Abstract
Aggression and impulsivity are linked to suicidal behaviors, but their relationship to the suicidal crisis remains unclear. This magnetoencephalography (MEG) study investigated the link between aggression, impulsivity, and resting-state MEG power and connectivity. Four risk groups were enrolled: high-risk (HR; n = 14), who had a recent suicidal crisis; lower-risk (LR; n = 41), who had a history of suicide attempts but no suicide attempt or ideation in the past year; clinical control (CC; n = 38), who had anxiety/mood disorders but no suicidal history; and minimal risk (MR; n = 28), who had no psychiatric/suicidal history. No difference in resting-state MEG power was observed between the groups. Individuals in the HR group with high self-reported aggression and impulsivity scores had reduced MEG power in regions responsible for sensory/emotion regulation vs. those in the HR group with low scores. The HR group also showed downregulated bidirectional glutamatergic feedback between the precuneus (PRE) and insula (INS) compared to the LR, CC, and MR groups. High self-reported impulsivity was linked to reduced PRE to INS feedback, whereas high risk-taking impulsivity was linked to upregulated INS to postcentral gyrus (PCG) and PCG to INS feedback. These preliminary findings suggest that glutamatergic-mediated sensory and emotion-regulation processes may function as potential suicide risk markers.
Keywords: suicide, magnetoencephalography (MEG), aggression, impulsivity, sensory dysregulation
Introduction
Suicide is a serious and complex health threat. In the USA, it is the 12th leading cause of overall mortality and the second and third leading cause of death in adolescents and young adults, respectively (Centers for Disease Control and Prevention 2020). Despite its high prevalence and significant impact on public health, identifying objective risk factors for suicidal behaviors remains challenging, largely because current risk assessment relies on self-reported records. Understanding the timing of suicidal behaviors is also crucial for assessing prognosis (Zuromski et al. 2019), perhaps because lifetime suicidal measures may not capture ongoing cognitive and neurobiological indicators of suicidal behavior (Lamontagne et al. 2023). In this context, developing objective neurobiological markers of suicide risk could improve both assessment and prevention efforts.
Aggression and impulsivity have consistently been linked to suicidal behaviors (Mann 2003, Gvion et al. 2014). In particular, high impulsivity has been associated with both increased aggression (Zouk et al. 2006) and a history of suicide attempts (Liu et al. 2017, Huang et al. 2023). Aggression and impulsivity can lead to dysregulated emotions and stress responses in individuals with suicidal behaviors (Stanley et al. 2019, Drachman et al. 2022) as well as decreased interoceptive sensitivity (DeVille et al. 2020, Smith et al. 2021) and higher levels of endocrinological biomarkers such as cortisol (O’Connor et al. 2020) and testosterone (Sher 2017). Nevertheless, few studies have explored impulsivity and aggression as potential neurobiological markers for suicidal behaviors. One study observed reduced brain activity in the ventral medial prefrontal cortex and paralimbic areas during a reinforcement learning task in older individuals with a history of suicide attempts and higher impulsivity (Dombrovski et al. 2013). Another study found that individuals with high self-reported impulsivity displayed decreased connectivity between sensory processing brain regions, including the right lateral occipital cortex and the sensorimotor network (Herman et al. 2020). Regarding the timing of suicidal behaviors, the cognitive performance of adolescents with a history of suicide attempts varied with regard to the recency of suicidal behaviors rather than the types of suicidal behaviors (Bridge et al. 2012, Pan et al. 2013), particularly in relation to impulsivity (Millner et al. 2020). Because aggression and impulsivity appear to play a critical role in suicidal behaviors and in the potential relationships surrounding the temporal dynamics of the suicidal event, investigating their neurobiological basis is essential to improving our understanding of suicide and potential interventions.
Previous studies identified several brain regions associated with suicidal behaviors (Benedetti et al. 2011). For instance, individuals who died by suicide were found to exhibit hypoperfusion in the subgenual anterior cingulate cortex, right precuneus, postcentral gyrus, and insula (Willeumier et al. 2011). Furthermore, individuals with a history of suicide attempts had reduced brain activity in the dorsolateral prefrontal (Alacreu-Crespo et al. 2020) and ventromedial prefrontal cortices (Brown et al. 2020) as well as altered brain activity in the insula and precuneus (Cao et al. 2015, Sankar et al. 2022). Suicidal ideation was also found to be associated with resting-state gamma source-level power in the anterior insula during a suicide implicit association task (Ballard et al. 2019, Gilbert et al. 2020). Interestingly, exploratory dynamic causal modeling (DCM) analysis suggested reduced connectivity from the early visual cortex to the insula, indicating potential sensory and emotion regulation failure contributing to an insula-oriented mechanism of suicidal behaviors (Ballard et al. 2020a).
Suicidal behaviors have also been linked to reduced brain function in regions associated with sensory input, emotional regulation, and decision-making (Lalovic et al. 2022). Individuals with a history of suicide attempts showed hypersensitivity to negative emotional cues in the dorsolateral prefrontal, somatosensory, and temporal cortices as well as the anterior cingulate gyrus and insula (Pan et al. 2013). Because disrupted connectivity between prefrontal brain regions and the insula may lead to impaired emotional responses (Gilbert et al. 2022), impaired regulation may contribute to maladaptive decision-making, reward-processing, and interoception (Alexander et al. 2019, Schmaal et al. 2020). The dysregulated sensory/emotion-regulating system, closely tied to insular function (DeVille et al. 2020), may trigger self-harming behaviors (Cummins et al. 2021). Thus, it is possible that individuals struggling with suicidal crisis—and particularly those exhibiting higher levels of impulsivity and aggression—may exhibit downregulation in brain regions related to sensory/emotion regulation. Collectively, the studies suggest that suicide may exhibit a negative association with brain function with regard to sensory and emotional regulation and that this relationship might be negatively moderated by aggression and impulsivity.
This study used resting-state magnetoencephalography (MEG) to investigate the electrophysiological correlates of the recent suicidal crisis in four risk groups: individuals experiencing a recent suicidal crisis; individuals with a history of suicide attempts but no suicide attempt or ideation in the past year; individuals with mood or anxiety disorders but no history of suicidal behaviors; and individuals with no psychiatric disorder or history of suicidal behaviors. The hypothesis was that, compared to other groups, individuals undergoing a recent suicidal crisis would show diminished source-level MEG power, specifically in brain regions associated with sensory and emotional regulation. It was also hypothesized that the relationship between risk groups and MEG power would be influenced by this reduction, interacting with both self-reported and task-oriented measures of aggression and impulsivity collected outside of the MEG scanner. DCM was used to explore the effective connectivity between these brain regions, including in those with high levels of aggression and impulsivity.
Materials and methods
Participants
One hundred and twenty-one participants took part in the study (NCT02543983). Participants were recruited through the Neurobiology of Suicide protocol; the sample recruitment pipeline has previously been documented (Ballard et al. 2020b). Participants were selected according to recency of suicidal thoughts and behaviors rather than specific psychiatric diagnoses. The participant groups included (I) those who had experienced a recent suicidal crisis, defined as attempted either suicide or suicidal ideation with intent to act within the past 2 weeks [high-risk (HR); n = 14: 6M/8F; mean age: 37.62 + 12.15]; (II) those with a history of suicide attempt but no suicide attempt or ideation in the past year [lower-risk (LR); n = 41: 12M/29F; mean age: 42.12 + 15.36]; (III) individuals diagnosed with anxiety or mood disorders but with no history of suicide attempt or ideation [clinical control (CC); n = 38: 20M/18F; mean age: 44.76 + 15.50]; and (IV) individuals with no personal or family history of either psychiatric disorder or suicidal behavior [minimum risk (MR); n = 28: 11M/17F; mean age: 34.53 + 11.65] (Fig. 1; Supplementary Table S1). All participants were between the ages of 18 and 70 years. Individuals with active suicide risk were managed in the research setting as per our previously published protocol (Ballard et al. 2020b).
