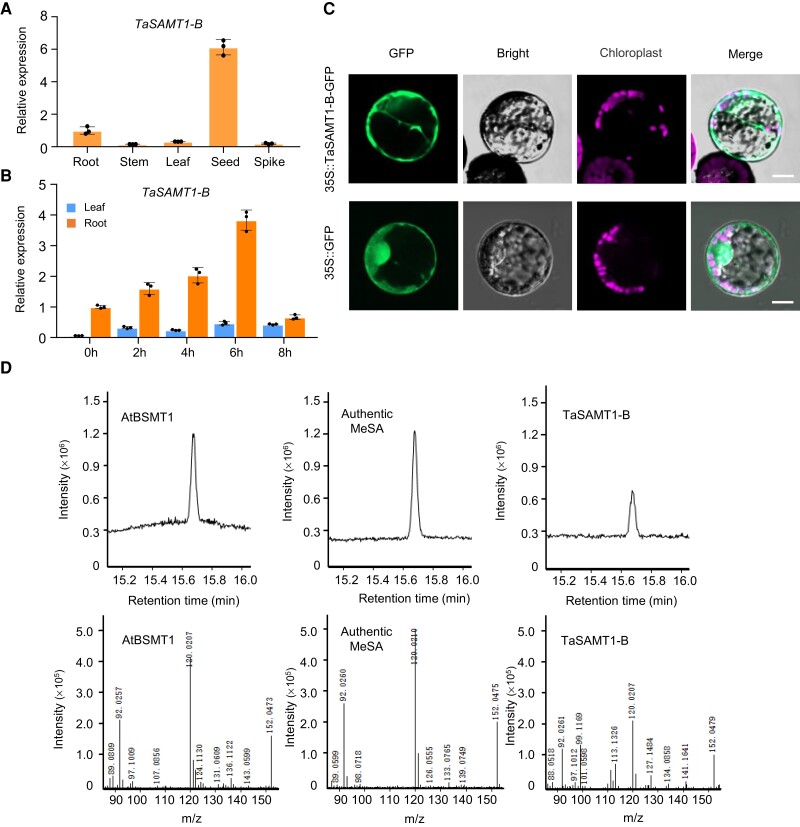
TaSAMT1 expression was highest in seeds, followed by roots, with low and similar levels in stems, leaves, and spikes (Fig. 1A). We also examined the response of TaSAMT1 transcript levels to low-temperature stress by exposing seedlings to −10°C for up to 8 h. TaSAMT1 expression was quickly induced in leaves and roots by freezing stress (Fig. 1B). To determine the subcellular localization of TaSAMT1, we transfected wheat protoplasts with a construct encoding a TaSAMT1-B-GFP fusion protein between TaSAMT1-B and green fluorescent protein (GFP). We detected green fluorescence signals mainly in the cytoplasm, suggesting that TaSAMT1-B is a cytosolic protein (Fig. 1C).
Figure 1.
TaSAMT1 expression pattern and enzyme activity of the encoded protein. A) Relative TaSAMT1 transcript levels in the indicated tissues, as determined by RT-qPCR. B) Relative TaSAMT1 transcript levels in response to treatment at −10°C for up to 8 h. Data were normalized to β-ACTIN and are shown as means ± standard deviation (SD, n = 3). C) Subcellular localization of TaSAMT1 in wheat leaf protoplasts. TaSAMT1-B (fused to GFP) localizes to the cytoplasm. Scale bars, 10 μm. D) Conversion of salicylic acid (SA) to methyl salicylate (MeSA) by TaSAMT1-B. Each enzymatic reaction was performed using SA as a substrate in the presence of S-adenosyl-L-methionine (SAM). Top, partial GC chromatograms of the MeSA standard and the reaction products from cell lysates containing AtBSMT1 or TaSAMT1, as analyzed by GC-MS; bottom, GC mass spectra of the authentic MeSA standard and the enzymatic products from cell lysates containing TaSAMT1.
To test whether TaSAMT1 has enzymatic function, we produced and purified recombinant TaSAMT1-B fused to glutathione S-transferase (GST) in Escherichia coli, together with GST alone, TaSAMT1-A-GST, and TaSAMT1-D-GST, using AtBSMT1-GST as a positive control (Chen et al. 2003; Supplementary Fig. S4A). We then incubated crude bacterial protein extracts in the presence of SA to test methylation activity, revealing efficient catalysis of SA methylation with S-adenosyl-L-methionine (SAM) as a methyl donor for the cell lysates containing TaSAMT1-B or AtBSMT1 (Fig. 1D), but not those with TaSAMT1-A or TaSAMT1-D (Supplementary Fig. S4B), consistent with the absence of a complete carboxyl methyltransferase domain for these latter proteins (Supplementary Fig. S3). These results suggest that TaSAMT1-B converts SA to MeSA and contributes to the regulation of the cold response in wheat.
Overexpression and knockout of TaSAMT1-B validate its role in freezing tolerance
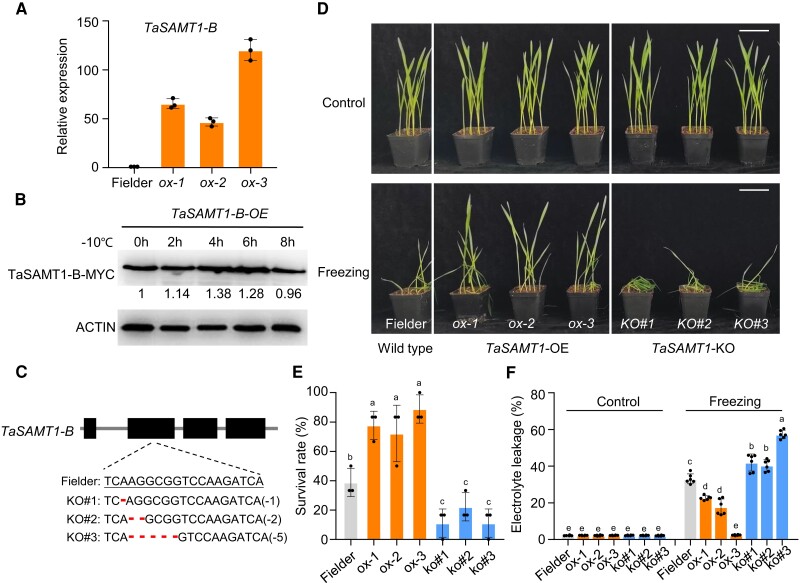
To investigate the role of TaSAMT1 in the cold tolerance of wheat, we generated transgenic wheat lines overexpressing TaSAMT1-B-MYC (TaSAMT1-B-OE). We obtained 11 putative TaSAMT1-B overexpression lines in the wheat cultivar Fielder and chose three independent TaSAMT1-B-OE lines (ox1, ox2, and ox3) with significantly elevated TaSAMT1-B transcript abundance for characterization (Fig. 2A). Immunoblot analysis with an anti-MYC antibody showed that TaSAMT1-B accumulates in the TaSAMT1-B-OE plants (Fig. 2B).
Figure 2.
TaSAMT1 improves freezing tolerance in wheat. A) Relative TaSAMT1-B transcript levels in wild-type (Fielder) and TaSAMT1-OE (ox-1, ox-2, and ox-3) plants. Data are shown as means ± SD (n = 3). B) Immunoblot analysis of TaSAMT1-B-MYC in TaSAMT1-OE lines under freezing stress. Ten-day-old seedlings grown in normal conditions were exposed to −10°C for 2, 4, 6, or 8 h, or collected prior to cold treatment (0 h). Total proteins were extracted and subjected to immunoblot analysis with anti-MYC antibodies. C)TaSAMT1-B sequences in the wild type (Fielder) and gene-edited mutants. The targeted sequence is underlined, and deleted nucleotides are indicated by red hyphens. The numbers to the right represent the number of deleted base pairs in each mutant. D) Ten-day-old wild-type Fielder, TaSAMT1-OE (ox-1, ox-2, and ox-3), and Tasamt1-B knockout (KO#1, KO#2, and KO#3) lines treated at −10°C for 1.5 h, followed by recovery for 24 h at 22°C. Representative photographs are shown. Scale bars, 5 cm. E) Survival rates of wild-type, TaSAMT1-OE, and Tasamt1-B lines after freezing treatment. Data are shown as means ± SD derived from measurements of at least 15 plants per genotype in three independent experiments. F) Electrolyte leakage of the wild-type Fielder, TaSAMT1-OE lines, and Tasamt1-B lines in control conditions and after freezing treatment. Data are shown as means ± SD (n = 6). The data in (E) and (F) were analyzed by one-way ANOVA followed by Tukey's test. Different lowercase letters indicate a significant difference (P < 0.05).
We also generated Tasamt1 mutants in Fielder using clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 9 (Cas9)-mediated gene editing. We designed a single guide RNA (sgRNA) targeting a specific region in the second exon of TaSAMT1-B, since only TaSAMT1-B contains a complete carboxyl methyltransferase domain. We obtained three null mutants of TaSAMT1-B (KO#1, with a 1-bp deletion; KO#2, with a 2-bp deletion; and KO#3, with a 5-bp deletion in TaSAMT1-B) (Fig. 2C). To assess the performance of the TaSAMT1-B-OE and Tasamt1 mutant lines under freezing stress conditions, we exposed 10-day-old seedlings (after germination) to −10°C for 1.5 h before returning them to normal conditions for recovery (Fig. 2D). When we scored the survival rates (SR) of all seedlings 24 h later, the TaSAMT1-B-OE plants had significantly higher SRs (83.3% in ox1, 75.0% in ox2, and 87.5% in ox3) than wild-type seedlings (∼33%) (Fig. 2, D and E). Conversely, when subjected to freezing stress, the SRs of the Tasamt1 lines (8.3% in KO#1, 16.7% in KO#2, and 15.3% in KO#3) were significantly lower than that of wild-type plants (Fig. 2, D and E).
Cell membrane stability and integrity under temperature stress are important indicators of cold tolerance in plants. Therefore, we examined changes in the relative electrolyte leakage (REL) of the TaSAMT1-B-OE and Tasamt1 lines. We observed no clear difference in REL between the wild-type, TaSAMT1-B-OE, and Tasamt1 lines when grown at 22°C (Fig. 2F). However, after exposure to freezing treatment (−10°C for 1.5 h), the REL of all TaSAMT1-B-OE lines was significantly lower, while that of the Tasamt1 lines was higher, compared to the wild type (Fig. 2F). Taken together, these results indicate that TaSAMT1-B is a positive regulator of cold tolerance in wheat.
TaSAMT1-B affects MeSA and SA accumulation under freezing stress
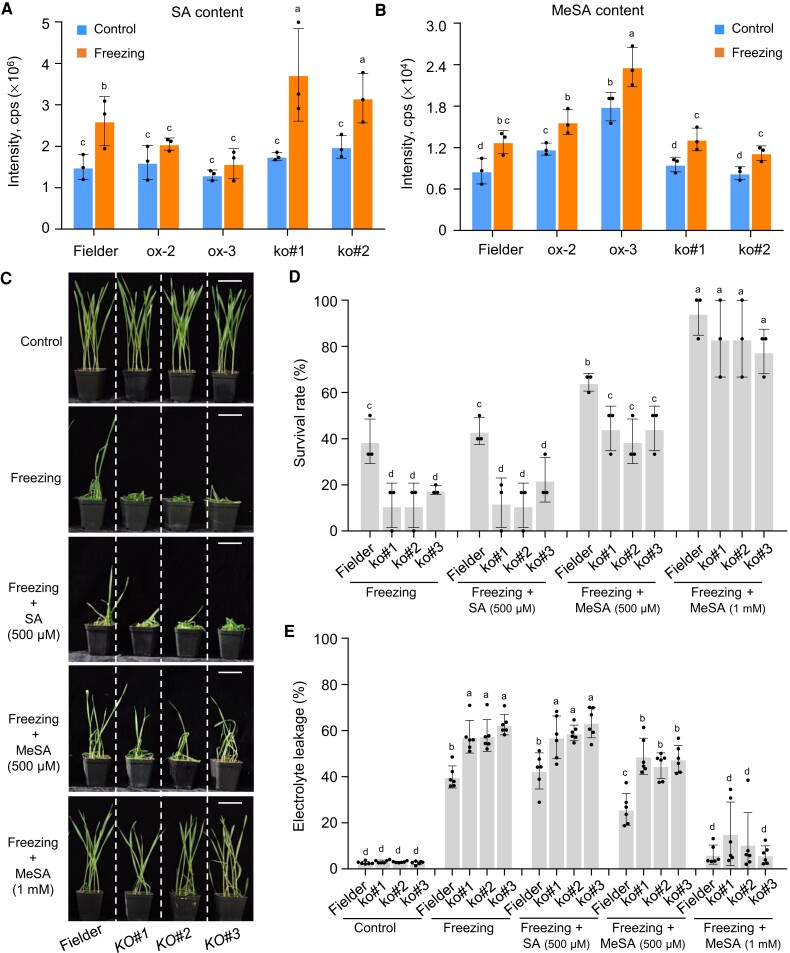
Multiple studies have proposed that MeSA may be an important messenger that plays a signaling role in regulating stress response and alleviating cold injury symptoms in crops (Park et al. 2007; Zhang et al. 2011; Siboza et al. 2014; Habibi et al. 2019). To investigate whether TaSAMT1-B affects endogenous MeSA levels to modulate tolerance under freezing stress, we measured the endogenous SA and MeSA levels in the wild type together with different TaSAMT1-B-OE and Tasamt1 lines experiencing freezing stress. We established that MeSA accumulates to significantly higher levels in the TaSAMT1-B-OE lines relative to wild-type plants under both control and freezing conditions, while SA levels did not significantly increase upon exposure to freezing stress, in contrast to the wild type (Fig. 3, A and B). Consistent with this finding, MeSA levels were substantially lower and SA contents higher in Tasamt1-B lines exposed to freezing stress compared to wild-type plants (Fig. 3, A and B), demonstrating that TaSAMT1 is a major SA methyltransferase that converts SA to MeSA in response to freezing stress.
