Abstract
Duplicate testes lined in series were observed in the right scrotum of a 6-week-old Sprague-Dawley rat in a single-dose toxicity study. Of the two right testicles, one was spherical and less than half the size of a normal testis. The other was oval-shaped, slightly smaller than a normal testis, and possessed clear, tortuous blood vessels similar to those of a normal testis. Each right testis was grossly separated but faced the intertesticular adipose tissue and was sparsely joined by thin cord-like structures. Only one epididymis covered or encompassed the two right testes. The caput epididymis was attached to the smaller spherical testis, whereas the cauda epididymis was attached to the oval testis. Histopathological examination revealed that the smaller spherical testis on the right side and the testis on the left side were normal. The oval-shaped testis on the right exhibited markedly dilated degenerative seminiferous tubules with one to two layers of Sertoli or germ cells, and almost no spermatogenesis was observed. Multinucleated germ cells were observed in the lumen of the degenerated seminiferous tubules. The right epididymis was morphologically normal and contained few sperm in the epididymal duct of the tail. The cord-like structures between duplicate testes comprised fibrous and adipose tissues. Single efferent ductules, ectopic cartilage, and skeletal muscle tissues were buried in the adipose tissue. To our knowledge, this is the first report of spontaneous polyorchidism in a rodent.
Keywords: polyorchism, triorchism, supernumerary testes, congenital disease, developmental abnormality, gonadal anomaly
Polyorchidism is a gonadal anomaly characterized by the presence of more than two testes. In general, the terms supernumerary testes and triorchidism (presence of three testes) are currently used interchangeably1. In domestic mammals, only a few cases of polyorchidism have been reported, including those in dogs2, 3, cats4, 5, 6, and horses7, 8, suggesting the rarity of this urogenital condition. Although reports involving birds are limited, it appears to be a more common occurrence among birds9, 10, 11. Only approximately 140 cases have been identified in humans, and these are considered rare malformation12. Several possible mechanisms for this congenital anomaly have been proposed (e.g., duplication or division of the genital ridge, degeneration of parts of the mesonephric component, and constriction by the development of the peritoneal band). However, many anatomical variations in polyorchidism have not been fully explained12, 13, 14. Herein, we report a case of spontaneous polyorchidism in a 6-week-old Sprague-Dawley rat that was incidentally observed in a toxicity study.
A 6-week-old male Crl:CD (SD) rat was assigned to a single-dose oral toxicity study. The rat was housed in a plastic cage in an environmentally controlled room (room temperature at 23 ± 3°C; relative humidity of 30‒60%; lighting cycle of 12 h light/12 h dark) and supplied a pellet diet and tap water ad libitum. All experimental procedures were conducted after the study was approved by the Institutional Animal Care and Use Committee at Shionogi Pharmaceutical Research Center. Upon study completion, rats were euthanized and necropsied. For further investigation, the bilateral testes, epididymides, kidneys, and ureters were collected, fixed in 10% neutral-buffered formalin (the testes and epididymides were prefixed with Bouin’s solution), and routinely processed. Paraffin sections were stained with hematoxylin and eosin, and histopathologically examined.
Duplicate testes aligned in series were observed in the right scrotum (Fig. 1). They differed in size and shape. One was spherical and less than half the size of a normal testis, while the other was oval-shaped and slightly smaller than a normal testis, with clear tortuous blood vessels similar to those of a normal testis. A vessel connection between the two testes was observed, and the pampiniform plexus was attached to the oval-shaped testis (Supplementary Fig.). Each right testis was grossly joined by thin cord-like structures and covered with abundant adipose tissue, partly connected to the adipose tissue of the testicular cord. Only one epididymis covered or encompassed the two right testes. The caput epididymis was attached to the smaller spherical testis, whereas the cauda epididymis was attached to the oval testis. No other morphological abnormalities were observed in the surrounding tissues such as the deferent duct, pampiniform plexus, kidneys, or ureters.
Fig. 1.
