Abstract
Lassa fever has been estimated to cause 5,000 deaths annually in West Africa. Recently, war in the zone where Lassa fever is hyperendemic has severely impeded control and treatment. Vaccination is the most viable control measure. There is no correlation between antibody levels and outcome in human patients, and inactivated vaccines produce high titers of antibodies to all viral proteins but do not prevent virus replication and death in nonhuman primates. Accordingly, we vaccinated 44 macaques with vaccinia virus-expressed Lassa virus structural proteins separately and in combination, with the object of inducing a predominantly TH1-type immune response. Following Lassa virus challenge, all unvaccinated animals died (0% survival). Nine of 10 animals vaccinated with all proteins survived (90% survival). Although no animals that received full-length glycoprotein alone had a high titer of antibody, 17 of 19 survived challenge (88%). In contrast, all animals vaccinated with nucleoprotein developed high titers of antibody but 12 of 15 died (20% survival). All animals vaccinated with single glycoproteins, G1 or G2, died, but all those that received both single glycoproteins (G1 plus G2) at separate sites survived, showing that both glycoproteins are independently important in protection. Neither group had demonstrable antibody levels prior to challenge. We demonstrate that in primates, immune responses to epitopes on both glycoproteins are required to protect against lethal challenge with Lassa virus without having untoward side effects and that this protection is likely to be primarily cell mediated. We show that an effective, safe vaccine against Lassa virus can and should be made and that its evaluation for human populations is a matter of humanitarian priority.
Lassa virus is endemic in rural West Africa. The prevalence of antibody to Lassa virus ranges from 5% in Guinea and 15 to 20% in Sierra Leone and Liberia to over 20% in Nigeria (7, 30). Lassa fever has been estimated to cause from 100,000 to 300,000 infections a year and several thousand deaths (30). The fatality rate for hospitalized patients is about 17%, but in certain groups of patients, such as pregnant women in their third trimester, more than 30% may die, and fetal or neonatal loss is about 88% (34). Deafness is a common complication of Lassa fever, affecting as many as 15% of patients and rendering an estimated 1 to 2% of the population hearing impaired in areas with high rates of infection (11). Treatment with intravenous ribavirin has been shown to be effective; however, it is not widely available in the areas where the disease is endemic and must be administered in the first week of illness for optimal efficacy (28). Recently, social and economic conditions have deteriorated in areas of high endemicity of eastern Sierra Leone and Liberia, and incidence and mortality have increased (R. Allan, R. Ladbury, K. Skinner, and S. Mardel, Abstr. Int. Conf. Emerg. Infect. Dis., abstr. 16, p. 21, 1998).
Lassa virus, an arenavirus, exhibits persistent, asymptomatic infection, with profuse urinary virus excretion in Mastomys natalensis, the ubiquitous and highly commensal rodent host (23, 31). Human infection is due to contact with rodents or infected patients. Widespread prevention of such contact is presently impractical, so provision of a vaccine for community and hospital use is an imperative public health need (19, 23, 30). We previously reported that a vaccinia virus expressing the Lassa virus glycoprotein protected four nonhuman primates against lethal challenge with Lassa virus (16). We now present data using standardized vaccination and challenge protocols which compare levels of protection afforded by vaccines expressing the full range of Lassa virus proteins for two different species of nonhuman primates.
MATERIALS AND METHODS
Nonhuman primates.
We studied 44 nonhuman primates: 28 Macaca mulatta (rhesus) and 16 Macaca fascicularis (cynomolgus) monkeys under protocols approved by the Centers for Disease Control and Prevention Animal Care and Use Committee. All procedures requiring animal handling were performed with the monkeys being under light ketamine anesthesia. Immediately before Lassa virus challenge, animals were moved from biosafety level 2 to biosafety level 4 facilities, where they were housed in Bioclean laminar-flow animal containment hoods (BiochemGARD, Sanford, Maine), and daily inspections were made to record changes in appetite, water consumption, behavior, and general condition. Some animals were sacrificed in extremis for humanitarian reasons (minimal responses to stimuli, hypothermia, and hypotension). Antibody to simian retrovirus (SRV) was measured in animals which were from a colony in the facility.
Lassa vaccine candidates.
The viruses used to immunize were NYBH strains of vaccinia virus either expressing Lassa genes, not expressing these genes as a negative control, or expressing Mopeia virus genes (MOP) as a positive control (41; M. P. Kiley, J. V. Lange, and K. M. Johnson, Letter, Lancet ii:738, 1979). Lassa virus is an arenavirus and has an ambisense S segment coding for structural proteins and an L segment coding for the viral polymerase (2). We therefore used vaccinia viruses expressing the following S-segment Lassa structural proteins: (i) the full-length glycoprotein (V-LSG), (ii) the nucleoprotein (V-LSN), (iii) the full-length glycoprotein and nucleoprotein in the same construct (V-LSG/N), and finally (iv) single glycoproteins (V-LSG1 [containing residues 1 to 296] and V-LSG2 [with a deletion of residues 67 to 234]) (1, 31, 33). Sequences used were derived from the Josiah strain of Lassa virus, isolated from a patient in Sierra Leone. Among 10 negative control animals, 3 received NYBH and 7 were unvaccinated.
We vaccinated 34 animals (Table 1). Two received V-LSG1, and two received V-LSG2. Eleven received V-LSN. Nine received either the full-length glycoprotein expressed singly (seven were vaccinated with V-LSG) or the separate glycoproteins expressed in combination (two were vaccinated with V-LSG1 plus V-LSG2). A further eight animals were vaccinated with constructs expressing all the protein products of the small segment of the Lassa virus genome; six were vaccinated simultaneously with V-LSG and V-LSN, and two were vaccinated with a single construct, V-LSG/N. Two animals received 104 PFU of Mopeia virus subcutaneously.
TABLE 1.