Figure 3.
TaSAMT1 affects SA and MeSA accumulation under freezing stress. A, B) The content of endogenous SA (A) and MeSA (B) levels in wild-type Fielder, TaSAMT1-OE lines (ox-2 and ox-3), and Tasamt1-B lines (KO#1 and KO#2) under control and freezing treatment. C) Ten-day-old wild-type and Tasamt1-B (KO#1, KO#2, and KO#3) lines were sprayed with SA (500 μM) or MeSA (500 μM, 1 mM) before being exposed to −10°C for 1.5 h, followed by recovery for 24 h. Representative photographs are shown. Scale bars, 5 cm. D) Survival rates of the wild-type and Tasamt1-B lines after freezing treatment alone or together with spraying of 500 μM SA, 500 μM MeSA, or 1 mM MeSA. Data are shown as means ± SD derived from measurements of at least 15 plants per genotype in three independent experiments. E) Electrolyte leakage of the wild-type and Tasamt1-B lines in control and freezing conditions alone or together with spraying of 500 μM SA, 500 μM MeSA, or 1 mM MeSA. Data are shown as means ± SD (n = 6). The data in (D) and (E) were analyzed by one-way ANOVA followed by Tukey's test. Different lowercase letters indicate a significant difference (P < 0.05).
Exogenous MeSA application rescues the compromised freezing tolerance of the Tasamt1 mutant
To determine whether TaSAMT1 modulates freezing tolerance via MeSA accumulation, we assessed the effect of exogenous MeSA application on the freezing tolerance of Tasamt1 mutants and wild-type plants (Fig. 3C). To this end, we measured SRs and cell membrane stability in the wild-type Fielder and Tasamt1 lines exposed to freezing conditions alone or together with a prior spray of SA or MeSA at different concentrations (Fig. 3, D and E). When 500 μM MeSA was applied before freezing treatment, the SR and cell membrane stability of both wild-type and Tasamt1 plants significantly improved over those seen following freezing stress treatment alone (Fig. 3, C to E). This response was dosage dependent, as an application of 1 mM MeSA fully rescued the SR and cell membrane stability defects seen in the Tasamt1 mutants following freezing treatment, and comparable to wild-type Fielder under freezing stress (Fig. 3, C to E). This finding is consistent with a role for TaSAMT1 in freezing tolerance that is dependent on MeSA accumulation.
Notably, an exogenous application of 500 µM SA before freezing stress treatment resulted in no significant effect in SR or cell membrane stability of either wild-type or Tasamt1 plants relative to plants experiencing freezing stress alone (Fig. 3, C to E). These results suggest that MeSA, but not SA, plays a vital role in freezing tolerance in wheat. Together, these results strongly indicate that TaSAMT1 is indeed involved in freezing tolerance through catalyzing SA methylation to produce MeSA.
TaBZR1 directly binds to the promoter of TaSAMT1
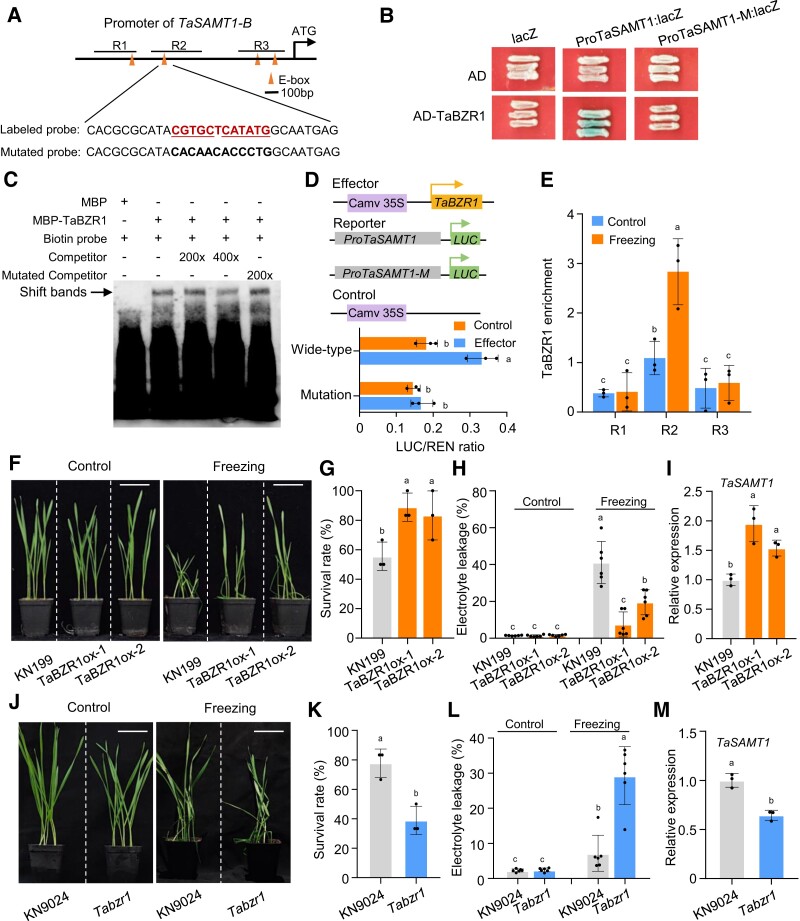
To investigate the molecular mechanism by which cold stress triggers TaSAMT1 expression, we performed a yeast one-hybrid screen using the TaSAMT1-B promoter as a bait and cDNA prepared from total wheat RNA extracted from leaves and roots as a library. We thus identified 91 potential upstream regulators of TaSAMT1-B transcription, based on their ability to bind to the TaSAMT1-B promoter (Supplementary Data Set 1). Among them, we noticed a putative BZR1 family member encoded by TraesCS2A02G187800, which we confirmed as binding to the TaSAMT1-B promoter in directed yeast one-hybrid assays (Fig. 4, A and B). Sequence alignment indicated that the protein encoded by TraesCS2A02G187800 contains a basic helix–loop–helix (bHLH)-like DNA-binding domain and shares the highest sequence identity with Os07g0580500 and AtBZR1; we thus named this protein TaBZR1 (Supplementary Fig. S5, A and B).
Figure 4.
TaBZR1 directly regulates TaSAMT1 transcription and contributes to wheat freezing tolerance. A) Diagram of the TaSAMT1-B promoter showing the positions of predicted TaBZR1-binding motifs and the sequences of the labeled probe (red font indicates E-box) and mutated probes used for electrophoretic mobility shift assay (EMSA). B) Yeast one-hybrid assays showing the binding of TaBZR1 to the TaSAMT1-B promoter. pLACZi2μ co-transformed with pB42AD was used as negative control. C) EMSA showing the binding of TaBZR1 to the TaSAMT1-B promoter. Biotin-labeled fragments representing parts of the TaSAMT1-B promoter containing the E-box were incubated with recombinant MBP or MBP-TaBZR1. Competitor or mutated competitor probes were added in 200× or 400× excess to analyze the specificity of binding. D) Transactivation of the TaSAMT1 promoter by TaBZR1 in wheat protoplasts. The two reporters represent wild-type (proTaSAMT1) and mutated (proTaSAMT1-M) 1-kb TaSAMT1 promoter fragments. LUC, firefly luciferase; REN, Renilla luciferase. Data are shown as means ± SD (n = 3). E) Chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR) validation of TaBZR1-binding sites on the TaSAMT1-B promoter. Enrichment of the R1, R2, and R3 fragments of the TaSAMT1-B promoter was determined in chromatin precipitated with anti-BZR1 antibodies. F) Ten-day-old seedlings from the wild-type KN199 and TaBZR1-OE lines (ox-1 and ox-2) were exposed to −10°C for 1.5 h, then allowed to recover for 24 h. Representative photographs are shown. Scale bars, 5 cm. G) Survival rates of KN199 and TaBZR1-OE lines after freezing treatment. Data are shown as means ± SD derived from measurements of at least 15 plants for each genotype in three independent experiments. H) Electrolyte leakage of KN199 and TaBZR1-OE lines in control and freezing conditions. Data are shown as means ± SD (n = 6). I) Relative TaSAMT1-B transcript levels in KN199 and TaBZR1-OE plants. Data were normalized to wheat TaACTIN and shown as means ± SD (n = 3). J) Ten-day-old wild-type KN9204 and Tabzr1 mutant seedlings were exposed to −10°C for 1.5 h, then allowed to recover for 24 h. Representative photographs are shown. Scale bars, 5 cm. K) Survival rates of KN9204 and Tabzr1 after freezing treatment. Data are shown as means ± SD derived from measurements of at least 15 plants per genotype in three independent experiments. L) Electrolyte leakage of KN9204 and Tabzr1 in control and freezing conditions. Data are shown as means ± SD (n = 6). M) Relative TaSAMT1-B transcript levels in KN9204 and Tabzr1 plants. Data were normalized to TaACTIN and are shown as means ± SD (n = 3). The data in (G, H, I) and (K, L, M) were analyzed by one-way ANOVA followed by Tukey's test. Different lowercase letters indicate a significant difference (P < 0.05).
In addition, RT-qPCR and immunoblot analyses indicated that TaBZR1 transcript levels and TaBZR1 protein abundance are significantly enhanced in wheat under freezing stress, with an expression pattern for TaBZR1 similar to that of TaSAMT1 (Supplementary Fig. S6, A and B). As a master transcriptional regulator in the BR signaling pathway, BZR1 binds to BR response elements (BRREs, CGTG(T/C)) and E-box (CANNTG) motifs in the promoters of its target genes (Sun et al. 2010; Nolan et al. 2020). We thus analyzed the sequences of the TaSAMT1-B promoter (defined as up to 1 kb upstream from the ATG) and identified several putative E-box motifs (Fig. 4A). We found the TaBZR1 could directly bind to the promoter of TaSAMT1 region R2 containing E-box motif from −679 bp to −387 bp (Fig. 4B).
To further test the binding of TaBZR1 to the R2 region of the TaSAMT1-B promoter, we performed an electrophoretic mobility shift assay (EMSA) using recombinant purified TaBZR1 fused to maltose-binding protein (MBP) and a labeled probe with an E-box motif from the TaSAMT1 promoter (−614 to −584 bp, in the center of the R2 region above). We determined that recombinant MBP-TaBZR1 binds to the labeled probe but not to a mutated version of the probe that abolishes the E-box motif (Fig. 4C). Y1H assays also showed no binding between a mutated version of the TaSAMT1-B promoter lacking the E-box motif with the R2 region and TaBZR1 (Fig. 4B). We also performed firefly luciferase (LUC) activity assays to examine the transactivation capacity of TaBZR1 toward the TaSAMT1-B promoter. To this end, we placed the LUC reporter gene under the control of the TaSAMT1-B promoter (1 kb upstream from the ATG, as above) and transfected the resulting proTaSAMT1-B:LUC construct into wheat protoplasts. LUC activity derived from the proTaSAMT1 construct increased significantly when co-transfected with an effector construct expressing TaBZR1, compared to the empty vector control (Fig. 4D). However, disruption of the E-box abolished TaBZR1-mediated transactivation of the TaSAMT1 promoter (Fig. 4D), indicating that TaBZR1 positively regulates TaSAMT1 transcription. Moreover, chromatin immunoprecipitation followed by qPCR (ChIP-qPCR) with an anti-BZR1 antibody confirmed that TaBZR1 binds directly to the E-box motif-containing segments within the TaSAMT1-B promoter regions. Specifically, freezing stress treatment enhanced the enrichment of the R2 region containing the E-box motif from the TaSAMT1-B promoter in the precipitated chromatin, in line with TaSAMT1-B elevated transcriptional activation compared to mock treatment (Fig. 4E). Together, these results indicate that TaSAMT1-B may be an important TaBZR1 target for freezing tolerance, thus connecting the BR and SA pathways.
TaBZR1 contributes to freezing tolerance in wheat
To investigate the possible role of TaBZR1 in freezing tolerance in wheat, we obtained TaBZR1 overexpression lines in wheat (TaBZR1-OE) (Yang et al. 2023). RT-qPCR analysis showed significantly higher TaBZR1 transcript levels in the TaBZR1-OE lines compared with wild-type plants (Supplementary Fig. S6C). Moreover, we identified a Tabzr1 mutant by screening a mutant library in the KN9204 (a wheat variety) background. The mutant carries a G-to-A transition in the second exon of TraesCS2A02G187800, resulting in the introduction of a premature translation termination codon (Supplementary Fig. S7A). Immunoblot analysis with an anti-BZR1 antibody revealed a 50% decrease in TaBZR1 abundance in the Tabzr1 mutant compared to the wild type (Supplementary Fig. S7B).
