Abstract
Γδ T cell infiltration into tumours usually correlates with improved patient outcome, but both tumour-promoting and tumoricidal effects of γδ T cells have been documented. Human γδ T cells can be divided into functionally distinct subsets based on T cell receptor (TCR) Vδ usage. Still, the contribution of these different subsets to tumour immunity remains elusive. Here, we provide a detailed γδ T cell profiling in colon tumours, using mass and flow cytometry, mRNA quantification, and TCR sequencing. δ chain usage in both the macroscopically unaffected colon mucosa and tumours varied considerably between patients, with substantial fractions of Vδ1, Vδ2, and non-Vδ1 Vδ2 cells. Sequencing of the Vδ complementarity-determining region 3 showed that almost all non-Vδ1 Vδ2 cells used Vδ3 and that tumour-infiltrating γδ clonotypes were unique for every patient. Non-Vδ1Vδ2 cells from colon tumours expressed several activation markers but few NK cell receptors and exhaustion markers. In addition, mRNA analyses showed that non-Vδ1 Vδ2 cells expressed several genes for proteins with tumour-promoting functions, such as neutrophil-recruiting chemokines, Galectin 3, and transforming growth factor-beta induced. In summary, our results show a large variation in γδ T cell subsets between individual tumours, and that Vδ3 cells make up a substantial proportion of γδ T cells in colon tumours. We suggest that individual γδ T cell composition in colon tumours may contribute to the balance between favourable and adverse immune responses, and thereby also patient outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03758-7.
Keywords: γδ T cells, Colon cancer, Tumour immunity, TCRδ chain
Background
Γδ T cells are unconventional T cells expressing a semi-variable T cell receptor (TCR) composed of a limited selection of γ and δ chains, which bind to invariant MHC I-like molecules, as well as other stress-induced cell surface proteins. Cognate TCR binding leads to immediate effector functions, such as cytotoxicity and cytokine secretion. Human γδ T cells are usually characterized based on δ chain usage, where Vδ1, Vδ2, and Vδ3 are the most common. Furthermore, the preferential pairing of different δ and γ chains divide γδ T cells into additional subsets [1, 2]. Oligoclonal populations of γδ T cells are present in different tissues, such as mucosal tissues, skin, and peripheral blood [3]. In humans, Vδ2 cells dominate in the circulation, while Vδ1 cells are more common in the intestinal mucosa [1–3]. In addition to the TCR, both Vδ1 and Vδ2 cells express various NK cell receptors that react to the expression of surface molecules induced in both infected and transformed cells. Especially the expression of NKp30 and NKp46 has been shown to delineate subsets of γδ T cells with increased cytotoxic capacity towards tumour cells [4, 5]. When activated, γδ T cells also produce pro-inflammatory cytokines in addition to their cytotoxic functions [6]. In a cancer setting, the infiltration of γδ T cells has been associated with an improved clinical outcome in studies across several types of haematological malignancies and solid tumours, including colorectal cancer (CRC) [7–9]. However, in studies with a CRC focus, γδ T cells were both positively and negatively correlated to a favourable patient outcome [8, 10, 11]. Generally, anti-tumour immunity and a beneficial patient response are commonly associated with cytotoxicity and the production of Th1 type cytokines [12–14]. As conventional T cells, γδ T cells can be divided into different subsets based on cytokine production. In tumour immunity, the two best described are γδ1 and γδ17 cells, with a cytokine profile similar to Th1 and Th17 cells, respectively, and the proportions of these cells detected in different studies vary considerably [15, 16].
There is currently a lack of understanding of which γδ T cell subsets contribute to a pro- or anti-tumour immune response, and how they distribute in individual tumours. In this study, we could show that γδ T cells-infiltrating colon tumours express Vδ1, Vδ2, or Vδ3 TCRδ chains and that these subsets are distinct from circulating γδ T cells. The proportions of these cells varied considerably among tumours, as did the clonotypes detected, which were all private to a single tumour. We identified a substantial presence of Vδ3 cells in colon tumours which had reduced anti-tumour effector functions and expressed several tumour-promoting mediators.
Material and methods
Patient samples
This study was performed at the Sahlgrenska Academy at the University of Gothenburg together with the Sahlgrenska University Hospital. All procedures and experiments were performed in accordance with the Declaration of Helsinki and were approved by the Regional Research Ethics Committee of western Sweden (reference no 249–15). Venous blood, macroscopically unaffected colon mucosa (collected at least 10 cm away from the tumour border), and tumour tissue were collected from 45 colon cancer patients (25 males and 20 females, aged 38 to 90, median age 75) undergoing resection surgery for stage I–IV tumours. Cells from 15 of these patients were used for mass cytometry, 27 for flow cytometry analyses, and 3 for both mass and flow cytometry. See Suppl. Table S1 for additional patient and tumour characteristics. In a separate set of 10 patients, comprising 7 males and 3 females, aged 51–89 (Suppl. Table S1), we analysed the TCR repertoire in resected tumour tissues. None of the patients had undergone radio- or chemotherapy during the last 2 years. Microsatellite status was determined as previously described using the microsatellite instability (MSI) Analysis System v.1.2 (ProMega) [17]. MSI-High (MSI-H) tumours were defined as tumours with more than 1 marker showing instability, MSI-Low (MSI-L) as tumours with one marker showing instability, and microsatellite stable (MSS) tumours as tumours with no markers showing instability.
Cell isolation and stimulation
The tissue material was collected during surgery and transported in ice-cold PBS before isolation of lymphocytes within two hours, and lamina propria lymphocytes were isolated as previously described [18]. Venous blood samples were collected in heparinized tubes during surgery, and peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Ficoll-Paque (GE Healthcare Bio-Sciences AB).
