ABSTRACT
Pregnancy is an excellent opportunity to provide medical interventions to women. It is also a stress test used to predict health. Numerous studies have demonstrated that the pre-pregnancy body mass index (BMI) and gestational weight gain (GWG) are critical factors for pregnancy complications such as hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), large or small gestational age infants, and spontaneous preterm birth (sPTB). These complications are associated with an increased risk of cardiovascular disease (CVD), which is a leading cause of mortality in women. In addition, complications adversely affect the short- and long-term prognoses of children. Optimal GWG to reduce complications is recommended based on pre-pregnancy BMI; however, racial differences should also be noted. The values in the Japanese guidelines are lower than those in the American Institute of Medicine guidelines. The Asian BMI thresholds for CVD risk are also lower than those in Europe. Therefore, weight management should be based on racial/genetic background. Interpregnancy weight gain or loss has also been reported to be associated with the risk of pregnancy complications; however, few studies have been conducted in Asian populations. Our previous reports suggested that avoiding an excess of 0.6 kg/m2/year of annual BMI gain may reduce the risk of HDP or GDM, and insufficient gain of < 0.25 kg/m2/year may increase sPTB recurrence. Annual BMI is useful for practical weight control during interpregnancy. Based on these findings, effective approaches should be established to improve the health of women and their offspring.
Key Words: interpregnancy, body mass index, gestational weight change, cardiovascular diseases
INTRODUCTION
Body weight management is essential for maintaining overall health throughout life. Maintaining a normal weight is particularly critical for preventing adverse complications in pregnant women, including hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), large or small for gestational age (LGA or SGA), and spontaneous preterm birth (sPTB). These complications have harmful effects on mortality and morbidity in both women and their offspring. First, these pregnancy complications, including HDP, GDM, sPTB, and SGA, are related to the risk of women’s health issues later in life, especially cardiovascular diseases (CVD), a leading cause of women’s morbidity and mortality.1-3 Besides CVD, HDP increases the risk of hypertension, heart failure, stroke, and cognitive decline.4,5 Consequently, these complications contribute to the reduction of women’s healthy life expectancy.6 Second, preterm neonates born to mothers with HDP and sPTB have neurological and respiratory problems, diminishing their quality of life. Third, their offspring are at a high risk of hypertension, obesity, and CVD in later life.7 These findings underscore the significant impact that the prevention of HDP, GDM, and sPTB can have on public health. Weight management strategies to prevent these complications can be implemented even in low-resource settings. However, establishing an efficient protocol for women remains challenging.
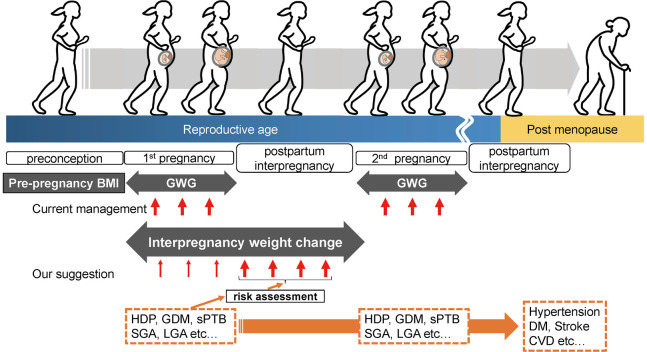
What is the optimal timing for interventions to reduce pregnancy complications: pre-conception, pregnancy, or post-partum/interpregnancy (between pregnancies)? Figure 1 summarizes the association between weight change and health problems. Obstetric management predominantly focuses on interventions during pregnancy. Optimal gestational weight gain (GWG) by pre-pregnancy body mass index (BMI) has been established, drawing from data on Japanese women.8 However, the short duration of intervention during pregnancy is often insufficient to achieve the desired outcome. Recognizing this limitation, the concept of preconception care has emerged, emphasizing the need for preventive measures prior to pregnancy to enhance pregnancy outcomes further.9 Despite the recommendation for all women of reproductive age to plan their pregnancies and adopt a healthier lifestyle that includes diet, sleep, physical exercise, and mental health,10,11 efficient interventions face challenges, given that half of all pregnancies are unintended.12,13 Recently, the importance of interpregnancy care has received increasing attention.2 Pregnancy is an excellent opportunity for women to engage with health care resources, and the postpartum/interpregnancy period is a strategic phase that provides comprehensive information and guidance. During the postpartum/interpregnancy period, women can gain insight into the potential risks in subsequent pregnancies and anticipate health issues in later life. This period is a critical juncture in which women acquire the knowledge and tools necessary to make informed decisions that positively impact their reproductive health and overall well-being. Despite the significance of this period, interpregnancy weight management in the United States is currently simplified, with a basic recommendation to achieve a normal weight2 (18.5–24.9 kg/m2). Moreover, there is a significant lack of evidence specifically addressing the needs of Asian women in this issue. Therefore, it is necessary to establish more practical protocols for interpregnancy weight management that address diverse populations of women.
