Abstract
Approximately 50% of Americans have hypertension, which significantly increases the risk of heart failure. In response to increased peripheral resistance in hypertension, intensified mechanical stretch in the myocardium induces cardiomyocyte hypertrophy and fibroblast activation to withstand increased pressure overload. This changes the structure and function of the heart, leading to pathological cardiac remodeling and eventual progression to heart failure. In the presence of hypertensive stimuli, cardiac fibroblasts activate and differentiate to myofibroblast phenotype capable of enhanced extracellular matrix secretion in coordination with other cell types, mainly cardiomyocytes. Both systemic and local renin-angiotensin-aldosterone system activation lead to increased angiotensin II stimulation of fibroblasts. Angiotensin II directly activates fibrotic signaling such as transforming growth factor β/SMAD and mitogen-activated protein kinase (MAPK) signaling to produce extracellular matrix comprised of collagens and matricellular proteins. With the advent of single-cell RNA sequencing techniques, heterogeneity in fibroblast populations has been identified in the left ventricle in models of hypertension and pressure overload. The various clusters of fibroblasts reveal a range of phenotypes and activation states. Select antihypertensive therapies have been shown to be effective in limiting fibrosis, with some having direct actions on cardiac fibroblasts. The present review focuses on the fibroblast-specific changes that occur in response to hypertension and pressure overload, the knowledge gained from single-cell analyses, and the effect of antihypertensive therapies. Understanding the dynamics of hypertensive fibroblast populations and their similarities and differences by sex is crucial for the advent of new targets and personalized medicine.
Keywords: cardiac fibroblasts, fibrosis, hypertension, hypertrophy, pressure overload
INTRODUCTION
Hypertension is an epidemic in the United States as it affects more than half of the population, and despite numerous antihypertensive therapies on the market, three-quarters of individuals with hypertension are poorly managed. According to the Centers for Disease Control and Prevention (CDC), 116 million of the United States population have been categorized as hypertensive with blood pressure higher than 130/80 mmHg (stage 1) and at least 37 million of these have uncontrolled blood pressure above 140/90 mmHg (stage 2) (1, 2). Hypertension is the primary risk factor for cardiovascular mortality and is a major stimulus for cardiac remodeling that leads to heart failure (3). Hypertension causes hemodynamic overload and induces gradual structural remodeling of the heart, including cardiomyocyte hypertrophy leading to thickening of the ventricular wall and increased fibrosis as a result of extracellular matrix (ECM) deposition in the perivascular regions and extending to the interstitium (4). These structural and morphological changes lead to gradual decline in left ventricular function and ultimately progression to heart failure.
Cardiac fibroblasts are the major matrix-producing cells in the heart (5, 6). Fibroblasts are responsible for the structural and functional homeostasis in a healthy heart and also for the pathophysiology of fibrosis during hypertension (7–9). In the setting of long-standing hypertension, fibroblasts extensively activate and proliferate, leading to increased perivascular and interstitial ECM deposition. Collagen, a structural stress-bearing component of a healthy heart, accumulates excessively in a hypertensive heart, leading to stiffness and diastolic dysfunction. Increased collagen content in the coronary vasculature impairs vasomotor function and increases the risk of myocardial infarction (MI). Disruption in excitation-contraction coupling in the heart resulting from fibrosis leads to arrythmias (10, 11). In general, increased ECM production by cardiac fibroblasts causes a multimodal impairment in cardiac function in response to hypertension.
The increased peripheral resistance in hypertension causes hemodynamic alterations and pressure overload in the left ventricle (LV) (7). Reactive fibrosis initiates in a pressure-overloaded heart where the cardiac fibroblasts respond to hemodynamic alterations. This is in contrast to the reparative fibrosis in ischemic injury, which involves a loss of cardiomyocytes and replacement with a fibrotic scar. In response to cardiac pressure overload, increased mechanical stretch leads to fibroblast activation and differentiation. In addition, vasoactive peptides such as angiotensin II (ANG II) and endothelin-1 (ET-1), reactive oxygen species (ROS), and growth factors such as transforming growth factor β (TGFβ) can induce fibroblast activation and are all increased during cardiac pressure overload (7, 12, 13). Fibroblast activation is a compensatory response to withstand increased hemodynamic overload. When activated, these cells express contractile proteins such as alpha smooth muscle actin (α-SMA), produce increased levels of collagen/fibronectin, and are migratory and proliferative (14). In addition, activated fibroblasts also secrete increased levels of matrix metalloproteinases (MMPs) such as MMP-9 and -14 along with various cytokines and chemokines, leading to increased overall collagen turnover with a net increase in ECM accumulation (15). Although fibroblast activation is initially a compensatory response to pressure overload, the persistent activation of these cells and ongoing ECM accumulation result in impaired cardiac function.
Although fibroblasts were once considered a homogeneous population, the advent of single-cell approaches has revealed a diversity in fibroblast populations within the healthy and pressure-overloaded heart (16). Multiple groups have shown differential clusters of cardiac fibroblasts with transcriptomes suggestive of distinct phenotypes (17–19). For example, we observed that fibroblasts in the left ventricle of hypertensive rats both before and after antihypertensive treatment are comprised of nine clusters, based on differential gene expression. These include homeostatic, proliferative, proinflammatory activated, and moderately fibrogenic inflammation-suppressing phenotypes. The actual density and activity of these fibroblasts define the fibrotic microenvironment and resultant fibrosis. Thus, shifting the activation state or proportions of specific fibroblast populations can alter cardiac remodeling (17).
Hypertension is the leading risk factor for cardiovascular diseases including myocardial infarction (MI) and heart failure. Clinically, up to 75% of patients presenting with MI have underlying hypertension (20). Understanding how fibroblasts change in the setting of chronic pressure overload and hypertension is critical for understanding the baseline activation state before cardiac injury. That is, understanding the drivers of activation as well as the shifts in activation states that occur after established hypertension is necessary for the development of future therapies that slow the pathological remodeling that underlies heart failure.
Although none of the antihypertensive drugs was designed to target fibroblasts directly, some have been shown to limit fibroblast accumulation and slow fibrosis (21). Specifically, angiotensin-converting enzyme (ACE) inhibitor (ACEi) and angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), and potentially beta blockers offer protection against fibrosis and cardiac remodeling. Moreover, we have shown that the ACE inhibitor enalapril can produce a phenotypic shift in fibroblast populations that persists even after stopping the treatment (17, 22). Therapies specific to fibroblasts are still lacking and have immense potential to prevent cardiac remodeling and heart failure among patients with hypertension. This review highlights the mechanisms by which fibroblasts become activated in the pressure-overloaded myocardium, what knowledge has been gained by fibroblast-specific targeting in transgenic mice and single-cell approaches, and the impact of relieving pressure overload on cardiac fibroblasts and ECM deposition. Finally, we highlight critical future areas of discovery to advance approaches to prevent and or reverse cardiac fibrosis associated with long-standing hypertension.
HYPERTENSION ACTIVATES MULTIPLE FIBROTIC SIGNALING PATHWAYS IN THE MYOCARDIUM
Hypertension increases mechanical workload in the myocardium and induces left ventricular hypertrophy through activation of hypertrophic pathways such as extracellular signal-regulated kinase (ERK), c-Jun-NH2-terminal kinase (JNK), p38/mitogen-activated protein kinases (MAPKs), and protein kinase B (PKB/AKT) pathway (23). Increased stretch in the pressure-overloaded heart activates cardiac fibroblasts and induces their differentiation toward activated and/or myofibroblast phenotype (24). Fibroblasts are sensitive to change in mechanical strain in the heart (25). Mechanical strain is directly proportional to collagen deposition by fibroblasts (26–30). Cardiomyocytes locally release ANG II in response to stretch and paracrine signaling, and cardiomyocyte-fibroblast communication through gap junctions activates cardiac fibroblasts to collagen fiber-producing myofibroblasts to increase contractile forces (30–33). Stretch induces fibroblasts to secrete ET-1 and TGFβ, which has been shown to amplify TGFβ1 expression in stationary cardiomyocytes, further implicating paracrine signaling between cardiomyocyte and fibroblasts (34).
Fibroblast-specific targeting using transgenic mouse models has provided important insight into critical mediators of fibroblast activation and ECM deposition in various models of pressure overload. Cardiac fibroblast targeting has been most effectively achieved with the Tcf21cre mouse and more recently the Pdgfracre mouse. Development of the Postncre mouse has allowed for direct targeting of activated myofibroblasts. Specifically, the use of lineage-traced Tcf21cre and Postncre mice revealed that myofibroblasts primarily arise in the pressure-overloaded left ventricle as a result of activation of resident fibroblasts (35). These activated fibroblasts are then primarily responsible for the collagen deposition that occurs in the pathologically remodeling heart. A variety of signals trigger activation of fibroblasts and are highlighted briefly below and in Fig. 1.
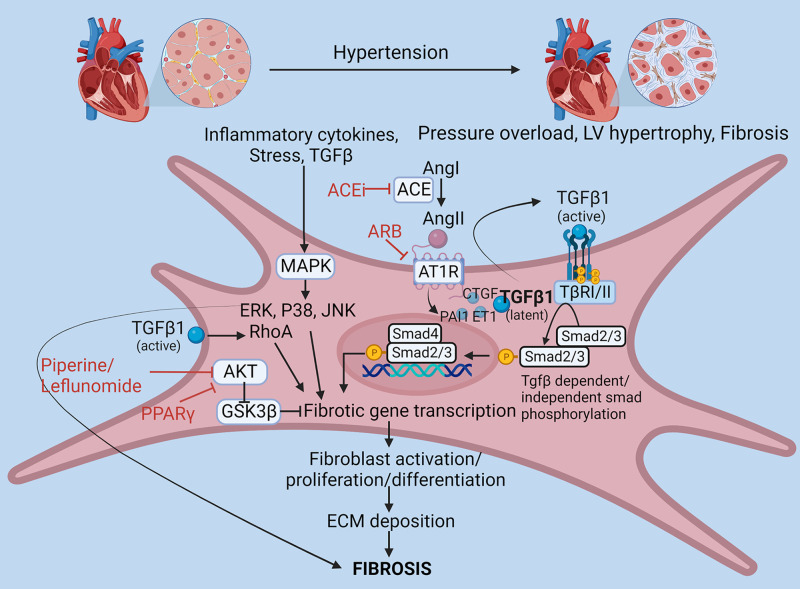
Figure 1.
Molecular mechanisms of fibroblast activation and fibrosis in hypertension. Hypertension induces pressure overload, left ventricular (LV) hypertrophy, and fibrosis through activation of fibrotic signaling in fibroblasts. Release of angiotensin II (ANG II) in hypertension releases growth factors like transforming growth factor β (TGFβ), connective tissue growth factor (CTGF), plasminogen activator inhibitor-1 (PAI1), and endothelin 1 (ET1), which can induce fibrotic gene through Smad phosphorylation and activation. TGFβ and other stimuli can also induce fibrosis through mitogen-activated protein kinase (MAPK) signaling. Angiotensin-converting enzyme (ACE) inhibitors (ACEis), angiotensin receptor blockers (ARBs), piperine/leflunomide, and peroxisome proliferator receptor (PPAR)-γ can block fibrotic signaling by acting at various target points. AT1R, AT1 receptor; ECM, extracellular matrix; RhoA, Ras homolog family member A. Images created with a licensed version of BioRender.com.
