Abstract
Chemokines are involved in recruitment and activation of hematopoietic cells at sites of infection and inflammation. The M3 gene of γHV68, a gamma-2 herpesvirus that infects and establishes a lifelong latent infection and chronic vasculitis in mice, encodes an abundant secreted protein during productive infection. The M3 gene is located in a region of the genome that is transcribed during latency. We report here that the M3 protein is a high-affinity broad-spectrum chemokine scavenger. The M3 protein bound the CC chemokines human regulated upon activation of normal T-cell expressed and secreted (RANTES), murine macrophage inflammatory protein 1α (MIP-1α), and murine monocyte chemoattractant protein 1 (MCP-1), as well as the human CXC chemokine interleukin-8, the murine C chemokine lymphotactin, and the murine CX3C chemokine fractalkine with high affinity (Kd = 1.6 to 18.7 nM). M3 protein chemokine binding was selective, since the protein did not bind seven other CXC chemokines (Kd > 1 μM). Furthermore, the M3 protein abolished calcium signaling in response to murine MIP-1α and murine MCP-1 and not to murine KC or human stromal cell-derived factor 1 (SDF-1), consistent with the binding data. The M3 protein was also capable of blocking the function of human CC and CXC chemokines, indicating the potential for therapeutic applications. Since the M3 protein lacks homology to known chemokines, chemokine receptors, or chemokine binding proteins, these studies suggest a novel herpesvirus mechanism of immune evasion.
Chemokines are chemoattractant and immunomodulatory molecules that play a central role in many inflammatory processes (30). They are divided into four structural groups based on the number and arrangement of conserved cysteines and are consequently named CC, CXC, C, and CX3C chemokines. CC chemokines generally regulate macrophages and lymphocytes; they include monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), and regulated upon activation of normal T-cell expressed and secreted (RANTES). CXC chemokines include interleukin-8 (IL-8), monokine induced by gamma interferon (Mig), macrophage inflammatory protein 2 (MIP-2), stromal cell-derived factor 1 (SDF-1), granulocyte chemotactic protein 2 (GCP-2), interferon-inducible protein 10 (IP-10), B-cell-attracting chemokine (BCA-1), and KC. While many CXC chemokines stimulate the activity of neutrophils, some regulate lymphocytes. The only members of the C and CX3C chemokine families are lymphotactin and fractalkine, respectively.
Given the importance of chemokines in the immune system, it is not surprising that viruses have evolved mechanisms for interacting with the chemokine system. Both poxviruses and herpesviruses use two known strategies for interacting with the chemokine system, one via virus-encoded chemokine receptor homologs and one via virus-encoded chemokine homologs (see, e.g., references 24 and 28; reviewed in references 16 and 19). An additional strategy, secretion of chemokine binding proteins with novel structures, has been shown for poxviruses but not to date for herpesviruses (see Discussion).
We considered the hypothesis that herpesviruses, like poxviruses, encode secreted chemokine binding proteins and tested this hypothesis using murine γHV68. γHV68 is a gamma-2 herpesvirus with homology to Epstein-Barr virus, herpesvirus saimiri, and Kaposi's sarcoma herpesvirus. γHV68 infects laboratory mice and can be genetically manipulated (5, 31), providing a unique tool for identifying host and viral factors that regulate herpesvirus infection (reviewed in references 35 and 42). γHV68 encodes a number of molecules that probably interact with the host immune system. These include a homolog of host G-protein-coupled receptors with strong homology to the IL-8 receptor (40), a protein with homology to poxvirus serpins (5, 31), a homolog of host D-type cyclins that causes cell cycle progression in lymphocytes and is encoded by an oncogene (39), and a homolog of host proteins that function as regulators of complement activation (14). The presence of these homologs suggests that γHV68 has multiple strategies for subverting host cellular machinery and immune responses.
We recently described an abundant secreted protein encoded by the γHV68 M3 gene (38). The M3 genomic region is transcribed during latency (32, 41), raising the intriguing possibility that a secreted protein could play a role in establishment or reactivation from latency. Since the M3 protein is secreted, we considered the possibility that it interacts with host inflammatory cell receptors or cytokines. In this report, we demonstrate that the M3 protein binds both mouse and human chemokines (designated m and h chemokines, respectively) with high affinity and blocks chemokine signaling. This demonstrates a novel third mechanism (in addition to encoding chemokine receptors and chemokines) by which herpesviruses interact with the chemokine system: secretion of a high-affinity chemokine binding protein that inhibits chemokine action.
MATERIALS AND METHODS
Production of γHV68-infected cell supernatants.
Murine 3T12 fibroblast cells were either mock infected or infected at a multiplicity of infection of 5 with γHV68 (WUMS strain) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). After 1 h, the monolayer was washed with phosphate-buffered saline (PBS), fresh DMEM containing 10% FCS was added, and infection was allowed to proceed for 8 h at 37°C. The monolayer was then washed with PBS, fresh DMEM without FCS was added, and the infection was allowed to proceed for 20 h at 37°C. The culture supernatant was passed through a 0.2-μm-pore-size filter and concentrated 120-fold at 4°C (Centriprep-10; Amicon, Inc., Beverly, Mass.). Concentrated supernatants were centrifuged at 150,000 × g for 3 h to remove residual free virus and then stored at 4°C. The concentration of the M3 protein was determined by densitometry of silver-stained 12.5% acrylamide gels with known amounts of purified bacterially expressed M3 protein (see below) as a standard. Densitometric comparison was performed with 1D Image Analysis software (Eastman Kodak Co., Rochester, N.Y.), and measurements were taken within the linear range of densitometrically determined band intensities.