Figure 1.
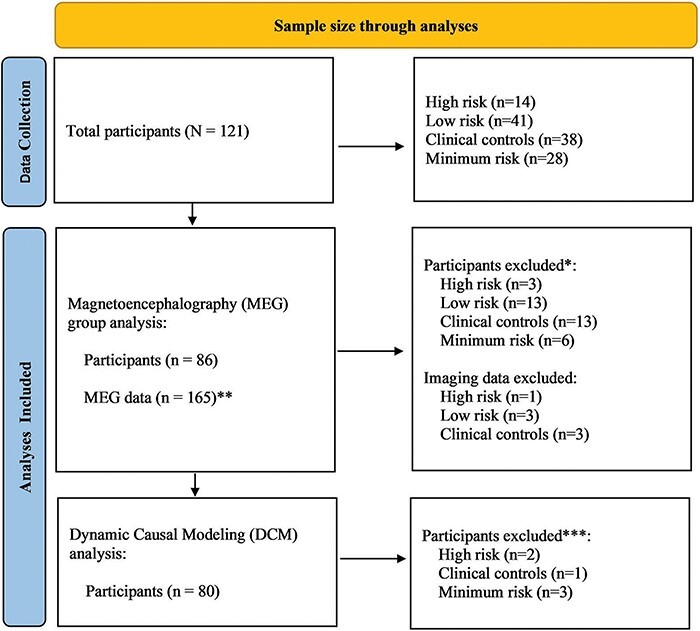
Flow chart of the sample size in the MEG analysis.
*Participants were excluded from the analysis if resting-state MEG data were not available.**Most participants had two resting-state imaging sessions. Seven of the participants had only one resting-state MEG scan.***Participants were included if a fit index for any individual DCMs was >0.8.
Participants were excluded from the study if they had current drug or alcohol dependence, severe or unstable medical conditions, or tested positive for human immunodeficiency virus. Non-English speakers, pregnant individuals, and individuals with schizophrenia were also excluded. The Combined Neuroscience Institutional Review Board approved the protocol at the National Institutes of Health in Bethesda, MD, and all participants provided written, informed consent.
Clinical ratings
General and suicidal clinical ratings
Mood and anxiety rating scales administered to assess the severity of psychiatric symptoms included the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979), the Hamilton Rating Scale for Depression 24 (HAMD-24) (Hamilton 1960), and the Hamilton Anxiety Rating Scale (HAMA) (Hamilton 1959). Clinicians interviewed participants and measured suicidal behaviors, including attempt history and ideation, with the Columbia Suicide Severity Rating Scale (Posner et al. 2011) and the Scale for Suicide Ideation (Beck et al. 1979).
Self-reported impulsivity
Self-reported assessments of impulsivity were conducted within 2 days of scanning using the Barratt Impulsiveness Scale (BIS), a well-validated 30-item measurement of different types of self-reported impulsivity (Patton et al. 1995), including attention, motor control, and non-planning.
Task-based impulsivity
Two reward-oriented tasks—the Monetary-Choice Questionnaire (MCQ) and the Balloon Analogue Risk Task (BART)—were also used to measure impulsivity and risk-taking. Additional details about these instruments can be found in the Supplementary material.
Aggression
The Buss–Perry Aggression (BPA) questionnaire (Buss and Perry 1992) measures different types of aggressive characteristics, such as physical aggression, verbal aggression, anger, and hostility.
MEG preprocessing
One or two 8-min eye-closed resting-state MEG scans were collected during the scanning session. Neuromagnetic data were collected using a CTF 275-channel whole-head system using first-order axial gradiometer MEG sensors and superconducting quantum interference devices (VSM MedTech Ltd, Coquitlam, bc, Canada). MEG data preprocessing was performed using the computational resources of the NIH Biowulf high-performance computing cluster (http://hpc.nih.gov). MEG data were cleaned for potential artifacts and localized to the source space. In the source space, beamformer weights were estimated, and the output power was projected at each voxel within five bandwidths: delta (2–4 Hz), theta (4–8 Hz), alpha (9–14 Hz), beta (15–29 Hz), and gamma (30–58 Hz). Source-level images were warped to Talairach space using Analysis of Functional NeuroImages (AFNI) (Cox 1996) software for group-level comparisons. Additional details are available in the Supplementary material.
Dynamic causal modeling
DCM, a generative model that seeks to find hidden neural states from measured brain responses using a Bayesian perspective (Stephan et al. 2010), was used to assess the extrinsic connectivity between sensory/emotion-regulating brain regions. Briefly, the “CMM_NMDA” model (a conductance-based neural mass model) was used to assess the effective connectivity between the regions of interest (ROIs) in the lateral postcentral gyrus, precuneus, inferior frontal gyrus, angular gyrus, and insula. Additional details are available in the Supplementary material.
Cross-spectral densities were modeled using local field potentials from each ROI, including the lateral postcentral gyrus (Montreal Neurological Institute (MNI) coordinates: x = 68, y = −12, z = 16), precuneus (MNI coordinates: x = 7, y = −62, z = 47), inferior frontal gyrus (MNI coordinates: x = 57, y = 17, z = 17), angular gyrus (MNI coordinates: x = 42, y = −62, z = 32), and insula (MNI coordinates: x = 37, y = 1, z = −1). The ROI time-series were segmented into two-second epochs, and the DCMs were fitted using a wide frequency range from 1 to 58 Hz. Individual DCMs were estimated, and their fit to the model was evaluated in the 86 participants for whom resting-state MEG data were available (Fig. 1). The selection of individual DCMs for subsequent analysis was based on the correlation coefficient between the spectral densities of the raw and modeled data. Individual DCMs with a correlation coefficient of >0.8 and a better correlation coefficient between the two recordings were chosen. Six additional participants were excluded from the analysis due to inadequate fit qualification, leaving 80 participants included in the DCM analysis (Fig. 1). At the group level, model parameters were assessed using parametric empirical Bayes (PEB) estimation (Zeidman et al. 2019).
Statistical analysis
Statistical analyses were conducted to compare the demographic and clinical characteristics of the HR, LR, CC, and MR groups. Analysis of variance (ANOVA) and chi-squared tests were used to evaluate demographic differences between the groups, with age, sex, and ethnicity entered as predictor variables. Differences in anxiety and depressive symptoms were analyzed using one-way ANOVA, with depressive (MADRS and HAMD-24) and anxiety symptom (HAMA) scores entered as predictor variables and age and sex as covariates. Differences in aggression and impulsivity measures between the groups were also studied, and impulsivity (BIS, MCQ, and BART) and aggression (BPA) scores were entered as predictor variables with age and sex as covariates. Because so few differences were observed between the CC and MR groups, these two were combined into a control (CL) group for subsequent analyses. The internal consistency of self-reported impulsivity scores was reasonable (α = 0.69 for BIS and α = 0.62 for BPA). However, there was notable heterogeneity among the task-driven impulsivity scores, such as the MCQ and BART (α = −0.01). This lack of internal consistency might indicate that self-reported and task-oriented impulsivity reflect different aspects of impulsivity. Task-oriented impulsivity, especially as measured by the BART, may reflect another construct, such as risk-taking (White et al. 2008). Kendall’s tau was used to explore the correlation between the aggression and impulsivity scales (BIS, MCQ, BART, and BPA) because the sample size was small and because a potential nonlinear association was assumed.