Angiotensin II
A major molecular pathway activated in hypertension is the renin-angiotensin-aldosterone system (RAS) (23, 36). ANG II is the key effector of the RAS and acts through angiotensin receptors AT1R and AT2R, with AT1R promoting and AT2R suppressing growth (37).
Along with the systemic RAS, which functions to increase blood pressure through increased peripheral vascular resistance and sodium and water retention, a local RAS exists in the myocardium (23, 38). Collectively, the cells within the myocardium express all components of the RAS including angiotensinogen, renin, and ACE. In addition, there are non-renin and non-ACE enzymes that can perform equivalent functions (e.g., cathepsin, chymase) (39). Both intracellular and extracellular RAS and ANG II secretion have been shown in cardiac fibroblasts (40, 41). ANG II has been shown to have mitogenic effects on and promote activation of cardiac fibroblasts either directly through its action on AT1R or through the secretion of various growth factors (42). Specifically, ANG II via the AT1R stimulates production of TGFβ, ET-1, natriuretic peptides, connective tissue growth factor (CTGF/CCN2), and plasminogen activator inhibitor-1 (PAI-1), which collectively create positive feedback for ongoing fibroblast activation and ECM deposition (43).
Transforming Growth Factor β
The transforming growth factor β (TGFβ)-Smad pathway is one of the key pathways for activation of fibrotic signaling and subsequent cardiac remodeling (Fig. 1) (9, 44, 45). TGFβ1 is the primary isoform mediating fibrosis and is upregulated in the remodeling myocardium (45, 46). TGFβ1 is secreted as a latent complex and is activated by various stimuli such as proteases (MMP-9, MMP-2, and plasmin), ECM protein (e.g., thrombospondin-1), and ROS. Active TGFβ1 binds TGFβ receptors (TβRs). TβRII on the cell surface is constitutively active, and the subsequent complex transphosphorylates TβRI. This interaction primarily leads to phosphorylation and activation of transcription factor Smads (Smad 2 and Smad 3), which form a complex with Smad 4 and translocate from cytoplasm to the nucleus, leading to transcription of target genes, including collagens and Ccn2 (47). Fibroblast-specific targeting of these pathways has revealed their major role in mediating fibrosis in the pressure-overloaded heart. Specifically, tamoxifen-induced deletion of Tgfbr1/2 or Smad3 in a PostnMCM mice shows reduced fibrosis in cardiac pressure overload after transverse aortic constriction (TAC) (48). Moreover, deletion of Smad2/3 in Postn+ fibroblasts blocks α-SMA expression and myofibroblast differentiation following pressure overload (48).
Besides TGFβ1-induced activation of Smad, several notable fibrotic pathways are activated, directly or indirectly, by TGFβ, including p38, ERK, JNK, and Ras homolog family member A (RhoA) (Fig. 1). Inhibition of p38 in cultured mouse right ventricle fibroblasts led to reduced TGFβ-induced collagen production, suppression of smad2/3 phosphorylation, and reduced fibroblast differentiation (49). Similarly, fibroblast-specific p38α knockout (KO) mice show protection against isoproterenol-induced myocardial damage by reduction in Il-6 and Tgfβ1 expression. Fibroblast-specific deletion of Mapk14 (p38α) in a tamoxifen-inducible cre recombinase model in PostnMCM mice blocks fibroblast-to-myofibroblast differentiation in ANG II-phenylephrine (PE)-induced cardiac hypertrophy. The study also showed similar effect after ischemic injury in Mapk14fl/flPostnMCM and Mapk14fl/flTcf21MCM mouse lines (50). In hypertension, activating transcription factor 3 is increased exclusively in fibroblasts as a protective mechanism to suppress p38/MAPK activity and reduce fibrotic remodeling (51).
Protein kinase B (AKT) signaling activation also occurs downstream of TGFβ in a RhoA-dependent manner. Blockade of AKT signaling and downstream glycogen synthase kinase 3β (GSK-3β) by piperine blocks activation and differentiation of fibroblasts to myofibroblasts by TGFβ or ANG II (52, 53). Transfection of neonatal rat cardiac fibroblasts with adenovirus expressing a GSK-3β mutant reduces GSK-3β phosphorylation and α-SMA-expressing fibroblasts (54). Fibroblast-specific deletion of GSK-3α (Gsk3afl/flTcf21MCM and Gsk3afl/flPostnMCM mice) results in attenuation of TAC-induced fibrosis via downregulation of ERK (55). GSK-3β is a major component of WNT/β-catenin signaling, which regulates cell proliferation, survival, and growth. Pressure overload results in upregulation of Wnt/β-catenin in cardiac fibroblasts, and the loss of β-catenin in resident cardiac fibroblasts reduces fibrotic response after pressure overload in mice with downregulated collagen and periostin expression (56). Peroxisome proliferator-activated receptor (PPAR)γ and WNT/β-catenin interact antagonistically, as PPARγ agonists increase inhibitors of WNT signaling such as GSK3β, Smad7 (inhibitory smad), and Dickkopf-related protein 1 (DKK1) and inhibit TGFβ-induced fibrotic signaling (57).
Sympathetic Nervous System Activation
Increased sympathetic drive leading to β-adrenergic activation is another potential driver of hypertension. Overactivation of β-adrenergic receptor (βAR) during hypertension results in cardiomyocyte apoptosis/hypertrophy and fibroblast activation (58, 59). Cardiac fibroblasts express βARs, and activation of βAR by the β-agonist isoproterenol induces a significant fibrotic response that is attenuated in β2AR KO mice (60). In the TAC model of pressure overload, rats showed upregulated proinflammatory cytokines such as IL-1β and IL-18 and components of inflammasome, i.e., NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3), which is attenuated by the βAR antagonist metoprolol. Also, fibroblasts treated with the α/β-adrenergic agonist norepinephrine showed inflammasome activation with increase in ROS, which led to downstream increase in collagen production (61). The degree to which β-adrenergic antagonists are effective antifibrotic therapies is discussed in select antihypertensive therapies prevent fibroblast activation and fibrosis.
FIBROBLASTS WORK IN COORDINATION WITH OTHER CELLS TO DRIVE FIBROSIS IN HYPERTENSION/PRESSURE OVERLOAD
Fibroblasts regulate cardiac remodeling after hypertension and pressure overload as an integrated communication with cardiomyocytes and immune cells through cytokines, growth factors, and matrix proteins/proteases (Fig. 2) (62). Although fibroblasts are the major cell type in producing extracellular matrix, the cardiomyocyte response to hemodynamic alterations and emergence of inflammation in the heart plays a crucial role in initiation and extent of fibrosis.
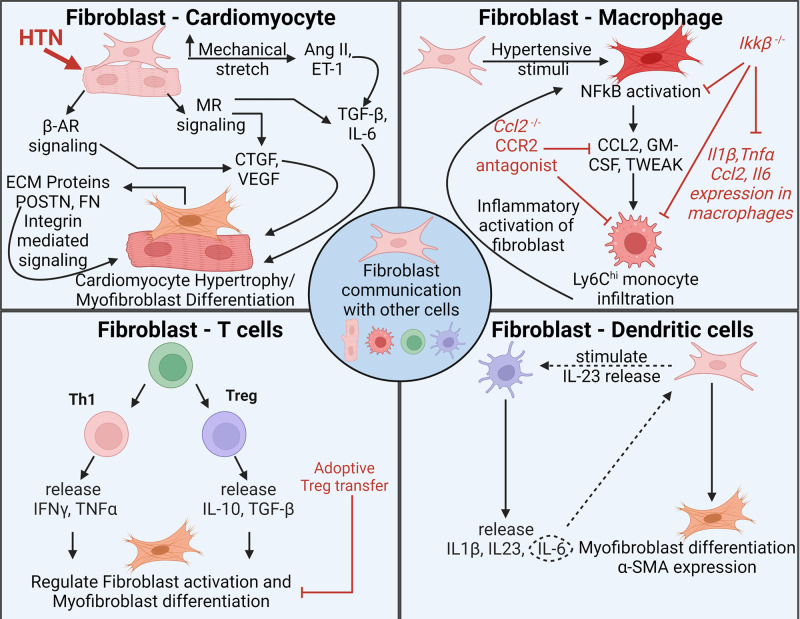
Figure 2.
Fibroblasts coordinate with other cell types in hypertension to regulate fibrosis. Fibroblasts communicate with different cell types in hypertension, such as cardiomyocytes, macrophages, T cells, and dendritic cells, and regulate fibrosis. All different cell types can directly or indirectly modulate fibroblast activation. Hypertensive stimuli (HTN) induce mechanical stretch and β-adrenergic receptor (β-AR) and mineralocorticoid receptor (MR) signaling and cause fibroblast activation and extracellular matrix (ECM) protein production, which can induce cardiomyocyte hypertrophy. Similarly, fibroblasts can secrete chemoattracts like CCL2, which brings in more proinflammatory macrophages to the heart and subsequently induce fibroblast activation as well. Cytokine released from T cells and dendritic cells can induce direct or indirect activation of fibroblasts. α-SMA, alpha smooth muscle actin; ET1, endothelin 1. Images created with a licensed version of BioRender.com.
Cardiomyocyte-fibroblast interaction is the most important cellular communication in hypertrophy and fibrosis signaling during hypertension. Cardiomyocytes and fibroblasts are coupled through gap junctions containing connexin 43/45 proteins and communicate via F-actin-containing membrane nanotubes and via paracrine signaling (31, 63). Cardiomyocytes and cardiac fibroblasts are electrically and mechanically coupled. Fibroblasts can affect electrical activity of cardiomyocytes. Unlike cardiomyocytes, fibroblasts cannot generate action potentials, but mechanical stretch is responsible for their membrane potential. As such, cardiomyocyte response to mechanical stress directly affects fibroblasts (24). In addition, cardiomyocytes can release growth factors through mineralocorticoid receptor signaling to initiate fibrosis (64). Both cardiomyocyte and fibroblasts produce TGFβ in response to ANG II and mechanical stretch in hypertension (65). TGFβ induces myofibroblast proliferation via Smad in fibroblasts, as it also induces cardiomyocyte hypertrophy and apoptosis through MAPK-associated pathways, as described above. ANG II-associated release of TGFβ and IL-6 from cardiac fibroblasts directly induces both cardiac hypertrophy and fibrosis (31). ECM proteins such as periostin, fibronectin, etc. produced by activated fibroblasts can directly stimulate cardiomyocyte hypertrophy through integrin-mediated signaling (66–68). Fibroblast activation is also acutely dependent on mechanical stretch, as discussed above (69). Sustained stimulation of β-adrenergic receptors (βARs) induces the release of CCN2/CTGF and vascular endothelial growth factor (VEGF) through cAMP-dependent and protein kinase A (PKA)-dependent signaling in cardiomyocytes.
