Abstract
Introduction:
Although the concept of hope is highly relevant for cancer patients, little is known about its association with cancer-relevant biomarkers. Here we examined how hope was related to diurnal cortisol and interleukin-6 (IL-6), a pro-inflammatory cytokine previously associated with tumor biology and survival in ovarian cancer. Secondly, we examined whether hope and hopelessness are distinctly associated with these biomarkers.
Method:
Participants were 292 high-grade ovarian cancer patients who completed surveys and provided saliva samples 4x/daily for 3 days pre-surgery to assess diurnal cortisol. Blood (pre-surgery) and ascites were assessed for IL-6. Hope and hopelessness were assessed using standardized survey items from established scales (Center for Epidemiological Studies Depression Scale; Profile of Mood States, Functional Assessment of Cancer Therapy). Two hopeless items were z-scored and combined into a composite for analysis. Regression models related these variables to nocturnal cortisol, cortisol slope, plasma and ascites IL-6, adjusting for cancer stage, BMI, age, and depression.
Results:
Greater hope was significantly related to a steeper cortisol slope, β = −0.193, p = 0.046, and lower night cortisol, β = −0.227, p = 0.018, plasma IL-6, β = −0.142, p = 0.033, and ascites IL-6, β = −0.290, p = 0.002. Secondary analyses including both hope and hopelessness showed similar patterns, with distinct relationships of hope with significantly lower nocturnal cortisol β = −0.233, p = 0.017 and ascites IL-6, β = −0.282, p = 0.003, and between hopelessness and a flatter cortisol slope, β = 0.211, p = 0.031.
Conclusions:
These data suggest a biological signature of hope associated with less inflammation and more normalized diurnal cortisol in ovarian cancer. These findings have potential clinical utility but need replication with more diverse samples and validated assessments of hope.
Keywords: Hope, Diurnal cortisol, Inflammation, Ovarian cancer, Interleukin-6, Hopelessness, Positive psychology
1. Introduction
Maintaining hope is a fundamental principle throughout the continuum of cancer care for patients, caregivers, and clinicians alike (Corn et al., 2020) although the concept of hope has also been controversial in oncology care (Corn et al., 2020). A recent review highlighted the importance of hope for cancer patients. Across 33 empirical studies, hope was associated with enhanced quality of life, social relationships, spiritual and existential well-being, and with less depression, distress, and symptomatology (Nierop-van Baalen et al., 2020). Hope was also related to more active coping and less helplessness in breast cancer patients (Hasson-Ohayon et al., 2009). In addition, an association has been found between high levels of hope and lower mortality among patients with advanced cancer receiving palliative care (Corn et al., 2022). Nevertheless, little is known about whether there is a relationship between hope and cancer-relevant biomarkers.
A recent review of hope in cancer research (Feldman and Corn, 2023) indicated that hope has been defined by two main approaches, Snyder’s “Hope Theory” (Snyder, 1994, 2002) and Herth’s model of hope (Herth, 1991). Hope theory conceptualizes hope as a cognitive construct combining goal-oriented expectations, the perception of the ability to find ways to reach goals (pathways) and agency, which is motivation to achieve goals. Herth’s hope model is based on a multi-dimensional approach to hope (Dufault and Hope, 1985) and recognizes affective, cognitive, behavioral, affiliative, temporal, and contextual components. The role of positive psychological approaches (including hope) in enhancing personal resources and coping has been highlighted for its potential long-term adaptive benefits (Fredrickson, 2001). In contrast, hopelessness has been described as the presence of negative expectations about the future accompanied by loss of motivation to make things different (Beck et al., 1974). Thus, hope has a component of agency that is not present in hopelessness. A recent factor analysis has shown that hopefulness and hopelessness are best conceptualized as two distinct but correlated constructs; therefore, the absence of hopelessness does not indicate the presence of hope (Huen et al., 2015). For example, regarding a decision to receive a second-line therapy, a patient may not be hopeless that a second-line therapy will work, and yet may not be particularly hopeful about it either.
There are minimal data on biological correlates of hope. A national survey of older US adults found that hope was associated with lower allostatic load (a composite of 7 neuroendocrine, metabolic, and inflammatory measures) among Whites and among Blacks who had previously experienced racial discrimination (Mitchell et al., 2020). However, among patients in the stressful surgical context of open heart surgery, hope was not related to the pro-inflammatory cytokine interleukin-6 (IL-6) (Ai et al., 2010). In contrast, hopelessness has been associated with flatter cortisol slopes, suggestive of poorer diurnal cortisol regulation (Pössel et al., 2015). Hopelessness has also been associated with higher levels of IL-6 following a laboratory based social stressor (Giletta et al.,2018).
Related positive psychological constructs such as positive affect and benefit finding have been associated with biomarkers including diurnal cortisol patterns and inflammatory markers in the general population (Bower et al., 2008; Stellar et al., 2015; Steptoe, 2019; Steptoe et al., 2012). However, with several exceptions (Cruess et al., 2000; Wang and Hoyt, 2018), there has been minimal investigation of positive psychological factors and biomarkers in cancer patients. Among cancer patients, psychosocial attributes such as depression, social isolation, and anxiety have been associated with negative changes in a variety of biomarkers including neuroendocrine and inflammatory markers linked with tumor progression (Antoni et al., 2011; Bower et al., 2018d; Cohen et al., 2012; Lutgendorf et al., 2018, 2020; Chang et al., 2022). However, we are not aware of any studies that have characterized the association between hope and cancer-relevant biomarkers in oncology patients.
