ABSTRACT
Androgen receptor signaling inhibitors combined with androgen deprivation therapy have become the standard of care for metastatic castration-sensitive prostate cancer (mCSPC), regardless of tumor volume or risk. However, survival of approximately one-third of these patients has not improved, necessitating further treatment escalation. On the other hand, for patients with oligometastatic mCSPC, there is an emerging role for local radiation therapy. Although data remain scarce, it is expected that treatment of both primary tumor as well as metastasis-directed therapy may improve survival outcomes. In these patients, systemic therapy may be de-escalated to intermittent therapy. However, precise risk stratification is necessary for risk-based treatment escalation or de-escalation. In addition to risk stratification based on clinical parameters, research has been conducted to incorporate genomic and/or transcriptomic data into risk stratification. In future, an integrated risk model is expected to precisely stratify patients and guide treatment strategies. Here, we first review the transition of the standard treatment for mCSPC over the last decade and further discuss the newest concept of escalating or de-escalating treatment using a multi-modal approach based on the currently available literature.
Key Words: prostate cancer, metastasis, systemic therapy, local therapy, metastasis-directed therapy
INTRODUCTION
The incidence of prostate cancer (PCa) has been increasing in Japan due to multiple factors, including the implementation of cancer screening and changes in dietary habits. In 2019, PCa was the most common cancer and the 6th leading cause of cancer-related deaths in Japanese males (https://ganjoho.jp/reg_stat/statistics/stat/summary.html). Even though prostate cancer specific antigen (PSA) screening is recommended by the Japanese Urological Association (JUA) guidelines and is available throughout the country, many men are still not screened, and more than 10% of newly diagnosed PCa patients present with metastasis at diagnosis. The treatment paradigm for metastatic PCa has undergone a major shift over the last decade, and multiple new combination therapies are currently being tested in clinical trials. In the present review, changes in the standard treatment profile for metastatic PCa over the last decade are summarized, and the latest concepts for escalating or de-escalating treatment using a multi-modal approach are discussed.
TRANSITION OF THE STANDARD SYSTEMIC TREATMENT FOR METASTATIC PCa
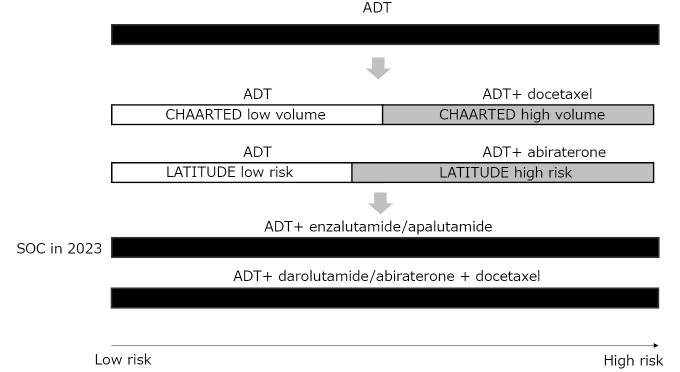
Over the last decade, the systemic therapies for metastatic PCa have changed dramatically (Fig. 1). PCa is driven by androgens, and more than 90% of PCa initially respond to androgen deprivation through surgical or medical castration. However, cancer cells eventually acquire resistance and become castration-resistant PCa (CRPC). Basic research has revealed that most CRPC are still dependent on the androgen receptor (AR) pathway through multiple mechanisms, such as AR amplification, AR mutation, production of ligand-independent splice variants, and use of adrenal or cancer cell-produced androgen.1 This has led to the discovery of potent androgen receptor signaling inhibitors (ARSIs) active against CRPC.2 Abiraterone is a CYP17A1 inhibitor that inhibits androgen production by the adrenal gland and cancer cells. The COU-AA-301 and COU-AA-302 trials showed the overall survival (OS) benefit of abiraterone in patients with pre- and post-chemotherapy metastatic CRPC.3,4 Enzalutamide and apalutamide are potent AR antagonists with similar chemical structures that inhibit the ligand binding of androgens and nuclear translocation of AR. The AFFIRM and PREVAIL trials showed an OS benefit of enzalutamide in patients with pre- and post-chemotherapy metastatic CRPC, respectively.5,6 In addition, the PROSPER and SPARTAN trials showed the OS benefits of enzalutamide and apalutamide, respectively, in non-metastatic CRPC.7,8 These trials have established the role of ARSIs in the treatment of CRPC.
Fig. 1.
