Abstract
A transient transfection-fusion assay was established to investigate membrane fusion mediated by pseudorabies virus (PrV) glycoproteins. Plasmids expressing PrV glycoproteins under control of the immediate-early 1 promoter-enhancer of human cytomegalovirus were transfected into rabbit kidney cells, and the extent of cell fusion was quantitated 27 to 42 h after transfection. Cotransfection of plasmids encoding PrV glycoproteins B (gB), gD, gH, and gL resulted in formation of polykaryocytes, as has been shown for homologous proteins of herpes simplex virus type 1 (HSV-1) (A. Turner, B. Bruun, T. Minson, and H. Browne, J. Virol. 72:873–875, 1998). However, in contrast to HSV-1, fusion was also observed when the gD-encoding plasmid was omitted, which indicates that PrV gB, gH, and gL are sufficient to mediate fusion. Fusogenic activity was enhanced when a carboxy-terminally truncated version of gB (gB-008) lacking the C-terminal 29 amino acids was used instead of wild-type gB. With gB-008, only gH was required in addition for fusion. A very rapid and extended fusion was observed after cotransfection of plasmids encoding gB-008 and gDH, a hybrid protein consisting of the N-terminal 271 amino acids of gD fused to the 590 C-terminal amino acids of gH. This protein has been shown to substitute for gH, gD, and gL function in the respective viral mutants (B. G. Klupp and T. C. Mettenleiter, J. Virol. 73:3014–3022, 1999). Cotransfection of plasmids encoding PrV gC, gE, gI, gK, and UL20 with gB-008 and gDH had no effect on fusion. However, inclusion of a gM-expressing plasmid strongly reduced the extent of fusion. An inhibitory effect was also observed after inclusion of plasmids encoding gM homologs of equine herpesvirus 1 or infectious laryngotracheitis virus but only in conjunction with expression of the gM complex partner, the gN homolog. Inhibition by PrV gM was not limited to PrV glycoprotein-mediated fusion but also affected fusion induced by the F protein of bovine respiratory syncytial virus, indicating a general mechanism of fusion inhibition by gM.
Virus-cell fusion events mediated by viral membrane glycoproteins constitute a crucial primary step in the infectious cycle of all enveloped viruses. All known viral fusion proteins or protein complexes have common features. They are composed of one or two type I integral membrane glycoproteins, contain extended ectodomains carrying N-linked carbohydrates, form higher-order oligomers, are present on the viral membrane at high surface density, and contain a fusion peptide in a membrane-anchored subunit. Many viral fusion proteins are tight complexes of two glycoprotein subunits that confer binding as well as fusion activity, and many are made as larger precursors which require proteolytic activation of their fusogenic potential (16). In general, it is suggested that a triggered conformational change exposes a previously cryptic fusion peptide, which is then able to insert into the lipid bilayer and thus initiate the fusion reaction by disturbing the membrane integrity. The best-studied example is fusion mediated by hemagglutinin (HA) of influenza virus. Although the crystal structures for both neutral and pH-activated forms of HA are known, the molecular mechanism underlying the merger of two membranes in HA-mediated fusion is still unclear (6).
Herpesviruses encode numerous different envelope glycoproteins whose functions are only slowly becoming clearer. In the alphaherpesviruses herpes simplex virus type 1 (HSV-1) and pseudorabies virus (PrV), 11 glycoproteins have been identified and characterized to some extent (26, 27, 37). gC and gD have been found to bind cell surface receptors (11, 27, 37, 39). Virus mutants lacking glycoprotein gB, gD, gH, or gL are unable to penetrate target cells, a defect which can be at least partially overcome by treatment with an artificial fusogen, polyethylene glycol, indicating that these proteins are involved in the fusion process (reviewed in references 27 and 37). However, no classical viral fusion protein has been identified for any member of the herpesviruses, although there are reports that constitutive expression of gB or gD in transgenic cells does increase polykaryocyte formation (4, 5). Glycoprotein gB, a highly conserved protein present in all subfamilies of the herpesviruses, is one of the most abundant proteins in the viral membrane and exhibits many features described for fusion proteins: it is a homodimeric type I N-glycosylated membrane protein, which in most herpesviruses is cleaved by a cellular protease into two disulfide-linked subunits (33). Sequence alignments of a hydrophobic region located near the transmembrane domain revealed intriguing similarity with known fusion peptides (35). However, attempts to induce cell fusion solely with gB were inconclusive, indicating either that gB is not the fusion protein or that fusion requires an additional viral protein(s).
Recently, in a transient expression-fusion assay, gB, gD, and the gH-gL complex of HSV-1 have been found to be necessary and sufficient to mediate membrane fusion in a Cos cell transfection system (38). This was the first successful assay to measure alphaherpesvirus-induced membrane fusion in the absence of virus infection. The data showed that all four proteins have to be present for fusion to occur. To establish common and divergent features in membrane fusion induced by alphaherpesviral glycoproteins, we established a fusion assay with PrV glycoproteins and analyzed the contribution of different viral glycoproteins to the fusion process.
MATERIALS AND METHODS
Construction of expression plasmids.