A linear mixed model (3dLMEr) in AFNI was used to compare resting-state MEG signals between the groups (HR vs. non-HR; HR vs. LR; HR vs. CL), with aggression and impulsivity measures as predictors and age and session as covariates. The significant threshold was set to α = 0.05, with cluster-based multiple comparison corrections using 3dClustSim used to identify significant findings. Interactions between the groups and the aggression and impulsivity measures were the primary measures of interest. One trend-level finding was reported and used in the DCM analysis because it may shed light on sensory regulation.
The fully connected DCM model with sensory/emotion-regulating regions was developed based on the group difference analysis results (Fig. 2a). Reduced models with three variations were then estimated (Fig. 2c and d). Model 1 included fully reciprocated feedforward and feedback connections between the insula and postcentral gyrus and between the insula and precuneus. In Models 2 and 3, the postcentral gyrus carried feedforward signals to the insula, with recurrent feedback connections from the insula to the postcentral gyrus. In Model 2, the insula had feedforward signals to the precuneus, whereas the precuneus carried feedforward signals to the insula in Model 3. The estimated negative free energy bound of the log-model evidence score of each model, suggesting the relative explained variance, was compared using the fixed-effect analysis of the Bayesian model selection. The model with the highest log-model evidence score was then selected for subsequent analyses.
Figure 2.
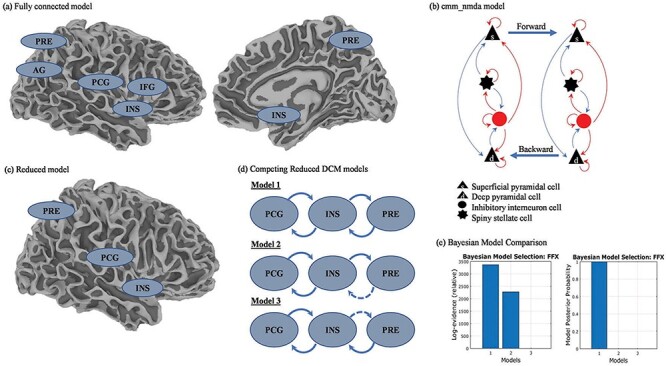
DCM of the effective connectivity in the HR group compared to the other groups*.
PRE: precuneus; PCG: postcentral gyrus; INS: insula; IFG: inferior frontal gyrus; AG: angular gyrus; FFX: fixed-effect analysis.*The other groups included the LR, CC, and MR groups.
Finally, PEB analysis was applied to explore group differences in glutamatergic connectivity among the specified brain regions. The study focused on feedforward and feedback α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)- and N-methyl-d-aspartate (NMDA)-mediated connectivity. The models tested for group differences between the HR and non-HR, HR and LR, and HR and CL groups. Parameters with a posterior probability (posteriorp) of >.95 were considered significant. Age was included as a covariate because of its association with brain volume in individuals with a history of suicide attempts (Gifuni et al. 2021). To assess whether impulsivity and aggression affected glutamatergic connectivity in the specified brain regions beyond the main effect of group differences, the main effect of aggression and impulsivity measures on connectivity was assessed while controlling for group differences and age.
Results
Participants
Demographic information can be found in Table 1. No significant differences in sex or ethnicity were noted, but participants in the CC group were older than those in the MR group (Supplementary Tables S1 and S2). The HR group had higher depression (MADRS and HAMD-24) and anxiety (HAMA) scores than the LR and MR groups (Supplementary Tables S1 and S2).
Table 1.
Demographic and clinical information.
| Comparison | Category | Variables | Statistics | |||
|---|---|---|---|---|---|---|
| χ 2 score | F-score | P-value | ||||
| Group difference between the HR, LR, CC, and MR groups | Demographic | Age | 3.12 | .029 | ||
| Ethnicity | White (vs. non-White) | 2.59 | .459 | |||
| Sex | Female (vs. male) | 4.51 | .211 | |||
| Primary mood diagnosis | Bipolar disorder (vs. MDD)a | 10.23 | .006 | |||
| Depressionand anxiety | MADRS | 58.92 | <.001 | |||
| HAMD-24 | 61.60 | <.001 | ||||
| HAMA | 42.27 | <.001 | ||||
| Impulsivity | MCQ | 0.48 | .696 | |||
| BART | 0.66 | .577 | ||||
| BIS | Total | 8.39 | <.001 | |||
| BIS | Attentional | 10.67 | <.001 | |||
| BIS | Motor | 4.28 | .007 | |||
| BIS | Non-planning | 8.87 | <.001 | |||
| Aggression | BPA | 4.89 | .003 | |||
| HR group vs. non-HR groupb | Impulsivity | MCQ | 0.50 | .483 | ||
| BART | 0.47 | .494 | ||||
| BIS | Total | 11.88 | <.001 | |||
| BIS | Attentional | 10.07 | <.001 | |||
| BIS | Motor | 4.53 | .036 | |||
| BIS | Non-planning | 6.85 | .010 | |||
| Aggression | BPA | 5.45 | .021 | |||
The primary bipolar diagnosis included Bipolar Disorder I, Bipolar Disorder II, and Bipolar Disorder not otherwise specified.
The non-HR group included the LR, CC, and MR groups.
Measures of impulsivity and aggression
The total and subscale scores for the BIS and BPA questionnaires differed between the groups (Table 1; Supplementary Table S1). The HR group showed higher levels of self-reported aggression and impulsivity than the MR group (P < .05) (Supplementary Table S2). The HR group also had significantly higher levels of aggression and impulsivity than the other three groups (Table 1; Supplementary Table S1). However, no differences were observed between the groups for the reward-oriented impulsivity tasks (MCQ and BART) (P > .10).
The relationship between aggression and impulsivity can be found in Table 2. Self-reported impulsivity measured by the BIS and its subscales (attentional, motor, and non-planning) correlated with aggression, as assessed via the BPA (P < .05). No such association was observed between the BIS or BPA with the reward-oriented MCQ and BART impulsivity tasks. Additional details about the aggression and impulsivity scales and their correlation with potential covariates such as alcohol consumption, substance use, medication history, and suicidal measures such as attempt history, frequency, and lethality can be found in Supplementary Table S3.
Table 2.
Kendall’s Tau score between the impulsivity and aggression scores.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| BIS total | ||||||
| BIS attentional | 0.63b | |||||
| BIS motor | 0.47b | 0.20a | ||||
| BIS non-planning | 0.69b | 0.45b | 0.25b | |||
| BPA | 0.30b | 0.30b | 0.18a | 0.24b | ||
| BART | −0.04 | −0.05 | −0.06 | 0.005 | 0.01 | |
| MCQ | 0.0002 | −0.09 | 0.01 | 0.06 | 0.02 | −0.12 |
P <.01; and
P <.001.
Electrophysiology—MEG results
Source-localized power in the delta, theta, alpha, beta, and gamma bands was analyzed using linear mixed models. The model examined the interaction between the groups (HR, LR, CC, and MR) and measures of aggression and impulsivity. No significant group-level differences were observed with self-reported aggression and impulsivity scores on source-localized power, including on the BIS and BPA.
Individuals in the HR group with high BPA scores had significantly reduced right hemispheric MEG power in the frontal and parietal regions compared to those in the HR group with low BPA scores (Fig. 3; Table 3). These regions included the angular gyrus and middle frontal gyrus in the alpha band and the lateral precuneus and inferior frontal gyrus in the beta band.
Figure 3.
