Abstract
Introduction
The variety of robotic surgery systems, training modalities, and assessment tools within robotic surgery training is extensive. This systematic review aimed to comprehensively overview different training modalities and assessment methods for teaching and assessing surgical skills in robotic surgery, with a specific focus on comparing objective and subjective assessment methods.
Methods
A systematic review was conducted following the PRISMA guidelines. The electronic databases Pubmed, EMBASE, and Cochrane were searched from inception until February 1, 2022. Included studies consisted of robotic-assisted surgery training (e.g., box training, virtual reality training, cadaver training and animal tissue training) with an assessment method (objective or subjective), such as assessment forms, virtual reality scores, peer-to-peer feedback or time recording.
Results
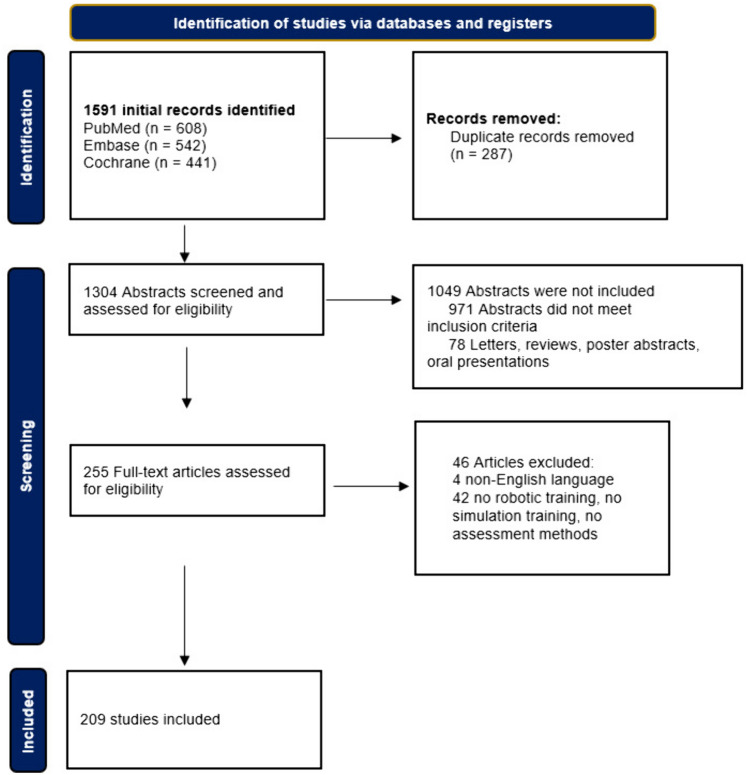
The search identified 1591 studies. After abstract screening and full-texts examination, 209 studies were identified that focused on robotic surgery training and included an assessment tool. The majority of the studies utilized the da Vinci Surgical System, with dry lab training being the most common approach, followed by the da Vinci Surgical Skills Simulator. The most frequently used assessment methods included simulator scoring system (e.g., dVSS score), and assessment forms (e.g., GEARS and OSATS).
Conclusion
This systematic review provides an overview of training modalities and assessment methods in robotic-assisted surgery. Dry lab training on the da Vinci Surgical System and training on the da Vinci Skills Simulator are the predominant approaches. However, focused training on tissue handling, manipulation, and force interaction is lacking, despite the absence of haptic feedback. Future research should focus on developing universal objective assessment and feedback methods to address these limitations as the field continues to evolve.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-024-10915-7.
Keywords: Robotic-assisted surgery, Assessment methods, Robotic surgery training, Simulation training, Objective assessment, Subjective assessment
The rapid growth of robotic-assisted surgery over the last decade, driven by intraoperative technical benefits and, to a lesser extent, significant benefits for patients, has caused a demand for adequate surgeon training [1–5]. Robotic-assisted surgery is a complex minimally invasive surgery technique that requires a different training approach compared to open or laparoscopic surgery, where mentoring surgeons can directly teach through hands-on methodsh in a direct hands-on fashion [6, 7].
Simulation training has emerged as a valuable method for acquiring the technical skills needed for robotic surgery in a safe environment [8, 9]. Robotic simulation demonstrates a significant learning effect, with acquired skills proving transferable to actual robotic procedures [10–12]. Individual feedback during robotic simulation is essential for developing efficient learning curves [13, 14]. Moreover, assessing performance provides trainees and their supervisors with a clear representation of skill progress and the achievement of clinical proficiency [15].
In 2000, the da Vinci Surgical System, developed by Intuitive Surgical Inc., became the first FDA-approved robotic-assisted surgery (RAS) system that used a minimally invasive surgical approach [16]. Over the past two decades, various training modalities have been developed for the da Vinci Surgical System and other robotic systems, including didactic lectures, box training, virtual reality (VR) training, wet lab training involving either animal or human cadavers, proctoring, and mentoring [17]. Furthermore, a range of robotic skill assessment tools has been developed, categorized as subjective and objective tools [18, 19]. These tools vary from subjective assessment forms such as GEARS, R-OSATS and GOALS to objective built-in scoring systems [20]. Advances in computer processing have also facilitated the development of built-in scoring systems that collect objective data from robotic simulators [21].
