Abstract
Lateral branches are important components of shoot architecture and directly affect crop yield and production cost. Although sporadic studies have implicated abscisic acid (ABA) biosynthesis in axillary bud outgrowth, the function of ABA catabolism and its upstream regulators in shoot branching remain elusive. Here, we showed that the MADS-box transcription factor AGAMOUS-LIKE 16 (CsAGL16) is a positive regulator of axillary bud outgrowth in cucumber (Cucumis sativus). Functional disruption of CsAGL16 led to reduced bud outgrowth, whereas overexpression of CsAGL16 resulted in enhanced branching. CsAGL16 directly binds to the promoter of the ABA 8′-hydroxylase gene CsCYP707A4 and promotes its expression. Loss of CsCYP707A4 function inhibited axillary bud outgrowth and increased ABA levels. Elevated expression of CsCYP707A4 or treatment with an ABA biosynthesis inhibitor largely rescued the Csagl16 mutant phenotype. Moreover, cucumber General Regulatory Factor 1 (CsGRF1) interacts with CsAGL16 and antagonizes CsAGL16-mediated CsCYP707A4 activation. Disruption of CsGRF1 resulted in elongated branches and decreased ABA levels in the axillary buds. The Csagl16 Csgrf1 double mutant exhibited a branching phenotype resembling that of the Csagl16 single mutant. Therefore, our data suggest that the CsAGL16–CsGRF1 module regulates axillary bud outgrowth via CsCYP707A4-mediated ABA catabolism in cucumber. Our findings provide a strategy to manipulate ABA levels in axillary buds during crop breeding to produce desirable branching phenotypes.
CsAGL16 positively regulates axillary bud outgrowth in cucumber by directly promoting CsCYP707A4-mediated ABA catabolism, and CsGRF1 interacts with CsAGL16 and antagonizes the CsAGL16-mediated CsCYP707A4 activation.
Introduction
Lateral branching is one of the most important components of shoot architecture that directly affects crop yield and production cost. In cereal crops such as rice (Oryza sativa) and wheat (Triticum aestivum), the number of branches determines the quantity of flowers and seeds, thereby influencing the final yield. In horticultural crops like cucumber (Cucumis sativus), tomato (Solanum lycopersicum), and watermelon (Citrullus lanatus), lateral branches often need to be manually removed to minimize nutrition competition and disease occurrence, which lead to an increase in production cost. Therefore, dissecting the regulatory mechanisms of lateral branch development is of considerable agronomic value for crop breeding with ideal shoot architecture.
Development of lateral branches is a highly plastic process that can be divided into 3 stages: axillary bud initiation, dormancy, and outgrowth (Leyser 2003). Under favorable conditions, certain axillary buds directly progress to the outgrowth stage immediately after initiation (Fu et al. 2014; Xu et al. 2016; Chen et al. 2019). Whether axillary buds enter the dormancy stage or continue to grow after initiation is coregulated by multiple hormone signaling pathways (Greb et al. 2003; Schmitz and Theres 2005). Auxin inhibits bud outgrowth by suppressing auxin efflux from these buds (Ongaro and Leyser 2007; Balla et al. 2011, 2016). Overexpression (OE) of the rice PIN-FORMED (PIN) auxin efflux carrier, OsPIN2, resulted in increased tiller formation (Chen et al. 2012). BRANCHED1/TEOSINTE BRANCHED1 (BRC1/TB1) is an important negative regulator for branching (Aguilar-Martínez et al. 2007; Wang et al. 2019; Long et al. 2022). In cucumber, CsBRC1 inhibits the expression of CsPIN3 in axillary buds, leading to impaired auxin efflux and inhibition of axillary bud outgrowth (Shen et al. 2019). Cytokinin was shown to downregulate the expression of PsBRC1 and stimulate bud outgrowth in pea (Pisum sativum) (Braun et al. 2012). The Arabidopsis (Arabidopsis thaliana) altered meristem program1 mutants exhibited elevated cytokinin levels, increased lateral branches, and decreased BRC1 expression levels (Helliwell et al. 2001). Strigolactones (SLs) inhibit axillary bud outgrowth through impeding auxin efflux in axillary buds (Crawford et al. 2010; Domagalska and Leyser 2011). Exogenous auxin application could upregulate the transcription of SL biosynthetic genes (Sorefan et al. 2003; Foo et al. 2005; Arite et al. 2009; Hayward et al. 2009). In Arabidopsis and rice, mutation of the SL biosynthesis gene DWARF27 (D27) led to increased lateral branches/tillers (Arite et al. 2007; Lin et al. 2009; Waters et al. 2012). DWARF14 (D14) is a receptor for SL, and its mutation resulted in enhanced branching in Arabidopsis (Yao et al. 2016; Seto et al. 2019). The repressor protein DWARF53 (D53) of the SL signaling pathway can interact with D14 and positively regulate tiller development (Jiang et al. 2013; Zhou et al. 2013). Moreover, SL can induce the expression of BRC1, and brc1 mutants show insensitivity to SL, indicating that BRC1 functions downstream of SL (Aguilar-Martínez et al. 2007; Dun et al. 2012). Therefore, complicated crosstalk exists among the auxin, cytokinin, and SL signaling pathways, and BRC1/TB1 serves as the critical hub for multiple signals during axillary bud outgrowth (Aguilar-Martínez et al. 2007; Leyser 2009; Wang et al. 2019).
The AGAMOUS-LIKE 17 (AGL17)-like clade is a small subset of the MADS-box transcription factor family. In Arabidopsis, there are 4 genes in the AGL17-like clade (Alvarez-Buylla et al. 2000; Par̆enicová et al. 2003), in which AGL16 and AGL17 regulate flowering through the APETALA1 and LEAFY pathways (Han et al. 2008; Hu et al. 2014; Dong et al. 2023). AGL16 also acts as a negative regulator in the salt and drought stress response of Arabidopsis (Zhao et al. 2020, 2021). There are 5 AGL17-like clade members in rice: OsMADS23, OsMADS25, OsMADS27, OsMADS57, and OsMADS61 (Puig et al. 2013). OsMADS23 promotes the biosynthesis of endogenous abscisic acid (ABA) by activating the transcription of target genes 9-CIS-EPOXYCAROTENOID DIOXYGENASE 2 (OsNCED2), OsNCED3, and OsNCED4, thereby enhancing drought and salt tolerance of rice (Li et al. 2021). OsMADS57 inhibits the expression of the SL receptor D14, thereby positively regulating rice tillering. Interestingly, OsTB1 interacts with OsMADS57 to alleviate the inhibitory effect on D14 during tiller development (Guo et al. 2013).
ABA has been well characterized and shown to play critical roles in plant stress responses such as drought and salt stresses (Waadt et al. 2022; Wang, Zhou, et al. 2023). In addition, several studies showed that there is a negative correlation between ABA content and bud activity (Tamas et al. 1979; Knox and Wareing 1984 ; Mader et al. 2003). Exogenous application of ABA inhibited the growth of axillary buds in pea (Arney and Mitchell 1969), Arabidopsis (Chatfield et al. 2000), and tomato (Cline and Oh 2006). Disruption of ABA biosynthesis resulted in increased lateral branching (Reddy et al. 2013; Yao and Finlayson 2015). In Arabidopsis, BRC1 directly activates the expression of HOMEOBOX PROTEIN21 (HB21), HB40, and HB53, which in turn promotes the transcription of NCED3, resulting in ABA accumulation in axillary buds and inhibition of bud outgrowth (González-Grandío et al. 2017). In cotton (Gossypium hirsutum), GhBRC1 directly binds to the promoter of GhNCED1 and activates its transcription to repress branch outgrowth (Zhan et al. 2021).
Notably, the ABA level in a plant depends on a delicate balance between biosynthesis and catabolism. A key step in ABA catabolism is ABA 8′-hydroxylation, which is catalyzed by the enzyme ABA 8′-hydroxylase encoded by CYP707A genes (Cutler and Krochko 1999; Nambara and Marion-Poll 2005). In Arabidopsis, there are 4 CYP707A genes that mainly function in seed dormancy (Kushiro et al. 2004; Millar et al. 2006; Okamoto et al. 2006). Similarly, in barley (Hordeum vulgare), RNAi lines targeting the CYP707A gene showed higher ABA level and increased grain dormancy (Gubler et al. 2008). CYP707A is also found to be involved in carotenoid content in cucumber fruit (Wang, Jin, et al. 2023). To date, the role of ABA catabolism and its upstream regulators during shoot branching remain mysterious.
Cucumber is a vegetable crop cultivated worldwide, with great nutritional and economic value. The majority of cucumbers are used for the fresh market, which requires indeterminant growth for continuous harvesting. To obtain maximum yield and minimum disease occurrence, lateral branches need to be manually removed, which dramatically enhances labor costs. Thus, dissecting the regulatory mechanism of lateral branch development is of critical importance for cucumber production. Here, we demonstrated that CsAGL16 promotes axillary bud outgrowth by stimulating the expression of the ABA 8′-hydroxylase gene CsCYP707A4 and thus reduces the ABA level in the axillary bud. We also revealed that General Regulatory Factor 1 (CsGRF1) interacts with CsAGL16 at the protein level, and such interaction attenuates the CsAGL16-mediated CsCYP707A4 transcriptional activation. Together, we identified a CsAGL16–CsGRF1 module regulating lateral branch outgrowth via ABA catabolism in cucumber.