Local inflammation through ROS from hypertrophic signaling in cardiomyocyte and apoptosis of cardiomyocytes brings immune cells to the heart that also potentially regulate fibroblast activation and myofibroblast differentiation (70). Proinflammatory activation of fibroblasts can further contribute to macrophage recruitment. We have shown that fibroblasts isolated from the left ventricle (LV) of NG-nitro-l-arginine methyl ester (l-NAME)-treated spontaneously hypertensive rats (SHR), compared with fibroblasts isolated from naive SHR LV, secrete higher levels of monocyte chemoattractant protein (MCP-1/CCL2) and granulocyte-macrophage colony-stimulating factor, both of which are involved in macrophage recruitment and proliferation (22) NF-κB is upregulated in cardiac fibroblasts after pressure overload which increases Ccl2 release and directly drives Ly6Chi monocyte recruitment to the heart (71). In ANG II-induced hypertension, Haudek et al. (19) showed that the collagen-producing myeloid-derived fibrocytes (CD45+) infiltrate to the heart in a CCL2-dependent manner, as Ccl2−/− mice showed reduction in interstitial fibrosis, expression of Col I/III, Tgfβ, and Tnf, and Mac2+ macrophages. CCL2 signals through chemokine receptor CCR2, whose expression is increased during hypertension (72). Fibroblast-induced macrophage infiltration is also observed through a proinflammatory cytokine, TNF-like weak inducer of apoptosis (TWEAK), as it binds to fibroblast growth factor inducible 14 (Fn14) on cell surface and induces CCL2 secretion from fibroblasts.
Similar to macrophages, T cells are integral mediators of fibrosis that directly interact with fibroblasts. Various subsets of T cells differentially regulate cardiac fibrosis through cytokine release and immune activation (73). Although cytokines released from Th1 cells are proinflammatory and have been shown to inhibit fibroblast activation, direct contact of Th1 cells with cardiac fibroblasts via α4-integrin promotes myofibroblast differentiation (74, 75). A recent paper shows that MyD88 deletion in T cells increases adhesion of Th1 cells to cardiac fibroblasts through vascular cell adhesion molecule (VCAM)-1 and their subsequent activation in their coculture (76). Treg cells directly produce TGFβ, which can promote cardiac fibroblast proliferation, while also inhibiting fibrotic response through IL-10 release (77, 78). In a pressure-overloaded heart, adoptive transfer of cultured splenic Tregs (CD4+CD25+) attenuates cardiac fibrosis with reduced myofibroblast number (79). Similarly, Treg transfer in ANG II-induced hypertensive mice shows reduction in oxidative stress marker in the heart (80).
Oxidative damage and inflammation bring dendritic cells, which secrete cytokines such as IL-1β, IL-6, and IL-23 to home proinflammatory T cells to the heart (81). Although direct action of dendritic cells to cardiac fibroblasts is unclear, it has been shown that IL-6 has direct implications for fibroblast transdifferentiation as IL-6 promotes α-SMA and collagen expression in fibroblasts (82, 83). Both ANG II and TGFβ induce IL-6 release in fibroblasts, and IL-6 further activates TGFβ-Smad3 signaling and fibrosis (83, 84). In coculture of dermal fibroblasts and dendritic cells, fibroblasts enhanced migratory potential in dendritic cells and induced MMP-9 release (85). Also, fibroblasts induce IL-23 release from dendritic cells, which activates Th17 T cells that are known to induce fibrosis (86, 87).
Fibroblast-cellular communication is still an underexplored area and requires further study. Most of the aforementioned studies are associative in nature yet indicate fibroblast cross talk with other cell types during progressing cardiac fibrosis. However, the direct relationship between fibroblasts and other cell types, especially immune cells, during hypertensive cardiac hypertrophy and fibrosis requires further studies to delineate specific roles of intercellular communication.
SINGLE-CELL APPROACH SHOWS A SHIFT IN CARDIAC FIBROBLAST PHENOTYPE IN REACTIVE FIBROSIS
Cardiac fibrosis in hypertension and pressure overload has been studied through various animal models. Chemical-induced hypertension such as ANG II, alone or in combination with phenylephrine, nitric oxide synthase inhibition with l-NAME, and TAC are all used to study cardiac remodeling resulting from pressure overload. Fibroblast activation, migration, and proliferation are seen in all models with induced hypertension or surgically induced pressure overload. The advent of single-cell RNA sequencing (scRNA-seq) has allowed for greater investigation of the cardiac fibroblasts that contribute to the fibrotic remodeling occurring within the heart. This has led to identification and understanding of the heterogeneity of these cells. Differences in animal species and model, the timing of assessments, the methods used for cell isolation, as well as the depth of sequencing result in a diversity of conclusions across studies. Nonetheless, there are common findings across multiple studies among major fibroblast clusters and their activated genetic signatures that are emerging.
Fibroblasts should ideally be identified based on expression of PDGFRa and/or Tcf-21 but are also commonly captured as noncardiomyocytes that lack expression of CD31 (endothelial cells) or CD45 (immune cells). In our previous single-cell RNA sequencing study (17), we isolated fibroblasts through plating to broadly select for collagen-producing cells. These cells were also found to be CD31 and CD45 negative (17).
Changes in fibroblast subpopulations in the pressure-overloaded myocardium as determined by single-cell approaches for each model are discussed below and in Table 1.
Table 1.
Identified fibroblast clusters from recent single-cell studies
| Disease Model | Methods of Fibroblast Identification | Sequencing Information | Number of Fibroblast Clusters/Activated Clusters | Primary Conclusions |
|---|---|---|---|---|
| Hypertension (ANG II, mouse) (18) | Differential gene expression (PDGFRa, Col1) | 10× chromium v2 kit, 12,000 cells (7,474 read from 2 hearts), Illumina Hiseq, 100,000 read/cell | 9/Fibro-Wif1, Fibro-Cilp, Fibro-Thbs4 | Two unique clusters: Fibro-Cilp and Fibro-Thbs4, identified after ANG II; Meox1: transcriptional switch for activation |
| Hypertension (ANG II/PE, mouse) (88) | FACS-sorted PDGFRa+ cells as FAP+ and FAP− cells | 10× chromium v3.1 kit, Illumina NovasEq. 6,000, 100,000 reads/cell | 10/Postn/Fap cluster, Cxcl1 cluster, Acta2/Tagln cluster | Activated fibroblast cluster identified with higher Postn/Fap expression; IL-1β monoclonal antibody treatment ↓ FAP+ fibroblasts |
| Hypertension (SHR) (17) | Col1a1+CD45−CD68−CD31− | 10× chromium v3 kit, (≈2,000 cells/sample read), 1 LV per sample, n = 7 per group, Illumina Hiseq4000 | 9/Proinflammatory (Ccl7, Ccl2), moderately fibrogenic (Ccn3), homeostatic, gateway | Transient ACE inhibition: ↑ proportion of cells in homeostatic cluster and ↓ proportion in gateway cluster |
| Transverse aortic constriction (mouse) (89) | Differential gene expression | 10× chromium, 35,551 total cells read (13,937 fibroblasts) | 9/POSTN/FAP cluster | Fibroblasts shifted toward sham-like phenotype after JQ1 treatment |
| Heart failure (human) (88) | Differential gene expression/cellular indexing of transcriptomes and epitopes (CITE) proteomics | 10× chromium v3.1 kit/ TotalseqA, 143,804 cells, Illumina NovasEq. 6,000 S4,104 read/cell | 13/POSTN/FAP cluster, CCL2/THBS1 cluster, ACTA2/TAGLN cluster | Postn/FAP cluster: major fibrogenic cluster in heart failure samples; CCL2 cluster: proinflammatory; Runx1 was major transcription factor for fibrosis |
ACE, angiotensin-converting enzyme; LV, left ventricle; SHR, spontaneously hypertensive rat.
ANG II-Induced Hypertension
ANG II is the most commonly used model for hypertension-induced cardiac stress. Cardiac fibrosis and activated fibroblast phenotype have been well noted in ANG II-induced hypertension. Recently, single-cell RNA sequencing (scRNA-seq) revealed nine cardiac fibroblast subsets after 2 weeks of ANG II administration. Of these, two subsets were characterized as distinct phenotypes of activated fibroblasts, named fibroblast-Cilp and fibroblast-Thbs4, which are absent in naive heart, with fibroblast-Cilp being the most abundant phenotype. Although these two were the only distinct hypertensive fibroblast clusters observed in the study, they were not identified by the authors as myofibroblasts based on a lack of upregulation of Acta2 (18). Fibroblast-Cilp cells are highly enriched for ECM organization, cellular adhesion, and cellular migration, whereas the fibroblast-Thbs4 cluster is highly enriched for cellular growth regulation and heart development. Compared with the seven other fibroblast clusters identified in the study, fibroblast-Cilp and fibroblast-Thbs4 are combined enriched for ECM remodeling and wound healing and are distinctive from other clusters by increased expression of Thbs4, Fmod, Cthrc1, and Cilp2 (18). The authors were able to identify distinct THBS4+ cells around interstitial fibrotic areas. Pseudotime analysis comparing RNA velocity shows that fibroblast-Thbs4 fibroblasts arise from fibroblast-Cilp cells, whereas fibroblast-Cilp arise from other fibroblast clusters (18).
In the ANG II-phenylephrine-induced hypertensive heart, at day 28, 10 clusters of fibroblasts are identified to make up the fibroblasts isolated from isotype-treated and IL-1β monoclonal antibody-treated hearts. Fibroblasts were sorted as PDGFRa+ cells by flow cytometry. A specific single-cell transcriptomic cluster of fibroblast activator protein/Periostin (Fap/Postn)-high fibroblasts are present in the heart that are specifically absent in sham-treated hearts. The study shows that IL-1β monoclonal antibody treatment reduces the abundance of FAP+PDGFRa+ cells in the hypertensive heart. In the same study the authors also show that human fibroblasts treated with TGFβ also show expansion of a FAP/POSTN cluster (88).
Spontaneously Hypertensive Rats
SHR exhibit primary hypertension similar to humans and are prone to adverse cardiac outcomes. These rats are normotensive at 3–4 wk of age and progressively develop higher blood pressure by 6–7 wk of age leading to sustained hypertension. Adult SHR exhibit left ventricular hypertrophy and a higher proportion of cardiac fibroblasts than their normotensive counterpart, the Wistar-Kyoto (WKY) rat (90).