Cross-linking assay.
The interaction of the M3 protein with various human chemokines was detected using a chemical cross-linking assay as described previously (37). Briefly, γHV68-infected cell supernatants were incubated with the appropriate chemokine for 2 h at room temperature. After incubation, the protein complexes were covalently cross-linked by the addition of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (Sigma, St. Louis, Mo.) to a final concentration of 40 mM for 30 min at room temperature, and the reaction was quenched by the addition of 1/10 volume of 1.0 M Tris (pH 7.5). Laemmli sample buffer containing 2-mercaptoethanol (sample buffer) was added to the mixtures, the samples were boiled for 3 min, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide), and transferred to a nitrocellulose membrane for immunoblotting. M3 protein complexes were detected by probing with a 1:5,000 dilution of anti-M3 polyclonal rabbit antiserum (Cocalico, Reamstown, Pa.) and a 1:5,000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G antibody.
Bacterial and baculovirus expression systems.
The following primers were used to amplify the genomic region of γHV68 corresponding to the secreted form of the M3 protein (38), adding a NdeI site and six histidine residues at the 5′ end and a XhoI site at the 3′ end: 5′-ACATATGCACCATCATCATCATCATCTTACTCTAGGTTTGGCACCTGCT-3′ and 5′-ACTCGAGTCTACTACTAATGATCCCCAAAATACTCCAGCCT-3′. The 1,187-bp fragment was sequenced and cloned into pet30a(+), and recombinant protein was induced and purified over a nickel column as specified by the manufacturer (Novagen, Madison, Wis.). For baculovirus expression, the full-length M3 open reading frame was amplified using the following primers: 5′-AGCGGCCGCATGGCCTTCC TATCCACATCTGTGCT-3′ (inserting a NotI site 5′ of the M3 start methionine) and 5′-ACTCGAGTCTACTACTAATGATCCCCAAAATACTCCAGCCT-3′ (inserting a XhoI site 3′ of the M3 stop codon). The 1,244-bp fragment was sequenced and cloned into the pFastBac vector, and recombinant baculovirus was generated by the Bac-to-Bac baculovirus expression system method (Life Technologies). A control β-galactosidase-expressing baculovirus was constructed at the same time by using unmanipulated pFastBac vector containing the lacZ gene. Supernatants from cells infected with the M3 protein-expressing and control baculoviruses were collected 4 days after infection of Sf9 cells, clarified by centrifugation (200 × g) for 5 min, and then stored at 4°C. The M3 protein was further purified by ion-exchange chromatography followed by size exclusion chromatography. The H2M protein, an isoelectric point- and size-matched control protein, was purified through the use of a concavalin A column followed by size exclusion chromatography (11).
Immunoprecipitation.
Either 100 μl of γHV68-infected (M3 protein at ∼3 μM) or mock-infected 3T12 cell supernatants, or 100 μl of Sf9 cell supernatants after infection with either M3 protein-expressing (M3 protein at ∼500 nM) or LacZ-expressing baculovirus, was incubated with 500 pmol of 125I-labeled hRANTES or IL-5 (Amersham, Arlington Heights, Ill.) for 30 min at 4°C. Samples were first immunoprecipitated by incubation with 3 μl of preimmune rabbit serum for 60 min at 4°C followed by addition of 15 μl of protein A-conjugated agarose beads (Calbiochem, La Jolla, Calif.) and incubation for an additional 120 min at 4°C. The beads were isolated by centrifugation for 15 min at 7,000 × g, washed three times with 500 μl of phosphate buffered saline containing 0.05% Tween 20, and resuspended in 20 μl of sample buffer (23). Supernatants were subsequently incubated with 5 μl of serum from a rabbit multiply immunized with bacterially expressed M3 protein and were then incubated with 30 μl of protein A-conjugated agarose beads, which were recovered and washed as above. Precipitated samples were resuspended in 20 μl of sample buffer, separated on a 20% acrylamide gel, and analyzed by autoradiography.
Binding assays.
Saturation analysis determination of the disassociation constant for 125I-labeled hRANTES binding to M3 protein was performed as follows. M3 protein (250 pmol) (as diluted γHV68 infected cell supernatants) was incubated with increasing amounts of 125I-labeled hRANTES for 60 min. Bound hRANTES was recovered by incubation with 5 μl of anti-M3 polyclonal antiserum, followed by incubation with 30 μl of protein A-conjugated agarose beads, which were recovered and washed as described above. Precipitated 125I-hRANTES was resuspended in 1 ml of scintillation fluid, and the counts per minute (cpm) were compared to the cpm of input radioactivity to determine the amount of 125I-hRANTES bound. Competitive inhibition with CC and CXC chemokines was demonstrated by incubating 250 pmol of M3 protein with 500 pmol of 125I-hRANTES and increasing amounts of unlabeled chemokine or IL-5 and precipitating the hRANTES as above. Unlabeled IL-5 and chemokines were purchased from R&D Systems, Minneapolis, Minn. All measurements were determined in triplicate and repeated in at least two independent experiments. The Kd values were determined using the GraphPad Prism data analysis software package (GraphPad Software, San Diego, Calif.).