The aim of this systematic review was to provide an overview of different training modalities and assessment methods for teaching and assessing surgical skills in robotic surgery. Specifically, the focus was on comparing the use of objective and subjective assessment methods.
Methods
Search strategy
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. The electronic clinical databases of Pubmed, EMBASE, and Cochrane were searched for original and primary articles from inception until March 1, 2023. The search consisted of a combination of free terms and database-specific index terms without applying any filters (Supplemental File A, Tables A1–A3). The search was limited to studies published in English.
All three electronic databases were independently consulted by two reviewers (M.R. & E.U.). Initially, the duplicate records of the three electronic databases were removed. Subsequently, a critical selection took place based on titles and abstracts. The full-text articles of the selected abstracts were critically studied and assessed for eligibility. If a full-text article was not available, the authors were contacted. Potential conflicts of inclusion between reviewers were discussed to reach a consensus. In case of discrepancies or disagreement regarding study inclusion, a third researcher (F.D.) was consulted.
Inclusion criteria
Currently used training and assessment modalities for robotic-assisted surgery, regardless of the aim or specialty of the training/curriculum were identified. Included studies involved robotic-assisted surgery simulation training (such as box training, virtual reality training, cadaver training, animal tissue training, live animal training, and artificial tissue training) along with an assessment method (objective or subjective), including assessment forms, virtual reality scores, sensors, measuring systems, peer-to-peer feedback, or time recording. Reviews, letters, editorials, and comments were excluded. Studies without assessment methods (unsupervised training, no feedback, no assessments) or outcome measures were excluded.
Data collection
To provide a comprehensible overview of the extensive range of robotic-assisted surgery training and assessments, the following data were collected per article: author, year of publication, title, training modality, assessment method, subjective/objective assessment, participants, and study findings.
Objective assessment was defined as an assessment expressed in objective values, such as time (s), motion (mm/cm), force (N), collisions, human performance (EEG, eye movement tracking, pupillary response, EMG), task-specific errors, clutch usage, and task trainer score (based on multiple objective parameters). Subjective assessment was defined as an assessment method not expressed in objective values, such as assessment forms (GEARS, OSATS, GOALS), video assessment feedback, or oral feedback (peer-to-peer or supervisor) after observation. Study data were collected and recorded in Microsoft Excel, and figures were created using GraphPad (Prism 9.0.0, San Diego, California USA).
Data analysis
The data were analyzed using a narrative synthesis approach, which involved summarizing and synthesizing the results of the included studies in a narrative format. The results of the studies were grouped by the type of training modality (e.g., dry lab, virtual reality) and by subjective or objective outcome measure (e.g., subjective forms, time and motion measures, force measure).
Basic and advanced courses
To illustrate the difference in the application of training modalities, the studies were divided into two groups: basic and advanced training. Basic courses encompassed fundamental skills, whereas advanced courses delved deeper into specific technical skills and procedural training. This distinction was made because the purpose of both training courses inherently impacts the training modality and, therefore, the assessment method. A novice robotic surgeon is more likely to begin with virtual training or dry lab, while a more advanced robotic surgeon is more likely to train in the wet lab with animal tissue models. Basic training aimed to introduce the trainee to the robotic surgery system, controls, user interface, camera, and to develop initial technical robotic surgery skills. Advanced training aimed to enhance pre-existing robotic surgery skills and apply them in high-fidelity models before applying the skills in practice.
Ethical committee review
As the study involved a systematic review focused on literature analysis, it was exempt from Ethical Committee review.
Results
The search yielded a total of 1591 studies. After abstract screening and examination of full-texts, 209 studies were identified that focused on robotic surgery training and included an assessment tool (Fig. 1). Multiple training modalities, assessment tools, and objective assessment parameters were identified.
Fig. 1.
Flow diagram of study inclusions
Training modalities
Three main training systems were identified in the studies: the da Vinci Surgical System was used in 91 studies with dry lab sessions and 30 studies with wet lab sessions, 36 studies using the dV-Trainer, and 64 studies utilized the da Vinci Surgical Skills simulator (Table 1). Some studies used a combination of training modalities to compare training outcomes or the effect on learning curves, such as a combination of virtual simulation and wet lab training, or a combination of dry lab training and hands-on cadaveric training.
Table 1.