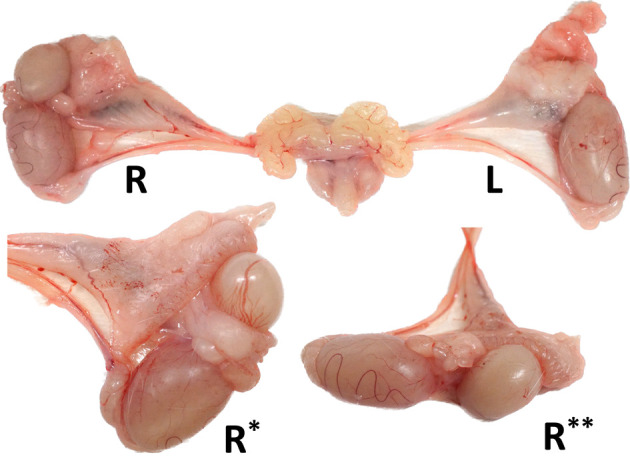
Macroscopic images of male reproductive organs in the present case. The right testis is duplicated and lined in series with a single attached epididymis (R*, opposite side of R; R**, lateral side of R). Each right testis is joined by thin cord-like structures and covered with abundant adipose tissue. The testis and epididymis on the left side, vas deferens and blood vessels on both sides, and accessory glands were normal.
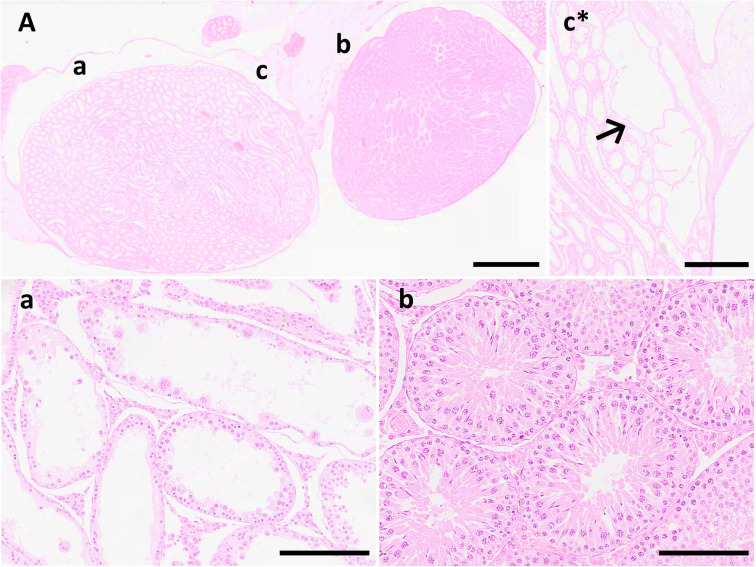
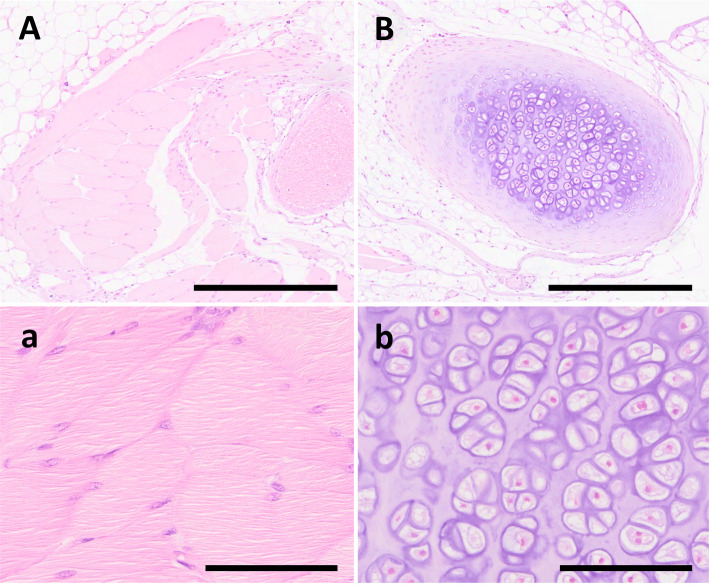
Histopathological examination revealed that the oval-shaped testes on the right exhibited markedly dilated degenerative seminiferous tubules with one to two layers of Sertoli or germ cells, and almost no spermatogenesis (Fig. 2a). Multinucleated germ cells were observed in the lumen of the degenerated seminiferous tubules. The right spherical testis was small and possessed few seminiferous tubules; however, all stages of spermatogenesis were present. Additionally, there were fewer sperms in the center of the seminiferous tubules than in the left testis (Fig. 2b). A dilated rete testis appeared at the caput side edge of the oval testis on the right side (Fig. 2c) along with dilated seminiferous tubules, suggesting a blind extremity of sperm flow that generally flowed from the caudal side of the testis to the caput side toward the caput epididymis. A rete testis was observed at the edge of the smaller spherical testis on the right side, which was histologically normal. The right epididymis was morphologically normal and included a small number of sperms in the epididymal duct of the tail, although fewer sperms were present than in the left normal testis, suggesting the presence of a flow connection. The cord-like structures between the duplicate testes consisted of fibrous and adipose tissues, and the ectopic cartilage and skeletal muscle tissues were buried in the adipose tissues (Fig. 3). Furthermore, the vas efferens was observed outside the tunica albuginea, adjacent to the smaller spherical testis on the right side. Based on morphological examination, the rat in this case was diagnosed with spontaneous polyorchidism with ectopic mesenchymal tissue.
Fig. 2.