Results
CsAGL16 positively regulates lateral branch outgrowth in cucumber
Lateral branching is an agronomic trait that adversely affects fresh cucumber production due to the elevated cost of manual removal of branches. The AGL17-like clade member OsMADS57 has been found to positively regulate axillary bud outgrowth in rice through the SL signaling pathway (Guo et al. 2013). Here, we explored the AGL17-like clade members in cucumber, in which CsAGL16 (CsaV3_3G048150) is a homologous gene of Arabidopsis AGL16 and rice OsMADS57 (Supplementary Fig. S1). CsAGL16 is highly expressed in lateral branches and flowers (Fig. 1A). In situ hybridization results further confirmed the enrichment of CsAGL16 transcripts in shoot apical meristem (SAM), lateral organ primordia (LOP), and flower buds (Fig. 1, B to E).
Figure 1.
Mutation of CsAGL16 resulted in inhibited lateral branch outgrowth in cucumber. A) Expression analyses of CsAGL16 by qRT-PCR in different tissues from R1461 inbred line. CsUBI was used as internal standard. B to E) In situ hybridization analysis of CsAGL16. The expression signals of CsAGL16 were detected in the SAM and LOP B) and flower buds C). The CsAGL16 sense probe SP6 was hybridized as a control D to E). Scale bars: 50 μm. F) Mutation forms of 2 homozygous Csagl16 mutants mediated by CRISPR/Cas9 system. The premature stop codons of Csagl16 #1 line occurs in the C-terminal domain, while that of Csagl16 #2 line occurs in the K-domain. Target sequences and PAM are marked in red and blue lines. Red dashes/nucleotides represent deletions or insertions, respectively. Deletion/insertion nucleotides are marked as a minus or plus above the sequence. The dashed box shows the corresponding protein premature termination. The green boxes on the right indicate CsAGL16 protein coding region, and the gray boxes indicate missense sequences resulting from frameshift mutations. G) Representative images of WI1983M and Csagl16 mutants grown in the autumn. Scale bars: 10 cm. H) Diagrammatic data showed the position and length of lateral branches of WI1983M and Csagl16 mutants. Each column represents an individual cucumber plant. Each layer specifies a node in the plant. Green squares represent no bud outgrowth, and yellow squares represent bud outgrowth longer than 4 cm. I) The average branch number per plant of WI1983M and Csagl16 mutants. J) The average lateral branch length at each node in the WI1983M and Csagl16 lines. K) The total lateral branch length of a single plant in the WI1983M and Csagl16 lines. Significance analysis was conducted with the 2-tailed Student's t-test (**P < 0.01). Values are means ± Sd (n = 3). aa, amino acid; LB, lateral branch; PE, petal primordium; PAM, protospacer-adjacent motif; ST, stamen primordium.
To investigate the biological function of CsAGL16, we employed the CRISPR/Cas9 system to perform gene editing, resulting in the generation of 2 homozygous lines. Csagl16 #1 and #2 displayed an insertion of 1 bp and a deletion of 536 bp, respectively, both leading to premature termination (Fig. 1F). Phenotypic characterization showed that Csagl16 mutants exhibited a significant reduction in the number and length of lateral branches compared with the wild-type WI1983M (Fig. 1, G and H). Statistical analysis showed that the average number of lateral branches per plant in the WI1983M line was 6.7, whereas only 3.0 and 3.7 in the 2 Csagl16 mutants (Fig. 1I). Furthermore, the average length of lateral branches at each node in the Csagl16 mutants was shorter than that of WI1983M plants (Fig. 1J). The total branch length per WI1983M plant was approximately 148.2 cm, whereas the Csagl16 mutant lines were 64.5 and 62.8 cm, respectively, exhibiting a 56.5% to 57.6% reduction (Fig. 1K). These results demonstrated that functional disruption of CsAGL16 greatly inhibits axillary bud outgrowth in cucumber.
In order to verify the role of CsAGL16 in branch development, we overexpressed CsAGL16 in the cucumber inbred line R1461, which produces few branches. Two independent lines were generated (OE #1 and OE #2). Compared with wild-type R1461, the expression of CsAGL16 was upregulated 44.1- and 37.5-fold in the OE lines (Fig. 2A). Immunoblot analysis confirmed the accumulation of CsAGL16 protein in OE lines (Fig. 2A). As expected, both OE lines exhibited a substantial increase in the number and length of lateral branches (Fig. 2, B and C). In R1461 plants, only 37.5% of lateral branches exceeded 50 cm in length, while 55.2% and 45.8% of lateral branches exceeded 50 cm in length in the OE lines (Fig. 2C). Quantification data showed that R1461 line averaged 5.3 lateral branches per plant, but OE #1 and OE #2 averaged 9.7 and 8.0 branches, respectively (Fig. 2D). The average length of lateral branches at each node in the R1461 was shorter than that of OE plants (Fig. 2E). The total lateral branch length per R1461 plant was 229.7 cm, whereas 546.5 and 365.0 cm in OE lines (Fig. 2F). Therefore, CsAGL16 serves as a positive regulator for lateral branch outgrowth in cucumber.
Figure 2.
OE of CsAGL16 promoted lateral branch outgrowth in cucumber. A) Expression and protein level analyses of CsAGL16 in OE plants. CsUBI was used as internal standard. The CsAGL16 protein was detected in OE lines using anti-Flag antibody. The Ponceau S of Rubisco large subunit was demonstrated as a loading control. B) Representative images of R1461 and CsAGL16-OE plants grown in the autumn. Scale bars: 10 cm. C) Diagrammatic data showed the position and length of lateral branches of R1461 and CsAGL16-OE lines. Each column represents an individual cucumber plant. Each layer specifies a node in the cucumber plant. Green squares represent no bud outgrowth, yellow squares represent bud outgrowth longer than 4 cm, and brown squares represent lateral branch length ≥ 50 cm. D) The average branch number per plant of R1461 and OE lines. E) The average lateral branch length at each node in the R1461 and OE lines. F) The total lateral branch length of a single plant in the R1461 and OE lines. Significance analysis was conducted with the 2-tailed Student's t-test (*P < 0.05 and **P < 0.01). Values are means ± Sd (n = 3). LB, lateral branch; OE, CsAGL16 overexpression line.
CsAGL16 directly promotes the expression of CsCYP707A4 in cucumber
To investigate the downstream targets of CsAGL16, we performed RNA-seq analysis using the OE lines as experimental material, resulting in the identification of 270 differentially expressed genes (DEGs) (Supplementary Data Set S1). Given the important role of ABA in branch development, we were particularly interested in the ABA 8′-hydroxylase gene (CsaV3_4G034320) in the upregulated DEGs, which is the homologous gene of CYP707A4 in Arabidopsis. Therefore, we designated this gene as CsCYP707A4 hereinafter. The expression of CsCYP707A4 was decreased to approximately 26% in the Csagl16 mutants (Fig. 3A), while increased about 3-fold in the OE lines (Fig. 3B), suggesting that CsAGL16 promotes the transcription of CsCYP707A4. Reverse transcription quantitative PCR (RT-qPCR) analysis showed that CsCYP707A4 exhibited the highest expression in axillary buds, followed by stems and plant tips (Fig. 3C). In situ hybridization assay showed that CsCYP707A4 was mainly expressed in LOP (Fig. 3C), partially overlapping with the expression pattern of CsAGL16.
Figure 3.
CsCYP707A4 acts downstream of CsAGL16. A, B) The relative expression of CsCYP707A4 in axillary buds of WI1983M and Csagl16 mutants A) and R1461 and OE lines B). C) Expression pattern analyses of CsCYP707A4 by RT-qPCR and in situ hybridization. CsUBI was used as internal standard in RT-qPCR. In in situ hybridization assay, the expression signals of CsCYP707A4 were detected in LOP and the CsCYP707A4 sense probe SP6 was hybridized as a control. Scale bars: 50 μm. D) Schematic diagram of the distribution of CArG-box in CsCYP707A4 promoter. Red asterisks represent the locations of putative CArG-box. P1, P2, P3, and P4 indicate the positions of P1 (429 bp), P2 (361 bp), P3 (356 bp), and P4 (360 bp) fragments used in yeast 1-hybrid. E) Yeast 1-hybrid assay indicates that CsAGL16 can bind to the P2 fragment. Blue bacterial plaque indicates the presence of interaction. F) ChIP-qPCR analyses showed that CsAGL16 binds to the P22 fragment of CsCYP707A4 in vivo. The Tubulin was used as internal standard. G) In dual-LUC reporter analysis, proCsCYP707A4:LUC and proCsCYP707A4△P2:LUC were used as the reporter, and 35S::CsAGL16 and 62-SK empty vector were used as the effector. H) Dual-LUC reporter analysis. The data indicated that CsAGL16 activates the expression of CsCYP707A4, but not proCsCYP707A4△P2. The empty vector 62-SK was used as the control. I, J) ABA concentration in axillary buds of Csagl16 mutants I) and OE lines J). Significance analysis was conducted with the 2-tailed Student's t-test (*P < 0.05 and **P < 0.01). Values are means ± Sd (n = 3 in A) to C), I), and J) and n = 9 in H). OE, CsAGL16 overexpression line.
To explore whether CsAGL16 can directly bind to CsCYP707A4, we analyzed the promoter region of 2,000-bp upstream of the start codon of CsCYP707A4 and identified 5 putative CsAGL16-binding sites (CArG-box). We further performed yeast 1-hybrid assays with the 4 fragments (P1 to P4) containing the binding sites (as shown in Fig. 3D). As shown in Fig. 3E, CsAGL16 can directly bind to the P2 fragment (Fig. 3E). Next, we divided the P2 fragment into 3 fragments: P21, P22, and P23, and performed ChIP-qPCR assay with these 3 fragments. The results showed that the P22 fragment was significantly enriched, rather than P21 and P23 (Fig. 3F). Further analysis by yeast 1-hybrid revealed that CsAGL16 was unable to bind to the P2 fragment without the P22 fragment, suggesting that the P22 fragment is required for CsAGL16 binding (Fig. 3E).