All PrV genes were derived from strain Kaplan (20). The open reading frame (ORF) for gB was amplified by PCR with specific primers (29) and cloned as a BamHI-EcoRI fragment into appropriately cleaved plasmid pcDNA3 (In Vitrogen, Groningen, The Netherlands) (29). pgB-008 expresses a C-terminally truncated derivative of gB which lacks the 29 C-terminal amino acids (29). Plasmid gDgI-CMV contains the bicistronic expression unit encompassing the gD and gI genes which are transcribed into a single mRNA (12). The gH ORF was amplified by PCR of PrV DNA using specific primers and ligated as an EcoRI-XhoI fragment into appropriately cleaved vector pcDNA3. The gL ORF was excised with BspEI and PstI from cloned fragment BamHI-6 and ligated into vector pRc/CMV (In Vitrogen). The gDH ORF was PCR amplified with specific primers from viral PrV-ΔgLPass DNA (23) and cloned via the EcoRI and XhoI sites into pcDNA3. The PrV gM (UL10) ORF was amplified with specific primers from cloned BamHI fragment 3 and inserted into pcDNA3. Construction of expression plasmids (vectors pcDNA3 and pRC/CMV) coding for the other (glyco)proteins tested has been described recently for UL20 and gK (8), gE and gI (2), gC (12), and gN (19). Plasmid DNA was prepared with the plasmid maxiprep kit (Qiagen, Hilden, Germany). Expression of proteins was verified by establishing stable cell lines and transcomplementation of corresponding deletion mutants and/or immunofluorescence with specific sera or monoclonal antibodies on stably or transiently transfected cells.
Plasmids encoding equine herpesvirus 1 (EHV-1) gM, EHV-1 gN, infectious laryngotracheitis virus (ILTV) UL10 (10), and ILTV UL49.5 were generously provided by Klaus Osterrieder, Christian Seyboldt, and Walter Fuchs (Insel Riems, Germany). A plasmid expressing a synthetic gene for the bovine respiratory syncytial virus (BRSV) F protein (Fsyn), in which putative splice sites were eliminated and the GC content was enhanced, was generously provided by Patricia König and Günther Keil (Insel Riems). All genes were expressed under the control of the immediate-early 1 promoter-enhancer complex of human cytomegalovirus (HCMV) except the Fsyn gene, which was cloned downstream of the murine CMV (MCMV) early promoter. To induce F protein expression, a plasmid encoding MCMV immediate-early protein 1 had to be cotransfected (P. König and G. Keil, personal communication).
Fusion assays.
Rabbit kidney (RK13) cells were seeded at approximately 8 × 105 cells per well in six-well culture dishes. Transfections were performed with SuperFect transfection reagent (Qiagen) using 2 μg of each glycoprotein-encoding plasmid. The amount of DNA was equalized by adding appropriate amounts of pcDNA3 vector. DNA was mixed in 150 μl of minimal essential medium without serum, and 10 μl of SuperFect reagent was added and mixed. After 10 min of incubation at room temperature, 830 μl of medium containing 10% fetal calf serum was added, and the transfection mixture was dispersed in duplicate wells onto the cell monolayer. Cells were incubated at 37°C for 18 h. Thereafter, the transfection mixture was replaced by medium with 10% fetal calf serum, and cells were further incubated at 37°C for 9 to 24 h as indicated. They were then fixed with 80% ethanol and incubated with a monoclonal antibody (MAb) directed against gB (A20-c26) (29) or a MAb directed against the BRSV F protein (kindly provided by G. Taylor, Compton, United Kingdom) followed by incubation with anti-mouse immunoglobulin G-fluorescein isothiocyanate conjugate (Daco Diagnostics, Hamburg, Germany).
Quantitation of fusion.
Nuclei in 100 polykaryocytes per assay were counted microscopically by two independent observers in two independent assays. Polykaryocytes were defined as cells containing more than one nucleus. Average values and standard deviation were then calculated.
RESULTS
Establishment of a transient expression-fusion assay.