Study characteristics
| Total studies | 209 |
| Total training modalities | 255 |
| da Vinci Surgical System | 121 (121/255; 47%) |
| Dry lab | 91 |
| Wet lab | 30 |
| Animal models (porcine, chicken) | 22 |
| Cadaver training | 2 |
| Live procedure | 6 |
| dV-trainer Mimic Technologies | 36 (14%) |
| da Vinci Surgical Skills simulator (dVSS) | 64 (25%) |
| Other Robotic Training modalities | 34 (13%) |
N.B. The mentioned training modalities are based on the names used in the studies. Other Robotic Training modalities include: RobotiX Mentor, Senhance Surgical System, ProMIS and AdLap
Basic and advanced courses
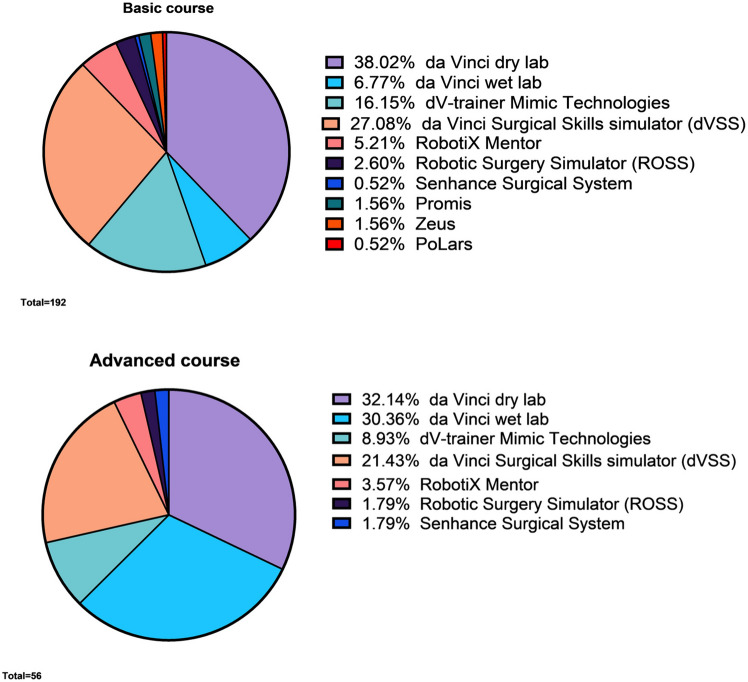
Out of the 209 studies, 165 (79%) consisted of basic courses, and 44 (21%) consisted of advanced courses. The majority of the basic courses consisted of dry lab on the da Vinci Surgical System (73/195; 37%) and a virtual simulator (83/195; 43%) (Table 2, Fig. 2 and Supplemental File A, Fig. A1). A significant proportion (n = 42) of the basic course studies involved medical students (42/165; 25%), comparing learning curves of novices between different training modalities. In 28 studies (28/165; 17%), the training group consisted exclusively of residents, in 21 studies (21/165; 13%) of experts (surgeons, urologists, and gynaecologists), and in 74 studies (74/165; 45%) there was a combination of different experience groups (novices, intermediates and experts).
Table 2.
Basic course characteristics
| Total studies | 165 (165/209; 79%) |
| Total training modalities | 195 |
| da Vinci Surgical System | 86 (86/195; 44%) |
| Dry lab | 73 |
| Wet lab | 13 |
| Animal models (porcine, chicken) | 12 |
| Cadaver training | 1 |
| dV-trainer Mimic Technologies | 31 (16%) |
| da Vinci Surgical Skills simulator (dVSS) | 52 (27%) |
| Other Robotic Training modalities | 26 (13%) |
N.B. Other Robotic Training modalities include: RobotiX Mentor, Senhance Surgical System, ProMIS and AdLap
Fig. 2.
Basic and advanced modalities
Regarding advanced courses, the largest training modality consisted of da Vinci dry lab training (18/60; 30%) and da Vinci wet lab training (17/60; 28%) on the da Vinci Surgical System (Table 3). Out of the 44 studies, 9 studies (9/44; 20%) had a training group consisting only of residents, 21 studies (21/44; 48%) had experts as participants, and 14 studies (14/44; 32%) had a combination of different experience groups.
Table 3.