Single-cell analyses revealed a number of fibroblast clusters in left ventricular fibroblasts isolated from adult male SHR similar to that observed in mouse models of ANG II-induced hypertension (17, 18). Fibroblasts isolated by selective plating from left ventricle were comprised of nine distinct clusters based on their transcriptome profile, with the majority of cells in seven clusters (17) Fibroblast clusters were characterized as homeostatic, proinflammatory activated, proliferative, moderately fibrogenic/inflammation suppressing, translationally active, low inflammation, and gateway phenotypes, with fibroblasts exhibiting variable inflammatory and fibrogenic profiles. Homeostatic fibroblasts are characterized by their least fibrogenic nature and low expression of inflammatory genes. Proinflammatory fibrogenic fibroblasts had highest expression of inflammatory genes such as Ccl2, Ccl7, and Lgals3 and are potentially involved in immune cell recruitment. This fibroblast cluster was the most activated and typical of the “myofibroblast” state with upregulated Acta2 expression. A “proliferative” fibroblast cluster showed the highest expression of cellular proliferation genes and had reduced inflammatory profile. A moderately fibrogenic and inflammation-suppressing cluster had reduced oxidative stress and increased expression of fibrotic markers such as Thbs4, Scx, and Spp1. And an intermediate gateway fibroblast cluster that, by pseudotime analysis, appears to feed the proinflammatory cluster is characterized by enrichment for wound healing, proliferation, and cellular adhesion. Two other intermediatory fibroblast clusters show increased protein translation along with slight reduction in fibrosis, inflammation, and proliferation signatures (17). Further understanding of the diverse fibroblast phenotypes and their activation states within the hypertensive left ventricle will be crucial in targeting fibrosis and adverse cardiac remodeling. The impact of transient angiotensin-converting enzyme inhibition on these fibroblast clusters is discussed in antifibrotic effects of select antihypertensive drugs can persist even after stopping treatment.
Pressure Overload
TAC is a commonly used animal model of pressure overload that exhibits classic signs of left ventricular hypertrophy, collagen accumulation, and cardiac dysfunction as seen in hypertensive hearts. However, unlike the pressure overload and gradual cardiac remodeling that occurs in hypertension, TAC produces acute overload and accelerated remodeling response. Fibroblast proliferation peaks between 4 and 7 days after the TAC surgery (91, 92), with sustained elevations in fibroblast density (92). In TAC-induced pressure-overloaded hearts, along with hypertrophy of the myocytes there is an accumulation of collagen fibers in the perivascular region that extends into the interstitium. Collagen content is highest at 7 days after TAC. Perivascular regions in the left ventricle show maximum fibrotic and inflammatory changes with increased adventitial layer thickness, collagen content, macrophage extravasation, and active stiffness (14, 93). The extent of LV hypertrophy and impaired cardiac physiology directly correlates with the interstitial fibrosis. Along with an increase in fibroblasts in the interstitium and their activity, cardiac dilation and vascular remodeling occur as an adaptive response and later lead to cardiac dysfunction. Increased expression of integrins and neurohormonal/growth factor secretion are seen at day 7 of pressure overload, which correlates with higher proliferation and migration of day 7 and 14 fibroblasts (94). Fibroblasts from pressure-overloaded hearts have higher tensile strength observed with increased collagen contraction in vitro (94).
Single-cell analyses on mouse hearts with heart failure after 2 months of pressure overload by TAC show nine fibroblast clusters with high Postn-expressing fibroblasts driving fibrosis. Transcription factor mesenchyme homeobox 1 (Meox1) enrichment and high expression in myofibroblasts is seen after TAC. Meox1 knockdown shows reduction in α-SMA fibers in TGFβ-treated fibroblasts, indicating its direct role in fibroblast plasticity and profibrotic action (89). MEOX1/RUNX1 is found to be specifically induced in an activated fibroblast single-cell cluster with high FAP/POSTN in patients with heart failure (89). The study targeted fibrosis through JQ1 treatment [small-molecule inhibitors of bromodomain and extraterminal domain (BET)] and found that JQ1 treatment directly shifted fibroblast single-cell clusters toward more sham-like whereas stopping the treatment had more similarity to cardiac pressure overload (89).
Heart Failure and Fibroblasts
Hypertension is one of the major risk factors, along with aging and diabetes, for nonischemic heart failure, especially heart failure with preserved ejection fraction (HFpEF) (95, 96). Up to 70% of patients with HFpEF have underlying hypertension (97). Fibrotic burden in HFpEF is directly associated with diastolic dysfunction (98). Therapeutically targeting early fibrotic signal and inflammatory cues could delay or prevent chronic heart failure development.
In a recent study, fibroblast populations from patients with heart transplants have been identified in nonischemic cardiomyopathy along with ischemic and acute MI fibroblasts (88). Patients with chronic heart failure, both ischemic and nonischemic, show 13 different subclusters of cardiac fibroblasts. Patients with chronic heart failure had accumulation of a specific cluster enriched for FAP and POSTN and a distinct cluster of myofibroblasts. Myofibroblasts are localized to perivascular regions, whereas the FAP/POSTN cluster shows higher localization in the area of high collagen expression. The FAP/POSTN cluster had higher cell surface expression of THY1, CD276, LAMP1, and CD63 and directly associates with fibrosis. The FAP/POSTN fibroblast cluster showed specific link to disease-associated fibrosis and is enriched for ECM remodeling, cellular adhesion, and inflammatory pathways such as phosphoinositide 3-kinase (PI3K)/AKT and advanced glycation end products/receptor for advanced glycation end products (AGE/RAGE) pathway. MEOX1 comes as the top transcription factor driving the FAP/POSTN lineage of fibroblasts (88). Compared with a ANG II-PE mouse model of hypertension and cardiac hypertrophy and a mouse model of pressure overload by TAC, single-cell fibroblast cluster distribution shows similarity with 11 out of 13 clusters present and the FAP/POSTN cluster as the rapidly expanding cluster as observed in other recent studies (88, 89).
Similarly, fibroblasts in patients with dilated cardiomyopathy (DCM) show enhanced plasticity and 10 clusters based on gene expression. Two fibroblast clusters were uniquely present in patients with DCM. Similar to animal models, proinflammatory CCL2-expressing fibroblasts and POSTN/THBS4-expressing fibroblasts were upregulated in patients with DCM, though their abundance was lower than that of fibroblasts with conserved gene signature (99).
CARDIAC FIBROSIS IS A SEXUALLY DIMORPHIC PHENOMENON
Sex differences in cardiac fibrosis have recently come to light, with differences seen in activation of fibroblasts between sexes and also differences in fibroblast phenotypes observed in single-cell analysis (100–102). β-Adrenergic activation and subsequent fibrosis is higher in males compared with females (58). Cardiac fibroblasts show divergence in the fibrosis mechanism, as males have higher activation to myofibroblast phenotype compared with females while females have higher proliferation when treated with a β-adrenergic agonist (103). In a comprehensive study of cardiac cells by McLellan and Skelly et al. (18), ANG II-induced fibrogenic cell abundance in the heart was dimorphic between males and females. Female mice show significantly reduced cardiac fibrotic area, and the Fibro-Thbs4 cluster is twofold higher in females compared with males. The Fibro-Cilp cluster showed the highest number of genes different by sex after ANG II treatment. Along with fibroblasts, ANG II also induces a higher overall macrophage number, with increase in MHCIIlo macrophages in males compared with females (18). These physiological differences seen in fibrosis and fibrogenic cells could be highly valuable for targeting therapies in men and women. There remains a paucity of studies directly evaluating the degree to which sex differences exist in fibroblasts and fibrosis and in hypertensive heart disease. There is a critical need to focus on this area of investigation.
SELECT ANTIHYPERTENSIVE THERAPIES PREVENT FIBROBLAST ACTIVATION AND FIBROSIS
Given the durability and long half-life of collagen fibers, once fibrosis is established it is not easily reversed. As such, current strategies are focused on target organ protection and prevention of pathological remodeling. Although all antihypertensive drugs are effective at lowering blood pressure, there is variation in their ability to impact the structural remodeling, including ECM deposition in the left ventricle. For example, drugs that inhibit the renin-angiotensin system are consistently found to be more effective in limiting fibrosis in hypertensive models. The degree to which antihypertensive agents can impact ECM deposition and fibroblast activation is summarized below and in Table 2.
Table 2.
Impact of antihypertensive drugs on fibroblasts and fibrosis
| Drugs | Effect on Fibrosis | Direct Action on Fibroblasts |
|---|---|---|
| Alamandine | ↓ (104) | Inhibit ANG II-induced fibroblast activation (104) |
| Calcium channel blockers | ↓ (105–112) | Fibroblast apoptosis (112, 113) |
| Angiotensin receptor blockers | ↓ (114–120) | Fibroblast apoptosis (112, 117, 121, 122) |
| Angiotensin-converting enzyme inhibitors | ↓ (105, 111, 114, 119, 120, 123–126) | Fibroblast apoptosis (112, 121), shift fibroblast clusters to lower activation states (17) |
| Beta blockers | ↓/↔ (105, 111, 127–129) (class specific) | Fibroblast apoptosis (112) |
| Neprilysin inhibitor (sacubitril) and angiotensin receptor blocker (valsartan) | ↓ (116) | PKG restoration and inhibition of Rho activation (116) |
As described above, ANG II has direct actions on cardiac fibroblasts to promote proliferation and activation and stimulate release of ECM components, cytokines, and chemokines. Thus, it is not surprising that drugs that target ANG II production or action are effective in limiting fibroblast activation. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have been widely shown to be the most effective antihypertensive drugs to limit cardiac fibrosis associated with hypertension and aging and following myocardial infarction (105, 114–118, 123–126). In addition, alamandine is a member of the RAS that has been shown to have antifibrotic effects in SHR through actions at the MrgD receptor (104).
Although the exact mechanisms by which these drugs limit fibrosis have not been fully elucidated, increased collagenase activity, decreased collagen production, and inhibition of TGF-β signaling resulting from reduced AT1 receptor stimulation have each been proposed (104, 119, 120, 130). However, other classes of drugs that do not target the RAS have also been shown to have antifibrotic effects, including calcium channel blockers (CCBs) (105–111). Studies in experimental models of hypertension have revealed that ARBs, ACE inhibitors, CCBs, and beta blockers each induce caspase-dependent apoptosis of left ventricular fibroblasts (112, 113, 121,122). Apoptosis is likely independent of blood pressure lowering, since hydralazine had no effect on fibroblast density (112). Akashiba et al. (117) have demonstrated that, compared with vehicle, ARB treatment induces fibroblast apoptosis concomitant with an overall reduction in collagen content. It may be that fibroblast apoptosis is an important mechanism by which these agents limit uncontrolled fibrosis in the remodeling heart.
The combination of the neprilysin inhibitor sacubitril with the ARB valsartan may provide additional antifibrotic benefit over ARB alone. Specifically, this combination protects against TAC-induced fibrosis via a reduction in fibroblast number as well as a decrease in the expression of genes involved in fibroblast activation and ECM production. Moreover, this combination was more effective than either drug alone in vitro to limit proliferation and activation of fibroblasts (116). The authors showed that atrial natriuretic factor was effective in attenuating TGFβ1-induced fibroblast activation in vitro, suggesting a mechanistic link to sacubitril and reduced fibrosis (116). Moreover, they determined, in vitro, that the antifibrotic mechanism of the sacubitril-valsartan combination was at least partly due to restoration of protein kinase G (PKG) signaling in heart failure patient-derived cardiac fibroblasts, which inhibited Rho activation associated with myofibroblast transition (116). The findings are consistent with prior work showing that nitric oxide, an upstream activator of PKG, induces cardiac fibroblast apoptosis by inhibiting AKT signaling (131).