Virus titers of the challenge virus at various days after challenge in vaccinated and unvaccinated monkeys challenged with lethal doses of Lassa virus
| Vaccinea | Monkey | Day of death | Outcomeb | Vacc/chal intervalc | Challenge titerd | Lassa virus titer (log10 PFU/ml) by day following inoculation
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||||||
| NYBH | RH1 | 15 | D | 40 | 104 | 2.0 | 4.0 | 3.0 | ||||||||||
| RH3 | 12 | D | 49 | 104 | 2.0 | 3.0 | 3.0 | 4.2 | ||||||||||
| CY1 | 19 | D | >900 | 104 | 0.0 | 2.0 | 2.8 | 4.4 | 4.5 | 5.2 | 6.0 | 6.5 | 6.8 | 6.3 | 6.5 | 6.8 | ||
| None | RH9 | 19 | D | 104 | 4.6 | |||||||||||||
| RH10 | 10 | D | 104 | 4.2 | 6.1 | |||||||||||||
| RH13 | 12 | D | 104 | 0.0 | 0.0 | 3.6 | 3.8 | 4.1 | 4.1 | 5.2 | 5.7 | 6.5 | 6.4 | |||||
| RH14 | 13 | D | 104 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.4 | 3.8 | 4.5 | 5.2 | 6.3 | |||||
| RH15 | 15 | D | 104 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.2 | 4.5 | 5.2 | 6.0 | 6.3 | |||||
| RH16 | 13 | D | 104 | 0.0 | 0.0 | 0.0 | 4.2 | 3.6 | 4.3 | 4.5 | 4.8 | 5.7 | 6.4 | 6.7 | ||||
| RH39 | 11 | D | 104 | 0.0 | 0.0 | 2.0 | 2.0 | 3.2 | 3.6 | 4.6 | 5.4 | 6.2 | ||||||
| V-LSG1 | CY17 | 14 | D | 116 | 103 | 0.0 | 0.0 | 0.0 | 2.7 | 2.8 | 3.8 | 4.2 | 4.7 | 6.8 | 6.8 | 6.9 | ||
| CY18 | 16 | D | 116 | 103 | 0.0 | 0.0 | 2.5 | 3.5 | 3.8 | 4.0 | 4.3 | 5.2 | 5.6 | 5.8 | 6.0 | |||
| V-LSG2 | CY15 | 13 | D | 116 | 103 | 0.0 | 0.0 | 2.0 | 2.2 | 3.0 | 3.9 | 4.0 | 4.3 | 5.2 | 5.8 | 6.4 | ||
| CY16 | 12 | D | 116 | 103 | 0.0 | 2.5 | 3.6 | 3.9 | 4.8 | 5.9 | 6.2 | 6.3 | 7.9 | 8.0 | ||||
| V-LSN | CY13 | 9 | D | 274 | 104 | 0.0 | 2.7 | 4.8 | 5.8 | 5.9 | 6.2 | 6.1 | ||||||
| CY14 | 12 | D | 274 | 104 | 0.0 | 0.0 | 4.2 | 4.5 | 4.8 | 5.3 | 5.5 | 7.3 | ||||||
| CY5 | 9 | D | 70 | 104 | 6.1 | 7.8 | 8.6 | |||||||||||
| CY6 | 11 | D | 75 | 104 | 6.0 | 6.3 | 8.1 | 8.1 | ||||||||||
| RH11 | 13 | D | 62 | 104 | 0.0 | 0.0 | 0.0 | 0.0 | 2.3 | 3.5 | 3.2 | 3.4 | 3.8 | 3.7 | 3.7 | |||
| RH12 | 13 | D | 62 | 104 | 0.0 | 0.0 | 0.0 | 3.1 | 3.2 | 3.6 | 4.1 | 5.1 | 6.1 | 5.1 | 3.7 | |||
| RH19 | 11 | D | 354 | 104 | 0.0 | 0.0 | 0.0 | 1.6 | 3.6 | 3.9 | 5.1 | 5.8 | 7.0 | |||||
| RH20 | 12 | D | 157 | 104 | 0.0 | 0.0 | 1.3 | 1.6 | 2.1 | 3.3 | 5.0 | 6.0 | 8.2 | 9.0 | ||||
| RH30 | S | 126 | 103 | 0.0 | 0.0 | 2.1 | 2.3 | 2.0 | 1.9 | 1.8 | 1.4 | 1.3 | 1.5 | |||||
| RH31 | S | 126 | 103 | 0.0 | 0.0 | 2.3 | 2.3 | 2.5 | 1.6 | 1.4 | 1.3 | 1.4 | 1.9 | |||||
| RH33 | S | 75 | 103 | 0.0 | 1.7 | 1.8 | 2.0 | 1.5 | 1.0 | 1.2 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| V-LSG | CY11 | S | 274 | 104 | 0.0 | 0.0 | 4.3 | 4.5 | 4.1 | 4.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| CY12 | S | 274 | 104 | 0.0 | 0.0 | 0.0 | 4.6 | 3.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| CY19 | 21 | D | 700 | 104 | 0.0 | 0.0 | 0.0 | 2.3 | 3.7 | 3.0 | 2.5 | |||||||
| RH4 | S | 270 | 104 | 0.0 | 3.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| RH5 | S | 270 | 104 | 0.0 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||
| RH6 | S | 270 | 104 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||
| RH8 | S | 36 | 104 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||
| V-LSG1 + V-LSG2 | RH38 | S | 82 | 104 | 0.0 | 0.0 | 2.3 | 3.0 | 3.3 | 3.2 | 3.7 | 4.0 | 4.1 | 3.5 | 2.9 | 3.0 | ||
| RH37 | S | 86 | 104 | 0.0 | 1.0 | 2.3 | 2.9 | 1.8 | 2.0 | 2.1 | 2.8 | 1.4 | 1.4 | 1.4 | 1.0 | |||
| V-LSG + V-LSN | RH17 | S | 354 | 104 | 0.0 | 0.0 | 0.0 | 0.0 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 | |||||
| RH18 | S | 354 | 104 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| CY10 | S | 189 | 104 | 0.0 | 0.0 | 0.0 | 3.1 | 3.4 | 4.2 | 3.7 | 3.1 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| CY7 | 11 | D | 488 | 104 | 0.0 | 3.3 | 3.8 | 5.3 | 5.2 | 6.1 | 6.4 | 7.0 | ||||||
| CY8 | S | 189 | 104 | 0.0 | 0.0 | 0.0 | 2.3 | 3.1 | 3.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| CY9 | S | 488 | 104 | 0.0 | 0.0 | 4.2 | 5.4 | 5.3 | 5.7 | 4.5 | 3.0 | 2.3 | 0.0 | |||||
| V-LSG/N | RH35 | S | 86 | 104 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| RH36 | S | 82 | 104 | 0.0 | 1.0 | 2.3 | 2.5 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Mopeia virus | RH2 | S | 36 | 104 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| RH7 | S | 36 | 104 | 2.2 | 0.0 | 0.0 | 0.0 | |||||||||||
Vaccines are indicated as follows. For the negative controls, “None” indicates no vaccine given and “NYBH” indicates the NYBH strain of vaccinia virus. For the positive control, the Mopeia virus was used. The Lassa fever vaccines were V-LSG, the uncleaved, full-length glycoprotein; V-LSN, the nucleoprotein; V-LSG/N; V-LSG/N plus V-LSG, the glycoprotein and nucleoprotein, which were administered in the same construct or in two separate constructs given at the same time; and V-LSG1 and V-LSG2, the cleaved glycoproteins 1 and 2, which were given separately or at the same time. Sequences used were derived from the Josiah strain of the Lassa virus.