Advanced course characteristics
| Total studies | 44 (44/209; 21%) |
| Total training modalities | 60 |
| da Vinci Surgical System | 35 (35/60; 58%) |
| Dry lab | 18 |
| Wet lab | 17 |
| Porcine models | 10 |
| Cadaver training | 1 |
| Live procedure | 6 |
| dV-trainer Mimic Technologies | 5 (8%) |
| da Vinci Surgical Skills simulator (dVSS) | 12 (20%) |
| Other Robotic Training modalities | 8 (13%) |
N.B. Other Robotic Training modalities include: RobotiX Mentor, Senhance Surgical System, ProMIS and AdLap
Assessment tools
A total of 44 articles were identified where robotic skills were determined using the GEARS score (Global Assessment of Robotic Skills). Other validated assessment forms commonly used in robotic surgery training included Objective Structured Assessment of Technical Skill (OSATS) and Global Operative Assessment of Laparoscopic Skills (GOALS) (Fig. 3). Vaccaro et al. [23] evaluated the effectiveness of virtual reality training in improving surgical skill, which was measured using R-OSATS, a modified OSATS-score for robotic surgery. R-OSATS was included in other studies as well [24–26]. Non-technical skills such as leadership, communication, and situational awareness were also assessed during robotic surgery training. Two reports included Non-Technical Skills for Surgeons (NOTTS) [27, 28], while other studies measured non-technical skills using Interpersonal and Cognitive Assessment for Robotic Surgery (ICARS) and Non-Technical Skills in Robotic Surgery Score (NTSRS) [29].
Fig. 3.
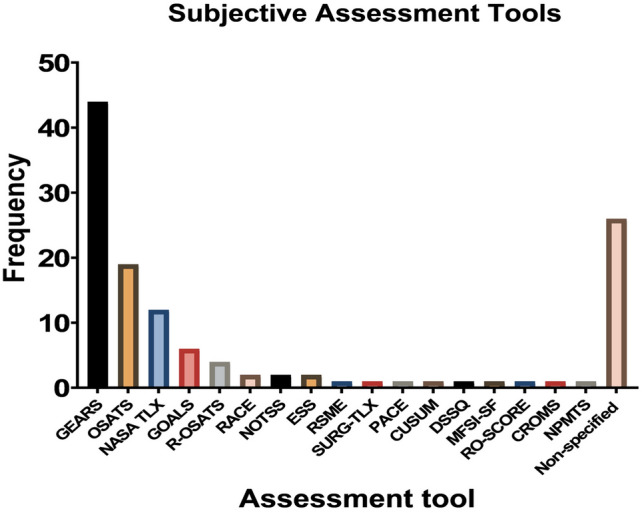
Subjective assessment tools. N.B. GEARS Global Assessment of Robotic Skills, OSATS Objective Structured Assessment of Technical Skill, NASA TLX Task Load Index, GOALS Global Operative Assessment of Laparoscopic Skills, R-OSATS Robotic—Objective Structured Assessment of Technical Skill, ESS Epworth Sleepiness Scale, NOTSS Non-Technical Skills for Surgeons, RACE Robotic Anastomosis Competence Evaluation, RSME Rating Scale for Mental Effort, SURG-TLX Task Load Index, PACE Prostatectomy Assessment and Competency Evaluation, ARCS Assessment of Robotic Console Skills, SARMS Structured Assessment of Robotic Microsurgical Skills, DSSQ Dundee Stress State Questionnaire, MFSI-SF Multidimensional Fatigue Symptom Inventory—Short Form, RO-SCORE Ottawa Surgical Competency Operating Room Evaluation, CROMS Clinically Relevant Objective Metrics of Simulators, NPMTS Numeric Psychomotor Test Score, NTSRS Non-Technical Skills in Robotic Surgery Score, ICARS Interpersonal and Cognitive Assessment for Robotic Surgery
Various subjective forms aimed to assess physical strain during robotic surgery. Overall, 12 articles measured workload using the validated NASA Task Load Index (TLX). Moore et al. [30] compared the workload between robotic-assisted tasks and laparoscopic tasks using the Rating Scale for Mental Effort (RSME) and the Surgery Task Load Index (SURG-TLX). Two articles focused on the effect of fatigue on robotic skills, where levels of fatigue were assessed using the Epworth Sleepiness Scale [31, 32].
A total of 120 articles were identified in which robotic skills were determined using a built-in scoring system that collects objective data from simulators (Fig. 4). These scoring systems were mostly available for the dVSS and the dV-trainer. The scoring systems calculated a cumulative total score based on multiple parameters such as time, motion, work space, collisions, excessive force, and instruments out of field. Total completion time was used as an objective parameter in all 120 articles, and in 13 of those (11%), the time to complete the task was the only objective outcome measured.
Fig. 4.
