Abstract
Combination chemotherapy administering multiple chemo-agents is widely exploited for the treatment of various cancers in the clinic. Specially for hepatocellular carcinoma (HCC), one of the most common malignancies, a co-administration of combinational cytostatic multi-kinase inhibitors and cytotoxic chemo-agents has been suggested as a potential curative approach. Here, Janus microcarriers were developed for the controlled local combination chemotherapy of HCC. The Janus microcarriers are composed of polycaprolactone (PCL) compartment and magnetic nanoparticles-loaded poly(lactide-co-glycolic acid) (PLGA) compartment which contain hydrophobic regorafenib and hydrophilic doxorubicin, respectively. Exploiting the magnetic anisotropy, rotational motion of the Janus microcarriers is controlled with magnetic field, which enables the active co-release of dual chemo-agents. Furthermore, Janus microcarriers exhibit magnetic resonance (MR) contrast effect, supporting the successful transcatheter intra-arterial delivery of the combination chemo-agents loaded the microcarriers to the targeted tumor. This Janus microcarriers potentially serve as a general combinational chemo-therapeutic platform for the co-delivery of various combinations of multi-chemo-agents.
Keywords: magnetic response, drug release, Janus microcarriers, cancer therapy, liver cancer
Graphical Abstract
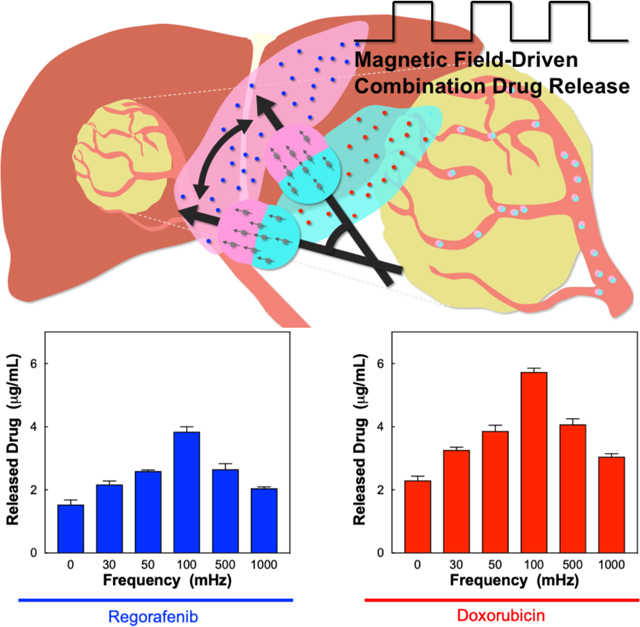
Magnetic Janus microcarriers are mirofluidically designed to simultaneously load dual chemo-agents and possess magnetic anisotropy. The microcarriers show rotational motion under alternating magnetic field, which causes the active release of the chemo-agents in local region. The microcarriers are promising for combinational chemo-therapy for the treatment of liver cancer through transcatheter intra-arterial delivery.
1. Introduction
Hepatocellular carcinoma (HCC) is the 5th most common malignancy in the world[1] and the 4th leading cause of cancer death in the US.[2] Developed countries have had an 80% increase in HCC incidence over the last 15 years.[3] Surgical resection and transplantation are the sole potentially curative treatments, but only 10–15% of patients are candidates.[4] Thus, doxorubicin-transcatheter arterial chemoembolization (DOX-TACE) as a mainstay of the treatment of HCC at intermediate stage has produced survival advantages by achieving higher concentrations of drugs within the targeted liver tumors with minimizing systemic exposure compared with systemic chemotherapy. However, possible activation of growth factors including hypoxia inducible factor-1a (HIF-1a) and vascular endothelial growth factor (VEGF) following TACE[5] may ultimately lead to recurrences after TACE.[6] Combination chemotherapy countering the stimulation of these growth factors may be critical to maximize the longitudinal efficacy of DOX-based TACE therapies for HCC.[7] Thus, anti-angiogenic cytostatic multi-kinase inhibitors (MKIs) in combination with DOX-TACE is now being a promising approach to maximize therapeutic outcomes by interfering angiogenesis and simultaneously inducing cancer cell apoptosis.[8] However, those therapies have been performed by a separate administration of systemic oral MKIs and DOX-TACE[8–9] due to the limitations of conventional single-compartment microcarriers. Side effect from nonspecificity of oral delivered anti-angiogenic MKI drug upon heterogeneous tumor environment limits the strategic and manageable combination chemotherapy[10] as noted failure of recent clinical phase-3 for combination of sorafenib with doxorubicin.[11] Thus, it is in high demand of controllable method for generating multi-featured drug carriers to encapsulate and release the combination chemo-agents.
Various nanocarriers including liposomes, dendrimers, polymers have demonstrated the potential for the combination drug delivery.[12] However, a controlled combination multi-drug delivery with an effective dosage in local area has been challenging. As drug eluting bead-transcatheter chemoembolization (DEB-TACE) has been used for localized drug delivery to HCC tumor region, a microscale drug eluting bead is a strong form of combination drug delivery carriers. Microfluidic technology has successfully produced uniform microstructures, composed of multiple components due to their high controllability over the size and composition. Microfluidically-designed Janus microcarriers possess two distinct compartments with different compositions,[13] which are beneficial for encapsulating and releasing combination multi-drugs. In addition, the electric or magnetic anisotropy of Janus microcarriers enables the external-field-controlled active motion, which is potentially advantageous for the triggered release of multi-drugs.
Here, we develop Janus microcarriers with a magnetic anisotropy whose two distinct compartments respectively contain anti-angiogenic MKI regorafenib and cytotoxic doxorubicin chemo-agents for magnetic-field-triggered local combinational chemotherapy of HCC (Figure 1a). The two distinct compartments are made of poly(lactide-co-glycolic acid) (PLGA) and polycaprolactone (PCL), which are successfully formed by a phase separation of the two biodegradable polymers in microfluidically-prepared emulsion droplets. During the phase separation, hydrophobic regorafenib and hydrophilic doxorubicin are loaded in PCL and PLGA compartments respectively, whereas magnetic nanoparticles are selectively loaded in PLGA compartment, rendering the microparticles magnetically anisotropic. Under alternating magnetic field, therefore, the Janus microcarriers show rotational motions, which drives the local release of the combination chemo-agents. In addition, the Janus microcarriers show a magnetic resonance (MR) contrast effect, which enables the monitoring of the transcatheter intra-arterial delivered microparticles to the targeted liver cancer.