D, died; S, survived the Lassa virus challenge.
Vacc/chal interval, interval between vaccination and challenge (days).
Challenge titer, titer of challenge virus on retesting after challenge (log10 PFU/ml).
Immunization and challenge procedures.
All animals received a single vaccination consisting of 0.2 ml of vaccine given intradermally and simultaneously at four separate sites (each forearm and the lateral aspect of each thigh) at a dilution which delivered to each animal a total dose of 109 PFU. When two vaccines were used, each vaccine was administered in one arm and one leg on the ipsilateral side. Typical vaccinia virus lesions were measured and recorded regularly. All animals were challenged subcutaneously with 103 to 104 PFU of the Josiah strain of Lassa virus in 0.5 ml of phosphate-buffered saline within 36 to 700 days (Table 1).
Laboratory procedures.
Lassa virus titers following challenge were measured in serum samples and tissue specimens by plaque assay on Vero E6 cells. Cocultivation was performed by mixing a trypsinized suspension of Vero E6 cells with an equal number of cells from a suspension of ground liver, spleen, or kidney. Anti-Lassa virus antibodies were measured by immunofluorescent antibody (IFA) techniques and by radioimmunoprecipitation (RIP) using [S]methionine and glucosamine radiolabels as previously described (16, 40). The intensities of the RIP bands were graded by the same observer using a scale of 1 to 4, with 1 being a faint band and 4 being a strong band. Reverse transcriptase PCR (RT-PCR) detection was applied to guanidine-extracted sera and tissues from infected monkeys to detect Lassa virus RNA during both the acute and the convalescent stages of infection as described previously (36). Primers were derived from a region of the small RNA segment of Lassa virus coding for the glycoprotein (36). Hemoglobin, hematocrit, platelet, leukocyte, and erythrocyte counts were performed using a Hycell 555 cell counter (Boehringer Mannheim, Houston, Tex.) or a Coulter Electronics Inc. (Hialeah, Fla.) model T660 counter. Some white cell and differential counts were performed manually. Clinical chemistries were performed using a Lambda 3 UV–visual-spectrum spectrophotometer (Perkin-Elmer Corporation, Norwalk, Conn.).
Autopsy and biopsy.
A unilateral nephrectomy with splenectomy and liver biopsies was performed on three animals to obtain serial biopsy material. Survivors were electively sacrificed when they were well and aviremic between days 21 and 966. Animals which died or which were sacrificed in extremis during acute Lassa virus infection were autopsied, and their livers, spleens, and kidneys were taken for virus isolation and RT-PCR. Effusions, when present, were sampled for virus titration. Cerebrospinal fluid (CSF) was taken after careful surgical exposure of the cisterna magna to avoid blood contamination.
Data analysis.
Data were collated using Paradox 3.0 data management systems and analyzed using EpiInfo 5.01a and SAS/PC software. Values are from an unconditional logistic regression analysis that controlled for monkey species, unless otherwise stated.
RESULTS
Survival following challenge.
Twenty of 44 animals survived Lassa virus challenge (Table 1). Among the survivors were 8 of 9 V-LSG-vaccinated animals and 9 of 10 monkeys which received the full S-segment products (Mopeia virus, V-LSG/N, or V-LSG plus V-LSN). This protection against death was significant when compared with the death rate of controls (P < 0.0005). The deaths were of the two animals with the longest vaccine-to-challenge intervals (488 and 700 days; range, 36 to 700 days; mean, 319 days). Survival diminished as the vaccine-to-challenge interval increased (P < 0.05; range, 36 to 700 days). A trend towards increasing duration of viremia (days) was also observed with increased intervals between vaccination and challenge.
The 24 animals that died or were sacrificed in extremis included all 10 negative (unprotected) controls and all 4 vaccinated with single glycoproteins (V-LSG1 or V-LSG2). In addition 8 of the 11 V-LSN-vaccinated animals died, which shows that this vaccine was not significantly protective (P > 0.10). The V-LSN animals appeared to have a shorter and more acute process than unprotected animals. The median day of death for V-LSN animals was day 11.5 (range, 9 to 13), compared with day 13 (range, 10 to 19) for control animals (P < 0.10). The highest individual terminal viremias were seen in V-LSN-vaccinated animals (highest viremia, 109 versus 106.7 PFU/ml for unprotected animals at death). This phenomenon of more severe disease and early death was, however, related to the challenge dose. Back titration of the challenge inoculum used in the final experiment showed that the titer had dropped from 104 to 103 PFU/ml. The three V-LSN-vaccinated animals in that experiment that received the lower challenge titer survived. These three animals were rhesus monkeys. In contrast, all four V-LSG1- and V-LSG2-vaccinated animals received the lower challenge dose but died regardless.
