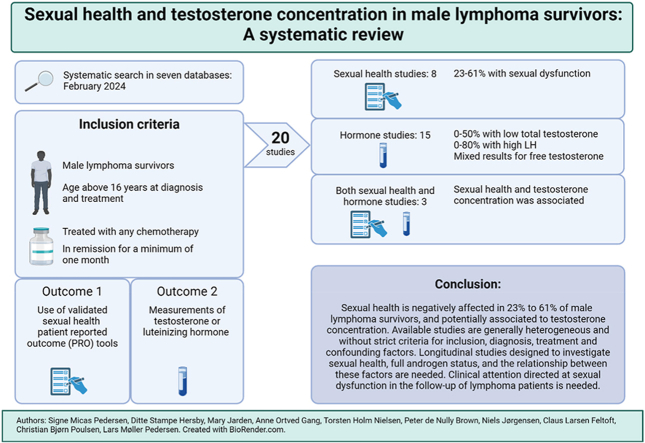
Abstract
Advancements in lymphoma treatment have increased the number of long-term survivors who may experience late effects such as impaired sexual function and testosterone deficiency. The aim of this review was to determine the prevalence of testosterone deficiency and sexual dysfunction among male lymphoma survivors; and associations between the two. A systematic search identified 20 articles for inclusion. The prevalence of low total testosterone was 0%–50 %, with mean values within reference levels, and for luteinizing hormone above reference levels in 0%–80 %. Four studies included SHBG and free testosterone, with mixed results. Compromised sexual health was found in 23%–61 %. Overall, total testosterone and sexual health were associated. The risk of bias (ROBINS-E and RoB 2) was high/very high, leading to low/very low overall confidence in the bulk of evidence (GRADE). Longitudinal studies evaluating biologically active testosterone and sexual health are needed, to develop evidence based standard procedures for follow-up of sexual health.
Keywords: Sexual health, Erectile dysfunction, Testosterone deficiency, Testosterone, Hypogonadism, Androgen, Lymphoma, Survivor
Graphical abstract
Highlights
-
•
Male lymphoma survivors suffer from erectile dysfunction after chemotherapy.
-
•
Compromised sexual health was found in half of male lymphoma survivors.
-
•
The prevalence of low total testosterone concentration was 0–50 %.
-
•
SHBG/free testosterone is rarely investigated when assessing testosterone deficiency.
-
•
Overall, total testosterone and sexual health were associated.
1. Introduction
Increasing survival rates among lymphoma patients [1], have emphasized the need to improve quality of life (QoL) of survivors. Sexual health has been shown to be associated with QoL [2], making it a significant aspect to address in efforts to improve QoL of lymphoma survivors. Cancer treatment may have great impact on multiple functions in the body, including the reproductive system. Thus, testosterone deficiency and sexual health issues can manifest as long-term consequences of treatment. While many male patients are informed of the possible sexual health implications of treatment at diagnosis [3], there is a need for follow-up on the subject after cessation of lymphoma treatment [[4], [5], [6]].
In 2009, one of the first studies investigating the relationship between serum testosterone concentration and sexual health in lymphoma survivors was conducted in Norway [7]. It found low serum total testosterone concentrations in 30 % of survivors. Furthermore, a significant relationship between hormonal status and sexual function was reported, and sexual function was more affected in survivors than in controls. However, the study included survivors of both childhood and adult lymphoma, and those treated with radiotherapy alone. In 2013, two large studies were performed based on the German Hodgkin Study Group's (GHSG) randomized Hodgkin Disease (HD) trials including more than 2700 male survivors [8] abiding to more stringent inclusion criteria. Subjects treated with ABVD (A - doxorubicin, B - bleomycin, V - vinblastine, d - dacarbazine) were found to be in better sexual health compared to those treated with BEACOPP (B – bleomycin, E − etoposide, A – doxorubicin, C – cyclophosphamide, O – vincristine, P - procarbazine, P – prednisolone) while remaining comparable to controls. They observed a 15–20 % prevalence of testosterone deficiency. However, with no increased prevalence of related symptoms of testosterone deficiency among survivors compared to controls using the Aging Male Symptom (AMS) Scale. Mean testosterone concentration was within reference levels. These studies have been important for our understanding of androgen status and sexual health after treatment for Hodgkin lymphoma (HL). Given the limited number of studies conducted of this size, it is important to provide an overview of all available data to evaluate how sexual health of lymphoma survivors is affected after cancer and treatment.
In recent years, reviews have explored the sexual and gonadal function of cancer survivors, including those with hematologic malignancies. However, there is a lack of recent systematic reviews of male lymphoma survivors evaluating sexual health, androgen status and the relationship between the two. Existing reviews have focused on mixed hematologic diagnoses [[9], [10], [11]], mixed sexes [12,13], or multiple cancer diagnoses [[14], [15], [16], [17]]. Several reviews have focused on fertility or are lacking more recent studies [14,[18], [19], [20], [21]]. Six of these reviews found that lymphoma survivors suffered from sexual dysfunction (18–61 %) addressed using different endpoints. Some studies recur in many reviews. However, the variation in included studies is still noticeable, probably due to variations in scopes and inclusion criteria of the reviews. One prior review has investigated the relationship between hormonal status and sexual health [19]. However, the usability of this review is constrained by the lack of more recently published studies.
The primary aim of this systematic review was to obtain current and valid data on impaired sexual health, testosterone deficiency, and the association between these factors in male lymphoma survivors using a systematic, structured and pre-planned synthesis of relevant studies in accordance with PRISMA guidelines.
2. Materials and methods
The current review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. An initial search of the literature investigating the sexual health and androgen status of lymphoma survivors was conducted in April 2022, with an update in June 2023 (two new articles were included) and in February 2024 (one new articles was included). Some overlap occurred because the updated searches were time limited by year. A study protocol was registered in the PROSPERO database [22]. Study screening and data extraction was performed by two reviewers (DH and SMP) using the online software Covidence (Covidence systematic review software 2022, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org).
The aim was addressed by the following research questions and utilizing the PICO (patient, intervention, comparator, outcome) framework:
2.1. Research questions
(i) What is the prevalence of patient reported sexual dysfunction, in adult male survivors of malignant lymphoma treated with chemotherapy in complete remission? (ii) What is the prevalence of low serum testosterone concentration/high luteinizing hormone (LH), in adult male survivors of malignant lymphoma treated with chemotherapy in complete remission? (iii) Is the carrier protein sexual hormone binding globulin (SHBG) affected in survivors of malignant lymphoma after treatment, altering the availability of biologically available free testosterone (FT)? (iv) Is there an association between serum testosterone/LH and sexual health? (v) What characterizes the identified patient groups in relation to lymphoma diagnosis, age, time after chemotherapy and type of treatment?
2.2. PICO: Participants
Male lymphoma survivors aged 16 years or older at diagnosis, who completed medical antineoplastic treatment, including chemotherapy with or without radiation therapy, who were in complete remission after treatment. Intervention: Antineoplastic treatment in the form of chemotherapy, with/without radiation therapy, both as first line treatment and treatment of relapse. Comparator: Studies with and without a comparator arm were considered eligible, as this review aimed to clarify the prevalence of a defined medical condition. Outcomes: (i) Sexual health measured by any standardized validated patient reported outcome questionnaire, evaluating erectile function, sexual QoL or sexual satisfaction. (ii) Evaluation of S-testosterone (at a minimum)/LH/SHBG levels after completion of treatment.
2.3. Search strategy
A systematic search was conducted in multiple databases, including MEDLINE, EMBASE, PsycInfo, Cinahl, Scopus, Web of Science, the Cochrane Library and www.clinicaltrials.gov. The search-terms used were Lymphoma AND (testosterone OR sexual health), including several synonyms for each term. Further information on the search strategy is described in the PROSPERO protocol.
