Abstract
Introduction:
Polysubstance use and misuse can increase risks for nonfatal and fatal drug overdose. To categorize drugs used in combination in nonfatal overdoses, we analyzed data from emergency department (ED) overdose-related visits in 18 states funded by CDC’s Enhanced State Opioid Overdose Surveillance (ESOOS) program.
Methods:
From 2017 to 2018, 120,706 ED visits included at least one hospital discharge code indicating acute drug poisoning for opioids, stimulants, hallucinogens, cannabis, anti-depressants, sedatives, alcohol, benzodiazepines, or other psychotropic drugs. Latent class analyses were conducted to determine the groupings of drug combinations in overdose visits.
Results:
Latent class analyses indicated a model of 5 classes – mostly heroin overdose (42.5% of visits); mostly non-heroin opioid overdose/use (27.3%); opioid, polysubstance (11.0%); female, younger (< 25 years), other non-opioid drugs (10.5%); female, older (> 55 years), benzodiazepine (8.0%). Findings indicated that heroin continues to be a large burden to EDs, yet EDs are also seeing overdose survivors with polydrug toxicity.
Conclusions:
Medication-assisted treatment could be initiated in the emergency department following overdose for patients with opioid use disorder, and post-overdose protocols, such as naloxone provision and linkage to treatment and harm reduction services, have the potential to prevent future overdose for those at risk.
Keywords: Drug overdose, Emergency department, Opioid, Polysubstance use
1. Introduction
Drug overdoses have substantial burden on morbidity and mortality in the United States (Hedegaard, Bastian, & Trinidad, 2018; Scholl, Seth, & Kariisa, 2018; Seth, Rudd, & Noonan, 2018), and the current drug overdose epidemic is projected to continue to worsen (Jalal,Buchanich, & Roberts, 1979). Opioid overdose mortality rates increased by 12.0% from 2016 to 2017, with 47,600 deaths involving opioids in the United States in 2017 (Centers for Disease Control and Prevention, 2017; Scholl et al., 2018). Sharp increases over time are often attributed to certain types of opioids. The first wave was marked by deaths from prescription opioids beginning in the 1990s; the second wave beginning in 2010 involved increases in heroin-involved deaths. Finally starting in 2013, increases in deaths involving synthetic opioids, likely from illicitly-manufactured fentanyl (IMF), were observed (Centers for Disease Control and Prevention, 2017; Gladden, Martinez, & Seth, 2016; Hedegaard, Warner, & Miniño, 2017; Kolodny, Courtwright, & Hwang, 2015; Rudd et al., 2014, 2016; Scholl et al., 2018).
There are also concurrent increases in other drug overdoses and polydrug use contributing to mortality. The most common in these overdose deaths included opioids, benzodiazepines, and stimulants (Warner, Trindad, & Bastian, 2016). In 2016, opioid-involved deaths had large percentages of concomitant drug involvement, for example 69.2% and 70.5% for fentanyl-related and heroin-related deaths, respectively (Hedegaard et al., 2018; Warner et al., 2016). The substantial increase in opioid-related deaths from 2002 to 2015 is attributed in part to co-occurring use of benzodiazepines and heroin (Kandel, Hu, & Griesler, 2017). An analysis from 2010 to 2016 identified increasing trends in deaths involving synthetic opioids other than methadone in addition to deaths related to the following drugs: other opioids (prescription and illicit), cocaine, psychostimulants, benzodiazepines, and other drug types (e.g., antidepressants, barbiturates) (Jones, Einstein, & Compton, 2018; Kariisa, Scholl, & Wilson, 2019).
Though it is essential to understand which drugs used in combination cause an overdose death, it is equally important to determine polysubstance use contributing to nonfatal overdose. The study of nonfatal opioid overdoses treated in emergency departments (EDs) are of paramount importance for overdose surveillance and intervention. Changes in trends of overdose-related ED visits can signal changing, perhaps worsening, patterns of substance use and misuse before overdose mortality data are available; therefore, nonfatal overdose data can provide an earlier indication of the need for public health action. Because patients who experience a nonfatal overdose are at much higher risk for subsequent nonfatal overdose and/or a fatal overdose (Olfson, Wall, & Wang, 2018), identifying the etiology of these opioid-related ED visits could potentially provide opportunities to intervene and save lives. Nonfatal opioid overdoses are also often associated with complications of pulmonary, cardiovascular, muscular, and renal systems (Darke & Hall, 2003).
Medicaid data show that among those who had a nonfatal opioid overdose, males, persons aged 34 years and older, those recently prescribed benzodiazepines, and those whose previous overdoses involving heroin were more likely to have a subsequent nonfatal or fatal drug overdose within one year (Olfson et al., 2018). Another study of ED visits involving opioid overdoses showed that while non-heroin opioid overdose discharges decreased during 2010–2014, heroin-involved discharges have increased (Guy, Pasalic, & Zhang, 2018).
Although several studies have analyzed ED visit discharge or billing data, there has been a substantial time lag from the date of visit to when data are available for analysis. The strength of ED syndromic surveillance data is rooted in the ability to identify changing disease patterns early, before diagnoses are confirmed, and to help mobilize a rapid response (Henning, 2004). Consequently, ED syndromic data can be analyzed to alert communities of meaningful changes in overdose-related ED visits, in concert with prevention and harm reduction strategies, because of the rapid availability of this information (Ising, Proescholdbell, & Harmon, 2016; Vivolo-Kantor, Seth, & Gladden, 2018). With the inclusion of discharge diagnosis codes, which provide standardized information on clinical care and diagnoses, we can draw from the perspective of medical professionals. Results on self-reported drug use may be biased, and our analysis of discharge codes will complement findings from studies drawing upon surveys (Meacham, Roesch, & Strathdee, 2018), specifically from the National Survey on Drug Use and Health (NSDUH) (Castaldelli-Maia, Andrade, & Keyes, 2016; Ghandour, Martins, & Chilcoat, 2008; Jones, Baldwin, & Compton, 2017; Kandel et al., 2017). Several of these studies have largely focused on defining subgroups within the larger population with opioid use disorder or with self-reported drug use behaviors. There is a lack of information on these persons identified in latent classes when they access emergency care, including emergency department visits and hospitalizations.
Recognizing that drug overdoses are heterogeneous events, the objective of this study is to describe typologies of emergency department visits involving suspected nonfatal drug overdoses. To our knowledge, this is the first latent class analysis to determine overlapping substance-related discharge diagnosis codes among patients treated in the emergency department for drug overdose. Previous research studies using latent class analyses have used participant self-report of drug use with no observation of actual behavior and have focused on persons who use drugs, but do not have necessarily have outcomes that require seeking healthcare (i.e., overdose) (Fong, Matusow, & Cleland, 2015; Kendler, Ohlsson, & Sundquist, 2013; Meacham et al., 2015, 2018; Monga, Rehm, & Fischer, 2007; Scherer, Romano, & Voas, 2018). Our analysis with available data from several states conducting drug overdose surveillance with emergency department data will be able to characterize the classes of patients affected by nonfatal drug overdoses including the combination of drugs used at the time of overdose.