Viremia and viral load in tissues and exudates.
Viremias following challenge are shown in Table 1. All but two animals experienced viremia, but in general, limitation of viremia correlated with outcome and thus protection. The highest titers were seen in the animals that had received the V-LSN recombinant vaccine, but this difference was not statistically significant when these animals were compared with the unvaccinated animals. On the other hand, the groups of animals which received the entire glycoprotein (V-LSG) or all the S-segment proteins (V-LSG/N or V-LSG plus V-LSN) showed significantly diminished mean virus titers compared with those of unvaccinated animals (P < 0.001 and 0.001, respectively; Student t test). The one V-LSG-vaccinated animal that died reached maximum viremia on day 8, and the last day on which virus was detected in serum was day 11. At autopsy on day 21 this animal had evidence of pericarditis and cardiac failure. Virus could not be recovered from postmortem blood samples, but there were virus-laden transudates. Virus was also recovered in low titers from liver, kidney, and CSF. This animal was coincidentally SRV antibody positive; however, overall analysis of SRV positivity among the animals showed no association with outcome following challenge.
Straw-colored pericardial fluid was obtained at autopsy from all animals that received V-LSN, the one that died after V-LSG vaccination, and five of the unvaccinated animals. Pleural fluid was available from one V-LSN vacinee, the one that died from V-LSN–V-LSG vaccination, and four unvaccinated animals. Titers in these fluids were almost uniformly higher than in serum (range, 103 to 108.3 PFU/ml). Titers in CSF were lower than in serum (range, <100 to 104.6 PFU/ml).
Persistence of Lassa virus.
The latest day on which virus could be recovered from serum was day 14, and that from tissues was day 21. Evidence for persistence elsewhere in tissues or fluids in survivors could not be found by cocultivation of tissues taken up to 112 days following challenge (Table 2). However, autopsy and biopsy material examined by RT-PCR revealed that viral RNA could be detected at least 112 days after challenge.
TABLE 2.
Detection of Lassa virus RNA by RT-PCR in tissues taken from monkeys surviving challenge
| Day postchallenge | Monkey | Vaccine received | RT-PCR result with biopsy and autopsy tissuesa
|
||
|---|---|---|---|---|---|
| Liver | Kidney | Spleen | |||
| 20 | RH17 | V-LSG/N | Positive | Positive | Positive |
| 20 | RH18 | V-LSG/N | Positive | Positive | Positive |
| 21 | RH30 | V-LSN | Bx positive | ||
| 21 | RH31 | V-LSN | Bx positive | ||
| 30 | RH4 | V-LSG | Positive | Positive | Negative |
| 30 | RH5 | V-LSG | Positive | Positive | Negative |
| 35 | RH33 | V-LSN | Negative | Positive | Positive |
| 35 | RH35 | V-LSG + V-LSN | Negative | Negative | Positive |
| 35 | RH37 | V-LSG1 + V-LSG2 | Negative | Positive | Positive |
| 35 | RH38 | V-LSG1 + V-LSG2 | Negative | Positive | Positive |
| 63 | RH30 | V-LSN | Negative | Negative | Positive |
| 63 | RH31 | V-LSN | Negative | Negative | Positive |
| 84 | CY11 | V-LSG | Negative | Negative | Negative |
| 84 | CY12 | V-LSG | Negative | Negative | Negative |
| 87 | CY9 | V-LSG+V-LSN | Bx positive | ||
| 112 | CY10 | V-LSG+V-LSN | Positive | Positive | Positive |
| 112 | CY8 | V-LSG+V-LSN | Positive | Positive | Positive |
Primers to the Lassa glycoprotein gene were used as described previously (36). Bx positive, positive for Lassa virus RNA. Rates of positivity were 43% with liver specimens (n = 14), 71% with kidney specimens (n = 17), and 71% with spleen specimens (n = 14).
Antibody experiments.
Survival or death did not correlate with the titer of IFA to whole Lassa virus antigens prior to challenge. Following vaccination and prior to challenge, low-titer IFA could be detected in V-LSN–V-LSG- and V-LSN-vaccinated animals, with titers ranging from 4 to 256. There was a decline in antibody titer with time, but challenge resulted in a rapid boost in titers. We performed RIP analyses to determine the pattern of responses against all viral proteins in order to search for a correlation with protection. RIP responses to the glycoprotein were seen only in animals receiving the whole S segment or the glycoprotein. There were barely detectable amounts of glycoprotein antibody by RIP assay after a single vaccination, but this increased briskly on challenge in protected animals (Fig. 1). IFA titers and strong nucleoprotein responses seen by RIP without responses to the glycoprotein were seen in those animals vaccinated with the corresponding vaccine. Repeated efforts failed to demonstrate the presence of plaque reduction neutralization in sera from vaccinated animals before challenge or in the sera of surviving animals after recovery from challenge.