Transition of standard treatment for metastatic castration-sensitive prostate cancer
ADT: androgen deprivation therapy
SOC: standard of care
More recent clinical trials have tested the activity of drugs used for CRPC in the earlier phase against metastatic castration-sensitive PCa (mCSPC), which has not been previously treated by androgen deprivation therapy (ADT). In 2015, it was reported by the CHAARTED study that chemo-hormonal therapy, involving the addition of 6 cycles of docetaxel to ADT prolonged OS by more than a year (57.6 months vs 44.0 months).9 The OS benefit was apparent in the high-volume group, defined by the presence of visceral metastases or ≥ 4 bone lesions with ≥ 1 beyond the vertebral bodies and pelvis, but not in the low-volume group. In 2017, the LATITUDE study showed that the addition of abiraterone to ADT prolonged OS in the high-risk group, as defined by the presence of at least two of the three high-risk factors associated with poor prognosis: a Gleason score (GS) of 8 or more, at least three bone lesions, and the presence of measurable visceral metastasis.10 These studies led to the notion that mCSPC can be risk-stratified based on tumor volume and GS, and that high-volume or high-risk patients can benefit from more intensive treatment in addition to ADT.
Next came a wave of studies showing the OS benefit of adding ARSI to ADT in all patients with mCSPC, regardless of tumor volume or risk. The TITAN study showed the OS benefit of adding apalutamide to ADT in unselected mCSPC patients.11 The ARCHES and ENZAMET studies showed the same results with enzalutamide.12,13 Subanalysis of these studies consistently showed similar hazard ratios for high-volume and low-volume diseases defined by the CHAARTED study criteria and for high-risk and low-risk diseases defined by the LATITUDE study criteria. These studies concluded that ADT plus ARSI doublet therapy is the standard of care (SOC) for all patients with mCSPC.
Overall, the survival of patients with mCSPC has been significantly prolonged by ARSI doublet therapy. However, it has become apparent that there is still a group of patients whose prognosis remains poor even with ARSI doublet therapy. The PSA nadir level is a surrogate of OS in mCSPC treated with ARSI doublet therapy. The patients who achieve PSA nadir ≤ 0.2 ng/mL by ARSI doublet show prolonged OS.14 However, approximately a third of mCSPC patients fail to achieve PSA nadir ≤ 0.2 ng/mL, and the OS of these patients is similar to those who don’t reach this PSA nadir level by ADT alone.15 Therefore, further treatment escalation or alternative treatments in addition to ARSI are necessary for these patients.
Recently, the results of the phase 3 ARASENS study investigating whether the addition of darolutamide, another potent ARSI, to chemo-hormonal therapy by ADT and docetaxel prolongs OS, was reported.16 The study was conducted in unselected, chemo-fit patients with mCSPC. This study showed that adding darolutamide to ADT and docetaxel significantly prolonged OS in all patients with mCSPC compared with that of ADT and docetaxel alone. Subanalysis showed a significant OS benefit in CHAARTED high-volume disease and a marginal difference in CHAARTED low-volume disease, but a consistent significant OS benefit in LATITUDE high- and low-risk disease.17 Similar results were obtained in another study, PEACE-1, which demonstrated the benefit of combining abiraterone with chemo-hormonal therapy.18 The results of these two studies raise two clinically important questions. The first question was whether there was an additional benefit of docetaxel over ARSI doublet. This remains an important question, since both the ARASENS and PEACE1 studies merely showed the benefit of adding ARSI to chemo-hormonal therapy, and not vice versa. The second question is whether docetaxel is necessary in all mCSPC patients, since data show that the OS of low-risk or low-volume mCSPC patients is already long with ARSI doublet. While OS benefits in all patients was shown, practitioners, including experts in the field, are still doubtful whether the triplet therapy of combining ARSI, ADT, and docetaxel is SOC for all patients with mCSPC. However, considering the cost of conducting phase 3 studies, it is unlikely that these questions will be addressed in another randomized controlled trial, and the accumulation of evidence from real-world data will be critical for determining the best combination of treatments for individual patients.