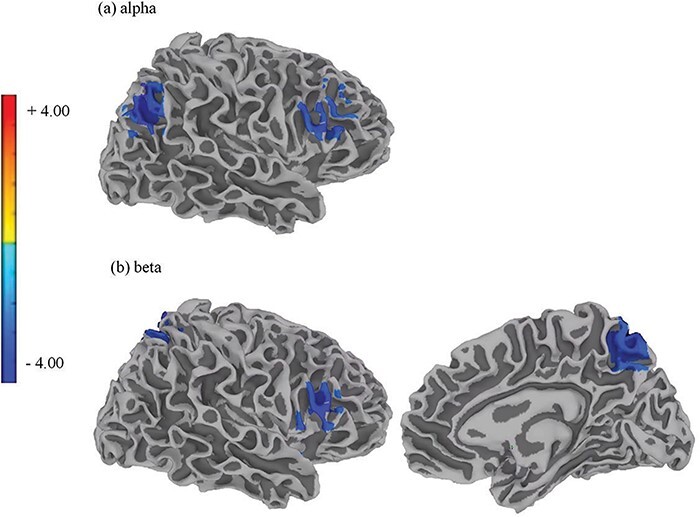
The band-specific, band-limited power of the MEG signals in individuals in the HR group who had experienced a recent suicidal crisis and had varying levels of self-reported aggression.
Reduced resting-state MEG power was observed in individuals who had recently experienced a suicidal crisis (the HR group) and possessed high self-reported aggression scores, as measured by the BPA scale, compared to individuals in the HR group with low self-reported aggression scores. Brain regions in the right hemisphere showing reduced resting-state MEG power included (a) the angular gyrus (voxel-based corrected P = .02) and middle frontal gyrus (voxel-based corrected P = .04) for the alpha band and (b) the inferior frontal gyrus (voxel-based corrected P = .04) and medial precuneus (voxel-based corrected P = .02) for the beta band.
Table 3.
Interactions between resting-state MEG power within the HR group and various self-reported aggression and impulsivity scores.
| Regressor | Bandwidth | Laterality | Regions of interest | Voxel size | MNI coordinates | Grp × Reg F-score | Grp × Reg P-value | Averaged beta | Corrected P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| BPA | Alpha | Right | Angular gyrus | 56 | 42 | −67 | 32 | 24.05 | .03 | −6.14 | .02 |
| Alpha | Right | Middle frontal gyrus | 40 | 52 | 22 | 22 | −5.13 | .04 | |||
| Alpha | Right | Inferior parietal lobule | 30 | 62 | −27 | 27 | −4.89 | .05 | |||
| Beta | Right | Precuneus | 69 | 7 | −62 | 47 | 19.70 | .01 | −5.04 | .02 | |
| Beta | Right | Inferior frontal gyrus | 38 | 57 | 17 | 17 | −5.75 | .04 | |||
| Beta | Right | Precuneus | 21 | 42 | −67 | 37 | −4.79 | .09 | |||
| Theta | Right | Angular gyrus | 20 | 47 | −62 | 32 | 16.21 | .06 | −5.16 | .10 | |
| BIS total | Alpha | Right | Inferior frontal gyrus | 19 | 42 | −67 | 32 | 21.04 | .01 | −10.51 | .09 |
| Beta | Right | Inferior frontal gyrus | 19 | 57 | 17 | 17 | 20.75 | <.01 | −9.51 | .10 | |
| BIS attentional | Alpha | Right | Angular gyrus | 79 | 42 | −62 | 32 | 28.63 | <.01 | −9.94 | .01 |
| Alpha | Right | Middle frontal gyrus | 72 | 52 | 22 | 22 | −8.54 | .01 | |||
| Alpha | Right | Inferior parietal lobule | 53 | 62 | −27 | 27 | −8.19 | .02 | |||
| Beta | Right | Precuneus | 185 | 22 | −62 | 42 | 23.32 | <.01 | −8.14 | .01 | |
| Beta | Right | Inferior frontal gyrus | 105 | 57 | 7 | 22 | −8.52 | .01 | |||
| Delta | Right | Precuneus | 34 | 52 | −57 | 32 | 18.95 | .01 | −7.84 | .04 | |
| Gamma | Right | Postcentral gyrus | 21 | 67 | −12 | 17 | 23.09 | .01 | −7.71 | .09 | |
| Theta | Right | Supramarginal gyrus | 62 | 52 | −52 | 27 | 20.78 | .01 | −8.06 | .02 | |
Interactions in resting-state MEG power occurred in individuals in the HR group with higher self-reported aggression and impulsivity scores compared to those in the HR group with low scores. Those interactions were estimated while contrasting the HR group to participants in the LR, CC, and MR groups. The BPA measured self-reported aggression and the BIS measured self-reported impulsivity. BIS attentional was a subscale of the BIS scores. Grp × Reg indicates the score differences between the groups (HR vs. others) in predicting MEG power.
Individuals in the HR group with high BIS attentional subscale scores had reduced MEG power in the right frontal and parietal regions compared to those with low BIS attentional subscale score (Fig. 4; Table 3). These regions included the angular gyrus, middle frontal gyrus, and inferior parietal lobule in the alpha band; the precuneus and inferior frontal gyrus in the beta band; the precuneus in the delta band; and the supramarginal gyrus in the theta band. Reduced gamma MEG power was also observed in the gamma band at trend-level significance (P = .09). In contrast, BPA and BIS scores did not affect MEG power differences for individuals in the LR, CC, or MR groups.
Figure 4.
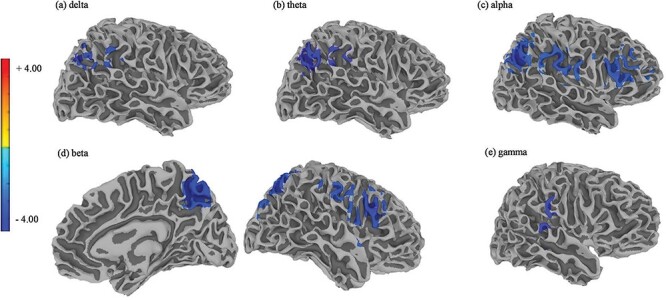
The band-specific, band-limited power of the MEG signals in individuals in the HR group who had experienced a recent suicidal crisis and had varying levels of attentional self-reported impulsivity (BIS attentional).
Reduced resting-state MEG power was observed in individuals who had recently experienced a suicidal crisis (the HR group) and had high self-reported attentional impulsivity scores, as estimated by the attentional subscales of the Barratt Impulsivity Scale (BIS), compared to individuals in the HR group with low self-reported impulsivity scores. Brain regions in the right hemisphere showing reduced resting-state MEG power included (a) the precuneus (voxel-based corrected P = .04) for the delta band, (b) the supramarginal gyrus (voxel-based corrected P = .02) for the theta band, (c) the angular gyrus (voxel-based corrected P = .01), middle frontal gyrus (voxel-based corrected P = .01), and inferior parietal lobule (voxel-based corrected P = .02) for the alpha band, (d) the precuneus (voxel-based corrected P = .01) and inferior frontal gyrus (voxel-based corrected P = .01) for the beta band, and (e) the postcentral gyrus (voxel-based corrected P = .09) for the gamma band.
Electrophysiology—DCM results
DCM was used to examine effective connectivity in the five specified regions (lateral postcentral gyrus, precuneus, inferior frontal gyrus, angular gyrus, and insula (i.e. the fully connected model, Fig. 2a). Compared to the non-HR, LR, and CL groups, the HR group showed downregulation of bidirectional AMPA feedback between the lateral postcentral gyrus and insula and the lateral postcentral gyrus and precuneus (posteriorp = 1; Supplementary Table S4). Based on the results, the right insula, precuneus, and lateral postcentral gyrus were included in the follow-up analysis.