2.4. Selection criteria
All study designs were accepted, both with and without a comparator. Case-reports, letters to the editor, conference abstracts, and reviews were excluded. The references of all included publications were reviewed for relevant articles. For articles that included more than five lymphoma survivors but did not meet inclusion criteria, attempts were made to obtain supplementary data by contacting the corresponding author by email, if contact information was available. Articles of all languages were included, and translated using google translate (French, German, Italian, Slovenian, Russian) or translated by native speaking person (Chinese). No fixed timeframe was used. Inclusion criteria were studies investigating (i) sexual health (using any validated Patient Reported Outcome Measure (PROM)) or (ii) androgen status (measured by serum testosterone, LH levels and/or SHBG), in (iii) male survivors with any lymphoma diagnosis, (iv) who received chemotherapy (v) after the age of 16 and (vi) who had been in complete remission for at least one month. Results had to be available as means or medians of PROM scores and/or concentration of androgenic steroid hormones, and preferably including a total percentage of deficient survivors. Exclusion criteria were: (i) use of unvalidated PROMs, (ii) results without subdivision regarding diagnosis, pediatric and adult populations including studies primarily of adults but with more than 5 % children, (iii) studies without hormone reference levels in the absence of a percentage of survivors with testosterone deficiency and (iv) lymphoma survivors undergoing allogeneic stem-cell transplantation.
2.5. Extraction
The two reviewers (SMP and DHS) independently extracted the following data; author, country, and year of publication, study design, year of evaluation, sample size, age at diagnosis and evaluation of survivors, length of follow-up, inclusion and exclusion criteria, study procedures, characteristics on cases (and controls), lymphoma diagnoses included and treatment specifications, PROM choice and definition of scoring system, hormonal/SHBG measurements and reference levels, mean/median for PROM and/or hormonal/SHBG levels, and percentage of survivors with deficiencies.
2.6. Hormone analyses
Serum total testosterone (TT) and LH were stated in different units, and for easier comparison, values were converted using the www.unitslab.com and www.endmemo.com calculators to nmol/l for TT and IU/L for LH. Mean values were calculated when patient level values were provided. However, four studies had units that were difficult to convert; Asbjørnsen et al. [23] and Kreuser et al. [24] reported LH in ng/ml which the authors of this review could not find any conversion for. Behringer et al. [8] and Kreuser et al. [24] reported TT levels in ng/L and ng/ml, respectively, which did not convert reliably. In this review, the results from Behringer et al. (7) were converted from the assumed correct unit of ng/ml to nmol/L, and for Kreuser et al. ng/dl converted to nmol/L.
2.7. Questionnaires from included studies
Four different questionnaires were used in the eight included studies investigating sexual health. The International Index of Erectile Function (IIEF) questionnaire exists in several forms with five or 15 questions covering five domains in the longest form (Erectile function, intercourse satisfaction, orgasmic function, sexual desire and overall satisfaction) and the short form only addressing the erectile function domain. The Brief Sexual Function Inventory (BSFI) consists of 11 questions grouped in five domains (Erection score, sexual drive, ejaculation score, problem assessment score and overall satisfaction score). The FLZMb-sex questionnaire is from the FLZ questionnaire (Life satisfaction questionnaire – Lebenszufriedenheit in German). FLZ is designed to evaluate life satisfaction across 10 domains, including the sexual satisfaction domain FLZ-sex. Each domain comprises seven questions with FLZ-sex having 6 categories (sexual attraction, sexual efficiency, sexual contacts, sexual response, sexual partner interaction, and communication), and one total score FLZMb-sex/partner relationship. The FLZMb-sex is used in this review. One study used the additional scale for sexual functioning from the Quality-of-Life Questionnaire for Survivors (QLQ-S). This subscale consists of three questions investigating changes in three areas (sexual interest, sexual activity, and satisfaction). Further definition can be found in the respective articles, as displayed in Table 1.
