Abstract
Tissue cross-reactivity (TCR) studies for the development of therapeutic antibodies are conducted to estimate any possible binding sites within the human body that can be affected by the antibody when assessing safety in humans. Any possible binding sites include specific binding sites of the antibody to its target antigen and nonspecific or off-target binding sites. In TCR studies the therapeutic antibodies and immunohistochemistry (IHC) of frozen tissues must be applied in assays. However, there are technical issues with applying a therapeutic antibody or test article to IHC, such as human-on-human staining, difficulty in applying the test article to IHC, and retention of the target antigen in frozen sections. In the current review, we introduce three case studies in which these technical issues were addressed, and propose a practical scheme for points to consider when conducting a TCR study. Information on the target antigen distribution obtained through robust assays and case-by-case strategies were found to be useful for understanding and assessing the relevance of toxic effects between animals and humans. Thus, we anticipate that by considering the points discussed in the current review and combining the data with information on the biological features of the target antigens and therapeutic antibodies, it will be possible to predict safety risks in humans with higher accuracy.
Keywords: tissue cross-reactivity, therapeutic antibody, immunohistochemistry
Introduction
Tissue cross-reactivity (TCR) studies for the development of therapeutic antibodies are conducted to estimate any possible binding sites for antibodies within the human body1, 2. Any possible binding sites include specific binding sites of the antibody to its target antigen and nonspecific binding that occurs independently of the target antigen, which is often referred to as off-target binding3. The organs and tissues that show binding are considered potential sites that can be affected by the antibody; therefore, the distribution of binding sites should be considered when assessing safety in humans.
To fulfil the objective of comprehensive screening for any possible binding sites in humans, the therapeutic antibodies must be applied in assays1, 2, and immunohistochemistry (IHC) of frozen tissues is the most generally selected method. However, there are several technical issues associated with the application of therapeutic antibodies or test articles in IHC.
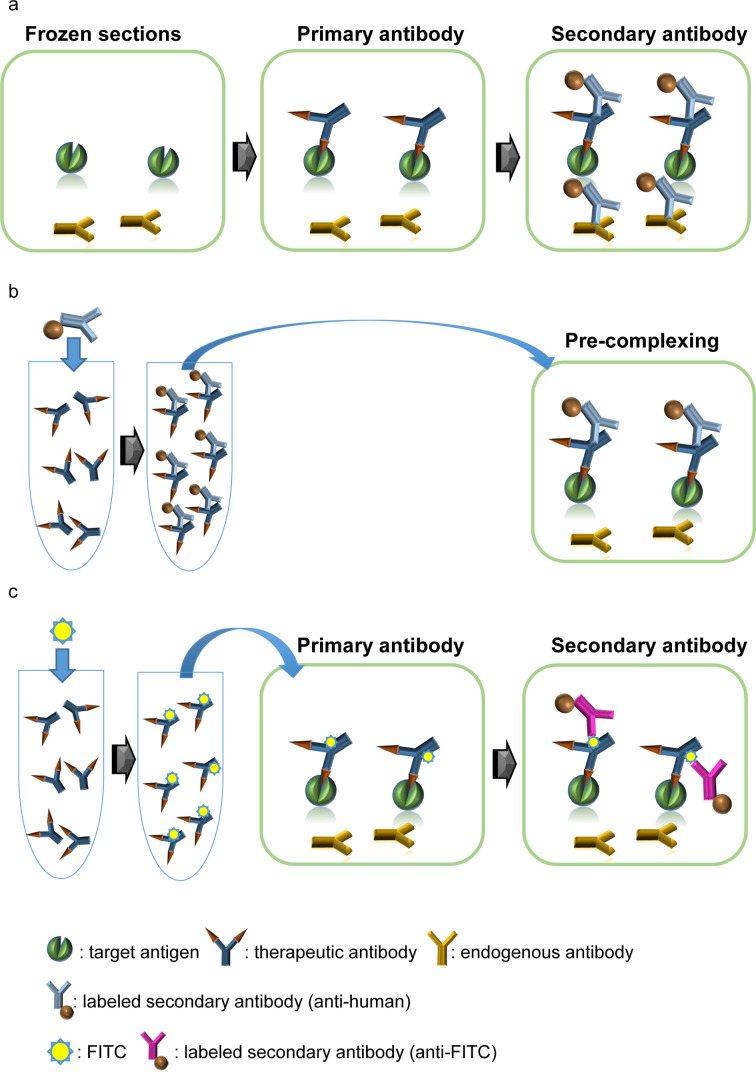
A common issue is that the test article is usually in a human or humanized format. With such antibodies, the specific detection of the test article by an anti-human immunoglobulin secondary antibody in human tissue can be compromised by endogenous human IgG (Fig. 1a). This can be overcome by employing a pre-complexing method: incubating the primary antibody with a secondary antibody before applying the antibody to the tissue (Fig. 1b). The pre-complexing method is effective for “human-on-human” staining in skilled facilities, but application of this method often results in reduced sensitivity compared to conventional methods. An alternative method is to label the primary antibody, which may be effective in retaining the sensitivity of the assay (Fig. 1c).