2. Methods
2.1. Dataset
Analyses are based on data from states participating in the Centers for Disease Control and Prevention’s (CDC) Enhanced State Opioid Overdose Surveillance (ESOOS) program (https://www.cdc.gov/drugoverdose/foa/state-opioid-mm.html) and the National Syndromic Surveillance Program (NSSP) (Gould, Walker, & Yoon, 2017). The ESOOS program was implemented to track and analyze ED data with the aim of establishing an early warning system to detect sharp increases (i.e., potential outbreaks) or decreases (e.g., to rapidly identify successful intervention efforts) in nonfatal overdoses including suspected drug overdoses, opioid overdoses, and heroin overdoses. NSSP partners with medical facilities, state and local health departments, vendors for electronic health records (EHR), or health information exchanges (HIE) to transmit data from internal medical record systems to the BioSense Platform so that they can be analyzed in the Electronic Surveillance System for the Early Notification of Community-Based Epidemics (ESSENCE, https://www.cdc.gov/nssp/biosense/onboarding.html). The data sent to NSSP by these partners provide near timely information primarily on visits to hospital EDs, but the system also includes data from outpatient clinics and ambulatory care centers (CDC NSSP, 2018). NSSP requires specific data elements, including information on the date and time of visit, the chief complaint, and discharge diagnosis codes to be shared using HL7 version 2.5.1 (CDC NSSP, 2016). Please see https://www.cdc.gov/nssp/biosense/publications.html for additional information on how NSSP processes data from HL7 messages.
2.2. Sample
Our current analysis includes 18 states from all four US Census regions, who allow the CDC ESOOS team access to their NSSP data for analysis in ESSENCE. A total of 5755 EDs, outpatient clinics, and ambulatory care centers from these states submitted data to NSSP for a total of 142,816,687 visits from January 1, 2017 through December 31, 2018 (Fig. 1). We selected only the 1384 emergency department facilities from these states. We also only selected patients who were treated in the emergency department within these facilities. We limited our sample to ED visits among those persons who were 11 years or older (N = 76,076,306 ED visits) because those over 11 account for most fatal overdoses (Centers for Disease Control and Prevention, 2017). Using this sample, we queried ESSENCE using all diagnosis codes assigned to a visit; each record had a minimum of 1 and maximum of 47 concurrent discharge codes (mean = 5.2; median = 3). Specifically, we queried discharges from the records for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes indicating an initial ED encounter from unintentional or undetermined poisonings from known “drugs of abuse”: T40, T42.3, T42.4, T42.6, T42.7, and T43 (Hedegaard & Johnson, 2019; National Center for Health Statistics, 2019).
Fig. 1.
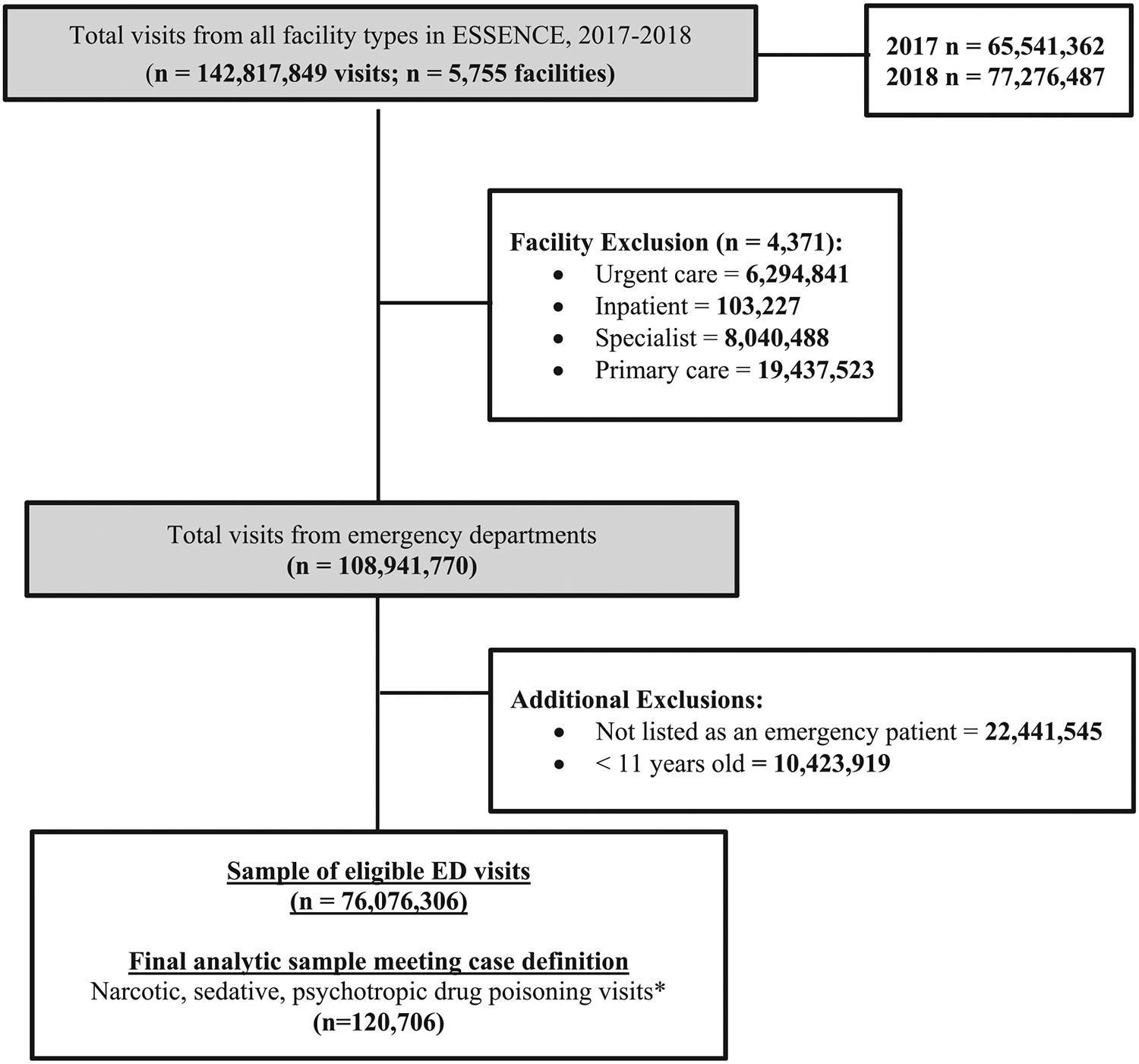
Inclusion criteria of latent class analysis involving Emergency Department visits, 2017–2018 ESOOS data.