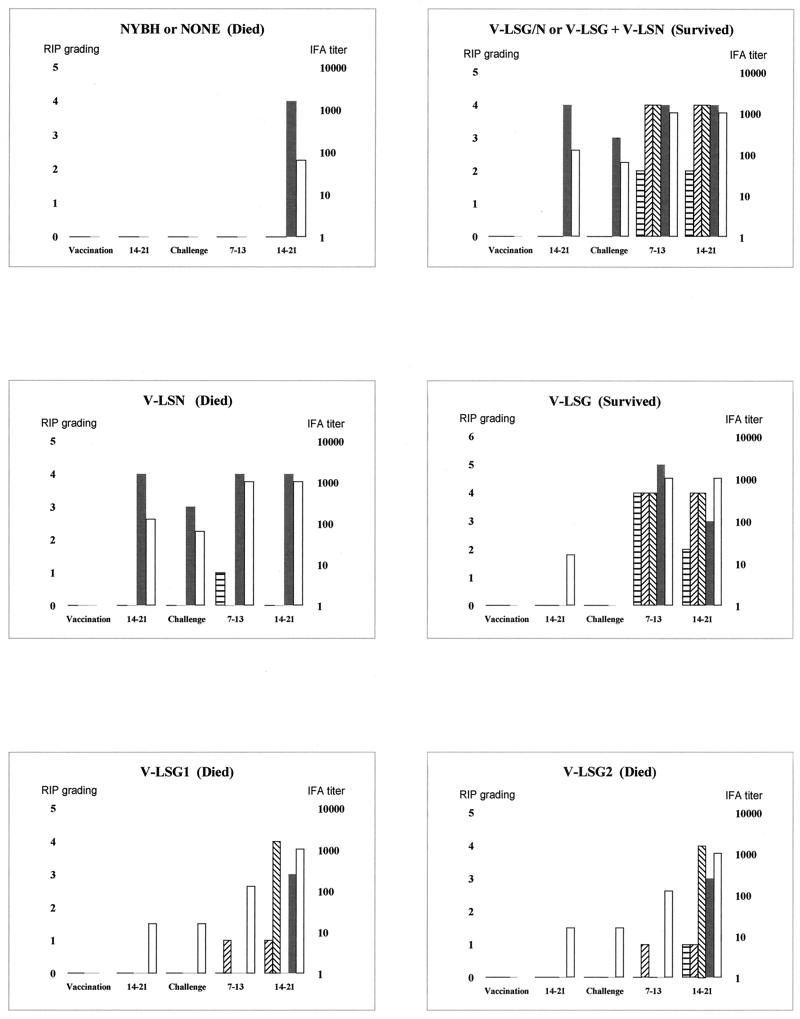
FIG. 1.
RIP readings and IFA titers in sera from several monkeys in each group following vaccination and challenge. The densities of the RIP bands against each nucleoprotein were scored from 0 to 5 subjectively by a single observer on the same occasion. The x axis denotes events and time following vaccination and challenge in days. Open bars denote antibody titer, and the other bars represent immune responses detected by RIP assay as follows:  , glycoprotein;
, glycoprotein;  , glycoprotein 1;
, glycoprotein 1;  , glycoprotein 2; and ■, nucleoprotein.
, glycoprotein 2; and ■, nucleoprotein.
Evidence of disturbances in clinical chemistry and hematology.
Following challenge, mean hemoglobin and hematocrit counts, cell volume, and red cell counts did not vary significantly with viremia. Platelet counts were moderately depressed in viremic compared with those in nonviremic animals, but this did not correlate with virus titer in serum or with the vaccine received. Changes in differential white cell counts (reduced lymphocyte and raised neutrophil counts) and minimal disturbances in hepatic enzymes did correlate with protection and thus outcome (data not shown). Consistent with a possible cytotoxic T lymphocyte dominant protective response was the observation of a prompt lymphocytic response to the virus challenge by day 4 in animals that had been protected with recombinants expressing all the S-segment proteins. This finding contrasts with the usual lymphopenia seen in primates fatally infected with Lassa virus (18). Furthermore, animals that died had significantly higher peak neutrophil counts than those that survived. Serum aspartate transaminase levels were highest in animals that had received the V-LSN vaccine and in the unprotected animals, which correlates with outcome. Animals protected by V-LSG or the full S segment showed little or no disturbance of liver function, even in the face of viremia. Overall, the V-LSN-vaccinated animals had marked lymphopenia and higher mean aspartate aminotransferase values than unvaccinated animals. The V-LSN-vaccinated animals were observed to be sicker and died earlier than unvaccinated animals.
DISCUSSION
Single administration of a vaccine expressing the full-length Lassa virus glycoprotein affords protection against Lassa fever in primates, with or without expression of the nucleoprotein. Vaccines expressing single glycoprotein genes, or the nucleoprotein alone, do not protect. This is the first convincing evidence that a protective immune response in nonhuman primates requires expression of the full-length Lassa virus glycoprotein. There was no difference in protection attributable to different macaque species, so it is reasonable to anticipate that similar protection can be achieved in humans.
Vaccination using the nucleoprotein may protect primates against a lower challenge dose of Lassa virus (103 PFU). On the other hand, we did not observe superior protection in animals given V-LSN and V-LSG together in terms of reductions in maximum virus titers or duration of viremia. V-LSN-vaccinated animals receiving the full Lassa challenge dose (104 PFU) suffered 100% mortality, earlier deaths (by 2 days), and the highest viremias (up to 109 PFU/ml). The three V-LSN survivors that received a lower challenge dose were rhesus monkeys. We do not know if difference in species may have influenced outcome. Against this is the observation that among the V-LSN fatalities, half were rhesus and half were cynomolgous monkeys, but numbers are too small to draw conclusions. We can conclude from the data currently available only that V-LSN is not required for protection.
The lack of relationship between high levels of preexisting high-titer antibody to the Lassa nucleoprotein and protection is consistent with findings for patients in Sierra Leone, for whom there was no correlation between titer of immunoglobulin G antibody on hospital admission and subsequent outcome (22). In this study we showed that the presence of high titers of anti-nucleoprotein antibody measured prior to challenge bears no relation to outcome. The nucleoprotein is produced in excess of other proteins during arenavirus replication, and this may explain the dominance of anti-nucleoprotein antibodies in test systems. While we observed little in the way of antibody to glycoprotein after vaccination with glycoprotein antigen, we did observe a brisk response after challenge (Fig. 1). However, we were not able to demonstrate neutralizing antibody activity in any postchallenge serum by the technique that uses a fixed amount of serum and various dilutions of virus (20). Unlike with Junin virus infection, Lassa fever in humans does not respond to treatment with high-titer human immune plasma, so the role of antibodies detected by IFA assay in virus clearance or in protection from initial infection is at least secondary (16, 25, 29). There are some experimental data indicating that protection is conferred by giving immune serum before challenge to monkeys, but neutralizing antibodies to Lassa virus are notoriously difficult to demonstrate (16, 20, 21). Attempts at treatment of monkeys after infection have been uniformly unsuccessful. Fatal choriomeningitis caused by intracranial inoculation of the closely related arenavirus lymphocytic choriomeningitis virus (LCMV) can be prevented (but not treated) in mice using a neutralizing monoclonal antibody to the LCMV G1 protein (3, 5, 6).