Leukocyte preparation and intracellular calcium measurements.
Human neutrophils were prepared from whole peripheral blood of healthy donors by Ficoll-Hypaque discontinuous gradient centrifugation and dextran sedimentation followed by hypotonic lysis of the remaining red blood cells. Murine leukocytes were obtained from C57BL/6 mice by washing the peritoneal cavity 3 h (>90% neutrophils) or 72 h (>90% macrophages) after instillation of thioglycolate. hRANTES and m-fractalkine were tested on cell lines expressing CCR5 (6) or CX3CR1 (7), respectively. Cells, at a concentration of 1.5 × 106 cells/ml, were loaded with 2.5 μM Fura II-AM (Molecular Probes, Eugene, Oreg.) in PBS for 45 min at 37°C. The cells were washed twice with PBS and suspended in PBS at a final concentration of 1.5 × 106 cells/ml. A 1-ml volume of cells was mixed with 1 ml of Hanks balanced salt solution for further analysis of calcium flux responses. The cuvette containing 2 ml of cells was continuously stirred at 37°C in an MS-III ratio fluorescence spectrophotometer (Photon Technology International, Inc., London, Ontario, Canada). The cells were stimulated with RANTES, mMIP-1α, mMCP-1, hSDF-1, m-fractalkine, IL-8 (Peprotech, Rocky Hill, N.J.), N-formyl-Met-Leu-Phe (fMLF) (Sigma), supernatants from insect cells expressing M3 protein or LacZ protein, and/or 100 nM of purified M3 protein or purified H2M protein. The calcium flux response was measured continuously every 200 ms as a relative fluorescence ratio of excitation at 340 nm and 380 nm with emission at 510 nm.
RESULTS
The M3 protein binds chemokines.
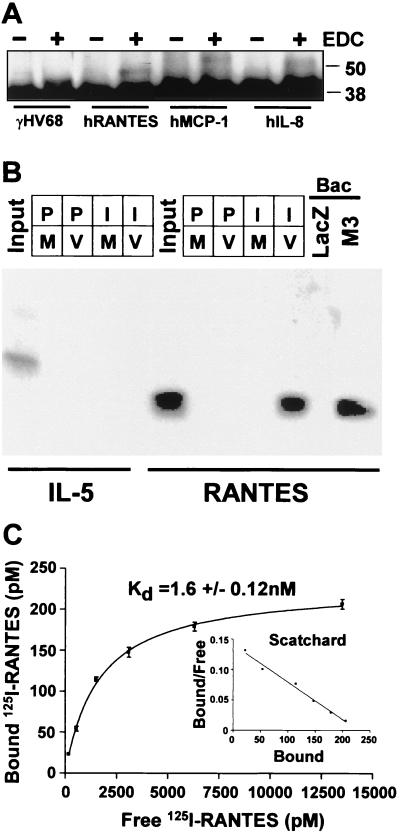
To detect chemokine binding proteins in the supernatant from γHV68-infected cells, supernatants were collected 24 h after γHV68 or mock infection, mixed with various chemokines, and chemically cross-linked. Binding of proteins to the M3 protein was detected after analysis by denaturing gel electrophoresis and Western blotting with anti-M3 polyclonal antiserum (Fig. 1A). A supershifted M3 protein-containing complex was observed when hRANTES, hMCP-1, or hIL-8 was added to supernatants from γHV68-infected cells. Similar complexes were not seen when mock-infected supernatants were used (data not shown). To further demonstrate that the M3 protein binds chemokines, infected cell supernatants were incubated with 100 pmol of 125I-hRANTES or 125I-IL-5 and immunoprecipitated with polyclonal rabbit serum raised against bacterially expressed M3 protein. 125I-hRANTES was precipitated by M3 protein-specific antiserum, but not by preimmune antibody, from infected cell supernatants but not from mock-infected supernatants (Fig. 1B). IL-5 was not precipitated from the γHV68 supernatants by anti-M3 antibody, demonstrating the specificity of M3 protein binding to hRANTES. In addition, we assayed supernatants harvested from Sf9 cells infected with baculoviruses expressing either the M3 protein or β-galactosidase. Anti-M3 protein antibody precipitated 125I-hRANTES from supernatants from cells infected with the M3 protein-expressing baculovirus but not the β-galactosidase-expressing baculovirus. Together, these experiments demonstrate that the γHV68 M3 protein specifically binds hRANTES in the absence of other γHV68-encoded proteins. To measure the affinity of the interaction between the M3 protein and both mouse and human chemokines, saturation binding and competition experiments were performed using 125I-hRANTES. The conditions of the assay were selected such that input M3 protein was quantitatively precipitated (data not shown). The interaction of 125I-hRANTES and M3 protein was saturable and of high affinity (Kd = 1.6 ± 0.12 nM) (Fig. 1C).
FIG. 1.