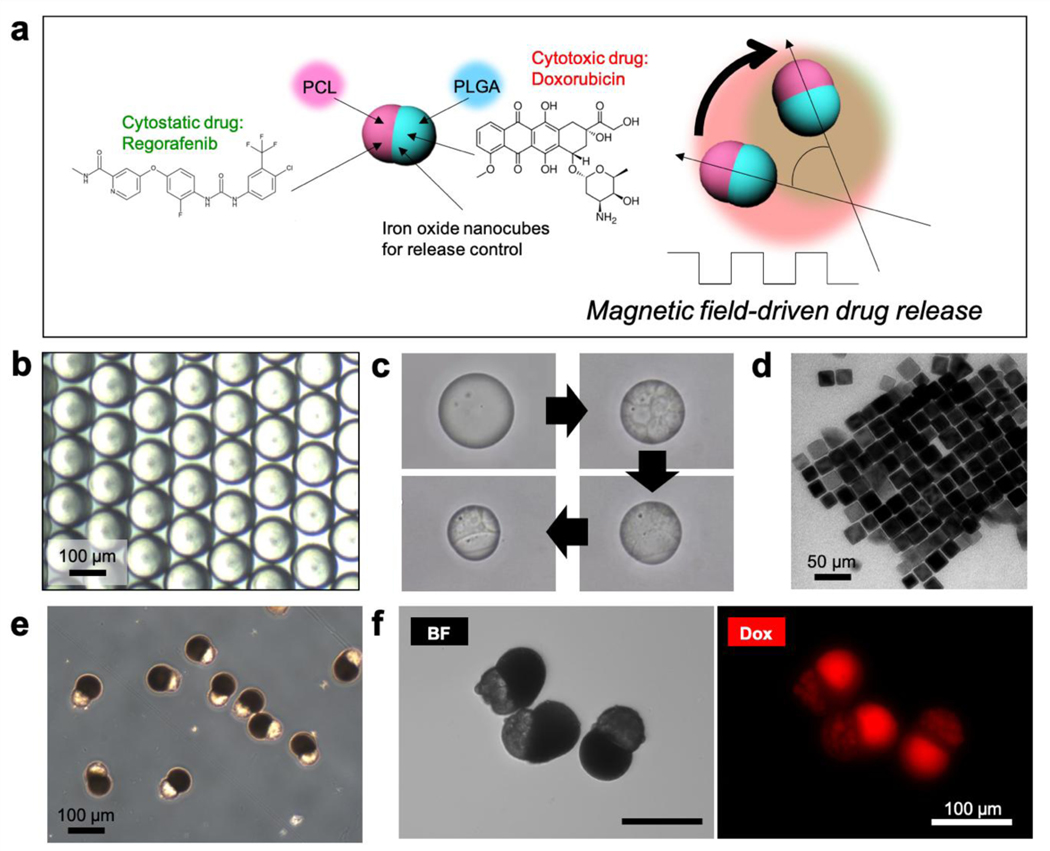
Figure 1.
(a) Schematic illustration of Janus microcarrier composed of regorafenib-loaded PCL compartment and doxorubicin-loaded PLGA compartment. The PLGA compartment contains iron-oxide nanocubes, which enables the rotational motion of the Janus microcarriers under alternating magnetic field and the active release of the drugs. (b) Optical microscopy (OM) image of the oil-in-water (O/W) emulsion drops. (c) Time series of OM images showing the phase separation in the emulsion droplet. (d) Transmission electron microscopy (TEM) image of iron-oxide nanocubes. (e) OM image of monodisperse Janus microcarriers. (f) OM and fluorescent microscopy images of Janus microcarriers containing regorafenib and fluorescent doxorubicin.
2. Results and Discussion
2.1. Janus Microcarriers for Magnetic-controlled Chemotherapy
To prepare Janus microcarriers with uniform size and composition, two immiscible biodegradable polymers of PLGA and PCL are dissolved in chloroform, which is emulsified in aqueous solution of poly(vinyl alcohol) (PVA) to form oil-in-water (O/W) emulsion droplets using a glass capillary microfluidic device (Figure S1a). The drops are initially spherical and have an average diameter of 120 μm (Figure 1b and S1b), which turn to Janus-shaped microparticles with average dimension of 88 μm along the long axis (Figure S1c). As the chloroform is gradually depleted by evaporation, two polymers are concentrated in the droplets, which causes the spinodal decomposition to PCL-rich and PLGA-rich microdomains above a threshold concentration.[10,11] The microdomains are further coalesced to minimize interfacial energy. Because the PCL-rich domain is more hydrophobic than PLGA-rich one, the small PCL-rich domains are embedded in a matrix of PLGA-rich domain in each droplet (Figure 1c). As the PCL-rich domains further coalesce, the growing core is exposed to water. This causes the dewetting of the PLGA-rich domain on the surface of PCL-rich core as PVA adsorbs at the PCL-water interface and reduces the interfacial energy (Figure 1c). Additional coalescence results in two distinct compartments formed at the opposite poles with a compartment boundary at the center and completely consolidated to solid microparticles.[12] The concentration of DMSO partially miscible with continuous water phase was critical to prepare stable Janus compartment. The DMSO concentration was adjusted to a volume ratio of 30:1:1 (polymer solution:nanocube dispersion:DMSO) (Figure S2). The emulsion drops are finally consolidated to solid microparticles, while maintaining the overall Janus configuration. The whole phase separation process is shown in Figure S3 and Movie S1 of the Supporting Information. For the magnetic anisotropy, iron-oxide nanocubes with an average size of 27 nm are dispersed in the emulsion drops. The iron oxide nanocubes are synthesized by thermal decomposition of iron-oleate complex in a mixture of sodium oleate, n-docosane, and 1-octadecene and the surface is capped with oleic acid (Figure 1d and Figure S4).[14] In addition, dual chemo-agents of regorafenib and doxorubicin are dissolved in the emulsion drops. The iron-oxide nanocubes with a ligand of oleic acid selectively migrate to the PLGA-rich compartment where having carboxyl end groups for coordination interaction with iron oxide nanocubes via hydrogen bonds during the consolidation period (Figure S3b).[15] The resulting microparticles have PCL-rich compartment with rough surface and PLGA-rich compartment with smooth surface, as shown in Figure 1e; PCL forms semicrystalline structure, which results in the rough surface.[16] The PLGA-rich compartments are rendered to be dark brown as the iron-oxide nanocubes are concentrated. The regorafenib and doxorubicin are concentrated in PCL- and PLGA-rich compartments, respectively. This compartment-selective loading is achieved by molecular affinity of the drugs to the compartments. The relatively hydrophobic drug, regorafenib, migrates to the PCL-rich compartment as it has a higher affinity to relatively hydrophobic PCL than the PLGA, whereas the relatively hydrophilic drug, doxorubicin, migrates to the PLGA-rich compartment as the affinity tendency is opposite. The selective loading of the doxorubicin in PLGA-rich compartment can be confirmed with a strong red fluorescence in the smooth compartment, as shown in the right panel of Figure 1f. The shape of Janus microparticles remains unchanged, even though the relative loading of regorafenib to doxorubicin is varied (Figure S5). The combination of two chemo-agents, regorafenib and doxorubicin, is selected as it exhibits synergistic anti-cancer effect against human HCC cell line, HepG2, when they are co-delivered. The synergism between regorafenib and doxorubicin is confirmed by combination index (CI) according to the isobologram equation of Chou-Talalay[17], where CI values determine the cellular inhibition of two combination chemo-agents: synergism (CI<1), additive effect (CI=1), and antagonism (CI>1) (see the Experimental Section for the details). Various relative doses of the two drugs show the value of CI smaller than 1, indicating that the combination is highly synergetic (Figure S6 and Table S1).