Table 1.
Overview of included articles.
| Study | Sample size | Country | No. of studies | Total sample size |
|---|---|---|---|---|
| Studies measuring androgen status | ||||
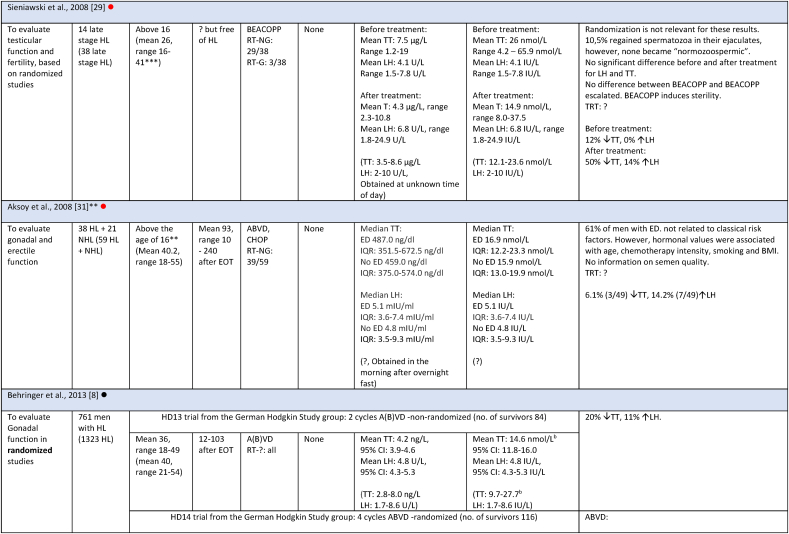
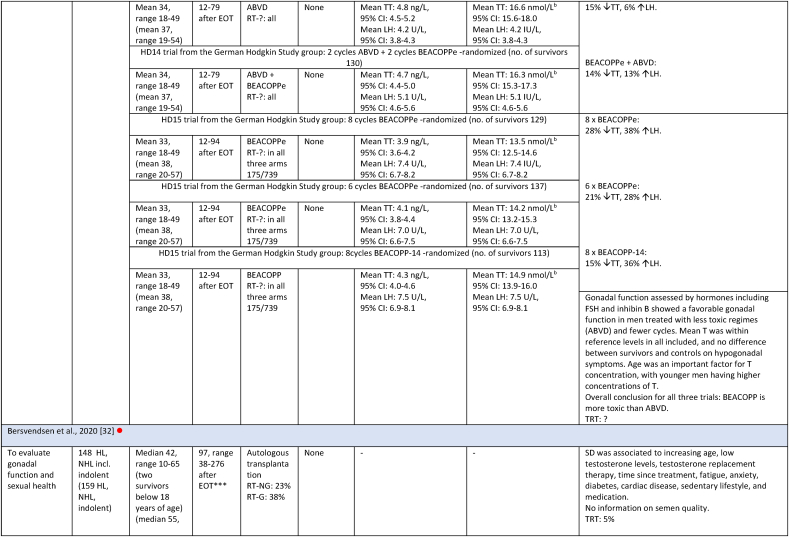
| Behringer [8] | 761 | Germany | 3 | 818 |
| Sieniawski [25] | 38 | |||
| Kreuser [24] | 19 | |||
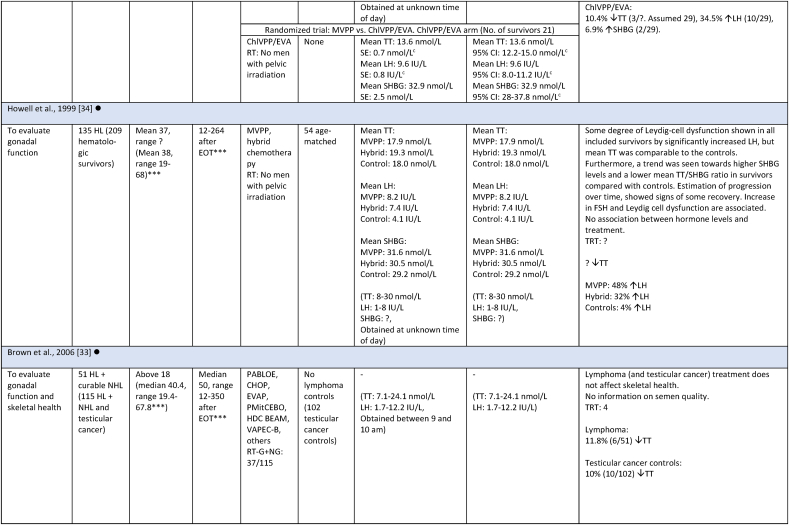
| Howell [26] | 135 | United Kingdom | 4 | 310 |
| Brown [27] | 51 | |||
| Clark [28] | 50 | |||
| Chapman [29] | 74 | |||
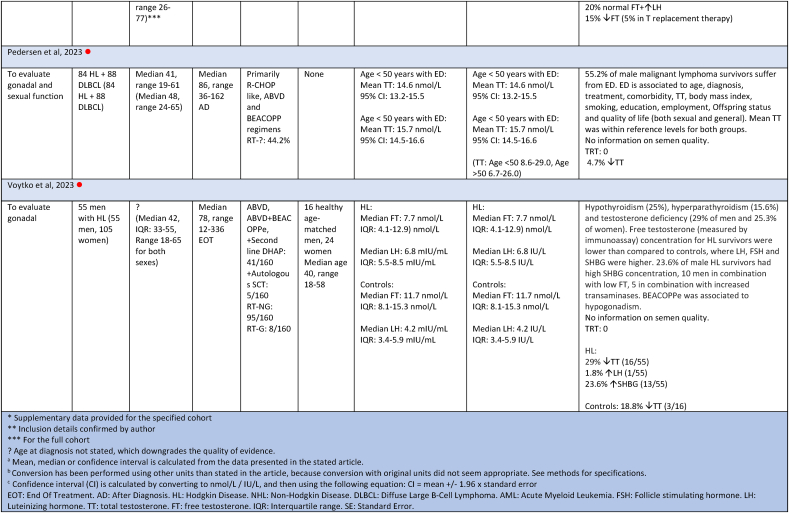
| Pedersen [30] | 172 | Denmark | 2 | 181 |
| Specht [31] | 9 | |||
| Asbjørnsen [23] | 8 | Norway | 2 | 167 |
| Bersvendsen [32] | 159 | |||
| Aksoy [33] | 59 | Turkey | 1 | 59 |
| Viviani [34] | 44 | Italy | 1 | 44 |
| Wang [35] | 10 | Hong Kong | 1 | 10 |
| Voytko [36] | 55 | Russia | 1 | 55 |
| 8 countries | 15 studies | 1644 survivors | ||
| European countries: 5a | 12 studies | 1520 survivors | ||
| Others: 3 | 3 studies | 124 survivors | ||
| Studies measuring sexual health | ||||
| Behringer [37] | 1826 | Germany | 2 | 1870 |
| Mütsch [38] | 44 | |||
| Pedersen [30] | 172 | Denmark | 1 | 172 |
| Bersvendsen [32] | 159 | Norway | 1 | 159 |
| Gan [39] | 104 | Malaysia | 1 | 104 |
| Aksoy [33] | 59 | Turkey | 1 | 59 |
| Tsatsou [40] | 50 | Greece | 1 | 50 |
| Eeltink [41] | 30 | The Netherlands | 1 | 30 |
| 7 countries | 8 studies | 2444 survivors | ||
| European countries: 5a | 6 studies | 2281 survivors | ||
| Others: 2 | 2 studies | 163 survivors | ||
Turkey is not counted as a European country in this review, due to the only small area within European territory.
2.8. Quality assessment
Risk of bias was assessed by the Risk of Bias in Non-randomized Studies of Exposure (ROBINS-E) instrument, and the Cochrane risk-of-bias tool for randomized studies (RoB 2). The bulk of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. A thorough description of the methods and results is provided in the supplementary data.
2.9. Data analysis
Since included studies applied different PROMs and investigated patients at different time-intervals after treatment, it was not possible to conduct meta-analyses on sexual health outcomes. Nor was a meta-analysis conducted for hormone and SHBG concentrations due to a pronounced heterogeneity in reference levels and variations in sample collection timing throughout the day. Most included studies had limited sample sizes, and results spanned more than 50 years of research, increasing the likelihood of measurement discrepancies. Therefore, the review relies on a narrative commentary synthesis of data.
3. Results
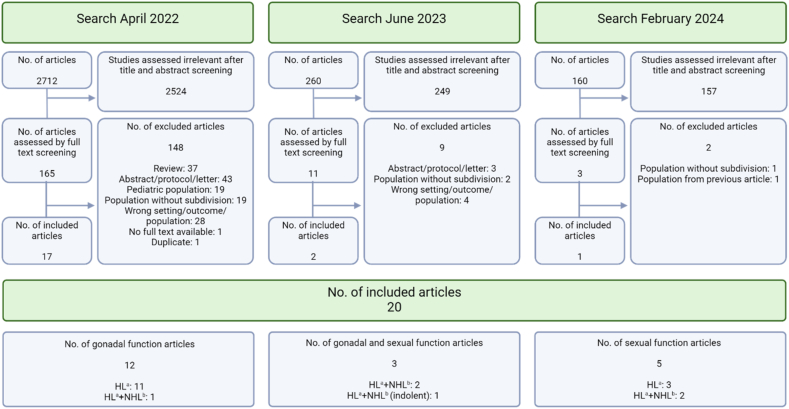
Out of the initial 165 articles eligible for full text screening, 29 did not entirely meet inclusion criteria, but corresponding authors were contacted for supplementary details. Nine authors responded, four provided relevant data and two confirmed details. Consequently, these studies were included in the review. In the updated time limited searches three additional studies were included. In total, 159 articles were excluded after full text screening. Twenty articles were included for review (Fig. 1); 12 studies investigated androgen status alone, five investigated sexual health, and three investigated both outcomes.
Fig. 1.
– Flowchart of screening and inclusion of articles. HL=Hodgkin Lymphoma, NHL=Non-Hodgkin Lymphoma. Created with Biorender.com. HL=Hodgkin Lymphoma, NHL=Non-Hodgkin Lymphoma. Created with Biorender.com.