Enrichment of CD45+ cells was performed before mass cytometry using the REAlease TIL MicroBead kit (Miltenyi). Cells were kept overnight at 37 °C for functional assays and mass cytometry analysis or at 4 °C for phenotypic analysis using flow cytometry. For cytokine production analyses, cells were incubated overnight in culture medium at 37 °C before polyclonal stimulation the following morning using 50 ng/mL of phorbol 12-myristate 13-acetate (PMA) and 680 ng/mL of ionomycin calcium salt (Sigma-Aldrich) for 4 h, together with a protein transport inhibitor (BD Golgi stop, BD Biosciences).
γδ TCR sequencing
Resected tissue samples were cut into smaller pieces and immediately frozen and stored in liquid nitrogen in advanced DMEM/F12 (Thermo Fisher) substituted with 100 U/ml of Penicillin and 100 µg /ml of Streptomycin, 10 mM HEPES, Glutamax according to the supplier’s recommendation (Gibco), and 10% dimethylsulphoxid (DMSO) until isolation of lymphocytes as previously described [19]. γδ TCR sequencing was performed on DNA extracted from the isolated lymphocytes with the QIAamp Blood Mini Kit (Qiagen), according to the manufacturer’s instructions. A total of 1.5 µg DNA was analysed using SiMSen-Seq [20], except for one patient (Patient#405) where only 1 µg DNA was available. Library quantity and size distribution were assessed on a Fragment Analyzer using HS NGS Fragment kit (Agilent Technologies). The libraries were pooled at equimolar concentration and purified with a Pippin Prepp using 2% agarose gel reagent kit (Sage Science). Final libraries were quantified with quantitative PCR and then sequenced on the MiniSeq Sequencing System using paired-end and 2 times 150 bp sequencing (Illumina). The raw sequencing data in fastq format have been deposited to the NCBI short read archive (SRA; https://www.ncbi.nlm.nih.gov/sra) with accession number PRJNA1107040.
The raw sequencing reads for γδ TCR sequencing were analysed with the MIGEC bioinformatics pipeline [21], including unique molecular identifier extraction, consensus read assembly, and annotation of the complementarity-determining region 3 (CDR3) region including annotation of V, D, and J segments, by blasting to known CDR3 sequences.
Mass cytometry
Mass cytometry analysis was performed as previously described [22], using live cell barcoding with CD45 antibodies conjugated to different isotopes to individually label cells from blood, unaffected colon mucosa, and tumour samples [23]. For a detailed antibody list, see Suppl. Table S2.
Flow cytometry
Single cell suspensions were stained with a live/dead exclusion dye followed by antibodies to surface antigens. For detection of cytokines and GrB, cells were fixed and permeabilised using the FoxP3 staining kit (eBioscience). For a detailed antibody list, see Suppl. Table S3. The samples were acquired on a BD LSR Fortessa. Samples with fewer than 50 cells of any of the investigated subsets (Vδ1, Vδ2, and non-Vδ1Vδ2 cells) were not included in the phenotypic or functional analyses.
mRNA quantification
Live Vδ1, Vδ2, and non-Vδ1Vδ2 cells from 4 colon tumours were sorted using a BD FACS-Aria Fusion. Multiplex mRNA quantification was performed using the nCounter Analysis system together with the nCounter human Immunology v2 panel (Nanostring) at KIGene (Karolinska Institutet, Stockholm). Nanostring data were normalized to adjust for platform-associated and sample input variations and thresholds were set according to Nanostring guidelines (0, 3–3 for the positive control normalization and 0, 1–10 for the housekeeping gene normalization). The Vδ1 cells from one patient were subsequently excluded from analysis due to low RNA quality. The normalized data have been deposited to Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) with accession number GSE266504.
Immunofluorescence
Four-μm cuts from formalin-fixed paraffin-embedded tissue blocks of unaffected mucosa and colorectal tumours were mounted on Superfrost Plus microscope slides. Sections were deparaffinized and rehydrated, and antigens were unmasked with pH9 Tris–EDTA buffer. Tissue was stained with CD3 (A0452, Agilent; Opal 570), CD8α (SP16, Thermo Fisher Scientific; Opal 620), TCRδ (H41, Santa Cruz Biotechnologies; Opal 690), pan-cytokeratin (KRT/1877R, Abcam; Opal 520), respectively, using the Opal Polaris 7-Color Manual IHC Kit (Akoya Biosciences). Subsequently, nuclei were stained with spectral DAPI (Akoya Biosciences) and slides were mounted with ProLong Glass Antifade Mounting media (Thermo Fisher Scientific). Tissue sections were scanned with the Metafer Slide Scanning Platform (Axio Imager.Z2 Microscope and 20x/0.8/air objective, Zeiss) equipped with a SpectraSplit filter system (Kromnigon). Images were analysed with Strataquest (TissueGnostics).
Data processing and statistical analysis
Mass cytometry data were analysed using OMIQ version 10. All clusters that contributed with less than 1% of all γδ T cells were excluded from the analysis. Data from the multiplex mRNA quantification were analysed using the Nsolver software (version 4). Flow cytometry data were analysed using FlowJo version 10 and OMIQ version 10. Gini-Simpson diversity index was calculated using the Diverse package in R (version 0.1.5). Statistical analyses of paired data were performed using two-tailed Wilcoxon matched-pairs signed rank test and of unpaired data using two-tailed Mann–Whitney test. When comparing three groups of matched data, the Friedman test followed by Dunn’s post-test was used to achieve multiplicity adjusted P values. Statistical tests were performed using GraphPad PRISM version 9. P-values < 0.05 were considered statistically significant.