2.2. Rotational Motion of Microcarriers on Alternating Magnetic Field
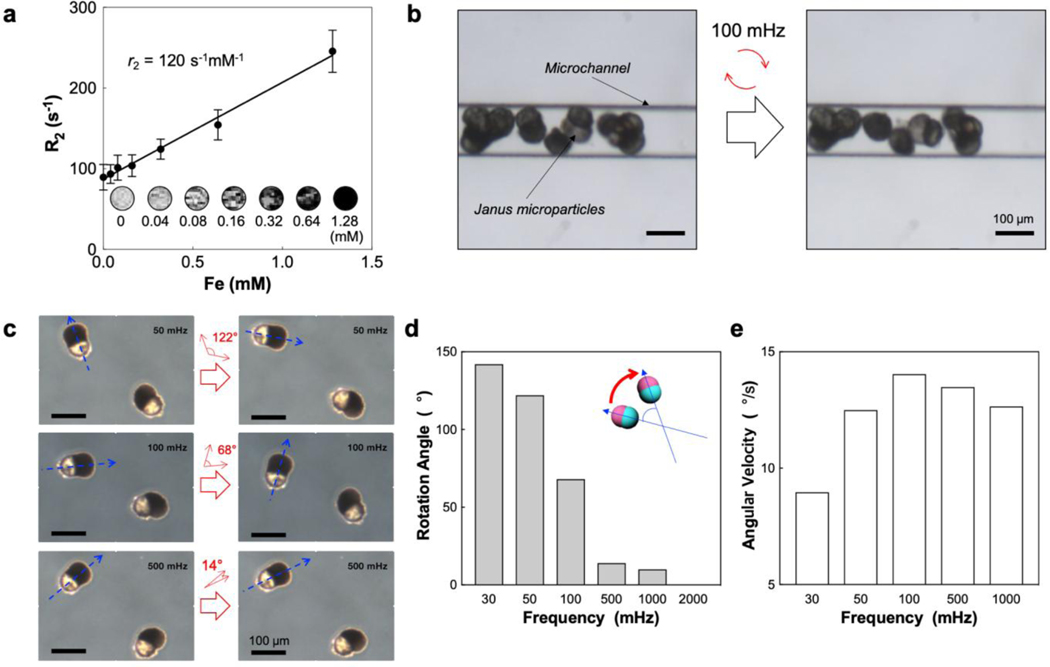
MR contrast effect of the Janus microcarriers is characterized with T2-weighted MR images (Figure 2a). The signal decays along with the concentration of iron, where the concentration is varied by adjusting the number of the Janus microcarriers in agar phantom. The value of r2 relaxivity is 120 s−1mM−1 at a field strength of 7 T at 300 K, which is sufficient to visualize the microparticles using MRI. To demonstrate the embolization and magnetic field–responsive motion of the microparticles in the hepatic artery, the microparticles are injected into the microchannel with a dimension of 130 μm while flowing the fluids through the channel. The microparticles are stacked in the channel and show the rotational motion when alternating magnetic field is applied (Figure 2b). The microparticles repeatedly rotate clockwise and counter-clockwise, synchronized with the alternation of the field, rather than rotating in one direction (Movie S2 of the Supporting Information). The rotation angle decreases as the frequency of the alternating field increases (Figure 2c, 2d and Figure S7). The angle is 142° at the frequency of 30 mHz, which decreases to 68° at 100 mHz, 14° at 500 mHz, and 7° at 1000 mHz. Above 100 mHz, the range of rotational angle is decreased and close to the vibration rather than rotation. No angle is measured at 2000 mHz. This reduction of the angle along with the frequency is caused by the reduction of rotation time in one direction which is consistent to the half cycle of the alternating field (Figure S8). The angular velocity increases as the frequency is raised from 0 to 100 mHz, which then decreases as the frequency is further raised (Figure 2e). This reduction of the rotational motion is caused by off-resonance between fast magnetic field frequency and Janus microcarriers related with liquid drag force.[18]
Figure 2.
(a) R2 value of Janus microcarriers in 1% agar phantoms as a function of iron concentration at 7 T. Insets are T2-weighted MR images. (b) OM images before and after applying alternating magnetic field at 100 mHz to Janus microcarriers in a microchannel while flowing the suspension medium. (c) OM images before and after applying alternating magnetic fields at 50, 100, and 500 mHz, respectively. The long axes of the Janus microcarriers are indicated with blue arrows and the angle variations are denoted with red arrows. (d) Rotation angle as a function of frequency. (e) Angular velocity as a function of frequency.