To further verify whether CsAGL16 can activate the expression of CsCYP707A4, we conducted a dual-luciferase (LUC) reporter analysis. Compared with the control group of 62-SK, a significant increase in LUC enzyme activity was detected upon CsAGL16 and ProCsCYP707A4:LUC coinjection into Nicotiana benthamiana leaves (Fig. 3, G and H). Consistently, CsAGL16 was unable to activate the expression of CsCYP707A4 without the P2 fragment (Fig. 3, G and H). These results indicated that CsAGL16 can directly bind to the P2 fragment of CsCYP707A4 promoter and activate its expression.
CsCYP707A4 regulates ABA levels through catabolic pathways (Cutler and Krochko 1999; Nambara and Marion-Poll 2005). Therefore, we detected the ABA content in the axillary buds of Csagl16 mutants and OE lines. Compared with WT, the level of ABA in Csagl16 mutants increased by 28.2% and that of OE lines decreased by 15.5% (Fig. 3, I and J). These results implied that CsAGL16 may promote branch outgrowth through stimulating CsCYP707A4-mediated ABA catabolism in cucumber.
Knockdown of CsCYP707A4 inhibits lateral branch outgrowth of cucumber
To investigate the role of CsCYP707A4 in regulating axillary bud outgrowth, we generated gene-edited lines of CsCYP707A4 and obtained 2 independent transgenic lines, Cscyp707a4 #1 and Cscyp707a4 #2. Both mutations occurred at the second target site, resulting in deletions of 13 and 160 bp, respectively, leading to premature termination (Fig. 4A). Compared with the wild-type WI1983M, the length and number of lateral branches were significantly reduced in Cscyp707a4 mutants (Fig. 4, B and C). WI1983M exhibited an average of 15.7 lateral branches per plant, with 53.2% of them exceeding 50 cm in length, while Cscyp707a4 #1 and Cscyp707a4 #2 had an average of 12.3 and 11.3 lateral branches per plant, with only 37.8% and 23.5% branches longer than 50 cm, respectively (Fig. 4, C and D). The average lateral branch length at each node was substantially shorter in the Cscyp707a4 mutants (Fig. 4E). We calculated the total length of lateral branches for each plant. The results showed that the total length of lateral branches in Cscyp707a4 #1 and Cscyp707a4 #2 decreased 43.6% and 59.1% compared with wild-type WI1983M (Fig. 4F). Consistent with the role in ABA catabolism, the ABA level in the axillary buds of Cscyp707a4 mutants increased 33.0% compared with WI1983M (Fig. 4G). These data suggested that CsCYP707A4 positively regulates axillary branch outgrowth through ABA catabolism in cucumber.
Figure 4.
Cscyp707a4 mutants displayed reduced lateral branches in cucumber. A) Mutation forms of 2 homozygous Cscyp707a4 mutants mediated via CRISPR/Cas9 system. Target sequences and PAM sites were indicated below the red and blue lines. Red dashes represent base deletions, and deletion nucleotides are marked as a minus above the sequence. The dashed box on the right shows the corresponding premature terminated protein. The red boxes indicate CsCYP707A4 protein coding region, and the gray indicates missense sequences resulting from frameshift mutations. B) Representative images of WI1983M and Cscyp707a4 mutants grown in the spring. Scale bars: 10 cm. C) Diagrammatic data show the position and length of lateral branches of WI1983M and Cscyp707a4 mutants. Each column represents an individual cucumber plant. Each layer represents a node in the cucumber plant. Green squares: no bud outgrowth; yellow squares: bud outgrowth ≥ 4 cm; brown squares: lateral branch length ≥ 50 cm. D) The average branch number per plant of WI1983M and Cscyp707a4 mutants. E) The average lateral branch length at each node in the WI1983M and Cscyp707a4 mutants. F) The total lateral branch length of a single plant in WI1983M and Cscyp707a4 mutants. G) ABA concentration in axillary buds of WI1983M and Cscyp707a4 mutants. Significance analysis was conducted with the 2-tailed Student's t-test (*P < 0.05 and **P < 0.01). Values are means ± Sd (n = 3). aa, amino acid; FW, fresh weight; LB, lateral branch; PAM, protospacer-adjacent motif.
Elevated expression of CsCYP707A4 can rescue axillary bud outgrowth in Csagl16 mutants
To explore the genetic relationship between CsAGL16 and CsCYP707A4 during branch development, we employed CsAGL16 promoter driving the expression of CsCYP707A4 in the Csagl16 mutant background. Two independent lines, pAC (ProCsAGL16:CsCYP707A4 Csagl16) #1 and #2 (Fig. 5, A and B), were obtained. RT-qPCR analysis showed that the expression of CsCYP707A4 in pAC #1 and #2 was 5.0 and 6.7 times higher than that in the Csagl16 mutants and 2.3- and 3.1-fold increase compared with wild-type WI1983M, respectively (Fig. 5C). Phenotypic observations showed that the elevated expression of CsCYP707A4 substantially promoted the outgrowth of axillary buds in the Csagl16 mutants (Fig. 5, A and B). The Csagl16 mutant had an average of 5.3 lateral branches per plant, while pAC #1 and #2 had 13.7 and 14.7 lateral branches (Fig. 5D). Similarly, the average lateral branch length at each node in the pAC plants was longer than that in Csagl16 mutants (Fig. 5E). The total length of lateral branches per plant in pAC #1 and #2 lines were 2.7 and 2.9 times higher than that in Csagl16 mutants, accounting for 66.2% and 70.2% of wild-type WI1983M (Fig. 5F). Therefore, the elevated expression of CsCYP707A4 can largely rescue the branching phenotype in the Csagl16 mutants, indicating that the function of CsAGL16 is dependent on the CsCYP707A4-mediated ABA catabolism pathway during branch outgrowth in cucumber.
Figure 5.
OE of CsCYP707A4 in Csagl16 mutants can partially restore the lateral branch phenotype. A) Representative images of WI1983M, Csagl16 mutants, and pAC (proCsAGL16:CsCYP707A4 Csagl16) plants grown in the spring. Scale bars: 10 cm. B) Diagrammatic data show the position and length of lateral branches of WI1983M, Csagl16 mutants, and pAC lines. Each column represents an individual cucumber plant. Each layer represents a node in the cucumber plant. Green squares represent no bud outgrowth, yellow squares represent bud outgrowth longer than 4 cm, and brown squares represent lateral branch length ≥ 50 cm. C) The relative expression analyses of CsCYP707A4 in WI1983M, Csagl16, and pAC plants by RT-qPCR. CsUBI was used as internal standard. D) The average branch number per plant of WI1983M, Csagl16, and pAC plants. E) The average lateral branch length at each node in Csagl16 and pAC plants. F) The total lateral branch length of a single plant in WI1983M, Csagl16, and pAC plants. Significance analysis was conducted with the 2-tailed Student's t-test (**P < 0.01). Values are means ± Sd (n = 3). LB, lateral branch; ns, no significant difference.
CsAGL16 functions through the ABA metabolism pathway in cucumber
To further validate the effect of ABA on lateral branch development, we exogenously applied Na2WO4 or ABA in cucumber. Na2WO4 was reported to be an inhibitor of ABA biosynthesis in plants (Milborrow and Lee 1997; Hansen and Grossmann 2000). Considering that the ABA level was increased in the Csagl16 mutants, we treated the axillary buds of Csagl16 mutants with Na2WO4. Following Na2WO4 treatment, both the number and length of lateral branches increased in wild-type WI1983M and Csagl16 (Fig. 6, A and C). In the untreated control group, WI1983M and Csagl16 had approximately 10.7 and 4.3 lateral branches, respectively. However, in the Na2WO4-treated group, WI1983M and Csagl16 exhibited 12.0 and 8.0 lateral branches, respectively (Fig. 6C). For the total lateral branches length per plant, Na2WO4 treatment resulted in an increase of 31.1% and 134.1% in WI1983M and Csagl16 mutant lines, respectively (Fig. 6D). Therefore, exogenous application of Na2WO4 partially alleviated the inhibitory effect of ABA on axillary bud outgrowth in the Csagl16 mutants.
Figure 6.
ABA level in axillary buds affects the outgrowth of lateral branch in CsAGL16 transgenic lines. A, B) Representative images of WI1983M and Csagl16 plants treated with Na2WO4A) and R1461 and OE plants treated with ABA B) grown in the spring. Scale bars: 10 cm. C) Diagrammatic data showed the position and length of lateral branches in different lines. Each column represents an individual cucumber plant. Each layer represents a node in the cucumber plant. Green squares: no bud outgrowth; yellow squares: bud outgrowth ≥ 4 cm; brown squares: lateral branch length ≥ 50 cm. D, E) The total branch length of each plant upon Na2WO4D) or ABA treatment E). Through 1-way ANOVA analysis with Tukey's Studentized Range (HSD) test, different letters indicate significant differences (P < 0.05). Values are means ± Sd (n = 3). LB, lateral branch; OE, CsAGL16 overexpression line.
Considering that the ABA level was decreased in the CsAGL16-OE plants, we applied ABA to the axillary buds of R1461 and OE plants. The statistical results showed that spraying ABA significantly inhibited the outgrowth of axillary buds in R1461 and OE plants (Fig. 6, B and C). In the control, R1461 and OE plants had 4.3 and 6.3 lateral branches, respectively. However, following ABA treatment, the number of lateral branches in R1461 and OE plants decreased to 2.7 and 3.0, respectively (Fig. 6C). The total lateral branch length of OE lines was significantly greater than that of the WT before ABA treatment (control), while the total branch length was comparable between WT and OE lines after ABA treatment, suggesting that ABA can rescue the phenotype of OE lines (Fig. 6E). These results indicated that ABA inhibits axillary bud outgrowth, and CsAGL16 functions through the ABA metabolism pathway in cucumber.