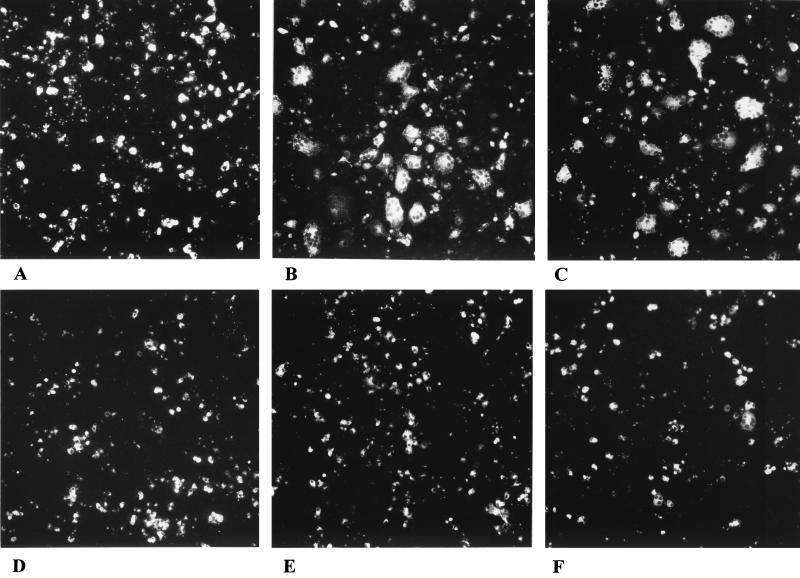
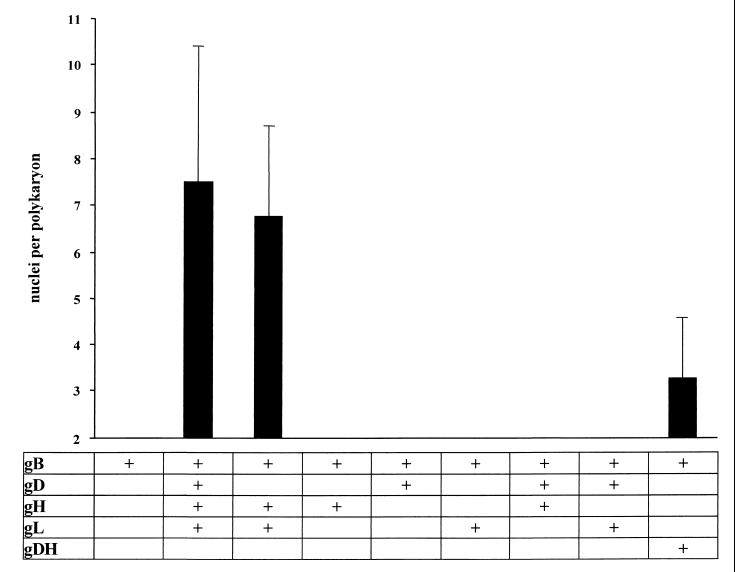
To investigate fusion induced by PrV glycoproteins, plasmids encoding gB, gD, gH, and gL were cotransfected into RK13 cells, and fusion was analyzed 42 h after transfection. As shown in Fig. 1A, after transfection of only the gB-expressing plasmid, fluorescent single cells and no polykaryocytes were observed, which indicates that gB alone is not sufficient to promote cell fusion. In contrast, cotransfection of plasmids coding for gB, gD, gH, and gL resulted in cell-cell fusion, demonstrated by emergence of polynucleated cells (Fig. 1B). Thus, in this system, expression of these four viral glycoproteins is sufficient to induce fusion of RK13 cells, although not all fluorescing cells were involved in syncytia. Polykaryon formation was also observed when the gD expression plasmid was omitted (Fig. 1C). Thus, gD is not required for fusion in the PrV system, which mimics the situation found for direct cell-to-cell spread during virus infection. While gD is dispensable for direct cell-to-cell spread of PrV, this process does not occur in HSV-1 in the absence of gD (25, 32, 34). In contrast, in the absence of gL (Fig. 1D and E) or gH (Fig. 2), fusion did not occur. Also, we never observed any fusion in the absence of gB (data not shown). When plasmids encoding gD, gH, and gL were replaced with a plasmid encoding the gDH hybrid protein (Fig. 1F) obtained from PrV-ΔgLPass (23), formation of polykaryocytes was also observed, although only at a low level (Fig. 2). The gDH protein has been shown to compensate for the absence of gL, but also complements the gD and gH defects in the respective viral mutants (23). These results were quantitated by counting nuclei in 100 polykaryocytes per assay in duplicate assays by two independent observers. Data are shown in Fig. 2.
FIG. 1.
PrV glycoprotein-mediated cell fusion. RK13 cells were transfected with plasmids coding for gB (A); gB, gD, gH, and gL (B); gB, gH, and gL (C); gB and gH (D); gB, gD, and gH (E); or gB and gDH (F). Cells were microscopically examined, and pictures were taken 42 h posttransfection. Magnification, ×65.
FIG. 2.
Quantitation of PrV glycoprotein-mediated cell fusion. As a measurement for the extent of fusion induced after transfection of expression plasmids for gB and the indicated glycoproteins, nuclei within 100 polykarya per assay were counted in two parallel assays by two independent observers, and average values and standard deviations were calculated.
Enhancement of fusion by C-terminally truncated gB.
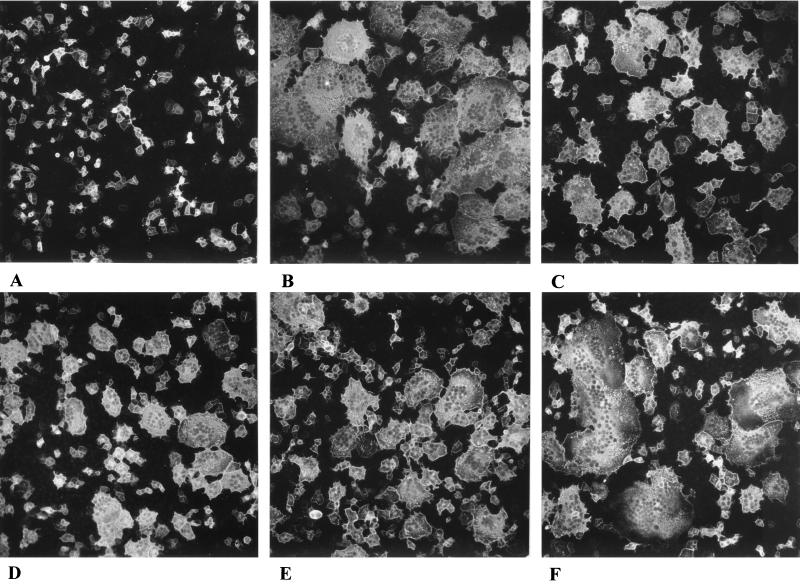
Although fusion was readily observed in our assay system, we tried to enhance fusion by inclusion of a plasmid encoding a C-terminally truncated version of gB, gB-008, in which the C-terminal 29 amino acids were deleted. A similar deletion in HSV-1 gB had been shown to cause a syncytial phenotype, indicative of increased fusogenic activity (1). Cells expressing gB-008 complement gB-negative PrV mutants, but plaques exhibit a syncytial phenotype (29). The results are shown in Fig. 3 and summarized in Fig. 4.