Photomicrographs of the present case. (A) Spectacle magnification of duplicate testes observed in the right scrotum. The oval-shaped testis exhibit dilatation of the seminiferous tubules. Scale bar=3 mm. (a) Markedly degenerative seminiferous tubules with one to two cell layers of Sertoli and germ cells. Multinucleated germ cells are also observed inside the lumen. Scale bar=200 μm. (b) Smaller spherical testis exhibiting normal histological structures of seminiferous tubules. Scale bar=200 μm. (c) Dilated rete testis is observed at the caput side edge of the oval-shaped testis (Serial section of A) suggesting blind extremity of sperm flow. Scale bar=1 mm.
Fig. 3.
Photomicrographs of the present case. (A) and (B) Spectacle magnification of ectopic mesenchymal tissues of uncertain origin buried in the adipose tissue of cord-like structures between duplicate testes. Scale bar=1 mm. (a) Higher magnification of (A). Ectopic skeletal muscle tissue composed of mature myocytes. Scale bar=100 μm. (b) Higher magnification of (B). Ectopic cartilage tissue composed of mature chondrocytes. Scale bar=100 μm.
Anomalous polyorchidism is defined as the presence of more than two testes. Many anatomical variations of polyorchidism have been reported in the field of human medicine12, 15, 16. The patterns were as follows: each duplicate testis possessed its own vas deferens and epididymis, its own epididymis but no vas efferens, or its own vas efferens but no epididymis. There were also patterns with a common single epididymis and vas efferens attached across duplicate testes (consistent with the present case) or a common single vas efferens attached to duplicate testes with its own epididymis. The number of testes varies between three and five, although triorchidism (three testes) is most common in humans and domestic animals1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 14.
During drug development, embryo-fetal developmental (EFD) toxicity studies are conducted to detect the developmental effects of a compound during organogenesis17. Rats are often used as animal models in these studies, and their fetuses are evaluated for malformations immediately before birth. Therefore, it is possible to refer to a large amount of historical control data regarding external, skeletal, and visceral anomalies. In historical control data of visceral anomalies in rat EFD toxicity studies, performed from 1994–2015, the only listed testicular anomalies were malpositioned, undescended, and absence of testis18, 19; polyorchidism being an extremely rare anomaly in rats.