RISK STRATIFICATION OF mCSPC PATIENTS
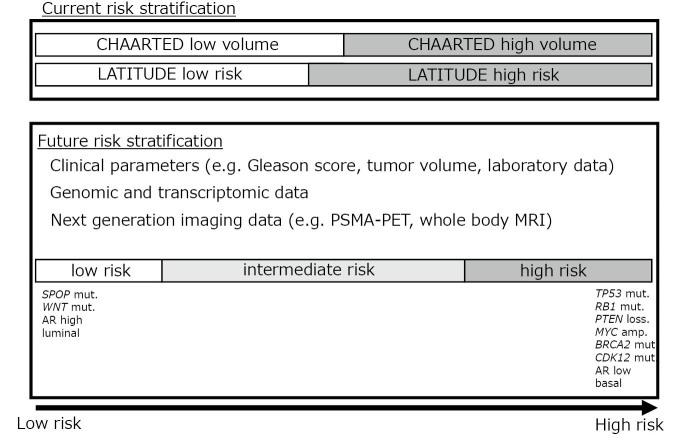
Glass et al reported the oldest prognostic model for mCSPC in 2003.19 The model differentiated three prognostic groups based on the localization of bone disease (appendicular or axial skeleton), performance status ([PS] 0 or 1–3), PSA (≥ 65 ng/mL or < 65 ng/mL), and GS (≥ 8 vs < 8). More recently, Gravis et al identified alkaline phosphatase (ALP), pain intensity, hemoglobin, lactate dehydrogenase (LDH), and bone metastasis as independent prognostic factors in mCSPC.20 Notably, the discriminatory ability of ALP alone was superior to that of the Glass risk model. Other reports have identified the extent of disease (EOD) score, GS ≥ 8, bone pain, PS, tumor volume (≤ 2 bone metastasis vs ≥ 3 bone/visceral metastasis), PSA > 100 ng/mL, Gleason pattern 5 on biopsy, and cT4 disease as poor prognostic factors in mCSPC.21-24 Currently, the CHAARTED study tumor volume group and the LATITUDE study risk group are the two most frequently used criteria for stratifying patients with mCSPC. However, unlike the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk score for kidney cancer,25 the parameters that constitute these models were not selected based on multivariate analysis, including potential prognostic factors reported previously. To establish a more precise prognostic model, a large retrospective study was conducted in Japan.26 Data from 304 consecutive patients with mCSPCs treated at a single institution were reviewed. Univariate and multivariable analyses including potential prognostic factors were performed, and EOD score ≥ 2 or the presence of liver metastasis, LDH, and a primary Gleason pattern of 5 were identified to be independent prognostic factors predicting OS. There was a strong correlation between ALP level and EOD score, and ALP did not remain as an independent prognostic factor. A risk prediction model was constructed using logistic regression analysis, and the patients were divided into three risk groups (low, intermediate, and high) according to their risk score. At a median follow-up period of 46 months, the median OS for the high- and intermediate-risk groups was 28 and 59 months, respectively, and was not reached for the low-risk group. The model was subsequently validated in an independent cohort of 520 Japanese patients. The median OS for the high- and intermediate-risk groups were 41 and 63 months, respectively, and was not reached in the low-risk group. Harrell’s C-index was 0.649. Notably, the authors identified that of the 153 patients classified as high-volume by the CHAARTED criteria, 56 and 24 were reclassified into the intermediate- and low-risk groups with a median OS of 60 and 121 months, respectively, suggesting that CHAARTED high-volume patients consist of heterogeneous populations with significantly different OS. The limitation of this study was that both the discovery and validation cohorts consisted of heterogeneous populations and included patients who were treated before ARSI and taxane chemotherapy became widely available in Japan. A study is currently ongoing to update the model using a contemporary cohort and to examine whether the risk model is clinically useful in the current era, where the ARSI doublet is the SOC. Another recent study from Japan retrospectively compared the effectiveness of ARSI doublet and classic combined androgen blockade (CAB) in LATITUDE high-risk mCSPC patients and demonstrated that a significant OS benefit by abiraterone was only observed in patients without the Gleason pattern 5,27 suggesting the importance of risk stratification even in the ARSI doublet era.
GENOMIC AND TRANSCRIPTOMIC MARKERS FOR RISK STRATIFICATION
While prognostic models based on clinical variables can stratify mCSPC to a certain extent, the inference from clinical variables alone is limited. With wide availability of next-generation sequencing technology, genomic and transcriptomic landscape of advanced PCa has been revealed,28 and is expected to more precisely guide treatment (Fig. 2). For example, aberrations in certain genes, such as AR, TP53, cell cycle-associated genes (eg, RB1), and MYC are known to be enriched in tumors with a worse prognosis, whereas alterations in SPOP and WNT pathways occur in tumors with better prognosis.29 Although most of these alterations are prognostic and not predictive, molecular classification may help identify patients who require more or less intensified treatments. SPOP is an E3 ubiquitin ligase that ubiquitinates the AR, leading to its degradation. It has been shown that SPOP mutations inhibit its activity, leading to AR accumulation. SPOP mutation is also known to be a driver of PCa carcinogenesis, along with TMPRSS2-ERG gene fusion and FOXA1 mutations. Approximately 10% of PCa patients are known to harbor SPOP mutations. One of the clinical characteristics of PCa with SPOP mutations is a good response to treatments targeting the AR. A recent study showed that the time to castration resistance was significantly longer in patients with SPOP mutations than in those with wild-type SPOP.30 In addition, a retrospective study of mCSPC patients who were treated with either ARSI doublet or chemo-hormonal therapy as the first-line systemic treatment revealed that in the ARSI cohort, the presence of SPOP mutation was associated with a longer time to castration resistance and OS compared to wild-type SPOP.31 In contrast, SPOP mutation status was not associated with either endpoint in the chemo-hormonal therapy cohort, suggesting that SPOP status may serve as a predictive marker in addition to its role as a prognostic marker. For patients with SPOP mutations, the ARSI doublet may be the preferred option and docetaxel can be spared until disease progression. Another study focused on alterations in major tumor suppressor genes (TP53, PTEN, RB1) and clinical outcomes of mCSPC.32 This study identified that progression-free survival (PFS) was significantly shorter in patients with tumor suppressor gene alterations who were initially treated with ADT and abiraterone. For patients initially treated with chemo-hormonal therapy, there was no difference between the groups, suggesting that patients without tumor suppressor gene alterations may benefit the most from ARSI doublet therapy.