CsAGL16-mediated transcriptional activation of CsCYP707A4 was attenuated by CsGRF1
In order to search for interacting proteins of CsAGL16, we performed immunoprecipitation MS (IP-MS) analysis. Among the results obtained, we found a gene with the Gene Symbol LOC101210822 (Supplementary Data Set S2), is classified as a 14-3-3 family member (CsaV3_3G047630) in the Cucurbitaceae genome database. Through phylogenetic analysis, we found that CsaV3_3G047630 is a homologous gene of Arabidopsis GENERAL REGULATORY FACTOR1 (AtGRF1), AtGRF2, and AtGRF4 (Supplementary Fig. S2). Consequently, we named it CsGRF1 hereinafter. Previous studies showed that GRFs are ubiquitously expressed and exist in almost all eukaryotic organisms, mediating the activities of metabolic enzymes and transcription factors (Ichimura et al. 1987; Robinson et al. 1994).
To validate the interaction between CsGRF1 and CsAGL16, we conducted a yeast 2-hybrid assay. The result indicated that yeast cells cotransformed with CsGRF1 and CsAGL16 can grow in the synthetic dropout nutrient medium (SD/-Trp-Leu-His-Ade) (QDO) (Fig. 7A). Next, we performed a firefly LUC complementation imaging assay in N. benthamiana, which revealed an interaction between CsGRF1 and CsAGL16 (Fig. 7B). Moreover, coimmunoprecipitation (co-IP) tests were performed, and the data showed that nFlag-CsAGL16 can be identified when coexpressed with CsGRF1-GFP in N. benthamiana leaves, but not with negative control GFP (Fig. 7C). These results demonstrated that CsGRF1 and CsAGL16 can interact at the protein level both in vitro and in vivo.
Figure 7.
CsGRF1 interacts with CsAGL16 at protein level. A) Yeast 2-hybrid assay indicated that CsGRF1 interacts with CsAGL16. T-AD and 53-BD were used as positive controls. B) Firefly LUC complementation assay demonstrated the interaction between CsGRF1 and CsAGL16. The transition from blue to green indicates a gradual enhancement of the interaction signal. C) Immunoblotting assay in which the CsGRF1 and CsAGL16 protein in total and precipitated proteins were detected using anti-GFP or anti-Flag antibody. D) Diagram of the dual-LUC reporter analysis. The proCsCYP707A4:LUC was used as the reporter, 35S::CsAGL16, 35S::CsGRF1, and 62-SK empty vector were used as effector. E) Dual-LUC reporter analysis indicated that CsGRF1 antagonizes the transcriptional activation of CsCYP707A4 by CsAGL16. The empty vector 62-SK was used as the control. Through 1-way ANOVA analysis with Tukey's Studentized Range (HSD) test, different letters indicate significant differences (P < 0.01). Values are means ± Sd (n = 9). F) The protein levels of CsAGL16 and CsGRF1 used for dual-LUC reporter analysis were detected by immunoblot. AD, GAL4 activation domain; BD, GAL4 DNA binding domain.
In order to analyze the biological significance of the interaction between CsGRF1 and CsAGL16, we conducted dual-LUC reporter assay. The results showed that the expression of proCsCYP707A4:LUC was unaffected by CsGRF1. However, when CsGRF1 and CsAGL16 were cotransformed, the activity of proCsCYP707A4:LUC was significantly reduced compared with that of CsAGL16 alone (Fig. 7, D and E). Immunoblot analysis indicated that the protein level of CsAGL16 and CsGRF1 in cotransformation was similar to that of single transformation (Fig. 7F). These results suggested that CsGRF1 interacts with CsAGL16 and antagonizes the transcriptional activation of CsCYP707A4 by CsAGL16.
CsGRF1 negatively regulates lateral bud outgrowth through ABA catabolism
To validate the function of CsGRF1 during branch development, we analyzed the spatiotemporal expression pattern of CsGRF1. RT-qPCR analysis showed that CsGRF1 had the highest expression level in lateral branches (Fig. 8A). In situ hybridization data indicated that strong signals of CsGRF1 expression were detected in the SAM and LOP. In addition, high expression of CsGRF1 was found in the vascular system of leaf primordia and stem (Fig. 8, B and C). Next, we constructed knockout lines of CsGRF1 using the CRISPR/Cas9 system and obtained 2 independent lines (Csgrf1 #1 and Csgrf1 #2). Csgrf1 #1 and #2 lost 1 and 16 bp, respectively, both resulting in premature termination (Fig. 8D). Compared with wild-type WI1983M, Csgrf1 mutants showed an increase in axillary bud outgrowth (Fig. 8, E and F). The average number of lateral branches per plant was 5.3 in WI1983M, while 6.7 and 6.3 in Csgrf1 #1 and Csgrf1 #2, respectively (Fig. 8G). The branch length at each node of Csgrf1 lines was also greater than that of WI1983M (Fig. 8H). The total length of lateral branches per plant in Csgrf1 #1 and Csgrf1 #2 was 2.2 and 1.7 times that of WI1983M plants (Fig. 8I). We next detected the expression level of CsCYP707A4 in the Csgrf1 mutants. The results showed that the expression of CsCYP707A4 was upregulated 2.8- and 2.6-fold in Csgrf1 #1 and Csgrf1 #2, respectively, compared with WI1983M (Fig. 8J). Consistently, the ABA level in the axillary buds decreased by 20.2% in Csgrf1 mutants compared with WI1983M (Fig. 8K). These data demonstrated that CsGRF1 inhibits axillary bud outgrowth through attenuating the CsCYP707A4-mediated ABA catabolism in cucumber.
Figure 8.
Mutation of CsGRF1 stimulated lateral branch outgrowth in cucumber. A) Expression analyses of CsGRF1 by RT-qPCR in different tissues of R1461 inbred line. CsUBI was used as internal standard. B, C) In situ hybridization analysis of CsGRF1. The expression signals of CsGRF1 were detected in the SAM and LOP B). The CsGRF1 sense probe SP6 was hybridized as a control C). Scale bars: 50 μm. D) Mutation forms of CsGRF1 mediated by CRISPR/Cas9 system. Target sequences and PAM are indicated below the red and blue lines, respectively. Red dashes represent base deletions, and deletion nucleotides are marked as a minus above the sequence. The dashed box on the right shows the corresponding protein premature termination. The blue boxes indicate CsGRF1 protein coding region, and gray boxes represent missense sequences resulting from frameshift mutations. E) Representative images of WI1983M, Csgrf1 #1 and Csgrf1 #2 plants grown in the autumn. Scale bars: 10 cm. F) Diagram of the position and length of lateral branches of WI1983M, Csgrf1 #1 and Csgrf1 #2 lines. Each column represents an individual cucumber plant. Each layer represents a node in the cucumber plant. Green squares: no bud outgrowth. Yellow squares: bud outgrowth ≥ 4 cm. Brown squares: lateral branch length ≥ 50 cm. G) The average branch number per plant of WI1983M and Csgrf1 mutants. H) The average lateral branch length at each node in the WI1983M, Csgrf1 #1 and Csgrf1 #2 lines. I) The total lateral branch length of a single plant in the WI1983M, Csgrf1 #1 and Csgrf1 #2 lines. J) The relative expression of CsCYP707A4 in axillary buds of WI1983M, Csgrf1 #1 and Csgrf1 #2 lines. K) ABA concentration in axillary buds of WI1983M and Csgrf1 mutants. Significance analysis was conducted with the 2-tailed Student's t-test (*P < 0.05 and **P < 0.01). Values are means ± Sd (n = 3). aa amino acid; FW, fresh weight; LB, lateral branch; PAM, protospacer-adjacent motif.
Genetic analysis of CsAGL16 and CsGRF1 in cucumber
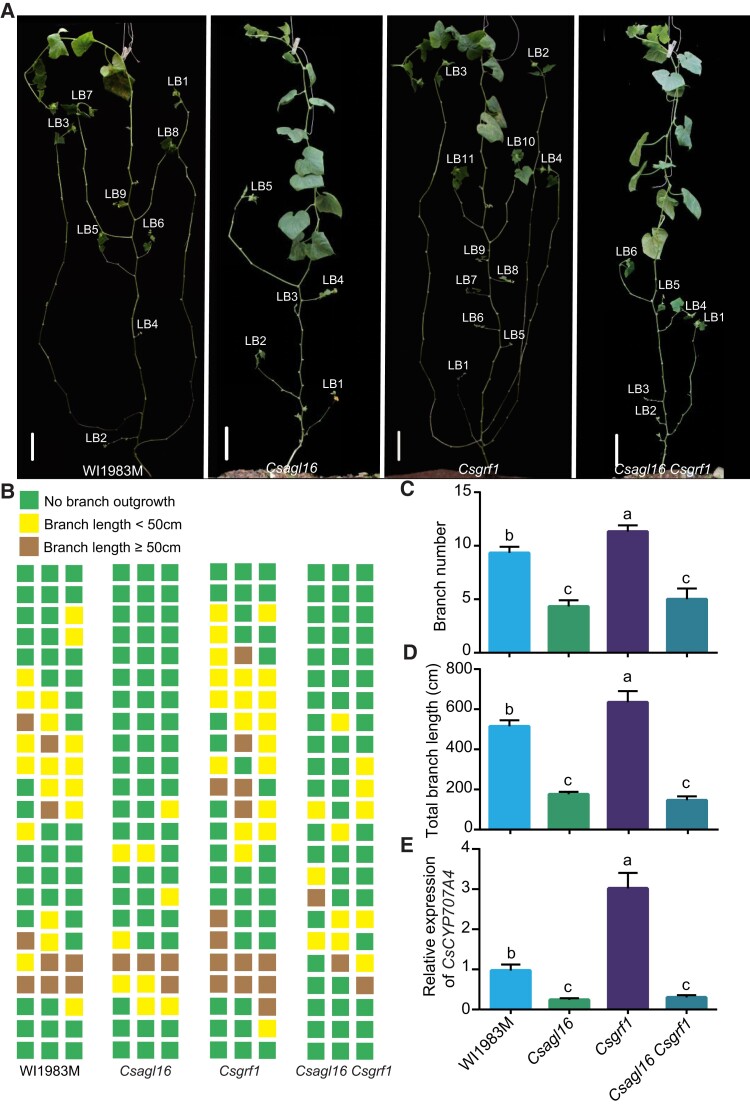
To explore the genetic relationship between CsAGL16 and CsGRF1, we constructed the double mutant of Csagl16 Csgrf1. Consistent with the antagonistic relationship between CsAGL16 and CsGRF1, the single mutants Csagl16 and Csgrf1 displayed opposite phenotypes with regard to branch number and branch length, as well as contrasting CsCYP707A4 expression (Fig. 9). The Csagl16 Csgrf1 double mutant exhibited similar branching phenotypes to that of Csagl16 mutant (Fig. 9, A and B). The average number of lateral branches per plant was 4.3 and 5.0 in Csagl16 and Csagl16 Csgrf1, respectively, which was significantly reduced compared with WI1983M or Csgrf1 (Fig. 9C). Similarly, the total branch length of Csagl16 Csgrf1 resembled that of the Csagl16 single mutant (Fig. 9D). In contrast to the enhanced CsCYP707A4 expression of Csgrf1 single mutant, the expression of CsCYP707A4 in Csagl16 Csgrf1 double mutants was dramatically reduced, mimicking that of Csagl16 single mutant (Fig. 9E). These data suggested that CsAGL16 and CsGRF1 act in the same genetic pathway, and CsGRF1 functions genetically upstream of CsAGL16 during lateral branch development in cucumber.