The M3 protein is capable of binding chemokines. (A) Infected cell supernatants were incubated with various chemokines, cross-linked (with EDC), and analyzed by immunoblotting with anti-M3 polyclonal antiserum. Uncomplexed M3 protein is present as the 44-kDa band, while a supershifted band indicates M3 protein covalently complexed with the indicated chemokine. (B) Coimmunoprecipitation of M3 protein and RANTES. Either 100 μl of γHV68-infected (V) or mock-infected (M) 3T12 cell supernatants or 100 μl of Sf9 cell supernatants after infection with either M3 protein- or LacZ-expressing baculovirus (Bac) was incubated with 500 pmol of 125I-labeled human RANTES or IL-5. Samples precipitated with preimmune sera are designated P, while those precipitated with sera from a rabbit multiply immunized with bacterially expressed M3 protein are designated I. The first and sixth lanes (Input) contain 5 fmol of 125I-IL-5 or 125I-labeled human RANTES, respectively. (C) Saturation binding and Scatchard analysis of M3 protein binding to 125I-labeled human RANTES. The mean and standard error of the mean for specific binding of triplicate samples is shown.
The M3 protein binds multiple CC chemokines, fractalkine, and lymphotactin, but not murine CXC chemokines, with high affinity.
Competition for binding of 125I-hRANTES to M3 protein was used to determine if the M3 protein binds other chemokines and to determine the binding affinity of unlabeled hRANTES, as well as several murine CC and CXC chemokines. Preincubation of 125I-hRANTES with increasing amounts of the unlabeled CC chemokines hRANTES, mMCP-1, and mMIP-1α led to dose-dependent inhibition of binding of 125I-hRANTES to M3 protein (Fig. 2A), while increasing amounts of unlabeled IL-5 had no effect (data not shown). Similar competition experiments with m-fractalkine and m-lymphotactin demonstrated that both of these chemokines bound with high affinity (Fig. 2B). Interestingly, while the M3 protein bound the human CXC chemokine IL-8 (Fig. 1, 2C), seven murine CXC chemokines failed to efficiently compete for binding of 125I-hRANTES to the M3 protein (Kd > 1.0 μM for all seven [Fig. 2C]). These data show that the M3 protein binds with high affinity to (i) the CC chemokines hRANTES, mMIP-1α, and mMCP-1, (ii) the murine C and CX3C chemokines lymphotactin and fractalkine, and (iii) the CXC chemokine hIL-8, but not the tested murine CXC chemokines.
FIG. 2.
Chemokine binding properties of the M3 protein. (A) Competitive inhibition of 125I-RANTES binding to the M3 protein by CC chemokines. The percentage of maximal binding (mean and standard error of the mean) refers to binding in the absence of competitor (average value of maximal binding, 110,000 cpm). The Kd values determined are indicated. (B) Competitive inhibition of 125I-RANTES binding to the M3 protein by C and CX3C chemokines. (C) Competitive inhibition of 125I-RANTES binding to the M3 protein by CXC chemokines.
The M3 protein blocks chemokine-mediated calcium flux.
To test the functional consequence of M3 protein chemokine binding, we monitored calcium flux in human neutrophils, mouse macrophages and neutrophils, and cell lines expressing CCR5 or CX3CR1, stimulated with various chemokines. As a control, we examined the effect of M3 on signaling by the nonchemokine chemoattractant fMLF or ATP, which activate G-protein-coupled receptors distinct from the chemokine receptors. The various chemokine receptors, as well as the fMLF and ATP receptors, are coupled to calcium mobilization, which can be monitored in real time in Fura II-AM-loaded cells. This calcium mobilization is a proximal event in chemokine receptor signaling.
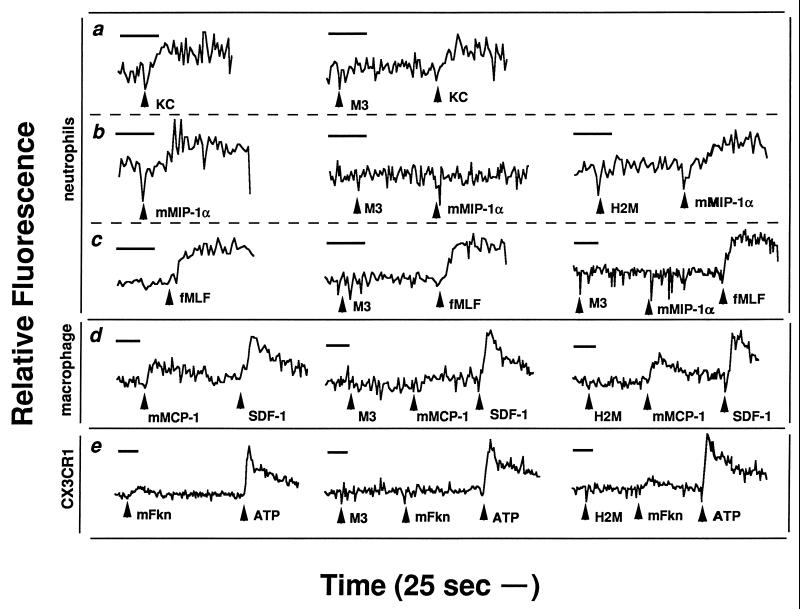
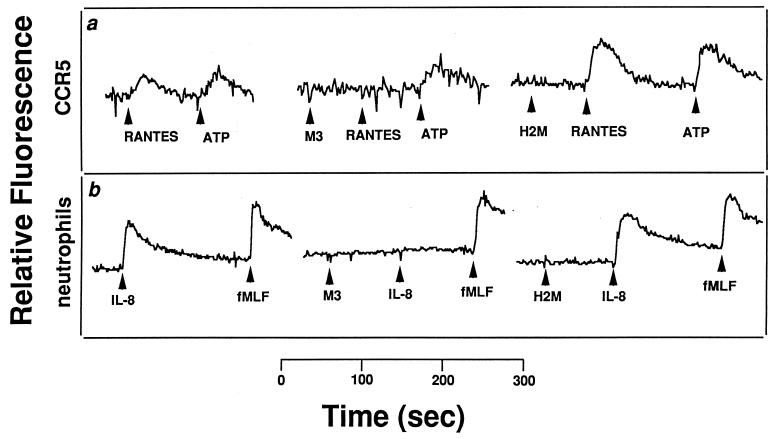