As with the TaSAMT1-B-OE and Tasamt1-B lines above, we assessed the performance of the TaBZR1-OE and Tabzr1 mutant lines under freezing stress conditions (Fig. 4, F and J). When subjected to freezing treatment, the SR was significantly higher in TaBZR1-OE lines and lower in the Tabzr1 mutant relative to the wild type (Fig. 4, G and K). While there was no clear difference in REL among the wild-type, TaBZR1-OE, and Tabzr1 plants when grown at 22°C (Fig. 4, H and L), the REL after freezing treatment decreased in the TaBZR1-OE lines but increased in the Tabzr1 mutant, compared to the wild type (Fig. 4, H and L). These results indicate that TaBZR1 participates in wheat freezing tolerance.
We assessed the relative TaSAMT1-B expression levels in the TaBZR1-OE lines and Tabzr1 mutant plants. TaSAMT1-B expression was upregulated in the TaBZR1-OE lines but downregulated in the Tabzr1 mutant compared to the wild type (Fig. 4, I and M). Accordingly, MeSA accumulated to significantly higher levels in TaBZR1-OE lines compared to wild-type plants exposed to freezing stress (Supplementary Fig. S8). These results are consistent with our hypothesis that TaBZR1 functions as a positive regulator of TaSAMT1-B expression and MeSA accumulation, leading to elevated freezing tolerance.
TaSAMT1-B is required for the role of the TaBZR1-mediated BR pathway in freezing tolerance
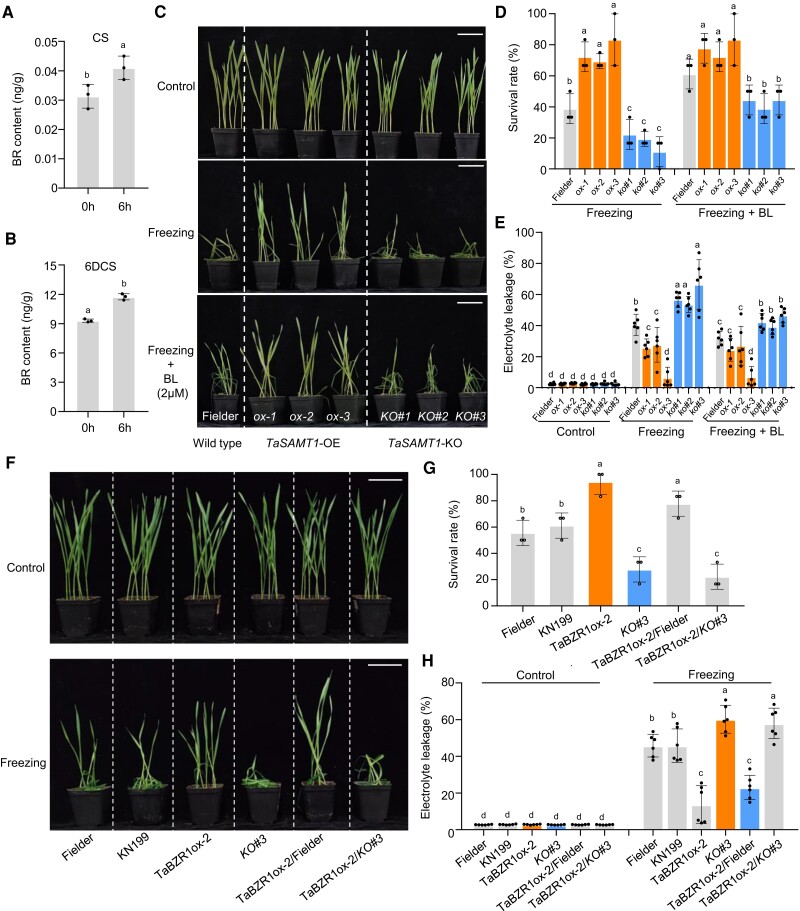
To check whether the BR pathway is involved in the cold response of wheat, we measured the endogenous levels of BRs in wheat seedlings under freezing stress. We detected higher levels of endogenous BRs, including castasterone and 6-deoxocastasterone, in wild-type seedlings under cold stress (Fig. 5, A and B). Moreover, relative TaBZR1 transcript levels were upregulated after BL treatment (Supplementary Fig. S9A), as were those of TaSAMT1 (Supplementary Fig. S9B). To further examine whether the BR signaling pathway regulates cold tolerance synergistically with the SA pathway via TaSAMT1 in wheat, we exposed wild-type, TaSAMT1-B-OE, and Tasamt1-B plants to freezing stress alone or following a prior spray with brassinolide (BL) (Fig. 5C).
Figure 5.
TaSAMT1-B is required for the TaBZR1-mediated involvement of the BR pathway in freezing tolerance. A, B) Levels of castasterone (CS) and 6-deoxocastasterone (6DCS) in wild-type Fielder under control and freezing conditions. Data are shown as means ± SD (n = 3 biologically independent samples). C) Ten-day-old wild-type Fielder, TaSAMT1-OE (ox-1, ox-2, and ox-3), and Tasamt1-B (KO#1, KO#2, and KO#3) lines sprayed with 2 μM brassinolide (BL), exposed to −10°C for 1.5 h, then allowed to recover for 24 h. Scale bars, 5 cm. D) Survival rates of wild-type Fielder, TaSAMT1-OE, and Tasamt1-B lines following freezing treatment alone or together with prior spraying with 2 μM BL. Data are shown as means ± SD derived from measurements of at least 15 plants per genotype in three independent experiments. E) Electrolyte leakage of the wild-type, TaSAMT1-OE, and Tasamt1-B lines after freezing treatment and/or spraying with 2 μM BL. Data are shown as means ± SD (n = 6). F) Phenotypes of Fielder, KN199, TaBZR1 ox-2, one Tasamt1-B line (KO#3), TaBZR1 ox-2 in Fielder, and TaBZR1ox-2 in Tasamt1-B KO#3 under freezing treatment (−10°C for 1.5 h, followed by recovery for 24 h). Representative photographs are shown. Scale bars, 5 cm. G) Survival rates of the indicated genotypes after freezing treatment. Data are shown as means ± SD derived from measurements of at least 15 plants per genotype in three independent experiments. H) Electrolyte leakage of the indicated genotypes in control and freezing conditions. Data are shown as means ± SD (n = 6). The data were analyzed by one-way ANOVA followed by an LSD test. Different lowercase letters indicate a significant difference (P < 0.05).
The effects of BL application on survival rate and cell membrane stability under cold stress were negligible in TaSAMT1-B-OE lines between freezing stress alone and combined freezing stress and BL treatment, consistent with the strong cold tolerance of these lines (Fig. 5, C to E). By contrast, an application of BL onto wild-type and Tasamt1-B seedlings before freezing stress treatment improved their survival rate and cell membrane stability compared to freezing stress treatment alone. Notably, the application of BL only partially alleviated the freezing sensitivity of Tasamt1-B seedlings and was not sufficient to return to wild-type levels (Fig. 5, C to E), suggesting the TaSAMT1-B functions downstream of the BR pathway and is important for BR-mediated freezing tolerance.
To test this hypothesis genetically, we crossed a TaBZR1-OE line (ox-2) to a Tasamt1-B mutant line (KO#3) and identified plants homozygous for the mutation of TaSAMT1-B and overexpressing TaBZR1. This Tasamt1-B TaBZR1-OE line shared the same decreased SR and cell membrane integrity phenotypes as the Tasamt1-B mutant under freezing treatment (Fig. 5, F to H), indicating that TaSAMT1-B functions downstream of TaBZR1 and modulates freezing tolerance. Collectively, these results suggest that TaSAMT1-B is required for the BR-regulated pathway and plays a vital role in freezing tolerance in wheat.
TaBZR1 interacts with the histone acetyltransferase TaHAG1
To explore the regulatory mechanisms of TaBZR1 in the transcriptional regulation of TaSAMT1-B, we performed a yeast two-hybrid screen using TaBZR1 as bait against a wheat cDNA library prepared from total RNA extracted from seedling leaves and roots of wheat. We identified 192 potential TaBZR1-interacting proteins (Supplementary Data Set 2). Among them, a putative plant histone acetyltransferase protein TaHAG1 (also named GENERAL CONTROL NONDEREPRESSIBLE 5 [GCN5]) encoded by TraesCS1D02G134200 caught our attention; we confirmed its interaction with TaBZR1 in a targeted yeast two-hybrid assay (Supplementary Fig. S10A).
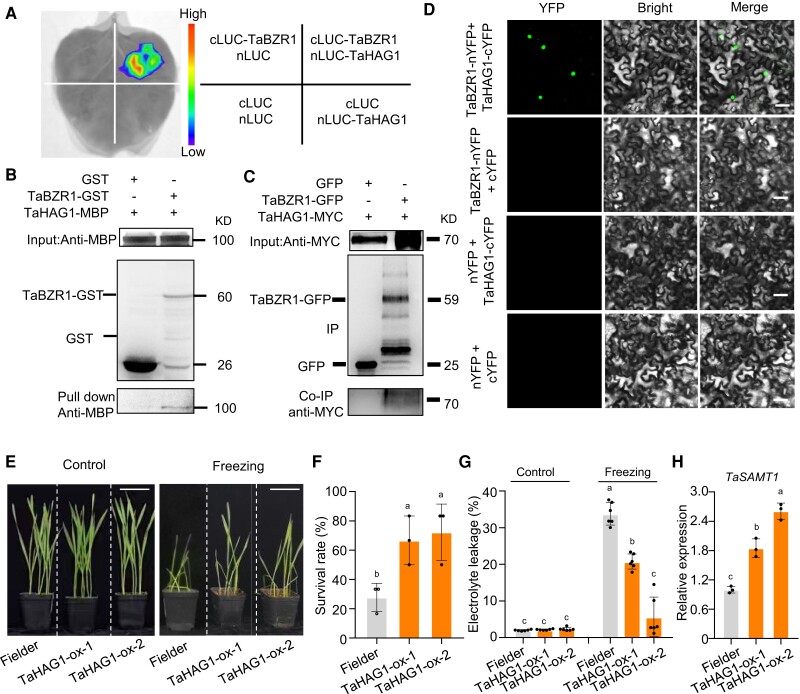
To corroborate the interaction between TaBZR1 and TaHAG1 in planta, we performed luciferase complementation imaging (LCI) assays, which showed that co-expression of cLUC-TaBZR1 and nLUC-TaHAG1 in the leaves of Nicotiana benthamiana plants yields strong luciferase activity; however, we detected no signal in leaves expressing the empty control constructs or when co-expressing cLUC-TaBZR1 with nLUC-TaSRT1 (SIRTUIN 1) or co-expressing nLUC-TaHAG1 with cLUC-TaDT-A (Drought Tolerant-A), although cLUC-TaDT-A interacts with nLUC-TaSRT1 (Fig. 6A and Supplementary Fig. S10B). GST pull-down assays also showed that TaBZR1-GST directly interacts with TaHAG1-MBP (Fig. 6B). Furthermore, we conducted co-immunoprecipitation (Co-IP) assays of protein extracts from N. benthamiana leaves expressing TaBZR1-GFP, TaHAG1-MYC, and the GFP alone via Agrobacterium (Agrobacterium tumefaciens)-mediated infiltration. Following an IP with an anti-GFP antibody, an immunoblot analysis with an anti-MYC antibody detected a band corresponding to TaHAG1-MYC in the samples co-expressing TaBZR1-GFP and TaHAG1-MYC, but not in those expressing TaHAG1-MYC and GFP (Fig. 6C). We then confirmed the TaBZR1–TaHAG1 interaction using bimolecular fluorescence complementation (BiFC) assays in which an excited YFP reconstitution signal was observed using a laser scanning confocal fluorescence microscope when the fusion constructs TaBZR1-nYFP and TaHAG1-cYFP were present, but not when those transformed with the corresponding control were present (Fig. 6D). Meanwhile, no interaction was detected between TaBZR1-nYFP and TaSRT1-cYFP or TaHAG1-cYFP and TaDT-A-nYFP, although TaSRT1 interacts with TaDT-A in nuclei (Supplementary Fig. S10C), suggesting that the TaBZR1–TaHAG1 interaction in vivo is specific. Collectively, these results demonstrate the TaBZR1–TaHAG1 interaction in vitro and in vivo, suggesting that TaHAG1 might participate in the TaBZR1 pathway under freezing stress in wheat.
Figure 6.