Fig. 1.
Common technical issues and solutions for human-on-human staining. (a) Interference of endogenous immunoglobulin in the detection of the primary antibody. The test article is usually a humanized or human immunoglobulin. As an anti-human immunoglobulin antibody is used as a secondary antibody with a conventional indirect method, the secondary antibody will bind endogenous immunoglobulins in human tissue. (b) The pre-complexing method. By reacting the primary antibody with the secondary antibody prior to applying to the tissue, it is possible to avoid binding of the secondary antibody to endogenous immunoglobulin. (c) Use of a labeled antibody. A labeled antibody can be detected either directly, or indirectly with a secondary antibody against the labeling agent. FITC: fluorescein isothiocyanate.
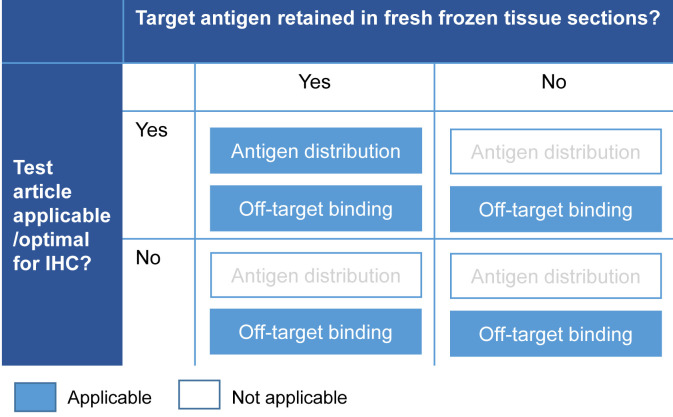
Technical issues in study design are also common (Fig. 2). As therapeutic antibodies are not developed for IHC use, the test article is often unsuitable for IHC3. Similarly, reduced or lost retention of the target antigen in frozen sections can be an issue3. In such cases, although it is possible to evaluate off-target binding, valuable information on the distribution of the target antigen cannot be obtained. Therefore, it may be necessary to incorporate additional methods.
Fig. 2.
Common technical issues in study designs. IHC: immunohistochemistry.
As the features of a target antigen and its therapeutic antibody vary, there is no one-size-fits-all approach. Here, we present some cases that we encountered and discuss some of the technical challenges we faced in conducting TCR studies for the development of therapeutic antibodies.
Case Studies
Case 1: Selecting the optimal antibody for target distribution
The first case was a TCR study for the development of an anti-human tissue factor (TF) antibody4. The test article was a human IgG4 antibody. As endogenous human IgG4 levels in the human body are very low3, 5, 6, a conventional indirect IHC method was judged to be applicable in this case.
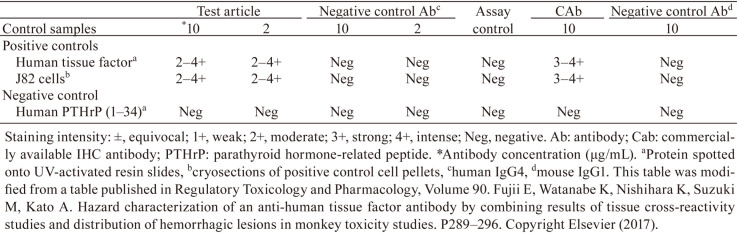
In a preliminary study, we found that the staining intensity in the positive controls was low despite the use of a conventional high-sensitivity detection method. Thus, we designed a study to compare the test article with a commercially available IHC antibody, and found that the IHC antibody detected human TF with robust sensitivity (Table 1). Based on these results, we decided to evaluate any possible binding with the test article and analyse the target distribution with the IHC antibody in human and cynomolgus monkey tissues.