To address this knowledge gap, we investigated how hope was associated with biomarkers previously associated with tumor progression and survival in ovarian and other cancers, namely the proinflammatory cytokine IL-6 (Lane et al., 2011; Browning et al., 2018) and the anti-inflammatory neuroendocrine hormone cortisol (Cohen et al., 2012; Schrepf et al., 2015; Sephton et al., 2013; Sephton et al., 2000). IL-6 is produced by a variety of cells including immune cells, fibroblasts, endothelial cells, and ovarian tumor cells c (Nilsson et al., 2005; Balkwill and Mantovani, 2001) and is considered to be one of the major immunoregulatory cytokines in the ovarian tumor microenvironment (Browning et al., 2018). IL-6 activates signaling pathways leading to ovarian tumor proliferation, angiogenesis (the process by which tumors stimulate formation of blood vessels that support their growth), metastatic spread, and resistance to chemotherapy (Browning et al., 2018; Isobe et al., 2015) and has also been linked to ovarian cancer progression and survival (Dobrzycka et al., 2013; Tempfer et al., 1997). Cortisol has been shown to increase invasiveness of ovarian cancer cells in vitro (Sood et al., 2006), and ascites-derived cortisol is associated with higher levels of cytokines promoting tumor growth coupled with inhibition of molecules supporting cellular immunity (Knochenhauer et al., 2021). We have previously reported higher levels of nocturnal cortisol and diminished cortisol variability in ovarian cancer patients compared to patients with benign gynecologic disease and healthy controls (Weinrib et al., 2010). Additionally, high levels of nocturnal cortisol and a flatter diurnal cortisol slope have both been associated with poorer survival in ovarian cancer (Schrepf et al., 2015). Disrupted cortisol rhythms have also been related to poorer survival in breast, lung, and renal cell cancer patients (Cohen et al., 2012; Sephton et al., 2013; Sephton et al., 2000).
We hypothesized that hope would be associated with better diurnal cortisol regulation (lower nocturnal cortisol and steeper slope) and less inflammatory activity (lower levels of plasma and ascites IL-6). A secondary question was whether hopelessness would have any distinctive relationship with these biomarkers relative to hope. We hypothesized that hope and hopelessness would share a certain amount of overlapping variance, but they would each have distinctive associations with biological outcomes. Because fatigue is known to be associated with both IL-6 and cortisol (Bower and Lamkin, 2013; Costanzo et al., 2005) and is also linked with depression (Jacobsen et al., 2003; Targum and Fava, 2011), we also examined the potential contribution of fatigue to these associations in ancillary analyses.
2. Methods
2.1. Participants
Women with suspected ovarian cancer were prospectively recruited at their initial clinic visit at two Midwestern academic medical centers and one academic medical center in the Southeastern United States as part of a larger study of biobehavioral factors in ovarian cancer. The study was IRB-approved at all sites, and informed consent was obtained during the initial visit prior to primary surgery or neoadjuvant chemotherapy. Participants with primary epithelial ovarian, peritoneal, or fallopian tube carcinomas were eligible following histologic confirmation of disease. Women with benign disease, non-epithelial malignancies, tumors of low malignant potential (LMP), previous cancer within five years, use of systemic corticosteroids in the last month, and age below 18 years were excluded. To maximize homogeneity, for the present analyses, only those with high grade disease (poorly differentiated and more aggressive tumors) receiving their primary surgery before chemotherapy were included. Of the 342 patients meeting these conditions, 26 had missing values for the hope/hopelessness items and 24 were missing values for both cortisol and IL-6; therefore, 292 patients were included in the final sample.
2.2. Procedure
Participants completed surveys and provided saliva 4 times daily for 3 days pre-surgery to assess cortisol. Patients were instructed to refrigerate samples at home until returned to the clinic at the time of their surgery; samples were stored at −80 °C until analysis. Peripheral blood was sampled pre-surgery, and ascites was collected during surgery. Both were collected in heparinized tubes (BD Biosciences, San Jose, CA) and centrifuged at 2200 rpm at 4 °C for 5 min and frozen at −80 °C for later analysis.
2.3. Measures
2.3.1. Center for Epidemiologic Studies Depression Scale
(CESD) (Radloff, 1977) is a well-validated 20-item self-report scale measuring the frequency of depressive symptoms over the last week on a 0–3-point scale, ranging from (0) indicating symptoms occurring rarely or not at all to (3) indicating symptoms occurring most or all of the time. Four subscales representing facets of depressive symptoms (vegetative depression, depressed mood, positive mood, and interpersonal relationships) have been confirmed by factor analysis (Sheehan et al., 1995). The depressed mood subscale was used as a covariate in regression analyses as a conservative measure to control for potential effects of negative affectivity. This subscale does not contain any items related to hope or hopelessness. The CESD has been frequently used in cancer patients (Hann et al., 1999).
2.3.2. The Functional Assessment of Cancer Therapy
The FACT-G is a well-validated 27-item measure assessing physical, social, functional, and emotional well-being in cancer patients over the last week. Items are measured on a 5-point scale from (0) not at all to (4) very much (Webster et al., 2003; Basen-Engquist et al., 2001).
2.3.3. The Profile of Mood States (short form)
The POMS-SF (Curran et al., 1995) is a 37-item self-report scale assessing mood over the past week. It includes 6 subscales: anxiety, dysphoria, anger, vigor, fatigue, and confusion. Items are words or phrases that are endorsed on a scale from 0 (not at all) to 4 (extremely). This scale has been validated previously in cancer patient populations (Thornton et al., 2008). The fatigue subscale was used in ancillary analyses examining the potential contribution of fatigue to the relationships of hope and hopelessness with the biological outcomes.
2.3.4. Hope and hopelessness
Hope and hopelessness were indexed using 3 face-valid items from existing scales: a hopeful item from the CESD (“I felt hopeful about the future”), a hopeless item from the FACT (“I am losing hope in the fight against my illness”), and a hopeless item from the POMS-SF (the adjective “hopeless”). Because the presence of hope is not equivalent to the absence of hopelessness and these items have been shown to be distinct constructs in factor analyses (Huen et al., 2015), “hopeful” and “hopeless” items were analyzed separately. Each item was z-scored and the two hopeless items were combined into a composite for analysis. Only those patients with both hope and hopeless data were included in the sample.
2.3.5. Demographic and clinical information
Demographic information was obtained by self-report. Clinical information was abstracted from medical records. Stage was divided into early (I-II) and advanced (III-IV).
2.3.6. Cortisol
Cortisol was collected upon waking (4–9 am), within 30 min of waking, between 4 and 6:30 pm, and at bedtime (8–12 pm). Participants were instructed not to eat, drink caffeine, or exercise for 30 min prior to sample collection, and to refrigerate salivettes (Starstedt) after collection. Salivettes were returned prior to surgery or NAC. Collection time was noted on salivettes by participants; this practice was shown previously to be reliable (Kraemer et al., 2006). Assays were performed in the laboratory of Clemens Kirschbaum at the Technical University of Dresden using a commercial chemiluminescence immunoassay (IBL, Hamburg, Germany). The lower limit of detection is 0.41 nmol/L and inter-assay and intra-assay coefficients of variance are < 10 %. Values were natural log transformed to normalize their distribution. The cortisol awakening sample (waking + 30 mins) was not used in these analyses as our primary variables of interest from previous analyses were nocturnal cortisol and cortisol slope which were both related to survival (Schrepf et al., 2015). Cortisol was collected at only 2 of the 3 sites. More negative (steeper) cortisol slopes indicate more rapid salivary cortisol declines over the course of the day and are considered to represent better diurnal cortisol regulation.