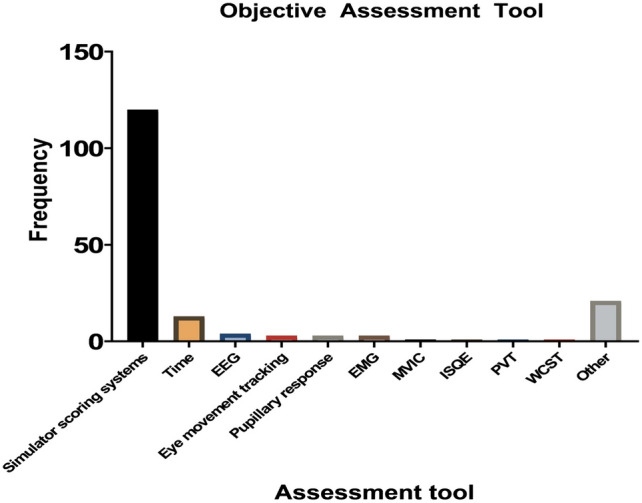
Objective assessment tools. EMG eye movements, MVIC maximum voluntary isometric muscle contractions, ISQE Indirect Sealing Quality Evaluation, PVT Psychomotor Vigilance Task, WCST Wisconsin Card Sorting Test
Multiple studies focused on cognitive metrics during robotic training. Wu et al. [33] determined the correlation between cognitive and behavioral metrics with skill level. In this study, EEG and pupillary response were indicators of cognition and workload. Other reports measured the pupil diameter as well [34, 35].
Out of the total 209 studies, 47 studies (23%) used subjective assessment methods such as assessment forms, while 102 articles (49%) used objective assessment tools (Supplemental File A, Fig. A2). 60 studies (28%) used a combination of subjective and objective assessment methods. The majority of the objective assessment methods consisted of scoring systems inherent to the specific training modality in question.
Timeline of training modalities and assessment methods
From 2005 to 2014, a majority of studies included a virtual or digital training modality. These studies primarily focused on initial learning curves, with the dominant assessment method being the dVSS built-in scoring system. Additionally, some initial studies involving dry lab were conducted, where performance was assessed using the OSATS form.
From 2014 onwards, a shifts was observed in the studies, with a primarily focus on analyzing the transfer of virtual and dry lab skills to the wet lab and operating room. Furthermore, training was not only limited solely to virtual or digital training, but a majority of the studies incorporated a second more invasive modality such as animal tissue, human cadaver labs, or direct practice in the operating room. Increasingly, studies incorporated an additional assessment form alongside the dVSS to assess both general procedural skills and procedure-specific skills. The dominant assessment form in this regard was the GEARS assessment form.
Transferability of skill
In total, 45 studies primarily focused on the transferability of simulation skills to the operating room. In 42 (93%) of the studies, the initial training and assessment consisted of virtual training on the dVSS with dVSS metrics as assessment, and dry lab sessions with GEARS assessment. Performance in the operating room was assessed either in real-time or with videos using the GEARS form. The control group received no simulation training. In 37 (82%) of the studies, simulation performance was correlated with better performance in the operating room.
Discussion
This systematic review provides a comprehensive overview of training in robotic-assisted surgery and the corresponding assessment methods. Since the da Vinci Surgical System has been the most widely used robotic surgery system in the past years, training has primarily focused on this system, particularly through dry lab training using suture pads and artificial tissue. Dry lab training offers a high-fidelity simulation environment that enables trainees to practice and hone their technical skills in a safe and controlled setting [28, 36, 37]. Box trainers enable the training of advanced technical skills with visual tissue interaction. There is an absence of haptic feedback, but this is compensated for by the visual component of instrument and tissue interaction forces. Through visual cues and tissue manipulation, a trainee learns to deal with the absence of haptic feedback. In addition, dry lab training with bio tissue and 3D-printed tissue models can also be used in conjunction with objective assessment methods, such as force measurements. This can provide valuable information about a trainee’s ability to handle and manipulate tissue during surgery, thus enhancing assessment accuracy. Assessment in box training commonly involves subjective assessment forms like the validated GEARS and OSATS forms [38–40]. While these forms have been validated by experts and have various modified versions, their generalizability is limited. Continuous validation is necessary for new trainings tasks or procedures, and assessment is dependent on the presence or post hoc evaluation of an expert, and interrater variability has to be taken into account.
The second most commonly used training modality is the da Vinci Skills Simulator (dVSS). Virtual reality (VR) training allows improvement in basic technical skills, procedural training tasks, and familiarization with the user interface, controls, and camera [41–43]]. VR training provides a convenient and cost-effective way to train and assess surgical skills. Similar to dry lab training, a challenge of VR training is the absence of haptic feedback. Current technology lacks the ability to simulate realistic virtual tissue and instrument interaction forces. As a results, the feedback and assessment incorporated in VR trainers are limited to parameters like time, motion, and task-specific errors. Another challenge of VR training is the limited availability of the master console, requiring trainees to train outside of regular working hours in the hospital. With the dVSS being a major training modality, the dVSS assessment score is also the most commonly used feedback and assessment method.
Ideally, one training modality should not replace the other due to their inherent drawbacks, but instead they should complement each other. A curriculum ideally comprises a step-by-step methodology, progressively incorporating more invasive modalities and increasing difficulty. For instance, basic training could commence with theory, including e-learning, narrated videos, or presentations. Subsequently, system training could be conducted using the digital training platform of the surgical robot to familiarize the trainee with the user interface, controls, cameras, and technicalities. Following this, the initial technical skills such as bimanual dexterity, depth perception, and tissue manipulation can be honed in the digital trainer. Upon achieving validated benchmarks, dry lab can commence. Here, objective force and motion sensors provide feedback on technical skills, while assessment tools such as GEARS for general skills and modified assessment forms for specific procedures offer personalized feedback to trainees.