Table 1. Comparison of Staining between a Test Article Antibody and an IHC Antibody for Human Tissue Factor.
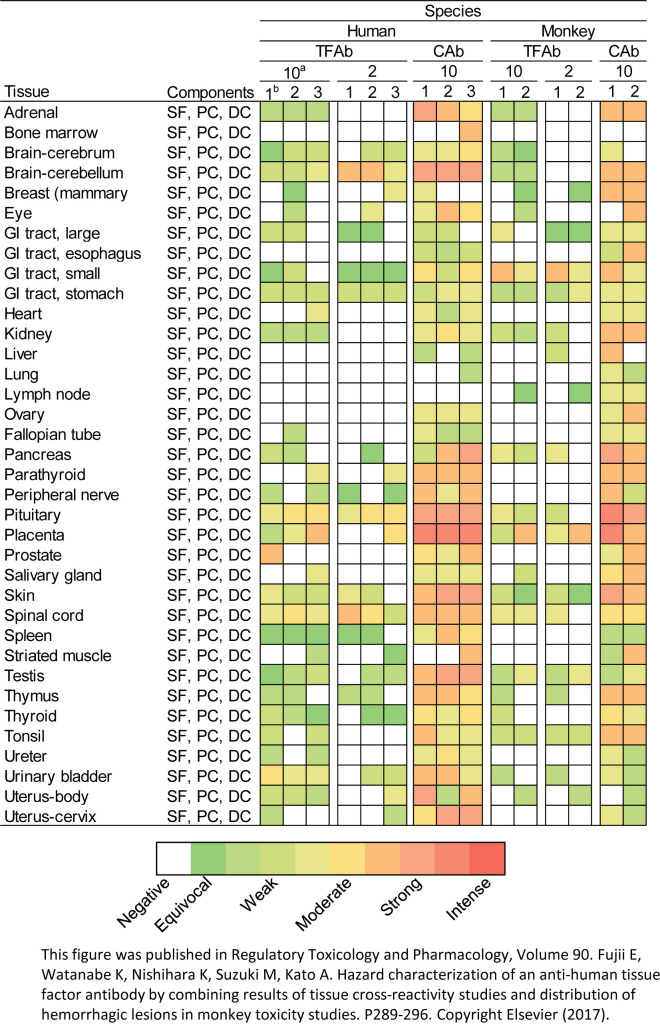
The distribution of positive staining was similar between the test article and the IHC antibody; however, the IHC antibody tended to yield a more consistent staining pattern (Fig. 3). For example, the staining in the heart and urinary bladder with the IHC antibody tended to be more intense and consistent between individuals (Fig. 3). In these organs, the test article was found to cause haemorrhagic lesions in cynomolgus monkey toxicity studies4. From the TCR study, we were able to judge that the binding sites would be similar between cynomolgus monkeys and humans, and thus, the risk of toxicity would also be similar.
Fig. 3.
Results in representative organs from a tissue cross-reactivity study for antibodies against human tissue factor. The level of staining is shown as a heat map in which each square represents one tissue block. All the blocks were obtained from different individuals. a, concentration in μg/mL; b, serial number of each tissue block. TFAb: therapeutic anti-human TF antibody; Cab: commercially available anti-human TF antibody; GI: gastrointestinal; SF: stromal fibroblast; PC: perithelial cell; DC: dendritic cell. This figure was published in Regulatory Toxicology and Pharmacology, Volume 90. Fujii E, Watanabe K, Nishihara K, Suzuki M, Kato A. Hazard characterization of an anti-human tissue factor antibody by combining results of tissue cross-reactivity studies and distribution of hemorrhagic lesions in monkey toxicity studies. P289–296. Copyright Elsevier (2017).