3.1. Androgen status
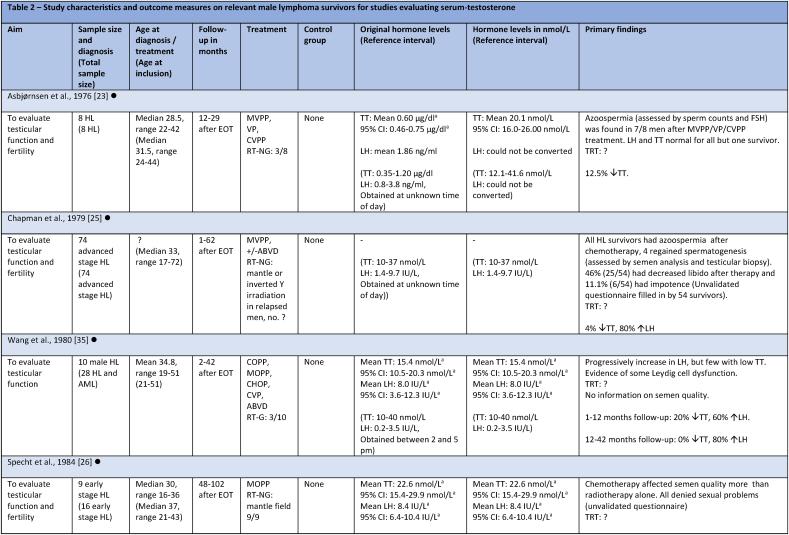
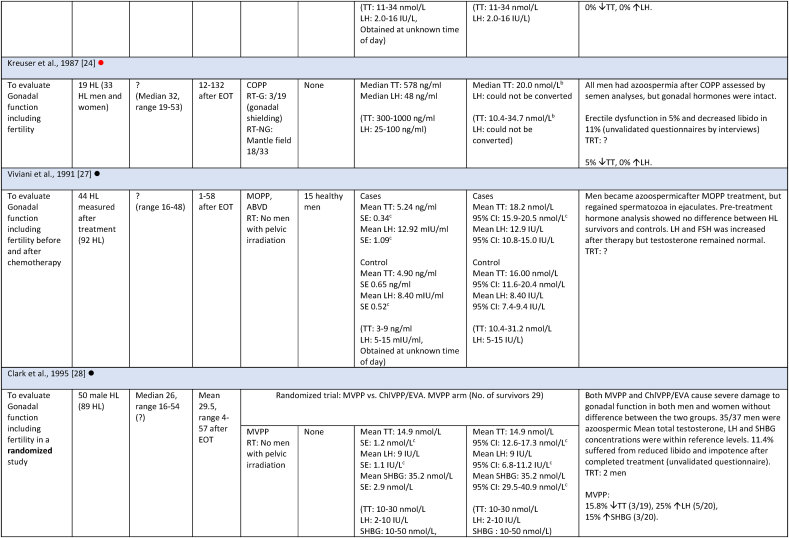
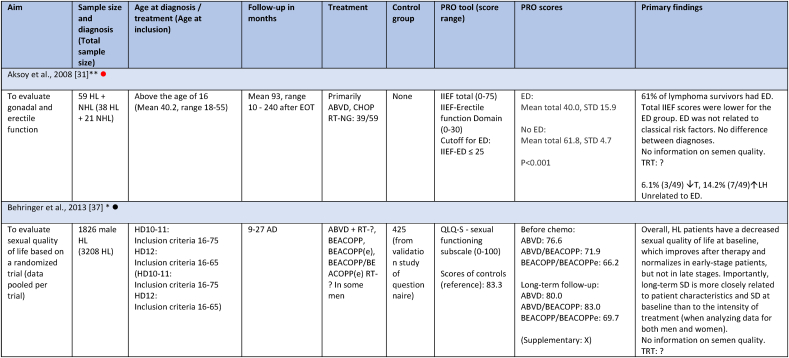
Studies evaluating androgen status included 1644 survivors, with the majority (1520) residing in European countries (Table 1). Among the 15 studies, seven had fertility as primary outcome [[23], [24], [25], [28], [29], [31], [34]]. Four included both HL and NHL survivors [[27], [30], [32], [33]], while the remaining 10 only included HL survivors [8,[23], [24], [25], [28], [29], [31], [34],[26], [35], [36]]. One of the four studies included both curable and indolent (incurable) NHL survivors [32], two studies only curable, and one study did not specify whether indolent lymphoma survivors were included. Four of the 15 studies only included late-stage HL, one only early-stage HL, six included all stages, and four did not specify stages included. The age range of the included survivors was between 16 and 65 years at time of diagnosis, and between 16 and 77 years at follow-up. Follow-up was between 1 and 350 months. Four studies included HL survivors earlier than 6 months after therapy, and for one study follow-up was not specified [25]. In eight studies survivors were treated with outdated treatment regimens. Survivors in all studies were treated with a curative intent, except for treatment of the indolent lymphoma subgroup of 15 survivors in one study [32], and one study not specifying neither subgroups of NHL nor the treatment of 9.5 % of the participants, who did not receive a CHOP regimen [33]. For an overview of included studies see Table 2.
Table 2.
Study characteristics and outcome measures on relevant male lymphoma survivors for studies evaluating serum-testosterone.
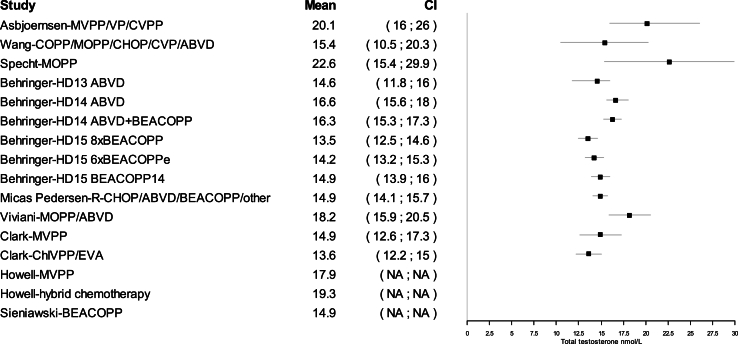
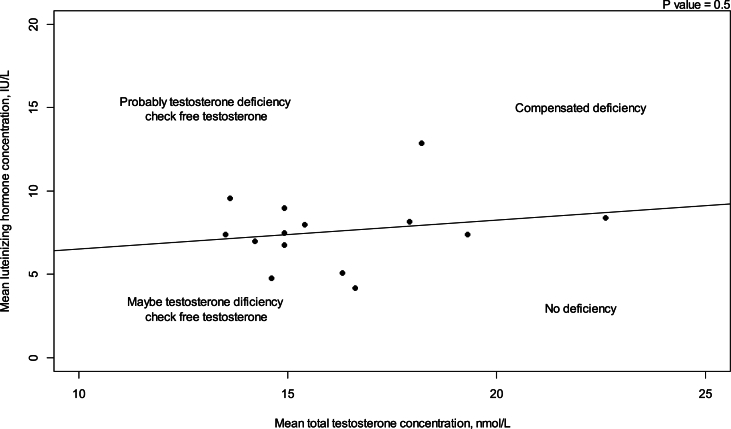
All mean TT concentrations were within the provided reference level for each study (Fig. 2). Older studies providing means in relation to obsolete treatment regimens had wider confidence intervals and were thereby less certain. Mean TT was lower for survivors treated with a BEACOPP regimen according to Behringer et al. [8], although still within reference levels. Reported prevalence for TT below reference levels was more heterogenous and found to be between 0 % and 50 %. Mean LH levels were also heterogenous and not found to be related to low concentrations of TT in several studies. If mean TT was a result of a compensated state, concentrations in the lower half of the reference levels would be accompanied by high LH. This was not found (Fig. 3). Four studies evaluated FT through measuring SHBG. Two studies investigating current treatment regimens provided prevalence of low free androgen index in15 % (TTx100/SHBG), and found 5 % already in replacement therapy [32], and 29 % with low FT (direct immunoassay) and 18.2 % in combination with high SHBG levels. Two studies investigated outdated treatment regimens. One found approximately 15 % with low TT and 15 % with increased SHBG levels, but mean hormone concentrations within reference levels [28] (no calculation of free testosterone was done). The other found a trend towards increased TT/SHBG ratio [26]. No obvious relationship between diagnosis or treatment and low FT was found in three studies, where one study found low FT concentrations associated to the BEACOPPe regimen [36].
Fig. 2.
Mean total testosterone concentrations in nmol/L (nanomole/liter) and 95 % confidence intervals (CI) for included studies providing mean serum total testosterone concentrations.
See footnotes of Table 2 for treatment abbreviations.
Fig. 3.