A total of 120,706 ED visits in 2017 and 2018 met this criteria and were included in the analysis (Fig. 1). We did not include ED visits with only ICD-10-CM diagnosis codes that did not provide information on the type of drug (e.g., T50.901A or poisoning by unspecified drugs, medicaments and biological substances) and/or drugs not usually abused to get a euphoric or analgesic sensation (e.g., T37.0X1A or poisoning by sulfonamides). The specific variables included to create the indicators are described below; however, to be included in this analysis a visit had to be assigned at least one acute drug poisoning ICD–10–CM diagnosis code. Visits included in our analysis could have one ICD-10-CM acute poisoning code (e.g., only anti-depressant overdose), multiple ICD-10-CM acute poisoning codes (e.g., heroin and cocaine overdose codes), or at least one ICD-10-CM acute poisoning code and at least one ICD-10-CM code for mental and behavioral disorders due to psychoactive substance use (i.e., “F codes”). Records with mental and behavioral disorder codes without any acute poisoning codes (i.e., “T codes”) were not included in our analysis. Visits were only included if they had at least one of the T codes listed above. All future discussion of poisoning and use disorder codes will be referred to as overdose/use.
2.3. Variables
Using both the ICD-10-CM acute poisoning diagnosis codes (i.e., “T codes”) and drug, alcohol, or tobacco use codes (i.e., “F codes”) a total of 13 drug indicators were identified: heroin, non-heroin opioids, marijuana, benzodiazepines, other depressants/sedatives, anti-depressants, cocaine, other stimulants, hallucinogens, alcohol, tobacco, inhalants, and psychoactive/psychotropic drugs (see Table 1). Binary indicators were created to denote the presence of each of the codes associated with the drug indicators. For example, if a visit included codes for heroin poisoning (i.e., T40.1X1A) and benzodiazepine use (i.e., F13) then the visit was coded as yes to “opioid” and yes to “benzodiazepine.”
Table 1.
Description and frequencies of ICD-10-CM diagnosis codes included in ED visit sample at discharge (N = 120,706).
| Drug | ICD-10-CM | Description of code | N (%)a |
|---|---|---|---|
| Heroin | T40.1X1A; T40.1X4A | Poisoning by heroin | 55,298 (45.8) |
| Non-Heroin, opioids | F11 | Opioid-related disorders | |
| T40.0X1A; T40.0X4A | Poisoning by opium | ||
| T40.2X1A; T40.2X4A | Poisoning by other opioids | ||
| T40.3X1A; T40.3X4A | Poisoning by methadone | ||
| T40.4X1A; T40.4X4A | Poisoning by other synthetic narcotics | ||
| T40.601A; T40.604A | Poisoning by other and unspecified narcotics | ||
| T40.691A; T40.694A | Poisoning by other narcotics | 47,864 (39.7) | |
| Tobacco | F17 | Nicotine dependence | |
| O9933-O99335 | Tobacco use disorder complicating pregnancy, childbirth, and the puerperium | 24,126 (20.0) | |
| Benzodiazepines | T42.4X1A; T42.4X4A | Poisoning by benzodiazepines | 11,336 (9.4) |
| Psychoactive/Psychotropic | F19 | Other psychoactive substance-related disorders | |
| T43.8 | Poisoning by other psychotropic drugs | ||
| T43.9 | Poisoning by unspecific psychotropic drug | 8756 (7.3) | |
| Cocaine | F14 | Cocaine-related disorders | |
| T40.5X1A; T40.5X4A | Poisoning by cocaine | 7704 (6.4) | |
| Alcohol | F10 | Alcohol-related disorders | |
| T51.8X1A; T51.8X4A | Toxic effect of alcohol | ||
| T51.9X1A; T51.9X4A | Toxic effect of unspecified alcohol | ||
| Y904 | Blood alcohol level of 80–99 mg/100 ml | ||
| Y905 | Blood alcohol level of 100–119 mg/100 ml | ||
| Y906 | Blood alcohol level of 120–199 mg/100 ml | ||
| Y907 | Blood alcohol level of 200–239 mg/100 ml | ||
| Y908 | Blood alcohol level of 240 mg/100 ml or more | ||
| Y909 | Presence of alcohol in blood, level not specified | 7483 (6.2) | |
| Marijuana | F12 | Cannabis-related disorders | |
| T40.7X1A; T40.7X4A | Poisoning by cannabis | 6945 (5.8) | |
| Other stimulants | F15 | Other stimulant-related disorders | |
| T43.601A; T43.604A | Poisoning by unspecified psychostimulant | ||
| T43.611A; T43.614A | Poisoning by caffeine | ||
| T43.621A; T43.624A | Poisoning by amphetamines | ||
| T43.631A; T43.634A | Poisoning by methylphenidate | ||
| T43.691A; T43.694A | Poisoning by other psychostimulants | 6730 (5.6) | |
| Others | F13 | Sedative, hypnotic, or anxiolytic related disorders | |
| depressants/sedative | T42.3X1A; T42.3X4A | Poisoning by barbiturates | |
| T42.6X1A; T42.6X4A | Poisoning by other antiepileptic and sedative-hypnotic drugs | ||
| T42.7X1A; T42.7X4A | Poisoning by unspecified antiepileptic and sedative-hypnotic drugs | 5805 (4.8) | |
| Anti-depressants | T43.011A; T43.014A | Poisoning by tricyclic antidepressants | |
| T43.021A; T43.024A | Poisoning by tetracyclic antidepressants | ||
| T43.1X1A; T43.1X4A | Poisoning by monoamine-oxidase-inhibitor antidepressants | ||
| T43.2X1A; T43.2X4A | Poisoning by other and unspecified antidepressants | ||
| T43.201A; T43.204A | Poisoning by unspecified antidepressants | ||
| T43.211A; T43.214A | Poisoning by selective serotonin and norepinephrine reuptake inhibitors | ||
| T43.221A; T43.224A | Poisoning by selective serotonin reuptake inhibitors | ||
| T43.291A; T43.294A | Poisoning by other antidepressants | ||
| T43.3X1A; T43.3X4A | Poisoning by phenothiazine antipsychotics and neuroleptics | ||
| T43.4X1A; T43.4X4A | Poisoning by butyrophenone and thiothixene neuroleptics | ||
| T43.501A; T43.504A | Poisoning by unspecified antipsychotics and neuroleptics | ||
| T43.591A; T43.594A | Poisoning by other antipsychotics and neuroleptics | 5807 (4.8) | |
| Hallucinogens | F16 | Hallucinogen-related disorders | |
| T40.8X1A; T40.8X4A | Poisoning by lysergide (LSD) | ||
| T40.901A; T40.904A | Poisoning by unspecified psychodysleptics | ||
| T40.991A; T40.994A | Poisoning by other psychodysleptics | 1025 (0.9) | |
| Inhalants | F18 | Inhalant-related disorders | 42 (0.03) |
Not mutually exclusive categories. Percentages will not add up to 100.
We included two socio-demographic characteristics: sex (male or female) and age group, in years (11–14, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and 85 or older).