2.3. Release of Dual Chemo-agents induced by Magnetic-responsive Rotational Motion
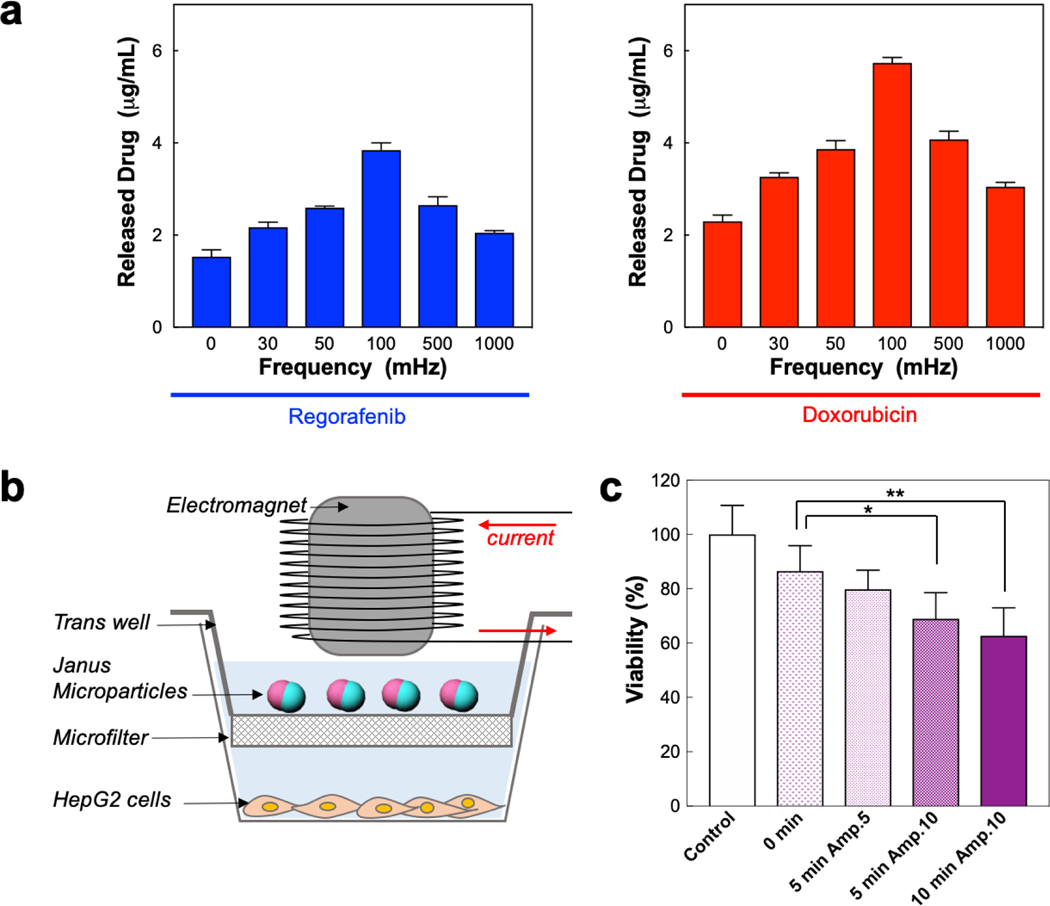
The active co-release of dual chemo-agents is induced by the magnetic-responsive rotational motion of the Janus microcarriers. The frequency of magnetic field directly affects the release amount of both chemo-agents (Figure 3a). The microparticles show the highest release rates at the frequency of 100 mHz for both regorafenib and doxorubicin up to 32% and 72% from the microcarriers (drug loading content: 1.2 wt% for regorafenib and 0.8 wt% for doxorubicin), respectively (Figure S9). This frequency is consistent with that of highest angular velocity, indicating that the release is accelerated by the rotational motion. To study the influence of the magnetic field-driven drug release from the Janus microcarriers on the cellular death, we specially design the double-stacked reservoir with a microfilter in the middle. The filter allows the transport of the drugs while separating the microparticles above the filter from HepG2 cells below the filter (Figure 3b). The microparticles are subjected to an alternating magnetic field with a frequency of 100 mHz using an electromagnet, where the exposure time and intensity of magnetic field are controlled. The longer exposure and higher amplitude of the magnetic field increases the cytotoxicity to the cells (Figure 3c). The exposure for 5 and 10 minutes at 10 Oe shows significant reduction of the cell viability, compared to a control group that is the cells with no magnetic field, with the P-values less than 0.05 in the Welch’s t-test, where *P = 0.0189 for 5 minutes and **P = 0.0051 for 10 minutes, respectively.
Figure 3.
(a) The concentrations of regorafenib (left) and doxorubicin (right) in the suspension medium as a function of frequency of alternating magnetic field, where Janus microcarriers are incubated in PBS for 4 h after the field exposure for 10 min. (b) Schematic illustration for the experimental setup to study the magnetic-field-trigged release of drugs from Janus microcarriers and the anticancer effect against human liver cancer cell lines, HepG2 cells. (c) Cell viability of HepG2 cells treated with different amplitude and exposure time at the frequency of 100 mHz. (*P = 0.0189 and **P = 0.0051 in the Welch’s t-test, n = 6).
