Abstract
Pancreatic ductal adenocarcinoma (PDAC) has a dismal prognosis with a 5-year overall survival of 11%. The disease is usually diagnosed at advanced stages, and systemic chemotherapy is the standard-of-care treatment for the majority of patients with PDAC. Although novel treatment options, such as targeted therapy and immunotherapy, have achieved substantial progress leading to practice-changing results, with FDA approvals for several solid tumors so far, the progress achieved for PDAC is relatively limited. Recent studies uncovered potential therapeutic targets for patients with PDAC, and potential therapeutic opportunities are currently being further examined. Herein, we review recent advances in systemic therapy regimens, including cytotoxic agents, targeted therapies, immunotherapy, and novel therapeutic options for managing patients with PDAC. We also elaborate on molecular profiling to guide treatment and existing therapeutic opportunities that may further advance the clinical care of patients with this devastating disease.
Keywords: Pancreatic adenocarcinoma, Immunotherapy, KRAS12C, Fusion genes, Targeted therapy
Introduction
The incidence of pancreatic cancer has been increasing significantly over the last decade with a concerning trend in mortality, particularly due to limited therapeutics for management.1 With the current trend, pancreatic cancer is expected to be the second leading cause of cancer-related death in the US, driven by both the increasing incidence of cases and the aggressive biological nature of this disease.2 Currently, the 5-year overall survival (OS) for advanced-stage pancreatic ductal adenocarcinoma (PDAC) is approximately 2.5% to 5%, indicating an unmet need for developing novel therapeutics to improve outcomes for patients with pancreatic cancer, particularly those with advanced-stage disease.3
The biology of pancreatic cancer carries unique characteristics. The molecular underpinning of pancreatic adenocarcinoma remains one of the key drivers of the aggressive biology of this cancer.4 One of the key molecular features of pancreatic cancer is the high incidence of KRAS and TP53 mutations, leading to dysregulation in both oncogenic and tumor suppressor pathways.5 Several other tumor suppressor genes, such as SMAD4 and CDKN2A (p16), are also frequently mutated in PDAC and are also considered to be founder alterations that contribute to the aggressive nature of PDAC and its resistance to chemotherapy.6,7 Nonetheless, systemic combination chemotherapeutics remain the mainstay treatment of advanced-stage pancreatic cancer. Although novel targeted therapy or immunotherapy agents improve OS rates in certain cancer types (eg, NSCLC, renal cell carcinoma, and malignant melanoma), the majority of these drugs yielded limited clinical benefits in pancreatic cancer.5,8 Therefore, these tumors represent an unmet clinical need.
Despite the challenges in drug development for PDAC, there has been promising progress in targeted therapeutics for pancreatic cancer, and research efforts continue to investigate immunotherapeutics to change the course of this aggressive disease. Herein, we discuss recent advances in treatments of patients with PDAC and future therapeutic opportunities for novel therapeutics.
Systemic Chemotherapy
Standard-of-care treatment for metastatic pancreatic cancer (mPDAC) has significantly evolved over the last decade. The study by the PRODIGE group investigated the role of folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) for patients with mPDAC.9 In this study, 342 patients with mPDAC with an ECOG status 0 to 1 were randomized to receive FOLFIRINOX or single-agent gemcitabine. The median progression-free survival (PFS) and OS for FOLFIRINOX and gemcitabine were 6.4 months versus 3.3 months (HR, 0.47 [95% CI, 0.37-0.59]; P <.001) and 11.1 months versus 6.8 months (HR, 0.57 [95% CI; 0.45-0.73]; P < .001), respectively. These findings are consistent with improved outcomes with the use triplet regimen over single-agent gemcitabine and resulted in practice change after a long period of time without significant progress. This study was followed by a clinical trial that investigated gemcitabine and albumin-bound paclitaxel (nab-paclitaxel) combination.10 In this study, patients with a Karnofsky Performance Status Score of 70 or above (ECOG 0-1) were randomized to receive either gemcitabine (1000 mg/m2) and nab-paclitaxel (125 mg/m2) weekly for 3 weeks, followed by a week off or single-agent gemcitabine (1000 mg/m2) weekly 7 out of 8 weeks, followed by days 1, 8, and 15 every 4 weeks. In this study, patients who received the combination therapy had a median PFS of 5.5 months and OS of 8.5 months, while patients who received gemcitabine single agent had a median PFS of 3.7 months and OS of 6.7 months (HR for PFS and OS [0.69 and 0.62]; P < .001 for PFS and OS). This study established gemcitabine and nab-paclitaxel as alternative first-line therapy for patients with mPDAC. Although a retrospective comparative study suggested improved OS and PFS rates with FOLFIRINOX as compared to gemcitabine and nab-paclitaxel, at this time, there is no prospective data to confirm the superior efficacy of FOLFIRINOX over the gemcitabine–nab-paclitaxel combination.11 Given relatively better tolerance with the use of doublet regimens, for patients who are not eligible to receive modified FOLFIRINOX due to poor performance status, a gemcitabine-based regimen can be considered.12 Biweekly dosing of gemcitabine and nab-paclitaxel is an effective alternative for patients with relatively poor performance status.13 Importantly, patients with germline or sporadic BRCA1, BRCA2, and PALB2 alterations should be considered for platinum-based chemotherapies as first-line treatment, including gemcitabine and cisplatin.14,15
Second-line chemotherapy for patients with PDAC and otherwise no actionable gene has been established in the NAPOLI trial. In this 3-arm randomized phase III trial, patients who previously had disease progression with gemcitabine-based first-line therapy received either nanoliposomal irinotecan alone or in combination with fluorouracil plus folinic acid or fluorouracil plus folinic acid alone.16 Patients who received combination therapy with nanoliposomal irinotecan had significantly improved OS (6.1 months) compared to patients who received fluorouracil plus folinic acid alone (4.2 months) (HR, 0.67 [95% CI 0.49-0.92]; P = .012). Although the therapeutic value of nanoliposomal irinotecan is not well established for patients with prior irinotecan therapy (such as FOLFIRINOX), the benefit appears to be more pronounced for patients with no prior irinotecan-based therapy.17 Other systemic chemotherapy options include FOLFOX18 or gemcitabine-based combinations, depending on prior line of therapy.