Results
γδ T cells in colon tumours
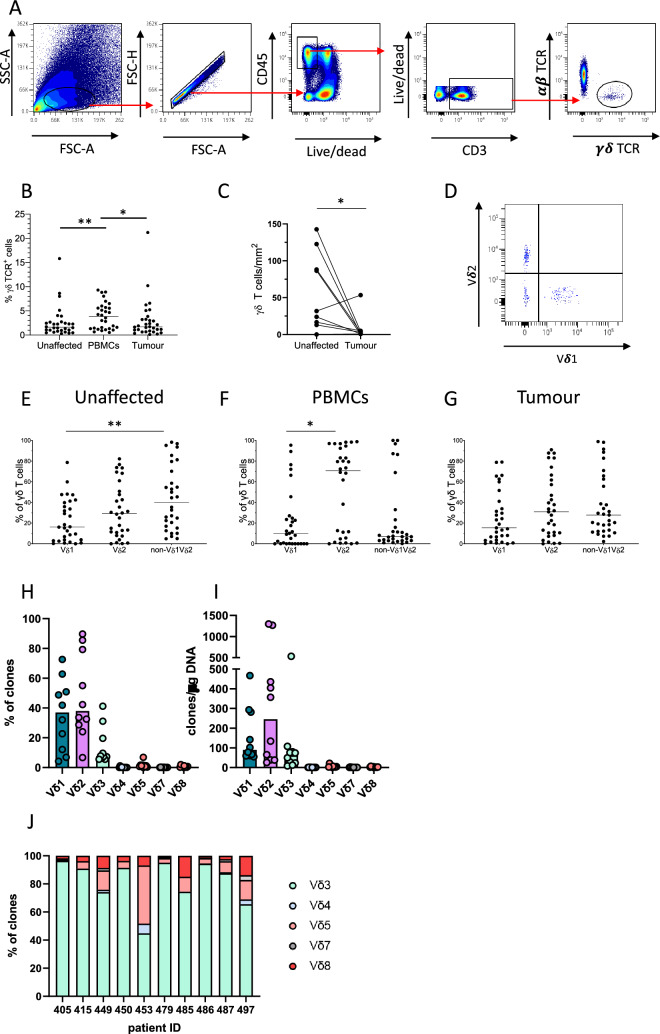
To investigate the subsets of γδ T cells present in colon tumours, we used fresh samples recovered from patients undergoing resection surgery. Using flow cytometry, γδ T cells were identified as CD3+ cells stained by a pan-γδ-TCR antibody but not by a pan-αβ-TCR antibody (Fig. 1A). γδ T cell frequencies were significantly lower in both the tumours and the macroscopically unaffected colon compared to in the blood (Fig. 1B). Using fluorescence microscopy, we also analysed the numbers of γδ T cells present in the tumours and unaffected colon mucosa. Here, the numbers of γδ T cells were also significantly reduced (p < 0.05) in the tumour compared to the unaffected colon mucosa from the same individuals (Fig. 1C, Suppl. Fig. S1). These analyses also showed that γδ T cells in the tumours were primarily positioned in the stroma rather than in the tumour epithelium.
Fig. 1.
Identification of tumour-infiltrating γδ T cells. Single cell suspensions were isolated from tumours, corresponding unaffected colon mucosa, and blood, and the frequencies of γδ T cells among the CD3+ T cells were analysed using flow cytometry, immunofluorescence, and CDR3 sequencing. A Gating strategy from a representative tumour sample. B Frequencies of γδ T cells among all CD3+ lymphocytes determined by flow cytometry. C Density of γδ T cells determined by fluorescence microscopy in sections from formalin-fixed tumours and corresponding unaffected colon mucosa. D Flow cytometry staining of Vδ1 and Vδ2 in a representative tumour sample of γδ T cells gated as in (a). E–G Usage of the Vδ1 and Vδ2 chains by γδ T cells was determined by flow cytometry in cell suspensions from unaffected colon mucosa (E), blood (F), and tumour (G). H Vδ chain usage was determined by CDR3 sequencing in γδ T cells isolated from colon tumours and the percentage of clones using the respective Vδ segments or (I) the number of clones using each Vδ segment per μg of DNA is shown for each patient. In (I) values less than 1 were set at 1 to improve visualization. J Distribution of non-Vδ1Vδ2 clones in the individual tumours. Symbols represent individual values and lines the median. In (C), symbols are connected to show corresponding values from the same patients. Data in (B), (E), (F), and (G) were analysed using two-tailed Friedman test followed Dunn’s post-test and in (C) using two-tailed Wilcoxon test. *p < 0.05 and ** < 0.01. n = 10 for immunofluorescence and CDR3 sequencing, and n = 30 for flow cytometry analyses
To further define the TCRs of tumour-infiltrating γδ T cells, we analysed the Vδ chain usage (Fig. 1D). The dominating subset in the circulation was Vδ2 cells. In the tissue, there were also considerable numbers of Vδ2 cells in both unaffected mucosa and tumours, while Vδ1 cells were less numerous in most patients. We could also document a substantial proportion of γδ T cells that did neither express Vδ1 nor Vδ2. These non-Vδ1Vδ2 cells were present in both tumour and unaffected mucosa from all patients (Fig. 1E–G). Quantitative Vδ CDR3 sequencing analyses clearly showed that the large majority of non-Vδ1 Vδ2 cells in the tumours used Vδ3. Only one out of ten patients displayed a sizeable Vδ5 population alongside the Vδ3 cells (Fig. 1H, I, J).