2.3. Magnetic-controlled Combination Chemotherapy Effect In Vivo
To study in-vivo therapeutic effect of co-delivery of dual chemo-agents and visualize the Janus microcarriers using MRI, we use the orthotopic HCC rat model. McA-RH7777 rat hepatoma cells are inoculated into Sprague Dawley rats and after 1 week of tumor growth, the Janus microcarriers are infused through a transcatheter to the intra-arterial region with x-ray DSA selective catheterization.[19] Transcatheter hepatic intra-arterial infusion relies upon differences in the blood supply between HCC and normal liver tissues. Hepatic arteries supply ~90% of blood flow to HCC but only ~25% of flow to normal liver[19a, 20], most of the Janus microcarriers injected into the hepatic artery are delivered to the HCC region.[21] Because of a preferential tumor region accumulation of the Janus microcarriers, the peripheral and proximal tumor regions show a significant reduction of T2 signal relative to the surrounding normal liver region (Figure 4a). The deposition of the Janus microcarriers in the rim region of tumor is further confirmed by the presence of iron in tissues stained with Prussian Blue (Figure 4b). The distribution of Janus microcarriers are good agreement with previous results of IA infused microspheres.[14, 19] The deposited Janus microcarriers in tumor-feeding arteries permit a controlled release of drugs into the tumor blood vessels. The release of regorafenib and doxorubicin from the Janus microcarriers is triggered by the alternating magnetic field at 100 mHz and the potential therapeutic effect of co-delivery of dual chemo-agents is studied by H&E, TUNEL and CD34 immuno-histological analysis of the tumor tissues (Figure 4b). The released cytotoxic doxorubicin and anti-angiogenic regorafenib significantly enhanced TUNEL positive apoptosis and decreased micro-vessel development in HCC tumor region including the rim area. In addition, the biodegradation of PCL and PLGA components[22] would contribute to prevent possible tumor recurrence[5] by permanent embolization in long-term. Further in-vivo optimization, long-term therapeutic response and survival rate studies are warranted to demonstrate enhanced synergistic anti-cancer effect of co-delivered combination chemo-agents by the magnetic-field-triggered drug release from IA-infused Janus microcarriers.
Figure 4.
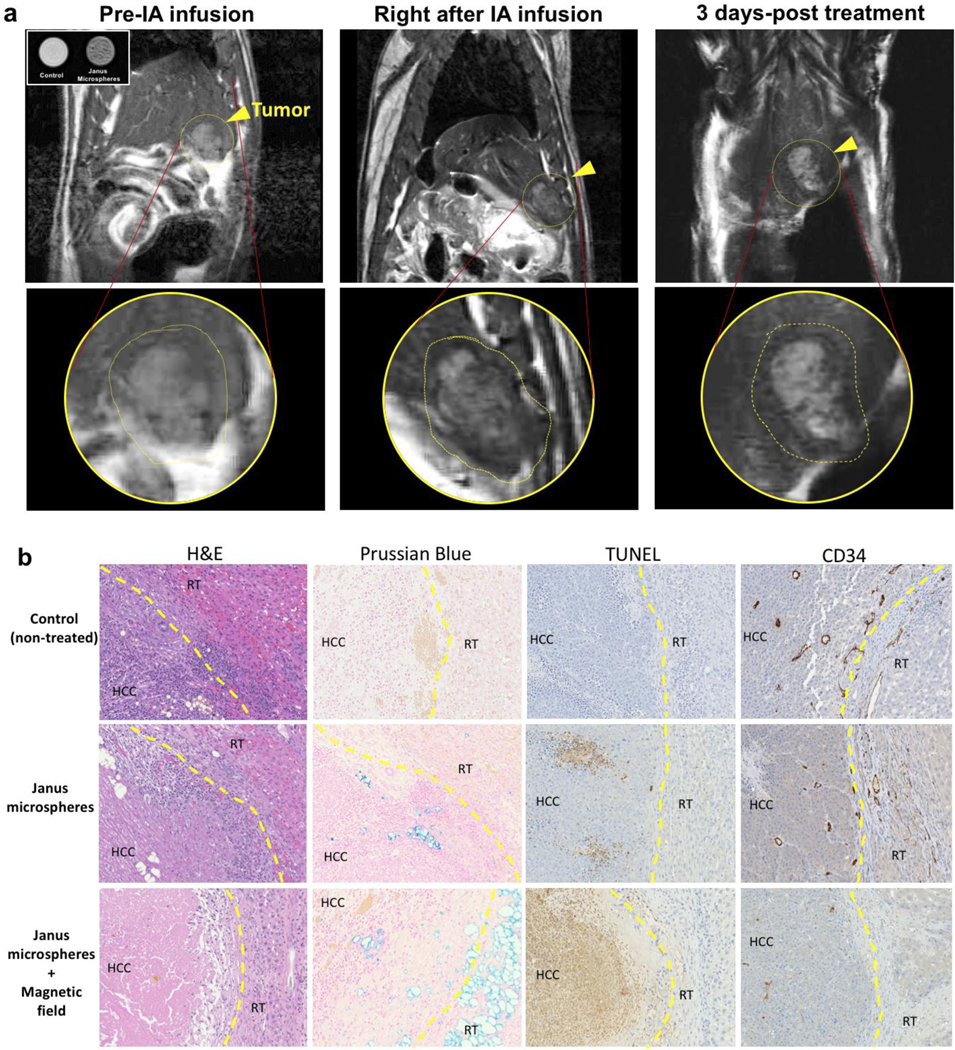
(a) MR T2 images of McA-Rh7777 HCC rat treated with IA infusion of Janus microcarriers (inset) MR contrast effect of Janus microcarriers (0.5 mg/ml) dispersed in 1% agarose phantom (control: only phantom). Tumors are delineated with yellow circles and the tumor regions are magnified in bottom row. Dark contrast in tumors is showing deposited Janus microcarriers after the IA infusion, (b) Optical microscope image of tissues samples stained with H&E, Prussian blue, TUNEL and CD34 after euthanizing at 3 day-post treatment. The brown color in TUNEL and CD34 staining indicate TUNEL-positive apoptotic cells and micro-vessels in the tumor tissues, respectively. (HCC: HCC tumor region and RT: rim of tumor)
3. Conclusion
In conclusion, we report Janus microcarriers for on-demand co-delivery of combinational chemo-agents in a local tumor region. Dual compartments are integrated in each Janus microcarrier by the phase separation of two immiscible biocompatible/biodegradable polymers in O/W emulsion droplets. The uniform size and composition of the Janus microcarriers are secured by the microfluidic technology. In each Janus microcarriers, iron-oxide nanocubes are preferentially segregated in one compartment, which provides the magnetic anisotropy for rotational motion under alternating magnetic field. Dual combination chemo-agents are loaded in their own compartments in the Janus microcarriers, which are released by the magnetic-field-induced rotation. Furthermore, the Janus microcarriers exhibit the MR contrast effect due to the iron-oxide nanocubes, which supports the successful administration of the Janus microcarriers to the targeting location. As a combination chemo-agents, we select anti-angiogenetic MKI regorafenib and cytotoxic doxorubicin in this work, which shows synergistic anti-cancer effect. We prove the potential utility of our microcarriers for the interventional treatment of HCC through in-vitro and in-vivo experiments. We believe that the Janus microcarriers can serve as a combinational chemo-therapeutic platform for the controllable delivery of various combinations of multi-chemo-agents.