Figure 9.
Phenotypic analysis of Csagl16 Csgrf1 double mutant. A) Representative images of WI1983M, Csagl16, Csgrf1 and Csagl16 Csgrf1 plants grown in the autumn. Scale bars: 10 cm. B) Diagrammatic data showed the position and length of lateral branches of WI1983M, Csagl16, Csgrf1, and Csagl16 Csgrf1 lines. Each column represents an individual cucumber plant. Each layer represents a node in the cucumber plant. Green squares: no bud outgrowth; yellow squares: bud outgrowth ≥ 4 cm; brown squares: lateral branch length ≥ 50 cm. C) The average branch number per plant of WI1983M, Csagl16, Csgrf1, and Csagl16 Csgrf1 mutant plants. D) The total lateral branch length of a single plant in the WI1983M, Csagl16, Csgrf1, and Csagl16 Csgrf1 lines. E) The relative expression of CsCYP707A4 in axillary buds of WI1983M, Csagl16, Csgrf1, and Csagl16 Csgrf lines. Through 1-way ANOVA analysis with Tukey's Studentized Range (HSD) test, different letters indicate significant differences (P < 0.05). Values are means ± Sd (n = 3). LB, lateral branch.
Discussion
CsAGL16 promotes axillary bud outgrowth via CsCYP707A4-mediated ABA catabolism in cucumber
MADS-box transcription factors were found to function in flowering regulation, fruit ripening, and abiotic stress responses (Theissen et al. 2000; Fang and Fernandez 2002; Vrebalov et al. 2002; Par̆enicová et al. 2003; Arora et al. 2007). In this study, we characterized the function CsAGL16 in cucumber, the homologous gene of Arabidopsis AGL16 and rice OsMADS57. AGL16 was reported to regulate flowering, as well as salt and drought response in Arabidopsis (Hu et al. 2014; Zhao et al. 2020, 2021; Dong et al. 2023). OsMADS57 was shown to promote rice tillering through inhibiting the expression of SL receptor D14 (Guo et al. 2013). Unlike its homologs, we found that CsAGL16 stimulates axillary bud outgrowth via ABA catabolism. Functional disruption of CsAGL16 led to reduced bud outgrowth, whereas OE of CsAGL16 resulted in enhanced branching (Figs. 1 and 2). Further, we demonstrated that CsAGL16 directly binds to the promoter of ABA 8′-hydroxylase gene CsCYP707A4 and promotes its expression (Fig. 3, D to H). Loss of function of CsCYP707A4 led to inhibited axillary bud outgrowth (Fig. 4). Elevated expression of CsCYP707A4 or treatment with ABA biosynthesis inhibitor Na2WO4 can largely rescue the Csagl16 mutant phenotype (Figs. 5 and 6). Therefore, CsAGL16 promotes axillary bud outgrowth via CsCYP707A4-mediated ABA catabolism in cucumber.
Previous studies of ABA in regulating branch development have focused on ABA biosynthesis genes NCEDs. Elevated expression of OsNCED1 has been shown to inhibit tiller growth in rice (Luo et al. 2019). BRC1 inhibits axillary bud outgrowth through indirectly promoting the expression of NCED3 (González-Grandío et al. 2017), whereas GhBRC1 directly activates GhNCED1 expression in cotton (Zhan et al. 2021). Here, we provide evidence that CsAGL16 stimulates shoot branching via promoting ABA catabolism in cucumber. Notably, the branching phenotype in the Cscyp707a4 mutants was less severe compared with Csagl16 mutants (Figs. 1 and 4). OE of CsCYP707A4 in the Csagl16 mutants only partially recovered the axillary bud outgrowth (Fig. 5), suggesting that additional downstream targets other than ABA pathway exist in the CsAGL16-mediated shoot branching in cucumber.
Interestingly, trans-β-carotene is a common precursor substance of ABA and SL biosynthesis that is regulated by D27 protein family (Tolnai et al. 2023). IDEAL PLANT ARCHITECTURE1, as a direct downstream target gene of SL, directly promotes the expression of TB1 in rice (Lu et al. 2013; Kerr and Beveridge 2017). TB1/BRC1 can stimulate ABA biosynthesis by promoting the expression of ABA biosynthesis genes NCEDs (González-Grandío et al. 2017; Zhan et al. 2021). Therefore, there is a complex regulatory network between ABA and SL signaling pathways during lateral branch development. Here, our data demonstrated that CsAGL16 positively regulates cucumber bud outgrowth through directly stimulating CsCYP707A4-mediated ABA catabolism. Considering the CsAGL16 homologous gene OsMADS57 in rice promotes tiller development through inhibiting the transcription of SL receptor D14 (Guo et al. 2013), it is plausible to speculate that AGL16/MADS57 may act as an important hub of ABA and SL signaling pathways during lateral branch development.
CsGRF1 interacts with CsAGL16 to attenuate CsCYP707A4 activation during cucumber axillary bud outgrowth
GRF proteins were found to participate in multiple metabolic and signaling processes through interacting with target proteins in a sequence-specific and phosphorylation-dependent manner (Muslin et al. 1996; Sehnke et al. 2001; Bridges and Moorhead 2004). The Arabidopsis GRF proteins regulate the activities of metabolic enzymes including starch synthase (Sehnke et al. 2001), glucose synthase, peroxidase, affeate o-methyl transferase (Finnie et al. 1999), and plasma membrane H+-ATPase (Korthout and de Boer 1994; Marra et al. 1994; Oecking et al. 1994). GRF proteins can specifically interact with phosphorylated form of BRASSINAZOLE-RESISTANT 1 protein, thereby playing an important role in brassinosteroid (BR) signal transduction (Gampala et al. 2007). Here, we found that Csgrf1 mutants displayed elongated branches (Fig. 8), elevated expression of CsCYP707A4, and decreased ABA in the axillary buds (Fig. 8, J and K). However, CsGRF1 was unable to directly inhibit CsCYP707A4 expression (Fig. 7E). Instead, CsGRF1 interacts with CsAGL16 at the protein level (Fig. 7, A to C), and such interaction attenuated the transcriptional activation of CsCYP707A4 by CsAGL16 (Fig. 7E). Csagl16 Csgrf1 double mutant exhibited branching phenotype resemble of Csagl16 single mutant (Fig. 9), suggesting CsGRF1 functions genetically upstream of CsAGL16. Thus, our data suggested that CsGRF1 regulates lateral bud outgrowth by interacting with CsAGL16 and antagonizing the CsAGL16-mediated CsCYP707A4 activation to fine-tune ABA level in cucumber. It needs further investigation whether CsGRF1 mediates BR signaling pathway and what is the link between BR and ABA pathways during shoot branching in cucumber.
Excessive lateral branching in cucumber can adversely affect planting density, nutrient competition, and ultimately yield. Our study identified the CsAGL16–CsGRF1 module regulating axillary bud outgrowth through ABA catabolism in cucumber. CsAGL16 directly activates the expression of CsCYP707A4, which in turn promotes ABA catabolism in axillary buds. This leads to a reduction in ABA level and promotion of axillary bud outgrowth (Fig. 10A). CsGRF1 can inhibit CsAGL16-mediated CsCYP707A4 activation by interaction with CsAGL16, resulting in an increase in ABA level and inhibition of bud outgrowth (Fig. 10B). Thus, CsGRF1 antagonizes CsAGL16 to fine-tune ABA level during branch development in cucumber. In the future, we can manipulate ABA level in axillary buds by modulating the expression of AGL16, CYP707A4, and GRF1 during crop breeding with desired branching. To obtain crops with more branches, we can elevate the expression of AGL16 and CYP707A4 or knockdown of GRF1 through gene editing in the promoter or coding region. On the contrary, downregulation of AGL16 and CYP707A4 or upregulation of GRF1 can be utilized in crop breeding with less branches.
Figure 10.