Targeted Therapies
Next-generation sequencing (NGS) has revolutionized the care of patients with cancer. Aguirre et al demonstrated that up to 25% of patients with PDAC may have potentially targetable genomic alterations (Figure 1).19 According to the Know Your Tumorinitiative, the median OS of patients with PDAC with actionable alterations on matched therapy is approximately 2.5 years when compared to 1.5 years among patients on unmatched therapies, indicating that targeting therapy for potentially actionable genes should be evaluated for all patients with advanced-stage pancreatic cancer.20 Given that growing evidence suggests that survival benefit matched therapies, genome-driven clinical decisions should be implemented in routine patient care, which is discussed further in the following sections.
Figure 1.
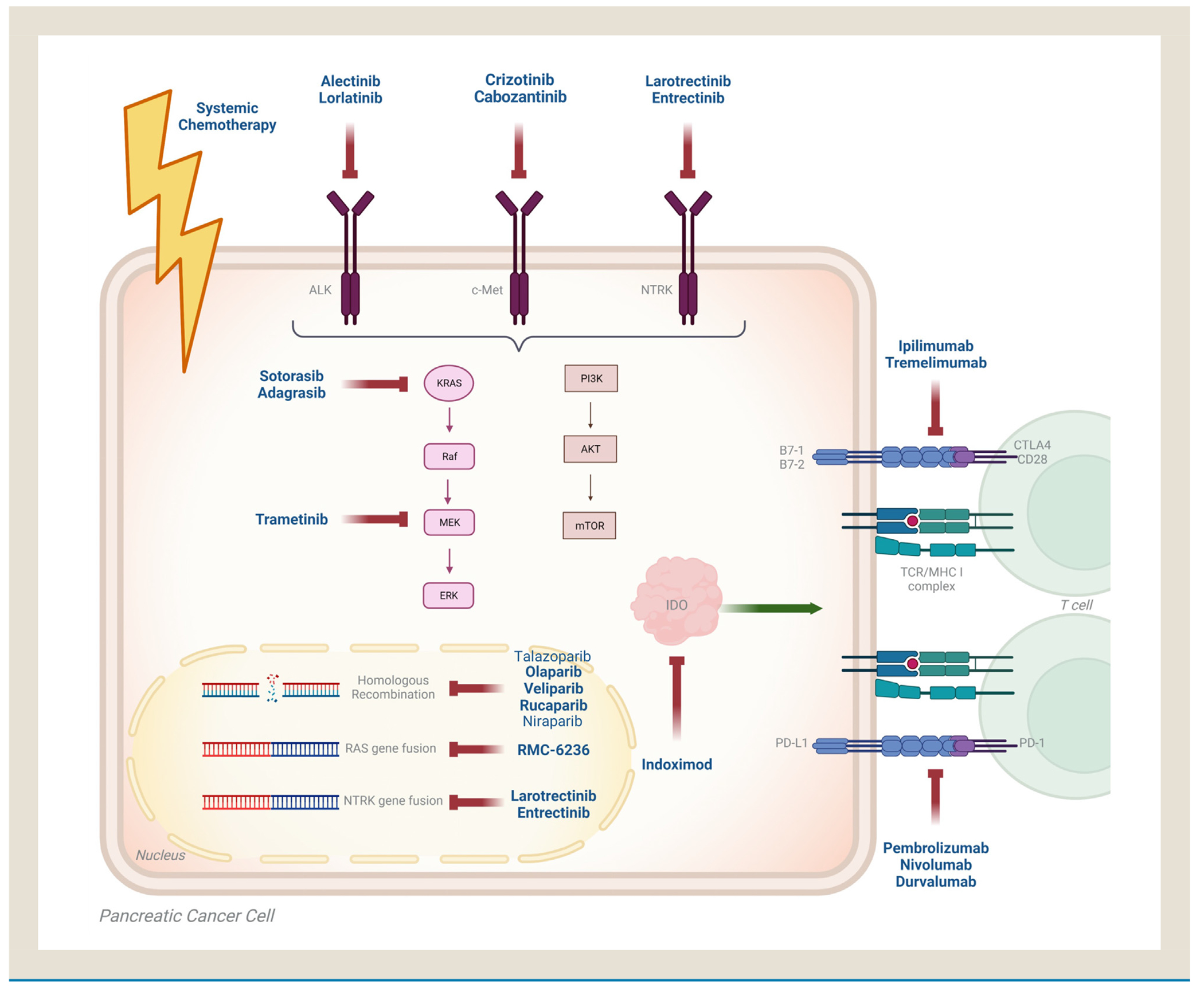
Potentially actionable genetic alterations in pancreatic ductal adenocarcinoma
Homologous Recombination Deficiency
Genomic integrity is maintained by DNA-damage response and repair pathway. BRCA1, BRCA2, and PALB2 are key genes that play a significant role in DNA-damage repair, which is triggered by DNA-damage response molecules, including ATM and ATR.21 Homologous recombination is one of the most important and precise DNA-damage repairs in humans, and this pathway is orchestrated by BRCA1, BRCA2, and PALB2 proteins. Patients whose tumors harbor homologous repair deficiency (HRD) due to loss of function alterations in their key genes are known to be more sensitive to platinum-based chemotherapy regimens.22 Important to note that several cohort studies evaluating outcomes of patients with HRD consistently reported improved outcomes with the use of platinum-based chemotherapeutics as a first-line therapy.23 A recent clinical trial investigated cisplatin-based chemotherapy with or without veliparib, which is a poly (ADP-ribose) polymerase inhibitor(PARPi).24 In this study, although veliparib did not add any significant value to platinum therapy, cisplatin and gemcitabine combination resulted in an ORR of 74.1% and a median PFS of 10.1 months, creating this combination as an alternative standard of care to FOLFIRINOX, which also contains oxaliplatin. At this time, there is no comparative study of cisplatin-based doublet and FOLFIRINOX first line, and both combinations can be considered for patients with pancreatic cancer and HRD. Notably, there is also growing evidence irinotecan which also induces cytotoxic DNA damages, may have a clinical advantage for the treatment of patients with HRD.25
Olaparib, a PARPi, was investigated in patients with germline BRCA1/2 alterations and as maintenance therapy. In this randomized phase III trial, patients received olaparib 300 mg twice daily or a placebo after induction platinum-based chemotherapy if their disease did have progression.26 Although there was not any difference in OS, patients who received olaparib had improved median PFS (7.4 months vs. 3.8 months HR, [0.53]; P = .004). Based on this evidence, olaparib was approved by the FDA for the maintenance treatment of patients with pancreatic cancer with a germline BRCA1/2 mutation in early 2020.26 Moreover, rucaparib, a PARPi, has been demonstrated to be effective in patients whose tumors harbor either germline PALB2 or somatic BRCA2 mutations in a phase II trial.27 In this single-arm study, a total of 46 patients were enrolled and received platinum-based chemotherapy for at least 16 weeks, and if no progression was noted, then they received rucaparib 600 mg twice daily. In this study, median PFS and OS were 13.1 and 23.5 months, respectively. Although the results of this study are promising, the lack of randomization limits our understanding of the true additive benefit of this therapy to platinum-based induction chemotherapy in the maintenance setting. Nonetheless, both agents are already included in NCCN guidelines as therapeutic approaches for patients with pancreatic cancer harboring HRD.28 The progress on PARPi continues to evolve with novel approaches and combinations. A recent study investigated the combination of niraparib, a parp inhibitor, with nivolumab or ipilimumab, which are immune checkpoint inhibitors in a phase 1b/2 trial.29 Interestingly, patients who received niraparib and ipilimumab achieved promising outcomes (6-month PFS rate of 59.6% 95% CI 44·3–74·9; P = .045) while patients who received niraparib with nivolumab had inferior outcomes (6-month PFS rate of 20·6% 95% CI 8·3-32·9; P = .0002). This biological dilemma warrants further translational studies to better define conflicting outcomes noted with 2 classes of immune checkpoint inhibitors. There is an ongoing NCI-sponsored phase II clinical trial assessing whether the addition of pembrolizumab to olaparib would be more beneficial in patients with metastatic PDAC with germline BRCA1 or BRCA2 mutations (NCT04548752).