The impact of beta-blocker treatment on fibrosis in models of hypertension has been mixed, with some showing beneficial effects (111, 127) and others showing no impact (128). Ye et al. (129) found class-specific differences in antifibrotic effects, with nebivolol showing greater beneficial effects compared with atenolol, potentially because of a greater restoration of miR27a and -29a (linked to a decrease in Sp1 expression) and miR-133a (linked to a decrease in Cdc42 expression) in cardiac fibroblasts.
Future strategies must be focused on identifying the mechanisms by which select antihypertensive agents protect against or potentially reverse fibrosis through direct actions on fibroblasts. Fibroblast-specific cre drivers will allow for selective knockdown of angiotensin or adrenergic receptors or L-type calcium channels, thus separating the overall cardiac or hemodynamic response from fibroblast effects.
NONPHARMACOLOGICAL REVERSAL OF PRESSURE OVERLOAD
Although antihypertensive drugs have been shown to induce changes in fibroblasts and extracellular matrix, it has been challenging to separate the antihypertensive and thus reduction in pressure overload effects from the drug-specific effects on fibroblasts. Recent studies have begun to investigate the impact of relieving pressure overload in a TAC/unTAC model. In these models, the TAC procedure is performed and after cardiac hypertrophy and fibrosis have been established the suture is removed from the aorta, resulting in a rapid reversal of the pressure-overloaded state. This model mimics and recapitulates clinical settings such as replacement of a stenotic aortic valve leading to a reduction in cardiac pressure overload. In these cases, up to 6 wk after removal of the aortic constriction (unTAC), there was a modest, yet incomplete, regression of collagen fibrils and myocardial stiffness remained elevated compared with control levels (132). Nonetheless, the study observed an increase in collagen hybridizing peptide activity and matrix metalloprotease activity 2–4 wk after unTAC, which gradually reduced over time, suggesting a transient initiation of collagen degradation early after LV unloading. Using a similar model, Ruppert et al. (101) found that females exhibit a more profound regression of myocardial fibrosis after debanding than males. In addition, in the female hearts there was a greater normalization of the myocardial proteome after the debanding, suggesting a greater ability to adapt and return to baseline.
What remains to be determined are assessments of direct changes in fibroblasts. Studies that are geared toward evaluating fibroblast phenotype and/or transcriptome in the overloaded versus unloaded heart will be informative as to the degree to which reduction in pressure alone can stimulate a reversal of the activated state. Moreover, evaluating the composition of ECM after reversal of pressure overload will be of great interest.
ANTIFIBROTIC EFFECTS OF SELECT ANTIHYPERTENSIVE DRUGS CAN PERSIST EVEN AFTER STOPPING TREATMENT
It has been challenging to identify mechanistic links between antihypertensive therapies and antifibrotic effects because of the confounding influence of reductions in blood pressure, direct actions of the drugs to block or reduce activation of receptor systems, and in some cases regression of left ventricular hypertrophy. As previously reviewed, drugs that inhibit the renin-angiotensin system are uniquely effective in altering the trajectory of aging-related fibrosis in hypertensive rats (133). Several studies have investigated the impact of transient antihypertensive therapy on future fibrosis. Specifically, when fibrosis development was evaluated up to 30 wk after stopping therapy, drugs that inhibit the RAS outperformed calcium channel blocker with respect to the degree of collagen accumulation over the off-treatment period (133). More recent studies have provided more insight into the mechanisms underlying the persistent protection.
The antifibrotic effects of ACE inhibitors have been shown to persist even after cessation of treatment. ACE inhibition has been shown to persistently alter fibroblast physiology in a manner that protects against future activation and fibrosis (22, 134, 135). Specifically, the beneficial effects of ACE inhibition persist after a washout of the drug, resulting in an attenuated fibrotic and inflammatory response to nitric oxide synthase inhibition. Employing a washout period allows for the evaluation of the consequences of transient RAS inhibition without the confounder of ongoing antihypertensive effects and ongoing reduction in ANG II signaling. Interestingly, when isolated from an actively remodeling left ventricle, cardiac fibroblasts from rats that had been previously transiently treated (e.g., 2-wk treatment, 2-wk washout before l-NAME challenge) displayed a phenotype that was less fibrogenic when evaluated in culture. Specifically, they secreted lower levels of chemokines, expressed lower levels of Col1a1 gene expression, and had lower proliferation rates (22). This was the first demonstration that at least some of the antifibrotic effects of ACE inhibitor may be due to persistent changes in the cardiac fibroblasts.
Recently, single-cell analysis was performed to evaluate the transcriptional changes induced in fibroblasts after transient ACE inhibition in adult SHR. Garvin et al. (17) showed that there was an overall downregulation of genes involved in ECM production, wound healing, and fibroblast activation in left ventricular fibroblasts isolated from male adult SHR 2 wk after stopping ACE inhibitor treatment. This is consistent with the persistent beneficial effects previously reported after cessation of ACE inhibitor treatment. In addition, this transient ACE inhibition resulted in an increase in the proportion of homeostatic fibroblasts that are characterized by a low activation profile. This is accompanied by a reduction in the proportion of fibroblasts that are precursors to activation (17). In general, fibroblasts isolated from rats previously treated with an ACEi show a reduced fibrotic profile with decreased expression of ECM genes such as Col1a1, Col3a1, Timp1, Lgals3, Ccn1, Ccn3, Fn1, Lox, S100a4, Fbln2, etc. compared with fibroblasts from control rats. Thus, transient ACE inhibition resulted in a persistent downregulation of genes involved in fibrosis and fibroblast activation as well as a shift in the proportions of subsets of fibroblasts leading to the homeostatic population as the dominant cluster. Collectively this led to an overall fibroblast phenotype that was in a low activation state that appears to be more resistant to activation when stimulated in vivo. Although future studies are required to identify the mechanisms by which ACE inhibition (and potentially other therapeutics) produces these persistent changes in fibroblasts, the findings do suggest that it is possible to change the trajectory of fibrosis without ongoing pharmacotherapy.
FUTURE DIRECTIONS
Collectively, in three different models of pressure overload (e.g., TAC, ANG II, SHR) there are consistently 9–10 clusters of fibroblasts identified in the left ventricle. In all cases there are approximately two clusters that, based on the transcriptome, show some degree of activation. Based on the work of Kanisicak et al. (35), these cells are likely all arising from the same lineage and thus the various clusters likely represent cells along a spectrum of activation. Trajectory analyses/pseudotime analyses have provided insight into the transition states that may be most targetable for future interventions. Identifying key nodes and pathways that facilitate transition from one activation state to the next holds promise for developing future therapeutics.
Although these studies have been incredibly helpful in understanding fibroblast heterogeneity and their in vivo activities, there are some noteworthy limitations. Consolidatory bioinformatic approaches are necessary to evaluate the data generated from the various models and animal strains to determine the degree to which the transcriptomes of the various clusters are similar. Moreover, localization is likely very important when it comes to fibroblast activation and function. Fibroblasts are highly responsive to their microenvironment, and thus it will be important to determine the degree to which different activation states are localized to interstitial versus perivascular regions and how these shift with disease progression. As spatial genomics improves to a single-cell level, this will be important for the development of future therapeutics for cardiac fibrosis.
Multiomic analyses, such as proteomic validation, are essential to identify what genomic clusters from bioinformatics differential gene expression represent an actual in vivo phenotype of fibroblasts that can potentially be targeted. Given the posttranscriptional and posttranslational modifications that are known to be important in regulating fibroblast function, a layered approach is necessary to truly evaluate the various clusters of fibroblasts. Moreover, proteins like collagen have a very long half-life; thus change in collagen gene expression does not truly reflect the actual degree of change in the ECM.
There remains a major challenge of targeting therapeutics directly to cardiac fibroblasts. Given that hypertension itself is a major risk factor for heart disease and fibrosis, it may be that adjunctive therapies to antihypertensive treatment are necessary. That is, targeting fibroblasts without also reducing the afterload on the heart will likely do more harm than good. These therapeutic approaches will also need to consider the sex and hormonal status of the patients. There is some promise in small-molecule targeting of fibrotic signaling pathways and drugs that inhibit histone deacetylases (HDACs) (136). Given that activation pathways are not unique to fibroblasts and represent fundamental signaling pathways in many cell types, careful evaluation of off-site actions will be important for minimizing adverse events.
Heart failure with preserved ejection fraction (HFpEF) is an important condition for which there are no effective therapies. There remains an ongoing challenge to develop an animal model that effectively mimics this condition. A major challenge is that in all models the elevated blood pressure results from an ongoing treatment to maintain that high blood pressure that is not necessarily similar to what is causing primary hypertension in humans. Although these models may be helpful to identify mechanisms that may underlie fibrosis or pathological remodeling, there will be challenges in identifying treatment strategies that improve blood pressure and fibrosis.
CONCLUSIONS
Hypertension induces a pressure overload of the left ventricle that results in hypertrophy and fibrotic remodeling. Associated with and driving these structural changes are hyperplasia and activation of resident cardiac fibroblasts. Given that hypertension is a major risk factor for future cardiac events, it is critical to understand the fibroblast milieu in this pressure-overloaded state. That is, at the time of myocardial infarction most individuals will have already had long-standing hypertension and left ventricular hypertrophy. Thus, the fibroblasts driving the wound healing response will likely arise from cells that are already activated to a degree. This likely contributes to the worse outcomes and exaggerated responses observed in myocardial infarction in the setting of hypertension. As more studies involving single-cell analyses are performed in fibroblasts from pressure-overloaded hearts, it will be important to evaluate the degree to which shifts in fibroblast subpopulations are common to pressure overload rather than model specific. Moreover, understanding the degree to which fibroblasts may be altered during antihypertensive therapies may reveal novel future targets to inhibit or reverse fibrosis development. Critically, the influence of sex and gonadal hormones on each of these processes must be a focus of future research.
GRANTS
T. M. Hale acknowledges funding from National Heart, Lung, and Blood Institute Grant R01HL153112.
DISCLAIMERS
This manuscript is the sole responsibility of the authors and does not represent the official views of the funding agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M.H. conceived and designed research; U.C. prepared figures; U.C. and T.M.H. drafted manuscript; U.C. and T.M.H. edited and revised manuscript; U.C. and T.M.H. approved final version of manuscript.