TaBZR1 physically interacts with histone acetyltransferase TaHAG1. A) Luciferase complementation imaging (LCI) assays. The construct encoding nLUC-tagged TaHAG1 was co-infiltrated into the leaves of N. benthamiana plants along with a construct encoding cLUC-targeted TaBZR1. B) MBP pull-down assay showing the interaction of recombinant TaHAG1-MBP with TaBZR1-GST. TaBZR1-GST was detected using anti-GST antibody. TaBZR1-GST was incubated with immobilized MBP or TaHAG1-MBP. Immunoprecipitated fractions were probed by immunoblotting with an anti-MBP and anti-GST antibody. Protein size (kD) is provided to the right of the gel. C) Co-immunoprecipitation (Co-IP) showing the physical interaction of TaBZR1 and TaHAG1. Protein extracts from the leaves of N. benthamiana plants (Input) and after IP with GFP-trap beads were probed by immunoblotting using an anti-MYC antibody. D) Bimolecular fluorescence complementation (BiFC) assay showing that TaBZR1 interacts with TaHAG1 in nuclei. Constructs encoding TaBZR1 and TaHAG1 fused to the N terminus or the C terminus of yellow fluorescent protein (YFP) were co-infiltrated into the leaves of N. benthamiana plants. Scale bars, 50 μm. E) Ten-day-old wild-type Fielder and TaHAG1-OE (ox-1 and ox-2) lines were exposed to −10°C for 1.5 h, followed by recovery for 24 h. Scale bars, 5 cm. F) Survival rates of the wild-type and TaHAG1-OE lines after freezing treatment. Data are shown as means ± SD derived from measurements of at least 15 plants per genotype in three independent experiments. G) Electrolyte leakage of the wild-type and TaHAG1-OE lines in control and freezing conditions. Data are shown as means ± SD (n = 6). H) Relative TaSAMT1 transcript levels in wild-type Fielder and TaHAG1-OE plants. Data were normalized to TaACTIN and are shown as means ± SD (n = 3). The data in (F), (G), and (H) were analyzed by one-way ANOVA followed by an LSD test. Different lowercase letters indicate a significant difference (P < 0.05).
TaHAG1 positively regulates freezing stress tolerance in wheat
To determine whether TaHAG1 responds to cold tolerance, we checked the expression pattern of TaHAG1 under freezing stress. RT-qPCR analysis showed that TaHAG1 transcript levels are upregulated by up to 50% under freezing stress relative to control conditions (Supplementary Fig. S11A). To characterize TaHAG1 function in the wheat response to freezing stress, we obtained TaHAG1 overexpression lines with significantly higher transcript levels compared to Fielder (Supplementary Fig. S11B; Zheng et al. 2021). Under freezing treatment, the SR of TaHAG1-OE lines was significantly higher than that of the wild type (Fig. 6, E and F). In addition, while the REL was comparable between TaHAG1-OE lines and wild-type seedlings grown under normal conditions, the REL values of the TaHAG1-OE lines were lower than that of the wild type following exposure to freezing conditions (Fig. 6G). These results indicate that TaHAG1 participates in the freezing tolerance of wheat.
TaHAG1 physically associates with the TaSAMT1 promoter and facilitates its transcription by modulating histone acetylation
The above findings that TaBZR1 targets and facilitates TaSAMT1-B transcription, together with the physical interaction of TaBZR1 and TaHAG1, prompted us to investigate whether TaSAMT1-B is a direct target of TaHAG1. To this end, we examined TaSAMT1-B transcripts levels in TaHAG1-OE and wild-type seedlings by RT-qPCR. TaSAMT1-B expression was upregulated in TaHAG1-OE lines compared to the wild type (Fig. 6H). Consistent with this observation, endogenous MeSA levels were significantly higher in TaHAG1-OE lines than in wild-type seedlings exposed to freezing stress conditions (Supplementary Fig. S12).
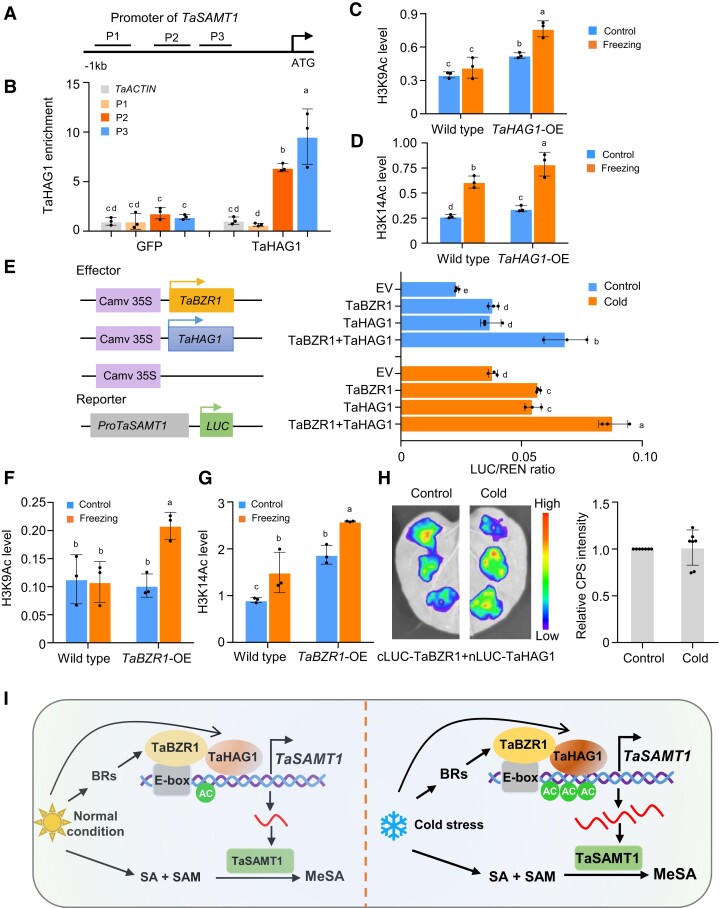
To determine whether TaHAG1 binds directly to the TaSAMT1-B promoter in vivo, we performed ChIP-qPCR using proUbi:TaHAG1-GFP seedlings. We detected a significant enrichment of the P2 and P3 fragments (−760 to −360 bp) of the TaSAMT1-B promoter in the chromatin precipitated with anti-GFP antibodies (Fig. 7, A and B). Since TaHAG1 is mainly involved in histone H3 lysine 9 and 14 acetylation in wheat (Zheng et al. 2021), we investigated the levels of H3K9 and H3K14 acetylation at the P3 fragment of TaSAMT1-B promoter. Accordingly, we performed ChIP-qPCR assays with antibodies against H3K9ac and H3K14ac using wild-type and TaHAG1-OE seedlings grown under control or freezing stress conditions. We observed low levels of the H3K9ac and/or H3K14ac modification over the TaSAMT1-B promoter in control wild-type seedlings, with significantly higher H3K14ac levels seen after freezing stress. Notably, H3K9ac and H3K14ac levels along the TaSAMT1-B promoter were significantly increased in TaHAG1-OE seedlings compared to wild-type seedlings under control and freezing stress conditions (Fig. 7, C and D).
Figure 7.
TaBZR1 and TaHAG1 cooperate to activate TaSAMT1-B transcription. A, B) ChIP-qPCR validation of TaHAG1-binding sites in the TaSAMT1-B promoter. Enrichment of the P1, P2, and P3 fragments of the TaSAMT1-B promoter was determined in chromatin precipitated with anti-GFP antibodies in TaHAG1-GFP lines. C, D) Levels of H3K9ac (C) and H3K14ac (D) modification along the TaSAMT1-B promoter region under control and freezing conditions. Data are shown as means ± SD (n = 3). E) Dual luciferase reporter assays in wheat protoplasts showing the ability of TaHAG1 and TaBZR1 to transactivate TaSAMT1-B promoter activity before and after freezing treatment (4°C in dark for 2 h). Left, diagrams of the effector and reporter constructs. Right, ratio of firefly luciferase (LUC) to Renilla luciferase (REN) indicating the transactivation ability of TaBZR1 and/or TaHAG1. Data are shown as means ± SD (n = 3). F, G) Enrichment of H3K9ac (F) and H3K14ac (G) in the TaSAMT1-B promoter region in wild-type KN199 and TaBZR1-OE lines under control and freezing conditions. Data are shown as means ± SD (n = 3). H) LCI assays. The construct encoding nLUC-tagged TaBZR1 was co-infiltrated into N. benthamiana leaves with a construct encoding cLUC-tagged TaHAG1 before and after cold treatment. Data are shown as means ± SD (n = 7). The relative luminescence values in control were set to 1. The data were analyzed by one-way ANOVA followed by an LSD test. Different lowercase letters indicate a significant difference (P < 0.05). I) A proposed working model for the TaHAG1–TaBZR1 cooperation in activating TaSAMT1 transcription for freezing tolerance. TaBZR1 alone establishes a basic transregulation system for activating the TaSAMT1 expression through its binding to the E-box; the interaction of TaHAG1 with TaBZR1 is necessary for enhanced transregulation via increasing histone acetylation. Higher TaSAMT1 levels in turn result in accumulation of MeSA and enhanced freezing tolerance.
We also used a transient expression system in wheat protoplasts to assess whether TaHAG1 induces TaSAMT1 transcription in vivo. The co-expression of TaHAG1 with a proTaSAMT1-B:LUC reporter construct resulted in significantly increased LUC activity compared to control samples with the empty vector control (Fig. 7E), indicating that TaHAG1 activates the transcription of TaSAMT1-B. Together, based on these findings, we propose that TaHAG1 directly binds to the TaSAMT1-B promoter to trigger epigenetic changes, which in turn facilitate its transcription in response to freezing stress.
TaBZR1 and TaHAG1 cooperatively activate TaSAMT1 transcription
The in vitro and in vivo protein interaction assays suggested that TaBZR1 and TaHAG1 may form a protein complex, which may bind to the TaSAMT1-B promoter and induce transcription. We therefore tested whether co-expression of TaHAG1 affected the TaBZR1-mediated transcriptional regulation of TaSAMT1-B in the same transient expression system used above with the proTaSAMT1-B:LUC reporter (Fig. 7E). Indeed, while the single expression of TaBZR1 or TaHAG1 activated transcription from the TaSAMT1-B promoter, their co-expression led to a significant increase in TaSAMT1-B promoter activation under control and freezing stress conditions. Moreover, the levels of H3K9ac at the TaSAMT1-B promoter were significantly increased in TaBZR1-OE seedlings compared to the wild type under freezing treatment; H3K14ac levels were significantly higher in TaBZR1-OE seedlings than in the wild type under both control and freezing stress conditions (Fig. 7, F and G). Thus, the overexpression of TaBZR1 triggers increased H3K9ac, H3K14ac modification, and transcriptional upregulation of the TaSAMT1-B locus under cold stress.
To connect the changes in histone acetylation and transcriptional activation of the TaSAMT1-B locus to the BR pathway, we performed a ChIP-qPCR assay for TaBZR1 on wild-type seedlings grown under control conditions or treated with BL. We observed an enrichment for TaBZR1 at the TaSAMT1-B promoter upon BL treatment (Supplementary Fig. S13A). In addition, we detected increased H3K14ac levels over the TaSAMT1 promoter following BL treatment relative to control seedlings; however, H3K9ac levels remained unchanged (Supplementary Fig. S13, B and C). Notably, these changes in acetylation levels were not caused by changes in the affinity between TaBZR1 and TaHAG1, as determined by LCI assays in N. benthamiana leaves co-expressing cLUC-TaBZR1 and nLUC-TaHAG1 exposed to control or freezing stress conditions (Fig. 7H). Taken together, these results indicate that TaBZR1 directly binds to the TaSAMT1-B promoter and further activates its transcription under cold stress by recruiting TaHAG1 and increasing the levels of histone H3 acetylation.
Discussion
Identifying the function of key modulators and exploring the regulatory mechanisms in plant cold stress responses are crucial to developing a strategy to improve cold tolerance and ensure crop productivity under adverse climate conditions. Genetic and molecular analyses have revealed major quantitative trait loci (QTLs) and genes that affect cold tolerance in crops (Fujino et al. 2008; Saito et al. 2010; Ma et al. 2015; Zhang et al. 2017; Liu et al. 2018; Mao et al. 2019; Zeng et al. 2021). However, the identity and nature of the underlying mechanisms remain to be fully dissected. This question is particularly challenging in common wheat, which carries a large hexaploid genome with limited gene editing efficiency. Here, we demonstrate that the SA methyl transferase TaSAMT1-B, which methylates SA into MeSA in vitro, plays a vital role in the freezing tolerance of wheat. We further reveal a key transcriptional regulatory node consisting of TaBZR1, the master regulator of BR signaling, interacting with the epigenetic modulator TaHAG1 to confer freezing tolerance to wheat through potentiating the transcriptional output of TaSAMT1-B.
SA and BR are two well-characterized signaling molecules in terms of their biosynthesis and signal transduction (Kim et al. 2022). SA plays key roles in plant immune responses, including resistance to pathogen attack, the hypersensitive response, cell death, and SAR (Ding and Ding 2020). Accumulating evidence indicates that SA is also involved in responses to abiotic stresses, including drought, salinity, and low temperature (Miura and Tada 2014). However, a mechanistic understanding of the potential interaction between the SA pathway and the cold stress response is extremely limited, although a recent study reported that the SA receptor NON-EXPRESSER OF PATHOGENESIS-RELATED GENES 1 (NPR1) plays an important role in freezing tolerance in Arabidopsis independently of the SA pathway (Olate et al. 2018).