Fig. 2.
Risk stratification of metastatic castration-sensitive prostate cancer
mCSPC can also be molecularly classified based on their transcriptome. One common classification is based on AR activity. The AR activity score was calculated based on the expression of a set of genes regulated by AR.33 Another classification was the luminal/basal classification determined by the PAM50 signature.34 This signature was originally developed for molecular subtyping of breast cancer. The Decipher Genomic Classifier is a PCa-specific classifier based on the expression of 22 transcripts.35 A correlative analysis of the CHAARTED study showed that the luminal B subtype was associated with a poorer prognosis with ADT alone but benefited significantly from the addition of docetaxel, in contrast to the basal subtype, where no OS benefit was demonstrated for chemo-hormonal therapy.36 Higher Decipher risk and lower AR activity scores were significantly associated with poorer OS. However, patients with higher Decipher risk showed greater improvements in OS with chemo-hormonal therapy. These data show the potential of transcriptome-based molecular subtyping for risk stratification of diseases and guide treatment. However, because of the cumbersome process of RNA-based analysis and lack of robustness, no transcriptome-based molecular subtyping has been clinically implemented in Japan.
TREATMENT ESCALATION FOR HIGH-RISK PATIENTS
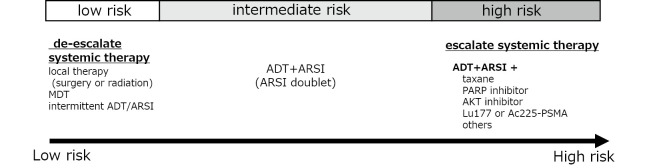
As described previously, a substantial number of patients show poor survival, even when initially treated with an ARSI doublet. To escalate systemic treatment, multiple novel classes of drugs that are active against CRPC are currently being tested for mCSPC in combination with the ARSI doublet. PI3K/AKT pathway is known to reciprocally sustain PCa growth with AR pathway.37 The ongoing CAPItello-281 trial (NCT04493853) is exploring whether the addition of an AKT inhibitor, capivasertib, to abiraterone and ADT improves survival in mCSPC patients with PTEN deficiency. Deficiency in DNA repair genes, especially those associated with homologous recombination repair, is another therapeutic target. The poly (ADP-ribose) polymerase (PARP) inhibitor olaparib has been shown to prolong the OS of patients with mCRPC with BRCA1 or BRCA2 mutations who are resistant to at least one ARSI38 and is approved worldwide. More recently, olaparib has been approved as a first-line treatment in combination with abiraterone for patients with mCRPC that harbor BRCA1 or BRCA2 mutations. Similar results have been obtained for talazoparib, another PARP inhibitor with a more potent PARP1 trapping function, in combination with enzalutamide, in mCRPC patients who have HRR-associated gene alterations.39 There is currently an ongoing phase 3 trial (NCT04821622) investigating whether this combination regimen, in addition to ADT, can prolong PFS in patients with mCSPC who have HRR-associated gene alterations. Prostate-specific membrane antigen (PSMA) is another therapeutic target. Cancer cells expressing PSMA can be targeted with antibodies or small molecules that bind to PSMA. This has enabled the development of positron emission tomography (PET) targeting PSMA. The molecular imaging using PSMA-PET allowed the development of “theranostics,” wherein a diagnostic tracer such as Ga-68 and F18 can be easily replaced by radioligands that emit alfa or beta particles such as Lu-177 and Ac-225 for ligand-specific targeted therapy. In the randomized phase 2 TheraP trial comparing the effectiveness of 177Lu-PSMA and cabazitaxel in patients with mCRPC resistant to both ARSI and docetaxel who were selected based on diagnostic PSMA-PET results, 177Lu-PSMA showed a superior response to cabazitaxel.40 Updated data allowing crossover at progression have recently been reported, showing that OS was similar between the two arms, indicating that both agents are active in this setting. The phase 3 VISION trial compared the effectiveness of 177Lu-PSMA with that of SOC in patients with mCRPC who were resistant to at least one ARSI and one taxane.41 This study met the primary endpoints of imaging-based PFS and OS, and the drug has been approved in several countries. The field is moving forward to test whether the addition of PSMA to the ARSI doublet can improve outcomes in patients with mCSPC (NCT04720157). With the results of ongoing clinical trials, the treatment of high-risk patients with mCSPC will likely be individualized based on molecular and imaging markers for appropriate treatment intensification.