Clearly the response to glycoprotein protects the animals from induction of fatal processes leading to disease and death. Route and infectious dose are important determinants of outcome, and the inoculum in this study was chosen to model the high-dose parenteral challenge for which we require protection (19). The immune response is the key to understanding control of pathogenesis in this disease. Severe illness seems to be a manifestation of disordered host responses rather than lytic viral destruction of cells and organs (4, 10, 17, 18). It seems reasonable from the experiments presented here to assume that the T-cell response is critical in controlling virus replication and preventing the cascade of fatal events that lead to death. Apoptosis of T cells has now been reported to correlate with fatal outcome in Ebola hemorrhagic fever. In Ebola virus, orderly T-cell responses with limited apoptosis correlate with survival and outcome is apparently determined very early in disease (4). Lymphopenia and impaired lymphocyte proliferation responses are characteristic of severe Lassa fever. It may be that apoptosis is likewise a central feature of the failure to control virus replication and hence fatal outcome.
Our data strongly implicate the primary role of cytotoxic T lymphocytes in protection from Lassa fever. The protection of our animals by Lassa virus glycoprotein expressed in vaccinia virus in the face of a low antibody response and the lack of protection by antibody alone led us to the conclusion that antibody alone does not provide protection (29). The immune response to the Lassa virus glycoprotein limits but does not eliminate virus replication after challenge. What we do know from our study is that epitopes on both glycoproteins are needed and that these are apparently able to induce protective immunity in concert but not independently. It is known that the proper folding of LCMV glycoprotein is critical for induction of the humoral response and that baculovirus- and vaccinia virus-expressed glycoproteins, such as we used in these experiments, are not posttranslationally processed or transported correctly to the membrane (12, 38, 39). Thus, the minimal measurable antibody response to glycoprotein after vaccination may be related to improperly folded glycoprotein. Despite the strong evidence implicating cell-mediated immunity, we still think we have to keep an open mind about some contribution to protective processes by antibodies.
Lassa virus has long been known to persist in humans, but secondary cases due to contact with recovered patients have never been reported (15, 28). We could not demonstrate persistent viremia but did detect persistence of Lassa virus RNA in tissues, particularly the spleen, at least 112 days after challenge. However, we could not recover virus from these tissues even using cocultivation techniques. Thus, it seems very unlikely that persistence at this level can have any public health importance. Indeed low-level persistence of viral RNA following natural infection may be important in protection against reinfection. By current estimates about 100,000 new Lassa virus infections are acquired naturally each year, and there is no evidence that such people, once recovered, infect others in their communities or in medical care facilities.
Not unexpectedly, we determined that vaccine-to-challenge intervals and challenge dose also influenced outcome. The two V-LSG- and V-LSG–V-LSN-vaccinated animals that died each had the longest vaccine-to-challenge intervals in their group (700 and 488 days, respectively). The single V-LSG death was atypical, in that the animal died late (day 19) with complications (pericarditis), seen occasionally in humans with Lassa fever (9, 28). V-LSN-vaccinated animals that received a 10-fold-lower challenge dose survived, even though this lower dose killed all the single-glycoprotein-vaccinated animals. Partial protection of guinea pigs vaccinated with the Lassa virus nucleoprotein constructs has also been observed (1, 8, 32). It is difficult and potentially misleading to compare data from disparate species, particularly from the rodent host, in which arenaviruses establish silent persistent infection, and from the accidental host, the primate, in which the host response is acute, severe, and often fatal. The “protection” seen in the three surviving monkeys vaccinated with nucleoprotein may, on the other hand, be purely a function of challenge. Persistently infected mastomys urine (about 102 to 103 Lassa virus particles/ml) is the most likely source of primary infections in humans (23, 30). In practice, however, virus titers in human blood may be as high as 107 to 109, so a vaccine for hospital staff must protect against high-dose challenge by parenteral routes such as needlesticks and accidental infusion (19, 22, 23).
Cross-protection against other West African strains of Lassa virus remains to be addressed. The protection afforded by the Mopeia virus from southern Africa certainly supports the idea that broad protection is achievable and desirable, particularly since some Nigerian strains may induce more severe and fatal illnesses than strains from further west (19, 23, 35). Monoclonal antibody mapping and molecular studies of the glycoproteins of African arenaviruses show a conserved B-cell epitope on G2 in all of the known African arenaviruses, including Mopeia and most South American arenaviruses (33). However, both G1 and G2 recognition is necessary based on the results of our experiments. We concluded from continuous observations over 14 years in Sierra Leone that a single natural infection provides long-term protective immunity against disease (27).
We are concerned that genetically engineered vaccines will protect only in the short term. With respect to the population that most needs this vaccine, a single shot of live attenuated candidate vaccine conferring life-long immunity is much preferred. Mopeia virus is a good illustration of an effective live attenuated vaccine, with the advantage that a single administration might induce life-long protection. Mopeia virus is unlikely ever to be acceptable, since it is presently classified as a BSL3 pathogen, even though data from Mozambique and southern Africa suggest that this virus is not pathogenic for humans (41). There are major sequence differences in the S segments of Lassa and Mopeia viruses, but no sites associated with virulence have been genetically mapped. Mopeia virus possesses not one but two hairpin loops connecting the ambisense S gene, but the G1 and G2 proteins have 74 and 80% amino acid sequence homology, respectively (37). Pathogenicity in LCMV has been mapped to the L gene, and the most divergent regions are found in the RdRp and Z proteins (14). RdRp analyses group Lassa virus and LCMV together as a separate arenavirus lineage (13, 24). Sequence data are not available for the Mopeia virus L gene, but geographically it is likely to also fall within this Old World arenavirus lineage. Despite this evidence, Mopeia virus is never going to achieve the safety standards required, and other solutions must be sought.