The relationship between mean luteinizing hormone and mean serum total testosterone concentration. Interpretation of testosterone in relationship to luteinizing hormone [42]. Dashed lines represent mean values for lower and upper reference interval limits based on reference intervals for included studies. Nmol/L = nanomole/liter. IU/L = International units/liter.
3.2. Sexual health
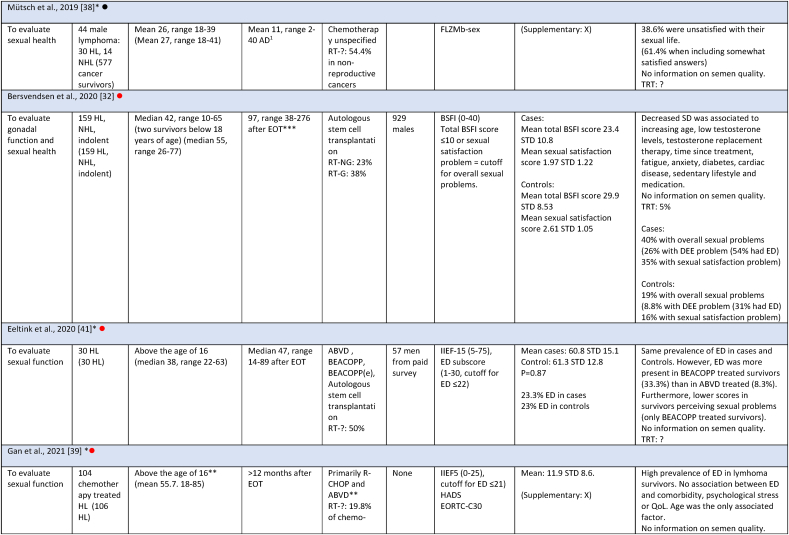
Studies evaluating sexual health consisted of 2444 survivors, with the majority (2281) residing in European countries. Of the eight studies, five included both HL and NHL survivors, with one also investigating indolent lymphoma survivors. Three studies only included HL. Five of the eight studies included all stages of disease, and three did not specify stages. Survivors were between 16 and 75 years at diagnosis and 16–85 years at follow-up. The length of follow-up was between 2 and 276 months for sexual health studies (one study included survivors earlier than 6 months after diagnosis). All studies included survivors who received current first-line treatment regimens with curative intent (except for treatment of the indolent lymphoma subgroup in one study (15 survivors), and one study that did not provide treatment details for 9.5 % of survivors). For an overview of included studies see Table 3.
Table 3.
Study characteristics and outcome measures for studies evaluating sexual health.
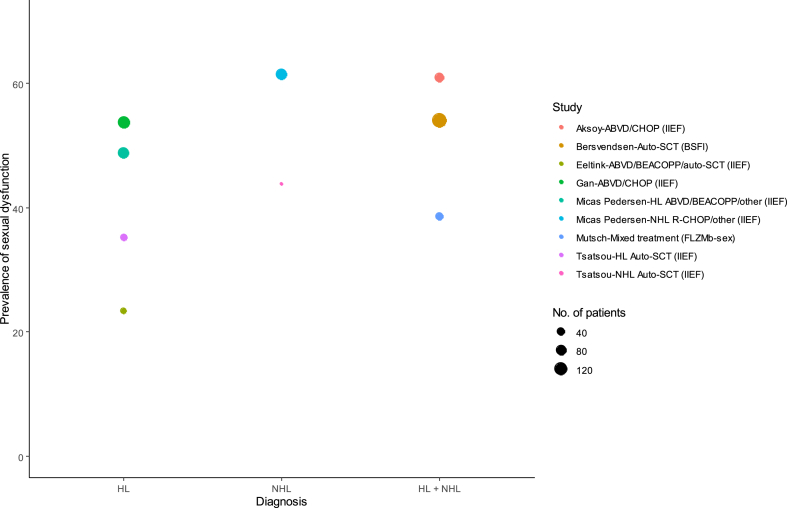
Sexual health was measured by four different PRO tools: BSFI, QLQ-S, FLZMb-sex and IIEF. The overall prevalence of survivors with sexual dysfunction (SD) ranged between 23 % and 61.4 % regardless of PRO tool (Table 3 and Fig. 4). Of the two studies that investigated other aspects of sexual health than erectile function and who reported prevalence of SD, the prevalence ranged between 40 % and 61.4 %. Five out of eight studies evaluated erectile function using the IIEF questionnaire, which made direct comparison possible. The prevalence of erectile dysfunction (ED) ranged between 23 % and 61 %. In some studies, ED was found to be associated with age, diagnosis, treatment, time since treatment, medication, physical activity, comorbidity, BMI, smoking, education/employment, having children, serum testosterone concentration, and quality of life. However, these findings were not consistent across all studies.
Fig. 4.
Prevalence of survivors with decreased sexual function by diagnosis See footnotes of Table 2, Table 3 for treatment abbreviations. HL: Hodgkin Lymphoma. NHL: Non-Hodgkin Lymphoma. IIEF: International Index of Erectile Function. BSFI: Brief Sexual Function Inventory. FLZMb-sex: Life Satisfaction Questionnaire-sexual domain. Prevalence is given in percentage of survivors with sexual dysfunction. NB: Should be provided in colors. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
One large randomized study, displaying results from three individual studies [37] did not provide prevalence of sexual dysfunction, but investigated the progression of sexual dysfunction over time. This study showed that sexual health at time of diagnosis was worse compared to controls. Over time, sexual health improved, but only to levels comparable to controls for ABVD treated survivors, whereas BEACOPP treated survivors improved but remained below the level of the controls (Supplementary material based on provided data from Behringer et al. [37]). In the current literature review, sexual health was comparable between HL and NHL survivors, with a slight tendency towards better sexual health among HL survivors (Fig. 4).
3.3. The association between androgens and sexual health
The relationship between sexual health and testosterone was investigated in three studies. Among these, two studies reported an association between testosterone concentrations and sexual function [30,32], while one study did not find such an association [33]. In a recently published article [30], an association between TT and ED was detected among 172 lymphoma survivors (HL and diffuse large B-cell lymphoma (DLBCL)), even though mean TT was within reference levels. Overt testosterone deficiency and ED, measured by the IIEF score, were not associated, perhaps due to a small subgroup size. In the study conducted by Bersvendsen et al. [32] measuring sexual health and hormonal levels in 159 survivors after autologous stem cell transplantation (auto-SCT), they found an association between low FT level and more affected sexual health (using the BSFI) in univariate analyses, but not after adjusting for social, psychological and physiological factors including fatigue, cardiovascular disease, sedentary lifestyle, use of medication with possible adverse effects on sexual function and testosterone replacement therapy. In contrast to the two previous studies, Aksoy et al. [33] did not find TT or testosterone deficiency to be associated with ED, as assessed by the IIEF score, among the 59 included survivors of HL and NHL. All three studies focused on male HL and NHL survivors, including a small subgroup of 15 indolent lymphoma survivors in one study, who were receiving current first-line treatment regimens.
3.4. Risk of bias
All included studies were evaluated for risk of bias using the ROBINS-E tool, and three randomized studies, were additionally evaluated with the RoB 2 tool. Nine studies with serum testosterone level as an outcome were assessed to be at very high risk of bias and six studies were at high risk of bias according to ROBINS-E. Two studies with sexual health as outcome were assessed to be at very high risk of bias and six studies high risk of bias according to ROBINS-E. All three randomized trials were evaluated to be at high risk of bias according to RoB 2. The complete risk of bias assessment is found in supplementary material.
3.5. GRADE - the certainty of evidence
According to GRADE, the certainty of evidence was found to be very low for hormonal outcomes, and low for sexual health outcomes. GRADE assessment is shown in supplementary material.