Consistent with the fact that M3 does not appreciably bind the CXC murine chemokine KC (Fig. 2C), addition of purified M3 protein to murine neutrophils did not inhibit the ability of KC to mobilize calcium (Fig. 3a). In contrast, the addition of equivalent amounts of M3 protein completely abrogated calcium mobilization in response to the murine CC chemokine MIP-1α, while equimolar amounts of a similarly purified control protein (H2M) did not (Fig. 3b). This was not due to any cytotoxic effects of the M3 protein, since signaling through the fMLF pathway was maintained (Fig. 3c). Similarly, the M3 protein blocked the ability of mMCP-1 to mobilize calcium within murine macrophages but did not block the function of hSDF-1 (Fig. 3d). In addition, M3 prevented m-fractalkine from inducing a calcium flux within a human embryonic kidney 293 cell line engineered to express human CX3CR1 (7). In additional experiments, hRANTES induction of calcium flux via activation of the hRANTES receptor CCR5, expressed in human embryonic kidney 293 cells (6), was completely abolished by pretreatment of the cells with M3 protein, whereas little to no effect was observed on calcium signaling by ATP through an endogenous nucleotide receptor (Fig. 4a). Similarly, the M3 protein, when added to human neutrophils, was able to specifically block the normal hIL-8-induced calcium flux, whereas it did not affect signaling by fMLF (Fig. 4b). In both cases, the control protein (H2M) did not exhibit these effects.
FIG. 3.
M3 protein blockade of murine chemokine activity. Shown are changes in the relative fluorescence of Fura II-AM-loaded cells, which monitors intracellular Ca2+ concentration. Test substances were added where indicated at 100 nM, except for fMLF and ATP, which were added at 1 μM. H2M is a control protein which was purified in a similar manner to M3; mFkn, m-fractalkine. Each tracing corresponds to the target indicated to the left of the row in which it is found. Each row of tracings corresponds to the same target chemokine. Neutrophils and macrophages represent cells harvested from C57BL/6 mice 3 and 72 h after instillation of thioglycolate. CX3CR1 represents a human embryonic kidney 293 cell line expressing human CX3CR1. Data are representative of at least two separate experiments for each chemokine. (a) KC; (b) mMIP-1α; (c) fMLF and mMIP-1α; (d) mMCP-1 and SDF-1; (e) m-fractalkine.
FIG. 4.
M3 protein blockade of human chemokine activity. Shown are changes in the relative fluorescence of Fura II-AM-loaded cells, which monitors intracellular Ca2+ concentration. (a) Addition of 100 nM M3 protein, 100 M H2M protein, 100 M hRANTES, or 1 μM ATP to a human embryonic kidney 293 cell line expressing human CCR5. (b) Addition of 10 nM M3 protein, 100 nM H2M protein, 10 nM hIL-8, or 10 nM fMLF to human neutrophils. Data are representative of at least two separate experiments for each chemokine.
Together, these data show that M3 blocks signaling by both human and murine chemokines in assays using both human and mouse cells and that this blockade is restricted to chemokines to which the M3 protein binds with high affinity (Table 1).
TABLE 1.
M3 interactions with chemokines
| Chemokine | Interaction with M3
|
|
|---|---|---|
| Binding affinitya | Inhibition of signalingb | |
| CC family | ||
| hRANTES | 9.4 nM | Yes |
| mMCP-1 | 4.1 nM | Yes |
| mMIP-1α | 6.4 nM | Yes |
| CXC family | ||
| hIL-8 | 18.7 nM | Yes |
| mKC | >1 μM | No |
| hSDF-1 | NDc | No |
| mSDF-1 | >1 μM | ND |
| mMIG | >1 μM | ND |
| mMIP-2 | >1 μM | ND |
| mGCP-2 | >1 μM | ND |
| mCRG-2 | >1 μM | ND |
| mBCA-1 | >1 μM | ND |
| C family | ||
| mLymphotactin | 9.3 nM | ND |
| CX3C family | ||
| mFractalkine | 12.1 nM | Yes |
DISCUSSION
The coordination of leukocyte recruitment into sites of infection is a critical aspect of the inflammatory response. Chemokines play a central role in this process through the activation and mobilization of macrophages, lymphocytes, dendritic cells, natural killer cells, and granulocytes (21). Secretion of a selective high-affinity chemokine binding protein by a herpesvirus suggests that soluble chemokines play a central role in herpesvirus infection. However, the selectivity pattern suggests that some chemokines are either unimportant (and thus not targeted for binding) or actually required for herpesvirus pathogenesis. The selectivity with which M3 binds chemokines suggests that M3 may have an application as an inhibitor of specific inflammatory processes involved in human disease. The demonstration that the experimentally manipulable gamma-2 herpesvirus γHV68 expresses a functional high-affinity chemokine scavenger opens an important new area of investigation in which the functional importance of chemokines to viral resistance can be explored using herpesvirus infection of a natural host.