During the embryonic stage, testes develop from the intermediate mesoderm, which runs along the posterior wall of the abdomen20. Primitive gonads are genital ridges with paired elevations in the ventral mesonephros. After primordial germ cells appear on the wall of the yolk sac, they move through the dorsal side of the hindgut and enter the space between the primordial germ cords of the genital ridge. Subsequently, the primordial germ cords form the testicular cords that form the basis of the seminiferous tubules. The attached portions of the efferent ducts originating from the mesonephric ducts (Wolffian ducts) form a convoluted duct that constitutes the epididymis. Possible mechanisms of polyorchidism have been proposed, including duplication or division of the genital ridge, degeneration of parts of the mesonephric component, or constriction by peritoneal band development12, 13, 14, which is somehow involved in the developmental process. Considering the many anatomical variations in polyorchidism, the direction (longitudinal or transverse) and location of the division (high or low, where the division includes the mesonephric duct)14 were inferred to contribute to these variations. In our case, the duplicate testes were clearly encapsulated by the tunica albuginea; one epididymis was attached to and encompassed the testes, and a flow connection between one of the duplicate testes and the epididymis was observed. Therefore, genital ridge duplication by transverse division, including the epididymis, in the early stages of development, is considered the most likely mechanism. However, there is also the possibility that torsion contributed to testicular division in our case, as the adipose tissues between the duplicate testes were partially connected to the adipose tissues of the testicular cord, which were in a condition likely to cause torsion. Polyorchidism is a risk factor for testicular torsion21.
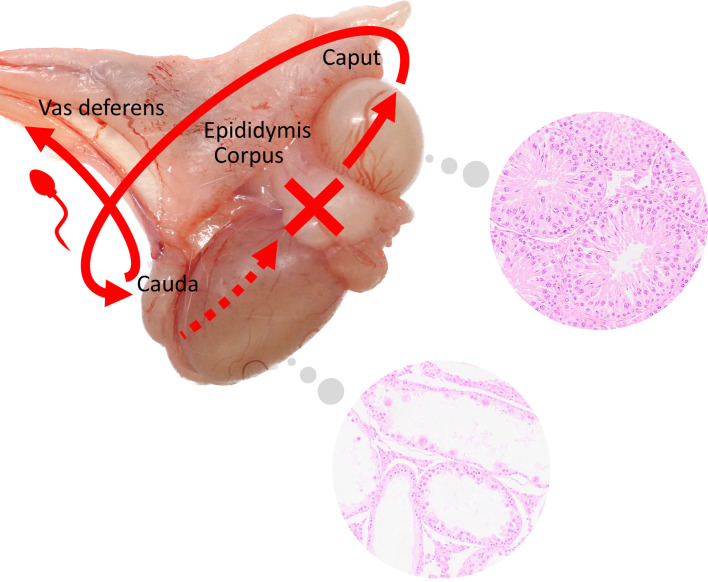
Previous studies have demonstrated that in the presence of an obstructed efferent duct lumen, blocked seminal fluid flow becomes a pressure that causes tubular dilatation and progresses to atrophy22. Another report revealed that disturbances in fluid homeostasis in the efferent and epididymal ducts caused by phosphodiesterase-4 inhibitors resulted in dilatation of seminiferous tubules and rete testes, followed by tubular degeneration and atrophy in rats23. A dilated rete testis appeared at the caput side edge of the oval testis on the right, and dilated seminiferous tubules (as observed in our case) suggested a blind extremity of sperm flow that generally flowed from the caudal side of the testis to the caput side toward the caput epididymis or close to that (Fig. 4). Few sperms were present in the epididymal duct of the tail, and the vas efferens was observed outside the tunica albuginea, adjacent to the smaller spherical testis on the right, thus indicating the presence of a flow connection between the testis and epididymis. In this case, the oval testis could have developed into a normally functioning testicle; however, the flow communication to the epididymis did not develop properly, resulting in abnormal spermatogenesis. In contrast, the smaller spherical testis was able to form sperm normally, as it was well connected to the epididymis.
Fig. 4.