In the present study, knocking out TaSAMT1-B resulted in lower MeSA accumulation and decreased tolerance to freezing stress, whereas overexpression of TaSAMT1-B led to elevated MeSA levels and enhanced tolerance to freezing stress. The higher content of MeSA and lower content of SA in TaSAMT1-B-OE lines indicate that TaSAMT1-B converts SA to MeSA in response to freezing stress (Fig. 3, A and B). We confirmed the positive effect of MeSA on cold tolerance when spraying seedlings with MeSA prior to imposing freezing conditions (Fig. 3, C to E). Therefore, our results demonstrate that the SA methyl transferase TaSAMT1-B is involved in MeSA accumulation upon freezing stress and is essential for cold tolerance in wheat.
The biological role of MeSA, a methyl ester form of SA, is not as well known as that of SA. SA is methylated to MeSA by SAMT, while MeSA is converted to SA by SA binding protein 2 (SABP2) (Kumar 2014). Since SA and MeSA are reciprocal precursors, it is often difficult to differentiate between their individual effects (Gondor et al. 2022). Several studies have shown that application of MeSA improves the cold stress tolerance of plants (Sayyari et al. 2011; Seydpour and Sayyari 2016; Fan et al. 2021; Zeng et al. 2022). Moreover, a recent study revealed that the volatile MeSA is emitted by cold-stressed tea (Camellia sinensis) plants and plays a pivotal role in priming cold tolerance for plant-to-plant communication via a CBF-dependent pathway (Zhao et al. 2020). MeSA is more hydrophobic and volatile than SA, and it can spread more easily and act faster than SA (Gondor et al. 2022). Together with our findings, we propose that MeSA might be a mobile signal in response to cold stress in plants. There is evidence that MeSA mediates cold signaling via the CBF-dependent pathway (Zhao et al. 2020); however, the mechanism by which MeSA promotes CBF expression and how MeSA modulates SA-mediated cold stress responses remain to be elucidated.
To mount protection against cold stress, endogenous BRs promote cold tolerance through the accumulation of the active unphosphorylated forms of BZR1 and BES1, leading to the activation of CBF-dependent or -independent signaling cascades (Li et al. 2017; Nolan et al. 2020). The relationships between BRs and other phytohormones have been widely studied and well established (De Vleesschauwer et al. 2012; Nolan et al. 2020). By contrast, less is known about whether BRs are engaged in physiological and molecular interactions with the SA signaling cascades to modulate plant growth and responses to stress. One study reported that BRs enhanced SA-mediated immune responses by inhibiting BRASSINOSTEROID-INSENSITIVE 2 (BIN2)-mediated phosphorylation of clade I TGA transcription factors in Arabidopsis (Kim et al. 2022).
In this study, a series of genetic and biochemical analyses revealed that the TaBZR1–TaSAMT1-B module plays an essential role in mediating the crosstalk between SA and BR signaling in response to cold stress. TaBZR1 directly targets the TaSAMT1-B promoter to play a key role in freezing tolerance in wheat (Fig. 4). Therefore, we propose that TaBZR1, which participates in the BR pathway, and TaSAMT1-B, involved in the SA pathway, form a crosstalk and improve freezing tolerance under cold stress. In addition, the expression pattern of TaSAMT1-B in the TaBZR1-OE and Tabzr1 mutant seedlings and the freezing tolerance phenotype of TaBZR1-OE and Tabzr1 plants both support a function for TaBZR1 in cold tolerance (Fig. 4), in agreement with a previous study (Li et al. 2017).
Switching from repressed to active chromatin status in epigenetic regulation is one of the means employed by plants to survive in an ever-changing environment. As a major epigenetic regulatory mechanism, histone acetylation plays an important role in transcriptional regulation (Servet et al. 2010; Kim et al. 2018; Zhao et al. 2019; Lin et al. 2022; Song et al. 2022). Multiple studies have suggested histone acetylation as an additional layer of regulation for transcriptional reprogramming upon cold stress response (Chinnusamy et al. 2007; Zhu et al. 2008; Moraga and Aquea 2015; Park et al. 2018). In this study, we revealed that TaBZR1 physically interacts with the histone acetyltransferase TaHAG1 and demonstrated that the TaSAMT1-B locus is a direct downstream target of TaHAG1 and plays a critical role in cold tolerance of wheat (Figs. 6 and 7).
TaHAG1 associates directly with the TaSAMT1-B promoter and deposits increased H3 acetylation levels in response to freezing stress, providing further epigenetic regulatory evidence that TaHAG1 contributes to freezing tolerance. Accordingly, we hypothesize that TaHAG1 expression upon freezing stress might induce a primed state characterized by enhanced histone acetylation at the promoter of cold-associated factors such as TaSAMT1-B, whose encoded protein then catalyzes more SA methylation to produce MeSA and improve freezing tolerance. Hence, we uncovered a regulatory mechanism between TaBZR1 and TaHAG1 for the regulation of TaSAMT1-B expression and freezing tolerance in wheat via TaBZR1 binding to the E-box motifs in the TaSAMT1-B promoter to recruit TaHAG1 and increase the levels of H3 acetylation.
It is worth noting that co-expression of TaBZR1 with TaHAG1 led to a significant increase in TaSAMT1-B promoter activation compared with the expression of TaBZR1 alone, reflecting the coactivation function of TaHAG1. Therefore, on the basis of the current and previous work, we present the following model to illustrate the interaction between BR and SA signaling in wheat response to freezing stress. TaBZR1, the master regulator of the BR signaling pathway, binds to the E-box motif in the TaSAMT1-B promoter to induce TaSAMT1 expression, which is further increased under freezing stress; the interaction of TaBZR1 with TaHAG1 is necessary for this enhanced transactivation (Fig. 7I). In addition, the Tasamt1-B mutants showed a lower tolerance to freezing stress that could not be rescued by exogenous BL application; mutation of TaSAMT1-B also suppressed the enhanced freezing stress tolerance of TaBZR1-OE seedlings (Fig. 5, F to H), suggesting that TaSAMT1-B functions downstream of the BR pathway and is important for BR-mediated freezing tolerance. Our findings also suggest that the synergistic mechanism between SA and BR pathways is more complex than what we revealed here. Future studies should include the identification of critical components with uncharacterized functions, analysis of functional redundancy between TaSAMT1 homologous genes, and dissection of potential crosstalk at the protein level to fully address how the SA and BR signaling pathways are involved in freezing tolerance.
Depending on the duration and severity of cold stress, plants must carefully coordinate growth and stress responses. An overactive response may impose a fitness and yield penalty, although it is not clear if growth limitation represents an energy trade-off or is simply concomitant with stress responses (Nolan et al. 2020). Therefore, identifying the target genes and elucidating the underlying mechanisms that coordinate growth and stress responses are crucial for engineering crops with optimized stress responses. We measured the performance of the wild type, TaSAMT1-B-OE, and Tasamt1-B mutants throughout their entire growth period. We detected comparable yield-related traits under field conditions for wild-type, TaSAMT1-B-OE, and Tasamt1-B plants for spike length, spikelet number per spike, and thousand-kernel weight (TKW) (Supplementary Fig. S14). These results suggest that manipulation of TaSAMT1 did not result in clear pleiotropic phenotypes or yield penalty under normal conditions but significantly increased freezing tolerance compared with wild-type plants under cold stress conditions. These findings suggest that TaSAMT1 could serve as a target for both genetic engineering and selection for improvement of wheat cold tolerance. The regulatory factors or genes involved in cold tolerance identified in this study may also be harnessed for genetic improvement of other crops.
Materials and methods
Plant materials and phenotyping of wheat freezing tolerance
Wheat (T. aestivum) plants with different genotypes were cultivated in the experimental field in Beijing (39°57′N, 116°17′E) during the natural growing seasons. The seedlings of different genotypes with similar germination rate and vigor were grown in cultivation pots (4 × 4 × 6 cm, length × width × depth) filled with uniformly mixed soil (Pindstrup turf to vermiculite in a v/v ratio of 1:1) in a greenhouse at a relative humidity of 70% and 26/20°C day/night temperatures, with a 16-h-light/8-h-dark photoperiod and a light intensity of 3,000 lux (Master GreenPower CG T 400W E40; Philips). Ten-day-old seedlings after germination were exposed to low-temperature conditions (−10°C) for 1.5 h and subsequently allowed to recover at 22°C for 24 h. The number of surviving seedlings with green and viable stems was recorded. Phenotypes were evaluated by survival rates. At least 15 seedlings for each line were assessed in each test; statistical analyses were based on data obtained from three independent experiments.
The N. benthamiana plants, used as host plants, were grown in a greenhouse at 24 to 25°C under a 16-h-light/8-h-dark cycle for 4 to 6 weeks before agroinfection. The Arabidopsis Col-0 plants were grown in a greenhouse at a relative humidity of 70% and 22/18°C day/night temperatures, with a 16-h-light/8-h-dark photoperiod for 3 weeks before total RNA extraction.
Electrolyte leakage assay
The relative electrolyte leakage (REL) assays were performed as described previously (Shi et al. 2012; Li et al. 2017). After freezing treatment, seedlings were placed into 15-mL tubes containing 10 mL of deionized water, whose electrical conductivity (EC) was S0, and then shaken for 15 min at 22°C to measure their EC as S1. After determining S1, the samples were boiled for at least 15 min and shaken at 22°C for another 20 min before their EC values were measured again, giving S2. REL was calculated as (S1–S0)/(S2–S0).
RNA extraction and RT-qPCR
Ten-day-old seedlings were grown under normal conditions and treated at −10°C for 2, 4, 6, or 8 h, with one more sample collected just before freezing treatment as a 0-h sample. Total RNA was extracted, followed by reverse transcription using HiScript II Q RT SuperMix (Vazyme, China). RT-qPCR was performed with Taq Pro Universal SYBR qPCR Master Mix (Vazyme, China) on a C1000 Touch Real-Time PCR system (Bio-Rad, USA) to detect the expression of specific genes.
Wheat protoplast isolation and transfection
Protoplasts were prepared from the wheat variety Fielder. Wheat protoplast isolation and transfection were performed as described (Wang et al. 2014). The Wizard Plus Midipreps DNA Purification System (Promega, USA) was used to extract plasmid DNA for protoplast transfection. Each plasmid (5 µg per construct) was introduced by polyethylene glycol (PEG)–mediated transfection. The transfected protoplasts were incubated at 22°C for 14 to 16 h.
Subcellular localization
The full-length coding sequence of TaSAMT1-B was cloned into XbaI-digested pCAMBIA1300-GFP vector using a Seamless Cloning and Assembly Kit (Biomed, China) to generate a fusion construct named pCAMBIA1300-GFP-TaSAMT1-B. The resulting vector and the empty vector (as control) were then separately transfected into wheat protoplasts as described above. After incubation at 23°C for 14 to 16 h in the dark, GFP and chlorophyll fluorescence were visualized using a confocal microscope (LSM880; Carl Zeiss; laser, 488 nm; intensity, 0.98%; collection bandwidth, 490 to 537 nm; gains: 805 v) and quantified using the image analysis software provided by the manufacturer.
Sequence alignment and phylogenetic analysis
To perform phylogenetic analysis, the amino acid sequences of SAMT1 and BZR1 from different plant species were downloaded from Ensembl-plants (http://plants.ensembl.org/). MEGA 7 was employed to construct the phylogenetic tree with the following programs: all protein sequences were aligned in Clustal W with default parameters; then the maximum likelihood (ML) method was used to build a phylogenetic tree with the Poisson model and 1,000 bootstrap replicates.
Production of recombinant TaSAMT1 and AtBSMT1 and enzyme assay
The full-length coding sequences of TaSAMT1-B, TaSAMT1-A, TaSAMT1-D, and AtBSMT1 were individually amplified from the cDNA prepared from total RNA extracted from seedling leaves of Fielder variety and Arabidopsis (Arabidopsis thaliana) accession Col-0 using specific primers (Supplementary Data Set 3) and 2×Hieff Canace Gold PCR Master Mix (Cat No.10149; Yeasen, Shanghai, China). The amplicons were cloned into BamHI-digested pGEX-6P-1 vector with a Seamless Cloning and Assembly Kit (Biomed, China). Escherichia coli Rosetta-gami2 (DE3) cells were transformed with each resulting construct. The production of each recombinant protein was induced with the addition of 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and incubated at 16°C for 16 h. The cells were collected by centrifugation at 6,297 × g for 5 min at 4°C and then resuspended and sonicated (39% amplitude, 20 min, 4°C) in lysis buffer (1× phosphate-buffered saline [PBS]). The supernatant from the resulting cell lysate was used as the crude enzyme extract. The reaction mixture for the enzyme activity assays comprised the following components: 4 mM MgCl2; 250 μM SAM (S-adenosyl-L-methionine); 100 μM adenosine triphosphate; 4 mM dithiothreitol; 1 mM substrates SA; and 900 μL crude proteins. The mixture was adjusted to a final volume of 1.0 mL and then incubated at 28°C for 1 h. The activity of recombinant proteins was analyzed using a gas chromatography–mass spectrometry (GC-MS) instrument (Agilent 7250). Briefly, 1 μL of each reaction mixture was injected into the GC-MS instrument and analyzed following the GC-MS temperature program: 50°C for 5 min, increased to 250°C at a rate of 10°C/min, and maintained for 2 min. Primer sequences are listed in Supplementary Data Set 3.