Comparison of poxvirus and herpesvirus chemokine binding proteins.
While this is the first report of a herpesvirus chemokine binding protein, there are two classes of known secreted poxvirus chemokine binding proteins (16, 19). These proteins are distinguished by the nature of their interactions with chemokines and thus the mechanisms by which they function. Type I chemokine binding proteins (CBP-I) include M-T7 from myxoma virus and S-T7 from Shope fibroma virus. These are 37-kDa members of the gamma interferon receptor family (37) that bind CC, CXC, and C chemokines with relatively low affinities (900 nM for hRANTES [16, 17, 19]) at the chemokine glycosaminoglycan binding (GAG binding) domain. The GAG binding domain of chemokines is thought to be involved in the binding of chemokines to GAGs in the extracellular matrix, thus allowing the establishment of stable gradients in tissue (17, 19, 46).
The original member of the CBP-II family is the myxoma virus M-T1 protein, and similar proteins have since been discovered in a variety of orthopoxviruses (13, 33). These are 35- to 40-kDa proteins without cellular homologs that bind CC chemokines with high affinity (7.2 nM for hRANTES [2]). The CBP-II family members bind chemokines at a site distinct from the GAG binding domain and interfere with the ability of the chemokine to signal through its receptor. Thus, both the site on the chemokine to which the CBPs bind and the affinity of the interaction distinguish the CBP-I and CBP-II families.
The M3 protein shares several characteristics with the poxvirus CBP-II family. Similar to the CBP-II family, the M3 protein binds chemokines with high affinity. The M3 protein also shares with the CBP-II family selective binding of CC chemokines, although the CBP-II members described to date have not shown any interactions with hIL-8 (2, 20, 33). It is interesting to speculate that the binding of human hIL-8 by the M3 protein suggests that M3 may bind as yet undescribed murine CXC chemokines important to gammaherpesvirus infection. Finally, both M3 protein and the CBP-II family prevent interactions between chemokines and their receptors that lead to signaling within the target cell (2).
Despite these functional similarities, there is no significant amino acid sequence homology between the CBP-II family and the M3 protein. The fact that these dissimilar viruses have evolved two distinct proteins with similar biochemical and functional activities highlights the importance of chemokines to the antiviral immune response. In addition, the fact that two different viral families have evolved high-affinity selective chemokine scavengers which are apparently unrelated at the primary sequence level argues for convergent evolution. This strongly supports the idea that significant evolutionary selective pressure has been exerted over time by host chemokines.
Implications for gammaherpesvirus pathogenesis.
While both poxviruses and herpesviruses express high-affinity chemokine binding proteins, the differences in pathogenesis between poxviruses and herpesviruses raise the possibility that these molecules may play different or overlapping roles in the pathogenesis of these two distinct DNA viruses. Poxvirus chemokine binding proteins are involved in regulating inflammation during acute infection. Myxoma viruses deficient in production of the CBP-I M-T7 show a dramatic reduction in disease symptoms and viral dissemination to secondary sites, and there is a marked increase of leukocyte infiltration into the site of infection (25). Myxoma viruses deficient in CBP-II M-T1 have a more subtle phenotype, with an increase in leukocyte infiltration but no significant difference in disease progression or mortality (13, 18).
Limitation of inflammatory chemokine action during acute infection may be similarly important for the pathogenesis of disease caused by γHV68. However, the herpesviruses also cause important chronic diseases including lymphomas and arteritis of the great vessels (reviewed for γHV68 in references 35 and 42), providing a unique opportunity to study the role of chemokines during chronic viral disease. For example, γHV68 causes a severe vasculitis of the great elastic arteries that is characterized by significant mononuclear cell infiltration of both the intima and adventitia of the great vessels (43), and MCP-1 and hIL-8 (to which M3 protein binds) can trigger adhesion of monocytes to damaged vascular endothelium. In addition, gammaherpesviruses have a unique relationship with hematopoietic cells that is not shared with poxviruses. Unlike poxviruses, gammaherpesviruses require hematopoietic cells for latency and long-term persistence in the host. For example, γHV68 latently infects both B cells and macrophages (36, 45) and B cells regulate the nature of γHV68 latency and reactivation (44). Given this, it is worth noting that the region of the genome encoding the M3 protein is transcriptionally active during latency (32, 41) and that the binding of chemokines by M3 protein is selective. M3 binds hIL-8, MCP-1, and RANTES, all three of which are important for inducing inflammation (see, e.g., reference 12). In contrast, SDF-1 and BCA-1, to which the M3 protein does not bind, are involved in B-cell lymphopoeisis and trafficking, respectively (8, 22). Since B cells and macrophages are latently infected by γHV68, it may be to the advantage of γHV68 to limit the infiltration of inflammatory cells by interacting with chemokines such as hIL-8, MCP-1, or RANTES while retaining the differentiating functions and homeostatic trafficking functions of SDF-1 and BCA-1.
It is interesting that lymphotactin and fractalkine are bound by the M3 protein. Little is known about the role of these chemokines in viral infection; however, their binding by the M3 protein suggests that they may have important functions in the host response to gammaherpesvirus infections.