Among the hemorrhagic fever-causing viruses, Lassa fever and hantaviruses afflict the greatest number of victims. Lassa fever could easily threaten communities in West Africa outside of its already broad area of endemicity. In the first three months of 2000, three fatal cases have been reported among travelers returning to Europe, and in April 2000 epidemics are again being seen in Nigeria, the most populous country in Africa. Since 1990, severe social disruption from conflicts and terror campaigns in Sierra Leone and Liberia have resulted in displacement of up to 2,000,000 people—half the population of the area—with a substantial increase in the already large number of Lassa fever cases and deaths (Allan et al., Int. Conf. Emerg. Infect. Dis., abstr. 134). An effective vaccine for the closely related hemorrhagic fever virus Junin virus has been made and has virtually eliminated disease caused by this virus in Argentina (26). We have shown that an effective vaccine for Lassa fever can be made. This vaccine needs to express the full-length glycoprotein, and the nucleoprotein is not required. We now need to pursue production and evaluation of a vaccine for human use as a matter of humanitarian concern and priority.
ACKNOWLEDGMENTS
We thank Anne Conaty, Lynette Brammer, Sam Trappier, Bertha Farrar, Gilda Perez-Oronoz, and Eddie Jackson for their contribution to the care of the animals and extensive technical assistance. We also acknowledge the contributions of D. Auperin and H. Morrison and thank them for providing us with the constructs used in our experiments. We are most grateful to Lindsay Whitton and Mark Girard for their helpful review of the manuscript.
REFERENCES
- 1.Auperin D D, Esposito J J, Lange J V, Bauer S P, Knight J, Sasso D R, McCormick J B. Construction of a recombinant vaccinia virus expressing the Lassa virus glycoprotein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 1988;9:233–248. doi: 10.1016/0168-1702(88)90033-0. [DOI] [PubMed] [Google Scholar]
- 2.Auperin D D, Sasso D R, McCormick J B. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology. 1986;154:155–167. doi: 10.1016/0042-6822(86)90438-1. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann M F, Zinkernagel R M. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 4.Baize S, Leroy E M, Georges-Courbot M C, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch S P, McCormick J B, Georges A J. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 5.Baldridge J R, Buchmeier M J. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J Virol. 1992;66:4252–4257. doi: 10.1128/jvi.66.7.4252-4257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldridge J R, McGraw T S, Paoletti A, Buchmeier M J. Antibody prevents the establishment of persistent arenavirus infection in synergy with endogenous T cells. J Virol. 1997;71:755–758. doi: 10.1128/jvi.71.1.755-758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch A. A serological survey of Lassa fever in Liberia. Bull W H O. 1978;56:811–813. [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg J C, Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guineapigs against Lassa fever. Lancet. 1987;ii:186–188. doi: 10.1016/s0140-6736(87)90767-7. [DOI] [PubMed] [Google Scholar]
- 9.Cummins D, Bennett D, Fisher-Hoch S P, Farrar B, McCormick J B. Electrocardiographic abnormalities in patients with Lassa fever. J Trop Med Hyg. 1989;92:350–355. [PubMed] [Google Scholar]
- 10.Cummins D, Fisher-Hoch S P, Walshe K J, Mackie I J, McCormick J B, Bennett D, Perez G, Farrar B, Machin S J. A plasma inhibitor of platelet aggregation in patients with Lassa fever. Br J Haematol. 1989;72:543–548. doi: 10.1111/j.1365-2141.1989.tb04321.x. [DOI] [PubMed] [Google Scholar]
- 11.Cummins D, McCormick J B, Bennett D, Samba J A, Farrar B, Machin S J, Fisher-Hoch S P. Acute sensorineural deafness in Lassa fever. JAMA. 1990;264:2093–2096. [PubMed] [Google Scholar]
- 12.Di Simone C, Buchmeier M J. Vaccines. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. Protection of mice from lethal LCMV infection by a reconstituted glycoprotein vaccine; pp. 33–37. [Google Scholar]
- 13.Djavani M, Lukashevich I S, Salvato M S. Sequence comparison of the large genomic RNA segments of two strains of lymphocytic choriomeningitis virus differing in pathogenic potential for guinea pigs. Virus Genes. 1998;17:151–155. doi: 10.1023/a:1008016724243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djavani M, Lukashevich I S, Sanchez A, Nichol S T, Salvato M S. Completion of the Lassa fever virus sequence and identification of a RING finger open reading frame at the L RNA 5′ end. Virology. 1997;235:414–418. doi: 10.1006/viro.1997.8722. [DOI] [PubMed] [Google Scholar]
- 15.Fisher-Hoch S P. Stringent precautions are not advisable when caring for patients with viral haemorrhagic fevers. Rev Med Virol. 1993;3:7–13. [Google Scholar]
- 16.Fisher-Hoch S P, McCormick J B, Auperin D D, Brown B G, Castor M, Perez G, Ruo S, Conaty A, Brammer L, Bauer S. Protection of rhesus monkeys from fatal Lassa fever by vaccination with a recombinant vaccinia virus containing the Lassa virus glycoprotein gene. Proc Natl Acad Sci USA. 1989;86:317–321. doi: 10.1073/pnas.86.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher-Hoch S P, McCormick J B, Sasso D, Craven R B. Hematologic dysfunction in Lassa fever. J Med Virol. 1988;26:127–135. doi: 10.1002/jmv.1890260204. [DOI] [PubMed] [Google Scholar]