2.4. Statistical analyses
A latent class analysis (LCA) approach was used to examine potential classes of concurrent drug, alcohol, and tobacco discharges. We used the package poLCA for R software (Linzer and Lewis, 2011) to perform these analyses by first fitting a null model as our baseline model. Next, we ran a series of nested models with k + 1 classes and used several model fit statistics to compare the k class model to the k + 1 class model. The Akaike’s Information Criterion (AIC), Bayesian Information Criterion (BIC), the log-likelihood (LL), and Model Likelihood Chi-squared were used to assess the best fit. To identify the most optimal model we considered both parsimony with good fit to the data and the most logical classes based on model interpretability to identify the distinct groups. Visits were assigned class membership based on the probability of their particular response profile (Masyn, 2013). All other data management was performed using Statistical Analysis Software, SAS software version 9.4 (Cary, NC).
3. Results
Among the 120,706 ED visits, the prevalence of drug overdose/use at discharge is shown in Table 1. Heroin (45.8%) and non-heroin opioids (39.7%) were the most commonly denoted drug indicators. Each of the other drugs existed in less than or equal to 20% of ED visits: tobacco (20.0%), benzodiazepines (9.4%), psychoactive/psychotropic (7.3%), cocaine (6.4%), alcohol (6.2%), marijuana (5.8%), other stimulants (5.6%), other depressants/sedatives (4.8%), anti-depressants (4.8%), hallucinogens (0.9%), and inhalants (0.03%). The range of drug overlap was 1 (only one drug discharge category indicated) to 9 drug categories during the same visit (mean = 1.6). Our sample was mostly male (61.4%), and persons aged 25–34 years (31.1%) had the most visits when compared to other age groups.
A latent class model consisting of five classes was selected based on fit statistics and by taking into consideration interpretation of the classes (see Appendix Table 3). Fit statistics, such as the BIC, showed improvement with decreasing values as the number of classes increased (1443.8–1372.7 in 2- and 7-class models, respectively). Among the models, the 4- and 5-class models had the lowest model likelihood ratio (LR), Chi-squared statistic (418.4 and 412.5), and similar AIC and BIC. Upon exploring the meaningful interpretation of the groupings of drug indicators in both the 4- and 5-class models, we selected the 5-class model because it had a more logical description (see Fig. 2).
Class 1 accounted for the largest proportion of our sample (42.5%). This group had the largest makeup of males (68.1%) and 25–34 year olds (39.2%), as seen in Table 2. This “mostly heroin overdose” class was distinguished from other classes because it was comprised of visits with 100% probability of heroin overdose. Visits in this class had relatively low percentages for the other drugs defined in this analysis. Class 2 made up 27.3% of the sample and had a slightly lower percentages of males (59.8%) and higher representation of age groups over 55 years (totaling 28.5%), compared to class 1, and had a 0% probability for heroin overdose. This class was differentiated by non-heroin opioid overdose/use (100%; “mostly non-heroin opioid overdose/use”). In addition, this class had relatively low levels of all other drug discharges defined in our analyses.
Table 2.
Characteristics of drugs used and socio-demographics, stratified by latent class (N = 120,706).
| Variable | Total | C1 (42.5%) | C2 (27.3%) | C3 (11.0%) | C4 (10.5%) | C5 (8.0%) |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 61.4 | 68.1 | 59.8 | 67.7 | 44.2 | 41.6 |
| Female | 38.4 | 31.8 | 40 | 32 | 55.5 | 58.1 |
| Age group | ||||||
| 11–14 years | 0.5 | 0 | 0.1 | 0 | 3.9 | 1.4 |
| 15–24 years | 14.4 | 12.9 | 9.7 | 13.7 | 30.3 | 18.9 |
| 25–34 years | 31.1 | 39.2 | 26.2 | 33.5 | 17.3 | 17.5 |
| 35–44 years | 19.2 | 20.7 | 17.9 | 21.5 | 15.5 | 15.6 |
| 45–54 years | 15.5 | 14.7 | 16.9 | 16.8 | 12.2 | 17 |
| 55–64 years | 12.4 | 9.8 | 16.4 | 12 | 10.2 | 16.5 |
| 65–74 years | 4.4 | 1.9 | 7.8 | 2.1 | 6 | 7.9 |
| 75–84 years | 1.3 | 0.1 | 2.8 | 0 | 2.7 | 3.3 |
| 85 years or older | 0.7 | 0.2 | 1.5 | 0 | 1.2 | 1.3 |
| Drug type | ||||||
| Heroin | 45.8 | 100 | 0 | 29.8 | 0 | 0 |
| Non-heroin, opioid | 39.7 | 12.3 | 100 | 46.9 | 2 | 17.2 |
| Tobacco | 20.0 | 15.9 | 11.7 | 51.1 | 14.1 | 19.2 |
| Benzodiazepines | 9.4 | 0.2 | 0 | 11.2 | 1 | 100 |
| Psychoactive/psychotropic | 7.3 | 5.4 | 5.6 | 17.9 | 3.8 | 7.3 |
| Cocaine | 6.4 | 1.3 | 0.7 | 36.9 | 2.1 | 0.8 |
| Alcohol | 6.2 | 2.6 | 3.6 | 16.9 | 6.7 | 13.8 |
| Marijuana | 5.8 | 0.1 | 0.1 | 25.1 | 16.5 | 4.4 |
| Other stimulants | 5.6 | 0.6 | 0.3 | 22.6 | 18.1 | 1.5 |
| Other depressants/sedatives | 4.8 | 0.1 | 1.1 | 9.6 | 22.8 | 10.1 |
| Anti-depressants | 4.8 | 0 | 0.3 | 1.7 | 41.6 | 3.2 |
| Hallucinogen | 0.9 | 0 | 0 | 1.5 | 6.1 | 0 |
| Inhalants | 0.03 | 0 | 0 | 0.1 | 0 | 0 |
| Number of Drug Types | ||||||
| 1 | 62.8 | 67.3 | 77.6 | 10.4 | 77.2 | 46.0 |
| 2 | 24.2 | 25.8 | 20.3 | 26.9 | 17.5 | 34.0 |
| ≥3 | 13.0 | 6.9 | 2.1 | 62.8 | 5.4 | 20.0 |
Note: Class 1: Mostly heroin overdose; Class 2: Mostly non-heroin opioid overdose/use; Class 3: Opioid, polysubstance; Class 4: Female, younger (< 25), other non-opioid drugs; Class 5: female, older (> 55 years), mostly benzodiazepine
Class 3 (11.0% of the sample) had substantial polysubstance use, 62.8% of individuals in this class had 3 or more drug types indicated at the time of the visit. In this class (“opioid, polysubstance”), we saw higher probabilities for males (67.7%), as well as discharges for: non-heroin opioid drug overdose/use (46.9%), marijuana (25.1%), other depressants/sedatives (9.6%), alcohol (16.9%), cocaine (36.9%), other stimulants (22.6%), tobacco (51.1%), and psychoactive/psychotropic drugs (17.9%).