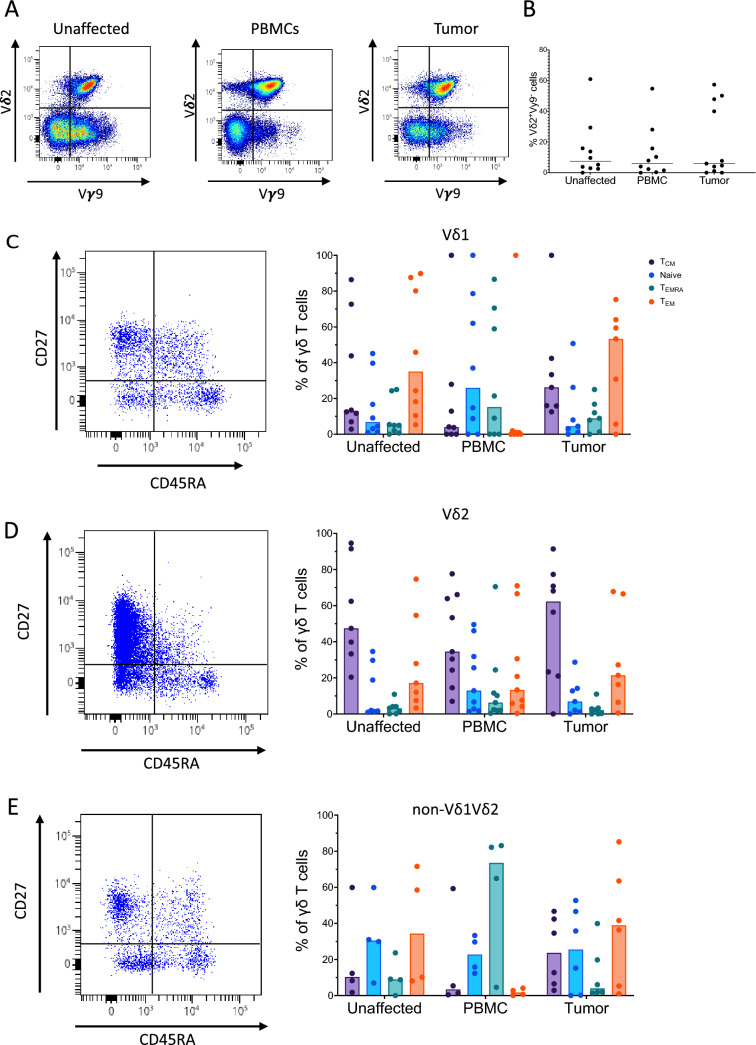
Vδ2 cells are divided into two main types based on their usage of the Vγ9 chain. The classical, innate-like Vγ9+Vδ2+ cells are the most common, while the rarer Vγ9−Vδ2+ cells have been described as a more adaptive-like cell type with a more diverse TCR [24]. γδ T cells expressing the Vγ9 chain were common in all tissues, and Vγ9 was most commonly paired with Vδ2 (Fig. 2A). However, we also found fractions of both Vδ1 and non-Vδ1Vδ2 cells in all tissues that expressed the Vγ9 chain (Suppl. Fig. S2). Vγ9−Vδ2+ cells were present to some extent in tissue samples and blood from most patients (Fig. 2B). We also detected low to moderate expression of CD8 in all subsets of γδ T cells investigated in all the tissues examined (Suppl Fig. S3).
Fig. 2.
Phenotype of tumour-infiltrating γδ T cells. Single cell suspensions were isolated from tumours, corresponding unaffected colon mucosa, and blood, and analysed using flow cytometry. A Vδ2 and Vγ9 staining in an unaffected tissue, blood, and tumour from a representative patient. B Frequencies of Vγ9− cells among all Vδ2 cells from the different tissues. C–E The frequencies of central memory, naïve, terminally differentiated effector memory, and effector memory cells among the Vδ1 (C), Vδ2 (D), and non-Vδ1Vδ2 (E) γδ T cells are shown alongside dot plots of Vδ1, Vδ2, and non-Vδ1Vδ2 cells from tumour tissue. Symbols represent individual values and lines and bars the median. n = 4–9
Different naïve and memory populations of γδ T cells can be distinguished based on the expression of CD45RA and CD27, defining naïve (CD45RA+CD27+), central memory (TCM, CD45RA−CD27+), effector memory (TEM, CD45RA−CD27−), and terminally differentiated effector memory (TEMRA, CD45RA+CD27−) cells [25]. This classification was originally devised for conventional αβ T cells and may not be directly applicable to γδ T cells, but we have used it here for convenience. The Vδ1 cells in the tumours and unaffected mucosa were usually dominated by TEM cells, while circulating Vδ1 cells were dominated by naïve and TEMRA cells (Fig. 2C). Vδ2 cells were similar to each other in all the examined locations and dominated by cells with a TCM phenotype (Fig. 2D). In the non-Vδ1Vδ2 cells in the colon mucosa and the tumours, the naïve cells were more prominent than in Vδ1 and Vδ2, and there was also a strong component of TEM cells in the non-Vδ1Vδ2 subset. In addition, the circulating non-Vδ1Vδ2 cells were dominated by TEMRA cells (Fig. 2E).
Taken together, these results show that γδ T cells do not infiltrate colon tumours to the same extent as the surrounding unaffected colon mucosa, but that there is a prominent subset of non-Vδ1Vδ2 cells primarily made up of Vδ3 cells in the tumours.