KRAS Pathway
Although KRAS mutations are the most prevalent genetic alterations in patients with PDAC, attempts to target KRAS have failed thus far. Recently, sotorasib received FDA approval for KRASG12C mutant NSCLC patients.30 However, this KRAS mutation accounts for only 1% of patients with KRAS mutant PDAC (Table 1).31,32 On the other hand, the vast majority of patients with KRAS mutant PDAC harbor KRASG12D mutation, and inhibitors specific to this mutation are yet to be investigated in clinical trials for PDAC. At this time, growing KRASG12C targeting approaches are being developed with highly promising outcomes for patients with PDAC.12,33 Sotorasib, one of the first specific, irreversible KRAS G12C small-molecule inhibitors, has been investigated for patients with pancreatic cancer in the CodeBreak100 trial. In this phase I/II study, 38 patients with chemotherapy-refractory advanced pancreatic cancer with a tumor harboring a KRASG12C mutation were enrolled and received sotorasib monotherapy.34 The authors have reported an objective response rate (ORR) of 21% with no fatal treatment-related adverse event and a disease control rate of 84.2%, indicating a highly promising response with monotherapy. This agent is now being investigated in combination with chemotherapy as second-line therapy for patients with metastatic PDAC (NCT05251038). Most recently, adagrasib, an irreversible and selective KRASG12C inhibitor, has been investigated in the KRYSTAL-1 trial (phase 1/2) for patients with solid tumors, including metastatic PDAC.35 In one of the cohorts of this phase II study, 12 patients with metastatic PDAC received adagrasib 600 mg twice daily. Ten patients were evaluable, 5 patients (50%) achieved partial response, and the disease control rate was 100%, indicating there is highly promising antitumor activity of this agent for patients with KRASG12C mutant PDAC. The median PFS was also promising and was noted to be 6.6 months. Although the numbers are relatively low in this study, early signals noted with both KRASG12C inhibitors carry significant future therapeutic opportunities for patients with PDAC. Other novel KRASG12C inhibitors are also being developed and investigated in solid tumors (NCT05009329).
Table 1.
Selected Clinical Trials With Targeted Therapeutics for the Management of Metastatic Pancreatic Cancer
| Drug | Target | Mechanism | Phase of Trial | Trial Design | Cancer Type | Primary Endpoint | Clinical Reference | Results | Adverse Events (Any Grade) | Current Status |
|---|---|---|---|---|---|---|---|---|---|---|
| Sotorasib | KRASG12C | Small molecule inhibitor of KRASG12C | I/II | Monotherapy or in combination with PD-1/PD-L1 inhibitors | Basket trial | Treatment-related adverse effects, objective response | NCT03600883 (CodeBreak 100) | 8 PR 21.1% ORR 32% DCR Median PFS 3.98 months Median OS 6.87 months |
Diarrhea (5.3%) Fatigue (5.3%) Abdominal pain (2.6%) |
Recruiting |
| Adagrasib | KRASG12C | Small molecule inhibitor of KRASG12C | I/II | Monotherapy or in combination with PD-1 or EGFR inhibitors | Basket trial | Treatment-related adverse effects, objective response | NCT03785249 (KRYSTAL-1) | 5 PR 100% DCR Median PFS 6.6 months |
Nausea (48%) Diarrhea (43%) Vomiting (43%) |
Recruiting |
| Olaparib | PARP | Small molecule inhibitor of PARP | III | Monotherapy or placebo | gBRCA mutated pancreatic cancer | Objective response | NCT02184195 | Median PFS 7.4 months Median OS 18.9 months Hazard Ratio 0.53 |
Fatigue (60%) Nausea (45%) Anemia (27%) |
Active, not recruiting |
| Rucaparib | PARP | Small molecule inhibitor of PARP | II | Monotherapy | BRCA1, BRCA2 or PALB2 mutated pancreatic cancer | Treatment-related adverse effects | NCT03140670 | Median PFS 13.1 months Median OS 23.5 months 41.7% ORR 66.7% DCR |
Anemia (74%) Nausea (48%) Increased ALT (47%) |
Active, not recruiting |
| Niraparib | PARP | Small molecule inhibitor of PARP | Ib/II | In combination with Ipilimumab or Nivolumab | Platinum-sensitive advanced pancreatic cancer | Treatment-related adverse effects, objective response | NCT03404960 | Niraparib + Nivolumab 6-month PFS 20.6% Niraparib + Ipilimumab 6-month PFS 59.6% |
Niraparib + Nivolumab Hypertension (8%) Anemia (4%) Thrombocytopenia (4%) Niraparib + Ipilimumab Fatigue (14%) Anemia (11%) |
Active, not recruiting |
| Entrectinib | TRK, ROS1 and ALK | Small molecule inhibitor of TRK, ROS1 and ALK | II | Monotherapy | Basket trial | Objective response | NCT02568267 | 2 PR 1 SD |
Arthralgias Myalgias Fatigue |
Recruiting |
| Zenocutuzumab | HER2 and HER3 | IgG1 bispecific antibody targeting HER2 and HER3 | I/II | Monotherapy | Basket trial | Objective response | NCT02912949 | ORR 39% | Asthenia, Diarrhea, Anemia Nausea |
Recruiting |
| Durvalumab and Tremelimumab | PD-L1 and CTLA4 | Monoclonal antibody against PD-L1 and CTLA4 | II | Combination therapy or placebo (in combination with Gemcitabine and Nab-Paclitaxel) | Metastatic pancreatic cancer | Objective response | NCT02879318 | Median OS 9.8 months Median PFS 5.5 months |
Fatigue (20%) Thromboembolic event (15%) Sepsis (11%) |
Active, not recruiting |
| Indoximod | IDO pathway | Small molecule inhibitor of IDO | I/II | In combination with Gemcitabine and Nab-Paclitaxel | Metastatic pancreatic cancer | Treatment-related adverse effects, objective response | NCT02077881 | ORR 46.2% CR 1% PR 45.2% Median OS 10.9 months |