Class 4 (10.5%) consisted mainly had a higher representation of females (55.5%), those ages younger than 25 years (34.2%), and with drugs other than opioids (“female, younger (< 25), other non-opioid drugs”). Compared to other latent classes, this class had the highest probabilities for overdose/use anti-depressants (41.6%) and other depressants/sedatives (22.8%). Finally, the fifth class (“female, older (> 55 years), benzodiazepine”) had the highest percentage among all latent classes for females (58.1%), and age groups above 55 years (29.0%), and other drug discharges such as benzodiazepines (100%), other depressants/sedatives (10.1%), and alcohol (13.8%).
4. Discussion
Our study provides a statistical approach for defining typologies of patient visits for suspected drug overdoses in ED facilities in 18 states throughout the US. Results corroborate the heterogeneous nature of patient visits into emergency departments, which have several different circumstances of drug discharges and characteristics. There were five latent classes identified: 1) mostly heroin overdose, 2) mostly non-heroin opioid overdose/use, 3) opioid, polysubstance, 4) female, younger (< 25 years), other non-opioid drugs, and 5) female, older (> 55 years), benzodiazepine. A better understanding of the groupings of patients based on drugs they have used solely or in combination allows emergency departments, public health entities, and treatment providers the opportunity to better target response and intervention strategies.
Our results indicated the largest latent class was made up of overdose visits involving heroin, almost exclusively, with very low percentages of co-occurring discharges for other drug overdose/use. This is a group at high risk for other diseases, due to routes of transmission from common drug administration behaviors. Heroin is usually injected, and sharing infected injection equipment may lead to increased risk of many adverse outcomes and sequelae, such as abscesses at sites of injection (Binswanger, Takahashi, & Bradley, 2008) and chronic infections from viruses, such as hepatitis C and Human Immunodeficiency Virus (HIV) (Degenhardt, Charlson, & Stanaway, 2016; Garfein, Vlahov, & Galai, 1996).
Class 2 constituted a 100% probability of opioid, non-heroin overdose/use. When compared to Class 1 in general, the individuals were older and had a slightly higher probability of being female. This suggests prescription opioid overdoses with a low probability of other drugs are a large burden of emergency department visits. In addition 98% of those in C2 had two or fewer drug discharges in a given visit. Despite decreases in opioid prescription rates in the United States after implementation of CDC prescribing guidelines (Bohnert, Guy, & Losby, 2018; Guy, Zhang, & Bohm, 2017), non-heroin opioid overdoses still persist.
Our third class had the second-largest probability of opioid, non-heroin overdose/use and had the highest percentage of cocaine overdose/use. Identifying this class as an important subgroup is consistent with recent evidence indicating many adverse health events after patients used cocaine laced with fentanyl (Jones et al., 2017, 2018; Kandel et al., 2017). It is possible that a cocaine-fentanyl drug overdose/use combination falls within this group, but without specific fentanyl overdose/use diagnosis codes in ICD–10–CM, we cannot confirm this hypothesis. Despite the inability to specifically define fentanyl from the ICD-10-CM codes in our analysis, our findings of this group may suggest the need for harm reduction messaging on the potential adulteration of cocaine with IMF or fentanyl analogs. This has important public health relevance because persons using drugs that are tainted with IMF are at higher risk of opioid-related hospitalizations and long term use (Deyo, Hallvik, & Hildebran, 2017) and potentially overdose (Tomassoni, Hawk, & Jubanyik, 2017).
Although demographic characteristics did not differ much between most of the classes, there were notable distinctions. Classes 4 and 5 included higher probabilities of women, and classes 2 and 5 had relatively higher percentages of persons ages over 55 years; in addition, class 5 percentages were higher than the study population for the following substances: benzodiazepine, other depressants, and alcohol. This combination is consistent with literature suggesting that among persons aged ≥65 years who suffer from substance use disorder, women, in particular, experienced more depressive and anxiety symptoms (Ros-Cucurull, Palma-Alvarez, & Daigre, 2018). Studies showed that women were prescribed benzodiazepines more often than men (Olfson, King, & Schoenbaum, 2015; Ros-Cucurull et al., 2018); survey data found that among national ambulatory care visits from 2003 to 2015, there are higher odds for women to use benzodiazepines: OR = 1.31 (95% CI: 1.24–1.38) and in patients ages 45–64 years: OR = 1.40 (95% CI: 1.33–1.48) (Agarwal & Landon, 2019). Co-prescribing of benzodiazepines with opioids may increase risk for long-term opioid use (Skurtveit, Furu, & Bramness, 2010) and fatal overdose (Darke & Hall, 2003). Published guidelines recommend that healthcare providers avoid co-prescribing opioids and benzodiazepines whenever possible (Bohnert, Valenstein, & Bair, 2011; Dowell, Haegerich, & Chou, 2016). The identified, distinct class may have substantial burden, as these overdoses may lead to more ED visits. This class also had a high probability of other depressants or sedatives (e.g. Eszopiclone or Lunesta) overdose/use and alcohol use. Though this group was distinguished by high probability of non-opioid drugs, this class did also have a 17.2% probability for non-heroin opioid overdose/use. Prescription drug monitoring programs (PDMPs) are a promising tool for prescribers to see the history of controlled substance prescribing. Armed with this knowledge, providers can make more informed decisions regarding the types of medications to prescribe to patients (Haegerich, Paulozzi, & Manns, 2014).
Class 4 featured young persons aged 11–24 years, with more discharges for marijuana, other depressants/sedatives, anti-depressants, hallucinogens, and other non-cocaine stimulants (e.g., methampheta-mine). Despite being a smaller latent class, it is important to consider this group because of their young ages. Even though primary prevention strategies may have failed to prevent initiation of drug use, secondary prevention efforts may curb the potential risk of overdose and chronic drug use/use. That said, this class had a low probability of opioid overdose/use discharges.
The classes identified and interpreted based on the latent class analysis results should be explored further. This study had the following limitations. First, the combinations of drug-related discharges may depend on how discharge codes are reported per state and/or ED facility. The poisoning codes used in available ED data can be influenced by patient self-report or observations by ED healthcare providers (Shah, Wood, & Dargan, 2011), therefore the inclusion of drug overdose/use codes in combination with others may be underestimated in ED data. Second, these visits involving patients with overdoses and concurrent use of drugs do not have consistent, uniform testing of biological samples (Wu & Broussard, 2003). The absence of this detailed information makes it difficult to identify drug overdose and use of fentanyl and other emerging drugs. Discharges in this analysis were defined by standardized ICD-10-CM codes, which are intended for billing purposes, and may not necessarily be an accurate clinical portrayal of the ED visit. Third, there were ED visits that were not included in the analysis with discharges such as T50.9, which denote an unknown drug overdose. The unknown drug discharges exist in about one-third of the visits reported to EDs; these overdose visits could not be categorized as a specific drug and may bias our results. This and other data quality issues may vary by state and/or reporting facility, and would likely impact identification of drug overdose/use visits. Fourth, the data lack patient identifiers which prohibits us from linking multiple records to an individual; thus, individuals may present multiple times in our data for repeat overdose. Finally, findings are not necessarily generalizable to jurisdictions not participating in NSSP or ESOOS.