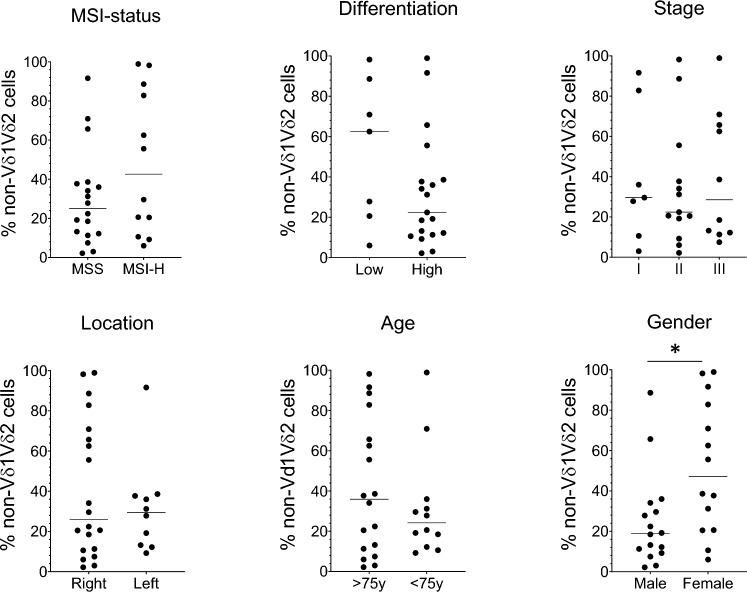
Infiltration of non-Vδ1Vδ2 cells in relation to clinicopathologic features
As the size of the non-Vδ1Vδ2 subset varied considerably between patients, we were interested to relate their presence to clinicopathologic features. However, in this relatively small material there was no correlation between non-Vδ1Vδ2 cell proportions and MSS/MSI status, tumour differentiation, stage, location (right vs left sided), or patient age. Only when comparing men and women could we find a significantly higher proportion of non-Vδ1Vδ2 cells in the tumours from female patients (Fig. 3).
Fig. 3.
Tumour-infiltrating non-Vδ1Vδ2 cells and tumour characteristics. Single cell suspensions were isolated from tumours, and the frequencies of non-Vδ1Vδ2 cells among the γδ T cells were determined by flow cytometry and related to MSI status, tumour differentiation, stage, and location, and patient age and gender. Symbols represent individual values and lines the median. Data were analysed using two-tailed Wilcoxon test. *p < 0.05. n = 30
Clonality of tumour-infiltrating γδ T cells
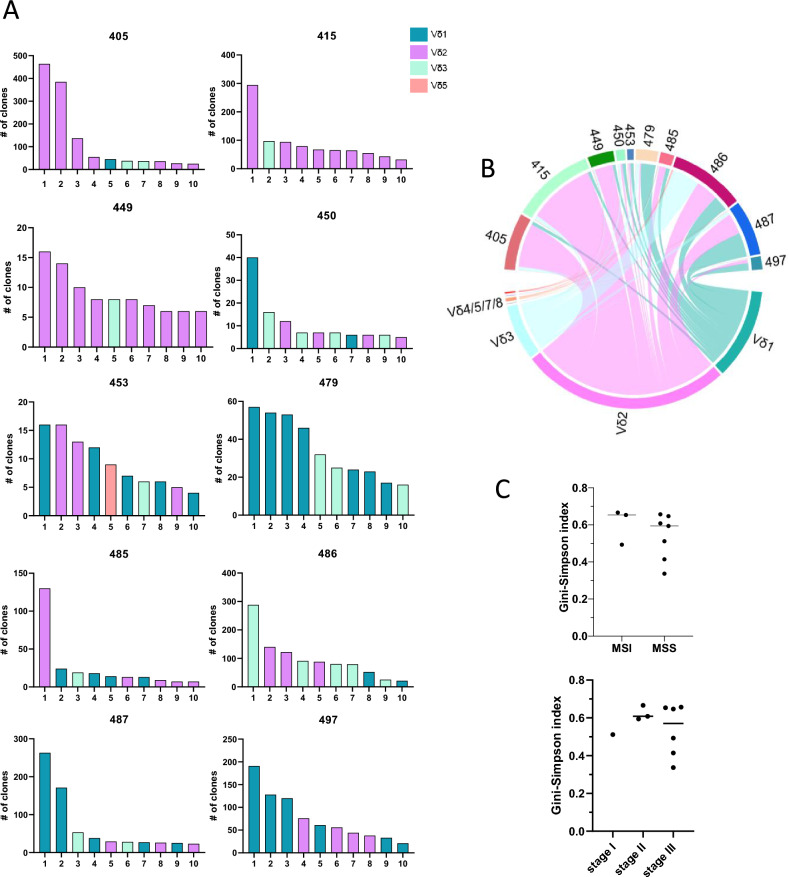
To determine clonality, and the potential of public, shared γδ clonotypes between patients, the δ chain CDR3 sequence was analysed. δ chain sequencing of 10 colon tumours resulted in a total of 9,403 productive recombinations, representing individual cells, where the CDR3 sequence was reliably determined, ranging from 175 to 2,167 recombinations from individual tumours. These recombinations were distributed between 2,092 clonotypes, containing between 1 and 464 recombinations per clonotype. The distribution of clonotypes differed markedly between individual tumours (Fig. 4A). Of note, the dominating clonotypes were either Vδ1, Vδ2, or Vδ3 in different tumours. However, in all but one of the patients, Vδ3 cells made up one to five of the ten dominating clonotypes (Fig. 4A, B). Unfortunately, we did not have access to unaffected tissue and blood from these individuals and could thus not investigate to which extent these clones were present in healthy tissues. The difference in γδ T cells between individual tumours was also reflected in the Gini-Simpson diversity index, which varied between 0.49 and 0.77 in the different tumours. There was no difference in diversity between cells from MSI-L/H and MSS tumours or between different stage tumours (Fig. 4C). Furthermore, there was no overlap between the clonotypes found in any patients, further emphasizing the large interindividual variation in γδ T cell composition between patients.