REFERENCES
- 1. Wall HK, Wright JS, Jackson SL, Daussat L, Ramkissoon N, Schieb LJ, Stolp H, Tong X, Loustalot F. How do we jump-start self-measured blood pressure monitoring in the United States? addressing barriers beyond the published literature. Am J Hypertens 35: 244–255, 2022. doi: 10.1093/ajh/hpab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138: e484–e594, 2018. doi: 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 3. Ritchey MD, Loustalot F, Wall HK, Steiner CA, Gillespie C, George MG, Wright JS. Million hearts: description of the national surveillance and modeling methodology used to monitor the number of cardiovascular events prevented during 2012-2016. J Am Heart Assoc 6: e006021, 2017. doi: 10.1161/JAHA.117.006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Czubryt MP, Hale TM. Cardiac fibrosis: pathobiology and therapeutic targets. Cell Signal 85: 110066, 2021. doi: 10.1016/j.cellsig.2021.110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, Pinto AR. Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep 22: 600–610, 2018. doi: 10.1016/j.celrep.2017.12.072. [DOI] [PubMed] [Google Scholar]
- 6. Ivey MJ, Tallquist MD. Defining the cardiac fibroblast. Circ J 80: 2269–2276, 2016. doi: 10.1253/circj.CJ-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Díez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens 9: 546–550, 2007. doi: 10.1111/j.1524-6175.2007.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chalise U, Becirovic-Agic M, Lindsey ML. The cardiac wound healing response to myocardial infarction. WIREs Mech Dis 15: e1584, 2023. doi: 10.1002/wsbm.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley JM, Spaletra P, Li Z, Sharp TE 3rd, Goodchild TT, Corral LG, Fung L, Chan KW, Sullivan RW, Swindlehurst CA, Lefer DJ. A novel fibroblast activation inhibitor attenuates left ventricular remodeling and preserves cardiac function in heart failure. Am J Physiol Heart Circ Physiol 315: H563–H570, 2018. doi: 10.1152/ajpheart.00603.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E. Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol 297: H1992–H2003, 2009. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schimmel K, Ichimura K, Reddy S, Haddad F, Spiekerkoetter E. Cardiac fibrosis in the pressure overloaded left and right ventricle as a therapeutic target. Front Cardiovasc Med 9: 886553, 2022. doi: 10.3389/fcvm.2022.886553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123: 255–278, 2009. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13. Matsuki K, Hathaway CK, Lawrence MG, Smithies O, Kakoki M. The role of transforming growth factor beta1 in the regulation of blood pressure. Curr Hypertens Rev 10: 223–238, 2014. doi: 10.2174/157340211004150319123313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jalil JE, Doering CW, Janicki JS, Pick R, Clark WA, Abrahams C, Weber KT. Structural vs. contractile protein remodeling and myocardial stiffness in hypertrophied rat left ventricle. J Mol Cell Cardiol 20: 1179–1187, 1988. doi: 10.1016/0022-2828(88)90597-4. [DOI] [PubMed] [Google Scholar]
- 15. Becirovic-Agic M, Chalise U, Daseke MJ 2nd, Konfrst S, Salomon JD, Mishra PK, Lindsey ML. Infarct in the heart: what’s MMP-9 got to do with it? Biomolecules 11: 491, 2021. doi: 10.3390/biom11040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garvin AM, Hale TM. Fibroblast shifts in the hypertensive heart: how single cell RNA-sequencing will accelerate advancements in anti-fibrotic therapies. J Mol Cell Cardiol 151: 44–45, 2021. doi: 10.1016/j.yjmcc.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garvin AM, De Both MD, Talboom JS, Lindsey ML, Huentelman MJ, Hale TM. Transient ACE (angiotensin-converting enzyme) inhibition suppresses future fibrogenic capacity and heterogeneity of cardiac fibroblast subpopulations. Hypertension 77: 904–918, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McLellan MA, Skelly DA, Dona MS, Squiers GT, Farrugia GE, Gaynor TL, Cohen CD, Pandey R, Diep H, Vinh A, Rosenthal NA, Pinto AR. High-resolution transcriptomic profiling of the heart during chronic stress reveals cellular drivers of cardiac fibrosis and hypertrophy. Circulation 142: 1448–1463, 2020. doi: 10.1161/CIRCULATIONAHA.119.045115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 49: 499–507, 2010. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Picariello C, Lazzeri C, Attanà P, Chiostri M, Gensini GF, Valente S. The impact of hypertension on patients with acute coronary syndromes. Int J Hypertens 2011: 563657, 2011. doi: 10.4061/2011/563657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45: 657–687, 2005. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 22. D’Souza KM, Biwer LA, Madhavpeddi L, Ramaiah P, Shahid W, Hale TM. Persistent change in cardiac fibroblast physiology after transient ACE inhibition. Am J Physiol Heart Circ Physiol 309: H1346–H1353, 2015. doi: 10.1152/ajpheart.00615.2015. [DOI] [PubMed] [Google Scholar]
- 23. Rysä J, Tokola H, Ruskoaho H. Mechanical stretch induced transcriptomic profiles in cardiac myocytes. Sci Rep 8: 4733, 2018. doi: 10.1038/s41598-018-23042-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abramochkin DV, Lozinsky IT, Kamkin A. Influence of mechanical stress on fibroblast-myocyte interactions in mammalian heart. J Mol Cell Cardiol 70: 27–36, 2014. doi: 10.1016/j.yjmcc.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 25. Saheera S, Krishnamurthy P. Cardiovascular changes associated with hypertensive heart disease and aging. Cell Transplant 29: 963689720920830, 2020. doi: 10.1177/0963689720920830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol 144: 666–672, 2008. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Yu W, Liu Y, Chen S, Huang Y, Li X, Liu C, Zhang Y, Li Z, Du J, Tang C, Du J, Jin H. Mechanical stretching stimulates collagen synthesis via down-regulating SO2/AAT1 pathway. Sci Rep 6: 21112, 2016. doi: 10.1038/srep21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herum KM, Choppe J, Kumar A, Engler AJ, McCulloch AD. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol Biol Cell 28: 1871–1882, 2017. doi: 10.1091/mbc.E17-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Garcia-Elias A, Benito B, Nattel S. The effects of cardiac stretch on atrial fibroblasts: analysis of the evidence and potential role in atrial fibrillation. Cardiovasc Res 118: 440–460, 2022. doi: 10.1093/cvr/cvab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neves JS, Leite-Moreira AM, Neiva-Sousa M, Almeida-Coelho J, Castro-Ferreira R, Leite-Moreira AF. Acute myocardial response to stretch: what we (don’t) know. Front Physiol 6: 408, 2015. doi: 10.3389/fphys.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall C, Gehmlich K, Denning C, Pavlovic D. Complex relationship between cardiac fibroblasts and cardiomyocytes in health and disease. J Am Heart Assoc 10: e019338, 2021. doi: 10.1161/JAHA.120.019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyon RC, Zanella F, Omens JH, Sheikh F. Mechanotransduction in cardiac hypertrophy and failure. Circ Res 116: 1462–1476, 2015. doi: 10.1161/CIRCRESAHA.116.304937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang P, Su J, Mende U. Cross talk between cardiac myocytes and fibroblasts: from multiscale investigative approaches to mechanisms and functional consequences. Am J Physiol Heart Circ Physiol 303: H1385–H1396, 2012. doi: 10.1152/ajpheart.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Wamel AJ, Ruwhof C, van der Valk-Kokshoorn LJ, Schrier PI, van der Laarse A. Stretch-induced paracrine hypertrophic stimuli increase TGF-beta1 expression in cardiomyocytes. Mol Cell Biochem 236: 147–153, 2002. doi: 10.1023/a:1016138813353. [DOI] [PubMed] [Google Scholar]
- 35. Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, Lin SC, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7: 12260, 2016. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang W, Xiong Y, Li X, Yang Y. Cardiac fibrosis: cellular effectors, molecular pathways, and exosomal roles. Front Cardiovasc Med 8: 715258, 2021. doi: 10.3389/fcvm.2021.715258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsutsumi Y, Matsubara H, Ohkubo N, Mori Y, Nozawa Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Moriguchi Y, Shibasaki Y, Kamihata H, Inada M, Iwasaka T. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res 83: 1035–1046, 1998. doi: 10.1161/01.res.83.10.1035. [DOI] [PubMed] [Google Scholar]
- 38. Sun Y. Intracardiac renin-angiotensin system and myocardial repair/remodeling following infarction. J Mol Cell Cardiol 48: 483–489, 2010. doi: 10.1016/j.yjmcc.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF, Dell Italia LJ. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol 311: H404–H414, 2016. doi: 10.1152/ajpheart.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garvin AM, Khokhar BS, Czubryt MP, Hale TM. RAS inhibition in resident fibroblast biology. Cell Signal 80: 109903, 2021. doi: 10.1016/j.cellsig.2020.109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol 294: H1675–H1684, 2008. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 42. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 98: 1627–1738, 2018. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 63: 423–432, 2004. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 44. Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106: 130–135, 2002. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 46. Nakajima H, Nakajima HO, Salcher O, Dittiè AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta1 transgene in the heart. Circ Res 86: 571–579, 2000. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 47. Attisano L, Lee-Hoeflich ST. The Smads. Genome Biol 2: REVIEWS3010, 2001. doi: 10.1186/gb-2001-2-8-reviews3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, Karch J, Molkentin JD. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 127: 3770–3783, 2017. doi: 10.1172/JCI94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kojonazarov B, Novoyatleva T, Boehm M, Happe C, Sibinska Z, Tian X, Sajjad A, Luitel H, Kriechling P, Posern G, Evans SM, Grimminger F, Ghofrani HA, Weissmann N, Bogaard HJ, Seeger W, Schermuly RT. p38 MAPK inhibition improves heart function in pressure-loaded right ventricular hypertrophy. Am J Respir Cell Mol Biol 57: 603–614, 2017. doi: 10.1165/rcmb.2016-0374OC. [DOI] [PubMed] [Google Scholar]