MULTI-MODAL TREATMENT FOR mCSPC AND POTENTIAL FOR DE-ESCALATION OF SYSTEMIC THERAPY IN LOW-RISK PATIENTS
A multi-modal approach to treat mCSPC with a low metastatic volume is also gaining significant attention. Although there is no consensus on the specific number of metastases that can be called oligometastases, in general, patients with three to five metastatic lesions on conventional imaging (CT and/or bone scan) are thought to be oligometastatic. Traditionally, it has been thought that treatment of the primary disease is futile in patients who are already metastatic. However, two phase 3 trials, STAMPEDE (Arm H)42 and HORRAD,43 were conducted to examine whether treatment of the primary tumor with radiation therapy in addition to ADT can improve survival outcomes in patients with mCSPC. In both trials, there was no additional benefit of radiation therapy in the intention-to-treat cohort. However, in the STAMPEDE trial, there was an OS benefit in patients with a low metastatic burden, and a similar trend was observed in the HORRAD trial. A meta-analysis of the two trials demonstrated 7% improvement in 3-year survival of patients with fewer than five bone metastases.44 Currently, ADT and local radiation therapy are listed as treatment options for oligometastatic mCSPC in the NCCN guidelines. However, it remains to be elucidated whether there is a similar benefit of local radiation therapy when added to ARSI doublet therapy.
There is also the question of whether radiation to the prostate is sufficient for oligometastatic mCSPC. Genomic trajectory analysis has shown that PCa can metastasize not only from the primary tumor but also from other metastatic sites.45 This indicates that additional survival benefits may be expected by adequately controlling metastatic sites with metastasis-directed therapy (MDT) using radiation therapy or surgery. However, evidence for MDT remains scarce. The only currently available evidence for MDT is metachronous oligometastasis, which develops in patients treated for localized PCa. In a randomized phase 2 trial (ORIOLE), MDT using stereotactic ablative radiation (SABR) prolonged the time to progression of metachronous oligometastatic PCa.46 Intriguingly, among those who received MDT, there was a significant difference in PFS when all PSMA-PET-avid lesions were ablated compared to when there was any untreated lesion, suggesting the importance of detecting and ablating all metastatic lesions. A small retrospective study from Japan included a cohort of patients encompassing those with oligometastatic mCSPC and those with localized PCa with pelvic lymph node metastasis. The study found that CRPC-free survival and cancer-specific survival (CSS) were longer in those who received MDT in combination with high-dose-rate prostate brachytherapy compared to those who did not receive MDT.47 All patients were scheduled to undergo adjuvant ADT for 36 months.
More recently, the EXTEND phase 2 randomized clinical trial showed that by combining MDT with intermittent hormone therapy (ADT +/- ARSI) in patients with metastatic PCa, the time to disease progression could be significantly prolonged.48 Intermittent ADT has been shown to be non-inferior to continuous ADT for non-metastatic PCa; nevertheless, it has also been reported to be inferior to continuous ADT for metastatic PCa.49 However, by controlling the primary and metastatic sites using local therapy and MDT, there may be a new role of intermittent ADT in the ARSI and MDT era in this disease setting. Considering the systemic side effects of long-term hormonal therapy and ARSI, de-escalation of hormonal therapy with intermittent hormonal therapy could be an excellent option for improving the quality of life of patients with oligometastatic mCSPC. Further research is needed to identify biomarkers and optimal imaging modalities to precisely identify patients who can be safely managed using this treatment strategy (Fig. 3).
Fig. 3.