Fig. 4.
Vδ chain usage in tumour-infiltrating γδ T cells. Single cell suspensions were isolated from frozen tumour specimens and the CDR3 region analysed with ultra-sensitive sequencing using unique molecular identifiers. A The number of clones in the ten most frequent clonotypes from each patient. Colour coding shows the Vδ usage in the respective clonotypes. B Chord diagram showing the distribution of clones using the different Vδ chains in individual patients. C Gini-Simpson index of diversity was calculated for each tumour and plotted as a function of microsatellite status and tumour stage. Symbols represent individual values and the line the median. n = 10
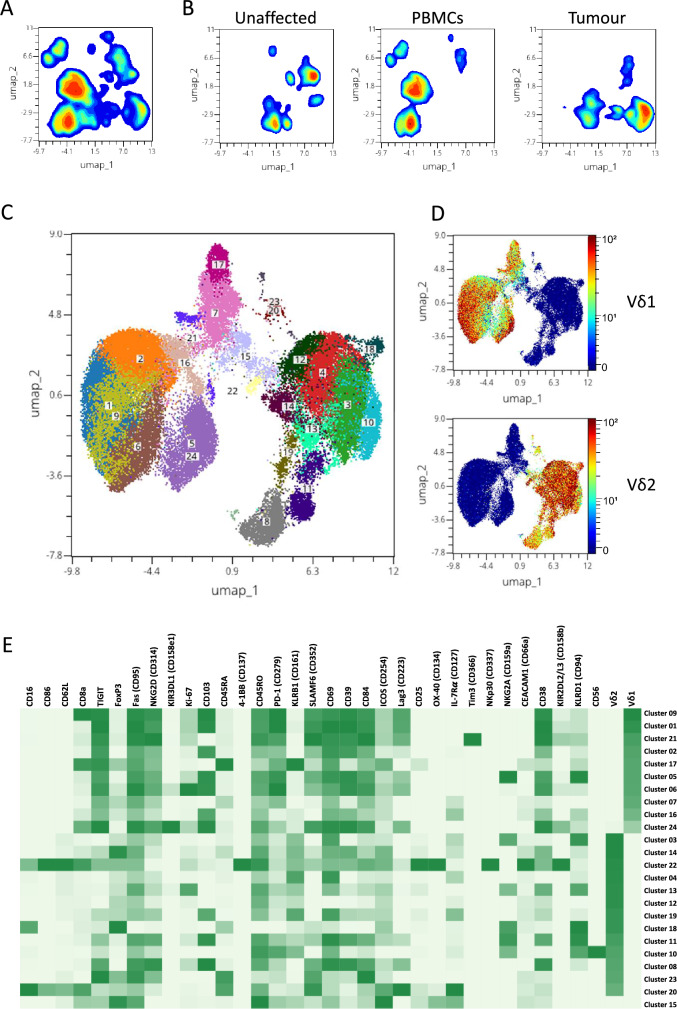
Mass cytometry and mRNA quantification reveal diverse clusters of tumour-infiltrating γδ T cells
To gain additional understanding of the different subsets of γδ T cells beyond δ chain usage, we employed a panel of antibodies focused on cytotoxicity and exhaustion markers and analysed ex-vivo isolated T cells using mass cytometry. γδ T cells were manually gated as live CD45+CD3+CD4−TCR γδ+ cells, and unsupervised dimensional reduction of the aggregated data from all patients was performed using the UMAP algorithm, followed by clustering using the phenograph algorithm. Initially, we clustered 59,110 γδ T cells from tumours, 38,275 from unaffected colon mucosa, and 204,561 from PBMC collected from 18 patients (Fig. 5A). From these analyses, it was clear that γδ T cells from blood and the colon tissue formed distinct clusters with low or no overlap (Fig. 5B). As the tumour-infiltrating lymphocytes presumably are the most relevant for anti-tumour immunity, we subsequently focused on their phenotype and effector functions. We thus performed unsupervised analysis of 59,110 tumour-infiltrating γδ T cells (Fig. 5C). Based on the expression of the Vδ1 or Vδ2 chain, 3 distinct groups containing Vδ1, Vδ2, and non-Vδ1Vδ2 cells were observed (Fig. 5D) In the tumours, we could identify 10 clusters of Vδ1 cells, 13 clusters of Vδ2 cells, and a single cluster of non-Vδ1Vδ2 cells (cluster 15). Expression of individual markers across the UMAP projection is shown in Suppl. Fig. S4. The contribution of cells from individual tumours to a certain cluster differed. Most clusters were made up of cells from all the tumours, while some clusters (e.g. cluster 18 and 20) consisted mainly of cells from a single tumour (Suppl. Fig. S5). While expression of several markers could be found in most clusters present in the tumours, other markers varied substantially in expression. For instance, the Vδ1 cells generally had a higher expression of CD103, CD38, and the exhaustion markers TIGIT, PD-1 (CD279), and CD39 compared to the other subsets. In contrast, the Vδ2 clusters were much more diverse with expression of several markers unique to only one or two clusters (Fig. 5E). The single non-Vδ1Vδ2 cluster present in the tumours had a high expression of CD45RO and Fas (CD95), and also a higher expression than most other clusters of several proteins expressed in activated cells, such as ICOS (CD254), OX-40 (CD134), CD25, and FoxP3 (Fig. 5E).
Fig. 5.