Case 2: FITC labelling to increase sensitivity
The second case was a TCR study for the anti-human interleukin 6 receptor (IL-6R) IgG1 antibody, tocilizumab (TCZ)7. In a preliminary study, we tested the pre-complexing method using the unlabeled antibody. However, as this method yielded no staining in the positive control tissues, we decided to label the test article with fluorescein isothiocyanate (FITC) to boost sensitivity.
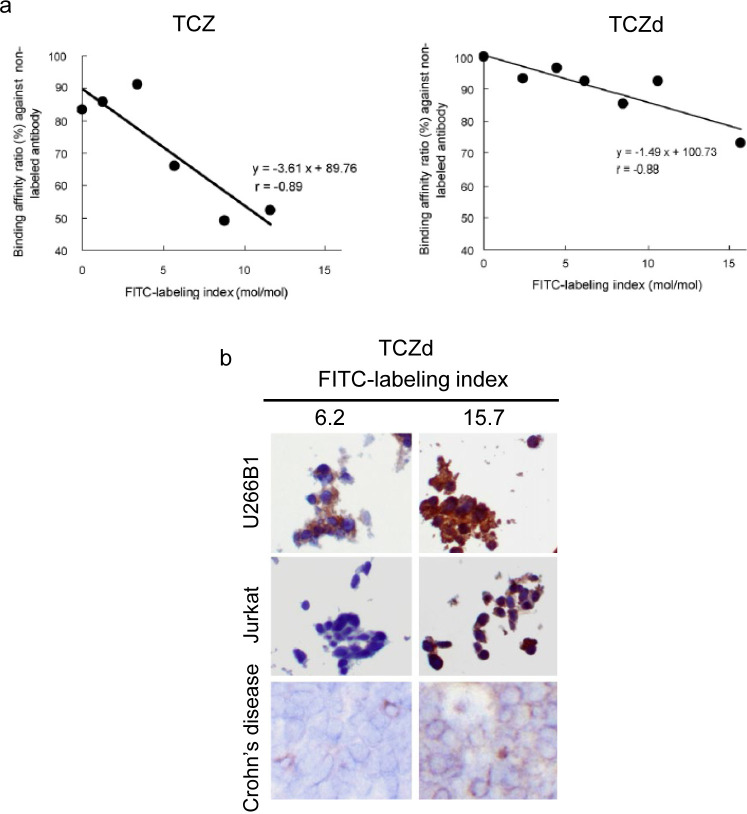
As a labeled antibody is expected to perform differently from an unlabeled antibody7, we first analyzed the correlation between the levels of labeling (labeling index, mol/mol) and binding affinity to the target antigen. In this study, we found that the labeling procedure itself caused the binding affinity to decrease to 80% of its original level. Additionally, we found that increasing the labeling index resulted in a steep drop in binding affinity compared to that of the unlabeled antibody (Fig. 4a).
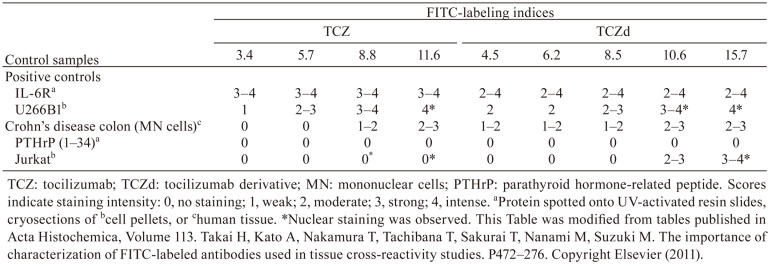
Fig. 4.
Features of labeled antibodies for tocilizumab (TCZ) and a tocilizumab derivative (TCZd). (a) Comparison of binding affinity and FITC-labeling index between TCZ and TCZd. (b) Representative images of staining results for the TCZd in cryosections. U266B1, IL-6R-expressing cells (positive control); Jurkat, IL-6R-negative cells (negative control); mononuclear cells in Crone’s disease colorectal tissue (positive control). This figure was published in Acta Histochemica, Volume 113. Takai H, Kato A, Nakamura T, Tachibana T, Sakurai T, Nanami M, Suzuki M. The importance of characterization of FITC-labeled antibodies used in tissue cross-reactivity studies. P472–276. Copyright Elsevier (2011).