It may be that gammaherpesviruses in general require selective activation and inhibition of specific parts of the chemokine system. Signaling through chemokine receptors is probably an important requirement for gammaherpesvirus pathogenesis, since KSHV, HVS, and γHV68 all encode homologs of host G-protein-coupled receptors with close homology to the hIL-8 receptor and since the HVS and KSHV homologs can signal either constitutively or after binding chemokines (1, 3, 29). Inhibition of specific aspects of chemokine signaling by KSHV, while controversial (34), may well be accomplished by secreting different chemokine homologs, some of which are agonists and some of which are antagonists for specific host chemokine receptors (4, 9, 10, 15, 26). Further studies of the γHV68 M3 protein, especially using mutants lacking the M3 protein, will be required to elucidate the function of this novel protein in modulating inflammation and in gammaherpesvirus pathogenesis and latency.
ACKNOWLEDGMENTS
This work was supported by NIH grant CA74730 to H.W.V. and S.H.S. In addition, H.W.V. was supported by NIH grant AI39616 and ACS grant RP6-97-134-01-MBC, S.H.S. was supported by NIH grants CA43143, CA52004, and CA58524, and V.V.B. was supported by NIH grant GM07200. G.M. is supported by operating grants from the MRC and NCI of Canada.
We thank David Leib, the members of his laboratory, and the members of the Speck and Virgin laboratories for their helpful comments during the course of this research. We thank Joan Sechler for technical assistance.
ADDENDUM
While this paper was under review, Parry et al. published a paper showing that the M3 protein binds a number of chemokines, blocks chemokine interactions with cells, and prevents chemokine signaling (27).
REFERENCES
- 1.Ahuja S K, Murphy P M. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem. 1993;268:20691–20694. [PubMed] [Google Scholar]
- 2.Alcami A, Symons J A, Collins P D, Williams T J, Smith G L. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160:624–633. [PubMed] [Google Scholar]
- 3.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 4.Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Schweickart V L, Siani M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 5.Clambey E T, Virgin IV H W, Speck S H. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J Virol. 2000;74:1973–1984. doi: 10.1128/jvi.74.4.1973-1984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combadiere C, Ahuja S K, Murphy P M. Cloning and functional expression of a human eosinophil CC chemokine receptor. J Biol Chem. 1996;271:11034. [PubMed] [Google Scholar]
- 7.Combadiere C, Salzwedel K, Smith E D, Tiffany H L, Berger E A, Murphy P M. Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J Biol Chem. 1998;273:23799–23804. doi: 10.1074/jbc.273.37.23799. [DOI] [PubMed] [Google Scholar]
- 8.Cyster J G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 9.Dairaghi D J, Fan R A, McMaster B E, Hanley M R, Schall T J. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J Biol Chem. 1999;274:21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- 10.Endres M J, Garlisi C G, Xiao H, Shan L, Hedrick J A. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J Exp Med. 1999;189:1993–1998. doi: 10.1084/jem.189.12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fremont D H, Crawford F, Marrack P, Hendrickson W A, Kappler J. Crystal structure of mouse H2-M. Immunity. 1998;9:385–393. doi: 10.1016/s1074-7613(00)80621-4. [DOI] [PubMed] [Google Scholar]
- 12.Gerszten R E, Garcia-Zepeda E A, Lim Y C, Yoshida M, Ding H A, Gimbrone M A J, Luster A D, Luscinskas F W, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 13.Graham K A, Lalani A S, Macen J L, Ness T L, Barry M, Liu L Y, Lucas A, Clark-Lewis I, Moyer R W, McFadden G. The T1/35kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology. 1997;229:12–24. doi: 10.1006/viro.1996.8423. [DOI] [PubMed] [Google Scholar]
- 14.Kapadia S B, Molina H, van Berkel V, Speck S H, Virgin H W., IV Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J Virol. 1999;73:7658–7670. doi: 10.1128/jvi.73.9.7658-7670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kledal T N, Rosenkilde M M, Coulin F, Simmons G, Johnsen A H, Alouani S, Power C A, Luttichau H R, Gerstoft J, Clapham P R, Clark-Lewis I, Wells T N C, Schwartz T W. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 16.Lalani A S, Barrett J, McFadden G. Modulating chemokines: more lessons from viruses. Immunol Today, 2000;21:100–106. doi: 10.1016/s0167-5699(99)01556-x. [DOI] [PubMed] [Google Scholar]
- 17.Lalani A S, Graham K, Mossman K, Rajarathnam K, Clark-Lewis I, Kelvin D, McFadden G. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J Virol. 1997;71:4356–4363. doi: 10.1128/jvi.71.6.4356-4363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalani A S, Masters J, Graham K, Liu L, Lucas A, McFadden G. Role of the myxoma virus soluble CC-chemokine inhibitor glycoprotein, M-T1, during myxoma virus pathogenesis. Virology. 1999;256:233–245. doi: 10.1006/viro.1999.9617. [DOI] [PubMed] [Google Scholar]
- 19.Lalani A S, McFadden G. Secreted poxvirus chemokine binding proteins. J Leukoc Biol. 1997;62:570–576. doi: 10.1002/jlb.62.5.570. [DOI] [PubMed] [Google Scholar]