The working model of CsAGL16–CsGRF1 regulates axillary bud outgrowth via CsCYP707A4-mediated ABA catabolism in cucumber. A) CsAGL16 directly binds to the promoter of CsCYP707A4 and activates its expression, resulted in elevated ABA catabolism and stimulation of axillary bud outgrowth. B) CsGRF1 can interact with CsAGL16 at the protein level, which inhibits the transcriptional activation of CsCYP707A4 by CsAGL16, leading to a reduction of catabolized ABA, increased ABA level in axillary buds and inhibition of lateral bud outgrowth. Arrow represents promotion. T-shaped represents inhibition.
Materials and methods
Plant materials and growth conditions
Cucumber (C. sativus) inbred lines R1461 (with few lateral branches) and WI1983M (with multiple lateral branches) were used for gene expression analysis and genetic transformation. After the soaked seeds germinate in the dark at 28 °C, they are cultured in a growth chamber (660 to 700 LUX, LED bulb, 16-h light/8-h dark) until the second true leaf fully expanded and then transplanted to a standard greenhouse of China Agricultural University. Water and fertilizer management and pest control are carried out according to standard conditions. N. benthamiana plants were grown in a 25 °C growth chamber under long-day condition (660 to 700 LUX, LED bulb, 16-h light/8-h dark) for 6 wk and used for biochemical analysis.
Phylogenetic analysis
In order to analyze the homologous proteins of the target gene, all amino acid sequences encoding the target gene in Arabidopsis (A. thaliana) were used as queries for BLASTp search on NCBI. Use the Muscle 3.6 program (http://www.drive5.com/muscle/) to align the full-length amino acid sequences of all proteins, and the alignment obtained was manually adjusted in GeneDoc 3.2. In MEGA6.0, ClusterW was used to align amino acid sequences and a phylogenetic tree was generated using the neighbor-joining method with 1,000 bootstrap replications (Tamura et al. 2013). The gene information is listed in Supplementary Table S1 and alignments and machine-readable tree files for phylogenetic analysis are provided in Supplementary Files S1 to S6.
RNA extraction and expression analysis
Samples were thoroughly grinded in liquid nitrogen, and the total RNA was extracted using Eastep Super Total RNA Extraction Kit (Promega, Madison, WI, USA) and reverse-transcribed into cDNA using FastKing gDNA Dispelling RT SuperMix Kit (Tiangen, Beijing, China). RT-qPCR analysis was performed using TB Green Premix Ex TaqTM II (Takara, Kyoto, Japan) on CFX384 Real-Time PCR System (BIO-RAD, Hercules, CA, USA). The cucumber Ubiquitin gene was used as an internal reference gene (Wan et al. 2010). Three biological replicates were performed for each gene, and each biological replicate contains 3 technical replicates. The primer information is listed in Supplementary Table S2.
Genetic transformation of cucumber
In order to obtain the CRISPR/Cas9 gene-editing plants of target genes, specific sgRNA target sites were designed and selected using the CRISPR-P 2.0 website (http://crispr.hzau.edu.cn/CRISPR2/). Using vector pCBC-DT1T2 as a template, a PCR fragment containing 2 sgRNA target sites was generated using the amplification method of 4 partially overlapping primers and then inserted into the vector pKSE402 using Bsa I site and T4 ligase (Xing et al. 2014; Hu et al. 2017). To obtain OE lines of CsAGL16, the CsAGL16 full-length coding sequence was constructed into the intermediate vector pUC19-nflag using Sal I and Pst I sites to generate pUC19-nflag-CsAGL16 construct. Then, the nflag-CsAGL16 fragment was amplified by PCR using the recombinant vector as a template and connected to the pCAMBIA1305 vector using Xba I and BstEⅡ sites to generate the final OE vector pro35S:nflag-CsAGL16. Similarly, in order to obtain OE plants of CsCYP707A4, the full-length CsCYP707A4 coding sequence was constructed into the intermediate vector pUC19-cflag using Xho I and BstB I sites to generate the vector pUC19-CsCYP707A4-cflag. Then, the vector was used as a template for PCR amplification to produce a CsCYP707A4-cflag fragment, and the promoter fragment of 2,000-bp upstream of CsAGL16 start codon regions (proCsAGL16) was amplified by PCR. Next, CsCYP707A4-cflag and proCsAGL16 fragments were constructed into the pCAMBIA1305 vector (Sal I and BstEⅡ sites) using a multifragment recombinant enzyme, resulting in the final OE vector proCsAGL16:CsCYP707A4-cflag. The resultant vectors were transformed into Agrobacterium (Agrobacterium tumefaciens) strain EHA105, and genetic transformation of cucumber was carried out using the Agrobacterium-mediated cotyledon transformation method (Hu et al. 2017). All constructed vectors carry GFP fluorescent markers. The primer information is listed in Supplementary Table S2.
In situ hybridization
The shoot apexes of 23-d-old cucumber seedlings were harvested and quickly fixed in 3.7% (v/v) formal-acetic-alcohol fixing solution (50-ml anhydrous ethanol, 5-ml glacial acetic acid, 10-ml 37% formaldehyde, and 35-ml DEPC-H2O) and stored them at 4 °C until use. In situ hybridization follows the previously published method (Zhang et al. 2013; Gu et al. 2018). Probe primers were designed in the specific regions of target genes, and sense and antisense probes were amplified using SP6 and T7 RNA polymerase, respectively, according to the instructions of the DIG RNA Labeling Kit (Roche, Basel, Switzerland). The primer sequences are listed in Supplementary Table S2.
Yeast 1-hybrid assay
The full-length coding sequence of CsAGL16 was cloned into vector pB42AD using EcoR I and Xho I sites. The CArG-box elements on the 2,000-bp promoter sequence upstream of CsCYP707A4 coding regions were searched and constructed the P1 (429 bp), P2 (361 bp), P3 (356 bp), P4 (360 bp), and P2 without P22 (P2△P22) into pLacZi2u using EcoR I and Xho I sites. Then, the CsAGL16-AD recombinant plasmid and the LacZ reporter driven by fragments of CsCYP707A4 were cotransformed into the yeast (Saccharomyces cerevisiae) strain EGY48. The yeast transformants were coated on SD/-Trp-Ura screening agar plate and cultured at 30 °C for 4 d to screen yeast-cotransformed strains. Subsequently, the screened cotransformed strains were transferred to SD/Gal/Raf/-Trp-Ura agar plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) to verify their interaction. The colonies harboring a bait–prey pair that can interact will turn blue (Wang et al. 2021). Yeast transformation refers to the Yeast Protocols Handbook (Clontech) (Li et al. 2010). The primer information is listed in Supplementary Table S2.
Yeast 2-hybrid assay
The full-length coding sequence of CsAGL16 and CsGRF1 was constructed into the pGADT7 or pGBKT7 vector using EcoR I and BamH I sites. The activation domain (AD) and binding domain (BD) recombinant plasmids were cotransformed into yeast strain Y2Hgold. Yeast transformation refers to the Yeast Protocols Handbook (Clontech) (Li et al. 2010). Yeast transformants were grown on synthetic dropout nutrient medium (SD/-Trp-Leu) (DDO) screening agar plates and cultured at 30 °C for 4 d to screen yeast cotransformant strains. Then, the screened cotransformed strains were transferred to synthetic dropout nutrient medium (SD/-Trp-Leu-His-Ade) (QDO) to verify their interaction. The primer information is listed in Supplementary Table S2.
Dual-LUC reporter analysis
The CsAGL16 and CsGRF1 full-length coding sequences were cloned into pGreenII 62-SK (BamH I and Hind Ⅲ sites) as effectors. The 2,000-bp promoter sequence upstream of CsCYP707A4 coding regions and proCsCYP707A4ΔP2 was constructed into pGreenII0800-LUC (Hind Ⅲ and BamH I sites) as reporters. The recombinant plasmid was transformed into Agrobacterium GV3101 (pSoup-p19). Agrobacterium-containing effectors and reporters were mixed in a 9:1 ratio and injected into N. benthamiana leaves (half leaf). Agrobacterium-containing pGreenII 62-SK empty plasmid and reporter genes were mixed and injected into the other half leaf of N. benthamiana as a negative control. The Molecular Devices SpectraMax i3x instrument was used to read the activity values of firefly LUC and Renilla reniformis luciferase (REN). The average of 9 sets of LUC/REN values was calculated to obtain the final result. The primer information is listed in Supplementary Table S2.
co-IP analysis
The coding sequence of CsGRF1 without the termination codon was constructed into the pCAMBIA1300-GFP vector using Sal I and Kpn I sites. The CsAGL16 protein expression vector served as the OE vector (pro35S:nflag-CsAGL16). The recombinant plasmids were transformed into Agrobacterium strain GV3101 and cotransfected with P19 Agrobacterium into N. benthamiana leaves. After 48 h of transfection, samples were collected and ground into powder in liquid nitrogen, and then extraction buffer was added. IP was performed with anti-GFP nanobody-coated agarose beads (KT-HEALTH, China, Catalog No. KTSM1301) at 4 °C for 3 h and the precipitate was collected and washed 3 to 5 times with washing buffer, denatured the precipitate at 100 °C. Subsequently, the precipitate was separated by SDS–PAGE and detected by immunoblotting with anti-GFP (TransGen Biotech, Beijing, China, catalog number HT801) or anti-FLAG (Sigma Aldrich, Burlington, MA, USA; catalog number F3165) antibodies. The primer information is listed in Supplementary Table S2.
Na2WO4 or ABA treatment in cucumber
Na2WO4 or ABA spraying treatment was applied when cucumber plants grown in the field reached 15 nodes. The axillary buds of wild-type WI1983M and Csagl16 mutants were sprayed with 6 mM Na2WO4, spraying once every 2 d for a total of 3 treatments. Phenotypic analysis was performed after 21 d of treatment. ABA was sprayed on the axillary buds of R1461 and CsAGL16 OE lines at a concentration of 10 μM, once every 2 d for a total of 5 treatments. After 21 d of treatment, phenotypes analysis was performed.