FIG. 3.
C-terminally truncated gB enhances fusion. RK13 cells were transfected with plasmids coding for gB-008 (A); gB-008, gD, gH, and gL (B); gB-008, gH, and gL (C); gB-008 and gH (D); gB-008, gD, and gH (E); or gB-008 and gDH (F). Cells were examined microscopically and pictures were taken 36 h posttransfection. Magnification, ×65.
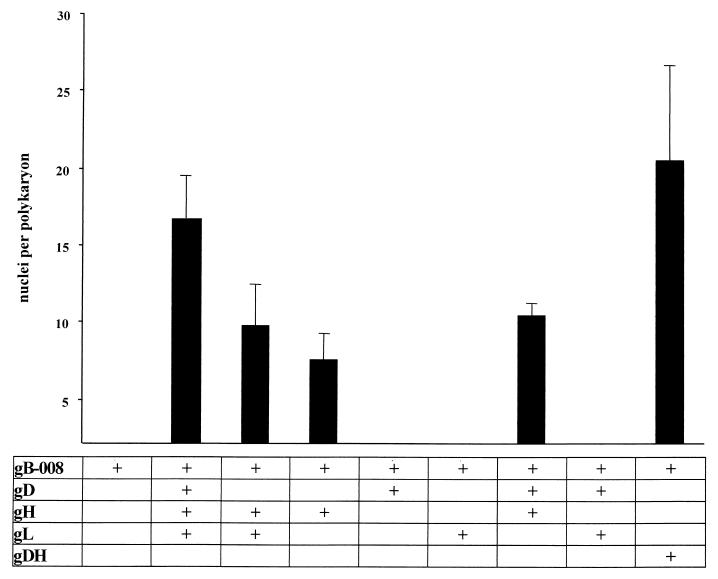
FIG. 4.
Quantitation of fusion mediated by C-terminally truncated gB. After transfection of expression plasmids for gB-008 and the indicated glycoproteins, fusion was quantitated as described in the legend to Fig. 2.
Fusion is enhanced after expression of gB-008 (substituting for gB), gD, gH, and gL (Fig. 3B) compared to transfections of gB, gD, gH, and gL (Fig. 1B). Transfection of gB-008 alone did not result in the formation of polynucleated cells (Fig. 3A), indicating that, like gB, gB-008 alone is not sufficient to mediate cell-cell fusion. Omission of the gD-expressing plasmid (Fig. 3C) reduced fusion (Fig. 4), but polykaryocyte formation still occurred efficiently. Surprisingly, fusion was also observed when gL was omitted from the assay (Fig. 3E) or when only gB-008 and gH were expressed (Fig. 3D). Interestingly, expression of gB-008 and gDH led to extended fusion (Fig. 3F).
Thus, the mutated gB and gH together seem to be sufficient to trigger fusion, i.e., the merger of membranes. In all our assays, we never detected fusion activity in the absence of either gB (mutated or wild type) or gH. This again is in good agreement with data obtained with virus mutants.
Requirement for gB and gH in cis or trans for fusion.
Next we tested whether gB and gH have to be present on the same membrane (in cis) to induce fusion or whether fusion also occurs when both are present on separate plasma membranes (in trans). To this end, cells were transfected either with the plasmid expressing gB-008 or with the gH expression vector. One day posttransfection, cells were trypsinized and mixed in different ratios. At no time after coseeding was fusion observed. In contrast, when expression plasmids for gB-008 and gH were cotransfected, and transfected and nontransfected cells were subsequently trypsinized and mixed, numerous polynucleated cells developed (data not shown). This indicates that both proteins have to be present on the same membrane in cis to induce fusion, which has also been postulated for HSV-1-induced fusion (7).
Effect of expression of other membrane (glyco)proteins on fusion.