The reduced model with those three brain regions (insula, precuneus, and postcentral gyrus) had three possible model architectures (Fig. 2c) that were compared to find the best-fitting model. Model 1 provided the best fit; this model included fully reciprocated feedforward and feedback connections between the insula and lateral postcentral gyrus and between the insula and precuneus (posteriorp = 1; Fig. 2d and e). Using PEB to test for group effects, the HR group showed downregulation of bidirectional AMPA feedback between the insula and precuneus (posteriorp > .95; Table 4). Individuals in the HR group also showed downregulation of AMPA feedback from the insula to the precuneus (Est = −1.05, posteriorp = .993) and from the precuneus to the insula (Est = −1.20, posteriorp = 1).
Table 4.
Results of the DCM analysis using Model 1.
| Extrinsic connectivity | vs. LR, CC, and MR groups | vs. LR group | vs. CC and MR groups | |||||
|---|---|---|---|---|---|---|---|---|
| Glutamatergic connectivity | From | To | Parameter estimation | Posterior probability | Parameter estimation | Posterior probability | Parameter estimation | Posterior probability |
| AMPA feedback | INS | PCG | 0.21 | .481 | 0.44 | .686 | ||
| AMPA feedback | INS | PRE | −1.1 | .993 | −0.97 | 1 | −1.14 | 1 |
| AMPA feedback | PCG | INS | 0.81 | .915 | −0.70 | .922 | −1.04 | .993 |
| AMPA feedback | PRE | INS | −1.20 | 1 | −1.09 | 1 | −1.25 | 1 |
Abbreviations: INS: insula; PRE: precuneus; PCG: postcentral gyrus.
With regard to the role of impulsivity and aggression in the context of the suicidal crisis, high total BIS scores were associated with downregulation of AMPA feedback from the precuneus to the insula in the overall sample, regardless of group (Est = −0.36, posteriorp = .994; Supplementary Table S5). In the HR group, the association between high BIS total scores and downregulation of AMPA feedback from the precuneus to the insula remained significant (Est = −0.31, posteriorp = .976). In addition, participants in the HR group with high total BIS scores exhibited upregulated AMPA feedback from the lateral postcentral gyrus to the insula (Est = 0.97, posteriorp = 1). Interestingly, in the overall sample, participants with high non-planning BIS subscale scores showed downregulated AMPA feedback from the precuneus to the insula compared to those with low non-planning BIS subscale scores (Est = −0.32, posteriorp = .978) and the HR group only (Est = −0.29, posteriorp = .954).
No significant differences in connectivity strength were observed between the insula, precuneus, and lateral postcentral gyrus based on the degree of aggression and reward-oriented impulsivity, measured by the BPA (Supplementary Table S6) and MCQ, respectively. However, significant glutamatergic connectivity among those brain regions was noted with the BART, another reward-oriented and risk-taking impulsivity task (Supplementary Table S7). In the overall sample of participants, high BART scores were associated with upregulation of AMPA forward and feedback connectivity from the insula to the precuneus (Est = 0.40, posteriorp = .983) and from the lateral postcentral gyrus to the insula (Est = 0.30, posteriorp = .961), respectively. This suggests that a tendency toward high risk-taking may be associated with hyperactivity of the sensory/emotion-regulating brain regions. In the HR group, individuals with high BART scores had upregulated AMPA forward connectivity from the insula to the precuneus (Est = 0.42, posteriorp = 1), upregulated AMPA feedback from the insula to the lateral postcentral gyrus (Est = 0.35, posteriorp = 1), and upregulated AMPA feedback from the lateral postcentral gyrus to the insula (Est = 0.33, posteriorp = 1) compared to those with low BART scores.
Discussion
This study used MEG and DCM to investigate aggression and impulsivity as potential neurobiological markers of the suicidal crisis. Individuals who experienced a recent suicidal crisis had higher depression, anxiety, and self-reported impulsivity/aggression scale scores than the comparison groups. Contrary to our expectations, no significant main effects were noted with regard to source-level MEG power when comparing those who had experienced a recent suicidal crisis to those who had a past—but no recent—history of suicide attempts or the two groups with no history of suicidal behaviors. However, those experiencing a recent suicidal crisis exhibited a negative association between self-reported aggression and impulsivity levels and source-level MEG power in brain regions involved in sensory/emotion regulation. These regions includes portions of the default mode network (DMN)—the precuneus, postcentral gyrus, and inferior frontal gyrus (Ordaz et al. 2018, Chin Fatt et al. 2021)—as well as the dorsal attention network (DAN) (Mehta et al. 2023), such as the angular gyrus. In addition, our DCM analysis found AMPA-mediated glutamatergic downregulation between the insula and precuneus, specifically in the HR group, indicating a lack of top-down attention/emotion regulation that could contribute to the association between impulsivity and suicide risk.
Interestingly, individuals with high scores on the BART task had AMPA-mediated glutamatergic upregulation from the insula to the precuneus and between the insula and lateral postcentral gyrus, suggesting heightened sensitivity to sensory information processing in the brain and its connection to risk-taking. However, no such connection was observed with the MCQ, another assessment of impulsive risk-taking. This discrepancy underscores the inconsistent relationship between different impulsivity matrices and the suicidal crisis and may hint at the heterogeneity of features and behavioral manifestations associated with impulsivity. No significant relationship was observed between aggression and glutamatergic affect connectivity among sensory/emotion-regulating brain regions.
A correlation was also observed between self-reported aggression and impulsivity, suggesting a shared individual trait related to suicide risk. These preliminary findings also support the presence of a neurobiomarker related to aggression in individuals with a recent suicidal crisis, as evidenced by less source-level MEG power in brain regions responsible for sensory/emotion regulation within the DMN. These findings differ from those observed in previously identified aggression-related brain regions, such as decreased top-down processing in the prefrontal cortex and increased activity in the limbic system, including the amygdala and cingulate gyrus (Siever 2008, Alegria et al. 2016). The findings also suggest a negative correlation between self-reported impulsivity and source-level resting-state MEG power in the sensory/emotion-regulating brain regions, specifically within the DMN, echoing our observations regarding self-reported aggression.
The reduced resting-state MEG power observed here in conjunction with high self-reported impulsivity and aggression scores in individuals who experienced a recent suicidal crisis suggests that the suicidal crisis may be linked to dysregulated brain circuitry in several key areas related to the somatosensory system, including (i) inhibition and attentional control (inferior frontal gyrus, middle frontal gyrus, angular gyrus, and precuneus; Hampshire et al. 2010, Japee et al. 2015, Aryutova et al. 2021); (ii) sensory perception (postcentral gyrus; DiGuiseppi and Tadi 2023); (iii) sensory integration and its relationship to motor behavior and memory (supramarginal gyrus, angular gyrus, and precuneus; Brechet et al. 2018, Aryutova et al. 2021); (iv) higher-order functioning (superior parietal lobule and precuneus; Cavanna and Trimble 2006, Alahmadi 2021); and (v) emotion regulation (inferior frontal gyrus, postcentral gyrus, angular gyrus, and insula; Kropf et al. 2019, Moon et al. 2022, Chan et al. 2023). Dysregulation in the somatosensory system might negatively affect cognition, including attentional shifting (Fiebelkorn and Kastner 2019), sensation-seeking, and risk-taking (Gable et al. 2015). Accurate risk assessment, mediated by the insula and precuneus, could also be impaired (Alacreu-Crespo et al. 2020). These cognitive impairments could affect how sensory information is regulated, potentially contributing to poor risk assessment of the suicide attempt or its consequences. These intriguing preliminary results suggest that cognitive deficits are key to suicidal processes and that additional research is needed to understand the relationship between the temporal dynamics of suicide risk, aggression, impulsivity, and the attention/emotion-regulating system (Minzenberg et al. 2015).