Next, advanced training is conducted to train both technical and procedural skills on ex-vivo animal tissue or live animals, again with feedback provided through GEARS. Furthermore, procedural skills could also be trained and assessed with human cadaver labs. A proctored procedure can then follow, and after doing a number of cases, a learning session can be scheduled to review the experiences of the robot learning curve and evaluate tips and tricks with peers.
Another observation is the limited variety in study protocols and methods. A significant number of studies compared VR training to no training and assessed performance using subjective forms in VR or box training. However, what is lacking is a comparison of different training methods. Future training modalities should incorporate objective parameters for assessing skill acquisition and reaching preset proficiency levels. Objective feedback should not only focus on time and motion but also on tissue manipulation, handling, interaction forces on the tissue, and specific quality assessment like sutures. Furthermore, to optimize training effectiveness and efficiency, prediction models and machine learning could be used to predict the necessary training load for each trainee. Objective assessment would also enable the comparison of learning curves on different robotic-assisted surgery systems. As the number of robotic surgery systems increases, a multi-RAS platform for training and assessment will be essential for training and evaluating proficiency across different robotic systems.
Conclusion
This systematic review provides an overview of training modalities and assessment methods in robotic-assisted surgery. Dry lab training on the da Vinci Surgical System and virtual training on the da Vinci Skills Simulator are the most commonly used approaches. However, focused training on tissue handling, manipulation, and force interaction is lacking, considering the absence of haptic feedback. Future research should focus on the development of universal objective assessment and feedback methods to address these limitations as the field continues to evolve.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Dr. A. Masie Rahimi, Ezgi Uluç, Dr. Sem F. Hardon, Prof. Dr. H. Jaap Bonjer, Prof. Dr. Donald van der Peet and Dr. Freek Daams received no funding for this work.
Declarations
Disclosures
Dr. A. Masie Rahimi, Ezgi Uluç, Dr. Sem F. Hardon, Prof. Dr. H. Jaap Bonjer, Prof. Dr. Donald van der Peet and Dr. Freek Daams have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson EB. The evolution of robotic general surgery. Scand J Surg. 2009;98(2):125–129. doi: 10.1177/145749690909800208. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed K, Khan MS, Vats A, Nagpal K, Priest O, Patel V, et al. Current status of robotic assisted pelvic surgery and future developments. Int J Surg. 2009;7(5):431–440. doi: 10.1016/j.ijsu.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Ashrafian H, Clancy O, Grover V, Darzi A. The evolution of robotic surgery: surgical and anaesthetic aspects. Br J Anaesth. 2017;119(December):i72–84. doi: 10.1093/bja/aex383. [DOI] [PubMed] [Google Scholar]
- 4.Rassweiler J, Hruza M, Teber D, Su LM. Laparoscopic and robotic assisted radical prostatectomy—critical analysis of the results. Eur Urol. 2006;49(4):612–624. doi: 10.1016/j.eururo.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 5.Rocco B, Albo G, Coelho RF. From leonardo to da Vinci: the history of robot-assisted surgery in urology. BJU Int. 2011;108(11):1714. doi: 10.1111/j.1464-410X.2011.10600.x. [DOI] [PubMed] [Google Scholar]
- 6.Abboudi H, Khan MS, Aboumarzouk O, Guru KA, Challacombe B, Dasgupta P, et al. Current status of validation for robotic surgery simulators a systematic review. BJU Int. 2013;111(2):194–205. doi: 10.1111/j.1464-410X.2012.11270.x. [DOI] [PubMed] [Google Scholar]
- 7.Sevdalis N, Hull L, Birnbach DJ. Improving patient safety in the operating theatre and perioperative care: obstacles, interventions, and priorities for accelerating progress. Br J Anaesth. 2012;109(SUPPL1):i3–16. doi: 10.1093/bja/aes391. [DOI] [PubMed] [Google Scholar]
- 8.Albani JM, Lee DI. Virtual reality-assisted robotic surgery simulation. J Endourol. 2007;21(3):285–287. doi: 10.1089/end.2007.9978. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher AG, Traynor O. Simulation in surgery: opportunity or threat? Ir J Med Sci. 2008;177(4):283–287. doi: 10.1007/s11845-008-0204-5. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee M, Siu KC, Suh IH, Klutman A, Oleynikov D, Stergiou N. A virtual reality training program for improvement of robotic surgical skills. Stud Health Technol Inform. 2009;142:210–214. [PubMed] [Google Scholar]