Risk-based treatment escalation/de-escalation strategy
MDT: metastasis-directed therapy
ADT: androgen deprivation therapy
ARSI: androgen receptor signaling inhibitor
CONSIDERATION FOR RACIAL DIFFERENCES
Race is one of the most well-known risk factors of PCa. Genome-wide association studies have revealed that many ethnicity-specific genetic loci are associated with PCa.50-53 The susceptibility to PCa as well as its biology, such as its response to hormonal therapy, differs according to race. For example, a retrospective study from a single institution in Hawaii showed that among PCa patients who received ADT at the hospital, CSS and OS were longer in Japanese patients compared to that in Caucasians, even though all patients were treated similarly with hormonal therapy.54 Concordantly, the subanalysis of the LATITUDE study focusing on Japanese subjects showed that for both the abiraterone and placebo arms, radiographic PFS (rPFS) was longer for the Japanese compared to that of the entire study population.55 Genetic polymorphisms have been shown to partially explain the differential responses to hormonal therapy among ethnicities. For example, a single nucleotide polymorphism (SNP) in HSD3B1 gene is known to be associated with response to hormonal therapy, including ARSI.56 Although the effect of the risk variant was the same across ethnicities, the frequency of the risk allele was much lower in Japanese compared to Caucasians.57 Furthermore, it has been reported that a SNP in 7p14.3 (rs1376350) is specifically associated with SPOP mutant PCa.58 PCa with SPOP mutations is associated with a better response to hormonal therapy and longer OS.30 Interestingly, the allele frequency of the variant associated with the SPOP mutation was five times higher in East Asians than in Caucasians. Further research is needed to examine the association between this SNP and the frequency of PCa with SPOP mutations in Asians. Since the decision to escalate or de-escalate systemic therapy for PCa largely depends on the effectiveness and duration of treatment with hormonal therapy, including ARSI, the optimal treatment strategy may differ by race and needs to be individualized. Further accumulation of data for each ethnic group is necessary.
CONCLUSIONS
ARSI doublet has become the SOC for all patients with mCSPC. However, with the emergence of new drugs and imaging modalities, escalation or de-escalation of systemic treatments combined with local ablative therapy, including MDT, can now be considered. Appropriate patient selection is key to a personalized approach, and further accumulation of data and the development of new biomarkers are warranted.
CONFLICT OF INTEREST
The authors declare no conflict of interest regarding the contents of the manuscript.
Editorial Note
This article has been selected as an “Editors’ Choice” by the editorial board.
Abbreviations
- PCa
prostate cancer
- CRPC
castration-resistant prostate cancer
- PSA
prostate specific antigen
- ARSI
androgen receptor signaling inhibitor
- mCSPC
metastatic castration-sensitive prostate cancer
- ADT
androgen deprivation therapy
- OS
overall survival
- MDT
metastasis-directed therapy
- AR
androgen receptor
- PSMA
prostate-specific membrane antigen
REFERENCES
- 1.Kobayashi T, Inoue T, Kamba T, Ogawa O. Experimental evidence of persistent androgen-receptor-dependency in castration-resistant prostate cancer. Int J Mol Sci. 2013;14(8):15615–15635. doi: 10.3390/ijms140815615. [DOI] [PMC free article] [PubMed]
- 2.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed]
- 3.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed]
- 4.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed]
- 5.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed]
- 6.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed]
- 7.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2018;378(26):2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed]
- 8.Small EJ, Saad F, Chowdhury S, et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol. 2019;30(11):1813–1820. doi: 10.1093/annonc/mdz397. [DOI] [PMC free article] [PubMed]
- 9.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed]
- 10.Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed]
- 11.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed]
- 12.Armstrong AJ, Azad AA, Iguchi T, et al. Improved Survival With Enzalutamide in Patients With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2022;40(15):1616–1622. doi: 10.1200/JCO.22.00193. [DOI] [PMC free article] [PubMed]