Fatigue Nausea Anemia |
Completed |
| Tarextumab | Notch Signaling | Monoclonal antibody against Notch receptor | Ib/II | Monotherapy or placebo (in combination with nab-paclitaxel and gemcitabine) | Previously untreated stage 4 pancreatic cancer | Treatment-related adverse effects, objective response | NCT01647828 | Median OS 6.4 months Median PFS 3.7 months ORR 20.2% |
Diarrhea (72%) Fatigue (52%) Thrombocytopenia (49%) |
Completed |
| Vismodegib | Hedgehog Pathway | Small molecule inhibitor of PTCH and SMO | Ib/II | Monotherapy or placebo (in combination with gemcitabine) | Metastatic pancreatic cancer | Objective response | NCT01064622 | Median PFS 4.0 months Median OS 6.9 months PR 8% SD 51% |
Neutropenia (28%) Fatigue (13%) Anorexia (9%) |
Completed |
| PEGPH20 | Hyaluronic Acid | Recombinant form of human hyaluronidase | Ib/II | Monotherapy or placebo (in combination with FOLFIRINOX) | Metastatic pancreatic cancer | Treatment-related adverse effects, objective response | NCT01959139 | Median OS 7.7 months Median PFS 4.3 months RR 33% |
Nausea (25%) Diarrhea (24%) Vomiting (22%) |
Active, not recruiting |
Given that the vast majority of PDAC patients harbor KRAS mutations, pan-RAS inhibitors might be attractive candidates to lead durable responses. RMC-6236, a pan-RAS inhibitor against G12D, G12V, and G12R mutations, demonstrated durable complete responses in combination with anti–PD-1 treatment in preclinical PDAC models.36 Another pan-RAS inhibitor, BI 1701963, that impairs KRAS and SOS protein interaction is currently being investigated in early phase clinical trials.37 Other potent mutation-specific KRAS inhibitors, including KRAS G12D, demonstrated promising preclinical signals for further development.38 Another approach for patients with KRAS mutations is cancer vaccine development by utilization of lipid nanoparticle mRNA-based vaccine strategies to target mutations such as KRAS G12D, G12V, and G13D could accelerate the development of effective treatment options for these undruggable targets. There is an ongoing phase I clinical trial to assess the safety of the mRNA-based vaccine against KRAS G12D, G12V, G13D, and G12C mutations in combination with pembrolizumab (NCT03948763) in patients with NSCLC, colorectal cancer, and pancreatic cancer. ELI-002 is a novel lymph node-targeted, AMP-modified therapeutic vaccine targeting KRAS-driven cancers currently being investigated in early phase clinical trials for patients who have minimal residual cancer.39 Recently a proof of concept case study reported successful tumor regression for a patient whose T cells were engineered to express T cell receptors targeting KRAS 12D mutation.40 This study suggests autologous T cell therapy may have the potential to generate clinically meaningful antitumor immunity. Further studies with expanded cohorts are warranted.
The unmet need to target KRAS mutations led researchers to pursue inhibition of upstream activator proteins such as SHP2 or SOS1.41 There are several SHP2 inhibitors utilized in clinical trials as a single or combinatorial agent in different cancer types. However, the majority of the SOS1 inhibitors are in the preclinical phase except for BI1701963 (NCT04975256).
Fusion Genes
The majority of patients with PDAC patients harbor KRAS mutations. However, KRAS wild-type ~10%) tumors have alternate fusion genes to drive tumor progression. To date, BRAF, ROS, NTRK, ALK, and RAF1 fusion genes have been identified to drive oncogenic transformation.42,43 In a study, patients with RAS wild-type disease were found to be enriched with fusion genes; BRAF (6.6%), Fibroblast growth factor receptor 2 (FGFR2) (5.2%), ALK (2.6%), RET (1.3%), and neuregulin 1 (NRG1) (1.3%).43 Although fusion genes are relatively uncommon overall population, they are potentially actionable. For example, a patient with MET gene fusion received crizotinib, a multikinase inhibitor with potent activity against MET, and achieved a complete radiological response, and the duration of response was continuing at the time of report over 12 months.44 RAF1 fusion genes are common in pancreatic acinar cell carcinomas (14.3%-18.5%).45 In a multicenter study, 4 out 5 patients with PDAC and with a RAF fusion gene achieved response top MEK inhibitor monotherapy (trametinib). Although there is a growing body of evidence demonstrating that RAF1 gene rearrangements are sensitive to MEK inhibitors, it is context-dependent. Contrary to the aforementioned case with a remarkable response to trametinib, a patient with pancreatic acinar cell carcinoma whose tumor harbors the GATM-RAF1 fusion gene did not show significant clinical improvement on trametinib (2 mg/day), indicating disease heterogeneity.46
Larotrectinib and entrectinib, both NTRK inhibitors, received accelerated approval by FDA in a tumor-agnostic manner in 2018 and 2019, respectively.12, 47 NTRK fusions are also potentially actionable with these novel agents for patients with pancreatic adenocarcinoma. In a case series, patients with NTRK gene fusions who were treated with entrectinib and 2 out 3 patients achieved radiological response to therapy.48 In another report, a patient with pancreatic acinar cell carcinoma achieved a deep and durable response with larotrectinib 100 mg twice daily, indicating NTRK fusions are highly actionable for patients with PDAC as well.