This study has important public health implications, and can assist with the response to drug overdoses in the United States. The categorization of persons who overdose on and use multiple drugs can help target and tailor strategies to prevent future drug overdose. Early intervention in the ED is warranted for patients who receive a multiple drug overdose diagnosis or a single drug overdose diagnosis with other drug use disorder. This is particularly true for younger populations who, in previous research indicated they were unaware of the risks of poly-substance use such as overdose (Lankenau, Teti, & Silva, 2012) and believed individuals overdose only by “taking too much.” (Daniulaityte, Falck, & Carlson, 2012). These findings on the demographic and discharge profiles could help identify the groups at high risk for the various drug combinations so that public health professionals and health care providers could potentially intervene earlier. Post overdose protocols have been implemented in EDs – including the provision of naloxone to those who are at risk for a future opioid overdose (Houry, Haegerich, & Vivolo-Kantor, 2018), on-site, ED-based medication assisted treatment (MAT) with buprenorphine for those with opioid use disorder (D’Onofrio, O’Connor, & Pantalon, 2015), and linkage to other types of care including harm reduction and mental health services (Centers for Disease Control and Prevention, 2018).
In addition to response efforts following an overdose while the patient is still in the ED, health care providers are of utmost importance for the prevention of multiple drug overdose/use. For example, in our analysis, the classes with polysubstance-related visits are prime candidates to benefit from additional health care provider education on the risks of prescribing multiple drugs at once. It is crucial for mid-level providers, such as nurses and other health care providers, to educate patients on the benefits and risks of prescription opioids for better managed care of pain symptoms (Guy & Shults, 2018). Use of multiple substances simultaneously can be dangerous and can increase risks of future nonfatal overdose, fatal overdose, or other adverse health outcomes (Bohnert, Walton, & Cunningham, 2018; Cone, Fant, & Rohay, 2004; Jones, 2013; Olfson et al., 2018).
The description of drug overdose/use typologies is an important step in communicating the drug overlap the demographic characteristics of those who visit EDs for overdose. Future studies of patients seeking care in EDs could allow for the detection of new trends in nonfatal drug overdoses or surrounding circumstances of drug overdoses in the United States. In addition, the identification of patients seeking treatment in EDs for overdoses from multiple types of drugs would allow for a more tailored and rapid response in the ED to prevent future overdose. In addition, a better understanding of how ED visits for polysubstance overdose/use change over time will provide communities an indication of where and when to implement effective prevention strategies.
HIGHLIGHTS.
National syndromic surveillance found 120,706 suspected overdoses, 2017–2018.
Five classes of patients were determined from the emergency department visits.
3 of the classes were heroin overdose; non-heroin opioid overdose/use; polysubstance.
2 of the classes were young females, non-opioid drugs; older female, benzodiazepine.
Classes can target initiation of treatment for patient with drug use disorder.
Acknowledgements
The authors would like to thank the state health departments participating in CDC’s National Syndromic Surveillance Program (NSSP), and the Enhanced State Opioid Overdose Surveillance (ESOOS) program, and members of the Epidemiology and Surveillance Branch in the Division of Overdose Prevention, National Center for Injury Prevention and Control, CDC. In particular, we would also like to thank Dr. Lawrence Scholl in the Division of Overdose Prevention, National Center for Injury Prevention and Control, CDC and Aaron Kite-Powell in the Division of Health Informatics and Surveillance, Center for Surveillance, Epidemiology, and Laboratory Services, CDC for consultation on this manuscript.
Role of funding sources
There are no funding sources for this analysis.
Abbreviations:
- AIC
Akaike’s Information Criterion
- BIC
Bayesian Information Criterion
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- ED
Emergency department
- HER
electronic health records
- ESOOS
Enhanced State Opioid Overdose Surveillance
- ESSENCE
Electronic Surveillance System for the Early Notification of Community-Based Epidemics
- HIE
health information exchanges
- HIV
Human Immunodeficiency Virus
- ICD-10-CM
International Classification of Diseases, Tenth Revision, Clinical Modification
- IMF
illicitly-manufactured fentanyl
- LCA
latent class analysis
- LL
log-likelihood
- LR
likelihood ratio
- NSDUH
National Survey on Drug Use and Health
- NSSP
National Syndromic Surveillance Program
- OR
odds ratio
- PDMP
prescription drug monitoring programs
- SAS
Statistical Analysis Software
Appendix
Fig. 2.
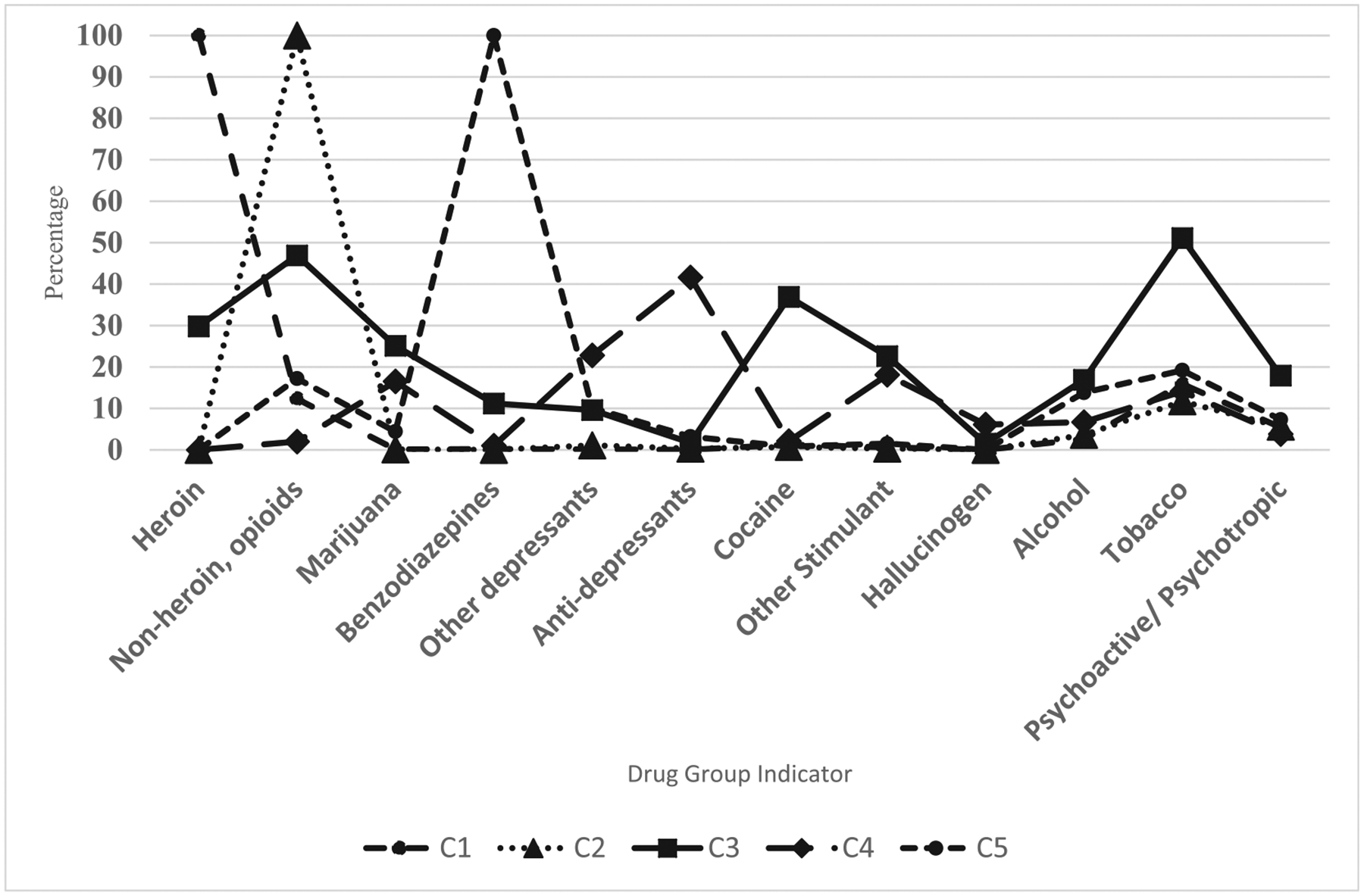
Probability of drug overdose/use diagnosis in the five latent classes identified. Note: Class 1: Mostly heroin overdose; Class 2: Mostly non-heroin opioid overdose/use; Class 3: opioid, polysubstance; Class 4: female, younger (< 25 years), other non-opioid drugs; Class 5: female, older (> 55 years), benzodiazepine.