- 18.Fisher-Hoch S P, Mitchell S W, Sasso D R, Lange J V, Ramsey R, McCormick J B. Physiological and immunologic disturbances associated with shock in a primate model of Lassa fever. J Infect Dis. 1987;155:465–474. doi: 10.1093/infdis/155.3.465. [DOI] [PubMed] [Google Scholar]
- 19.Fisher-Hoch S P, Tomori O, Nasidi A, Perez Oronoz G I, Fakile Y, Hutwagner L, McCormick J B. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. Br Med J. 1995;311:857–859. doi: 10.1136/bmj.311.7009.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahrling P B, Peters C J. Passive antibody therapy of Lassa fever in cynomolgus monkeys: importance of neutralizing antibody and Lassa virus strain. Infect Immun. 1984;44:528–533. doi: 10.1128/iai.44.2.528-533.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahrling P B, Peters C J, Stephen E L. Enhanced treatment of Lassa fever by immune plasma combined with ribavirin in cynomolgus monkeys. J Infect Dis. 1984;149:420–427. doi: 10.1093/infdis/149.3.420. [DOI] [PubMed] [Google Scholar]
- 22.Johnson K M, McCormick J B, Webb P A, Smith E S, Elliott L H, King I J. Clinical virology of Lassa fever in hospitalized patients. J Infect Dis. 1987;155:456–464. doi: 10.1093/infdis/155.3.456. [DOI] [PubMed] [Google Scholar]
- 23.Keenlyside R A, McCormick J B, Webb P A, Smith E, Elliott L, Johnson K M. Case-control study of Mastomys natalensis and humans in Lassa virus-infected households in Sierra Leone. Am J Trop Med Hyg. 1983;32:829–837. doi: 10.4269/ajtmh.1983.32.829. [DOI] [PubMed] [Google Scholar]
- 24.Lukashevich I S, Djavani M, Shapiro K, Sanchez A, Ravkov E, Nichol S T, Salvato M S. The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. J Gen Virol. 1997;78:547–551. doi: 10.1099/0022-1317-78-3-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiztegui J, Feuillade M, Briggiler A. Progressive extension of the endemic area and changing incidence of Argentine hemorrhagic fever. Med Microbiol Immunol. 1986;175:149–152. doi: 10.1007/BF02122437. [DOI] [PubMed] [Google Scholar]
- 26.Maiztegui J I, McKee K T, Jr, Barrera Oro J G, Harrison L H, Gibbs P H, Feuillade M R, Enria D A, Briggiler A M, Levis S C, Ambrosio A M, Halsey N A, Peters C J. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. AHF Study Group. J Infect Dis. 1998;177:277–283. doi: 10.1086/514211. [DOI] [PubMed] [Google Scholar]
- 27.McCormick J B. Arenaviruses. In: Fields B N, Knipe D M, editors. Fields virology. New York, N.Y: Raven Press; 1990. pp. 1245–1267. [Google Scholar]
- 28.McCormick J B, King I J, Webb P A, Johnson K M, O'Sullivan R, Smith E S, Trippel S, Tong T C. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis. 1987;155:445–455. doi: 10.1093/infdis/155.3.445. [DOI] [PubMed] [Google Scholar]
- 29.McCormick J B, King I J, Webb P A, Scribner C L, Craven R B, Johnson K M, Elliott L H, Belmont Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 30.McCormick J B, Webb P A, Krebs J W, Johnson K M, Smith E S. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 31.Monath T P, Newhouse V F, Kemp G E, Setzer H W, Cacciapuoti A. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science. 1974;185:263–265. doi: 10.1126/science.185.4147.263. [DOI] [PubMed] [Google Scholar]
- 32.Morrison H G, Bauer S P, Lange J V, Esposito J J, McCormick J B, Auperin D D. Protection of guinea pigs from Lassa fever by vaccinia virus recombinants expressing the nucleoprotein or the envelope glycoproteins of Lassa virus. Virology. 1989;171:179–188. doi: 10.1016/0042-6822(89)90525-4. [DOI] [PubMed] [Google Scholar]
- 33.Morrison H G, Goldsmith C S, Regnery H L, Auperin D D. Simultaneous expression of the Lassa virus N and GPC genes from a single recombinant vaccinia virus. Virus Res. 1991;18:231–241. doi: 10.1016/0168-1702(91)90021-m. [DOI] [PubMed] [Google Scholar]
- 34.Price M E, Fisher-Hoch S P, Craven R B, McCormick J B. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. Br Med J. 1988;297:584–587. doi: 10.1136/bmj.297.6648.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruo S L, Mitchell S W, Kiley M P, Roumillat L F, Fisher-Hoch S P, McCormick J B. Antigenic relatedness between arenaviruses defined at the epitope level by monoclonal antibodies. J Gen Virol. 1991;72:549–555. doi: 10.1099/0022-1317-72-3-549. [DOI] [PubMed] [Google Scholar]
- 36.Trappier S G, Conaty A L, Farrar B B, Auperin D D, McCormick J B, Fisher-Hoch S P. Evaluation of the polymerase chain reaction for diagnosis of Lassa virus infection. Am J Trop Med Hyg. 1993;49:214–221. doi: 10.4269/ajtmh.1993.49.214. [DOI] [PubMed] [Google Scholar]
- 37.Wilson S M, Clegg J C. Sequence analysis of the S RNA of the African arenavirus Mopeia: an unusual secondary structure feature in the intergenic region. Virology. 1991;180:543–552. doi: 10.1016/0042-6822(91)90068-m. [DOI] [PubMed] [Google Scholar]
- 38.Wright K E, Salvato M S, Buchmeier M J. Neutralizing epitopes of lymphocytic choriomeningitis virus are conformational and require both glycosylation and disulfide bonds for expression. Virology. 1989;171:417–426. doi: 10.1016/0042-6822(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 39.Wright K E, Spiro R C, Burns J W, Buchmeier M J. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology. 1990;177:175–183. doi: 10.1016/0042-6822(90)90471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wulff H, Lange J V. Indirect immunofluorescence for the diagnosis of Lassa fever infection. Bull W H O. 1975;52:429–436. [PMC free article] [PubMed] [Google Scholar]
- 41.Wulff H, McIntosh B M, Hamner D B, Johnson K M. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull W H O. 1977;55:441–444. [PMC free article] [PubMed] [Google Scholar]