Schema indicating the possible condition of the right duplicate testes in the present case. The red arrow indicates the direction of sperm flow in the testes and epididymis. Between duplicate testes (red cross), there is possibly a blind extremity of sperm flow that generally flowed from the caudal side of the testis to the caput side toward the caput epididymis.
Ectopically mature skeletal muscle and cartilage tissues were buried in the adipose tissue of the cord-like structures between duplicate testes. These two tissues were partially connected by thin connective tissue, suggesting a common origin (Fig. 3, serial sections). Histomorphological analysis revealed no cellular dysplasia within the component cells, and mesenchymal stem cells in adipose tissue may be the origin of these tissues. However, the precise cause of these ectopic tissues and their relationship to testicular anomalies remain unknown. One speculation is that morphological anomalies such as those in our case may have contributed to the ectopic tissues surrounding the male gonads by arranging the environment to cause ectopic lesions. To the best of our knowledge, as the ectopic tissue around the male gonads, the ectopic adrenal tissues of the testicular cord have been reported in humans24; however, there are no reports associating testicular anomalies with the pathogenesis of ectopic tissue, including animal reports. Further accumulation of such cases is needed.
This is an extremely rare case of spontaneous polyorchidism in a young Sprague-Dawley rat and it should be considered when conducting toxicity studies.
Disclosure of Potential Conflicts of Interest
The authors have no competing interests to disclose.
Supplementary Material
Acknowledgments
We would like to thank Rumiko Mochizuki, Chie Yabuuchi, and Hirotada Murayama (Shionogi TechnoAdvance Research Co., Ltd.) for supporting pathological specimen preparation, and the Kansai Conference on Toxicologic Pathology (KCTP) members for their helpful advice.
References
- 1.Foster RA. Male genital system. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed. Vol. 3, MG Maxie (ed). Elsevier, Missouri. 465–510. 2016. [Google Scholar]
- 2.Atkinson MC. Polyorchidism in a dog. Vet Rec. 145: 711–712. 1999. [PubMed] [Google Scholar]
- 3.Tamminen TM, Leinonen MR, Käck H, and Andersson M. A polyorchid dog. Reprod Domest Anim. 47: e26–e28. 2012. [DOI] [PubMed] [Google Scholar]
- 4.Milwright RD, and Smith KC. Polyorchidism in a cat. Vet Rec. 145: 679–680. 1999. [PubMed] [Google Scholar]
- 5.Roca-Ferrer J, Rodríguez E, Ramírez GA, Moragas C, and Sala M. A rare case of polyorchidism in a cat with four intra-abdominal testes. Reprod Domest Anim. 50: 172–176. 2015. [DOI] [PubMed] [Google Scholar]
- 6.Lohr BR, Lieske DE, and Parry NM. Polyorchidism in a cat. J Vet Diagn Invest. 34: 1020–1022. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earnshaw RE. Polyorchidism. Can J Comp Med Vet Sci. 23: 66. 1959. [PMC free article] [PubMed] [Google Scholar]
- 8.Davies EV. Polyorchidism in a horse. Vet Rec. 167: 310. 2010. [DOI] [PubMed] [Google Scholar]
- 9.Hocking PM. Bilateral testicular asymmetry and supernumerary testes in the domestic fowl (Gallus domesticus). Br Poult Sci. 33: 455–460. 1992. [DOI] [PubMed] [Google Scholar]
- 10.Witt CC, and Bautista E. Triorchidism in a Hummingbird. Wilson J Ornithol. 123: 632–635. 2011. [Google Scholar]