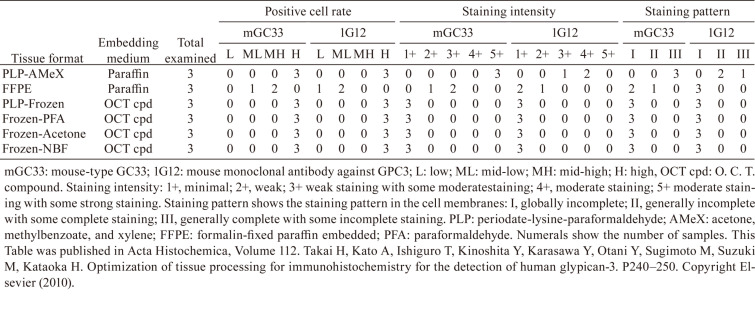
The IHC staining profiles were tested with the same TCZ-FITC antibodies using various positive and negative controls (Table 2). IL-6R protein spots were specifically stained, with no staining in the negative control parathyroid hormone-related peptide (1–34) spots for all tested labeling indices. In the positive control U266B1 cells, staining in the cytoplasm and/or cell membrane was observed with increased intensity, according to the higher labeling indices. However, nonspecific nuclear staining was observed in the negative control Jurkat cells at higher labeling indices. For the positive control Crohn’s disease tissues, staining was observed with higher indices, but no staining was observed with lower indices. Based on these results, we judged that optimal staining conditions could not be achieved.
Table 2. The Staining Intensity of IHC with FITC-labeled Test Article in Control Samples.
These results were compared to those of an IgG2a-type derivative of TCZ (TCZd). We found a less steep drop in binding affinity with FITC labeling (Fig. 4a), and with this antibody, we were able to find staining conditions at the lower labeling indices that could specifically stain the positive controls with no non-specific staining in the negative controls (Fig. 4b, Table 2). This shows that the effects of labeling can vary among antibodies, even when the target antigen is the same.
Although we succeeded in increasing the sensitivity of the assay by labeling the test article, the detection levels were lower than those of an IHC antibody. Therefore, we additionally conducted an antigen distribution study using the IHC antibody and identified several on-target binding sites that could not be detected with the labeled test article8. In this study, there was a discrepancy in staining in the liver between species: positive staining was observed in the human liver, but not in cynomolgus monkeys. This discrepancy is thought to reflect a difference in response to drug treatment between monkeys and humans. There were no test article-related changes in monkey toxicity studies; however, in a clinical trial, there were adverse events related to liver function, including increases in total cholesterol, HDL-C, triglycerides, and liver enzymes. Thus, the TCR results may have been the only pre-clinical information that had the potential to predict toxicity in humans.
Case 3: The influence of tissue format
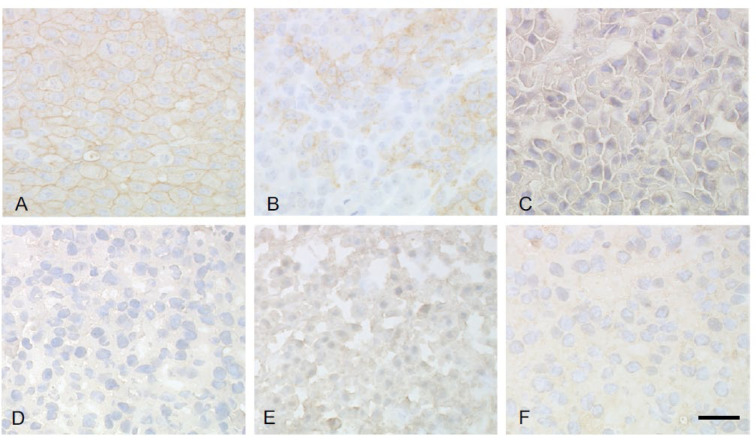
The third case involved a TCR study of an anti-glypican-3 (GPC3) antibody (GC33). Initially, a mouse-type GC33 antibody (mGC33) was tested and compared to a commercially available anti-human GPC3 antibody (1G12) to determine its target distribution in cancer tissues as a potential biomarker9. To this end, various tissue preparation formats, including frozen and formalin-fixed paraffin-embedded (FFPE) tissue, were tested to determine the method that yielded the highest sensitivity for target detection (Table 3). We found that staining of FFPE sections was more robust, but the staining was weaker and less clear in frozen tissue formats (Table 3, Fig. 5). Similar results were obtained for straining with the 1G12 antibody (Table 3). Based on these results, a TCR study was designed to evaluate any possible binding sites by performing a pre-complexing method with GC33 in cryosections, along with the evaluation of target distribution with mGC33 in FFPE sections. Although several binding sites were identified in these studies, there were no signs of toxicity related to the binding sites in humans or cynomolgus monkeys.