NRG1 fusions are also actionable genes for patients with cancer, and it has been demonstrated among patients with PDAC.49 Pathogenic NRG fusion products function as a ligand for epidermal growth factor receptor 3 (HER3), resulting in heterodimerization of HER3 with HER2 receptor with aberrant epidermal growth factor receptor (ERBB) downstream activity. In a precision medicine study, 2 patients with KRAS wild-type PDAC were found to have NRG1 gene fusion.49 Patients were treated with ERBB inhibitors (afatinib and erlotinib/pertuzumab), and they achieved radiological response.49 Currently, several agents are being developed to target NRG1-driven oncogenesis, including seribantumab (HER3 blockade, NCT04790695) and zenocutuzumab50 (bispecific HER2/HER3 blockade, NCT02912949). More prospective data are needed to further define the benefits of these agents and the actionability of this pathway for patients with PDAC.
Immune Checkpoint Inhibitors
It has been previously demonstrated that patients with microsatellite instability-high (MSI-H) tumors better respond to immune checkpoint inhibitors (ICIs). Currently, both pembrolizumab (humanized anti-programmed cell death protein 1 (PD-1) antibody), nivolumab (human IgG4 monoclonal antibody targeting PD-1), and dostarlimab (humanized anti-PD-1 antibody) have been approved for patients with metastatic MMR-D/MSI-H pancreatic cancer. However, MSI-H disease is seen in approximately 2% of patients with PDAC. Given the low prevalence of MSI pancreatic cancer, it is critical to determine which subgroup of patients should be screened for microsatellite instability. In a comparative study, Luchini et al demonstrated that patients with MSI PDAC show medullary and mucinous/colloid histology, KRAS/TP53 wild-type background, and more common JAK mutations.51
While observed responses among patients with MSI-H PDAC are highly encouraging, anti–PD-1 and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) monoclonal antibodies have not shown any signal for efficacy for patients with microsatellite stable (MSS) PDAC.52 Durvalumab (anti-PD1 blockade) and tremelimumab (anti-CTLA4) have been investigated for patients with PDAC whose disease has progressed on first-line systemic therapy.53 In this study, randomized phase II patients received either durvalumab and tremelimumab combination or durvalumab monotherapy, and unfortunately, the ORR was only 3.1%, while there was no objective response in the monotherapy arm, indicating a very poor response to ICI therapy. The authors reported a median PFS of 1.5 months for both arms, and the 6-month PFS rate was only 9.4%. ICIs have also been combined with chemotherapeutics to achieve a synergistic approach. The study by the Canadian Cancer Trials Group (PA.7) investigated the combination of durvalumab and tremelimumab doublet with gemcitabine and nab-paclitaxel for patients with metastatic PDAC.54 In this randomized phase II trial, patients were randomized to receive durvalumab and tremelimumab in combination with gemcitabine and nab-paclitaxel or chemotherapy alone. The authors reported no significant improvement in median PFS (5.5 months vs. 5.4 months, HR = 0.98) or OS (9.8 vs. 8.8 months, HR = 0.94) and ORR (30.3% vs. 23.0%), suggesting a lack of synergistic effect with combination of chemotherapy and ICIs.
To sensitize patients with MSS pancreatic cancer to ICIs, ICIs were combined with stroma-modifying agents. In a randomized phase II trial, patients received atezolizumab in combination with PEGylated recombinant human hyaluronidase (PEGPH20), which is a stroma-depleting agent or standard-of-care of chemotherapy (the MORPHEUS trial).55 In the interventional arm, the ORR was only 6.1%, and the median PFS was 1.5 months, consistent with the absence of a promising clinical signal with this approach. In another study, Parikh et al conducted a phase II clinical trial to assess the efficacy of radiotherapy along with immune checkpoint blockades (anti-PD1 and anti-CTLA4) for patients with MSS PDAC and colorectal cancer to convert their immune cold biology to an immune hot one.56 This proof-of-concept study showed the feasibility and safety of combining immunotherapy and radiation therapy with modest activity, and the lack of randomization limits conclusions on the true additive benefit of immunotherapy to radiation therapy. In this study, the authors did not detect any change in tumor-mutation burden before and after therapy; however, notably, elevated levels of NK cells prior to treatments were found to be associated with improved response. Given that it was the first proof-of-concept study showing the effectiveness of ICIs in combination with radiotherapy in one of the hard-to-treat cancers such as PDAC, further studies are warranted to evaluate scheduling and sequence of treatment modalities to improve outcomes in patients with PDAC. Further studies are needed to change the immune cold nature of PDAC to make it more sensitive to immunotherapy.
Other Targeted Approaches and Novel Therapeutic Avenues
The unmet research need in pancreatic cancer has triggered several novel approaches to identify new therapeutic opportunities. Indoleamine 2,3-dioxygenase 1 (IDO), a key enzyme that catalyzes L-tryptophan, has been associated with immune evasion of cancer cells and has become an interest of cancer research as a therapeutic target (Table 2).57 Based on preclinical data that supported the IDO1 inhibition to enhance anticancer immune response,58,59 indoximod, an IDO1 inhibitor, was investigated in clinical trials. A single-arm phase II study of indoximod, in combination with gemcitabine and nab-paclitaxel, did not meet the predetermined primary goal of a 30% reduction in HR;60 however, this combination provided a promising overall response rate of 46.2% and increased intratumoral CD8 T-cell density. Given that indoximod improved intratumoral CD8 density, particularly among responders, the addition of ICIs should be further considered as a therapeutic opportunity for a chemoimmunotherapy approach.
Table 2.