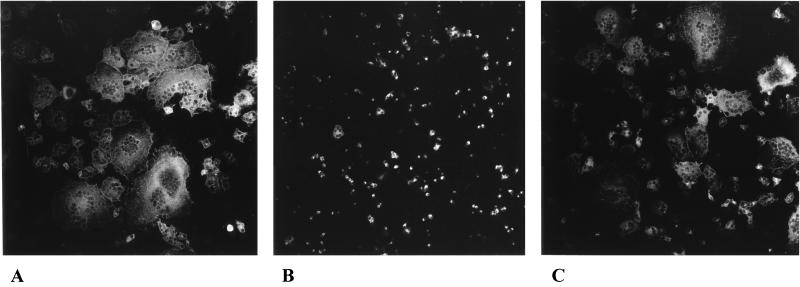
Studies with viral mutants revealed that in HSV-1 and PrV gB, gD, gH, and gL are required for penetration. The same proteins are essential for direct cell-to-cell spread of HSV-1, while in PrV gD is not necessary for this process (25, 32, 34). The essential glycoprotein gK is involved in cell-cell spread but not in penetration (17, 18, 22), and mutants lacking gM, gE, and gI are deficient in polykaryocyte formation, implying a role for these proteins in plasma membrane fusion but not in virion entry (7, 38). Since requirements for fusion in our transfection-fusion assay parallel those for cell-to-cell spread rather than those for penetration, it was of interest to analyze whether any of the other known PrV membrane (glyco)proteins has an effect on fusion. Therefore, cotransfections were performed using expression plasmids for gB-008 and gDH, which resulted in the most extensive fusion, and different combinations of plasmids encoding gC, gE, gI, gK, gM, gN, or UL20. No or only slight inhibition of fusion activity was observed after cotransfection with gC, gE, gK, gN, or UL20 plasmids; with gE and gI, which form a complex (44); or with gK and UL20, which have been shown to functionally interact (8) (data not shown). However, cotransfection with a gM-expressing plasmid resulted in drastically reduced fusion (Fig. 5B). Only a very few polykaryocytes were detectable, in contrast to extensive fusion after cotransfection of gB-008 and gDH expression plasmids without gM (Fig. 5A) or with a gN expression plasmid instead of gM (Fig. 5C). Quantitation is shown in Fig. 6.
FIG. 5.
Glycoprotein M inhibits fusion. RK13 cells were transfected with plasmids coding for gB-008 and gDH (A); gB-008, gDH, and gM (B); or gB-008, gDH, and gN (C). Pictures were taken 27 h after transfection. Magnification, ×65.
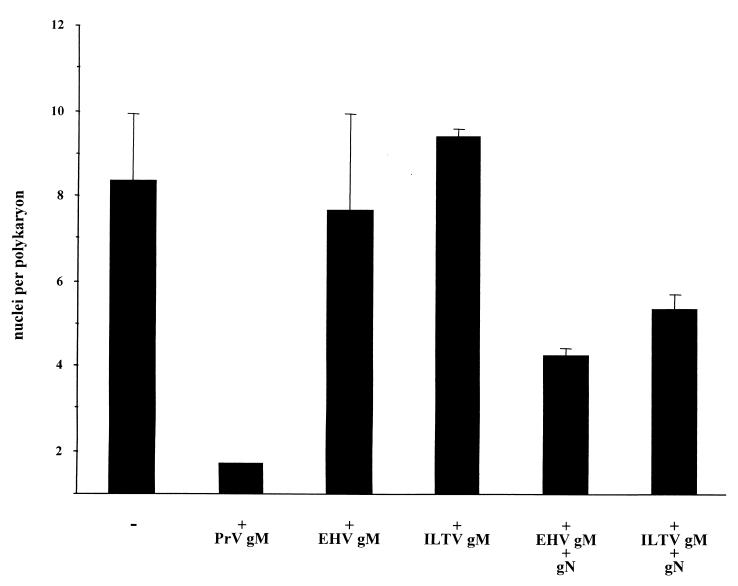
FIG. 6.
Quantitation of fusion inhibition by gM. Cells were cotransfected with expression plasmids for gB-008, gDH, and the indicated glycoproteins. Extent of fusion was analyzed as described in the legend to Fig. 2.
Inhibition of membrane fusion by gM and gN homologs of other herpesviruses.
To test whether the observed inhibition of membrane fusion is specific for PrV gM, we replaced PrV gM with the homologs from EHV-1 (gM) or ILTV (UL10). The gM-homologous ILTV UL10 gene product is not glycosylated and therefore cannot be designated gM (10). Inclusion of these plasmids had no inhibitory effect on fusion (Fig. 6). Since it had been shown for different herpesviruses that gM forms a complex with gN, the product of the UL49.5 gene (19, 24, 42), we tested the effect of cotransfection of both protein-encoding plasmids. Inclusion of the gN homologs with the gM of EHV-1 or ILTV (Fig. 6) reduced fusion, although not as efficiently as PrV gM alone. For PrV, the inclusion of gN in addition to gM did not influence the result.
Inhibitory effect of gM on fusion is not herpesvirus specific.