4. Experimental Section
Materials:
All reagents and solvents were obtained commercially and used without further purification. The poly(D, L-lactide-co-glycolide) (lactide:glycolide = 50:50, PLGA), polycaprolactone (PCL), poly(vinyl alcohol) (Mw 89,000–98,000, 99+% hydrolyzed, PVA), octadecylamine, Iron(III) chloride hexahydrate (≥98%), chloroform (CHCl3), dimethyl sulfoxide (DMSO), n-docosane (99%), 1-octadecene (90%), n-hexane, and ethanol were purchased from Sigma-Aldrich Co (Milwaukee, WI, USA). Sodium oleate (>97%) was purchased from TCI America (Portland, OR, USA). Regorafenib and doxorubicin HCl were purchased from LC Laboratories (Woburn, MA, USA).
Preparation and morphology analysis of iron oxide nanocubes:
Iron oxide nanocubes were prepared by thermal decomposition of iron (III) oleate in the presence of sodium oleate by following the protocol previously reported.[23] In a 50 mL three-neck round-bottom flask with 11 mL of 1-octadecene, the mixture of iron oleate (1.57 g, 1.75 mmol), n-docosane (6.0 g, 19.32 mmol), and sodium oleate (0.53 g, 1.74 mmol) was heated to 120°C under high vacuum for 1 h while stirring the mixture. The reaction mixture was then heated to 337°C with the heating rate of 3°C/min under nitrogen atmosphere and stirred for 30 min. Then, the reaction mixture was cooled to 80 °C. Iron oxide nanocubes were precipitated in a mixture of n-hexane and ethanol in a volume ratio of 2:3. The supernatant was discarded and the precipitates were re-dispersed in n-hexane and washed with ethanol. The washing process was repeated for three times. Finally, iron oxide nanocubes was dried under vacuum and re-dispersed in chloroform. The morphology of iron oxide nanocubes was observed with a transmission electron microscopy (TEM 120 kV, FEI Tecnai Spirit G2, Hillsboro, OR, USA) and the size distribution was determined by counting 500 iron oxide nanocubes with ImageJ.
Preparation and observation of Janus microcarriers:
The dispersed phase of the emulsion droplets contained PLGA, PCL, iron-oxide nanocubes, regorafenib, and doxorubicin. To prepare the dispersed phase, a polymer solution, a dispersion of the nanocubes, and a drug solution were mixed in the volume ratio of 30:1:1. The polymer solution was prepared by dissolving 0.5 w/v% PLGA and 0.5 w/v% PCL in chloroform. The iron-oxide nanocubes were dispersed in chloroform at the concentration of 15 mg/mL. The drug solutions were prepared by dissolving regorafenib and doxorubicin in dimethyl sulfoxide (DMSO), where the total drug molar concentration remained for 2 mM with the molar ratios were set to 10:1, 7:1, 5:1, 3:1, and 1:1, respectively. The continuous phase of the emulsion droplets was 4 w/w% aqueous solution of PVA. Emulsion droplets were produced by a glass capillary microfluidic device. The device was composed of two tapered cylindrical capillaries assembled in a square capillary.[12] The flow rates of the dispersed and continuous phases were set to 50 μL/min and 1 mL/min using syringe pumps (New Era NE-1000, NY, USA). The emulsion droplets were collected in the glass reservoir filled with the 4 w/w% aqueous solution of PVA and left undisturbed for 1 day for the solvent evaporation. Finally, the Janus microcarriers were washed with PBS. We stored the Janus microcarrier in the refrigerator (4°C) to preserve the drug integrity. The drug contents in the Janus microcarrier were characterized before every use by dissolving the part of the microcarriers with the chloroform and then measuring the amounts of drugs using HPLC for regorafenib and fluorescence for doxorubicin. The microparticles were observed by using an optical microscopy (CKX41, Olympus, Japan) and fluorescent microscopy (Cytation3, BioTek, Winooski, VT, USA) with a green filter.
Motion control of Janus microcarriers using controlled magnetic field:
Janus microcarriers were suspended in an aqueous solution of 1% Tween® 20 at the concentration of 10 mg/mL. The alternating magnetic field was applied to Janus microcarriers using a cylinder electromagnet (DC 12 V, 25 × 25 mm) and the frequency was adjusted to 30, 50, 100, 500, 1000, and 2000 mHz. The motion of Janus microcarriers was observed with the optical microscopy (CKX41, Olympus, Japan) and recorded with the video camera (QColor5, Olympus, Japan). The embolization of the microparticles was observed in the microchannel (130 μm × 130 μm) while flowing the aqueous solution of 1% Tween® 20 at the flow rate of 2 mL/min. The magnetic field was applied to the microchannel at the frequency of 100 mHz to see the motion of the microparticles.
Characterization of T2 relaxivity property of Janus microcarriers:
R2 relaxivity was measured by using 7T MRI scanner (BioSpec, Bruker, Billerica, MA, USA). The 1% agarose phantoms containing Janus microcarriers with various concentrations (0–15.4 mg/mL) was prepared. The iron content (Fe) in each phantom was determined by using inductive couple plasma mass spectrometry (ICP-MS, Perkin Elmer, Waltham, MA, USA). The phantoms were scanned using A Carr–Purcell–Meiboom–Gill (CPMG) sequence (TR = 1s, 1 mm slice thickness, 6 TE ranging from 10 to 60 ms) and R2 time constants of the samples were determined by fitting signal decay curves to the mono-exponential function: S(TE) = Moe−TE/T2. The Pearson correlation coefficient was calculated between the iron concentration and R2 values. The slope of the resulting linear least squares fit line provided the R2 relaxivity in units of S−1mM-1.