- 20.Lalani A S, Ness T L, Singh R, Harrison J K, Seet B T, Kelvin D J, McFadden G, Moyer R W. Functional comparisons among members of the poxvirus T1/35kDa family of soluble CC-chemokine inhibitor glycoproteins. Virology. 1998;250:173–184. doi: 10.1006/viro.1998.9340. [DOI] [PubMed] [Google Scholar]
- 21.Luster A D. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 22.Ma Q, Jones D, Borghesani P R, Segal R A, Nagasawa T, Kishimoto T, Bronson R T, Springer T A. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald M R, Burney M W, Resnick S B, Virgin H W., IV Spliced mRNA encoding the murine cytomegalovirus chemokine homolog predicts a beta chemokine of novel structure. J Virol. 1999;73:3682–3691. doi: 10.1128/jvi.73.5.3682-3691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald M R, Li X-Y, Virgin H W., IV Late expression of a β chemokine homolog by murine cytomegalovirus. J Virol. 1997;71:1671–1678. doi: 10.1128/jvi.71.2.1671-1678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mossman K, Nation P, Macen J, Garbutt M, Lucas A, McFadden G. Myxoma virus M-T7, a secreted homolog of the interferon-gamma receptor, is a critical virulence factor for the development of myxomatosis in European rabbits. Virology. 1996;215:17–30. doi: 10.1006/viro.1996.0003. [DOI] [PubMed] [Google Scholar]
- 26.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H G, Hayward G S, Reitz M S. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 27.Parry B C, Simas J P, Smith V P, Stewart C A, Minson A C, Efstathiou S, Alcami A. A broad spectrum secreted chemokine binding protein encoded by a herpesvirus. J Exp Med. 2000;191:573–578. doi: 10.1084/jem.191.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfold M E, Dairaghi D J, Duke G M, Saederup N, Mocarski E S, Kemble G W, Schall T J. Cytomegalovirus encodes a potent alpha chemokine. Proc Natl Acad Sci USA. 1999;96:9839–9844. doi: 10.1073/pnas.96.17.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenkilde M M, Kledal T N, Brauner-Osborne H, Schwartz T W. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J Biol Chem. 1999;274:956–961. doi: 10.1074/jbc.274.2.956. [DOI] [PubMed] [Google Scholar]
- 30.Schall T J, Bacon K B. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 31.Simas J P, Bowden R J, Paige V, Efstathiou S. Four tRNA-like sequences and a serpin homologue encoded by murine gammaherpesvirus 68 are dispensable for lytic replication in vitro and latency in vivo. J Gen Virol. 1998;79:149–153. doi: 10.1099/0022-1317-79-1-149. [DOI] [PubMed] [Google Scholar]
- 32.Simas J P, Swann D, Bowden R, Efstathiou S. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J Gen Virol. 1999;80:75–82. doi: 10.1099/0022-1317-80-1-75. [DOI] [PubMed] [Google Scholar]
- 33.Smith C A, Smith T D, Smolak P J, Friend D, Hagen H, Gerhart M, Park L, Pickup D J, Torrance D, Mohler K, Schooley K, Goodwin R G. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits beta-chemokine activity yet lacks sequence homology to known chemokine receptors. Virology. 1997;236:316–327. doi: 10.1006/viro.1997.8730. [DOI] [PubMed] [Google Scholar]
- 34.Sozzani S, Luini W, Bianchi G, Allavena P, Wells T N, Napolitano M, Bernardini G, Vecchi A, D'Ambrosio D, Mazzeo D, Sinigaglia F, Santoni A, Maggi E, Romagnani S, Mantovani A. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood. 1998;92:4036–4039. [PubMed] [Google Scholar]
- 35.Speck S H, Virgin H W., IV Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr Opin Microbiol. 1999;2:403–409. doi: 10.1016/s1369-5274(99)80071-x. [DOI] [PubMed] [Google Scholar]
- 36.Sunil-Chandra N P, Efstathiou S, Nash A A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 37.Upton C, Mossman K, McFadden G. Encoding a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 38.van Berkel V, Preiter K, Virgin IV H W, Speck S H. Identification and initial characterization of a murine gammaherpesvirus 68 gene encoding an abundantly secreted protein. J Virol. 1999;73:4524–4529. doi: 10.1128/jvi.73.5.4524-4529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dyk L F, Hess J L, Katz J D, Jacoby M, Speck S H, Virgin H W., IV The murine gammaherpesvirus 68 v-cyclin is an oncogene that promotes cell cycle progression in primary lymphocytes. J Virol. 1999;73:5110–5122. doi: 10.1128/jvi.73.6.5110-5122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virgin H W, IV, Presti R M, Li X-Y, Liu C, Speck S H. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virgin H W, IV, Speck S H. Unraveling immunity to gammaherpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr Opin Immunol. 1999;11:371–379. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 43.Weck K E, Dal Canto A J, Gould J D, O'Guin A K, Roth K A, Saffitz J E, Speck S H, Virgin H W., IV Murine gammaherpesvirus 68 causes large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [DOI] [PubMed] [Google Scholar]
- 44.Weck K E, Kim S S, Virgin IV H W, Speck S H. B cells regulate murine gammaherpesvirus 68 latency. J Virol. 1999;73:4651–4661. doi: 10.1128/jvi.73.6.4651-4661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weck K E, Kim S S, Virgin IV H W, Speck S H. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witt D P, Lander A D. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol. 1994;4:394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]