No connection was observed between aggression and glutamatergic connectivity in the sensory input and sensory/emotion-regulating regions, suggesting that neurotransmitters beyond glutamate might play a role in driving aggression and its connection to suicidal behavior. Serotonin deficiency in emotion-regulating brain regions, such as the prefrontal and anterior cingulate cortices, has been associated with aggression (Olivier 2004, Seo et al. 2008). Other neurotransmitters like dopamine, epinephrine, and norepinephrine could also act as biomarkers for aggression via oxidative stress regulation (Miczek et al. 2002, Patki et al. 2015). Testosterone, a male sex hormone, has been linked to aggression and might influence suicidal behaviors (Stefansson et al. 2016). Future research is needed to clarify these relationships.
In contrast, although reward-oriented impulsivity tasks (BART and MCQ) were not confirmed as neurobiomarkers for a recent suicidal crisis based on source-level resting-state MEG power in the present study, a relationship was nevertheless observed between glutamatergic connectivity and impulsivity on the BART. High risk-taking/reward-seeking led to increased sensory processing and regulation, indicated by positive AMPA effective connectivity between the lateral postcentral gyrus and the insula, as well as from the insula to the precuneus. Increased sensory processing and regulation were more prominent in the HR group than in the other groups. These findings suggest that high risk-taking, as assessed by the BART, was positively associated with somatosensory processing, especially in those with a recent suicidal crisis. While acknowledging the poor internal consistency between the BART and MCQ reported here, it should be noted that the BART may not be an optimal measure for capturing general impulsivity, especially considering its moderate to poor test–retest reliability in estimating brain activity (Korucuoglu et al. 2020, Li et al. 2020); instead, it may better capture risk-taking propensity.
Risk-taking might lead to hyposensitivity to somatosensory cues like pain (Cummins et al. 2021), particularly in those at risk for suicide (Van Heeringen 2018, Miglani et al. 2021). However, the relationship between risk-taking and suicidal behaviors remains unclear; while some studies found a link (Dougherty et al. 2009, Li et al. 2021), others found no such association, particularly during a suicidal crisis (Cole et al. 2019, Dillahunt et al. 2022). Future studies investigating this relationship and its neurobiomarkers should consider temporal dynamics surrounding the suicidal event.
Our DCM analysis found decreased AMPA-mediated glutamatergic connectivity between the insula and precuneus in the overall sample of participants and those with a recent suicidal crisis specifically. The insula is linked to interoception, emotion, learning, and value functioning (Namkung et al. 2017, Frey and McCabe 2020, Deng et al. 2021, Olvera and Miranda 2022), while the precuneus is responsible for consciousness, higher-order functioning, episodic memory retrieval, and social decision-making (Cavanna and Trimble 2006, Dubey et al. 2020). Downregulation of connectivity between these regions could influence somatosensory regulation, which may in turn downregulate emotional and cognitive functions.
Our study also identified no significant AMPA- or NMDA-mediated connectivity differences based on recent suicidal crisis and delay discounting impulsivity, as assessed by delay discounting scores derived from the MCQ. This finding is consistent with research showing weak or no links between delay discounting impulsivity and suicide (Bridge et al. 2015, McHugh et al. 2019). The discrepancy between the two risk-taking impulsivity measures in estimating suicide risk might be due to learning factors in the tasks (Ballard and McClure 2019). Additional research is needed to define various categories of impulsivity and investigate latent learning factors in the context of suicide research.
Although these preliminary findings are intriguing, several limitations bear mention. First, the sample size was relatively small, especially for the HR group, suggesting that our findings may not be generalizable to other populations; a larger longitudinal study is needed to validate these results. Second, resting-state MEG power was used to measure brain activity and connectivity characteristics at rest. However, this method cannot capture neurobiological changes that may have occurred in response to an ongoing event that induced aggression or impulsivity. Future studies could mitigate this limitation by using task-oriented or real-time measurement of neurobiomarkers associated with suicide risk. Third, while most of our participants had a diagnosis of major depressive disorder (MDD), a subset were diagnosed with bipolar disorder. The primary diagnosis (bipolar disorder vs. MDD) could potentially have influenced the relationship between the recency of suicidal events, neural electricity, and aggression, as well as impulsivity. Fourth, participants either abstained from or were maintained on medications during the study based on clinicians’ decisions; these ongoing medications may have introduced complexity into the observed relationships. Fifth, lifestyle factors, including smoking, alcohol consumption, and substance use, could indirectly modulate these relationships. These factors warrant consideration in future research.
Despite these limitations, the findings provide valuable insights into the relationship between the recent suicidal crisis and aggression and impulsivity as neurobiological markers, as measured through electrophysiological signals, and highlight the potential usefulness of studying electrophysiological activity and connectivity in suicide research.
Conclusion
This study examined the relationship between aggression, impulsivity, suicide risk, and brain regions involved in sensory/emotional regulation. The preliminary findings suggest that aggression and impulsivity may contribute to reduced activity and effective connectivity within brain regions associated with sensory/emotional regulation brain regions in individuals who recently experienced a suicidal crisis. The findings thus underscore the importance of temporal dynamics in neurobiological suicide research. In this context, different types of impulsivity may influence the direction of glutamatergic connectivity between the precuneus and insula, as observed here in individuals who recently experienced a suicidal crisis. Understanding the nonlinear relationship between aggression, impulsivity, and suicide risk through these specific neurobiomarkers holds promise for predicting and preventing suicide attempts.
Supplementary Material
Acknowledgements
The authors thank the 7SE research unit and staff for their support. Ioline Henter (National Institute of Mental Health) provided invaluable editorial assistance.
Contributor Information
Yoojin Lee, Experimental Therapeutics and Pathophysiology Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, United States.
Jessica R Gilbert, Experimental Therapeutics and Pathophysiology Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, United States.
Laura R Waldman, Experimental Therapeutics and Pathophysiology Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, United States.
Carlos A Zarate , Jr, Experimental Therapeutics and Pathophysiology Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, United States.
Elizabeth D Ballard, Experimental Therapeutics and Pathophysiology Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892, United States.
Supplementary data
Supplementary data is available at SCAN online.
Conflict of interest:
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the US government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Funding
This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (ZIAMH002927 and NCT02543983).
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The data are not publicly available due to privacy or ethical restrictions.