Accession numbers
The accession numbers for genes in this study are as follows: CsAGL16 (CsaV3_3G048150), CsCYP707A4 (CsaV3_4G034320), CsGRF1 (CsaV3_3G047630), and CsUBI (CsaV3_5G031430).
Supplementary Material
Acknowledgments
We are grateful to Professor Sanwen Huang (Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences) for giving CRISPR/Cas9 vectors.
Contributor Information
Jiacai Chen, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Liu Liu, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Guanghui Wang, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Guangxin Chen, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Xiaofeng Liu, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Min Li, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Lijie Han, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Weiyuan Song, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Shaoyun Wang, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Chuang Li, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Zhongyi Wang, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Yuxiang Huang, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Chaoheng Gu, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Zhengan Yang, College of Landscape and Horticulture, Yunnan Agricultural University, Kunming, Yunnan 650201, China.
Zhaoyang Zhou, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Jianyu Zhao, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Xiaolan Zhang, Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops, Department of Vegetable Sciences, China Agricultural University, Beijing 100193, China.
Author contributions
X.Z. and J.C. designed the research; J.C., L.L., G.W., and G.C. performed the experiments; J.C., X.L., and M.L. analyzed the data and participated in the experimental design; X.Z. and J.C. wrote the paper; L.H., W.S., S.W., C.L., Z.W., Y.H., C.G., Z.Y., Z.Z., and J.Z. provided experimental assistance; and all the authors read and approved the manuscript.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1 . Phylogenetic analysis of CsAGL16.
Supplementary Figure S2 . Phylogenetic analysis of CsGRF1.
Supplementary Table S1 . Accession numbers of genes in this study.
Supplementary Table S2 . Primers used in this study.
Supplementary Data Set S1 . The results of RNA-seq analysis.
Supplementary Data Set S2 . The results of IP-MS analysis.
Supplementary Data Set S3 . Statistical data.
Supplementary Files S1 to S6 . Alignments and machine-readable tree files for phylogenetic analysis.
Funding
This work is supported by grants from the National Natural Science Foundation of China (32025033, 32372699, and 31930097), Expert Workstation in Yunnan Province (202205AF150021), and Pinduoduo-China Agricultural University Research Fund (PC2023B01002).
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007:19(2):458–472. 10.1105/tpc.106.048934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla ER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, Vergara-Silva F, Yanofsky MF. MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. Plant J. 2000:24(4):457–466. 10.1046/j.1365-313x.2000.00891.x [DOI] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007:51(6):1019–1029. 10.1111/j.1365-313X.2007.03210.x [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J. D14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009:50(8):1416–1424. 10.1093/pcp/pcp091 [DOI] [PubMed] [Google Scholar]
- Arney SE, Mitchell DL. The effect of abscisic acid on stem elongation and correlative inhibition. New Phytol. 1969:68(4):1001–1015. 10.1111/j.1469-8137.1969.tb06500.x [DOI] [Google Scholar]
- Arora R, Agarwal P, Ray S, Singh AK, Singh VP, Tyagi AK, Kapoor S. MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genomics. 2007:8(1):242. 10.1186/1471-2164-8-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J. 2011:65(4):571–577. 10.1111/j.1365-313X.2010.04443.x [DOI] [PubMed] [Google Scholar]
- Balla J, Medveďová Z, Kalousek P, Matiješčuková N, Friml J, Reinöhl V, Procházka S. Auxin flow-mediated competition between axillary buds to restore apical dominance. Sci Rep. 2016:6(1):35955. 10.1038/srep35955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP, Boutet-Mercey S, Dalmais M, Antoniadi I, Li X, Maia-Grondard A, Le Signor C, Bouteiller N, et al. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 2012:158(1):225–238. 10.1104/pp.111.182725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE. 2004:2004(242):re10. 10.1126/stke.2422004re10 [DOI] [PubMed] [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 2000:24(2):159–169. 10.1046/j.1365-313x.2000.00862.x [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan X, Song W, Zhang Y, Xu G. Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1. Plant Biotechnol J. 2012:10(2):139–149. 10.1111/j.1467-7652.2011.00637.x [DOI] [PubMed] [Google Scholar]
- Chen MX, Zhu FY, Wang FZ, Ye NH, Gao B, Chen X, Zhao SS, Fan T, Cao YY, Liu TY, et al. Alternative splicing and translation play important roles in hypoxic germination in rice. J Exp Bot. 2019:70(3):817–833. 10.1093/jxb/ery393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG, Oh C. A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann Bot. 2006:98(4):891–897. 10.1093/aob/mcl173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010:137(17):2905–2913. 10.1242/dev.051987 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE. Formation and breakdown of ABA. Trends Plant Sci. 1999:4(12):472–478. 10.1016/S1360-1385(99)01497-1 [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol. 2011:12(4):211–221. 10.1038/nrm3088 [DOI] [PubMed] [Google Scholar]
- Dong X, Zhang LP, Tang YH, Yu D, Cheng F, Dong YX, Jiang XD, Qian FM, Guo ZH, Hu JY. Arabidopsis AGAMOUS-LIKE16 and SUPPRESSOR OF CONSTANS1 regulate the genome-wide expression and flowering time. Plant Physiol. 2023:192(1):154–169. 10.1093/plphys/kiad058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012:158(1):487–498. 10.1104/pp.111.186783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang SC, Fernandez DE. Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiol. 2002:130(1):78–89. 10.1104/pp.004721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie C, Borch J, Collinge DB. 14-3-3 proteins: eukaryotic regulatory proteins with many functions. Plant Mol Biol. 1999:40(4):545–554. 10.1023/A:1006211014713 [DOI] [PubMed] [Google Scholar]
- Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell. 2005:17(2):464–474. 10.1105/tpc.104.026716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XL, Xiao W, Wang DL, Chen M, Tan Q P, Li L, De Chen X, Gao DS. Roles of endoplasmic reticulum stress and unfolded protein response associated genes in seed stratification and bud endodormancy during chilling accumulation in Prunus persica. PLoS One. 2014:9(7):e101808. 10.1371/journal.pone.0101808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampala SS, Kim TW, He JX, Tang W, Deng Z, Bai MY, Guan S, Lalonde S, Sun Y, Gendron JM, et al. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell. 2007:13(2):177–189. 10.1016/j.devcel.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Grandío E, Pajoro A, Franco-Zorrilla JM, Tarancón C, Immink RG, Cubas P. Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc Natl Acad Sci U S A. 2017:114(2):E245–E254. 10.1073/pnas.1613199114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb T, Clarenz O, Schafer E, Muller D, Herrero R, Schmitz G, Theres K. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 2003:17(9):1175–1187. 10.1101/gad.260703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Liu XF, Zhao WS, Yan SS, Sun LH, Wu BN, Zhang XL. Functional characterization of the promoter and second intron of CUM1 during flower development in cucumber (Cucumis sativus L.). Hortic Plant J. 2018:4(3):103–110. 10.1016/j.hpj.2018.03.004 [DOI] [Google Scholar]
- Gubler F, Hughes T, Waterhouse P, Jacobsen J. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008:147(2):886–889. 10.1104/pp.107.115469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Xu Y, Liu H, Mao Z, Zhang C, Ma Y, Zhang Q, Meng Z, Chong K. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat Commun. 2013:4(1):1566. 10.1038/ncomms2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, García-Ponce B, Fonseca-Salazar G, Alvarez-Buylla ER, Yu H. AGAMOUS-LIKE 17, a novel flowering promoter, acts in a FT-independent photoperiod pathway. Plant J. 2008:55(2):253–265. 10.1111/j.1365-313X.2008.03499.x [DOI] [PubMed] [Google Scholar]
- Hansen H, Grossmann K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000:124(3):1437–1448. 10.1104/pp.124.3.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A, Stirnberg P, Beveridge C, Leyser O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009:151(1):400–412. 10.1104/pp.109.137646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Chin-Atkins AN, Wilson IW, Chapple R, Dennis ES, Chaudhury A. The Arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. Plant Cell. 2001:13(9):2115–2125. 10.1105/TPC.010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Li D, Liu X, Qi J, Gao D, Zhao S, Huang S, Sun J, Yang L. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant. 2017:10(12):1575–1578. 10.1016/j.molp.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Hu JY, Zhou Y, He F, Dong X, Liu LY, Coupland G, Turck F, de Meaux J. miR824-regulated AGAMOUS-LIKE16 contributes to flowering time repression in Arabidopsis. Plant Cell. 2014:26(5):2024–2037. 10.1105/tpc.114.124685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Isobe T, Okuyama T, Yamauchi T, Fujisawa H. Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+, calmodulin-dependent protein kinase II. FEBS Lett. 1987:219(1):79–82. 10.1016/0014-5793(87)81194-8 [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature. 2013:504(7480):401–405. 10.1038/nature12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr SC, Beveridge CA. IPA1: a direct target of SL signaling. Cell Res. 2017:27(10):1191–1192. 10.1038/cr.2017.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP, Wareing PF. Apical dominance in Phaseolus vulgaris L. J Exp Bot. 1984:35(2):239–244. 10.1093/jxb/35.2.239 [DOI] [Google Scholar]