Fig. 1.
The overview of body weight management and health issues during women’s life stages
BMI: body mass index
CVD: cardiovascular disease
DM: diabetes mellitus
GDM: gestational diabetes mellitus
GWG: gestational weight gain
HDP: hypertensive disorders of pregnancy
LGA: large for gestational age
SGA: small for gestational age
sPTB: spontaneous preterm birth
In this review, we examined previous studies on the effects of body weight on pregnancy complications across distinct phases of women’s lives: preconception, pregnancy, and postpartum/interpregnancy. This review provides a basic understanding of the relationship between body weight and the health of women and their offspring during critical life stages. Management issues and limitations specific to preconception and pregnancy are also discussed. In light of this, we outlined the potential benefits of interpregnancy weight management based on our recent findings. This lays the foundation for future research aimed at developing more practical protocols that will contribute to improve the health of both women and their offspring.
PRECONCEPTION—PRE-PREGNANCY BMI AND ADVERSE PERINATAL EFFECTS
BMI, calculating weight (kg) divided by squared height in meters (m2), is usually categorized14 as underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30.0 kg/m2). Pre-pregnancy overweight and obese are associated with an increased risk of HDP and GDM.15-17 On the contrary, pre-pregnancy underweight increases sPTB18 and SGA.19 Therefore, having a normal weight prior to conception minimizes HDP, GDM, and preterm birth risks, and women are recommended to achieve and maintain normal weight before they attempt pregnancy.10 However, approximately 40–45% of pregnancies are unintended, even in developed countries such as the United States12 and Japan.13 Thus, challenges remain in implementing preconception care.20
DURING PREGNANCY—WHAT IS RECOMMENDED IN GWG MANAGEMENT?
Emerging evidence has demonstrated that excess and insufficient GWG adversely affects mothers and their offspring.21 Guidelines on the optimal GWG are provided during pregnancy based on the women’s pre-pregnancy BMI categories8,22 (Table 1). The guidelines by the American Institute of Medicine (IOM), created using data from Caucasians, are used worldwide, but racial differences should be considered before application to Japanese women. The Asian BMI cutoff point for a higher risk of type 2 DM and CVD is lower than that of the Europeans, suggesting that Asian overweight14 is defined as ≥ 23.0 kg/m2. Thus, the optimal GWG that may minimize SGA, preterm birth, complicated delivery, and HDP, have been evaluated in Japanese women.8 In all the strata of pre-pregnancy BMI, especially in the obesity group, the values recommended by the Japanese Ministry of Health, Labor and Welfare (https://www.mhlw.go.jp/seisakunitsuite/bunya/kodomo/kodomo_kosodate/boshi-hoken/ninpu-02.html) are low compared to the IOM (Table 1). Therefore, appropriate GWGs may vary according to racial or genetic backgrounds, highlighting the importance of developing guidelines for Asian populations. The intervention to limit GWG could minimize the risks for GDM and LGA,23 which has also been shown in Asians.24 On the contrary, insufficient GWG (< 2.75 kg) may increase the prevalence of SGA in obese (≥ 25 kg/m2) Japanese women with GDM.25 Strict controls of GWG may be harmful for them. Moreover, appropriate interventions to support weight gain in underweight women remain unestablished.26 These are the issues that need to be addressed in the future.