4. Discussion
This review finds that male lymphoma survivors experience impaired sexual health after lymphoma disease and treatment. Some studies found sexual health associated to age, diagnosis, treatment, duration since treatment, medication, physical activity, comorbidity, BMI, smoking, education/employment, having children, TT/FT level and quality of life. In contrast, overall low TT and elevated LH levels were heterogeneously distributed across the studies, and therefore the conclusions are less certain, especially, as only four studies evaluated the biologically available FT or free fraction of testosterone (and SHBG), with mixed results. Three studies evaluated associations between sexual health and hormonal status, where two found an association and one did not. Testosterone deficiency, a clinical diagnosis with symptoms accompanied by low testosterone concentration, suggests that future studies should address both issues.
Selection bias was observed in some of the included studies. The included randomized studies are not representative of real-life survivors as an entity. Some did not provide results for each randomization group, but instead for all included survivors as an entity, which constitutes a problem when assessing prevalence in a very selected subgroup of survivors. All studies primarily included survivors who were diagnosed and treated at or above the age of 16 years. This ensures that no more than 5 % of survivors had received treatment before or during puberty, which leads to different problems than in those treated as an adult [43], even though evidence is scarce. Survivors were up to 77 years old at follow-up in studies investigating hormonal status and up to 85 years old in sexual health studies. Both gonadal hormones and sexual function decreases with increasing age [44,45], which could result in overestimation of the negative effect of chemotherapy treatment on sexual health compared to hormonal level. Furthermore, several studies evaluated sexual health and androgen status within the first year after therapy, possibly being too early to detect a chronic state, since studies have shown that the negative effects can decrease over time [35,37].
Mean TT reported in the studies was found to be within stated reference intervals, and only two studies found low TT in conjunction with elevated LH, indicating compensated Leydig-cell deficiency [26,35]. However, caution should be taken when comparing TT across different assays, because of more reliable analysis methods and assays in newer studies [46] and inter-laboratory and reference intervals differences. Furthermore, only four of 15 studies took the biologically available FT into account, by measuring SHBG in conjunction with TT [28,32,26,36]. All studies used different approaches; the free androgen index, the TT/SHBG ratio, FT measured by immunoassay, and one did not mention the bioavailable testosterone, but only overall SHBG concentrations, making comparison of results difficult. This presents a challenge in the available literature since a TT measurement provides an accurate assessment only if SHBG concentration is unaltered. SHBG can be affected by various factors including obesity (an increasing problem in the general population [47]), smoking and liver disease. In three of four studies, elevated SHBG levels were found in male lymphoma survivors, indicating that available evidence may be associated with inaccurate interpretations of hormonal status. SHBG could hypothetically be influenced after treatment due to discrete chronic damage to the liver [36] after chemotherapy or metabolic syndrome after steroid treatment. Only one study investigated the relationship between testosterone and BMI and found an association [33]. Such associations would be interesting to explore further in cancer survivors.
An association was found between TT and sexual function in two studies [30,32], indicating that even levels in the lower normal range are associated with SD. Whether this association reflects causation remains to be elucidated. The clinical utility of TT in the follow-up of lymphoma patients needs to be further investigated in future cohort studies, where we account not only for TT but also FT, BMI and other confounding factors such as smoking, fasting before sampling, time of day for sampling, metabolic syndrome and neuropathy among other comorbidities. The latter, which can be a result of lymphoma treatment, was found to be important in some included studies [32], but not in others [33,39]. An important factor is the age variation among survivors in sexual health and androgen status studies. In general, studies on sexual health included older survivors compared to studies evaluating androgen status. Since both gonadal function and sexual function can decrease in elderly men, the higher prevalence of SD compared to low TT could be a result of this difference.
Based on the available data, we did not find any specific diagnoses or treatment associated with a greater impact on sexual health than others. However, Behringer et al. [37] and Eeltink et al. [41] found BEACOPP treated survivors were more affected than those treated with ABVD. Behringer et al. [8] and Voytko et al. [36] also found testosterone to be lower in BEACOPP treated survivors indicating a potential association between treatment intensity and long-term SD. However, sexual health after auto-SCT has not been found to be more severely affected than survivors treated with standard first-line treatment alone. Perhaps due to a combination of younger age and less comorbidity permitting high dose treatment. Other studies lacked rigorous data on gonadal dysfunction related to specific treatments. Therefore, the available data did not allow for an analysis of the incidence of gonadal dysfunction as a late effect in relation to specific treatment regimens or dose intensity. We only found a tendency towards a better sexual function among HL survivors. Nevertheless, the findings of Behringer et al. showing different progression through time based on treatment, should be taken into consideration because of a large sample size. Our review provides no specific explanations for the relationship between diagnosis and the occurrence of SD, though a higher average age in the group of NHL patients could play a role. Both age itself and increased age-related comorbidity burden can have an impact on sexual health. It is well established that sexual health can be affected in older men, e.g., because of reduced testosterone levels, cardiovascular disease, diabetes and obesity [45,48]. This suggests that age might be more important for the outcome than diagnosis. It is important to note that other factors also contribute to the affected sexual health including body image, fatigue, and relationship status.
Studies included were primarily conducted in Europe. Therefore, the generalizability of the findings to cancer survivors worldwide may be limited due to cultural differences in perceptions of sexual health issues.
Studies evaluating hormonal status focused primarily on HL survivors, with only a few including NHL survivors. Both HL and NHL were represented in studies evaluating sexual health. Due to the higher age of NHL survivors, sexual health could appear more affected because of an older population being evaluated. One study also included indolent lymphomas and one did not specify NHL diagnoses. Indolent lymphomas are of a more chronic nature treated with less toxic treatment regimens and could represent bias if highly represented. However, this did not seem to be the case in this review. None of the included studies had conflicts of interest or problematic funding. Bias introduced by not including all stages in all studies is accounted for, as standard treatments vary based on disease stage.
A wide range of assessment tools are available for evaluating sexual health, with some tools evaluating sexual desire, some erectile function, and some sexual satisfaction or information needs. This heterogeneity of tools poses a problem and limits the comparability of studies. It would be beneficial for future research to utilize fewer validated tools to increase comparability. However, in the current review, five out of eight studies used one of two available IIEF scoring systems (the gold standard [49,50]), and therefore the comparability between studies was acceptable. Unfortunately, the largest study measuring sexual health [37], did not use the IIEF, but instead a score based on three questions, validated on testicular cancer patients only.
All included studies had a high or very high individual risk of bias, and the certainty of the majority of evidence was low for the sexual health outcome, and very low for hormonal outcomes. This poses a severe limitation to the reliability of the available evidence. Often confounding is not appropriately considered, missing data is not accounted for and sexual function and hormonal status prior to disease and treatment are not measured. Several studies in this review had fertility as primary endpoint, and not androgen status, thereby neglecting confounding factors relevant for erectile function. In the future, preference should be given to studies with androgen status and sexual health as primary endpoints.
Clinical implications:5.1: Survivors of lymphoma who have received chemotherapy, immunotherapy and/or autologous/allogenic stem cell transplantation are often left with both physical and psychological sequelae. In this review, we find that approximately half of male survivors of lymphoma are struggling with impaired sexual function, potentially influenced by hormonal changes. The impairment can be a result of treatment, underscoring the importance of incorporating focus on sexual health of survivors into standard follow-up programs. Despite survivors expressing a need for information and action, sexual health remains a challenging topic to address due to social taboos [15,51]. The approach to addressing sexual health concerns can begin with a simple conversation to investigate potential causes, such as psychological factors (e.g., depression), or physical factors (e.g., decreased sensibility, obesity, smoking, cardiac disease, medication, testosterone deficiency), followed by relevant blood tests to assess total testosterone, LH and SHBG. Consideration of a trial of PDE5-inhibitors or referral to relevant specialists may be warranted.