- Korthout HA, de Boer AH. A fusicoccin binding protein belongs to the family of 14-3-3 brain protein homologs. Plant Cell. 1994:6(11):1681–1692. 10.1105/tpc.6.11.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004:23(7):1647–1656. 10.1038/sj.emboj.7600121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. Regulation of shoot branching by auxin. Trends Plant Sci. 2003:8(11):541–545. 10.1016/j.tplants.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Leyser O. The control of shoot branching: an example of plant information processing. Plant Cell Environ. 2009:32(6):694–703. 10.1111/j.1365-3040.2009.01930.x [DOI] [PubMed] [Google Scholar]
- Li J, Li G, Gao S, Martinez C, He G, Zhou Z, Huang X, Lee JH, Zhang H, Shen Y, et al. Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome A signaling. Plant Cell. 2010:22(11):3634–3649. 10.1105/tpc.110.075788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu B, Wu Q, Min Q, Zeng R, Xie Z, Huang J. OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet. 2021:17(8):e1009699. 10.1371/journal.pgen.1009699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009:21(5):1512–1525. 10.1105/tpc.109.065987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Zhao TZ, Xu LL, Zhang W, Huang FY, Wang JJ, Hou XL, Li Y. Bcbrc1a is a negative regulator for tillering in non-heading Chinese cabbage. Veg Res. 2022:2(1):98–105. 10.48130/VR-2022-0011 [DOI] [Google Scholar]
- Lu Z, Yu H, Xiong G, Wang J, Jiao Y, Liu G, Jing Y, Meng X, Hu X, Qian Q, et al. Genome-wide binding analysis of the transcription activator ideal plant architecture1 reveals a complex network regulating rice plant architecture. Plant Cell. 2013:25(10):3743–3759. 10.1105/tpc.113.113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Takahashi M, Kameoka H, Qin R, Shiga T, Kanno Y, Seo M, Ito M, Xu G, Kyozuka J. Developmental analysis of the early steps in strigolactone-mediated axillary bud dormancy in rice. Plant J. 2019:97(6):1006–1021. 10.1111/tpj.14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader JC, Emery RJN, Turnbull CGN. Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiol Plantarum. 2003:119(2):295–308. 10.1034/j.1399-3054.2003.00179.x [DOI] [Google Scholar]
- Marra M, Fullone MR, Fogliano V, Pen J, Mattei M, Masi S, Aducci P. The 30-kilodalton protein present in purified fusicoccin receptor preparations is a 14-3-3-like protein. Plant Physiol. 1994:106(4):1497–1501. 10.1104/pp.106.4.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milborrow BV, Lee HS. Endogenous biosynthetic precursors of (+)-abscisic acid. IV. Biosynthesis of ABA from [2Hn] carotenoids by a cell-free system from avocado. Funct Plant Biol. 1997:24(6):715–726. 10.1071/PP96100 [DOI] [Google Scholar]
- Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J. 2006:45(6):942–954. 10.1111/j.1365-313X.2006.02659.x [DOI] [PubMed] [Google Scholar]
- Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996:84(6):889–897. 10.1016/S0092-8674(00)81067-3 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005:56(1):165–185. 10.1146/annurev.arplant.56.032604.144046 [DOI] [PubMed] [Google Scholar]
- Oecking C, Eckerskorn C, Weiler EW. The fusicoccin receptor of plants is a member of the 14-3-3 superfamily of eukaryotic regulatory proteins. FEBS Lett. 1994:352(2):163–166. 10.1016/0014-5793(94)00949-X [DOI] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006:141(1):97–107. 10.1104/pp.106.079475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongaro V, Leyser O. Hormonal control of shoot branching. J Exp Bot. 2007:59(1):67–74. 10.1093/jxb/erm134 [DOI] [PubMed] [Google Scholar]
- Par̆enicová L, de Folter S, Kieffer M, Horner DS, Favalli C, Busscher J, Cook HE, Ingram RM, Kater MM, Davies B, et al. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell. 2003:15(7):1538–1551. 10.1105/tpc.011544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Meynard D, Khong GN, Pauluzzi G, Guiderdoni E, Gantet P. Analysis of the expression of the AGL17-like clade of MADS-box transcription factors in rice. Gene Expr Patterns. 2013:13(5-6):160–170. 10.1016/j.gep.2013.02.004 [DOI] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA. Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol. 2013:163(2):1047–1058. 10.1104/pp.113.221895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Jones D, Patel Y, Martin H, Madrazo J, Martin S, Howell S, Elmore M, Finnen MJ, Aitken A. Mechanism of inhibition of protein kinase C by 14-3-3 isoforms. 14-3-3 isoforms do not have phospholipase A2 activity. Biochem J. 1994:299(3):853–861. 10.1042/bj2990853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G, Theres K. Shoot and inflorescence branching. Curr Opin Plant Biol. 2005:8(5):506–511. 10.1016/j.pbi.2005.07.010 [DOI] [PubMed] [Google Scholar]
- Sehnke PC, Chung HJ, Wu K, Ferl RJ. Regulation of starch accumulation by granule-associated plant 14-3-3 proteins. Proc Natl Acad Sci U S A. 2001:98(2):765–770. 10.1073/pnas.98.2.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto Y, Yasui R, Kameoka H, Tamiru M, Cao M, Terauchi R, Sakurada A, Hirano R, Kisugi T, Hanada A, et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat Commun. 2019:10(1):191. 10.1038/s41467-018-08124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zhang Y, Ge D, Wang Z, Song W, Gu R, Che G, Cheng Z, Liu R, Zhang X. CsBRC1 inhibits axillary bud outgrowth by directly repressing the auxin efflux carrier CsPIN3 in cucumber. Proc Natl Acad Sci U S A. 2019:116(34):17105–17114. 10.1073/pnas.1907968116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003:17(12):1469–1474. 10.1101/gad.256603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas IA, Ozbun JL, Wallace DH. Effect of fruits on dormancy and abscisic acid concentration in the axillary buds of Phaseolus vulgaris L. Plant Physiol. 1979:64(4):615–619. 10.1104/pp.64.4.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013:30(12):2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Münster T, Winter KU, Saedler H. A short history of MADS-box genes in plants. Plant Mol Biol. 2000:42(1):115–149. 10.1023/A:1006332105728 [DOI] [PubMed] [Google Scholar]
- Tolnai Z, Sharma H, Soós V. D27-like carotenoid isomerases: at the crossroads of strigolactone and ABA biosynthesis. J Exp Bot. 2023;75(4):1148–1158. 10.1093/jxb/erad475.:erad475 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002:296(5566):343–346. 10.1126/science.1068181 [DOI] [PubMed] [Google Scholar]
- Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, Schroeder JI. Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol. 2022:23(10):680–694. 10.1038/s41580-022-00479-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Zhao Z, Qian C, Sui Y, Malik AA, Chen J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal Biochem. 2010:399(2):257–261. 10.1016/j.ab.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Wang X, Jin B, Yan W, Wang J, Xu J, Cai C, Qi X, Xu Q, Yang X, Xu X, et al. Cucumber abscisic acid 8′-hydroxylase Csyf2 regulates yellow flesh by modulating carotenoid biosynthesis. Plant Physiol. 2023:193(2):1001–1015. 10.1093/plphys/kiad383 [DOI] [PubMed] [Google Scholar]
- Wang M, Le Moigne MA, Bertheloot J, Crespel L, Perez-Garcia MD, Ogé L, Demotes-Mainard S, Hamama L, Davière JM, Sakr S. BRANCHED1: a key hub of shoot branching. Front Plant Sci. 2019:10;76. 10.3389/fpls.2019.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhong Y, Liu X, Zhao C, Zhao J, Li M, Ul Hassan M, Yang B, Li D, Liu R, et al. Cis-regulation of the amino acid transporter genes ZmAAP2 and ZmLHT1 by ZmPHR1 transcription factors in maize ear under phosphate limitation. J Exp Bot. 2021:72(10):3846–3863. 10.1093/jxb/erab103 [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou Y, Ding Y, Chen C, Chen X, Su N, Zhang X, Pan Y, Li J. Novel flavin-containing monooxygenase protein FMO1 interacts with CAT2 to negatively regulate drought tolerance through ROS homeostasis and ABA signaling pathway in tomato. Hortic Res. 2023:10(4):uhad037. 10.1093/hr/uhad037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant physiol. 2012:159(3):1073–1085. 10.1104/pp.112.196253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014:14(1):327. 10.1186/s12870-014-0327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Truong TT, Barrero JM, Jacobsen JV, Hocart CH, Gubler F. A role for jasmonates in the release of dormancy by cold stratification in wheat. J Exp Bot. 2016:67(11):3497–3508. 10.1093/jxb/erw172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Finlayson SA. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 2015:169(1):611–626. 10.1104/pp.15.00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Ming Z, Yan L, Li S, Wang F, Ma S, Yu C, Yang M, Chen L, Chen L, et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature. 2016:536(7617):469–473. 10.1038/nature19073 [DOI] [PubMed] [Google Scholar]
- Zhan J, Chu Y, Wang Y, Diao Y, Zhao Y, Liu L, Wei X, Meng Y, Li F, Ge X. The miR164-GhCUC2-GhBRC1 module regulates plant architecture through abscisic acid in cotton. Plant Biotechnol J. 2021:19(9):1839–1851. 10.1111/pbi.13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou Y, Ding L, Wu Z, Liu R, Meyerowitz EM. Transcription repressor HANABA TARANU controls flower development by integrating the actions of multiple hormones, floral organ specification genes, and GATA3 family genes in Arabidopsis. Plant Cell. 2013:25(1):83–101. 10.1105/tpc.112.107854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao PX, Miao ZQ, Zhang J, Chen SY, Liu QQ, Xiang CB. Arabidopsis MADS-box factor AGL16 negatively regulates drought resistance via stomatal density and stomatal movement. J Exp Bot. 2020:71(19):6092–6106. 10.1093/jxb/eraa303 [DOI] [PubMed] [Google Scholar]
- Zhao PX, Zhang J, Chen SY, Wu J, Xia JQ, Sun LQ, Ma SS, Xiang CB. Arabidopsis MADS-box factor AGL16 is a negative regulator of plant response to salt stress by downregulating salt-responsive genes. New Phytol. 2021:232(6):2418–2439. 10.1111/nph.17760 [DOI] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013:504(7480):406–410. 10.1038/nature12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.