Table 1.
Recommended GWG based on pre-pregnancy BMI
| Japan (kg) | United States (kg) | |
| ≤ 18.4 (underweight) | 12.0–15.0 | 12.5–18.0 |
| 18.5–24.9 (normal weight) | 10.0–13.0 | 11.5–16.0 |
| 25.0–29.9 (overweight) | 7.0–10.0 | 7.0–11.5 |
| ≥ 30.0 (obese) | ≤5.0 | 5.0–9.0 |
BMI: body weight mass index
GWG: gestational weight gain
WHAT IS INTERPREGNANCY CARE?
Women who develop pregnancy complications must be informed regarding future pregnancies and health risks. However, these problems have often been ignored in clinical practice since most complications spontaneously resolve after delivery. Interpregnancy care has not been described in the Guidelines for Obstetrical Practice in Japan,27 although interpregnancy care starting seamlessly after delivery is recognized as a good opportunity to optimize healthy conditions to improve subsequent pregnancy outcomes and future health in the United States.2 Weight management as interpregnancy care can be tailored to specific pregnancy outcomes of previous pregnancies and may be more effectively implemented than preconception care. Besides weight management, interpregnancy care includes recommendations for breastfeeding for the first six months of life, avoiding interpregnancy intervals shorter than six months, mental health care, and managing medical and social conditions.2
INTERPREGNANCY—HOW INTERPREGNANCY WEIGHT CHANGE AFFECTS COMPLICATIONS IN A SUBSEQUENT PREGNANCY?
Several studies have reported an association between weight change during interpregnancy and subsequent pregnancy outcomes. In a Swedish large cohort study, a weight gain of ≥ 3 kg/m2 during interpregnancy increased the risks of HDP, GDM, cesarean section, and LGA.28 The results from the meta-analyses also revealed that interpregnancy BMI gain is associated with the risks of those complications.21,29-31 Furthermore, interpregnancy BMI gain most impacted pregnancy outcomes in women with BMI < 25 kg/m2 (normal or underweight) before the first pregnancy,29 which is similar to our findings.32 Thus, normal or underweight women also need to be advised to avoid gaining too much weight after delivery. In contrast, interpregnancy BMI loss is associated with risks of SGA and preterm birth.21 Unfortunately, these findings have been established in Western countries, and few studies have been conducted on Asian women. Additionally, as mentioned above, practical weight management protocols are not shown.2 Bariatric surgery may be considered for women with a BMI greater than 35 kg/m2, with at least one obesity-related severe morbidity and who are refractory to medical intervention.2 It improves pregnancy outcomes but also increases the risk of SGA.2 There are a few reports on the effect of bariatric surgery in Japan.33 Thus, recommending bariatric surgery appears premature as part of the interpregnancy management.