2.3.7. Interleukin-6
Interleukin-6 was assessed in plasma and ascites using an enzyme linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) with results calculated using the standard curve provided with the kit. The minimum detectable level is less than 0.7 pg/mL and inter assay variability ranges from 3.3 % to 6.4 %. IL-6 samples below the sensitivity of the regular assay were assessed with the R&D Systems High Sensitivity (HS) ELISA. To normalize the distribution, all values were log (10) transformed. Nine plasma samples with values under the detectable limit were not able to be assayed using the HS kit and were assigned the lowest detectable value (0.7 pg/mL). Sensitivity analyses for plasma IL-6 were also performed eliminating these 9 samples.
2.4. Statistical analyses
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 29.0. (IBM, Armonk, NY) Distributions were examined for outliers and normality before analysis. Cortisol data reduction was performed in the following manner. Sampling time outliers for cortisol were removed before analyses. Acceptable sampling time ranges were established to fit the maximum number of participants while maintaining a certain amount of homogeneity. The initial morning sample was collected at the participant’s normal awakening time, which is associated with a rise in cortisol (Kirschbaum et al., 2000). Acceptable sampling times were 0400 to 0900 h for morning cortisol, 1600 to 1830 h for pm cortisol, and 2000 to 2400 h for nocturnal cortisol. Samples were excluded if they were more than four standard deviations above the mean of their specific time point. Mean cortisol values were calculated at each time-point and the natural logarithm (ln) transformation was used to normalize their distribution. The slope of diurnal change in cortisol was calculated by regressing the 9 cortisol values on the hour of sample collection, with data pooled over the 3 collection days for each patient, consistent with prior research (Sephton et al., 2000; Sephton et al., 2009). For the primary analysis, regression models were used to determine associations between the hope item and the biological variables, adjusting for age, stage, body mass index (BMI), and the CESD depressed mood subscale. Secondary analyses were performed to address the question of whether hope and hopelessness have any distinctive relationships with the biological variables. These secondary models included both hope and hopelessness as predictors of the biological variables, adjusting for all covariates as above. Ancillary analyses examined whether fatigue might contribute to the associations seen in these analyses. For these analyses fatigue was included as a covariate along with age, stage, BMI, and mood, and including both hope and hopelessness as independent predictors.
3. Results
3.1. Participant characteristics
As seen in Table 1, participants had a mean age of 60.28 (±11.06) years and were largely married or partnered (78.08 %), White (95.89 %), and non-Hispanic (98.29 %). The mean level of depressive mood (CESD = 16.53, ±10.01) was in a range consistent with moderate clinical depression; 49.3 % of patients were in a range considered clinically depressed (CESD ≥ 16); 21.3 % were in the severely depressed range (CESD ≥ 24). There was substantial variability in self-reports of hope, with 38.7 % of participants reporting feeling hopeful most of the time, 34.6 % much of the time, 21.2 % occasionally, and 5.5 % rarely. There was more consistency in reports of “losing hope in the fight against my illness,” with 75.8 % reporting not at all and 15.8 % a little bit, and for the POMS “hopeless” item, 70.1.% reported that they were not at all hopeless and 17.4 % reported that they were a little hopeless. There was no significant difference in hope or hopelessness between patients with and without biomarker data, p values > 0.075. Neither hope nor hopelessness was related to potential demographic confounds such as marital status (p values > 0.10) or education (p values > 0.40). Hope and hopelessness were moderately correlated with each other (r = −0.365, p < 0.001) and with the depressed mood subscale of the CESD (hope: r = −0.386, p < 0.001; hopelessness: r = 0.455, p < 0.001).
Table 1.
Demographic and Clinical Information for High Grade Ovarian Cancer Patients.
| Patients | |
|---|---|
| N = 292 | |
| Age, M (SD) years | 60.28 (11.06) |
| Race, N, (%) | |
| Non-white | 10 (3.42%) |
| White | 280 (95.89%) |
| Ethnicity, N, (%) | |
| Non-Hispanic | 287 (98.29%) |
| Hispanic | 5 (1.71%) |
| Education, N, (%) | |
| High School or Less | 113(38.70%) |
| Some College/Trade School | 87 (29.79%) |
| College Degree | 65 (22.26%) |
| Advanced Degree | 26 (8.90%) |
| Missing | 1 (0.34%) |
| Relationship Status, N, (%) | |
| Not Married | 64(21.92%) |
| Married/Living with Partner | 228 (78.08%) |
| Stage, N, (%) | |
| I | 37 (12.67%) |
| II | 21 (7.19%) |
| III | 208 (71.23%) |
| IV | 26 (8.90%) |
| Grade | |
| High | 292 (100%) |
| Histology, N, (%) | |
| Non-Serous | 61 (20.89%) |
| Serous | 231 (79.11%) |
| Mean (SD) | |
| Body Mass Index, kg/m2 n=292 | 28.46 (6.96) |
| Plasma IL-6 (log 10) (pg/mL) n=265 | 1.06 (.59) |
| Ascites IL-6 (log 10) (pg/mL) n=133 | 3.65 (.52) |
| Nocturnal cortisol (In) nmol/L n= 144 | 1.65 (.80) |
| Cortisol Slope n=139 | −.104 (.05) |
| Depression (CESD total score) 292 | 16.53(10.01) |
Note: Percentages are in parentheses unless mean and standard deviation are specifically indicated. IL-6 = interleukin-6. CESD: Center for Epidemiological Studies Depression Scale.
3.2. Regression models
All regression models adjusted for cancer stage, BMI, age, and depressed mood. As seen in Table 2, hope was significantly related to lower night cortisol, β = −0.227 p = 0.018, and steeper cortisol slope, β = −0.193, p = 0.046, lower plasma IL-6, β = −0.142, p = 0.033 and ascites IL-6, β = −0.290, p = 0.002. (Fig. 1A and 1B). Sensitivity analyses excluding 9 participants with estimated levels of plasma IL-6 showed even stronger associations between hope and plasma IL-6, β = −208, p = 0.002.
Table 2.