Table 3. Variation in Immunoreactivity for Anti-GPC3 Antibodies (GC33, 1G12) with Different Tissue Formats.
Fig. 5.
Representative images of glypican-3 staining with mouse-type GC33 in various tissue formats Periodate-lysine-paraformaldehyde (PLP)-fixed and AMeX (acetone, methyl benozoate, and xylene) method-embedded (A), Formalin-fixed, paraffin embedded (FFPE) (B), PLP fixed-frozen (C), Frozen-paraformaldehyde (PFA) fixed (D), Frozen-acetone fixed (E), Frozen-formalin fixed (F). Bar=30 μm. This figure was published in Acta Histochemica, Volume 112. Takai H, Kato A, Ishiguro T, Kinoshita Y, Karasawa Y, Otani Y, Sugimoto M, Suzuki M, Kataoka H. Optimization of tissue processing for immunohistochemistry for the detection of human glypican-3. P240–250. Copyright Elsevier (2010).
Discussions and Conclusions
We have encountered several technical challenges in conducting TCR studies concerning human-on-human staining including application of the test article to IHC and retention of the target antigen in frozen sections. By carefully considering these issues for each case, it was possible to conduct TCR studies effectively.
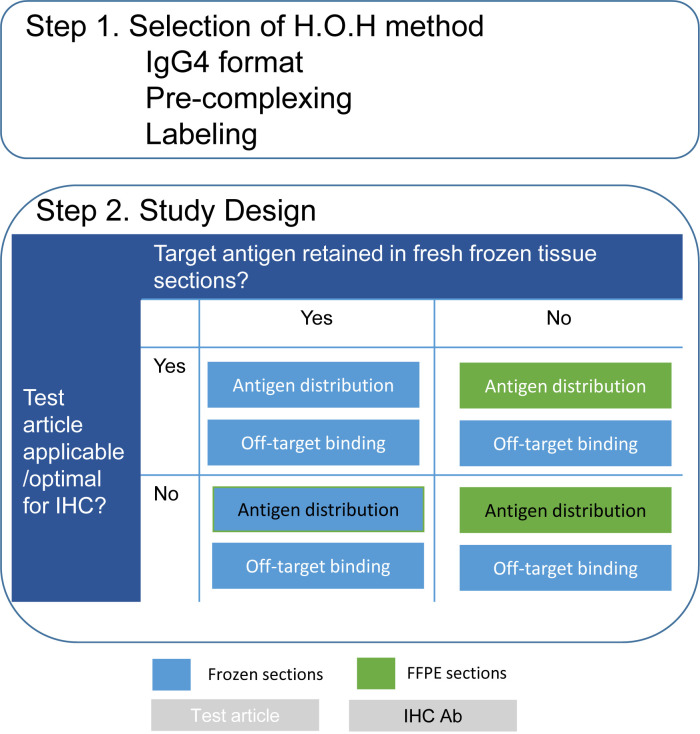
Based on our experience, we propose a scheme for practical points to consider (Fig. 6). The objective of a TCR study is to evaluate any possible binding sites, including on-target binding to the target antigen and off-target binding. First, a human-on-human method to avoid detection of endogenous human immunoglobulin with sufficient sensitivity should be selected (Step 1, Fig. 6). Second, if the therapeutic antibody is not applicable or not optimal for IHC, the use of an alternative sensitive antibody should be considered (Step 2, Fig. 6). Similarly, if the target antigen is not detectable in frozen sections, the use of FFPE sections may be effective (Step 2, Fig. 6). Since the use of an alternative antibody or FFPE sections is mainly for evaluating the distribution of the target antigen, studies on frozen sections with a test article may be necessary to obtain information concerning off-target binding.