Recently, we have investigated the associations using annual BMI changes (kg/m2/year) to establish a practical approach to minimize subsequent pregnancy outcomes.32,34,35 Annual BMI changes, which we have first reported in the interpregnancy period, are possible indicators for a specific, measurable, achievable, relevant, timely (SMART) goal setting in practical weight management. The SMART goal setting has been used in a weight management program.36 The risks for HDP, GDM, and sPTB have been recalculated from the dataset in previous reports.32,34,35 The results show a positive association between annual BMI change and the risks for HDP and GDM (Figure 2A and B, respectively) and a negative association between annual BMI change and sPTB (Figure 2C). By applying this formula, the risk of developing these complications in subsequent pregnancies can be calculated using the estimated weight shortly before the subsequent pregnancy and the desired timing for the subsequent pregnancy. Additionally, the risk in women with a history of these complications (Figure 2A–C, orange lines) was higher than that in women without such a history (Figure 2A–C, blue lines). This finding suggests that women should be managed based on the presence or absence of a history of complications. Furthermore, we have reported that avoiding an excess of 0.6 kg/m2/year of annual BMI increase may reduce the risk of HDP or GDM.32,35 For women who develop sPTB, an annual BMI change of ≥ 0.25 kg/m2/year may prevent the recurrence of sPTB.34 A recent Japanese study37 suggests that women with a pre-pregnancy BMI of less than 25 kg/m2 have an increased risk of LGA in their subsequent pregnancies if their interpregnancy weight gain exceeds 1 kg/m2. Thus, the importance of interpregnancy BMI change is becoming increasingly recognized in Japan. We propose a seamless weight management from pregnancy to postpartum/interpregnancy and risk assessment based on pregnancy complications (Figure 1) using annual BMI.
Fig. 2.
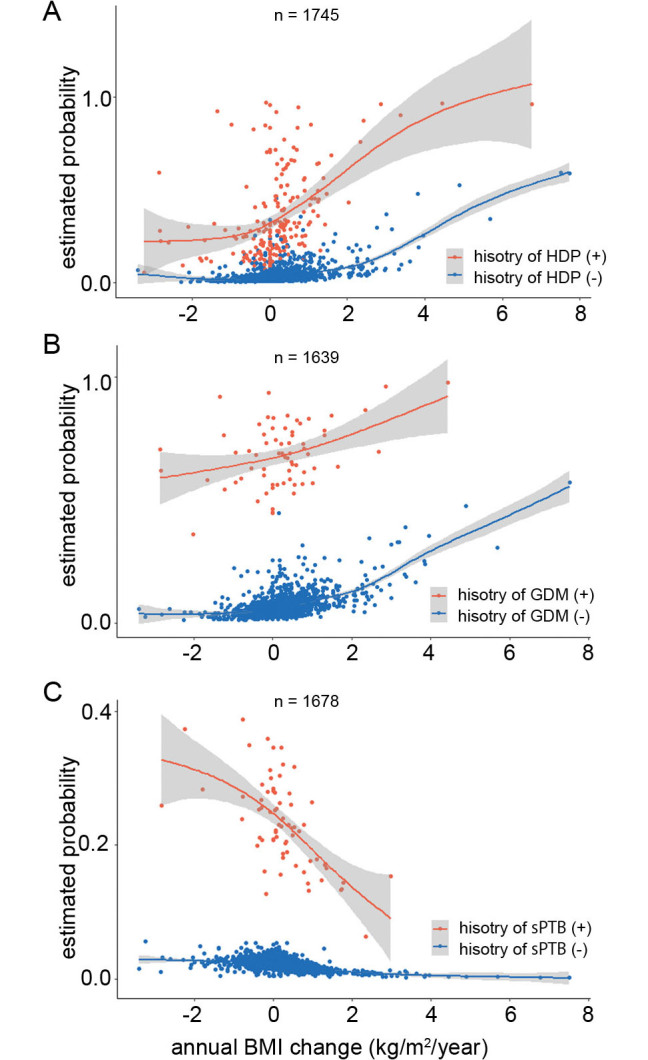
Estimated probabilities for HDP, GDM, and sPTB by annual BMI change during interpregnancy
The fitted values (solid lines) and 95% confidence intervals (shaded areas) for HDP (A), GDM (B), and sPTB (C) were calculated using a generalized additive model (R, version 4.1.3 [https://cran.r-project.org/]). The orange and blue lines indicate the presence and absence of history of pregnancy-related complications, respectively.