- 50. Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, Gunaje J, Otsu K, Davis J. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation 136: 549–561, 2017. doi: 10.1161/CIRCULATIONAHA.116.026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y, Li Z, Zhang C, Li P, Wu Y, Wang C, Bond Lau W, Ma XL, Du J. cardiac fibroblast-specific activating transcription factor 3 protects against heart failure by suppressing MAP2K3-p38 signaling. Circulation 135: 2041–2057, 2017. doi: 10.1161/CIRCULATIONAHA.116.024599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma ZG, Yuan YP, Zhang X, Xu SC, Wang SS, Tang QZ. Piperine attenuates pathological cardiac fibrosis via PPAR-gamma/AKT pathways. EBioMedicine 18: 179–187, 2017. doi: 10.1016/j.ebiom.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma ZG, Zhang X, Yuan YP, Jin YG, Li N, Kong CY, Song P, Tang QZ. A77 1726 (leflunomide) blocks and reverses cardiac hypertrophy and fibrosis in mice. Clin Sci (Lond) 132: 685–699, 2018. doi: 10.1042/CS20180160. [DOI] [PubMed] [Google Scholar]
- 54. Lal H, Ahmad F, Zhou J, Yu JE, Vagnozzi RJ, Guo Y, Yu D, Tsai EJ, Woodgett J, Gao E, Force T. Cardiac fibroblast glycogen synthase kinase-3beta regulates ventricular remodeling and dysfunction in ischemic heart. Circulation 130: 419–430, 2014. doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Umbarkar P, Tousif S, Singh AP, Anderson JC, Zhang Q, Tallquist MD, Woodgett J, Lal H. Fibroblast GSK-3alpha promotes fibrosis via RAF-MEK-ERK pathway in the injured heart. Circ Res 131: 620–636, 2022. doi: 10.1161/CIRCRESAHA.122.321431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiang FL, Fang M, Yutzey KE. Loss of beta-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun 8: 712, 2017. doi: 10.1038/s41467-017-00840-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vallée A, Lecarpentier Y, Guillevin R, Vallée JN. Interactions between TGF-beta1, canonical WNT/beta-catenin pathway and PPAR gamma in radiation-induced fibrosis. Oncotarget 8: 90579–90604, 2017. doi: 10.18632/oncotarget.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peter AK, Walker CJ, Ceccato T, Trexler CL, Ozeroff CD, Lugo KR, Perry AR, Anseth KS, Leinwand LA. Cardiac fibroblasts mediate a sexually dimorphic fibrotic response to beta-adrenergic stimulation. J Am Heart Assoc 10: e018876, 2021. doi: 10.1161/JAHA.120.018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cuspidi C, Ciulla M, Zanchetti A. Hypertensive myocardial fibrosis. Nephrol Dial Transplant 21: 20–23, 2006. doi: 10.1093/ndt/gfi237. [DOI] [PubMed] [Google Scholar]
- 60. Imaeda A, Tanaka S, Tonegawa K, Fuchigami S, Obana M, Maeda M, Kihara M, Kiyonari H, Conway SJ, Fujio Y, Nakayama H. Myofibroblast beta2 adrenergic signaling amplifies cardiac hypertrophy in mice. Biochem Biophys Res Commun 510: 149–155, 2019. doi: 10.1016/j.bbrc.2019.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dang S, Zhang ZY, Li KL, Zheng J, Qian LL, Liu XY, Wu Y, Zhang CY, Zhao XX, Yu ZM, Wang RX, Jiang T. Blockade of beta-adrenergic signaling suppresses inflammasome and alleviates cardiac fibrosis. Ann Transl Med 8: 127, 2020. doi: 10.21037/atm.2020.02.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bursac N. Cardiac fibroblasts in pressure overload hypertrophy: the enemy within? J Clin Invest 124: 2850–2853, 2014. doi: 10.1172/JCI76628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res 107: 1011–1020, 2010. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rickard AJ, Morgan J, Bienvenu LA, Fletcher EK, Cranston GA, Shen JZ, Reichelt ME, Delbridge LM, Young MJ. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension 60: 1443–1450, 2012. doi: 10.1161/HYPERTENSIONAHA.112.203158. [DOI] [PubMed] [Google Scholar]
- 65. Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res 106: 47–57, 2010. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Camus V, Bigenwald C, Ribrag V, Lazarovici J, Jardin F, Sarkozy C. Pembrolizumab in the treatment of refractory primary mediastinal large B-cell lymphoma: safety and efficacy. Expert Rev Anticancer Ther 21: 941–956, 2021. doi: 10.1080/14737140.2021.1953986. [DOI] [PubMed] [Google Scholar]
- 68. Konstandin MH, Völkers M, Collins B, Quijada P, Quintana M, De La Torre A, Ormachea L, Din S, Gude N, Toko H, Sussman MA. Fibronectin contributes to pathological cardiac hypertrophy but not physiological growth. Basic Res Cardiol 108: 375, 2013. doi: 10.1007/s00395-013-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 114: 616–625, 2014. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol 176: 1818–1828, 2019. doi: 10.1111/bph.14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Abe H, Tanada Y, Omiya S, Podaru MN, Murakawa T, Ito J, Shah AM, Conway SJ, Ono M, Otsu K. NF-kappaB activation in cardiac fibroblasts results in the recruitment of inflammatory Ly6C(hi) monocytes in pressure-overloaded hearts. Sci Signal 14: eabe4932, 2021. doi: 10.1126/scisignal.abe4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vilanova JR, Norenberg MD, Stuard ID. Thrombotic thrombocytopenic purpura. Systemic embolization from nonbacterial thrombotic endocarditis. N Y State J Med 75: 2246–2248, 1975. [PubMed] [Google Scholar]
- 73. Bradshaw AD, DeLeon-Pennell KY. T-cell regulation of fibroblasts and cardiac fibrosis. Matrix Biol 91-92: 167–175, 2020. doi: 10.1016/j.matbio.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Blanton RM, Carrillo-Salinas FJ, Alcaide P. T-cell recruitment to the heart: friendly guests or unwelcome visitors? Am J Physiol Heart Circ Physiol 317: H124–H140, 2019. doi: 10.1152/ajpheart.00028.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, Alcaide P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med 214: 3311–3329, 2017. doi: 10.1084/jem.20161791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bayer AL, Smolgovsky S, Ngwenyama N, Hernández-Martínez A, Kaur K, Sulka K, Amrute J, Aronovitz M, Lavine K, Sharma S, Alcaide P. T-cell MyD88 is a novel regulator of cardiac fibrosis through modulation of T-cell activation. Circ Res 133: 412–429, 2023. doi: 10.1161/CIRCRESAHA.123.323030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu L, Hu J, Lei H, Qin H, Wang C, Gui Y, Xu D. Regulatory T cells in pathological cardiac hypertrophy: mechanisms and therapeutic potential. Cardiovasc Drugs Ther, 2023. doi: 10.1007/s10557-023-07463-y. [DOI] [PubMed] [Google Scholar]
- 78. Kim HJ, Hwang SJ, Kim BK, Jung KC, Chung DH. NKT cells play critical roles in the induction of oral tolerance by inducing regulatory T cells producing IL-10 and transforming growth factor beta, and by clonally deleting antigen-specific T cells. Immunology 118: 101–111, 2006. doi: 10.1111/j.1365-2567.2006.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kanellakis P, Dinh TN, Agrotis A, Bobik A. CD4+CD25+Foxp3+ regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J Hypertens 29: 1820–1828, 2011. doi: 10.1097/HJH.0b013e328349c62d. [DOI] [PubMed] [Google Scholar]
- 80. Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 81. Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Luckett LR, Gallucci RM. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol 156: 1163–1171, 2007. doi: 10.1111/j.1365-2133.2007.07867.x. [DOI] [PubMed] [Google Scholar]
- 83. O’Reilly S, Ciechomska M, Cant R, van Laar JM. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-beta (TGF-beta) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem 289: 9952–9960, 2014. doi: 10.1074/jbc.M113.545822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gordon DJ, Milner AE, Beaney RP, Grdina DJ, Vaughan AT. Cellular radiosensitivity in V79 cells is linked to alterations in chromatin structure. Int J Radiat Oncol Biol Phys 19: 1199–1201, 1990. doi: 10.1016/0360-3016(90)90228-c. [DOI] [PubMed] [Google Scholar]
- 85. Saalbach A, Klein C, Schirmer C, Briest W, Anderegg U, Simon JC. Dermal fibroblasts promote the migration of dendritic cells. J Invest Dermatol 130: 444–454, 2010. doi: 10.1038/jid.2009.253. [DOI] [PubMed] [Google Scholar]
- 86. Schirmer C, Klein C, von Bergen M, Simon JC, Saalbach A. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 116: 1715–1725, 2010. doi: 10.1182/blood-2010-01-263509. [DOI] [PubMed] [Google Scholar]
- 87. Myer RG, El Mezayen R, High KP. Prostaglandin E2-dependent IL-23 production in aged murine dendritic cells. Exp Gerontol 45: 834–841, 2010. doi: 10.1016/j.exger.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lavine K, Amrute J, Luo X, Penna V, Bredemeyer A, Yamawaki T, Yang S, Kadyrov F, Heo G, Shi S, Lee P, Koenig A, Kuppe C, Jones C, Kopecky B, Hayat S, Ma P, Terada Y, Fu A, Furtado M, Kreisel D, Stitziel N, Li CM, Kramann R, Liu Y, Ason B. Targeting immune-fibroblast crosstalk in myocardial infarction and cardiac fibrosis (Preprint). Res Sq rs.3.rs-2402606, 2023. doi: 10.21203/rs.3.rs-2402606/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Alexanian M, Przytycki PF, Micheletti R, Padmanabhan A, Ye L, Travers JG, Gonzalez-Teran B, Silva AC, Duan Q, Ranade SS, Felix F, Linares-Saldana R, Li L, Lee CY, Sadagopan N, Pelonero A, Huang Y, Andreoletti G, Jain R, McKinsey TA, Rosenfeld MG, Gifford CA, Pollard KS, Haldar SM, Srivastava D. A transcriptional switch governs fibroblast activation in heart disease. Nature 595: 438–443, 2021. doi: 10.1038/s41586-021-03674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thorin-Trescases N, deBlois D, Hamet P. Evidence of an altered in vivo vascular cell turnover in spontaneously hypertensive rats and its modulation by long-term antihypertensive treatment. J Cardiovasc Pharmacol 38: 764–774, 2001. doi: 10.1097/00005344-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 91. Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 124: 2921–2934, 2014. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Souders CA, Borg TK, Banerjee I, Baudino TA. Pressure overload induces early morphological changes in the heart. Am J Pathol 181: 1226–1235, 2012. doi: 10.1016/j.ajpath.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 131: 471–481, 2009. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stewart JA Jr, Massey EP, Fix C, Zhu J, Goldsmith EC, Carver W. Temporal alterations in cardiac fibroblast function following induction of pressure overload. Cell Tissue Res 340: 117–126, 2010. doi: 10.1007/s00441-010-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sweeney M, Corden B, Cook SA. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: mirage or miracle? EMBO Mol Med 12: e10865, 2020. doi: 10.15252/emmm.201910865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ekström M, Hellman A, Hasselström J, Hage C, Kahan T, Ugander M, Wallén H, Persson H, Linde C. The transition from hypertension to hypertensive heart disease and heart failure: the PREFERS Hypertension study. ESC Heart Fail 7: 737–746, 2020. doi: 10.1002/ehf2.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 13: 18–28, 2011. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131: 1247–1259, 2015. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dachuan Y, Jinyu Q. The physiological response of ectomycorrhizal fungus Lepista sordida to Cd and Cu stress. PeerJ 9: e11115, 2021. doi: 10.7717/peerj.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Angelini A, Ortiz-Urbina J, Trial J, Reddy AK, Malovannaya A, Jain A, Entman ML, Taffet GE, Cieslik KA. Sex-specific phenotypes in the aging mouse heart and consequences for chronic fibrosis. Am J Physiol Heart Circ Physiol 323: H285–H300, 2022. doi: 10.1152/ajpheart.00078.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ruppert M, Barta BA, Korkmaz-Icöz S, Loganathan S, Oláh A, Sayour AA, Benke K, Nagy D, Bálint T, Karck M, Schilling O, Merkely B, Radovits T, Szabó G. Sex similarities and differences in the reverse and anti-remodeling effect of pressure unloading therapy in a rat model of aortic banding and debanding. Am J Physiol Heart Circ Physiol 323: H204–H222, 2022. doi: 10.1152/ajpheart.00654.2021. [DOI] [PubMed] [Google Scholar]