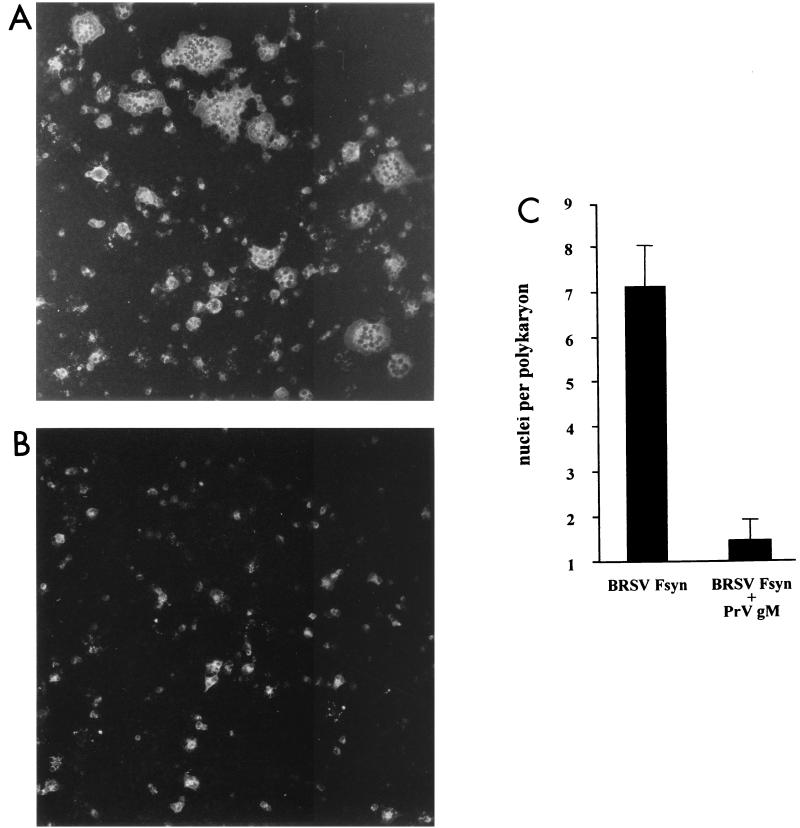
To investigate whether the inhibition of fusion by PrV gM is limited to fusion induced by herpesviral glycoproteins, a plasmid directing expression of a synthetic ORF encoding the F protein of BRSV was transfected into RK13 cells. For successful expression of the F protein via the nucleus, putative splice sites had been deleted and the original GC content had been raised (König and Keil, personal communication). Transient expression of this protein led to significant cell fusion (Fig. 7A), as expected from this bona fide fusion protein. Interestingly, after inclusion of the PrV gM-encoding plasmid (Fig. 7B), fusion was again drastically reduced (Fig. 7C). This result implies that fusion inhibition by gM is a general mechanism and not specific for fusion induced by herpesvirus glycoproteins.
FIG. 7.
BRSV F protein-induced fusion is inhibited by PrV gM. RK13 cells were transfected with Fsyn plasmid either with the inducer plasmid only (A) or in combination with gM (B). Fluorescing cells were examined, and pictures were taken 42 h after transfection. Magnification, ×100. (C) For quantitation, the average number of nuclei per polykaryon was determined by two independent observers.
DISCUSSION
During herpesvirus infection, several membrane fusion processes occur. For entry, the virion envelope fuses with the plasma membrane. Intranuclear capsids bud through the inner nuclear membrane into the perinuclear cisterna, which requires fusion of the inner nuclear membrane to give rise to a primary viral envelope. As shown for varicella-zoster virus and PrV, by subsequent fusion with the outer nuclear membrane or the membrane of the endoplasmic reticulum, naked capsids are released into the cytoplasm. Secondary envelopment takes place in proximity to trans-Golgi vesicles, which again involves membrane fusion. Mature virus particles are transported in vesicles to the plasma membrane and are released by fusion of vesicle and plasma membranes (13, 14, 40, 43). A similar mechanism has recently also been suggested for HSV-1 (3, 41). Direct transfer of infectivity from primary infected cells to adjacent uninfected neighbors, which results in plaque formation in cell culture, is also thought to involve limited fusion between the plasma membranes of the adjoining cells. Despite the importance of membrane fusion events during herpesvirus infection, the mechanisms guaranteeing controlled and directional fusion are only poorly understood.
For HSV-1, gB, gD, gH, and gL are necessary for entry and cell-to-cell spread, suggesting similar or even identical requirements for both processes. However, in PrV these membrane fusion events can be differentiated, since gD is essential for entry, whereas cell-to-cell spread occurs efficiently in the absence of gD in vitro and in vivo (15, 28, 32, 34). The transient transfection-fusion system described here resembles cell-to-cell spread in its requirements since fusion occurs efficiently in the absence of gD, while for the HSV-1 system all four wild-type proteins have be present (38). Thus, the in vitro fusion assay mimics the natural process during virus infection and demonstrates that PrV gB, gH, and gL are necessary and sufficient to mediate fusion.
Using mutated glycoproteins, either the hybrid gDH in combination with gB or C-terminally truncated gB-008 in combination with gH, we were able to induce fusion with only two viral components. The complexity of the fusion system has thus been reduced to a level which appears to be more amenable to detailed biochemical analyses than those involving three or four functional units. In both two-component fusion systems in PrV, gL is not required for induction of fusion. This may not be surprising, since a gL-negative mutant is able to directly spread from cell to cell, although to a very limited extent (21). gL forms a complex with gH, and gL is anchored in the membrane via this interaction (21, 36). However, whereas in HSV-1 gL is required for virion localization of gH, PrV gH is incorporated into virions in the absence of gL. PrV virions lacking gL are noninfectious, a defect which can be overcome by polyethylene glycol-induced fusion, indicating that gL plays an essential role in penetration but is less important for cell-to-cell spread, which further separates the two processes. It will be interesting to test whether a viral mutant expressing gB-008 instead of full-length gB is able to infect cells in a gL-independent manner.
The limited ability of the PrV gL− mutant for direct cell-to-cell spread was used for passaging, which resulted in the isolation of a mutant virus which proved to be infectious even in the absence of gL. In this PrV-ΔgLPass mutant, a protein consisting of the 271 N-terminal amino acids of gD fused to the 590 C-terminal amino acids of gH compensates for the lack of gL. Moreover, the gDH hybrid protein was shown to substitute for gH, gD, or gL and also for simultaneous lack of gD and gH (23). In our fusion assay, gDH also functionally substituted for gH and gL. Thus, gL function can be compensated for by alterations in gH. For induction of fusion, gDH requires only gB as an additional viral component. The most fusogenic combination in our assays was gDH and gB-008, which resulted in rapid and extensive fusion. This high fusogenic activity in combination with gB-008 may suggest that gL and/or the gL-binding domain of gH, which seems to be lost in gDH, negatively controls and restricts fusion. We never observed fusion when plasmids expressing either gB or gH or modified versions thereof were omitted, which parallels the inability to isolate any gB- or gH-negative infectious mutants. Thus, these two proteins appear to be central to the fusion process.