5. Conclusion
This systematic review finds that sexual health is negatively affected in 23 %–61 % of male lymphoma survivors, and potentially associated to testosterone concentration. Available studies are generally heterogeneous and lack strict criteria for inclusion, diagnosis, treatment, and confounding factors. Longitudinal studies designed to investigate sexual health, full androgen status, and the relationship between these factors are needed. This review points to a need for clinical attention to be directed towards sexual dysfunction in the follow-up of lymphoma patients. Longitudinal studies designed to investigate sexual health, full androgen status, and the relationship between these factors are needed. Such studies should be designed to account for age as a significant confounding variable. Furthermore, it should be considered to analyze a larger cohort of patients with different cancer diagnoses treated with uniform modalities to obtain a sufficient patient material.
Funding
Funding was obtained from the Department of Hematology Copenhagen University Hospital Research Funds, Einar Willumsens Scholarship, Jens and Maren Thestrups Scholarship, and Johannes Fog Fund.
Data availability statement
Data associated to this study is freely available in the originally published articles. If additional data has been provided by the authors, it can be found in supplementary data for this current publication.
CRediT authorship contribution statement
Signe Micas Pedersen: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Ditte Stampe Hersby: Writing – review & editing, Visualization, Resources, Methodology, Formal analysis, Data curation, Conceptualization. Mary Jarden: Writing – review & editing, Visualization, Methodology, Formal analysis. Torsten Holm Nielsen: Writing – review & editing, Supervision, Resources, Funding acquisition, Formal analysis. Anne Ortved Gang: Writing – review & editing, Supervision, Resources, Funding acquisition, Formal analysis. Christian Bjørn Poulsen: Writing – review & editing, Visualization, Supervision, Resources, Funding acquisition, Formal analysis. Peter de Nully Brown: Writing – review & editing, Resources, Formal analysis. Niels Jørgensen: Writing – review & editing, Supervision, Resources, Formal analysis. Claus Larsen Feltoft: Writing – review & editing, Resources, Formal analysis. Lars Møller Pedersen: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Formal analysis, Conceptualization.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interestsSigne Micas Pedersen reports equipment, drugs, or supplies was provided by Besins Healthcare. Peter de Nully Brown reports a relationship with Roche that includes: consulting or advisory. Peter de Nully Brown reports a relationship with Gilead Sciences Inc that includes: consulting or advisory. Peter de Nully Brown reports a relationship with Swedish Orphan Biovitrum that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
During the preparations, the data collection and the writing of this systematic review, guidance has been provided by Professor Hanne Tønnesen from the Parker Institute in Denmark. The authors of this systematic review would like to express great gratitude for this assistance. Furthermore, appreciation is extended to all authors of the included articles who have spent time and efforts in providing supplementary data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31915.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Marianne Steding-Jessen N.M.C. “Malignt Lymfom og CLL national årsrapport. 2021. www.rkkp.dk [Online]. Available:
- 2.Kim I.R., et al. Sexual problems in male vs. female non-Hodgkin lymphoma survivors: prevalence, correlates, and associations with health-related quality of life. Ann. Hematol. 2017;96(5):739–747. doi: 10.1007/s00277-017-2940-y. [DOI] [PubMed] [Google Scholar]
- 3.Bergström C., et al. Do young adults with cancer receive information about treatment-related impact on sex life? Results from a population-based study. Cancer Med. 2023;12(8):9893–9901. doi: 10.1002/CAM4.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond C., Beckjord E.B., Arora N., Bellizzi K., Jeffery D., Aziz N. Non-Hodgkin’s lymphoma survivors' fertility and sexual function-related information needs. Fertil. Steril. 2008;90(4):1256–1258. doi: 10.1016/j.fertnstert.2007.08.081. [DOI] [PubMed] [Google Scholar]
- 5.Frick M., et al. Patient-reported survivorship care practices and late effects after treatment of Hodgkin (HL) and non-Hodgkin lymphoma (NHL) J. Clin. Oncol. 2018;36(7_suppl) doi: 10.1200/jco.2018.36.7_suppl.119. 119–119, [DOI] [PubMed] [Google Scholar]
- 6.Mayo S.J., Brennenstuhl S., Panesar P., Bryant A.L. Patterns of concerns among hematological cancer survivors. Cancer Nurs. 2022;45(6):447–456. doi: 10.1097/NCC.0000000000001060. [DOI] [PubMed] [Google Scholar]
- 7.Kiserud C.E., et al. Do male lymphoma survivors have impaired sexual function? J. Clin. Oncol. 2009;27(35):6019–6026. doi: 10.1200/JCO.2009.23.2280. [DOI] [PubMed] [Google Scholar]
- 8.Behringer K., et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin study group HD13 to HD15 Trials. J. Clin. Oncol. 2013;31(2):231–239. doi: 10.1200/JCO.2012.44.3721. [DOI] [PubMed] [Google Scholar]
- 9.Eeltink C., et al. Sexual problems in patients with hematological diseases: a systematic literature review. Support. Care Cancer. 2022;30:4603–4616. doi: 10.1007/s00520-021-06731-7. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K.H., Schjødt I., Jarden M. The impact of hematopoietic stem cell transplantation on sexuality: a systematic review of the literature. Bone Marrow Transplant. 2012;47(5):716–724. doi: 10.1038/bmt.2011.169. [DOI] [PubMed] [Google Scholar]
- 11.Liptrott S.J., Shash E., Martinelli G. Sexuality in patients undergoing haematopoietic stem cell transplantation. Int. J. Hematol. 2011;94(6):519–524. doi: 10.1007/s12185-011-0960-2. [DOI] [PubMed] [Google Scholar]
- 12.Xu P., Choi E., White K., Yafi F.A. Low testosterone in male cancer patients and survivors. Sex. Med. Rev. 2021;9(1):133–142. doi: 10.1016/j.sxmr.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Brydøy M., Fosså S.D., Dahl O., Bjøro T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol. (Madr) 2007;46(4):480–489. doi: 10.1080/02841860601166958. [DOI] [PubMed] [Google Scholar]
- 14.Poorvu P.D., et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr. 2019;3(1):pkz008. doi: 10.1093/jncics/pkz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmann V., Laan E.T.M., den Oudsten B.L. Sexual health-related care needs among young adult cancer patients and survivors: a systematic literature review. J. Cancer Surviv. 2022;16(4):913–924. doi: 10.1007/S11764-021-01084-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzol D., et al. Prevalence of erectile dysfunction in male survivors of cancer: a systematic review and meta-analysis of cross-sectional studies. Br. J. Gen. Pract. 2021;71(706):E372–E380. doi: 10.3399/bjgp20X714197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasappa L., Sirsath N., Lakshmaiah K., Suresh T., Suresh Babu M., Babu K. Obstacles of cancer survivorship: sexuality issues - need to break communication barriers. Clin. Cancer Investig. J. 2014;3(6):459. doi: 10.4103/2278-0513.142614. [DOI] [Google Scholar]
- 18.Amin M., et al. ABVD and BEACOPP regimens' effects on fertility in young males with Hodgkin lymphoma. Clin. Transl. Oncol. 2021;23(6):1067. doi: 10.1007/S12094-020-02483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arden-Close E., Eiser C., Pacey A. Sexual functioning in male survivors of lymphoma: a systematic review. J. Sex. Med. 2011;8(7):1833–1840. doi: 10.1111/j.1743-6109.2011.02209.x. [DOI] [PubMed] [Google Scholar]
- 20.Drechsel K.C.E., et al. Reproductive ability in survivors of childhood, adolescent, and young adult Hodgkin lymphoma: a review. Hum. Reprod. Update. 2023;29(4):486–517. doi: 10.1093/humupd/dmad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabanegh E.S., Ragheb A.M. Male fertility after cancer. Urology. 2009;73(2):225–231. doi: 10.1016/j.urology.2008.08.474. [DOI] [PubMed] [Google Scholar]
- 22.S. Micas Pedersen, “PROSPERO protocol,” ID: CRD42022323169. Accessed: August. 17, 2023. [Online]. Available: https://www.crd.york.ac.uk/prospero.