- 102. Garvin AM, Hale TM. Sex matters in the aging heart: implications for antifibrotic therapies. Am J Physiol Heart Circ Physiol 323: H360–H361, 2022. doi: 10.1152/ajpheart.00340.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lu Y, Xia N, Cheng X. Regulatory T cells in chronic heart failure. Front Immunol 12: 732794, 2021. doi: 10.3389/fimmu.2021.732794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang L, Liu C, Chen X, Li P. Alamandine attenuates long-term hypertension-induced cardiac fibrosis independent of blood pressure. Mol Med Rep 19: 4553–4560, 2019. doi: 10.3892/mmr.2019.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brilla CG. Regression of myocardial fibrosis in hypertensive heart disease: diverse effects of various antihypertensive drugs. Cardiovasc Res 46: 324–331, 2000. doi: 10.1016/s0008-6363(99)00432-0. [DOI] [PubMed] [Google Scholar]
- 106. Sevilla MA, Voces F, Carron R, Guerrero EI, Ardanaz N, San Roman L, Arevalo MA, Montero MJ. Amlodipine decreases fibrosis and cardiac hypertrophy in spontaneously hypertensive rats: persistent effects after withdrawal. Life Sci 75: 881–891, 2004. doi: 10.1016/j.lfs.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 107. Lu JC, Cui W, Zhang HL, Liu F, Han M, Liu DM, Yin H-N, Zhang K, Du J. Additive beneficial effects of amlodipine and atorvastatin in reversing advanced cardiac hypertrophy in elderly spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 36: 1110–1119, 2009. doi: 10.1111/j.1440-1681.2009.05198.x. [DOI] [PubMed] [Google Scholar]
- 108. Nakamura T, Fukuda M, Kataoka K, Nako H, Tokutomi Y, Dong YF, Yamamoto E, Yasuda O, Ogawa H, Kim-Mitsuyama S. Eplerenone potentiates protective effects of amlodipine against cardiovascular injury in salt-sensitive hypertensive rats. Hypertens Res 34: 817–824, 2011. doi: 10.1038/hr.2011.35. [DOI] [PubMed] [Google Scholar]
- 109. Sandmann S, Claas R, Cleutjens JP, Daemen MJ, Unger T. Calcium channel blockade limits cardiac remodeling and improves cardiac function in myocardial infarction-induced heart failure in rats. J Cardiovasc Pharmacol 37: 64–77, 2001. doi: 10.1097/00005344-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 110. Jia Y, Xu J, Yu Y, Guo J, Liu P, Chen S, Jiang J. Nifedipine inhibits angiotensin II-induced cardiac fibrosis via downregulating Nox4-derived ROS generation and suppressing ERK1/2, JNK signaling pathways. Pharmazie 68: 435–441, 2013. [PubMed] [Google Scholar]
- 111. Del Mauro JS, Prince PD, Donato M, Fernandez Machulsky N, Morettón MA, González GE, Bertera FM, Carranza A, Gorzalczany SB, Chiappetta DA, Berg G, Morales C, Gelpi RJ, Taira CA, Höcht C. Effects of carvedilol or amlodipine on target organ damage in L-NAME hypertensive rats: their relationship with blood pressure variability. J Am Soc Hypertens 11: 227–240, 2017. doi: 10.1016/j.jash.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 112. Tea BS, Dam TV, Moreau P, Hamet P, deBlois D. Apoptosis during regression of cardiac hypertrophy in spontaneously hypertensive rats. Temporal regulation and spatial heterogeneity. Hypertension 34: 229–235, 1999. doi: 10.1161/01.hyp.34.2.229. [DOI] [PubMed] [Google Scholar]
- 113. Duguay D, Pesant S, Deschepper CF, deBlois D. Fibroblast apoptosis precedes cardiomyocyte mass reduction during left ventricular remodeling in hypertensive rats treated with amlodipine. J Hypertens 25: 1291–1299, 2007. doi: 10.1097/HJH.0b013e3280e126d5. [DOI] [PubMed] [Google Scholar]
- 114. Weidenbach R, Schulz R, Gres P, Behrends M, Post H, Heusch G. Enhanced reduction of myocardial infarct size by combined ACE inhibition and AT1-receptor antagonism. Br J Pharmacol 131: 138–144, 2000. doi: 10.1038/sj.bjp.0703544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Stein M, Boulaksil M, Jansen JA, Herold E, Noorman M, Joles JA, van Veen TA, Houtman MJ, Engelen MA, Hauer RN, de Bakker JM, van Rijen HV. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin-aldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol 299: H310–H321, 2010. doi: 10.1152/ajpheart.01137.2009. [DOI] [PubMed] [Google Scholar]
- 116. Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail 12: e005565, 2019. doi: 10.1161/CIRCHEARTFAILURE.118.005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Akashiba A, Ono H, Ono Y, Ishimitsu T, Matsuoka H. Valsartan improves L-NAME-exacerbated cardiac fibrosis with TGF-beta inhibition and apoptosis induction in spontaneously hypertensive rats. J Cardiol 52: 239–246, 2008. doi: 10.1016/j.jjcc.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 118. Zong M, Zhao H, Li Q, Li Y, Zhang J. Irbesartan ameliorates myocardial fibrosis in diabetic cardiomyopathy rats by inhibiting the TGFbeta1/Smad2/3 pathway. Exp Ther Med 20: 117, 2020. doi: 10.3892/etm.2020.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Guo C, Wang Y, Liang H, Zhang J. ADAMTS-1 contributes to the antifibrotic effect of captopril by accelerating the degradation of type I collagen in chronic viral myocarditis. Eur J Pharmacol 629: 104–110, 2010. doi: 10.1016/j.ejphar.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 120. Meng G, Wu F, Yang L, Zhu H, Gu J, He M, Xu J. Synergistic attenuation of myocardial fibrosis in spontaneously hypertensive rats by joint treatment with benazepril and candesartan. J Cardiovasc Pharmacol 54: 16–24, 2009. doi: 10.1097/FJC.0b013e3181a98b31. [DOI] [PubMed] [Google Scholar]
- 121. Der Sarkissian S, Marchand EL, Duguay D, Hamet P, deBlois D. Reversal of interstitial fibroblast hyperplasia via apoptosis in hypertensive rat heart with valsartan or enalapril. Cardiovasc Res 57: 775–783, 2003. doi: 10.1016/s0008-6363(02)00789-7. [DOI] [PubMed] [Google Scholar]
- 122. Der Sarkissian S, Tea BS, Touyz RM, deBlois D, Hale TM. Role of angiotensin II type 2 receptor during regression of cardiac hypertrophy in spontaneously hypertensive rats. J Am Soc Hypertens 7: 118–127, 2013. doi: 10.1016/j.jash.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 123. Ito N, Ohishi M, Yamamoto K, Tatara Y, Shiota A, Hayashi N, Komai N, Yanagitani Y, Rakugi H, Ogihara T. Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am J Hypertens 20: 792–799, 2007. doi: 10.1016/j.amjhyper.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 124. Katoh M, Egashira K, Mitsui T, Chishima S, Takeshita A, Narita H. Angiotensin-converting enzyme inhibitor prevents plasminogen activator inhibitor-1 expression in a rat model with cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis. J Mol Cell Cardiol 32: 73–83, 2000. doi: 10.1006/jmcc.1999.1053. [DOI] [PubMed] [Google Scholar]
- 125. Liang B, Leenen FH. Prevention of salt induced hypertension and fibrosis by angiotensin converting enzyme inhibitors in Dahl S rats. Br J Pharmacol 152: 903–914, 2007. doi: 10.1038/sj.bjp.0707472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Peng H, Carretero OA, Vuljaj N, Liao TD, Motivala A, Peterson EL, Rhaleb NE. Angiotensin-converting enzyme inhibitors: a new mechanism of action. Circulation 112: 2436–2445, 2005. doi: 10.1161/CIRCULATIONAHA.104.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pacca SR, de Azevedo AP, De Oliveira CF, De Luca IM, De Nucci G, Antunes E. Attenuation of hypertension, cardiomyocyte hypertrophy, and myocardial fibrosis by beta-adrenoceptor blockers in rats under long-term blockade of nitric oxide synthesis. J Cardiovasc Pharmacol 39: 201–207, 2002. doi: 10.1097/00005344-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 128. Ciulla MM, Paliotti R, Esposito A, Dìez J, López B, Dahlöf B, Nicholls MG, Smith RD, Gilles L, Magrini F, Zanchetti A. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation 110: 552–557, 2004. doi: 10.1161/01.CIR.0000137118.47943.5C. [DOI] [PubMed] [Google Scholar]
- 129. Ye H, Ling S, Castillo AC, Thomas B, Long B, Qian J, Perez-Polo JR, Ye Y, Chen X, Birnbaum Y. Nebivolol induces distinct changes in profibrosis microRNA expression compared with atenolol, in salt-sensitive hypertensive rats. Hypertension 61: 1008–1013, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00892. [DOI] [PubMed] [Google Scholar]
- 130. Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA 93: 156–160, 1996. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tian B, Liu J, Bitterman P, Bache RJ. Angiotensin II modulates nitric oxide-induced cardiac fibroblast apoptosis by activation of AKT/PKB. Am J Physiol Heart Circ Physiol 285: H1105–H1112, 2003. doi: 10.1152/ajpheart.01139.2002. [DOI] [PubMed] [Google Scholar]
- 132. Neff LS, Zhang Y, Van Laer AO, Baicu CF, Karavan M, Zile MR, Bradshaw AD. Mechanisms that limit regression of myocardial fibrosis following removal of left ventricular pressure overload. Am J Physiol Heart Circ Physiol 323: H165–H175, 2022. doi: 10.1152/ajpheart.00148.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hale TM. Persistent phenotypic shift in cardiac fibroblasts: impact of transient renin angiotensin system inhibition. J Mol Cell Cardiol 93: 125–132, 2016. doi: 10.1016/j.yjmcc.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 134. Biwer LA, Broderick TL, Xu H, Carroll C, Hale TM. Protection against L-NAME-induced reduction in cardiac output persists even after cessation of angiotensin-converting enzyme inhibitor treatment. Acta Physiol (Oxf) 207: 156–165, 2013. doi: 10.1111/j.1748-1716.2012.02474.x. [DOI] [PubMed] [Google Scholar]
- 135. Biwer LA, D’Souza KM, Abidali A, Tu D, Siniard AL, DeBoth M, Huentelman M, Hale TM. Time course of cardiac inflammation during nitric oxide synthase inhibition in SHR: impact of prior transient ACE inhibition. Hypertens Res 39: 8–18, 2016. doi: 10.1038/hr.2015.107. [DOI] [PubMed] [Google Scholar]
- 136. Travers JG, Wennersten SA, Peña B, Bagchi RA, Smith HE, Hirsch RA, Vanderlinden LA, Lin YH, Dobrinskikh E, Demos-Davies KM, Cavasin MA, Mestroni L, Steinkühler C, Lin CY, Houser SR, Woulfe KC, Lam MP, McKinsey TA. HDAC inhibition reverses preexisting diastolic dysfunction and blocks covert extracellular matrix remodeling. Circulation 143: 1874–1890, 2021. doi: 10.1161/CIRCULATIONAHA.120.046462. [DOI] [PMC free article] [PubMed] [Google Scholar]