Deletion mutants of glycoproteins gE, gI, gM, gK, and the putative membrane protein encoded by the UL20 gene exhibit a decrease in plaque size, which could indicate a role in fusion. However, inclusion of gE or gE plus gI, which form a noncovalently linked complex (44), or gK, UL20, or gK and UL20, which functionally interact (8), had no obvious effect on the extent of fusion. In contrast, inclusion of a PrV gM-expressing plasmid provoked a drastic inhibition of fusion compared to controls without gM. Thus, PrV gM negatively influences fusion in this assay. gM is one of the few nonessential glycoproteins which is conserved in the Herpesviridae. Deletion of gM in wild-type PrV resulted in only a slight decrease in plaque size and a ca. 100-fold reduction in viral titers (9). In contrast, we recently noted that deletion of gM from a gE/gI-negative virus mutant caused accumulations of nucleocapsids in association with tegument protein, and only very few virus particles were released. This correlated with a drastically reduced capacity to spread directly from cell to cell. Thus, we suggested that gE-gI and gM may have overlapping functions in late stages of virion morphogenesis, prior to final envelopment (2). As concerns the inhibitory function of gM in our fusion assays, none of our gM deletion mutants exhibited signs of deregulated fusion. The role of gM in the in vitro fusion assay with only a limited number of specific viral components thus appears to be different from the situation during viral replication, in which an abundance of viral proteins which are involved not only in fusion but also, e.g., in virion morphogenesis, is expressed in a coordinated manner. Therefore, the role of gM during virus infection may be more complex than measured in the fusion assay.
Still, inhibition of fusion by gM seems to be a function which is conserved at least within the alphaherpesviruses, since a reduction in PrV glycoprotein-induced cell fusion was also observed after addition of plasmids encoding gM homologs from EHV-1 or ILTV. However, in contrast to PrV, for these two viruses the product of the UL49.5 gene, which forms a complex with gM, had to be coexpressed. In PrV (19), Epstein-Barr virus (24), and ILTV (W. Fuchs, personal communication), the UL49.5 gene product is glycosylated and was named gN. Virion localization of PrV gN is dependent on the presence of gM, but gM is found properly processed in the envelope of a gN-negative PrV mutant (19). In contrast, EHV-1 and ILTV gM is not properly processed and transported in the absence of gN (30; Fuchs, personal communication). Thus, the requirements for gN in in vitro fusion inhibition are similar to those observed for gM processing in virus-infected cells.
Surprisingly, gM inhibited not only fusion induced by PrV glycoproteins but also fusion mediated by the BRSV F protein (31). gM is predicted to be a highly hydrophobic protein, consisting of seven or eight transmembrane domains. It is conceivable that this tight integration of gM into membranes reduces their fluidity and consequently their ability to fuse. Thus, the biochemical properties of membranes after insertion of herpesvirus gM proteins need to be closely analyzed.
ACKNOWLEDGMENTS
We thank Walter Fuchs, Volker Gerdts, Günther Keil, Alice Kollert, Patricia König, Klaus Osterrieder, and Christian Seyboldt from the Federal Research Centre for Virus Diseases of Animals for generously providing expression plasmids and Geraldine Taylor, Compton, United Kingdom, for the anti-F antibody. The expert technical assistance of Uta Hartwig and Nadine Müller is gratefully acknowledged.
This work was supported by the Deutsche Forschungsgemeinschaft (grant Me 854/4-1).