Characterization of Regorafenib and doxorubicin release from Janus microcarriers:
The drug release from Janus microcarriers before and after applying various magnetic field frequencies (0, 30, 50, 100, 500, and 1000 mHz), was analyzed by a high-performance liquid chromatography (HPLC, Agilent 1260 Infinity Quaternary LC, Santa Clara, CA, USA) equipped with a Zorbax C18 column (Agilent, 5 μm, 9.4 × 250 mm) for regorafenib and a fluorescent microplate reader (Cytation3, BioTek, Winooski, VT, USA) for doxorubicin. The seven samples with 1 mg/mL microparticles were prepared in the 1 mL of 10 mM PBS solution (pH 7.4 or 6.5, 0.02 wt% Tween 80). The supernatant from one sample was collected by sedimentation of the microparticles with the centrifugation (1000 rpm, 3 min). Then, the other six samples were exposed to magnetic frequencies of 0, 30, 50, 100, 500, and 1000 mHz, respectively for 10 min and left for 4 h. The supernatant from these samples was collected after centrifugation of the microparticles. Then, regorafenib content was measured with HPLC at a wavelength of 255 nm, while running the mobile phase of acetonitrile and ammonium acetate (60:40) pumped at a constant flow rate of 1 mL/min in the C18 column. The amount of regorafenib content was determined by a reference linear curve of regorafenib concentration vs. height of the HPLC signal. Doxorubicin content was analyzed by fluorescent signals from the samples (excitation 490 nm, emission 550 nm). The amount of doxorubicin was determined by a reference linear curve of doxorubicin concentration vs. fluorescent signal.
Cell culture:
The human liver cancer cell line, HepG2 (ATCC, Manassas, VA, USA) was cultured in Eagle’s Minimum Essential Medium (EMEM) with 10% fetal bovine serum (FBS) and supplemented with 1% penicillin at 37°C, under 5% CO2.
In vitro cytotoxicity test against Regorafenib and doxorubicin for combination index (CI):
100 μL of HepG2 cells with the concentration of 1 × 105 /mL was plated in each well of a 96-well plate and cultured for 12 h. 20 μL solutions of Regrorafenib and doxorubicin in various concentrations (0–10 μM) in DMSO were treated onto the cells and incubated for 24 h. The cell media was discarded and cells were washed with fresh PBS solutions to remove supernatant and debris. Then, cells were treated with 50 μL MTT solution (5 mg/mL solution in PBS) and incubated for 2 h. After incubation, add 100 μL of DMSO into the wells. The plate was sealed with aluminum foil to prevent light exposure and left for 30 min to dissolve the MTT formazan. The plate was read at 590 nm (O.D.) with the plate reader (BioTek Inc., Crawfordsville, IN, USA). After obtaining the concentration dependent cellular viability curves of regorafenib and doxorubicin, respectively, the cellular inhibitory concentration of each drug was determined. At various combination ratios of inhibitory concentrations for regorafenib and doxorubicin, the cytotoxicity test was performed by following the aforementioned method. Then, CI values were determined by using the multiple drug-effect equation of Chou-Talalay method[17]:
where indicates the dose of drug 1 in combination, indicates the dose of drug 2 in combination, and represent the dose of drug 1 and drug 2 alone when inhibiting , respectively.
In vitro cytotoxicity test against magnetic field-driven drug release:
100 μL of HepG2 cells with the concentration of 1 × 105 /mL was plated in each well of a 96-well plate (EMD Millipore MultiScreen-Mesh filter plates with receiver plates, Burlington, MA, USA) and cultured for 12 h. The 20 μL solutions of 5 mg/mL Janus microcarriers in PBS solution were treated onto the cells through receiver plates (microfilter). The magnetic field was applied to the microparticles with the frequency of 100 mHz in various exposure times and amplitudes, then, the cells were incubated for 4 h. The cell media was discarded and cells were washed with fresh PBS solutions to remove supernatant and debris. The cells were treated with 50 μL MTT solution (5 mg/mL solution in PBS) and incubated for 2 h. After incubation, add 100 μL of DMSO into the wells. The plate was sealed with aluminum foil to prevent light exposure and left for 30 min to dissolve the MTT formazan. The plate was read at 590 nm (O.D.) with the plate reader (BioTek Inc., Crawfordsville, IN, USA).
Animal Study:
For hepatic artery catheterization in HCC rat model, after laparotomy, a cotton-tipped applicator was used to expose the common hepatic artery (CHA), proper hepatic artery (PHA), and gastroduodenal artery (GDA). A micro bulldog clamp was placed on the CHA (World Precision Instruments, Sarasota, FL) to prevent bleeding during catheterization. 4–0 Vetacryl absorbable polyglycolic acid suture (Webster Veterinary, Devens, MA) was then used to ligate the GDA distally in order to control retrograde bleeding during catheterization. Next, a 24G SurFlash polyurethane catheter (Terumo Medical Co., Somerset, NJ) was inserted into the GDA, advanced into the PHA and then distally into the LHA. X-ray DSA using a C-ARM angiographic system (Siemens, Germany) was used to confirm catheter placement (24G × 3/4”). After selective catheterization, the Janus microcarriers (100 μL, 100 mg/mL) were vortexed for dispersion in PBS and the microcarriers were IA infused through the catheter; after infusion, blood flow to liver was restored by releasing bulldog clamp after catheter removal and proximal ligation of GDA; finally, abdomen was closed using 2-layer technique. Rats was transferred from x-ray DSA to MRI scanners in adjacent rooms. For magnetic field triggered drug release, an electro magnet that can focus magnetic field at specific region was used. Rats after IA infusion of Janus microcarriers were treated with an alternating magnetic field for 10 min. At 3-day post treatment, rats were euthanized to harvest liver and tumor tissues for immunohistology analysis.
Supplementary Material
Acknowledgements
This work was supported by grants R21CA173491, R01CA218659 and R01EB026207 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering. We also appreciate a partial support from the National Research Foundation (NRF) of Korea (NRF-2015K1A1A2033054).
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Soojeong Cho, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois 60611, USA.
Nam Gi Min, Department of Chemical and Biomolecular Engineering, KAIST, Daejeon, South Korea..
Dr. Wooram Park, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois 60611, USA.
Shin-Hyun Kim, Department of Chemical and Biomolecular Engineering, KAIST, Daejeon, South Korea..
Dong-Hyun Kim, Robert H. Lurie Comprehensive Cancer Center, Chicago, Illinois 60611, USA.; Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois 60611, USA.