Plasmid construction and plant transformation
To obtain knockout constructs for the candidate gene, the target sequences for the single guide RNAs (sgRNAs) were selected according to the specific region within the first or second exon using the E-Crispr Design Website (www.e-crisp.org/E-CRISP/designcrispr). The corresponding sgRNA sequences were synthesized (BGI, China), digested, and inserted into the binary vector pBUE411 via T4 DNA ligase (M0202, New England Biolabs, USA). The full-length coding sequence of TaSAMT1-B was amplified from the cDNA of seedling leaves of Fielder and cloned into BamHI-digested pMWB110 vector to obtain the proUbi:TaSAMT1-B construct. The resulting vectors harboring the desired inserts were transformed into Agrobacterium (A. tumefaciens) strain EHA105 and transformed into wheat cultivar Fielder by Agrobacterium-mediated transformation. The primers used for the identification of the knockout transgenic plants are listed in Supplementary Data Set 3.
SA, MeSA, and BL treatment
Salicylic acid (SA; Yuanye Bio-Technology, China), methyl salicylate (MeSA; Macklin, China), and brassinolide (BL; Coolaber, China) were dissolved in ethanol to proper concentrations as storage solutions. Each chemical was applied evenly on the leaf surface of 10-day-old seedlings to fully evaporated before freezing stress treatment using an identical volume of solvent (ethanol) as a mock treatment. After freezing treatment, the seedlings were allowed to recover at 22°C for 24 h before measuring the survival rates of seedlings.
SA and MeSA quantification
Ten-day-old seedlings of different genotypes were exposed to −10°C for the indicated times. All seedlings were then dried in a low-temperature freeze dryer (REVO-RV53B5); the resulting dry samples were triturated before the addition of solvent (1 ppm 70% [v/v] HPLC-grade methanol with the addition of the internal standard acyclovir and 0.5 ppm roxithromycin). The mixture was sonicated for 30 min, and the supernatant was filtered through a 0.22-μm filter. The filtrate was checked on an LC-electrospray ionization (ESI)-MS/MS system (HPLC, Shim-pack UFLC SHIMADZU CBM20A system; MS, Applied Biosystems 5500 Q-TRAP) as previously described (Chen et al. 2013). The detected metabolites were analyzed on a mass spectrometry platform (HPLC, Shim-pack UFLC SHIMADZU CBM20A system; MS, Applied Biosystems Triple TOF 5600). The metabolites were quantified using a previously described method (Chen et al. 2013).
BR quantification
BR content was determined by Nanjing Convinced-test Technology Co., Ltd. Ten-day-old seedlings were exposed to −10°C for 6 h using seedlings not exposed to freezing as a 0-h control sample. The plant tissues were frozen with liquid nitrogen in a mortar and ground into powder. To each 15-mL tube containing 1 g frozen plant material, 8 μL of a 1-μg/mL working solution of internal standards was added, followed by the addition of 10 mL extraction solvent consisting of isopropanol/H2O/concentrated HCl (2:1:0.002, v/v/v). The tubes were placed on a shaker at a speed of 100 rpm for 30 min at 4°C. Dichloromethane (5 mL) was added to each sample and shaken for 30 min in a cold room at 4°C. The samples were centrifuged at 13,000 × g for 5 min at 4°C. After centrifugation, the solvent from the lower phase was transferred to a screw-cap vial using a Pasteur pipette. The solvent mixture was concentrated but not completely dried using a nitrogen evaporator with nitrogen flow. The samples were redissolved in 0.4 mL methanol. For each sample, 2 µL was injected into a reverse-phased C18 Gemini HPLC column for HPLC-ESI-MS/MS analysis.
Yeast one-hybrid assays
The promoter fragment for TaSAMT1-B was amplified from the genomic DNA of Fielder and cloned into KpnI/XhoI-digested pAbAi vector (BGI, China). The construct was linearized with the restriction enzyme BstBI and then transformed into yeast (Saccharomyces cerevisiae) Y1HGold cells to generate a bait-specific reporter strain. The transformants were selected on synthetic defined medium lacking Ura and Leu (SD −Ura −Leu) and containing 400 ng/mL aureobasidin A (AbA).
The full-length coding sequence of TaBZR1 was cloned into the BamHI/XhoI-digested pB42AD (pJG4-5) vector, yielding the construct pB42AD-TaBZR1. The TaSAMT1-B promoter from Fielder was cloned into the BamHI/XhoI-digested pLACZi2μ vector. These two resulting plasmids were co-transformed into yeast strain EGY48. Y1H assays were performed according to the manufacturer's recommendations (Clontech, Japan). Transformants were selected on SD medium lacking Trp and Ura and containing X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) for blue color development (Li et al. 2010). The primers for Y1H were listed in Supplementary Data Set 3.
Transient dual luciferase reporter assay
The wild-type or mutated promoter sequences of TaSAMT1 were cloned into BamHI-digested pGreen II 0800-LUC vector to generate the reporter plasmids. The full-length coding sequences of TaBZR1 and TaHAG1 were individually cloned into XbaI-digested pUC18-35S vector to yield effector constructs. Relative LUC activity assays were performed in wheat protoplasts, with different combinations of constructs co-transfected. Wheat protoplasts were then incubated in darkness for 15 h at 22°C. For cold treatment, wheat protoplasts were incubated in darkness for 13 h at 22°C and then treated at 4°C for 2 h. The detection of firefly luciferase (LUC) and Renilla luciferase (REN) activity was performed as described previously (Zheng et al. 2021). Each sample consisted of three independent transfection reactions; the primers used for cloning are listed in Supplementary Data Set 3.
Electrophoretic mobility shift assay
The full-length coding sequence of TaBZR1 was cloned into EcoRI/SalI-digested pCold-MBP vector using a Seamless Cloning and Assembly Kit (Biomed, China) to produce an MBP fusion protein. MBP alone was produced from the empty vector and purified for use as a negative control. For EMSA, a 30-bp promoter fragment of TaSAMT1-B containing one E-box motif was synthesized (BGI, China) as biotin-labeled probe (Supplementary Data Set 3). Unlabeled probes containing the wild-type or mutated E-box motif were used as competitors. The assays were erformed using a Light Shift Chemiluminescent EMSA Kit (20148; Thermo Fisher Scientific) following the manufacturer's instructions. Recombinant MBP-TaBZR1 or MBP was added to the reaction mixture (1× binding buffer, 50 ng poly(dl·dC), 2.5% [v/v] glycerol, 0.05% [v/v] NP-40, 50 mM KCl, 5 mM MgCl₂, 2 mM EDTA [pH 8.0], and 1 nM biotin-labeled probe), followed by incubation at 25°C for 20 min. The reaction products were separated by 6% PAGE and transferred to a nylon membrane; detection of biotin-labeled DNA was performed according to the manufacturer's instructions.
Yeast two-hybrid assay
TaBZR1 was cloned into pGBKT7 vector and used as a bait to screen a yeast two-hybrid library. Different combinations of constructs were co-transformed into yeast strain AH109. Transformants were selected on SD medium lacking Leu and Trp. Liquid cultures started from positive transformants were then serially diluted and plated onto SD medium lacking Leu, Trp, and His and containing 60 mM 3-amino-1,2,4-triazole (3-AT).
Luciferase complementation imaging assays
The LCI assay was carried out between TaBZR1 and TaHAG1 as described previously (Song et al. 2022). Briefly, the full-length coding sequences of TaBZR1 and TaHAG1 were cloned into KpnI/SalI-digested pCAMBIA1300-nLUC (nLUC) or KpnI/BamHI-digested pCAMBIA1300-cLUC (cLUC) vector, respectively. The resulting constructs were transformed into Agrobacterium strain GV3101; Agrobacterium cultures were then resuspended in infiltration buffer to an OD at 600 nm of 0.2, mixed in a 1:1 ratio (v/v), and then infiltrated into the leaves of N. benthamiana plants. Firefly LUC signal was collected after 2 days using a cooled charge-coupled device camera (NightSHADE LB 985; Berthold Technologies, Bad Wildbad, Germany) after spraying 1 mM of D-luciferin onto the leaves (Coolaber; CL6928, China). For each assay, three independent leaves of the same plant were assessed for statistical analysis of relative LUC activity. The pCAMBIA1300 n-LUC and pCAMBIA1300 c-LUC vectors were used as negative controls.
In vitro pull-down assay
The recombinant proteins GST-TaBZR1 and MBP-TaHAG1 were purified from E. coli BL21 star (DE3). 10 μL GST-TaBZR1 or GST was incubated with glutathione beads (20507ES10; YEASEN, China) at 4°C for 2 h, followed by incubation with MBP-TaHAG1 for 2 h. The mixture was collected by centrifugation at 500 × g for 5 min at 4°C, followed by washing with PBS five times. Proteins bound to glutathione beads were separated on 10% (w/v) SDS-PAGE and detected with anti-GST (1:5,000, HT601, TransGen Biotech, China) and anti-MBP antibodies (1:5,000, AE016; ABclonal, China).
Co-IP assay
For the Co-IP assay, the TaBZR1-GFP and TaHAG1-MYC fusion constructs were prepared. The full-length coding sequences of TaBZR1 and TaHAG1 were individually cloned into XbaI-digested pCAMBIA1300-GFP or SmaI-digested pCAMBIA1300-MYC vector, respectively. Each construct was transformed into Agrobacterium strain GV3101 for infiltration of N. benthamiana leaves via Agrobacterium-mediated infiltration as described above. Total proteins were isolated from different infiltrated leaves and incubated with anti-GFP beads at 4°C for 4 to 6 h with gentle shaking. The beads were washed with five times before the immunoprecipitated proteins were eluted in IP buffer. Input and eluted protein were then analyzed by immunoblotting using anti-GFP (mouse, 1:2,000; Abclonal, AE012, China) or anti-c-MYC antibodies (mouse, 1:2,000; TransGen Biotech, HT101-01, China). Primer sequences are listed in Supplementary Data Set 3.
BiFC assay
For the BiFC assays, the full-length coding sequences of TaHAG1 and TaBZR1 were amplified and cloned individually into BamHI/XbaI-digested cYFP and nYFP vectors, respectively. The resulting constructs and the empty nYFPN and cYFP vectors were transformed into Agrobacterium strain GV3101 and mixed in the appropriate combinations for co-infiltration into N. benthamiana leaves. Two days later, the YFP fluorescence signals were detected with a confocal microscope (LSM880; Carl Zeiss, Heidenheim, Germany; laser, 488 nm; intensity, 0.98%; collection bandwidth, 490 to 598 nm; gains: 700 v).
ChIP assay
ChIP assays were performed as described previously (Zheng et al. 2021). Briefly, 2 g of 10-day-old wheat seedlings was collected and subjected to vacuum infiltration in 1% (v/v) formaldehyde for 20 min at 25°C. After isolation and lysis of nuclei, chromatin was sonicated (20% amplitude, 30 min, 4°C) and incubated with anti-BZR1 antibody (ABclonal, China), anti-GFP antibody (Millipore, USA), anti-H3K9ac antibody, and H3K14ac antibody (Abcam, Britain). The immunoprecipitated DNA fragments were then purified, and the enriched DNA fragments were analyzed by qPCR. Amplified DNA from the chromatin fractions prior to antibody incubation were used as controls. The relative enrichment at each target site was normalized to the input sample. Relevant primer sequences are listed in Supplementary Data Set 3.
Statistical analysis
The statistical significance of differences was evaluated by Student's t test and one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test (Supplementary Data Set 4). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01). Different lowercase letters indicate significant differences (P < 0.05).
Accession numbers
The sequences for all wheat genes used in this study are available in the Ensemb-plants (https://plants.ensembl.org/Triticum_aestivum/Info/Index). The accession numbers of the key genes mentioned are as follows: TaSAMT1-B (TraesCS3B02G445100), TaBZR1 (TraesCS2A02G187800), and TaHAG1 (TraesCS1D02G134200).
Supplementary Material
Acknowledgments
We thank Prof. Jiaqiang Sun (Institute of Crop Sciences, Chinese Academy of Agricultural Sciences) for providing the TaBZR1 transgenic lines. We thank Prof. Wei Chen and Dr. Man Feng (Huazhong Agricultural University) for SA and MeSA quantification. We thank Dr. Junna He (China Agricultural University) and Dr. Xinye Liu (Hebei Normal University) for helpful discussions and comments on the text.
Contributor Information
Wei Chu, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Shumin Chang, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Jingchen Lin, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Chenji Zhang, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Jinpeng Li, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Xingbei Liu, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Zehui Liu, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Debiao Liu, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Qun Yang, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Danyang Zhao, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Xiaoyu Liu, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Weilong Guo, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Mingming Xin, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Yingyin Yao, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Huiru Peng, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Chaojie Xie, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Zhongfu Ni, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Qixin Sun, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Zhaorong Hu, Frontiers Science Center for Molecular Design Breeding/Key Laboratory of Crop Heterosis and Utilization (MOE)/Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, No. 2 Yuanmingyuan Xi Road, Haidian District, Beijing 100193, PR China.
Author contributions
W.C. and S.M.C. performed the experiments with help from J.C.L, C.J.Z., J.P.L., X.B.L., Z.H.L., D.B.L., Q.Y., D.Y.Z., and X.Y.L.; W.L.G., M.M.X., Y.Y.Y., H.R.P., C.J.X., Z.F.N., and Q.X.S. contributed to materials; Z.R.H. conceived the research and analyzed the data; Z.R.H. wrote the article with input from Z.F.N. and Q.X.S.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1. Heatmap representation of the expression levels of TaSAMT family members at 4°C and 23°C (Supports Fig. 1).