Regression models of hope predicting cortisol and IL-6 in ovarian cancer patients.
| Variable | Unstandardized Coefficients | Standardized Coefficients | p | Variable | Unstandardized Coefficients | Standardized Coefficients | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Nocturnal Cortisol1 | B | Std. Error | Beta | Plasma IL-63 | B | Std. Error | Beta | ||
| (Constant) | 0.763 | 0.602 | 0.207 | (Constant) | 0.431 | 0.316 | 0.174 | ||
| Age | 0.008 | 0.006 | 0.121 | 0.168 | Age | −0.001 | 0.033 | −0.024 | 0.706 |
| Stage | 0.095 | 0.168 | 0.049 | 0.574 | Stage | 0.236 | 0.093 | 0.159 | 0.012 |
| BMI | 0.011 | 0.011 | 0.089 | 0.305 | BMI | 0.010 | 0.005 | 0.110 | 0.080 |
| CESD depress subscale | −0.015 | 0.024 | −0.059 | 0.532 | CESD depress subscale | 0.003 | 0.012 | 0.017 | 0.806 |
| Hope Cortisol Slope 2 | −0.205 | 0.086 | −0.227 | 0.018 | Hope Ascites IL-6 4 | −0.085 | 0.040 | −0.142 | 0.033 |
| (Constant) | −0.100 | 0.037 | 0.008 | (Constant) | 4.248 | 0.471 | <0.001 | ||
| Age | 0.000 | 0.000 | 0.043 | 0.645 | Age | −0.005 | 0.004 | −0.109 | 0.221 |
| Stage | 0.005 | 0.011 | 0.042 | 0.644 | Stage | −0.126 | 0.147 | −0.076 | 0.391 |
| BMI | −0.001 | 0.001 | −0.085 | 0.344 | BMI | −0.003 | 0.007 | −0.036 | 0.687 |
| CESD depress subscale | −0.001 | 0.002 | −0.061 | 0.525 | CESD depress subscale | 0.005 | 0.014 | 0.029 | 0.753 |
| Hope | −0.011 | 0.005 | −0.193 | 0.046 | Hope | −0.166 | 0.053 | −0.290 | 0.002 |
Notes:
n = 143,
n = 140
n = 264,
n = 132; Stage is early/advanced, hope and hopelessness are z-scored; BMI = Body Mass Index CESD = Center for Epidemiologic Studies Depression Scale (depressive subscale).
Fig. 1.
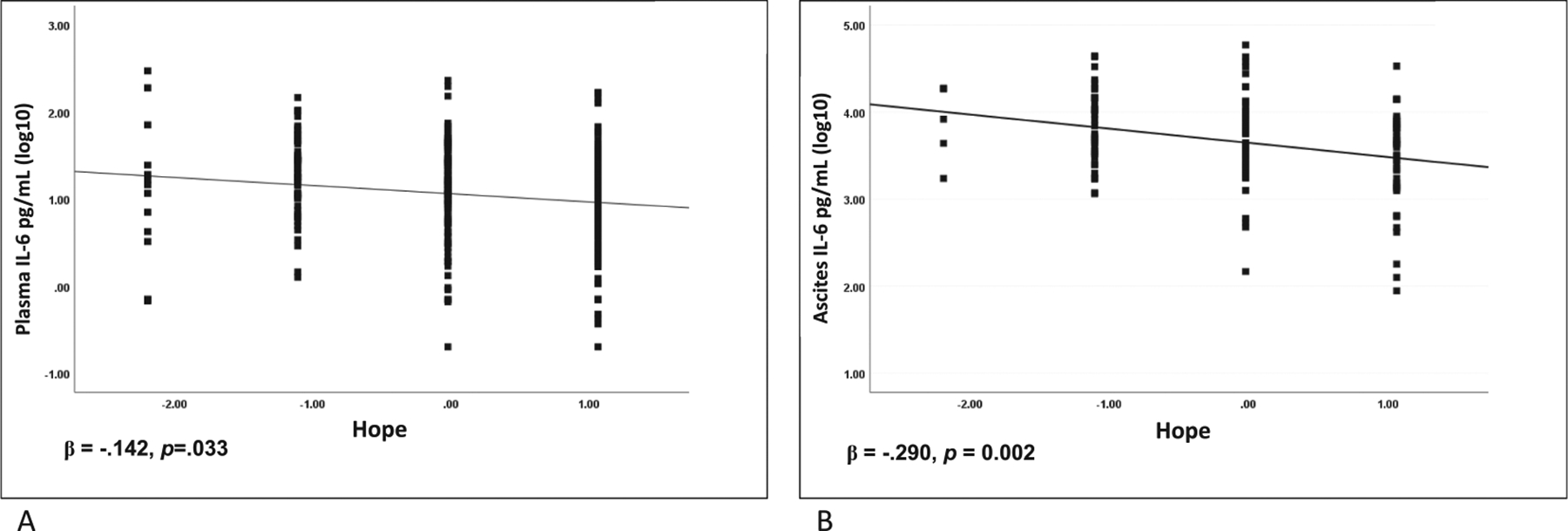
A) Hope and plasma IL-6 pg/mL in ovarian cancer patients. B) Hope and ascites IL-6 pg/mL in ovarian cancer patients. Hope and hopeless items are z-scored.
Secondary analyses including both hope and hopelessness, with covariates as above are shown in Table 3. In general, we observed the same directionality of effects as in the analyses with hope alone, but in some cases the effects of hope were attenuated due to shared variance with hopelessness. Over and above the non-significant effects of hopelessness, hope was significantly related to lower nocturnal cortisol, β = −0.233, p = 0.017 and to lower ascites IL-6, β = −0.282, p = 0.003. In contrast, when both hope and hopelessness were in the model together, hopelessness predicted a significantly flatter cortisol slope, β = 0.211, p = 0.031, whereas hope was associated with a steeper slope, but was no longer significant β = −0.152, p = 0.118. For plasma IL-6, hopelessness was associated with somewhat higher IL-6, β = 0.122, p = 0.084, whereas hope was unrelated to plasma IL-6, β = −0.112, p = 0.103. Sensitivity analyses eliminating samples with estimated IL-6 levels demonstrated a significant inverse relationship between hope and plasma IL-6, β = −0.182, p = 0.009.
Table 3.