BMI: body mass index
GDM: gestational diabetes mellitus
HDP: hypertensive disorders of pregnancy
sPTB: spontaneous preterm birth
FUTURE PERSPECTIVE
Women are encouraged to set annual BMI goals autonomously for subsequent pregnancies and future health. Visualizing their risks seems beneficial in this regard. We are currently advancing our program, wherein risks are presented upon entering various relevant factors, including age, pre-pregnancy BMI, and the history of pregnancy complications. Women can then determine their annual weight goals based on their individual risks and desired timing for the next pregnancy. Although further investigation is required to confirm the effectiveness of this intervention, it has the potential to enhance motivation for weight management.
CONCLUSION
Changes in the pre-pregnancy BMI, GWG, and interpregnancy BMI have a significant impact on the health of women and their offspring. Importantly, these risk factors are modifiable. These findings underscore the need to establish the most effective approach to enhance the health of women and their offspring. Implementation of such measures would yield substantial benefits for public health promotion.
ACKNOWLEDGEMENTS
We thank all our collaborators in previous studies.32,34,35 We also thank the lab icons for providing illustrations (https://lab-icons.com/blog) and Editage (www.editage.jp) for English language editing.
CONFLICT OF INTEREST
The authors declare no conflicts of interest regarding this review.
Abbreviations
- BMI
body mass index
- GDM
gestational diabetes mellitus
- GWG
gestational weight gain
- HDP
hypertensive disorders of pregnancy
- SGA
small for gestational age
- sPTB
spontaneous preterm birth
REFERENCES
- 1.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol. 2016;215(4):484.e1–484.e14. doi: 10.1016/j.ajog.2016.05.047. [DOI] [PubMed]
- 2.American College of Nurse-Midwives and the National Association of Nurse Practitioners in Women’s Health; American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, Louis JM, et al. Interpregnancy Care. Am J Obstet Gynecol. 2019;220(1):B2–B18. doi: 10.1016/j.ajog.2018.11.1098. [DOI]
- 3.Minissian MB, Kilpatrick S, Eastwood JA, et al. Association of Spontaneous Preterm Delivery and Future Maternal Cardiovascular Disease. Circulation. 2018;137(8):865–871. doi: 10.1161/circulationaha.117.031403. [DOI] [PMC free article] [PubMed]
- 4.Mielke MM, Frank RD, Christenson LR, Fields JA, Rocca WA, Garovic VD. Association of Hypertensive Disorders of Pregnancy With Cognition in Later Life. Neurology. 2023;100(19):e2017–e2026. doi: 10.1212/wnl.0000000000207134. [DOI] [PMC free article] [PubMed]
- 5.Hansen AL, Søndergaard MM, Hlatky MA, et al. Adverse Pregnancy Outcomes and Incident Heart Failure in the Women’s Health Initiative. JAMA Netw Open. 2021;4(12):e2138071. doi: 10.1001/jamanetworkopen.2021.38071. [DOI] [PMC free article] [PubMed]
- 6.Hinkle SN, Schisterman EF, Liu D, et al. Pregnancy Complications and Long-Term Mortality in a Diverse Cohort. Circulation. 2023;147(13):1014–1025. doi: 10.1161/circulationaha.122.062177. [DOI] [PMC free article] [PubMed]
- 7.Herrera-Garcia G, Contag S. Maternal preeclampsia and risk for cardiovascular disease in offspring. Curr Hypertens Rep. 2014;16(9):475. doi: 10.1007/s11906-014-0475-3. [DOI] [PubMed]