References
- [1].a) Kew MC, Toxicology 2002, 181–182, 35; [DOI] [PubMed] [Google Scholar]; b) Schweitzer NM, Hu B, Das U, Kim H, Greeley J, Curtiss LA, Stair PC, Miller JT, Hock AS, ACS Catal. 2014, 4, 1091. [Google Scholar]
- [2].Di Bisceglie AM, J. Vasc. Interv. Radiol 2002, 13, S169. [DOI] [PubMed] [Google Scholar]
- [3].El-Serag HB, Davila JA, Petersen NJ, McGlynn KA, Ann. Intern. Med 2003, 139, 817. [DOI] [PubMed] [Google Scholar]
- [4].Marcos-Alvarez A, Jenkins RL, Washburn WK, Lewis WD, Stuart KE, Gordon FD, Kane RA, Clouse ME, Arch. Surg 1996, 131, 292. [DOI] [PubMed] [Google Scholar]
- [5].a) Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F, Am. J. Gastroenterol 2008, 103, 914; [DOI] [PubMed] [Google Scholar]; b) Li Z, Hu DY, Chu Q, Wu JH, Gao C, Zhang YQ, Huang YR, World J. Gastroenterol. 2004, 10, 1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Han G, Yang J, Shao G, Teng G, Wang M, Yang J, Liu Z, Feng G, Yang R, Lu L, Chao Y, Wang J, Future Oncol. 2013, 9, 403. [DOI] [PubMed] [Google Scholar]
- [7].Geschwind JF, Chapiro J, Clin Adv. Hematol. Oncol 2016, 14, 585. [PubMed] [Google Scholar]
- [8].a) Kerbel RS, Kamen BA, Nat. Rev. Cancer 2004, 4, 423; [DOI] [PubMed] [Google Scholar]; b) Ma J, Waxman DJ, Mol. Cancer Ther 2008, 7, 3670; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Teoh D, Secord AA, Inter. J. Gynecol. Cancer 2012, 22, 348. [DOI] [PubMed] [Google Scholar]
- [9].a) Chao Y, Chung YÄ, Han G, Yoon JÄ, Yang J, Wang J, Shao GÄ, Kim BI, Lee TÄ, Int. J. Cancer 2015, 136, 1458; [DOI] [PubMed] [Google Scholar]; b) Geschwind JF, J. Vasc. Interv. Radiol 2002, 13, 991; [DOI] [PubMed] [Google Scholar]; c) Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS, Am. J. Clin. Oncol 2003, 26, 344; [DOI] [PubMed] [Google Scholar]; d) Llovet JM, Bruix J, Hepatology 2003, 37, 429; [DOI] [PubMed] [Google Scholar]; e) Chamberlain MN, Gray BN, Heggie JC, Chmiel RL, Bennett RC, Br. J. Surg 1983, 70, 596; [DOI] [PubMed] [Google Scholar]; f) Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T, Cancer 2003, 97, 1253; [DOI] [PubMed] [Google Scholar]; g) Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J, Hepatology 2002, 35, 1164; [DOI] [PubMed] [Google Scholar]; h) Li X, Feng GS, Zheng CS, Zhuo CK, Liu X, World J. Gastroenterol. 2004, 10, 2878; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Zhu AX, Duda DG, Sahani DV, Jain RK, Nat. Rev. Clin. Oncol 2011, 8, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Welker MW, Trojan J, Cancer Manag. Res 2013, 5, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Abou-Alfa GK, Niedzwieski D, Knox JJ, Kaubisch A, Posey J, Tan BR, Kavan P, Goel R, Lammers PE, Bekaii-Saab TS, Tam VC, Rajdev L, Kelley RK, Siegel AB, Balletti J, Harding JJ, Howard SL, Goldberg RM, Bertagnolli MM, Venook AP, J. Clin. Oncol 2016, 34. [Google Scholar]
- [12].a) Lee JH, Nan A, Drug Deliv J. 2012, 2012, 915375; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang L, RadovicMoreno AF, Alexis F, Gu FX, Basto PA, Bagalkot V, Jon S, Langer RS, Farokhzad OC, ChemMedChem 2007, 2, 1268. [DOI] [PubMed] [Google Scholar]
- [13].Min NG, Ku M, Yang J, Kim SH, Chem. Mater 2016, 28, 1430. [Google Scholar]
- [14].Park W, Chen J, Cho S, Park SJ, Larson AC, Na K, Kim DH, ACS Appl. Mater. Interfaces 2016, 8, 12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Leadley SR, Watts JF, J. Adhesion 1997, 60, 175; [Google Scholar]; b) Chumpitaz LDA, Coutinho LF, Meirelles AJA, Am J. Oil Chem. Soc 1999, 76, 379. [Google Scholar]
- [16].Li WX, Dong H, Tang GN, Ma T, Cao XD, RSC Adv. 2015, 5, 23181. [Google Scholar]
- [17].Chou TC, Talalay P, Adv. Enzyme Regul 1984, 22, 27. [DOI] [PubMed] [Google Scholar]
- [18].Kim DH, Karavayev P, Rozhkova EA, Pearson J, Yefremenko V, Bader SD, Novosad V, J. Mater. Chem 2011, 21, 8422. [Google Scholar]
- [19].a) Kim DH, Chen J, Omary RA, Larson AC, Theranostics 2015, 5, 477; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim DH, Li W, Chen J, Zhang Z, Green RM, Huang S, Larson AC, Sci. Rep 2016, 6, 29653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ackerman NB, Cancer 1972, 29, 435. [DOI] [PubMed] [Google Scholar]
- [21].a) Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montana X, Llovet JM, Bruix J, Hepatol J. 2007, 46, 474; [DOI] [PubMed] [Google Scholar]; b) Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST, Clin. Gastroenterol. Hepatol 2007, 5, 1100. [DOI] [PubMed] [Google Scholar]
- [22].a) Sun H, Mei L, Song C, Cui X, Wang P, Biomaterials 2006, 27, 1735; [DOI] [PubMed] [Google Scholar]; b) Gentile P, Chiono V, Carmagnola I, Hatton PV, Int. J. Mol. Sci 2014, 15, 3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singh G, Chan H, Baskin A, Gelman E, Repnin N, Kral P, Klajn R, Science 2014, 345, 1149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.