Fig. 6.
Proposal of a scheme for practical points to consider in TCR studies. H.O.H.: human-on-human; IHC: immunohistochemistry.
There is an ongoing debate on the significance of TCR data for predicting the effects of antibodies in humans, because organs and tissues that are positive in TCR studies do not necessarily match the target organs of toxicity3, 10, 11. One reason for this discrepancy may be the difficulty in designing IHC assays that sufficiently achieve the objectives of TCR studies. In our experience, we have found that by developing robust assays and case-by-case strategies, information on antigen distribution, in combination with other studies can be utilized to understand toxic effects and assess the relevance between animals and humans.
We anticipate that by considering the points discussed in the current review and combining the data with information on the biological features of the target antigen and therapeutic antibody, safety risks in humans can be predicted with higher accuracy.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.USA Department of Health and Human Services, Food and Drug Administration: Points to consider in the Manufacture and Testing of Monoclonal Antibody Products for Human Use; February 1997.
- 2.ICH(R1) Guideline: Preclinical safety evaluation of biotechnology-derived pharmaceuticals; July 1997. [DOI] [PubMed]
- 3.Leach MW, Halpern WG, Johnson CW, Rojko JL, MacLachlan TK, Chan CM, Galbreath EJ, Ndifor AM, Blanset DL, Polack E, and Cavagnaro JA. Use of tissue cross-reactivity studies in the development of antibody-based biopharmaceuticals: history, experience, methodology, and future directions. Toxicol Pathol. 38: 1138–1166. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Fujii E, Watanabe K, Nishihara K, Suzuki M, and Kato A. Hazard characterization of an anti-human tissue factor antibody by combining results of tissue cross-reactivity studies and distribution of hemorrhagic lesions in monkey toxicity studies. Regul Toxicol Pharmacol. 90: 289–296. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Hall WC, Price-Schiavi SA, Wicks J, and Rojko JL. Tissue cross-reactivity studies for monoclonal antibodies: predictive value and use for selection of relevant animal species for toxicity testing. In: Preclinical Safety Evaluation of Biopharmaceuticals: a Science-Based Approach to Facilitating Clinical Trials. J Cavagnaro (ed). John Wiley and Sons, New Jersey. 207–240. 2008. [Google Scholar]
- 6.Rispens T, and Huijbers MG. The unique properties of IgG4 and its roles in health and disease. Nat Rev Immunol. 23: 763–778. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takai H, Kato A, Nakamura T, Tachibana T, Sakurai T, Nanami M, and Suzuki M. The importance of characterization of FITC-labeled antibodies used in tissue cross-reactivity studies. Acta Histochem. 113: 472–476. 2011. [DOI] [PubMed] [Google Scholar]
- 8.Kato A, Watanabe T, Yamazaki M, Deki T, and Suzuki M. IL-6R distribution in normal human and cynomolgus monkey tissues. Regul Toxicol Pharmacol. 53: 46–51. 2009. [DOI] [PubMed] [Google Scholar]
- 9.Takai H, Kato A, Ishiguro T, Kinoshita Y, Karasawa Y, Otani Y, Sugimoto M, Suzuki M, and Kataoka H. Optimization of tissue processing for immunohistochemistry for the detection of human glypican-3. Acta Histochem. 112: 240–250. 2010. [DOI] [PubMed] [Google Scholar]
- 10.Bussiere JL, Leach MW, Price KD, Mounho BJ, and Lightfoot-Dunn R. Survey results on the use of the tissue cross-reactivity immunohistochemistry assay. Regul Toxicol Pharmacol. 59: 493–502. 2011. [DOI] [PubMed] [Google Scholar]
- 11.MacLachlan TK, Price S, Cavagnaro J, Andrews L, Blanset D, Cosenza ME, Dempster M, Galbreath E, Giusti AM, Heinz-Taheny KM, Fleurance R, Sutter E, and Leach MW. Classic and evolving approaches to evaluating cross reactivity of mAb and mAb-like molecules—a survey of industry 2008-2019. Regul Toxicol Pharmacol. 121: 104872. 2021. [DOI] [PubMed] [Google Scholar]