- 8.Morisaki N, Nagata C, Jwa SC, et al. Pre-pregnancy BMI-specific optimal gestational weight gain for women in Japan. J Epidemiol. 2017;27(10):492–498. doi: 10.1016/j.je.2016.09.013. [DOI] [PMC free article] [PubMed]
- 9.Freda MC, Moos MK, Curtis M. The history of preconception care: evolving guidelines and standards. Matern Child Health J. 2006;10(5 Suppl):S43–S52. doi: 10.1007/s10995-006-0087-x. [DOI] [PMC free article] [PubMed]
- 10.ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133(1):e78–e89. doi: 10.1097/aog.0000000000003013. [DOI] [PubMed]
- 11.Shimoya K, Kotani T, Satoh S, et al. Clinical guide for women with mental health problems during the perinatal period. J Obstet Gynaecol Res. 2022;48(1):20–33. doi: 10.1111/jog.15068. [DOI] [PubMed]
- 12.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed]
- 13.Huynh ST, Yokomichi H, Akiyama Y, et al. Prevalence of and factors associated with unplanned pregnancy among women in Koshu, Japan: cross-sectional evidence from Project Koshu, 2011–2016. BMC Pregnancy Childbirth. 2020;20(1):397. doi: 10.1186/s12884-020-03088-3. [DOI] [PMC free article] [PubMed]
- 14.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/s0140-6736(03)15268-3. [DOI] [PubMed]
- 15.Sugimura R, Kohmura-Kobayashi Y, Narumi M, et al. Comparison of three classification systems of Prepregnancy Body Mass Index with Perinatal Outcomes in Japanese Obese Pregnant Women: A retrospective study at a single center. Int J Med Sci. 2020;17(13):2002–2012. doi: 10.7150/ijms.47076. [DOI] [PMC free article] [PubMed]
- 16.Samuels-Kalow ME, Funai EF, Buhimschi C, et al. Prepregnancy body mass index, hypertensive disorders of pregnancy, and long-term maternal mortality. Am J Obstet Gynecol. 2007;197(5):490.e1–490.e6. doi: 10.1016/j.ajog.2007.04.043. [DOI] [PMC free article] [PubMed]
- 17.Lara-Barea A, Sánchez-Lechuga B, Aguilar-Diosdado M, López-Tinoco C. Higher daytime systolic BP, prepregnancy BMI and an elevated sFlt-1/PlGF ratio predict the development of hypertension in normotensive pregnant women. Reprod Biol Endocrinol. 2022;20(1):175. doi: 10.1186/s12958-022-01050-w. [DOI] [PMC free article] [PubMed]
- 18.Girsen AI, Mayo JA, Carmichael SL, et al. Women’s prepregnancy underweight as a risk factor for preterm birth: a retrospective study. BJOG. 2016;123(12):2001–2007. doi: 10.1111/1471-0528.14027. [DOI] [PMC free article] [PubMed]
- 19.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed]
- 20.Black KI, Middleton P, LibSt G, Huda TM, Srinivasan S. Interconception Health: Improving Equitable Access to Pregnancy Planning. Semin Reprod Med. 2022;40(3–04):184–192. doi: 10.1055/s-0042-1744517. [DOI] [PubMed]
- 21.Nagpal TS, Souza SCS, Moffat M, et al. Does prepregnancy weight change have an effect on subsequent pregnancy health outcomes? A systematic review and meta-analysis. Obes Rev. 2022;23(1):e13324. doi: 10.1111/obr.13324. [DOI] [PubMed]
- 22.Rasmussen KM, Yaktine AL; Committee to Reexamine IOM Pregnancy Weight Guidelines Food and Nutrition Board Board on Children, Youth, and Families eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US); 2009. https://www.ncbi.nlm.nih.gov/books/NBK32799/#summary.s1. Accessed Jauary 22, 2024.