References
- Alacreu-Crespo A, Olie E, Le Bars E et al. Prefrontal activation in suicide attempters during decision making with emotional feedback. Transl Psychiatry 2020;10:1–9.doi: 10.1038/s41398-020-00995-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmadi AAS. Investigating the sub-regions of the superior parietal cortex using functional magnetic resonance imaging connectivity. Insights Imaging 2021;12:47.doi: 10.1186/s13244-021-00993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegria AA, Radua J, Rubia K. Meta-analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry 2016;173:1119–30.doi: 10.1176/appi.ajp.2016.15081089 [DOI] [PubMed] [Google Scholar]
- Alexander L, Gaskin PLR, Sawiak SJ et al. Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron 2019;101:307-320e306.doi: 10.1016/j.neuron.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryutova K, Paunova R, Kandilarova S et al. Differential aberrant connectivity of precuneus and anterior insula may underpin the diagnosis of schizophrenia and mood disorders. World J Psychiatry 2021;11:1274–87.doi: 10.5498/wjp.v11.i12.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Gilbert JR, Fields JS et al. Network changes in insula and amygdala connectivity accompany implicit suicidal associations. Front Psychiatry 2020a;11:577628.doi: 10.3389/fpsyt.2020.577628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Reed JL, Szczepanik J et al. Functional imaging of the implicit association of the self with life and death. Suicide Life Threat Behav 2019;49:1600–08.doi:doi: 10.1111/sltb.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Waldman L, Yarrington JS et al. Neurobiological research with suicidal participants: a framework for investigators. Gen Hosp Psychiatry 2020b;62:43–48.doi: 10.1016/j.genhosppsych.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard IC, McClure SM. Joint modeling of reaction times and choice improves parameter identifiability in reinforcement learning models. J Neurosci Methods 2019;317:37–44.doi: 10.1016/j.jneumeth.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 1979;47:343–52.doi: 10.1037/0022-006X.47.2.343 [DOI] [PubMed] [Google Scholar]
- Benedetti F, Radaelli D, Poletti S et al. Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J Affective Disorders 2011;135:139–47.doi: 10.1016/j.jad.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Brechet L, Grivaz P, Gauthier B et al. Common recruitment of angular gyrus in episodic autobiographical memory and bodily self-consciousness. Front Neurosci 2018;12:270.doi: 10.3389/fnbeh.2018.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, McBee-Strayer SM, Cannon EA et al. Impaired decision making in adolescent suicide attempters. J Am Acad Child Adolesc Psychiatry 2012;51:394–403.doi: 10.1016/j.jaac.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, Reynolds B, McBee-Strayer SM et al. Impulsive aggression, delay discounting, and adolescent suicide attempts: effects of current psychotropic medication use and family history of suicidal behavior. J Child Adolesc Psychopharmacol 2015;25:114–23.doi: 10.1089/cap.2014.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, Wilson J, Hallquist MN et al. Ventromedial prefrontal value signals and functional connectivity during decision-making in suicidal behavior and impulsivity. Neuropsychopharmacology 2020;45:1034–41.doi: 10.1038/s41386-020-0632-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol 1992;63:452–59.doi: 10.1037/0022-3514.63.3.452 [DOI] [PubMed] [Google Scholar]
- Cao J, Chen JM, Kuang L et al. Abnormal regional homogeneity in young adult suicide attempters with no diagnosable psychiatric disorder: a resting state functional magnetic imaging study. Psychiatry Res 2015;231:95–102.doi: 10.1016/j.pscychresns.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564–83.doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . WISQARS leading causes of death reports. CDC, 2020. [Google Scholar]
- Chan CC, Alter S, Hazlett EA et al. Neural correlates of impulsivity in bipolar disorder: a systematic review and clinical implications. Neurosci Biobehav Rev 2023;147:105109.doi: 10.1016/j.neubiorev.2023.105109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Fatt CR, Jha MK, Minhajuddin A et al. Dysfunction of default mode network is associated with active suicidal ideation in youths and young adults with depression: findings from the T-RAD study. J Psychiatr Res 2021;142:258–62.doi: 10.1016/j.jpsychires.2021.07.047 [DOI] [PubMed] [Google Scholar]
- Cole AB, Littlefield AK, Gauthier JM et al. Impulsivity facets and perceived likelihood of future suicide attempt among patients who recently attempted suicide. J Affective Disorders 2019;257:195–99.doi: 10.1016/j.jad.2019.07.038 [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73.doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cummins TM, English O, Minnis H et al. Assessment of somatosensory function and self-harm in adolescents. JAMA Network Open 2021;4:e2116853.doi: 10.1001/jamanetworkopen.2021.16853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Xiao X, Yang T et al. A genetically defined insula-brainstem circuit selectively controls motivational vigor. Cell 2021;184:6344-6360.e18.doi: 10.1016/j.cell.2021.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVille DC, Kuplicki R, Stewart JL et al. Diminished responses to bodily threat and blunted interoception in suicide attempters. eLife 2020;9:e51593.doi: 10.7554/eLife.51593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGuiseppi J, Tadi P. Neuroanatomy, Postcentral Gyrus. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] [Google Scholar]
- Dillahunt AK, Feldman DA, Thomas LR et al. Self-injury in adolescence is associated with greater behavioral risk avoidance, not risk-taking. J Clin Med 2022;11:1288.doi: 10.3390/jcm11051288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Clark L et al. Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiatry 2013;70:1020–30.doi: 10.1001/jamapsychiatry.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM et al. Impulsivity and clinical symptoms among adolescents with non-suicidal self-injury with or without attempted suicide. Psychiatry Res 2009;169:22–27.doi: 10.1016/j.psychres.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman R, Colic L, Sankar A et al. Rethinking “aggression” and impulsivity in bipolar disorder: risk, clinical and brain circuitry features. J Affective Disorders 2022;303:331–39.doi: 10.1016/j.jad.2022.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey I, Georgescu AL, Hommelsen M et al. Distinct neural correlates of social and object reward seeking motivation. Eur J Neurosci 2020;52:4214–29.doi: 10.1111/ejn.14888 [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, Kastner S. A rhythmic theory of attention. Trends Cogn Sci 2019;23:87–101.doi: 10.1016/j.tics.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AL, McCabe C. Impaired social learning predicts reduced real-life motivation in individuals with depression: a computational fMRI study. J Affective Disorders 2020;263:698–706.doi: 10.1016/j.jad.2019.11.049 [DOI] [PubMed] [Google Scholar]
- Gable PA, Mechin NC, Hicks JA et al. Supervisory control system and frontal asymmetry: neurophysiological traits of emotion-based impulsivity. Soc Cognit Affective Neurosci 2015;10:1310–15.doi: 10.1093/scan/nsv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifuni AJ, Chakravarty MM, Lepage M et al. Brain cortical and subcortical morphology in adolescents with depression and a history of suicide attempt. J Psychiatry Neurosci 2021;46:E347–E357.doi: 10.1503/jpn.200198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JR, Ballard ED, Galiano CS et al. Magnetoencephalographic correlates of suicidal ideation in major depression. Biol Psych 2020;5:354–63.doi: 10.1016/j.bpsc.2019.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JR, Gerner JL, Burton CR et al. Magnetoencephalography biomarkers of suicide attempt history and antidepressant response to ketamine in treatment-resistant major depression. J Affective Disorders 2022;312:188–97.doi: 10.1016/j.jad.2022.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvion Y, Horresh N, Levi-Belz Y et al. Aggression-impulsivity, mental pain, and communication difficulties in medically serious and medically non-serious suicide attempters. Compr Psychiatry 2014;55:40–50.doi: 10.1016/j.comppsych.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Health Psychol 1959;32:50–55.doi: 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg 1960;23:56–62.doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM et al. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage 2010;50:1313–19.doi: 10.1016/j.neuroimage.2009.12.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AM, Critchley HD, Duka T. Trait impulsivity associated with altered resting-state functional connectivity within the somatomotor network. Front Behav Neurosci 2020;14:111.doi: 10.3389/fnbeh.2020.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MH, Kuan YH, Tu PC et al. Brain structural abnormalities and trait impulsivity in suicidal and non-suicidal patients with bipolar disorder. J Affective Disorders 2023;333:10–17.doi: 10.1016/j.jad.2023.04.050 [DOI] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD et al. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 2015;9:23.doi: 10.3389/fnsys.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korucuoglu O, Harms MP, Astafiev SV et al. Test-retest reliability of fMRI-measured brain activity during decision making under risk. NeuroImage 2020;214:116759.doi: 10.1016/j.neuroimage.2020.116759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf E, Syan SK, Minuzzi L et al. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz J Psychiatry 2019;41:261–69.doi: 10.1590/1516-4446-2018-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalovic A, Wang S, Keilp JG et al. A qualitative systematic review of neurocognition in suicide ideators and attempters: implications for cognitive-based psychotherapeutic interventions. Neurosci Biobehav Rev 2022;132:92–109.doi: 10.1016/j.neubiorev.2021.11.007 [DOI] [PubMed] [Google Scholar]