REFERENCES
- 1.Baghian A, Huang L, Newman S, Jayachandra S, Kousoulas K. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J Virol. 1993;67:2396–2401. doi: 10.1128/jvi.67.4.2396-2401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brack A R, Dijkstra J M, Granzow H, Klupp B G, Mettenleiter T C. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J Virol. 1999;73:5364–5372. doi: 10.1128/jvi.73.7.5364-5372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne H, Bell S, Minson T, Wilson D. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher M, Raviprakash K, Ghosh H P. Acid pH-induced fusion of cells by herpes simplex virus glycoproteins. J Biol Chem. 1990;265:5862–5868. [PubMed] [Google Scholar]
- 5.Campadelli-Fiume G, Avitabile E, Fini S, Stirpe D, Arsenakis M, Roizman B. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology. 1988;166:598–602. doi: 10.1016/0042-6822(88)90533-8. [DOI] [PubMed] [Google Scholar]
- 6.Chernomordik L, Kozlov M M, Zimmerberg J. Lipids in biological membrane fusion. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- 7.Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–7590. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietz P, Klupp B G, Weiland E, Köllner B, Mettenleiter T C. Pseudorabies virus glycoprotein K requires the UL20 gene product for processing. J Virol. 2000;74:5083–5090. doi: 10.1128/jvi.74.11.5083-5090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkstra J M, Mettenleiter T C, Klupp B G. Intracellular processing of pseudorabies virus glycoprotein M (gM): gM of strain Bartha lacks N-glycosylation. Virology. 1997;237:113–122. doi: 10.1006/viro.1997.8766. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs W, Mettenleiter T C. DNA sequence of the UL6 to UL20 genes of infectious laryngotracheitis virus and characterization of the UL10 gene product as a nonglycosylated and nonessential virion protein. J Gen Virol. 1999;80:2173–2182. doi: 10.1099/0022-1317-80-8-2173. [DOI] [PubMed] [Google Scholar]
- 11.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 12.Gerdts V, Jöns A, Mettenleiter T C. Potency of an experimental DNA vaccine against Aujeszky's disease in pigs. Vet Microbiol. 1999;66:1–13. doi: 10.1016/s0378-1135(98)00300-9. [DOI] [PubMed] [Google Scholar]
- 13.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granzow H, Weiland F, Jöns A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heffner S, Kovacs F, Klupp B G, Mettenleiter T C. Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J Virol. 1993;67:1529–1537. doi: 10.1128/jvi.67.3.1529-1537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez L D, Hoffmann L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson L, Goldsmith K, Snoddy D, Gosh H, Graham F L, Johnson D C. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J Virol. 1992;66:5603–5609. doi: 10.1128/jvi.66.9.5603-5609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayachandra S, Baghian A, Kousoulas K G. Herpes simplex virus type 1 glycoprotein K is not essential for infectious virus production in actively replicating cells but is required for efficient envelopment and translocation of infectious virions from the cytoplasm to the extracellular space. J Virol. 1997;71:5012–5024. doi: 10.1128/jvi.71.7.5012-5024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jöns A, Dijkstra J, Mettenleiter T C. Glycoproteins M and N of pseudorabies virus form a disulfide-linked complex. J Virol. 1998;72:550–557. doi: 10.1128/jvi.72.1.550-557.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan A S, Vatter A E. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959;7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 21.Klupp B G, Fuchs W, Weiland E, Mettenleiter T C. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J Virol. 1997;71:7687–7695. doi: 10.1128/jvi.71.10.7687-7695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klupp B G, Baumeister J, Dietz P, Granzow H, Mettenleiter T C. Pseudorabies virus glycoprotein gK is a virion structural component involved in virus release but is not required for entry. J Virol. 1998;72:1949–1958. doi: 10.1128/jvi.72.3.1949-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klupp B G, Mettenleiter T C. Glycoprotein gL-independent infectivity is mediated by a gD-gH fusion protein. J Virol. 1999;73:3014–3022. doi: 10.1128/jvi.73.4.3014-3022.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lake C, Molesworth S, Hutt-Fletcher L. The Epstein-Barr virus (EBV) gN homolog BLFR1 encodes a 15-kilodalton glycoprotein that cannot be authentically processed unless it is coexpressed with the EBV gM homolog BBRF3. J Virol. 1998;72:5559–5564. doi: 10.1128/jvi.72.7.5559-5564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ligas M, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mettenleiter T C. Initiation and spread of α-herpesvirus infections. Trends Microbiol. 1994;2:2–4. doi: 10.1016/0966-842x(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 27.Mettenleiter T C. Pseudorabies (Aujeszky's disease) virus: the virus and molecular pathogenesis—state of the art June 1999. Vet Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- 28.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol. 1996;70:2191–2200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nixdorf R, Klupp B G, Karger A, Mettenleiter T C. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J Virol. 2000;74:7137–7145. doi: 10.1128/jvi.74.15.7137-7145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osterrieder N, Neubauer A, Fakler B, Brandmüller C, Seyboldt C, Kaaden O-R, Baines J D. Synthesis and processing of the equine herpesvirus 1 glycoprotein M. Virology. 1997;232:230–239. doi: 10.1006/viro.1997.8561. [DOI] [PubMed] [Google Scholar]
- 31.Pastey M K, Samal S K. Analysis of bovine respiratory syncytial virus envelope glycoproteins in cell fusion. J Gen Virol. 1997;78:1885–1889. doi: 10.1099/0022-1317-78-8-1885. [DOI] [PubMed] [Google Scholar]
- 32.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira L. Function of glycoprotein B homologues of the family herpesviridae. Infect Agents Dis. 1994;3:9–27. [PubMed] [Google Scholar]
- 34.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reschke M, Reis B, Noding K, Rohsiepe D, Richter A, Mockenhaupt T, Garten W, Radsak K. Constitutive expression of human cytomegalovirus glycoprotein B (gpUL55) with mutagenized carboxy-terminal hydrophobic domains. J Gen Virol. 1995;76:113–122. doi: 10.1099/0022-1317-76-1-113. [DOI] [PubMed] [Google Scholar]
- 36.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spear P. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 38.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 40.Whealy M E, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteley A, Bruun B, Minson T, Browne H. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J Virol. 1999;73:9515–9520. doi: 10.1128/jvi.73.11.9515-9520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S X, Zhu P, Letchworth G J. Bovine herpes virus 1 glycoprotein M forms a disulfide-linked heterodimer with the UL49.5 protein. J Virol. 1997;72:3029–3036. doi: 10.1128/jvi.72.4.3029-3036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuckermann F, Mettenleiter T C, Schreurs C, Sugg N, Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988;62:4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]