- 11.Suh IH, Siu KC, Mukherjee M, Monk E, Oleynikov D, Stergiou N. Consistency of performance of robot-assisted surgical tasks in virtual reality. Stud Health Technol Inform. 2009;142:369–373. [PubMed] [Google Scholar]
- 12.Brown-Clerk B, Siu KC, Katsavelis D, Lee I, Oleynikov D, Stergiou N. Validating advanced robot-assisted laparoscopic training task in virtual reality. Stud Health Technol Inform. 2008;132:45–49. [PubMed] [Google Scholar]
- 13.Kaul S, Shah NL, Menon M. Learning curve using robotic surgery. Curr Urol Rep. 2006;7(2):125–129. doi: 10.1007/s11934-006-0071-4. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez JD, Bann SD, Munz Y, Moorthy K, Datta V, Martin S, et al. Qualitative and quantitative analysis of the learning curve of a simulated surgical task on the da Vinci system. Surg Endosc Other Interv Tech. 2004;18(3):372–378. doi: 10.1007/s00464-003-9047-3. [DOI] [PubMed] [Google Scholar]
- 15.Curry M, Malpani A, Li R, Tantillo T, Jog A, Blanco R, et al. Objective assessment in residency-based training for transoral robotic surgery. Laryngoscope. 2012;122(10):2184–2192. doi: 10.1002/lary.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AA, Bandari J, Pelzman D, Davies BJ, Jacobs BL. Diffusion and adoption of the surgical robot in urology. Transl Androl Urol. 2021;10(5):2151–2157. doi: 10.21037/tau.2019.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun AJ, Aron M, Hung AJ. Novel training methods for robotic surgery. Indian J Urol. 2014;30(3):333–338. doi: 10.4103/0970-1591.128506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubin AK, Smith R, Julian D, Tanaka A, Mattingly P. A comparison of robotic simulation performance on basic virtual reality skills: simulator subjective versus objective assessment tools. J Minim Invasive Gynecol. 2017;24(7):1184–1189. doi: 10.1016/j.jmig.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Kutana S, Bitner DP, Addison P, Chung PJ, Talamini MA, Filicori F. Objective assessment of robotic surgical skills: review of literature and future directions. Surg Endosc. 2022;36(6):3698–3707. doi: 10.1007/s00464-022-09134-9. [DOI] [PubMed] [Google Scholar]
- 20.Chen IHA, Ghazi A, Sridhar A, Stoyanov D, Slack M, Kelly JD, et al. Evolving robotic surgery training and improving patient safety, with the integration of novel technologies. World J Urol. 2021;39(8):2883–2893. doi: 10.1007/s00345-020-03467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judkins TN, Oleynikov D, Stergiou N. Objective evaluation of expert and novice performance during robotic surgical training tasks. Surg Endosc Other Interv Tech. 2009;23(3):590–597. doi: 10.1007/s00464-008-9933-9. [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaccaro CM, Crisp CC, Fellner AN, Jackson C, Kleeman SD, Pavelka J. Robotic virtual reality simulation plus standard robotic orientation versus standard robotic orientation alone: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2012;18(5):S57. doi: 10.1097/SPV.0b013e3182a09101. [DOI] [PubMed] [Google Scholar]
- 24.Newcomb LK, Bradley MS, Truong T, Tang M, Comstock B, Li Y-JJ, et al. Correlation of virtual reality simulation and dry lab robotic technical skills. J Minim Invasive Gynecol. 2018;25(4):689–696. doi: 10.1016/j.jmig.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui NY, Galloway ML, Geller EJ, Green IC, Hur H-C, Langston K et al (2014) Validity and reliability of the robotic objective structured assessment of technical skills. Obs Gynecol 123:1193–1199. Available from: http://links.lww.com/AOG/A498 [DOI] [PMC free article] [PubMed]
- 26.Siddiqui NY, Tarr ME, Geller EJ, Advincula AP, Galloway ML, Green IC, et al. Establishing benchmarks for minimum competence with dry lab robotic surgery drills. J Minim Invasive Gynecol. 2016;23(4):633–638. doi: 10.1016/j.jmig.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Raison N, Ahmed K, Abe T, Brunckhorst O, Novara G, Buffi N, et al. Cognitive training for technical and non-technical skills in robotic surgery: a randomised controlled trial. BJU Int. 2018;122(6):1075–1081. doi: 10.1111/bju.14376. [DOI] [PubMed] [Google Scholar]
- 28.Raison N, Wood T, Brunckhorst O, Abe T, Ross T, Challacombe B, et al. Development and validation of a tool for non-technical skills evaluation in robotic surgery—the ICARS system. Surg Endosc. 2017;31(12):5403–5410. doi: 10.1007/s00464-017-5622-x. [DOI] [PubMed] [Google Scholar]