Supplementary Figure S2. Phylogenetic analysis and protein sequence alignment of TaSAMTs with homologs in wheat, Brachypodium distachyon, barley, and rice (Supports Fig. 1).
Supplementary Figure S3. Multiple amino acid alignment and protein structure of the three TaSAMT1 homoeologs (Supports Fig. 1).
Supplementary Figure S4. Production of recombinant TaSAMT1-A, TaSAMT1-B, TaSAMT1-D, and AtBSMT1 fused to GST in E. coli (Supports Fig. 1).
Supplementary Figure S5. Phylogenetic analysis and protein sequence alignment of TaBZR1 and its homologs in different species (Supports Fig. 4).
Supplementary Figure S6. TaBZR1 expression in response to freezing treatment and in wild-type KN199 and TaBZR1-OE lines (Supports Fig. 4).
Supplementary Figure S7. Identification of the Tabzr1 mutant (Supports Fig. 4).
Supplementary Figure S8. Endogenous MeSA levels in wild-type KN199 and TaBZR1-OE plants under freezing treatment (Supports Fig. 4).
Supplementary Figure S9. Relative transcript levels of TaBZR1 and TaSAMT1 in response to BL treatment (Supports Fig. 5).
Supplementary Figure S10. The interaction of TaBZR1 with TaHAG1 is specific (Supports Fig. 6).
Supplementary Figure S11. Relative TaHAG1 transcript levels in response to freezing treatment and in wild-type Fielder and TaHAG1-OE lines (Supports Fig. 6).
Supplementary Figure S12. Endogenous MeSA levels in wild-type Fielder and TaHAG1-OE plants under freezing treatment (Supports Fig. 6).
Supplementary Figure S13. Enrichment of TaBZR1, H3K9ac, and H3K14ac at TaSAMT1-B promoter regions in wheat under BL treatment (Supports Fig. 7).
Supplementary Figure S14. Agronomic traits of different TaSAMT1 transgenic lines and wild-type Fielder plants grown in the field (Supports Figs. 2 and 7).
Supplementary Data Set 1. List of the upstream regulators of TaSAMT1-B expression identified in Y1H assays.
Supplementary Data Set 2. Candidate TaBZR1-interacting proteins identified by Y2H screening.
Supplementary Data Set 3. Primer pairs used in this study.
Supplementary Data Set 4. Data analysis.
Funding
This work was supported by the National Natural Science Foundation of China (32130078, 32072001), the Major Program of the National Natural Science Foundation of China (31991210), the Young Elite Scientist Sponsorship Program by CAST (2015QNRC001), and the 2115 Talent Development Program of China Agricultural University.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008:20(8):2117–2129. 10.1105/tpc.108.058941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaran E, Zeier TE, Griebel T, Zeier J. Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell. 2009:21(3):954–971. 10.1105/tpc.108.063164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo-García S, de Lucas M, Martínez C, Espinosa-Ruiz A, Davière JM, Prat S. BR-dependent phosphorylation modulates PIF4 transcriptional activity and shapes diurnal hypocotyl growth. Genes Dev. 2014:28(15):1681–1694. 10.1101/gad.243675.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castroverde CDM, Dina D. Temperature regulation of plant hormone signaling during stress and development. J Exp Bot. 2021:72(21):erab257. 10.1093/jxb/erab257 [DOI] [PubMed] [Google Scholar]
- Chen F, D'Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E. An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant J. 2003:36(5):577–588. 10.1046/j.1365-313X.2003.01902.x [DOI] [PubMed] [Google Scholar]
- Chen W, Gong L, Guo Z, Wang W, Zhang H, Liu X, Yu S, Xiong L, Luo J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Mol Plant. 2013:6(6):1769–1780. 10.1093/mp/sst080 [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu J-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007:12(10):444–451. 10.1016/j.tplants.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Crimp SJ, Zheng B, Khimashia N, Gobbett DL, Chapman S, Howden M, Nicholls N. Recent changes in southern Australian frost occurrence: implications for wheat production risk. Crop Pasture Sci. 2016:67(8):801–811. 10.1071/CP16056 [DOI] [Google Scholar]
- De Vleesschauwer D, Van Buyten E, Satoh K, Balidion J, Mauleon R, Choi IR, Vera-Cruz C, Kikuchi S, Höfte M. Brassinosteroids antagonize gibberellin- and salicylate-mediated root immunity in rice. Plant Physiol. 2012:158(4):1833–1846. 10.1104/pp.112.193672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C-K, Wang CY, Gross KC, Smith DL. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002:214(6):895–901. 10.1007/s00425-001-0698-9 [DOI] [PubMed] [Google Scholar]
- Ding P, Ding Y. Stories of salicylic acid: a plant defense hormone. Trends Plant Sci. 2020:25(6):549–565. 10.1016/j.tplants.2020.01.004 [DOI] [PubMed] [Google Scholar]
- Ding Y, Shi Y, Yang S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019:222(4):1690–1704. 10.1111/nph.15696 [DOI] [PubMed] [Google Scholar]
- Ding Y, Yang S. Surviving and thriving: how plants perceive and respond to temperature stress. Dev Cell. 2022:57(8):947–958. 10.1016/j.devcel.2022.03.010 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Raguso RA, Wang J, Ross JR, Pichersky E. Floral scent production in Clarkia breweri. III. Enzymatic synthesis and emission of benzenoid esters. Plant Physiol. 1998:116(2):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004:42(1):185–209. 10.1146/annurev.phyto.42.040803.140421 [DOI] [PubMed] [Google Scholar]
- Eremina M, Rozhon W, Poppenberger B. Hormonal control of cold stress responses in plants. Cell Mol Life Sci. 2016a:73(4):797–810. 10.1007/s00018-015-2089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremina M, Unterholzner SJ, Rathnayake AI, Castellanos M, Khan M, Kugler KG, May ST, Mayer KF, Rozhon W, Poppenberger B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc Natl Acad Sci U S A. 2016b:113(40):E5982–E5991. 10.1073/pnas.1611477113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Du Z, Cui X, Ji W, Ma J, Li X, Wang X, Zhao H, Liu B, Guo F, et al. . Preharvest methyl salicylate treatment enhance the chilling tolerance and improve the postharvest quality of apricot during low temperature storage. Postharvest Biol Technol. 2021:177:111535. 10.1016/j.postharvbio.2021.111535 [DOI] [Google Scholar]
- Fang P, Yan M, Chi C, Wang M, Zhou Y, Zhou J, Shi K, Xia X, Foyer CH, Yu J. Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 2019:180(4):2061–2076. 10.1104/pp.19.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT . World Food and Agriculture—Statistical Yearbook 2020. Rome, Italy; 2020.
- Frederiks TM, Christopher JT, Sutherland MW, Borrell AK. Post-head-emergence frost in wheat and barley: defining the problem, assessing the damage, and identifying resistance. J Exp Bot. 2015:66(12):3487–3498. 10.1093/jxb/erv088 [DOI] [PubMed] [Google Scholar]
- Fujino K, Sekiguchi H, Matsuda Y, Sugimoto K, Ono K, Yano M. Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc Natl Acad Sci U S A. 2008:105(34):12623–12628. 10.1073/pnas.0805303105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993:261(5122):754–756. 10.1126/science.261.5122.754 [DOI] [PubMed] [Google Scholar]
- Gondor OK, Pál M, Janda T, Szalai G. The role of methyl salicylate in plant growth under stress conditions. J Plant Physiol. 2022:277:153809. 10.1016/j.jplph.2022.153809 [DOI] [PubMed] [Google Scholar]
- Habibi F, Ramezanian A, Rahemi M, Eshghi S, Guillén F, Serrano M, Valero D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J Sci Food Agric. 2019:99(14):6408–6417. 10.1002/jsfa.9920 [DOI] [PubMed] [Google Scholar]
- Holman JD, Schlegel AJ, Thompson CR, Lingenfelser JE. Influence of precipitation, temperature, and 56 years on winter wheat yields in Western Kansas. Crop Manage. 2011:10(1):1–13. 10.1094/CM-2011-1229-01-RS [DOI] [Google Scholar]
- Huang J, Zhao X, Bürger M, Chory J, Wang X. The role of ethylene in plant temperature stress response. Trends Plant Sci. 2023:28(7):808–824. 10.1016/j.tplants.2023.03.001 [DOI] [PubMed] [Google Scholar]
- Hu Y, Jiang L, Wang F, Yu D. Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell. 2013:25(8):2907–2924. 10.1105/tpc.113.112631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Shi Y, Peng Y, Jia Y, Yan Y, Dong X, Li H, Dong J, Li J, Gong Z, et al. . Cold-induced CBF-PIF3 interaction enhances freezing tolerance by stabilizing the phyB thermosensor in Arabidopsis. Mol Plant. 2020:13(6):894–906. 10.1016/j.molp.2020.04.006 [DOI] [PubMed] [Google Scholar]
- Jiang G, Hassan MA, Muhammad N, Arshad M, Chen X, Xu Y, Xu H, Ni Q, Liu B, Yang W, et al. . Comparative physiology and transcriptome analysis of young spikes in response to late spring coldness in wheat (Triticum aestivum L.). Front Plant Sci. 2022:13:811884. 10.3389/fpls.2022.811884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HM, Saltveit ME. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant. 2002:115(4):571–576. 10.1034/j.1399-3054.2002.1150411.x [DOI] [PubMed] [Google Scholar]
- Keuskamp DH, Sasidharan R, Vos I, Peeters AJ, Voesenek LA, Pierik R. Blue-light-mediated shade avoidance requires combined auxin and brassinosteroid action in Arabidopsis seedlings. Plant J. 2011:67(2):208–217. 10.1111/j.1365-313X.2011.04597.x [DOI] [PubMed] [Google Scholar]
- Kim J-M, Sasaki T, Ueda M, Sako K, Seki M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front Plant Sci. 2015:6:114. 10.3389/fpls.2015.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-Y, Yang W, Forner J, Lohmann JU, Noh B, Noh Y-S. Epigenetic reprogramming by histone acetyltransferase HAG1/AtGCN5 is required for pluripotency acquisition in Arabidopsis. EMBO J. 2018:37(20):e98726. 10.15252/embj.201798726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-W, Youn J-H, Roh J, Kim J-M, Kim S-K, Kim T-W. Brassinosteroids enhance salicylic acid-mediated immune responses by inhibiting BIN2 phosphorylation of clade I TGA transcription factors in Arabidopsis. Mol Plant. 2022:15(6):991–1007. 10.1016/j.molp.2022.05.002 [DOI] [PubMed] [Google Scholar]
- Klessig DF, Choi HW, Dempsey DA. Systemic acquired resistance and salicylic acid: past, present, and future. Mol Plant Microbe Interact. 2018:31(9):871–888. 10.1094/MPMI-03-18-0067-CR [DOI] [PubMed] [Google Scholar]
- Kumar D. Salicylic acid signaling in disease resistance. Plant Sci. 2014:228:127–134. 10.1016/j.plantsci.2014.04.014 [DOI] [PubMed] [Google Scholar]
- Lantzouni O, Alkofer A, Falter-Braun P, Schwechheimer C. GROWTH-REGULATING FACTORS interact with DELLAs and regulate growth in cold stress. Plant Cell. 2020:32(4):1018–1034. 10.1105/tpc.19.00784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ye K, Shi Y, Cheng J, Zhang X, Yang S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol Plant. 2017:10(4):545–559. 10.1016/j.molp.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Li J, Li G, Gao S, Martinez C, He G, Zhou Z, Huang X, Lee J-H, Zhang H, Shen Y, et al. . Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome A signaling. Plant Cell. 2010:22(11):3634–3649. 10.1105/tpc.110.075788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q-F, Wang C, Jiang L, Li S, Sun SS, He J-X. An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal. 2012:5(244):ra72. 10.1126/scisignal.2002908 [DOI] [PubMed] [Google Scholar]
- Li Q, Zheng Q, Shen W, Cram D, Fowler DB, Wei Y, Zou J. Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell. 2015:27(1):86–103. 10.1105/tpc.114.134338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G-H, Liu H, Yu K, Liu R, Shine MB, Fernandez J, Burch-Smith T, Mobley JK, McLetchie N, Kachroo A, et al. . The plant cuticle regulates apoplastic transport of salicylic acid during systemic acquired resistance. Sci Adv. 2020:6(19):eaaz0478. 10.1126/sciadv.aaz0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G-H, Shine MB, de Lorenzo L, Yu K, Cui W, Navarre D, Hunt AG, Lee J-Y, Kachroo A, Kachroo P. Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host Microbe. 2016:19(4):541–549. 10.1016/j.chom.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Lin J, Song N, Liu D, Liu X, Chu W, Li J, Chang S, Liu Z, Chen Y, Yang Q, et al. . Histone acetyltransferase TaHAG1 interacts with TaNACL to promote heat stress tolerance in wheat. Plant Biotechnol J. 2022:20(9):1645–1647. 10.1111/pbi.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ou S, Mao B, Tang J, Wang W, Wang H, Cao S, Schläppi MR, Zhao B, Xiao G, et al. . Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat Commun. 2018:9(1):3302. 10.1038/s41467-018-05753-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-P, Yang Y, Pichersky E, Klessig DF. Altering expression of benzoic acid/salicylic acid carboxyl methyltransferase 1 compromises systemic acquired resistance and PAMP-triggered immunity in Arabidopsis. Mol Plant Microbe Interact. 2010:23(1):82–90. 10.1094/MPMI-23-1-0082 [DOI] [PubMed] [Google Scholar]