Ongoing Selected Clinical Trials for Pancreatic Cancer
| NCT | Phase | Treatment | Current Status | Study Group |
|---|---|---|---|---|
| NCT05251038 | I/II | Sotorasib plus systemic chemotherapy | Not yet recruiting | KRAS G12C-mutant advanced pancreatic cancer with progression of disease after first line treatment |
| NCT05009329 | I/II | JAB21822 monotherapy | Recruiting | KRAS G12C-mutant advanced solid tumors |
| NCT04975256 | I/Ib | Adagrasib plus BI 1701963 | Active, not recruiting | KRAS G12C-mutant advanced solid tumors |
| NCT04548752 | II | Olaparib plus Pembrolizumab or Olaparib alone | Recruiting | Metastatic pancreatic cancer with germline BRCA1/2 mutations |
| NCT03948763 | I | mRNA-5671/V941 plus Pembrolizumab or mRNA-5671/V941 alone | Active, not recruiting | KRAS mutant advanced or metastatic NSCLC, CRC or Pancreatic Adenocarcinoma |
| NCT02077881 | I/II | Indoximod plus Gemcitabine and Nab-Paclitaxel | Completed | Metastatic pancreatic cancer |
| NCT04361162 | II | Nivolumab plus Ipilimumab and Radiation | Recruiting | Microsatellite stable pancreatic cancer |
| NCT04543071 | II | Cemiplimab and Motixafortide plus systemic chemotherapy | Recruiting | Metastatic treatment naïve pancreatic cancer |
| NCT02451982 | II | Nivolumab, Urelumab, BMS-986253, GVAX and systemic chemotherapy | Recruiting | Surgically resectable pancreatic cancer |
| NCT03816163 | II | Zolbetuximab plus nab-paclitaxel and gemcitabine | Recruiting | CLDN18.2 positive metastatic pancreatic cancer |
| NCT04111458 | I | BI 1701963 plus Trametinib or BI 1701963 alone | Active, not recruiting | Basket trial |
| NCT03935893 | II | Autologous Tumor Infiltrating Lymphocytes | Recruiting | Basket trial |
| NCT04853017 | I | ELI-002; Therapeutic cancer vaccine targeting KRAS | Recruiting | Microsatellite stable pancreatic cancer |
| NCT03193190 | Ib/II | Atezolizumab, Cobimetinib, PEGPH20, Motixafortide, Selicrelumab, Bevacizumab, Simlukafusp alfa, Etrumadenant, Tiragolumab, Tocilizumab and systemic chemotherapy | Recruiting | Metastatic pancreatic cancer |
Targeting cancer stem cells and pathways associated with cancer-cell stemness has also been interrogated in PDAC. In a randomized phase II trial, tarextumab, a notch receptor inhibitor that targets cancer-cell stemness, has been investigated in combination with gemcitabine and nab-paclitaxel and compared to standard-of-care chemotherapy alone arm for patients with PDAC.61 In this study, unfortunately, patients who received tarextumab had inferior survival outcomes compared to patients who received standard-of-care chemotherapy (median OS 6.4 months vs. 7.9 months), leading to disappointment.61 Wnt signaling, which is also associated with cancer-cell stemness, was considered to be a therapeutic target. In a phase Ib trial, ipafricept (a Wnt inhibitor), in combination with gemcitabine and nab-paclitaxel, was investigated among patients with PDAC.62 In this single-arm study, median PFS and OS were noted at 5.9 months and 9.7 months, respectively,62 and they were relatively similar to historical controls.10 In addition to oncogenic drivers, targeting the tumor microenvironment was an attractive strategy to improve the efficacy of mainstay treatments in PDAC. In particular, stromal cells and extracellular matrix comprise 90% of pancreatic cancer tissue.63 Therefore, modifying stroma has been of interest as a therapeutic target for patients with PDAC. However, the results from clinical and preclinical studies are discouraging. Clinical studies assessing the efficacy of stroma-targeting agents in combination with chemotherapy have not resulted in significant improvement in clinical outcomes.64 Sonic Hedgehog pathway, which has been shown to be involved in dense stroma formation and desmoplasia, has been targeted by the use of vismodegib65 and saridegib66 in combination with chemotherapies, which did not yield any significant clinical improvement.65,66 PEGPH20 was also used to target dense pancreatic stroma in combination with FOLFIRINOX67 and gemcitabine and nab-paclitaxel combinations,68 and neither approach resulted in clinical benefit and perhaps led to inferior outcomes when combined with FOLFIRINOX.67 A recent translation study also showed better outcomes with dense stroma and more aggressive features with low-density stroma, which has been consistent with the findings of these clinical trials.69 Therefore, at this time, targeting PDAC stroma has limited clinical offerings for future therapeutic approaches.
Future Perspective
Currently, the mainstay treatment for patients with advanced-stage PDAC remains to be systemic chemotherapy. Recent discoveries have further advanced potential druggable targets among subgroups of PDAC patients, particularly those with RAS wild-type disease, which includes HRD, MMR deficiency, and gene fusions and amplifications. Perhaps more exciting progress for patients with PDAC is the evolution of KRAS targeting, which is the most seen genetic alteration in PDAC. The development of RAS targeting will further open therapeutic pathways for this challenging disease and will play a locomotive role in drug development.
Although the role of immunotherapy is limited except for patients with MSI-H PDAC, the effective targeting of RAS will likely impact the horizons of immunotherapy in PDAC. RAS oncogenes are associated with the immune-exclusion process and recruitment of tumor-suppressive macrophages to the tumor micoenvironement.70–73 Effective therapeutic-level RAS inhibition may at least partially reverse the immune exclusion process that renders a haven for cancer cells and enhances T-cell infiltration to the tumor microenvironment, which may create an opportunity for therapeutic synergy. This approach should be further investigated for patients with advanced-stage PDAC. Similarly, targeting other oncogenic drivers of the mitogen-activated protein kinase pathway may also derive additional therapeutic synergism for immunotherapy. Importantly, transforming growth factor beta (TGF-β) is another dysregulated pathway, particularly due to increased SMAD4 alterations that result in upregulation of this pathway74. Notably, TGF-β signaling has also been associated with the immune-exclusion process,75 and the role of immunotherapy with TGF-β targeting remains to be seen for patients with PDAC. Currently, a study is investigating TGF-β blockade in combination with chemotherapy with or without immune checkpoint blockade (NCT04390763) (Table 2). Combinations of PARPis and IOs are currently in progress (NCT04548752) in the maintenance setting, and if found to be significant, then further exploration can be considered in the other clinical scenarios, including for patients with chemotherapy-refractory diseases. The claudin 18.2, a tight junction protein that promotes carcinogenesis, is currently being targeted in clinical trials. A study investigating zolbetuximab, a monoclonal antibody directed to claudin 18.2 to provoke antibody-dependent cellular cytotoxicity, is currently being investigated in combination with gemcitabine and nab-paclitaxel (NCT03816163).76 Collectively, these novel approaches may create more therapeutic opportunities and create new avenues for the management of patients with PDAC.
Conclusion
The therapeutic evolution of PDAC has been evolving slowly, particularly the molecular underpinnings of PDAC. Unlike other solid tumors so far, targeted therapy and immunotherapy have major limitations, and they have been effective among relatively uncommon subgroups of patients with PDAC with wild-type KRAS. However, as RAS targeting evolves, therapeutic avenues of PDAC will likely speed up progress in the treatment paradigm of PDAC, including immunotherapy. Perhaps targeting other oncogenic-driver pathways that directly involve pancreatic carcinogenesis, such as TGF-β, may also provide further therapeutic progress. Identifying pancreas-specific neoantigens, such as claudin 18.2, may accelerate advances in managing PDAC, particularly for cellular-based therapies. Collectively, although the progress has been slow, more opportunities and scientifically promising approaches are being developed to change the course of this aggressive disease, and there is hope on the horizon.