- 29.Manuguerra A, Mazeaud C, Hubert N, Eschwège P, Roumiguié M, Salleron J, et al. Non-technical skills in robotic surgery and impact on near-miss events: a multi-center study. Surg Endosc. 2021;35(9):5062–5071. doi: 10.1007/s00464-020-07988-5. [DOI] [PubMed] [Google Scholar]
- 30.Moore LJ, Wilson MR, McGrath JS, Waine E, Masters RSW, Vine SJ. Surgeons’ display reduced mental effort and workload while performing robotically assisted surgical tasks, when compared to conventional laparoscopy. Surg Endosc. 2015;29(9):2553–2560. doi: 10.1007/s00464-014-3967-y. [DOI] [PubMed] [Google Scholar]
- 31.Mark JR, Kelly DC, Trabulsi EJ, Shenot PJ, Lallas CD. The effects of fatigue on robotic surgical skill training in Urology residents. J Robot Surg. 2014;8(3):269–275. doi: 10.1007/s11701-014-0466-z. [DOI] [PubMed] [Google Scholar]
- 32.Robison W, Patel SK, Mehta A, Senkowski T, Allen J. Can fatigue affect acquisition of new surgical skills? A prospective trial of pre- and post-call general surgery residents using the da Vinci surgical skills simulator. Surg Endosc. 2018;32:1389–1396. doi: 10.1007/s00464-017-5820-6. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Cha J, Sulek J, Zhou T, Sundaram CP, Wachs J, et al. Eye-tracking metrics predict perceived workload in robotic surgical skills training. Hum Factors. 2020;62(8):1365–1386. doi: 10.1177/0018720819874544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen JH, Chen J, Marshall SP, Ghodoussipour S, Chen A, Gill IS, et al. Using objective robotic automated performance metrics and task-evoked pupillary response to distinguish surgeon expertise. World J Urol. 2020;38(7):1599–1605. doi: 10.1007/s00345-019-02881-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowan A, Chen J, Mingo S, Reddy SS, Ma R, Marshall S, et al. Virtual reality vs dry laboratory models: comparing automated performance metrics and cognitive workload during robotic simulation training. J Endourol. 2021;35(10):1571–1576. doi: 10.1089/end.2020.1037. [DOI] [PubMed] [Google Scholar]
- 36.Ghazi A, Melnyk R, Hung AJ, Collins J, Ertefaie A, Saba P, et al. Multi-institutional validation of a perfused robot-assisted partial nephrectomy procedural simulation platform utilizing clinically relevant objective metrics of simulators (CROMS) BJU Int. 2020;127(6):645–653. doi: 10.1111/bju.15246. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi S, Cho B, Huaulmé A, Tatsugami K, Honda H, Jannin P, et al. Assessment of surgical skills by using surgical navigation in robot-assisted partial nephrectomy. Int J Comput Assist Radiol Surg. 2019;14(8):1449–1459. doi: 10.1007/s11548-019-01980-8. [DOI] [PubMed] [Google Scholar]
- 38.Dilley J, Singh H, Pratt P, Omar I, Darzi A, Mayer E. Visual behaviour in robotic surgery-Demonstrating the validity of the simulated environment. Int J Med Robot. 2020;16(2):e2075. doi: 10.1002/rcs.2075. [DOI] [PubMed] [Google Scholar]
- 39.Egi H, Hattori M, Tokunaga M, Suzuki T, Kawaguchi K, Sawada H, et al. Face, content and concurrent validity of the Mimic® dV-Trainer for robot-assisted endoscopic surgery: a prospective study. Eur Surg Res. 2013;50(3–4):292–300. doi: 10.1159/000353435. [DOI] [PubMed] [Google Scholar]
- 40.Obek C, Hubka M, Porter M, Chang L, Porter JR. Robotic versus conventional laparoscopic skill acquisition: implications for training. J Endourol. 2005;19(9):1098–1103. doi: 10.1002/central/CN-00768239/full. [DOI] [PubMed] [Google Scholar]
- 41.Vaccaro CM, Crisp CC, Fellner AN, Jackson C, Kleeman SD, Pavelka J. Robotic virtual reality simulation plus standard robotic orientation versus standard robotic orientation alone: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2013;19(5):266–270. doi: 10.1097/SPV.0b013e3182a09101. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Zhang C, Guo F, Sheng X, ji J, Xu Y, Cao Z, Lyu J, Lu X, Yang B. The application of virtual reality training for anastomosis during robot-assisted radical prostatectomy. Asian J Urol. 2020;8(2):204–208. doi: 10.1016/j.ajur.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almarzouq A, Hu J, Noureldin YA, Yin A, Anidjar M, Bladou F, et al. Are basic robotic surgical skills transferable from the simulator to the operating room? A randomized, prospective, educational study. Can Urol Assoc J. 2020 doi: 10.1002/central/CN-02193571/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.