- 23.Asbjørnsen G., Molne K., Klepp O., Aakvaag A. Testicular function after combination chemotherapy for Hodgkin's disease. Scand. J. Hematol. 1976;16(1):66–69. doi: 10.1111/j.1600-0609.1976.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 24.Kreuser E.D., Xiros N., Hetzel W.D., Heimpel H. Reproductive and endocrine gonadal capacity in patients treated with COPP chemotherapy for Hodgkin's disease. J. Cancer Res. Clin. Oncol. 1987;113(3):260–266. doi: 10.1007/BF00396383. [DOI] [PubMed] [Google Scholar]
- 25.Sieniawski M., et al. Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: a report of the German Hodgkin Study Group (GHSG) Blood. 2008;111(1 PG-71–6):71–76. doi: 10.1182/blood-2007-02-073544. [DOI] [PubMed] [Google Scholar]
- 26.Howell S.J., Radford J.A., Ryder W.D.J., Shalet S.M. Testicular function after cytotoxic chemotherapy: evidence of Leydig cell insufficiency. J. Clin. Oncol. May 1999;17(5) doi: 10.1200/JCO.1999.17.5.1493. 1493–1493, [DOI] [PubMed] [Google Scholar]
- 27.Brown J.E. Effect of chemotherapy on skeletal health in male survivors from testicular cancer and lymphoma. Clin. Cancer Res. 2006;12(21):6480–6486. doi: 10.1158/1078-0432.CCR-06-1382. [DOI] [PubMed] [Google Scholar]
- 28.Clark S.T., Radford J.A., Crowther D., Swindell R., Shalet S.M. Gonadal function following chemotherapy for Hodgkin's disease: a comparative study of MVPP and a seven-drug hybrid regimen. J. Clin. Oncol. 1995;13(1):134–139. doi: 10.1200/jco.1995.13.1.134. [DOI] [PubMed] [Google Scholar]
- 29.Chapman R.M., Rees L.H., Sutcliffe S.B., Edwards C.R.W., Malpas J.S. Cyclical combination chemotherapy and gonadal function. Retrospective study in males. Lancet. 1979;313(8111):285–289. doi: 10.1016/S0140-6736(79)90701-3. [DOI] [PubMed] [Google Scholar]
- 30.Micas Pedersen S., et al. Sexual dysfunction is highly prevalent in male survivors of malignant lymphoma. Sex. Med. 2023;11(2):1–10. doi: 10.1093/sexmed/qfad021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Specht L., Geisler C., Mm H., Ne S. Testicular function in young men in long-term remission after treatment for the early stages of Hodgkin's disease. Scand. J. Haematol. 1984;33(4 PG-356–62):356–362. doi: 10.1111/j.1600-0609.1984.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 32.Bersvendsen H.S., et al. Sexual function in long-term male lymphoma survivors after high-dose therapy with autologous stem-cell transplantation. Bone Marrow Transplant. 2020;55(5):891–905. doi: 10.1038/s41409-019-0745-4. [DOI] [PubMed] [Google Scholar]
- 33.Aksoy S., et al. Erectile dysfunction in successfully treated lymphoma patients. Support. Care Cancer. 2008;16:291–297. doi: 10.1007/s00520-007-0307-y. [DOI] [PubMed] [Google Scholar]
- 34.Viviani S., et al. Testicular dysfunction in Hodgkin's disease before and after treatment. Eur. J. Cancer Clin. Oncol. 1991;27(11):1389–1392. doi: 10.1016/0277-5379(91)90017-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang C., Ng R.P., Chan T.K., Todd D. Effect of combination chemotherapy on pituitary-gonadal function in patients with lymphoma and leukemia. Cancer. 1980;45:2030–2037. doi: 10.1097/00006254-198104000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Voytko M.S., Klimontov V.V., Pospelova T.I., Shebunyaeva Y.Y., Fazullina O.N. Endocrine disorders after combined chemoradiotherapy in hodgkin lymphoma survivors. Probl. Endocrinol. 2023;69(2):16. doi: 10.14341/PROBL13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behringer K., et al. Sexual quality of life in Hodgkin lymphoma: a longitudinal analysis by the German Hodgkin study group. Br. J. Cancer. 2013;108(1):49–57. doi: 10.1038/bjc.2012.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mütsch J., et al. Sexuality and cancer in adolescents and young adults - a comparison between reproductive cancer patients and patients with non-reproductive cancer. BMC Cancer. 2019;19(1):1–13. doi: 10.1186/s12885-019-6009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gan G., Ng D.L.C., Leong Y.C. Erectile dysfunction in male lymphoma survivors in a Southeast Asian country. Singapore Med. J. 2022;63:376–380. doi: 10.11622/smedj.2021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsatsou I., et al. Sexual function of male survivors of hematological malignancy treated by autologous hematopoietic stem cell transplantation: a multicenter controlled observational study. J. Sex Marital Ther. 2023;49(6):1–13. doi: 10.1080/0092623X.2023.2167756. [DOI] [PubMed] [Google Scholar]
- 41.Eeltink C.M., et al. Self-reported sexual function in sexually active male Hodgkin lymphoma survivors. Sex. Med. 2020;8(3):428–435. doi: 10.1016/j.esxm.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tajar A., et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European male ageing study. J. Clin. Endocrinol. Metab. 2010;95(4):1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 43.Wallace W.H.B., Kelnar C.J.H. 2009. Endocrinopathy after Childhood Cancer Treatment. [Google Scholar]
- 44.Wu F.C.W., et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J. Clin. Endocrinol. Metab. 2008;93(7):2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 45.Bacon C.G., Mittleman M.A., Kawachi I., Giovannucci E., Glasser D.B., Rimm E.B. Sexual function in men older than 50 Years of age: results from the health professionals follow-up study. Ann. Intern. Med. 2003;139(3) doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 46.Groenestege W.M.T., et al. Accuracy of first and second generation testosterone assays and improvement through sample extraction. Clin. Chem. 2012;58(7):1154–1156. doi: 10.1373/clinchem.2011.181446. [DOI] [PubMed] [Google Scholar]
- 47.Development Initiatives . Global Nutrition Report 2017: Nourishing the SDGs. Development Initiatives; Bristol, UK: 2017. [Google Scholar]
- 48.Feldman H.A., et al. 2002. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. [DOI] [PubMed] [Google Scholar]
- 49.Althof S.E., Parish S.J. Clinical interviewing techniques and sexuality questionnaires for male and female cancer patients. J. Sex. Med. 2013;10(SUPPL):35–42. doi: 10.1111/jsm.12035. [DOI] [PubMed] [Google Scholar]
- 50.Arrington R., Cofrancesco J., Wu A.W. Questionnaires to measure sexual quality of life. Qual. Life Res. 2004;13(10):1643–1658. doi: 10.1007/s11136-004-7625-z. [DOI] [PubMed] [Google Scholar]
- 51.Holme I.K., et al. Sexual problems as late effects: awareness and information needs among 1870 long-term Norwegian childhood, adolescent, and young adult cancer survivors (the NOR-CAYACS study) J. Adolesc. Young Adult Oncol. 2023:1–10. doi: 10.1089/jayao.2023.0031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated to this study is freely available in the originally published articles. If additional data has been provided by the authors, it can be found in supplementary data for this current publication.