Clustering analysis of tumour-infiltrating γδ T cells. Single cell suspensions were isolated from tumours, corresponding unaffected colon mucosa, and blood, and analysed using mass cytometry. A γδ T cells were first analysed using the UMAP dimensional reduction algorithm in concatenated data combined from blood, tumour, and unaffected tissue. B Data from A are shown individually for unaffected tissue, PBMCs, and tumours. C Tumour-infiltrating γδ T cells were analysed separately using the UMAP dimensional reduction algorithm together with the phenograph clustering algorithm. The markers indicated in (E) were all used to generate the clustering algorithms. D Expression of Vδ1 and Vδ2 overlaid on the clustered tumour-infiltrating γδ T cells. The colour scale represents staining intensity, and the scale is based on the minimum to the maximum signal in each specific marker. E Heatmap of marker expression in the clusters identified in tumour-infiltrating γδ T cells using the UMAP dimensional reduction and phenograph clustering algorithms. The colour scale shows the median signal intensity of the respective marker in each cluster, and the scales were generated individually for each marker and based on the minimum to the maximum signal in each specific marker. n = 18
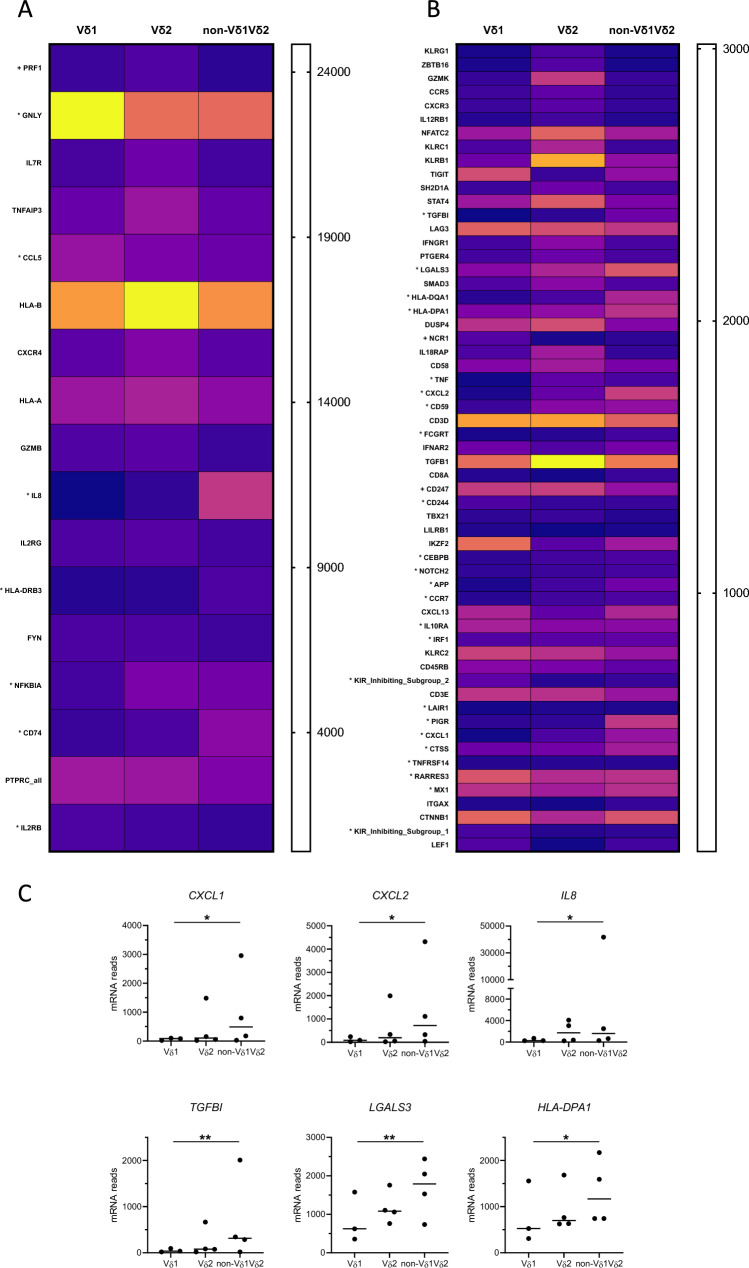
In a separate set of four patients, the tumour-infiltrating Vδ1, Vδ2, and non-Vδ1Vδ2 cells were sorted by flow cytometry immediately after isolation, and mRNA quantified. In total, we identified 76 genes that were differentially expressed between non-Vδ1Vδ2 and Vδ1 or Vδ2 cells (Suppl. Table S4). Vδ1 cells presented a signature consistent with cytotoxic effector functions, with a high expression of GNLY (Granulysin), PRF1 (Perforin), CD244 (2B4), and NCR1 (NKp46) (Fig. 6A, B). A cytotoxic effector signature could also be observed when comparing the Vδ2 and the non-Vδ1Vδ2 cells. However, in addition to PRF1, the Vδ2 transcriptome was dominated by expression of GRZK and GRZB (granzymes K and B), the killer cell lectin-like receptors KLRG1, KLRC1 (NKG2A), and KLRB1 (CD161; Fig. 6A, B). Interestingly, the non-Vδ1Vδ2 cells had a higher expression of genes associated with inflammatory and tumour-promoting responses, such as CXCL1 (GRO-α), CXCL2 (GRO-β), IL8 (IL-8), TGFBI (transforming growth factor-beta induced, TGFBI), and LGALS3 (Galectin-3), and several genes associated with antigen presentation (HLA-DPA1, HLA-DQA1, HLA-DRB3, and CD74), when compared to the other subsets (Fig. 6C).