Secondary Analyses: Regression models of hope and hopelessness predicting cortisol and IL-6 in ovarian cancer patients.
| Variable | Unstandardized Coefficients | Standardized Coefficients | p | Variable | Unstandardized Coefficients | Standardized Coefficients | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Nocturnal Cortisol1 | B | Std. Error | Beta | Plasma IL-63 | B | Std. Error | Beta | ||
| (Constant) | 0.711 | 0.623 | 0.256 | (Constant) | 0.560 | 0.323 | 0.085 | ||
| Age | 0.009 | 0.006 | 0.128 | 0.157 | Age | −0.002 | 0.003 | −0.040 | 0.526 |
| Stage | 0.097 | 0.169 | 0.050 | 0.568 | Stage | 0.219 | 0.093 | 0.147 | 0.020 |
| BMI | 0.011 | 0.011 | 0.090 | 0.301 | BMI | 0.009 | 0.005 | 0.104 | 0.097 |
| CESD depress subscale | −0.012 | 0.026 | −0.047 | 0.643 | CESD depress subscale | −0.005 | 0.013 | −0.026 | 0.715 |
| Hopelessness | −0.042 | 0.123 | −0.032 | 0.736 | Hopelessness | 0.090 | 0.052 | 0.122 | 0.084 |
| Hope Cortisol Slope 2 | −0.210 | 0.087 | −0.233 | 0.017 | Hope Ascites IL-64 | −0.067 | 0.041 | −0.112 | 0.103 |
| (Constant) | −0.077 | 0.038 | 0.045 | (Constant) | 4.335 | 0.480 | <0.001 | ||
| Age | − 1.179E–5 | 0.000 | −0.003 | 0.975 | Age | −0.005 | 0.004 | −0.116 | 0.192 |
| Stage | 0.004 | 0.011 | 0.031 | 0.733 | Stage | −0.133 | 0.147 | −0.081 | 0.365 |
| BMI | −0.001 | 0.001 | −0.101 | 0.259 | BMI | −0.004 | 0.007 | −0.046 | 0.606 |
| CESD depress subscale | −0.002 | 0.002 | −0.134 | 0.185 | CESD depress subscale | −0.003 | 0.017 | −0.022 | 0.838 |
| Hopelessness | 0.017 | 0.008 | 0.211 | 0.031 | Hopelessness | 0.056 | 0.059 | 0.097 | 0.347 |
| Hope | −0.008 | 0.005 | −0.152 | 0.118 | Hope | −0.161 | 0.053 | −0.282 | 0.003 |
Notes:
n = 143,
n = 138,
n = 264,
n = 132; Stage is early/advanced, hope and hopelessness are z-scored BMI = Body Mass Index CESD = Center for Epidemiologic Studies Depression Scale (depressive subscale).
Ancillary analyses, shown in Supplemental Table 1, examined the potential contribution of fatigue to the associations between hope, hopelessness, and the biological variables assessed. Fatigue was moderately correlated with both hopelessness, r = 0.38, p < 001 and hope, r = −0.286, p < 0.001. Including fatigue as a covariate slightly attenuated the association of hope with nocturnal cortisol, β = −0.212, p = 0.006 and ascites IL-6, β = −0.252, p = 0.009; however, both of these associations still remained significant over and above the effects of fatigue, depression, and hopelessness. Fatigue was associated with a significantly flatter cortisol slope (β =.266, p = 0.005) whereas hope, hopelessness, and depression became non-significant with fatigue in the model as a covariate. With fatigue in the model for plasma IL-6, the relationships of both hope and hopelessness were non-significant, whereas fatigue was significantly associated with higher plasma IL-6, β = 0.224, p = 0.001. In sensitivity analyses, greater hope was significantly related to lower plasma IL-6, β = −0.182, p = 0.010, over and above the significant effects of fatigue (p = 0.006) and the non-significant effects of hopelessness and depression.
4. Discussion
The main findings of this study are that among ovarian cancer patients prior to surgery, those reporting greater hope had lower IL-6 in peripheral blood and in the tumor microenvironment (ascites), lower levels of nocturnal cortisol, and a steeper (i.e., healthier) diurnal cortisol slope. When hope and hopelessness were included in the same model to examine their distinct effects, hope continued to be associated with lower levels of nocturnal cortisol and ascites IL-6, independent of the non-significant effects of hopelessness. In contrast, hopelessness was associated with a flatter cortisol slope independent of the effects of hope. When examined together, neither hope nor hopelessness showed a distinct association with plasma IL-6. Findings were robust while adjusting for age, stage, body mass index, and depressed mood. These data suggest that hope is associated with lower levels of inflammation and a healthier pattern of diurnal cortisol in ovarian cancer. These analyses also indicate that there are some distinct associations of hope and hopelessness with biological outcomes that emerge beyond their shared variance. Moreover, the effects of hope appear to be at least partially distinct from the effects of depression (i.e., hope remains associated with biological outcomes despite controlling for depressed mood).
Although the present study did not focus on clinical outcomes, prior research has shown that flatter cortisol slopes are related to poorer survival in ovarian and other cancers (Cohen et al., 2012; Schrepf et al., 2015; Sephton et al., 2013; Sephton et al., 2000); and that IL-6 is implicated in the biology of tumor progression (Browning et al., 2018) and related to poorer survival in ovarian cancer (Lane et al., 2011).
These findings are consistent with data on hope-related constructs. For example, a review of studies examining positive affect (e.g., happiness) and biological processes found a consistent association between higher levels of emotional well-being and both lower cortisol levels and a steeper salivary cortisol slope (Steptoe, 2019). In cancer patients, related constructs such as benefit finding (finding meaning in adversity), self-compassion, and positive affect have been related to steeper cortisol slopes (Ho et al., 2022; Wang and Hoyt, 2018) and lower overall cortisol levels (Cruess et al., 2000). The present findings are also consistent with prior research linking aspects of positive well-being with lower levels of inflammation (Moreno et al., 2016; Stellar et al., 2015; Steptoe et al., 2008, 2012).
The present data do not address the biobehavioral mechanisms underlying these findings, but we believe they likely involve the interaction of hope and coping strategies (Lazarus, 1999). For example, breast cancer patients high in hope had a lower fear of cancer recurrence 3 months post-diagnosis if they used the cognitive coping strategy of positive reinterpretation and growth (Stanton et al., 2002). Early-stage breast cancer patients who were high in hope and also used emotionally expressive coping reported lower distress and fewer cancer-related medical visits than their counterparts who were lower in emotional expression (Stanton et al., 2000, 2002). With respect to the agency component of hope, our findings are consistent with evidence of blunted cortisol (Bollini et al., 2004; O’Donnell et al., 2008), lower IL-6 reactivity to stress (Mausbach et al., 2011), and lower levels of catecholamines elicited by fear (Bandura et al., 1985) in individuals with high self-efficacy. Taken together, these findings suggest the possibility that hope, either via positive affect or more effective coping, may be associated with lower levels of both sympathetic and HPA-axis reactivity to stressors and allow for restorative activities (Bower et al., 2008), all of which can serve as a buffer to potential dysregulation of cortisol and stress-related amplification of inflammatory processes.