- 13.Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323–334. doi: 10.1016/S1470-2045(23)00063-3. [DOI] [PubMed]
- 14.Chowdhury S, Bjartell A, Agarwal N, et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Ann Oncol. 2023;34(5):477–485. doi: 10.1016/j.annonc.2023.02.009. [DOI] [PubMed]
- 15.Gebrael G, Sayegh N, Thomas VM, et al. Survival outcomes of real world patients with metastatic hormone-sensitive prostate cancer who do not achieve optimal PSA response with intensified androgen deprivation therapy with docetaxel or androgen receptor pathway inhibitors. Prostate Cancer Prostatic Dis. 2023. doi: 10.1038/s41391-023-00696-w. [DOI] [PubMed]
- 16.Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132–1142. doi: 10.1056/NEJMoa2119115. [DOI] [PMC free article] [PubMed]
- 17.Hussain M, Tombal B, Saad F, et al. Darolutamide Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J Clin Oncol. 2023;41(20):3595–3607. doi: 10.1200/JCO.23.00041. [DOI] [PubMed]
- 18.Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695–1707. doi: 10.1016/S0140-6736(22)00367-1. [DOI] [PubMed]
- 19.Glass TR, Tangen CM, Crawford ED, Thompson I. Metastatic carcinoma of the prostate: identifying prognostic groups using recursive partitioning. J Urol. 2003;169(1):164–169. doi: 10.1016/S0022-5347(05)64059-1. [DOI] [PubMed]
- 20.Gravis G, Boher JM, Fizazi K, et al. Prognostic Factors for Survival in Noncastrate Metastatic Prostate Cancer: Validation of the Glass Model and Development of a Novel Simplified Prognostic Model. Eur Urol. 2015;68(2):196–204. doi: 10.1016/j.eururo.2014.09.022. [DOI] [PubMed]
- 21.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 2006;24(24):3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed]
- 22.Millikan RE, Wen S, Pagliaro LC, et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol. 2008;26(36):5936–5942. doi: 10.1200/JCO.2007.15.9830. [DOI] [PMC free article] [PubMed]
- 23.Miyamoto S, Ito K, Miyakubo M, et al. Impact of pretreatment factors, biopsy Gleason grade volume indices and post-treatment nadir PSA on overall survival in patients with metastatic prostate cancer treated with step-up hormonal therapy. Prostate Cancer Prostatic Dis. 2012;15(1):75–86. doi: 10.1038/pcan.2011.47. [DOI] [PubMed]
- 24.Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61(1):195–202. doi:. [DOI] [PubMed]
- 25.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed]
- 26.Akamatsu S, Kubota M, Uozumi R, et al. Development and Validation of a Novel Prognostic Model for Predicting Overall Survival in Treatment-naïve Castration-sensitive Metastatic Prostate Cancer. Eur Urol Oncol. 2019;2(3):320–328. doi: 10.1016/j.euo.2018.10.011. [DOI] [PubMed]
- 27.Ueda T, Fujita K, Nishimoto M, et al. Predictive factors for the efficacy of abiraterone acetate therapy in high-risk metastatic hormone-sensitive prostate cancer patients. World J Urol. 2022;40(12):2939–2946. doi: 10.1007/s00345-022-04200-2. [DOI] [PubMed]
- 28.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed]
- 29.Stopsack KH, Nandakumar S, Wibmer AG, et al. Oncogenic Genomic Alterations, Clinical Phenotypes, and Outcomes in Metastatic Castration-Sensitive Prostate Cancer. Clin Cancer Res. 2020;26(13):3230–3238. doi: 10.1158/1078-0432.CCR-20-0168. [DOI] [PMC free article] [PubMed]
- 30.Swami U, Isaacsson Velho P, Nussenzveig R, et al. Association of SPOP Mutations with Outcomes in Men with De Novo Metastatic Castration-sensitive Prostate Cancer. Eur Urol. 2020;78(5):652–656. doi: 10.1016/j.eururo.2020.06.033. [DOI] [PMC free article] [PubMed]
- 31.Swami U, Graf RP, Nussenzveig RH, et al. SPOP Mutations as a Predictive Biomarker for Androgen Receptor Axis-Targeted Therapy in De Novo Metastatic Castration-Sensitive Prostate Cancer. Clin Cancer Res. 2022;28(22):4917–4925. doi: 10.1158/1078-0432.CCR-22-2228. [DOI] [PubMed]
- 32.Velez MG, Kosiorek HE, Egan JB, et al. Differential impact of tumor suppressor gene (TP53, PTEN, RB1) alterations and treatment outcomes in metastatic, hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2022;25(3):479–483. doi: 10.1038/s41391-021-00430-4. [DOI] [PMC free article] [PubMed]
- 33.Spratt DE, Alshalalfa M, Fishbane N, et al. Transcriptomic Heterogeneity of Androgen Receptor Activity Defines a de novo low AR-Active Subclass in Treatment Naïve Primary Prostate Cancer. Clin Cancer Res. 2019;25(22):6721–6730. doi: 10.1158/1078-0432.CCR-19-1587. [DOI] [PMC free article] [PubMed]
- 34.Bernard B, Muralidhar V, Chen YH, et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer. 2017;123(9):1536–1544. doi: 10.1002/cncr.30503. [DOI] [PMC free article] [PubMed]