Footnotes
Conflict of Interest
None of all authors.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Quante AS, Ming C, Rottmann M, et al. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016;5:2649–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet North Am Ed. 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- 4.Sahin IH, Iacobuzio-Donahue CA, O’Reilly EM. Molecular signature of pancreatic adenocarcinoma: an insight from genotype to phenotype and challenges for targeted therapy. Expert Opin Ther Targets. 2016;20:341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin IH, Askan G, Hu ZI, O’Reilly EM. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Ann Oncol. 2017;28:2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong J, Park YN, Park JS, Yoon D-S, Chi HS, Kim BR. Clinical significance of p16 protein expression loss and aberrant p53 protein expression in pancreatic cancer. Yonsei Med J. 2005;46:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24:1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun JW, Lee SH, Kim JS, et al. Comparison between FOLFIRINOX and gemcitabine plus nab-paclitaxel including sequential treatment for metastatic pancreatic cancer: a propensity score matching approach. BMC Cancer. 2021;21:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–123. [DOI] [PubMed] [Google Scholar]
- 13.Ahn DH, Krishna K, Blazer M, et al. A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Therap Adv Med Oncol. 2017;9:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wattenberg MM, Asch D, Yu S, et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Reilly EM, Lee JW, Zalupski M, et al. A randomized, multicenter, phase II trial of gemcitabine (G) , cisplatin (C) +/−veliparib (V) in patients with pancreas adenocarcinoma (PDAC) and a known germline (g) BRCA/PALB2 mutation. Am Soc Clin Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang-Gillam A, Li C-P, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet North Am Ed. 2016;387:545–557. [DOI] [PubMed] [Google Scholar]
- 17.Glassman DC, Palmaira RL, Covington CM, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer. 2018;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaanan A, Trouilloud I, Markoutsaki T, et al. FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer. 2014;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguirre AJ, Nowak JA, Camarda ND, et al. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov. 2018;8:1096–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahin IH, Lowery MA, Stadler ZK, et al. Genomic instability in pancreatic adenocarcinoma: a new step towards precision medicine and novel therapeutic approaches. Expert Rev Gastroenterol Hhepatol. 2016;10:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pishvaian MJ, Blais EM, Brody JR, et al. Outcomes in patients with pancreatic adenocarcinoma with genetic mutations in DNA damage response pathways: results from the know your tumor program. JCO Precis Oncol. 2019;3:1–10. [DOI] [PubMed] [Google Scholar]
- 23.Park W, Chen J, Chou JF, et al. Genomic methods identify homologous recombination deficiency in pancreas adenocarcinoma and optimize treatment selectiongenomic methods identify HRD and benefit to platinum in PDAC. Clin Cancer Res. 2020;26:3239–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly EM, Lee JW, Zalupski M, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol. 2020;38:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teo MY, O’Reilly EM. Is it time to split strategies to treat homologous recombinant deficiency in pancreas cancer? J Gastrointest Oncol. 2016;7:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA–mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiss KA, Mick R, O’Hara MH, et al. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol. 2021;39:2497–2505. [DOI] [PubMed] [Google Scholar]
- 28.Carlson RW, Koh W-J. The NCCN 2022 annual conference. J Natil Comprehensive Cancer Network. 2022;20:549–550. [DOI] [PubMed] [Google Scholar]
- 29.Reiss KA, Mick R, Teitelbaum U, et al. Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. Lancet Oncol. 2022;23:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waters AM, Der CJ. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb Perspect Med. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luchini C, Paolino G, Mattiolo P, et al. KRAS wild-type pancreatic ductal adenocarcinoma: molecular pathology and therapeutic opportunities. J Exp Clin Cancer Res. 2020;39:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreyer SB, Chang DK, Bailey P, Biankin AV. Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res. 2017;23:1638–1646. [DOI] [PubMed] [Google Scholar]
- 34.Strickler JH, Satake H, Hollebecque A, et al. First Data for Sotorasib in Patients With Pancreatic Cancer With KRAS P. G12C Mutation: A Phase I/II Study Evaluating Efficacy And Safety. American Society of Clinical Oncology; 2022. [Google Scholar]
- 35.Bekaii-Saab TS, Spira AI, Yaeger R, et al. KRYSTAL-1: Updated Activity and Safety of Adagrasib (MRTX849) in Patients (Pts) With Unresectable or Metastatic Pancreatic Cancer (PDAC) and Other Gastrointestinal (GI) Tumors Harboring A KRASG12C Mutation. American Society of Clinical Oncology; 2022. [Google Scholar]
- 36.Gustafson WC, Wildes D, Rice MA, et al. Direct targeting of RAS in pancreatic ductal adenocarcinoma with RMC-6236, a first-in-class, RAS-selective, orally bioavailable, tri-complex RASMULTI(ON) inhibitor. J Clin Oncol. 2022;40 591–591. [Google Scholar]
- 37.Gort E, Johnson ML, Hwang JJ, et al. A phase I, open-label, dose-escalation trial of BI 1701963 as monotherapy and in combination with trametinib in patients with KRAS mutated advanced or metastatic solid tumors. J Clin Oncol. 2020;38:TPS3651. [Google Scholar]
- 38.Christensen JG. Discovery and characterization of MRTX1133, a selective non-covalent inhibitor of KRASG12D. Paper presented at: AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics: Virtual; 2021. [Google Scholar]
- 39.Pant S, Furqan M, Abdul-Karim RM, et al. First-In-Human Phase 1 Trial of ELI-002 Immunotherapy as Treatment for Subjects With Kirsten Rat Sarcoma (KRAS)-Mutated Pancreatic Ductal Adenocarcinoma and Other Solid Tumors. Journal of Clinical Oncology. 2022;40(16):TPS2701. [Google Scholar]