- 23.Cantor AG, Jungbauer RM, McDonagh M, et al. Counseling and Behavioral Interventions for Healthy Weight and Weight Gain in Pregnancy: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(20):2094–2109. doi: 10.1001/jama.2021.4230. [DOI] [PubMed]
- 24.Bennett CJ, Walker RE, Blumfield ML, et al. Interventions designed to reduce excessive gestational weight gain can reduce the incidence of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2018;141:69–79. doi: 10.1016/j.diabres.2018.04.010. [DOI] [PubMed]
- 25.Saito Y, Kobayashi S, Ikeda-Araki A, et al. Association between pre-pregnancy body mass index and gestational weight gain and perinatal outcomes in pregnant women diagnosed with gestational diabetes mellitus: The Japan Environment and Children’s Study. J Diabetes Investig. 2022;13(5):889–899. doi: 10.1111/jdi.13723. [DOI] [PMC free article] [PubMed]
- 26.Hunter PJ, Muthiani Y, Näsänen-Gilmore PK, et al. A modular systematic review of antenatal interventions to address undernutrition during pregnancy in the prevention of low birth weight. Am J Clin Nutr. 2023;117 Suppl 2:S134–S147. doi: 10.1016/j.ajcnut.2023.01.024. [DOI] [PubMed]
- 27.Itakura A, Satoh S, Aoki S, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J Obstet Gynaecol Res. 2023;49(1):5–53. doi: 10.1111/jog.15438. [DOI] [PubMed]
- 28.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368(9542):1164–1170. doi: 10.1016/s0140-6736(06)69473-7. [DOI] [PubMed]
- 29.Timmermans YEG, van de Kant KDG, Oosterman EO, et al. The impact of interpregnancy weight change on perinatal outcomes in women and their children: A systematic review and meta-analysis. Obes Rev. 2020;21(3):e12974. doi: 10.1111/obr.12974. [DOI] [PMC free article] [PubMed]
- 30.Martínez-Hortelano JA, Cavero-Redondo I, Álvarez-Bueno C, Sanabria-Martínez G, Poyatos-León R, Martínez-Vizcaíno V. Interpregnancy Weight Change and Hypertension During Pregnancy: A Systematic Review and Meta-analysis. Obstet Gynecol. 2020;135(1):68–79. doi: 10.1097/aog.0000000000003573. [DOI] [PubMed]
- 31.Teulings NEWD, Masconi KL, Ozanne SE, Aiken CE, Wood AM. Effect of interpregnancy weight change on perinatal outcomes: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):386. doi: 10.1186/s12884-019-2566-2. [DOI] [PMC free article] [PubMed]
- 32.Tano S, Kotani T, Ushida T, et al. Annual body mass index gain and risk of hypertensive disorders of pregnancy in a subsequent pregnancy. Sci Rep. 2021;11(1):22519. doi: 10.1038/s41598-021-01976-y. [DOI] [PMC free article] [PubMed]
- 33.Watanabe A, Seki Y, Haruta H, Kikkawa E, Kasama K. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300(1):145–152. doi: 10.1007/s00404-019-05195-9. [DOI] [PubMed]
- 34.Tano S, Kotani T, Ushida T, et al. Optimal annual body mass index change for preventing spontaneous preterm birth in a subsequent pregnancy. Sci Rep. 2022;12(1):17502. doi: 10.1038/s41598-022-22495-4. [DOI] [PMC free article] [PubMed]
- 35.Tano S, Kotani T, Ushida T, et al. Annual Body Mass Index Gain and Risk of Gestational Diabetes Mellitus in a Subsequent Pregnancy. Front Endocrinol (Lausanne). 2022;13:815390. doi: 10.3389/fendo.2022.815390. [DOI] [PMC free article] [PubMed]
- 36.Coupe N, Cotterill S, Peters S. Enhancing community weight loss groups in a low socioeconomic status area: Application of the COM-B model and Behaviour Change Wheel. Health Expect. 2022;25(5):2043–2055. doi: 10.1111/hex.13325. [DOI] [PMC free article] [PubMed]
- 37.Shinohara S, Horiuchi S, Shinohara R, et al. Interpregnancy weight change as a potential risk factor for large-for-gestational-age infants: the Japan Environment and Children’s Study. J Matern Fetal Neonatal Med. 2023;36(1):2209251. doi: 10.1080/14767058.2023.2209251. [DOI] [PubMed]