Table 3.
Model fit indices and model classification diagnostics for up to seven class LCA (N = 120,706).
| Model (K-class) | LL† | Model LR Chi-squared† | df | P-value‡ | BIC† | AIC† |
|---|---|---|---|---|---|---|
| 2-Class | −721.6 | 4799.0 | 51 | < 0.00001 | 1443.8 | 1443.3 |
| 3-Class | −703.5 | 834.5 | 77 | < 0.00001 | 1407.9 | 1407.2 |
| 4-Class | −696.2 | 418.4 | 103 | < 0.00001 | 1393.6 | 1392.6 |
| 5-Class | −691.9 | 412.5 | 129 | < 0.00001 | 1385.4 | 1384.2 |
| 6-Class | −689.1 | 470.5 | 155 | < 0.00001 | 1380.0 | 1378.5 |
| 7-Class | −685.3 | 653.2 | 181 | < 0.00001 | 1372.7 | 1370.9 |
Statistics multiplied by 103.
The p-value is associated the Model LR Chi-squared test statistic.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agarwal S, & Landon BE (2019). Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Network Open, 2(1), e187399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Takahashi TA, Bradley K, et al. (2008). Drug users seeking emergency care for soft tissue infection at high risk for subsequent hospitalization and death. Journal of Studies on Alcohol and Drugs, 69(6), 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert A, Guy G, & Losby J (2018). Opioid prescribing in the united states before and after the centers for disease control and prevention’s 2016 opioid guidance. Annals of Internal Medicine, 169(6), 367–375. 10.7326/M18-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert AS, Valenstein M, Bair MJ, et al. (2011). Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA, 305(13), 1315–1321. [DOI] [PubMed] [Google Scholar]
- Bohnert AS, Walton MA, Cunningham RM, et al. (2018). Overdose and adverse drug event experiences among adult patients in the emergency department. Addictive Behaviors, 86, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldelli-Maia JM, Andrade LH, Keyes KM, et al. (2016). Exploring the latent trait of opioid use disorder criteria among frequent nonmedical prescription opioid users. Journal of Psychiatric Research, 80, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC NSSP (2016). CDC NSSP syndromic surveillance: Data elements of interest. [Accessed 12-01-2018] https://www.cdc.gov/nssp/biosense/docs/SYS-Data-Elements-of-Interest-10-27-2016.pdf.
- CDC NSSP (2018). NSSP update. [Accessed 12-01-2018] https://www.cdc.gov/nssp/news.html.
- Centers for Disease Control and Prevention (2017). National Center for Health Statistics. Underlying Cause of Death 1999–2016 on CDC WONDER Online Database. [Accessed April 10, 2018] https://wonder.cdc.gov/.
- Centers for Disease Control and Prevention (2018). Evidence-based strategies for preventing opioid overdose: What’s working in the United States. Accessed from National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; http://www.cdc.gov/drugoverdose/pdf/pubs/2018-evidence-based-strategies.pdf. [Google Scholar]
- Cone EJ, Fant RV, Rohay JM, et al. (2004). Oxycodone involvement in drug abuse deaths. II. Evidence for toxic multiple drug-drug interactions. Journal of Analytical Toxicology, 28(4), 217–225. [DOI] [PubMed] [Google Scholar]
- Daniulaityte R, Falck R, & Carlson R (2012). I’m not afraid of those ones just ‘cause they’ve been prescribed. International Journal of Drug Policy, 23(5), 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, & Hall W (2003). Heroin overdose: Research and evidence-based intervention. Journal of Urban Health, 80(2), 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Stanaway J, et al. (2016). Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: Findings from the Global Burden of Disease Study 2013. The Lancet Infectious Diseases, 16(12), 1385–1398. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Hallvik SE, Hildebran C, et al. (2017). Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naïve patients: A statewide retrospective cohort study. Journal of General Internal Medicine, 32(1), 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, O’Connor P, Pantalon M, et al. (2015). Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: A randomized clinical trial. JAMA, 313(16), 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, & Chou R (2016). CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recommendations and Reports, 65(1), 1–49. [DOI] [PubMed] [Google Scholar]
- Fong C, Matusow H, Cleland CM, et al. (2015). Characteristics of non-opioid substance misusers among patients enrolling in opioid treatment programs: A latent class analysis. Journal of Addictive Diseases, 34(2–3), 141–150. [DOI] [PubMed] [Google Scholar]
- Garfein R, Vlahov D, Galai N, et al. (1996). Viral infections in short-term injection drug users: The prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. American Journal of Public Health, 86(5), 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandour LA, Martins SS, & Chilcoat HD (2008). Understanding the patterns and distribution of opioid analgesic dependence symptoms using a latent empirical approach. International Journal of Methods in Psychiatric Research, 17(2), 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden R, Martinez P, & Seth P (2016). Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths – 27 states, 2013–2014. Morbidity and Mortality Weekly Report, 65, 837–843. [DOI] [PubMed] [Google Scholar]
- Gould DW, Walker D, & Yoon PW (2017). The evolution of BioSense: Lessons learned and future directions. Public Health Reports, 132(Suppl. 1), 7S–11S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP Jr., Pasalic E, & Zhang K (2018). Emergency department visits involving opioid overdoses, U.S., 2010–2014. American Journal of Preventive Medicine, 54(1), e37–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP Jr., & Shults R (2018). Opioid prescribing in the United States. Evidence-based information for nurses on the risks and benefits of prescription opioids. CDC Vital Signs. [Google Scholar]
- Guy GP Jr, Zhang K, Bohm MK, et al. (2017). Vital signs: Changes in opioid prescribing in the United States, 2006–2015. Morbidity and Mortality Weekly Report, 66(26), 697–704. 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegerich TM, Paulozzi LJ, Manns BJ, et al. (2014). What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug and Alcohol Dependence, 145, 34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Bastian B, Trinidad J, et al. (2018). Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. National vital statistics reports; vol. 67 no. 9. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Hedegaard H, & Johnson R (2019). An updated international classification of diseases, 10th revision, clinical modification (ICD-10-CM) surveillance case definition for injury hospitalizations. National health statistics reports. National Center for Health Statistics. [PubMed] [Google Scholar]