Ancillary analyses addressed the question of whether the associations of hope with biological outcomes might be a function of the patient’s fatigue, as fatigue is known to be related to both cortisol and IL-6 as well as to depression. Fatigue slightly attenuated the relationship of hope with all biological variables, although the association of hope with nocturnal cortisol and ascites IL-6 still remained significant independent of fatigue. Fatigue also attenuated the association of hopelessness and cortisol slope. It is possible that hope may wane as a consequence of the same disease progression biology that underlies greater fatigue (Browning et al., 2018; Bower and Lamkin, 2013), or that hopelessness may increase with increases in inflammatory mediators such as IL-6 that in turn produce fatigue (Bower and Lamkin, 2013). This should be examined in future research.
Recent research has also identified neurocognitive pathways that may potentially underlie these effects. For example, dispositional hope has been related to lower levels of low frequency fluctuations (fALFF) in the bilateral medial orbitofrontal cortex (mOFC), which is involved in motivation, goal directed behaviors, and reward processing. This spontaneous activity of the mOFC in individuals with trait hope was shown to have a protective role against anxiety (Wang et al., 2017). Activation of the brain’s positive reward system, which processes positive affect, expectations, and motivated behavior, has been shown to be related to decreased inflammation, and enhanced anti-tumor immunity (Ben-Shaanan et al., 2016, 2018; Dutcher et al., 2021). This represents another potential pathway by which an affective and motivational state such as hope may affect cancer-relevant biology.
With respect to hopelessness, although there is limited data related to biological outcomes, our findings are consistent with prior research showing that hopelessness is related to a flatter diurnal cortisol slope in a healthy middle-aged population (Pössel et al., 2015), although unlike the present study, previous findings reported that the effects of hopelessness were mediated through depression. We are not aware of studies that have examined links between hopelessness and biomarkers in cancer patients, but our finding that hopelessness was related to a flatter cortisol slope over and above the effects of hope, is consistent with previous research demonstrating associations between depression and flatter diurnal cortisol slopes in renal cancer patients (Cohen et al., 2012).
4.1. Limitations
Several limitations of this study should be noted. First, hope and hopelessness were assessed using individual, face-valid items. This approach has been used previously and has been associated with longer survival in advanced cancer patients in palliative care (Corn et al., 2022). However, additional research is warranted using more comprehensive assessments of these key constructs. Second, although the independence of the constructs of hope and hopelessness has previously been demonstrated (Huen et al., 2015), we have observed some overlapping variance in these two constructs, and the unique contribution that each construct makes to cancer-related biology needs more investigation. Third, it should be noted that inflammation can induce anhedonia and has been shown to inhibit functional connectivity within reward pathways in the brain (Felger et al.; Miller and Raison, 2016). Therefore, we cannot rule out the possibility that elevated levels of inflammation may have contributed to decreased motivation, negative affect, or fatigue that may be reflected as a lack of hope in this population. We adjusted for depressive mood in all analyses to minimize the possibility of this confound, and adjusted for fatigue in ancillary analyses, but these constructs should be further examined. Fourth, we did not have objective verification of cortisol sampling time; thus although participant labeling of sampling time has previously been shown to be reliable (Kraemer et al., 2006), there is the possibility of some measurement error without objective verification of sampling time. Additionally, we do not have other measures of positive affect and optimism, and thus cannot examine how distinctive hope would be compared to other measures of positive mood. Finally, the present study was correlational, and directionality and causality cannot be assumed.
4.2. Conclusions
Notwithstanding these limitations, the present findings are the first that we know of to characterize how hope and hopelessness are related to disease-relevant biomarkers in cancer. The findings indicate that hope is associated with less inflammation and more normalized cortisol profiles. There appear to be some distinct associations of hope and hopelessness with the biological outcomes assessed, with evidence of some overlap as well. These associations appear to be independent of depressive mood and may be somewhat influenced by fatigue. These findings support the potential biological relevance of positive psychological states such as hope in patients with cancer. The findings thus have potential clinical utility, particularly as interventions to enhance hope have been developed (Corn et al., 2023), but need to be replicated with more diverse samples, other assessments of hope, and other cancer-relevant biomarkers.
Supplementary Material
Funding sources
This project was supported in part by NIH grants CA193249, CA140933, CA246540 (SL); CA109298 and CA209904 (AKS), P30CA086862 (PI Weiner) and the American Cancer Society (AKS). GMS was supported by grant OPR21101 from the California Initiative to Advance Precision Medicine. BWC was supported by the Pamm Gross Kahane Research Institute of Lifesdoor.org. The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Thaker has served as a consultant for Immunon, has been on advisory boards for Astra Zeneca, Clovis Oncology, Glaxo Smith Kline, Seagen, Agenus, Immunon, Immunogen, Mersana, Novocure R-Pharm, Zentalis, Aadi Pharmaceuticals, Merck, Caris, and Iovance Biotherapeutics, and has had research funding from Merck and Glaxo Smith Kline, and is a Immunon shareholder; Dr. Lutgendorf is an Abbvie shareholder; Dr. Sood has done consulting for Merck, Astra Zeneca, Kiyatec, Glaxo Smith Kline, Onexo, ImmunoGen, Iylon, has a patent planned/pending for EGFL6 antibody, and is a Biopath shareholder; Dr. Corn is the Chief Medical Officer of Lutris-Pharma. All other authors report no conflict of interest.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2023.12.014.
Data availability
The authors do not have permission to share data.