- 40.Leidner R, Sanjuan Silva N, Huang H, et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N Engl J Med. 2022;386:2112–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann MH, Gerlach D, Misale S, Petronczki M, Kraut N. Expanding the reach of precision oncology by drugging All KRAS mutants. Cancer Discov. 2022;12:924–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gower A, Golestany B, Gong J, Singhi AD, Hendifar AE. Novel ALK fusion, PPFIBP1-ALK, in pancreatic ductal adenocarcinoma responsive to alectinib and lorlatinib. JCO Precision Oncology. 2020;4:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philip PA, Azar I, Xiu J, et al. Molecular characterization of KRAS wild–type tumors in patients with pancreatic adenocarcinoma. Clin Cancer Res. 2022;28(12):2704–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fusco MJ, Saeed-Vafa D, Carballido EM, et al. Identification of targetable gene fusions and structural rearrangements to foster precision medicine in KRAS wild–type pancreatic cancer. JCO Precision Oncology. 2021;5:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prall OWJ, Nastevski V, Xu H, et al. RAF1 rearrangements are common in pancreatic acinar cell carcinomas. Mod Pathol. 2020;33:1811–1821. [DOI] [PubMed] [Google Scholar]
- 46.McEvoy CR, Kee D, Prall OWJ, et al. MEK inhibitor therapy in carcinomas with RAF1 fusions: inferior response in a patient with pancreatic acinar cell carcinoma. JCO Precision Onco. 2019;3:1–2. [DOI] [PubMed] [Google Scholar]
- 47.Ayasun R, Guven DC, Gullu I. A novel agnostic tumor: NTRKoma. J Oncol Pharm Pract. 2021;27:802–803. [DOI] [PubMed] [Google Scholar]
- 48.Pishvaian MJ, Rolfo CD, Liu SV, Multani PS, Chow Maneval E, Garrido-Laguna I. Clinical Benefit of Entrectinib for Patients With Metastatic Pancreatic Cancer Who Harbor NTRK and ROS1 Fusions. American Society of Clinical Oncology; 2018. [DOI] [PubMed] [Google Scholar]
- 49.Heining C, Horak P, Uhrig S, et al. NRG1 fusions in KRAS wild-type pancreatic cancerNRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov. 2018;8:1087–1095. [DOI] [PubMed] [Google Scholar]
- 50.Schram AM, Odintsov I, Espinosa-Cotton M, et al. Zenocutuzumab, a HER2xHER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangementsTargeting cancers with NRG1 rearrangements. Cancer Discov. 2022;12(5):1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luchini C, Brosens LAA, Wood LD, et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications. Gut. 2021;70:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Reilly EM, Oh DY, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Reilly EM, Oh D-Y, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renouf D, Knox J, Kavan P, et al. LBA65 The Canadian Cancer Trials Group PA. 7 trial:rResults of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab-P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). Ann Oncol 2020;31:S1195. [Google Scholar]
- 55.Ko AH, Lee J, ALSINA M, et al. Phase Ib/II open-label, randomized evaluation of 2L atezolizumab (atezo)+ PEGPH20 versus control in MORPHEUS-pancreatic ductal adenocarcinoma (M-PDAC) and MORPHEUS-gastric cancer (M-GC). Journal of Clinical Oncology. 2020;38(15):4540. [Google Scholar]
- 56.Parikh AR, Szabolcs A, Allen JN, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2:1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang K, Wu Y-H, Song Y, Yu B. Indoleamine 2, 3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. 2021;14:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox E, Oliver T, Rowe M, et al. Indoximod: an immunometabolic adjuvant that empowers T cell activity in cancer. Front Oncol. 2018;8:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan Z, Sun J, Xu J, et al. Dual functional immunostimulatory polymeric prodrug carrier with pendent indoximod for enhanced cancer immunochemotherapy. Acta Biomater. 2019;90:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bahary N, Wang-Gillam A, Haraldsdottir S, et al. Phase 2 Trial of the IDO Pathway Inhibitor Indoximod Plus Gemcitabine/Nab-Paclitaxel for the Treatment of Patients With Metastatic Pancreas Cancer. American Society of Clinical Oncology; 2018. [Google Scholar]
- 61.Hu ZI, Bendell JC, Bullock A, et al. A randomized phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019;8:5148–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dotan E, Cardin DB, Lenz H-J, et al. Phase Ib study of WNT inhibitor ipafricept (IPA) with nab-paclitaxel (Nab-P) and gemcitabine (G) in patients (pts) with previously untreated stage IV pancreatic cancer (mPC). Journal of Clinical Oncology. 2019;37(4):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang H, Brekken RA. The next wave of stroma-targeting therapy in pancreatic cancer. Cancer Res. 2019;79:328–330. [DOI] [PubMed] [Google Scholar]
- 64.Uzunparmak B, Sahin IH. Pancreatic cancer microenvironment: a current dilemma. Clin Transl Med. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madden J. Infinity reports update from phase 2 study of saridegib plus gemcitabine in patients with metastatic pancreatic cancer. Infin Pharm. 2012. [Google Scholar]
- 67.Ramanathan RK, McDonough SL, Philip PA, et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol 2019;37:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tempero MA, Van Cutsem E, Sigal D, et al. HALO 109-301: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of Pegvorhyaluronidase Alfa (PEGPH20)+ Nab-Paclitaxel/Gemcitabine (AG) in Patients (pts) With Previously Untreated Hyaluronan (HA)-High Metastatic Pancreatic Ductal Adenocarcinoma (mPDA). American Society of Clinical Oncology; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Askan G, Sahin IH, Chou JF, et al. Pancreatic cancer stem cells may define tumor stroma characteristics and recurrence patterns in pancreatic ductal adenocarcinoma. BMC Cancer. 2021;21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahin I, Askan G, Hu Z, O’Reilly E. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Ann Oncol. 2017;28(12):2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sahin IH, Akce M, Alese O, et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. 2019;121(10):809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Datta J, Bianchi A, De Castro, Silva I, et al. Distinct mechanisms of innate and adaptive immune regulation underlie poor oncologic outcomes associated with KRAS-TP53 co-alteration in pancreatic cancer. Oncogene. 2022;41(28):3640–3654. [DOI] [PubMed] [Google Scholar]
- 73.Gu M, Gao Y, Chang P. KRAS mutation dictates the cancer immune environment in pancreatic ductal adenocarcinoma and other adenocarcinomas. Cancers. 2021;13:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmed S, Bradshaw A-D, Gera S, Dewan MZ, Xu R. The TGF-β/Smad4 signaling pathway in pancreatic carcinogenesis and its clinical significance. J Clin Med. 2017;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newsted D, Banerjee S, Watt K, et al. Blockade of TGF-β signaling with novel synthetic antibodies limits immune exclusion and improves chemotherapy response in metastatic ovarian cancer models. Oncoimmunology. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park W, O’Reilly EM, Furuse J, Kunieda F, Jie F, Kindler HL. Phase II, Open-Label, Randomized Study of First-Line Zolbetuximab Plus Gemcitabine and Nab-Paclitaxel (GN) in Claudin 18.2–Positive Metastatic Pancreatic Cancer (mPC). American Society of Clinical Oncology; 2020. [Google Scholar]