- Hedegaard H, Warner M, & Miniño A (2017). Drug overdose deaths in the United States, 1999–2016. Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Henning KJ (2004). What is syndromic surveillance? MMWR Supplement, 53, 5–11. [PubMed] [Google Scholar]
- Houry DE, Haegerich TM, & Vivolo-Kantor A (2018). Opportunities for prevention and intervention of opioid overdose in the emergency department. Annals of Emergency Medicine, 71(6), 688–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising A, Proescholdbell S, Harmon KJ, et al. (2016). Use of syndromic surveillance data to monitor poisonings and drug overdoses in state and local public health agencies. Injury Prevention, 22(Suppl. 1), i43–i49. [DOI] [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS, et al. (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science, 361(6408). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM (2013). Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002–2004 and 2008–2010. Drug and Alcohol Dependence, 132(1–2), 95–100. [DOI] [PubMed] [Google Scholar]
- Jones CM, Baldwin GT, & Compton WM (2017). Recent increases in cocaine-related overdose deaths and the role of opioids. American Journal of Public Health, 107(3), 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Einstein E, & Compton W (2018). Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010–2016. JAMA, 319(17), 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Hu M-C, Griesler P, et al. (2017). Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug and Alcohol Dependence, 178, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariisa M, Scholl L, Wilson N, et al. (2019). Drug overdose deaths involving cocaine and psychostimulants with abuse potential – United States, 2003–2017. Morbidity and Mortality Weekly Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, et al. (2013). A latent class analysis of drug abuse in a national Swedish sample. Psychological Medicine, 43(10), 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, et al. (2015). The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annual Review of Public Health, 36, 559–574. [DOI] [PubMed] [Google Scholar]
- Lankenau S, Teti M, Silva K, et al. (2012). Patterns of prescription drug misuse among young injection drug users. Journal of Urban Health, 89(6), 1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D, & Lewis J (2011). poLCA: An R package for polytomous variable latent class analysis. Journal of Statistical Software, 42(10). [Google Scholar]
- Masyn K (2013). Latent class analysis and finite mixture modeling. In Little T (Ed.). The Oxford handbook of quantitative methods (pp. 551–611). New York: Oxford University Press. [Google Scholar]
- Meacham MC, Roesch SC, Strathdee SA, et al. (2018). Latent classes of polydrug and polyroute use and associations with human immunodeficiency virus risk behaviours and overdose among people who inject drugs in Tijuana, Baja California, Mexico. Drug and Alcohol Review, 37(1), 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham MC, Rudolph AE, Strathdee SA, et al. (2015). Polydrug use and HIV risk among people who inject heroin in Tijuana, Mexico: A latent class analysis. Substance Use & Misuse, 50(10), 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga N, Rehm J, Fischer B, et al. (2007). Using latent class analysis (LCA) to analyze patterns of drug use in a population of illegal opioid users. Drug and Alcohol Dependence, 88(1), 1–8. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (2019). International classification of diseases, tenth revision, clinical modification (ICD-10-CM).“ Classification of diseases, functioning, and disability. Retrieved July 23, 2019, from https://www.cdc.gov/nchs/icd/icd10cm.htm.
- Olfson M, King M, & Schoenbaum M (2015). Benzodiazepine use in the United States. JAMA Psychiatry, 72(2), 136–142. [DOI] [PubMed] [Google Scholar]
- Olfson M, Wall M, Wang S, et al. (2018). Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug and Alcohol Dependence, 190, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros-Cucurull E, Palma-Alvarez RF, Daigre C, et al. (2018). Sex differences in an old adult sample with substance use disorder: A 6 months follow-up study. Psychiatry Research, 270, 1157–1165. [DOI] [PubMed] [Google Scholar]
- Rudd R, Paulozzi L, Bauer M, et al. (2014). Increases in heroin overdose deaths – 28 states, 2010 to 2012. Morbidity and Mortality Weekly Report, 63(39), 849–854. [PMC free article] [PubMed] [Google Scholar]
- Rudd R, Seth P, David F, et al. (2016). Increases in drug and opioid-involved overdose deaths – United States, 2010–2015. Morbidity and Mortality Weekly Report, 65, 1445014452. [DOI] [PubMed] [Google Scholar]
- Scherer M, Romano E, Voas R, et al. (2018). Latent classes of polydrug users as a predictor of crash involvement and alcohol consumption. Journal of Studies on Alcohol and Drugs, 79(3), 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, et al. (2018). Drug and opioid-involved overdose deaths – United States, 2013–2017. MMWR. Morbidity and mortality weekly report (pp. 67). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Rudd RA, Noonan RK, et al. (2018). Quantifying the epidemic of prescription opioid overdose deaths. American Journal of Public Health, 108(4), 500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Wood D, & Dargan P (2011). Survey of ICD-10 coding of hospital admissions in the UK due to recreational drug toxicity. QJM, 104, 779–784. [DOI] [PubMed] [Google Scholar]
- Skurtveit S, Furu K, Bramness J, et al. (2010). Benzodiazepines predict use of opioids– A follow-up study of 17,074 men and women. Pain Medicine, 11(6), 805–814. [DOI] [PubMed] [Google Scholar]
- Tomassoni AJ, Hawk KF, Jubanyik K, et al. (2017). Multiple fentanyl overdoses – New Haven, Connecticut, June 23, 2016. Morbidity and Mortality Weekly Report, 66(4), 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivolo-Kantor AM, Seth P, Gladden RM, et al. (2018). Vital signs: Trends in emergency department visits for suspected opioid overdoses – United States, July 2016–September 2017. Morbidity and Mortality Weekly Report, 67(9), 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Trindad J, Bastian B, et al. (2016). Drugs most frequently involved in drug overdose deaths: United States, 2010–2014. National vital statistics reports; Vol. 65 No. 1. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Wu AHMC, Broussard LA, et al. (2003). National academy of clinical biochemistry laboratory medicine practice guidelines: Recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clinical Chemistry, 49, 357–379. [DOI] [PubMed] [Google Scholar]