- Ma Y, Dai X, Xu Y, Luo W, Zheng X, Zeng D, Pan Y, Lin X, Liu H, Zhang D, et al. . COLD1 confers chilling tolerance in rice. Cell. 2015:160(6):1209–1221. 10.1016/j.cell.2015.01.046 [DOI] [PubMed] [Google Scholar]
- Mao D, Xin Y, Tan Y, Hu X, Bai J, Liu Z-Y, Yu Y, Li L, Peng C, Fan T, et al. . Natural variation in the HAN1 gene confers chilling tolerance in rice and allowed adaptation to a temperate climate. Proc Natl Acad Sci U S A. 2019:116(9):3494–3501. 10.1073/pnas.1819769116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci. 2014:5:4. 10.3389/fpls.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga F, Aquea F. Composition of the SAGA complex in plants and its role in controlling gene expression in response to abiotic stresses. Front Plant Sci. 2015:6:865. 10.3389/fpls.2015.00865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah K, Abdul Hamid NW, Abdul Rahman NSN. SA-mediated regulation and control of abiotic stress tolerance in rice. Int J Mol Sci. 2021:22(11):5591. 10.3390/ijms22115591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TM, Vukašinović N, Liu D, Russinova E, Yin Y. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell. 2020:32(2):295–318. 10.1105/tpc.19.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu J-Y, Wang Z-Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol. 2012:14(8):802–809. 10.1038/ncb2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olate E, Jiménez-Gómez JM, Holuigue L, Salinas J. NPR1 mediates a novel regulatory pathway in cold acclimation by interacting with HSFA1 factors. Nat Plants. 2018:4(10):811–823. 10.1038/s41477-018-0254-2 [DOI] [PubMed] [Google Scholar]
- Park J, Lim CJ, Shen M, Park HJ, Cha J-Y, Iniesto E, Rubio V, Mengiste T, Zhu J-K, Bressan RA, et al. . Epigenetic switch from repressive to permissive chromatin in response to cold stress. Proc Natl Acad Sci U S A. 2018:115(23):E5400–E5409. 10.1073/pnas.1721241115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-W, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007:318(5847):113–116. 10.1126/science.1147113 [DOI] [PubMed] [Google Scholar]
- Raza A, Charagh S, Najafi-Kakavand S, Abbas S, Shoaib Y, Anwar S, Sharifi S, Lu G, Siddique KHM. Role of phytohormones in regulating cold stress tolerance: physiological and molecular approaches for developing cold-smart crop plants. Plant Stress. 2023:8:100152. 10.1016/j.stress.2023.100152 [DOI] [Google Scholar]
- Ren Y, McGillen MR, Daële V, Casas J, Mellouki A. The fate of methyl salicylate in the environment and its role as signal in multitrophic interactions. Sci Total Environ. 2020:749:141406. 10.1016/j.scitotenv.2020.141406 [DOI] [PubMed] [Google Scholar]
- Saito K, Hayano-Saito Y, Kuroki M, Sato Y. Map-based cloning of the rice cold tolerance gene ctb1. Plant Sci. 2010:179(1-2):97–102. 10.1016/j.plantsci.2010.04.004 [DOI] [Google Scholar]
- Sato K, Abe F, Mascher M, Haberer G, Gundlach H, Spannagl M, Shirasawa K, Isobe S. Chromosome-scale genome assembly of the transformation-amenable common wheat cultivar ‘fielder’. DNA Res. 2021:28(3):dsab008. 10.1093/dnares/dsab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyari M, Babalar M, Kalantari S, Martínez-Romero D, Guillén F, Serrano M, Valero D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011:124(3):964–970. 10.1016/j.foodchem.2010.07.036 [DOI] [Google Scholar]
- Sayyari M, Babalare M, Kalantari S, Serranoc M, Valerod D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol. Technol. 2009:53(3):152–154. 10.1016/j.postharvbio.2009.03.005 [DOI] [Google Scholar]
- Scott IM, Clarke SM, Wood JE, Mur LAJ. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004:135(2):1040–1049. 10.1104/pp.104.041293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servet C, Conde e Silva N, Zhou D-X. Histone acetyltransferase AtGCN5/HAG1 is a versatile regulator of developmental and inducible gene expression in Arabidopsis. Mol Plant. 2010:3(4):670–677. 10.1093/mp/ssq018 [DOI] [PubMed] [Google Scholar]
- Seydpour F, Sayyari M. Chilling injury in cucumber seedlings amelioration by methyl salicylate. Int J Veg Sci. 2016:22(5):432–441. 10.1080/19315260.2015.1067938 [DOI] [Google Scholar]
- Shewry PR, Hey SJ. The contribution of wheat to human diet and health. Food Energy Secur. 2015:4(3):178–202. 10.1002/fes3.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ding Y, Yang S. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol. 2015:56(1):7–15. 10.1093/pcp/pcu115 [DOI] [PubMed] [Google Scholar]
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell. 2012:24(6):2578–2595. 10.1105/tpc.112.098640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siboza XI, Bertling I, Odindo AO. Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J Plant Physiol. 2014:171(18):1722–1731. 10.1016/j.jplph.2014.05.012 [DOI] [PubMed] [Google Scholar]
- Song N, Lin J, Liu X, Liu Z, Liu D, Chu W, Li J, Chen Y, Chang S, Yang Q, et al. . Histone acetyltransferase TaHAG1 interacts with TaPLATZ5 to activate TaPAD4 expression and positively contributes to powdery mildew resistance in wheat. New Phytol. 2022:236(2):590–607. 10.1111/nph.18372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui P, Jin J, Ye S, Mu C, Gao J, Feng H, Shen W-H, Yu Y, Dong A. H3k36 methylation is critical for brassinosteroid-regulated plant growth and development in rice. Plant J. 2012:70(2):340–347. 10.1111/j.1365-313X.2011.04873.x [DOI] [PubMed] [Google Scholar]
- Sun Y, Fan X-Y, Cao D-M, Tang W, He K, Zhu J-Y, He J-X, Bai M-Y, Zhu S, Oh E, et al. . Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell. 2010:19(5):765–777. 10.1016/j.devcel.2010.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taşgín E, Atící Ö, Nalbantoğlu B. Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul. 2003:41(3):231–236. 10.1023/B:GROW.0000007504.41476.c2 [DOI] [Google Scholar]
- Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H. Cold stress effects on reproductive development in grain crops: an overview. Environ Exp Bot. 2010:67(3):429–443. 10.1016/j.envexpbot.2009.09.004 [DOI] [Google Scholar]
- To TK, Nakaminami K, Kim J-M, Morosawa T, Ishida J, Tanaka M, Yokoyama S, Shinozaki K, Seki M. Arabidopsis HDA6 is required for freezing tolerance. Biochem Biophys Res Commun. 2011:406(3):414–419. 10.1016/j.bbrc.2011.02.058 [DOI] [PubMed] [Google Scholar]
- Trnka M, Rtter RP, Ruiz-Ramos M, Kersebaum KC, Olesen JE, Alud Z, Semenov MA. Adverse weather conditions for European wheat production will become more frequent with climate change. Nat Clim Change. 2014:4(7):637–643. 10.1038/nclimate2242 [DOI] [Google Scholar]
- Venzhik Y, Talanova V, Titov A. The effect of abscisic acid on cold tolerance and chloroplasts ultrastructure in wheat under optimal and cold stress conditions. Acta Physiol Plant. 2016:38(3):63. 10.1007/s11738-016-2082-1 [DOI] [Google Scholar]
- Verma V, Ravindran P, Kumar PP. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016:16(1):86. 10.1186/s12870-016-0771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell. 1994:6(7):959–965. 10.2307/3870006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Seller CA, Hsu P-K, Takahashi Y, Munemasa S, Schroeder JI. Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol. 2022:23(10):680–694. 10.1038/s41580-022-00479-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkowiak S, Gao L, Monat C, Haberer G, Kassa MT, Brinton J, Ramirez-Gonzalez RH, Kolodziej MC, Delorean E, Thambugala D, et al. . Multiple wheat genomes reveal global variation in modern breeding. Nature. 2020:588(7837):277–283. 10.1038/s41586-020-2961-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen X, Dong S, Jiang X, Wang L, Yu J, Zhou Y. Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol J. 2020:18(4):1041–1055. 10.1111/pbi.13272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Chen SJ, Kong WF, Li SH, Archbold DD. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006:41(3):244–251. 10.1016/j.postharvbio.2006.04.010 [DOI] [Google Scholar]
- Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu J-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol. 2014:32(9):947–951. 10.1038/nbt.2969 [DOI] [PubMed] [Google Scholar]
- Yang R, Yang Z, Xing M, Jing Y, Zhang Y, Zhang K, Zhou Y, Zhao H, Qiao W, Sun J. TaBZR1 enhances wheat salt tolerance via promoting ABA biosynthesis and ROS scavenging. J Genet Genomics. 2023:50(11):861–871. 10.1016/j.jgg.2023.09.006 [DOI] [PubMed] [Google Scholar]
- Ye K, Li H, Ding Y, Shi Y, Song C, Gong Z, Yang S. BRASSINOSTEROID-INSENSITIVE2 Negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell. 2019:31(11):2682–2696. 10.1105/tpc.19.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Cang J, Lu Q, Fan B, Xu Q, Li W, Wang X. ABA enhanced cold tolerance of wheat ‘dn1' via increasing ROS scavenging system. Plant Signal Behav. 2020:15(8):1780403. 10.1080/15592324.2020.1780403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Li L, Li L, Guo M, Chory J, Yin Y. Modulation of brassinosteroid-regulated gene expression by Jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc Natl Acad Sci U S A. 2008:105(21):7618–7623. 10.1073/pnas.0802254105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu X, Luo M, Yang S, Wu K. Involvement of histone modifications in plant abiotic stress responses. J Integr Plant Biol. 2013:55(10):892–901. 10.1111/jipb.12060 [DOI] [PubMed] [Google Scholar]
- Zeng R, Li Z, Shi Y, Fu D, Yin P, Cheng J, Jiang C, Yang S. Natural variation in a type-A response regulator confers maize chilling tolerance. Nat Commun. 2021:12(1):4713. 10.1038/s41467-021-25001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Wang L, Fu Y, Zuo J, Li Y, Zhao J, Cao R, Li J. Effects of methyl salicylate pre-treatment on the volatile profiles and key gene expressions in tomatoes stored at low temperature. Front Nutr. 2022:9:1018534. 10.3389/fnut.2022.1018534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Jing Y, Jiang Z, Lin R. The chromatin remodeling factor PICKLE integrates brassinosteroid and gibberellin signaling during skotomorphogenic growth in Arabidopsis. Plant Cell. 2014:26(6):2472–2485. 10.1105/tpc.113.121848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shen L, Li F, Meng D, Sheng J. Methyl salicylate-induced arginine catabolism is associated with up-regulation of polyamine and nitric oxide levels and improves chilling tolerance in cherry tomato fruit. J Agric Food Chem. 2011:59(17):9351–9357. 10.1021/jf201812r [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li J, Pan Y, Li J, Zhou L, Shi H, Zeng Y, Guo H, Yang S, Zheng W, et al. . Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat Commun. 2017:8(1):14788. 10.1038/ncomms14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Peng T, Chen C-Y, Ji R, Gu D, Li T, Zhang D, Tu Y-T, Wu K, Liu X. HY5 interacts with the histone deacetylase HDA15 to repress hypocotyl cell elongation in photomorphogenesis. Plant Physiol. 2019:180(3):1450–1466. 10.1104/pp.19.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Wang L, Wang J, Jin J, Zhang N, Lei L, Gao T, Jing T, Zhang S, Wu Y, et al. . Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. J Integr Plant Biol. 2020:62(10):1461–1468. 10.1111/jipb.12937 [DOI] [PubMed] [Google Scholar]
- Zheng B, Chapman SC, Christopher JT, Frederiks TM, Chenu K. Frost trends and their estimated impact on yield in the Australian wheatbelt. J Exp Bot. 2015:66(12):3611–3623. 10.1093/jxb/erv163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Lin J, Liu X, Chu W, Li J, Gao Y, An K, Song W, Xin M, Yao Y, et al. . Histone acetyltransferase TaHAG1 acts as a crucial regulator to strengthen salt tolerance of hexaploid wheat. Plant Physiol. 2021:186(4):1951–1969. 10.1093/plphys/kiab187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Jeong JC, Zhu Y, Sokolchik I, Miyazaki S, Zhu J-K, Hasegawa PM, Bohnert HJ, Shi H, Yun D-J, et al. . Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc Natl Acad Sci U S A. 2008:105(12):4945–4950. 10.1073/pnas.0801029105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.