- 35.Ross AE, Johnson MH, Yousefi K, et al. Tissue-based Genomics Augments Post-prostatectomy Risk Stratification in a Natural History Cohort of Intermediate- and High-Risk Men. Eur Urol. 2016;69(1):157–165. doi: 10.1016/j.eururo.2015.05.042. [DOI] [PubMed]
- 36.Hamid AA, Huang HC, Wang V, et al. Transcriptional profiling of primary prostate tumor in metastatic hormone-sensitive prostate cancer and association with clinical outcomes: correlative analysis of the E3805 CHAARTED trial. Ann Oncol. 2021;32(9):1157–1166. doi: 10.1016/j.annonc.2021.06.003. [DOI] [PMC free article] [PubMed]
- 37.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed]
- 38.Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383(24):2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed]
- 39.Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398):291–303. doi: 10.1016/S0140-6736(23)01055-3. [DOI] [PubMed]
- 40.Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed]
- 41.Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385(12):1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed]
- 42.Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed]
- 43.Boevé LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410–418. doi: 10.1016/j.eururo.2018.09.008. [DOI] [PubMed]
- 44.Burdett S, Boevé LM, Ingleby FC, et al. Prostate Radiotherapy for Metastatic Hormone-sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-analysis. Eur Urol. 2019;76(1):115–124. doi: 10.1016/j.eururo.2019.02.003. [DOI] [PMC free article] [PubMed]
- 45.Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547):353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed]
- 46.Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650–659. doi: 10.1001/jamaoncol.2020.0147. [DOI] [PMC free article] [PubMed]
- 47.Tsumura H, Ishiyama H, Tabata KI, et al. Long-term outcomes of combining prostate brachytherapy and metastasis-directed radiotherapy in newly diagnosed oligometastatic prostate cancer: A retrospective cohort study. Prostate. 2019;79(5):506–514. doi: 10.1002/pros.23757. [DOI] [PubMed]
- 48.Tang C, Sherry AD, Haymaker C, et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023;9(6):825–834. doi: 10.1001/jamaoncol.2023.0161. [DOI] [PMC free article] [PubMed]
- 49.Perera M, Roberts MJ, Klotz L, et al. Intermittent versus continuous androgen deprivation therapy for advanced prostate cancer. Nat Rev Urol. 2020;17(8):469–481. doi: 10.1038/s41585-020-0335-7. [DOI] [PubMed]
- 50.Haiman CA, Chen GK, Blot WJ, et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43(6):570–573. doi: 10.1038/ng.839. [DOI] [PMC free article] [PubMed]
- 51.Conti DV, Darst BF, Moss LC, et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021;53(1):65–75. doi: 10.1038/s41588-020-00748-0. [DOI] [PMC free article] [PubMed]
- 52.Akamatsu S, Takata R, Haiman CA, et al. Common variants at 11q12, 10q26 and 3p11.2 are associated with prostate cancer susceptibility in Japanese. Nat Genet. 2012;44(4):426–429,S1. doi: 10.1038/ng.1104. [DOI] [PubMed]
- 53.Takata R, Takahashi A, Fujita M, et al. 12 new susceptibility loci for prostate cancer identified by genome-wide association study in Japanese population. Nat Commun. 2019;10(1):4422. doi: 10.1038/s41467-019-12267-6. [DOI] [PMC free article] [PubMed]
- 54.Fukagai T, Namiki TS, Carlile RG, Yoshida H, Namiki M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97(6):1190–1193. doi: 10.1111/j.1464-410X.2006.06201.x. [DOI] [PubMed]
- 55.Suzuki H, Shin T, Fukasawa S, et al. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naive prostate cancer: final subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, phase 3 study. Jpn J Clin Oncol. 2020;50(7):810–820. doi: 10.1093/jjco/hyaa030. [DOI] [PMC free article] [PubMed]
- 56.Lu C, Terbuch A, Dolling D, et al. Treatment with abiraterone and enzalutamide does not overcome poor outcome from metastatic castration-resistant prostate cancer in men with the germline homozygous HSD3B1 c.1245C genotype. Ann Oncol. 2020;31(9):1178–1185. doi: 10.1016/j.annonc.2020.04.473. [DOI] [PubMed]
- 57.Shiota M, Narita S, Akamatsu S, et al. Association of Missense Polymorphism in HSD3B1 With Outcomes Among Men With Prostate Cancer Treated With Androgen-Deprivation Therapy or Abiraterone. JAMA Netw Open. 2019;2(2):e190115. doi: 10.1001/jamanetworkopen.2019.0115. [DOI] [PMC free article] [PubMed]
- 58.Romanel A, Garritano S, Stringa B, et al. Inherited determinants of early recurrent somatic mutations in prostate cancer. Nat Commun. 2017;8(1):48. doi: 10.1038/s41467-017-00046-0. [DOI] [PMC free article] [PubMed]