References
- Ai AL, Pargament KI, Appel HB, Kronfol Z, 2010. Depression following open-heart surgery: A path model involving interleukin-6, spiritual struggle, and hope under preoperative distress. J. Clin. Psychol 66 (10), 1057–1075. 10.1002/jclp.20716. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Blomberg B, et al. , 2011. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol. Psychiatry 71, 366–372. 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A, 2001. Inflammation and Cancer: Back to Virchow? Lancet 357 (9255), 539–545. 10.1016/s0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bandura A, Taylor CB, Williams SL, Mefford IN, Barchas JD, Jun 1985. Catecholamine secretion as a function of perceived coping self-efficacy. J. Consult. Clin. Psychol 53 (3), 406–414. 10.1037//0022-006x.53.3.406. [DOI] [PubMed] [Google Scholar]
- Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. , 2001. Reliability and validity of the Functional Assessment of Cancer Therapy-Ovarian. J. Clin. Oncol 19, 1809–1817. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L, 1974. The measurement of pessimism: The Hopelessness Scale. J. Consult. Clin. Psychol 42, 861–865. [DOI] [PubMed] [Google Scholar]
- Ben-Shaanan TL, Azulay-Debby H, Dubovik T, et al. , 2016. Activation of the reward system boosts innate and adaptive immunity. Nat. Med 22 (8), 940–944. 10.1038/nm.4133. [DOI] [PubMed] [Google Scholar]
- Ben-Shaanan TL, Schiller M, Azulay-Debby H, et al. , 2018. Modulation of anti-tumor immunity by the brain’s reward system. Nature. Communications 9 (1), 2723. 10.1038/s41467-018-05283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollini AM, Walker EF, Hamann S, Kestler L, 2004. The influence of perceived control and locus of control on the cortisol and subjective responses to stress. Biol. Psychol 67 (3), 245–260. 10.1016/j.biopsycho.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Bower JE, Lamkin DM, 2013. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav. Immun 30, S48–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J, Low C, Moskowitz J, Sepah S, Epel E, 2008. Benefit finding and physical health: Positive psychological changes and enhanced allostasis. Soc. Pers. Psychol. Compass 2 (1), 223–244. [Google Scholar]
- Bower JE, Shiao SL, Sullivan P, et al. , 2018d. Prometastatic Molecular Profiles in Breast Tumors From Socially Isolated Women. JNCI Cancer Spectr. Jul 2 (3). 10.1093/jncics/pky029pky0doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL, 2018. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag. Res 10, 6685–6693. 10.2147/cmar.S179189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Sloan EK, Antoni MH, Knight JM, Telles R, Lutgendorf SK, 2022. Biobehavioral Pathways and Cancer Progression: Insights for Improving Well-Being and Cancer Outcomes. Integr Cancer Ther 21. 10.1177/15347354221096081, 15347354221096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Cole SW, Sood AK, et al. , 2012. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One 7 (8), e42324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn BW, Feldman DB, Subbiah IM, et al. , 2023. Feasibility and Acceptability of an Online Intervention to Enhance Hopefulness Among Oncology Professionals. JNCI Cancer Spectr 7 (3), pkad030. 10.1093/jncics/pkad030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn BW, Feldman D, Schapira L, Steensma DP, Loprinzi CL, Bian J, 2020. Oncologists’ Reluctance to Use the Terms Hope and Cure: A Bibliometric Analysis of Articles From Two High-Impact Oncology Journals. JNCI Cancer Spectr. 4 (6) 10.1093/jncics/pkaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn BW, Feldman DB, Hull JG, O’Rourke MA, Bakitas MA, 2022. Dispositional hope as a potential outcome parameter among patients with advanced malignancy: An analysis of the ENABLE database. Cancer 128 (2), 401–409. 10.1002/cncr.33907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn BW, Feldman DB, Wexler I, 2020. The Science of Hope. Lancet Oncol. 21 (9), e452–e459. 10.1016/s1470-2045(20)30210-2. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM, 2005. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer 104, 305–313. 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- Cruess D, Antoni M, McGregor B, et al. , 2000. Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosom. Med 62, 304–308. 10.1097/00006842-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA, Studts JL, 1995. Short form of the profile of mood states (POMS-SF): Psychometric information. Psychol. Assess 7, 80–83. [Google Scholar]
- Dobrzycka B, Mackowiak-Matejczyk B, Terlikowska KM, Kulesza-Bronczyk B, Kinalski M, Terlikowski SJ, 2013. Serum levels of IL-6, IL-8 and CRP as prognostic factors in epithelial ovarian cancer. Eur. Cytokine Netw 24 (3), 106–113. 10.1684/ecn.2013.0340. [DOI] [PubMed] [Google Scholar]
- Dufault K, Hope MBC, 1985. Hope: Its Spheres and Dimensions. Nurs. Clin. North America 20 (2), 379–391. 10.1016/S0029-6465(22)00328-0. [DOI] [PubMed] [Google Scholar]
- Dutcher JM, Boyle CC, Eisenberger NI, Cole SW, Bower JE, 2021. Neural responses to threat and reward and changes in inflammation following a mindfulness intervention. Psychoneuroendocrinology 125, 105114. 10.1016/j.psyneuen.2020.105114. [DOI] [PubMed] [Google Scholar]
- Feldman DB, Corn BW, 2023. Hope and Cancer. Curr Opin Psychol. 49, 101506 10.1016/j.copsyc.2022.101506. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, et al. , Oct 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21 (10), 1358–1365. 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL., 2001. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 56 (3), 218–226. 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Slavich GM, Rudolph KD, Hastings PD, Nock MK, Prinstein MJ, Feb 2018. Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. J Child Psychol. Psychiatry 59 (2), 129–139. 10.1111/jcpp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D, Winter K, Jacobsen P, 1999. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J. Psychosom. Res 46 (5), 437–443. 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Hasson-Ohayon I, Braun M, Galinsky D, Baider L, 2009. Religiosity and hope: a path for women coping with a diagnosis of breast cancer. Psychosomatics 50 (5), 525–533. 10.1176/appi.psy.50.5.525. [DOI] [PubMed] [Google Scholar]
- Herth K, 1991. Development and refinement of an instrument to measure hope. Sch Inq Nurs Pract 5 (1), 39–51; 53–6. [PubMed] [Google Scholar]
- Ho RTH, Fong TCT, Wan AHY, 2022. Effects of Self-compassion on Diurnal Cortisol Pattern via Positive Affect in Colorectal Cancer Survivors. Mindfulness 13 (1), 211–221. 10.1007/s12671-021-01786-3. [DOI] [Google Scholar]
- Huen JM, Ip BY, Ho SM, Yip PS, 2015. Hope and Hopelessness: The Role of Hope in Buffering the Impact of Hopelessness on Suicidal Ideation. PLoS One 10 (6), e0130073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe A, Sawada K, Kinose Y, et al. , 2015. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PLoS One 10 (2), e0118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen P, Donovan KA, Weitzner MA, 2003. Distinguishig fatigue and depression in patients with Cancer. Sem Clin Neuropsychiatry. 8 (4), 229–240. 10.1016/S1084-3612(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH, 2000. Salivary cortisol. In: Fink G (Ed.), Encyclopedia of Stress. Academic Press. [Google Scholar]