- Lamontagne SJ, Zabala PK, Zarate CA Jr et al. Toward objective characterizations of suicide risk: a narrative review of laboratory-based cognitive and behavioral tasks. Neurosci Biobehav Rev 2023;153:105361.doi: 10.1016/j.neubiorev.2023.105361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Griffiths MD, Mei S et al. The mediating role of impulsivity and the moderating role of gender between fear of missing out and gaming disorder among a sample of Chinese university students. Cyberpsychol Behav Soc Netw 2021;24:550–57.doi: 10.1089/cyber.2020.0283 [DOI] [PubMed] [Google Scholar]
- Li X, Pan Y, Fang Z et al. Test-retest reliability of brain responses to risk-taking during the balloon analogue risk task. NeuroImage 2020;209:116945.doi: 10.1016/j.neuroimage.2019.116495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Trout ZM, Hernandez EM et al. A behavioral and cognitive neuroscience perspective on impulsivity, suicide, and non-suicidal self-injury: meta-analysis and recommendations for future research. Neurosci Biobehav Rev 2017;83:440–50.doi: 10.1016/j.neubiorev.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci 2003;4:819–28.doi: 10.1038/nrn1220 [DOI] [PubMed] [Google Scholar]
- McHugh CM, Chun Lee RS, Hermens DF et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res 2019;116:51–60.doi: 10.1016/j.jpsychires.2019.05.012 [DOI] [PubMed] [Google Scholar]
- Mehta K, Pines A, Adebimpe A et al. Individual differences in delay discounting are associated with dorsal prefrontal cortex connectivity in children, adolescents, and adults. Dev Cogn Neurosci 2023;62:101265.doi: 10.1016/j.dcn.2023.101265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF et al. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berlin) 2002;163:434–58.doi: 10.1007/s00213-002-1139-6 [DOI] [PubMed] [Google Scholar]
- Miglani M, Chavan BS, Gupta N. Pain threshold and pain tolerance as a predictor of deliberate self-harm among adolescents and young adults. Indian J Psychiatry 2021;63:142–45.doi: 10.4103/psychiatry.IndianJPsychiatry_348_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millner AJ, Lee MD, Hoyt K et al. Are suicide attempters more impulsive than suicide ideators? Gen Hosp Psychiatry 2020;63:103–10.doi: 10.1016/j.genhosppsych.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Lesh TA, Niendam TA et al. Control-related frontal-striatal function is associated with past suicidal ideation and behavior in patients with recent-onset psychotic major mood disorders. J Affective Disorders 2015;188:202–09.doi: 10.1016/j.jad.2015.08.049 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–89.doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- Moon H, Nam G, Hur JW. Neural correlates of affective theory of mind in medication-free nonsuicidal self-injury: an fMRI study. Front Psychiatry 2022;13:850794.doi: 10.3389/fpsyt.2022.850794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung H, Kim SH, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci 2017;40:200–07.doi: 10.1016/j.tins.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DB, Gartland N, O’Connor RC. Stress, cortisol and suicide risk. Int Rev Neurobiol 2020;152:101–30.doi: 10.1016/bs.irn.2019.11.006 [DOI] [PubMed] [Google Scholar]
- Olivier B. Serotonin and aggression. Ann NY Acad Sci 2004;1036:382–92.doi: 10.1196/annals.1330.022 [DOI] [PubMed] [Google Scholar]
- Olvera MJ, Miranda MI. Differential effects of N-methyl-D-aspartate receptors activation in the insular cortex during memory formation and updating of a motivational conflict task. Neuroscience 2022;497:39–52.doi: 10.1016/j.neuroscience.2022.02.035 [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, Goyer MS, Ho TC et al. Network basis of suicidal ideation in depressed adolescents. J Affective Disorders 2018;226:92–99.doi: 10.1016/j.jad.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Pan LA, Hassel S, Segreti AM et al. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol Med 2013;43:2129–42.doi: 10.1017/S0033291712002966 [DOI] [PubMed] [Google Scholar]
- Patki G, Atrooz F, Alkadhi I et al. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci Lett 2015;584:308–13.doi: 10.1016/j.neulet.2014.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;51:768–74.doi: 10.1002/1097-4679(199511)51:6 [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B et al. The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–77.doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar A, Scheinost D, Goldman DA et al. Graph theory analysis of whole brain functional connectivity to assess disturbances associated with suicide attempts in bipolar disorder. Transl Psychiatry 2022;12:1–10.doi: 10.1038/s41398-021-01767-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, van Harmelen AL, Chatzi V et al. Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry 2020;25:408–27.doi: 10.1038/s41380-019-0587-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Patrick CJ, Kennealy PJ. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav 2008;13:383–95.doi: 10.1016/j.avb.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L. Commentary: CSF and plasma testosterone in attempted suicide. Front Public Health 2017;5:92.doi: 10.3389/fpubh.2017.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry 2008;165:429–42.doi: 10.1176/appi.ajp.2008.07111774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Duffy ME, Joiner TE. Introduction to the special issue on interoception and suicidality. Behav Ther 2021;52:1031–34.doi: 10.1016/j.beth.2021.06.003 [DOI] [PubMed] [Google Scholar]
- Stanley B, Michel CA, Galfalvy HC et al. Suicidal subtypes, stress responsivity and impulsive aggression. Psychiatry Res 2019;280:112486.doi: 10.1016/j.psychres.2019.112486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson J, Chatzittofis A, Nordström P et al. CSF and plasma testosterone in attempted suicide. Psychoneuroendocrinology 2016;74:1–6.doi: 10.1016/j.psyneuen.2016.08.009 [DOI] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ et al. Ten simple rules for dynamic causal modeling. NeuroImage 2010;49:3099–109.doi: 10.1016/j.neuroimage.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heeringen K. What is suicidal behavior, and can it be prevented? In: The Neuroscience of Suicidal Behavior (Cambridge Fundamentals of Neuroscience in Psychology). Cambridge: Cambridge University Press, 2018, 1–22. [Google Scholar]
- White TL, Lejuez CW, de Wit H. Test-retest characteristics of the Balloon Analogue Risk Task (BART). Exp Clin Psychopharmacol 2008;16:565–70.doi: 10.1037/a0014083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeumier K, Taylor DV, Amen DG. Decreased cerebral blood flow in the limbic and prefrontal cortex using SPECT imaging in a cohort of completed suicides. Transl Psychiatry 2011;1:e28.doi: 10.1038/tp.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Jafarian A, Seghier ML et al. A guide to group effective connectivity analysis, part 2: second level analysis with PEB. NeuroImage 2019;200:12–25.doi: 10.1016/j.neuroimage.2019.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouk H, Tousignant M, Seguin M et al. Characterization of impulsivity in suicide completers: clinical, behavioral and psychosocial dimensions. J Affective Disorders 2006;92:195–204.doi: 10.1016/j.jad.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Zuromski KL, Bernecker SL, Gutierrez PM et al. Assessment of a risk index for suicide attempts among US Army soldiers with suicide ideation: analysis of data from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Network Open 2019;2:e190766.doi: 10.1001/jamanetworkopen.2019.0766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request. The data are not publicly available due to privacy or ethical restrictions.