- 11.Onu JE. Type B triorchidism in an adult indigenous fowl (Gallus domesticus) in Sokoto, Nigeri—case report. Sokoto J Vet Sci. 10: 32–35. 2012. [Google Scholar]
- 12.Bergholz R, and Wenke K. Polyorchidism: a meta-analysis. J Urol. 182: 2422–2427. 2009. [DOI] [PubMed] [Google Scholar]
- 13.Wilson WA, and Littler J. Polyorchidism; a report of two cases with torsion. Br J Surg. 41: 302–307. 1953. [DOI] [PubMed] [Google Scholar]
- 14.Nistal M. and Paniagua R. Non-neoplastic diseases of the testis. Urol Surg Pathol. 614–755. 2008. [Google Scholar]
- 15.Toyoda Y, Maruyama K, and Polyorchidism A. [Polyorchidism. A case report and review of literature]. Nihon Hinyokika Gakkai Zasshi. 65: 181–188. 1974. [PubMed] [Google Scholar]
- 16.Bergholz R, Koch B, Spieker T, and Lohse K. Polyorchidism: a case report and classification. J Pediatr Surg. 42: 1933–1935. 2007. [DOI] [PubMed] [Google Scholar]
- 17.Barrow P, and Clemann N. Review of embryo-fetal developmental toxicity studies performed for pharmaceuticals approved by FDA in 2018 and 2019. Reprod Toxicol. 99: 144–151. 2021. [DOI] [PubMed] [Google Scholar]
- 18.Kuwagata M, Sakai Y, Tanaka S, Takashima H, Katagiri R, Matsuoka T, Noritake K, Senuma M, Shimizu T, Hojo H, Ibi K, Kudo S, Oota T, Ube M, Miwa Y, Kajita S, Uesugi T, Yabe K, Tateishi T, Nakano N, Taniguchi T, Yamashita A, Hirano T, Kirihata Y, Sakai Y, Nishizawa S, Fujiwara M, Mineshima H, Horimoto M, and Ema M. Historical control data on developmental toxicity studies in rats. Congenit Anom (Kyoto). 59: 125–131. 2019. [DOI] [PubMed] [Google Scholar]
- 19.Ema M, Endoh K, Fukushima R, Fujii S, Hara H, Hirata-Koizumi M, Hirose A, Hojo H, Horimoto M, Hoshino N, Hosokawa Y, Imai Y, Inada H, Inawaka K, Itoh K, Katsumata Y, Izumi H, Kato H, Maeda M, Matsumoto K, Matsuo S, Matsuoka T, Matsuura I, Mineshima H, Miwa Y, Nakano N, Naya M, Noyori H, Ohta T, Oku H, Ono A, Shimizu T, Shimomura K, Takakura I, Tanaka R, Tateishi T, Tominaga Y, Uesugi T, Urakawa C, Yabe K, Yamashita A, Yamauchi T, and Yokoi R. Historical control data on developmental toxicity studies in rodents. Congenit Anom (Kyoto). 54: 150–161. 2014. [DOI] [PubMed] [Google Scholar]
- 20.Sadler TW. Urogenital system. In: Langman’s Medical Embryology, 12th ed., C Taylor (ed). Lippincott Williams & Wilkins, Baltimore. 232–259. 2012. [Google Scholar]
- 21.Xiaofei L, and Benzhang Z. Case report: testicular torsion in unilateral supernumerary testis. Front Pediatr. 10: 823374. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La DK, Creasy DM, Hess RA, Baxter E, Pereira ME, Johnson CA, Vinken P, and Snook SS. Efferent duct toxicity with secondary testicular changes in rats following administration of a novel leukotriene A4 hydrolase inhibitor. Toxicol Pathol. 40: 705–714. 2012. [DOI] [PubMed] [Google Scholar]
- 23.Heuser A, Mecklenburg L, Ockert D, Kohler M, and Kemkowski J. Selective inhibition of PDE4 in Wistar rats can lead to dilatation in testis, efferent ducts, and epididymis and subsequent formation of sperm granulomas. Toxicol Pathol. 41: 615–627. 2013. [DOI] [PubMed] [Google Scholar]
- 24.Mendez R, Tellado MG, Somoza I, Liras J, Sanchez-Abuin A, Pais E, and Vela D. Ectopic adrenal tissue in the spermatic cord in pediatric patients: surgical implications. Int Braz J Urol. 32: 202–207, discussion 207. 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.