- Knochenhauer H, Aquino-Acevedo A, Armaiz-Pena G, Previs R, 2021. Ascites-derived cortisol correlates with inflammatory and immunosuppressive cytokines in ovarian cancer patients. Int J Gyn Oncol 31 (4), A103–A104. [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, et al. , 2006. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatric Psychiatry. 14 (4), 325–333. [DOI] [PubMed] [Google Scholar]
- Lane D, Matte I, Rancourt C, Piché A, 2011. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 11, 210. 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, 1999. Hope: An emotion and a vital coping resource against despair. Soc. Res 653–678. [Google Scholar]
- Lutgendorf SK, Thaker PH, Arevalo JM, et al. , 2018. Biobehavioral modulation of the exosome transcriptome in ovarian carcinoma. Cancer 124 (3), 580–586. 10.1002/cncr.31078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Penedo F, Goodheart MJ, et al. , 2020. Epithelial-mesenchymal transition polarization in ovarian carcinomas from patients with high social isolation. Cancer 126 (19), 4407–4413. 10.1002/cncr.33060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, von Känel R, Roepke SK, et al. , 2011. Self-efficacy buffers the relationship between dementia caregiving stress and circulating concentrations of the proinflammatory cytokine interleukin-6. Am. J. Geriatr. Psychiatry 19 (1), 64–71. 10.1097/JGP.0b013e3181df4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16 (1), 22–34. 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell UA, Dellor ED, Sharif MZ, Brown LL, Torres JM, Nguyen AW, 2020. When Is Hope Enough? Hopefulness, Discrimination and Racial/Ethnic Disparities in Allostatic Load. Behav. Med 46 (3–4), 189–201. 10.1080/08964289.2020.1729086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno PI, Moskowitz AL, Ganz PA, Bower JE, 2016. Positive Affect and Inflammatory Activity in Breast Cancer Survivors: Examining the Role of Affective Arousal. Psychosom. Med 78 (5), 532–541. 10.1097/psy.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierop-van Baalen C, Grypdonck M, van Hecke A, Verhaeghe S, 2020. Associated factors of hope in cancer patients during treatment: A systematic literature review. J. Adv. Nurs 76 (7), 1520–1537. 10.1111/jan.14344. [DOI] [PubMed] [Google Scholar]
- Nilsson MB, Langley RR, Fidler IJ, 2005. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 65, 10794–10800. 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Badrick E, Kumari M, Steptoe A, 2008. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology 33 (5), 601–611. 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Pössel P, Mitchell AM, Sjögren E, Kristenson M, 2015. Do depressive symptoms mediate the relationship between hopelessness and diurnal cortisol rhythm? Int. J. Behav. Med 22 (2), 251–257. 10.1007/s12529-014-9422-6. [DOI] [PubMed] [Google Scholar]
- Radloff L, 1977. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas 1, 385–401. [Google Scholar]
- Schrepf A, Thaker PH, Goodheart MJ, et al. , 2015. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology 53, 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Dhabhar FS, Keuroghlian AS, et al. , 2009. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav. Immun 23, 1148–1155. 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Lush E, Dedert EA, et al. , 2013. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav. Immun 30, S163–S170. 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Sephton S, Sapolsky RM, Kraemer HC, Speigel D, 2000. Early mortality in metastatic breast cancer patients with absent of abnormal diurnal cortisol rhythms. J Nat Cancer Inst. 92, 994–1000. [DOI] [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H, 1995. The measurement structure of the Center for Epidemiologic Studies depression scale. J. Pers. Assess 64 (3), 507–521. [DOI] [PubMed] [Google Scholar]
- Snyder CR, 1994. The Psychology of Hope: You can get there from here. The psychology of hope: You can get there from here. Free. Press. [Google Scholar]
- Snyder CR, 2002. Hope Theory: Rainbows in the Mind. Psychol. Inq 13 (4), 249–275. [Google Scholar]
- Sood AK, Bhatty R, Kamat AA, et al. , 2006. Stress hormone mediated invasion of ovarian cancer cells. Clin. Cancer Res 12, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, Cameron CL, et al. , 2000. Emotionally expressive coping predicts psychological and physical adjustment to breast cancer. J. Consult. Clin. Psychol 68 (5), 875–882. [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, Huggins ME, 2002. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psychooncology 11 (2), 93–102. 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- Stellar JE, John-Henderson N, Anderson CL, Gordon AM, McNeil GD, Keltner D, 2015. Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion (Washington, DC). 15 (2), 129–133. 10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- Steptoe A, 2019. Happiness and Health. Annu. Rev. Public Health 40, 339–359. 10.1146/annurev-publhealth-040218-044150. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Demakakos P, de Oliveira C, Wardle J, 2012. Distinctive biological correlates of positive psychological well-being in older men and women. Psychosom. Med 74 (5), 501–508. 10.1097/PSY.0b013e31824f82c8. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M, 2008. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. Am. J. Epidemiol 167 (1), 96–102. 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Tempfer C, Zeisler H, Sliutz G, Haeusler G EH, Kainz C Serum evaluation of interleukin 6 in ovarian cancer patients. Gyn Oncol. 1997;66:27–30. [DOI] [PubMed] [Google Scholar]
- Targum SD, Fava M, 2011. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 8 (10), 40–43. [PMC free article] [PubMed] [Google Scholar]
- Thornton L, Andersen BL, Carson W, 2008. Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunol. Immunother 57, 1471–1481. 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AW, Hoyt MA, 2018. Benefit finding and diurnal cortisol after prostate cancer: The mediating role of positive affect. Psychooncology 27 (4), 1200–1205. 10.1002/pon.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xu X, Zhou M, et al. , 2017. Hope and the brain: Trait hope mediates the protective role of medial orbitofrontal cortex spontaneous activity against anxiety. Neuroimage 157, 439–447. 10.1016/j.neuroimage.2017.05.056. [DOI] [PubMed] [Google Scholar]
- Webster K, Cella D, Yost K, 2003. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 1, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrib A, Sephton SE, DeGeest K, et al. , 2010. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer 116, 4410–4419. 10.1002/cncr.25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.