Fig. 6.
mRNA expression in tumour-infiltrating γδ T cell subsets. Single cell suspensions were isolated from tumours and Vδ1, Vδ2, and non-Vδ1Vδ2 cells were sorted by flow cytometry and analysed using multiplex mRNA quantification. To facilitate interpretation of the data, the heatmap of differentially expressed genes has been divided into mRNAs with a high (A) and low (B) expression, respectively. Genes are presented in order of the highest to the lowest significance values of the difference between non-Vδ1Vδ2 cells and the other subsets within the two panels. The intensity scales indicate the normalized counts of mRNAs per cell. Genes marked with * indicate significant differences between non-Vδ1Vδ2 and Vδ1 cells, while no marking indicates significant differences between non-Vδ1Vδ2 and Vδ2 cells. C mRNA counts in cell subsets from individual tumours are shown for CXCL1, CXCL2, IL8, TGFBI, LGALS3, and HLA-DPA1. Data were analysed using two-tailed Friedman test without adjustment for multiple comparisons. *p < 0.05, **p < 0.01. n = 4
Taken together, these analyses show that Vδ1 and Vδ2 cells express markers that are associated with cytotoxic effector functions. In contrast, the non-Vδ1Vδ2 cells appear to have a more tumour-promoting function, as they express less NK cell receptors and cytotoxic effector molecules, but instead markers associated with an innate inflammatory immune response and direct tumour-promoting functions.
Cytokine production in tumour-infiltrating γδ T cells
To better understand the functional capacity of the non-Vδ1Vδ2 cell subset in colon cancer patients, we analysed the production of Th1 and Th17 associated cytokines and GrB following polyclonal stimulation. These experiments revealed that IFN-γ was highly expressed especially in Vδ2 cells from all tissues. In contrast, the non-Vδ1Vδ2 cells from the unaffected colon mucosa and the tumours only contained moderate frequencies of IFN-γ-producing cells (Fig. 7A). TNF production was considerably lower than that of IFN-γ and was also lower in the non-Vδ1Vδ2 subset compared to the Vδ2 subset in the cells present in both the tissue and the circulation (Fig. 7B). In contrast, IL-17A expression was only seen in non-Vδ1Vδ2 cells from some individuals, but virtually not in any of the other subsets of γδ T cells (Fig. 7C). This was similar to IL-8 expression, which was only detected in some patients and primarily in circulating non-Vδ1Vδ2 cells (Fig. 7D). GrB, on the other hand, was expressed at relatively high levels in cells from all tissues from all patients. Furthermore, there were no substantial differences in GrB production between the γδ T cell subsets from the tumours using different TCRs (Fig. 7E). Representative flow cytometry plots from one patient can be found in Suppl. Fig. S6–S8. The median fluorescence intensity of the cells staining positive for the respective cytokines was generally similar between the γδ T cells subsets, except for GrB staining intensity which was especially high in Vδ1 and non-Vδ1 Vδ2 cells from the circulation (Suppl. Fig. S9). In general, GrB production from γδ T cells was higher than in conventional αβ T cells, while TNF and IL-17 production was lower and IFN-γ and IL-8 production was similar to that in αβ T cells (Suppl. Fig. S10).
Fig. 7.
Cytokines and effector proteins in tumour-infiltrating γδ T cells. Single cell suspensions were isolated from tumours, corresponding unaffected colon mucosa, and blood, and stimulated with PMA and Ionomycin. Vδ1, Vδ2, and non-Vδ1Vδ2 cells were analysed for the expression of IFN-γ (A), TNF (B), IL-17A (C), IL-8 (D), and Granzyme B (E) by flow cytometry. Symbols represent individual values and the bars the median. n = 5–12
Discussion
Recent studies in CRC show the presence of different subsets of tumour-infiltrating γδ T cells with specific functions, which range from tumour-promoting to tumoricidal effects [11, 26]. This is likely context dependent and is yet to be fully understood. In this study, we used several strategies to delineate different subpopulations of tumour-infiltrating γδ T cells in colon cancer patients. We show that γδ T cell infiltration into tumours was reduced in most patients, compared to the surrounding unaffected colon mucosa, and that the tumour-infiltrating γδ T cells vary considerably between patients with regard to Vδ chain usage, phenotype, and functional properties.
Most research on human γδ T cells has focused on Vδ1 and Vδ2 cells, mainly due to the limited availability of antibodies to the other TCRs. However, in human tissues there is a considerable proportion of γδ T cells using other Vδ chains, both in tumours and the corresponding healthy tissue [5, 11, 26–28]. Here, we could document a similar accumulation of non-Vδ1Vδ2 cells in human colon tissues. In the tumours, TCR sequencing showed that these cells expressed Vδ3 to a very large extent and also contributed to the most expanded clonotypes in most of the patients. The Vδ3 cells in the tumours were often oligoclonal with one or a few dominating clones, and they may recognize tumour neoantigens or stress signals in the tumour cells, such as Annexin A2 [29]. The cognate ligands for Vδ3 cells also include the monomorphic MHC I-like molecules CD1d and MR1 [30, 31]. These molecules are also increased on the cell surface following endoplasmatic reticulum (ER) stress and inflammatory signals [32, 33], and reactivity against such antigens may also explain some of the clonal expansion of Vδ3 cells in the tumours.
We have used CD27 and CD45RA as markers of different memory populations, even though this nomenclature was originally devised for αβ T cells. Vδ1 and non-Vδ1 Vδ2 cells from colon, both unaffected and tumour tissue, harboured a large proportion of TEM-like cells which were not present among the circulating cells. This is similar to tissue-infiltrating γδ T cells in liver tissue and non-small cell lung cancer, where similar TEM-like Vδ1 cells have been documented [34, 35]. In the Vδ1 and non-Vδ1 Vδ2 cells, the distribution between memory subsets was conserved in colon mucosa and tumours, but different in blood, while Vδ2 cells were similar with regard to memory subsets in blood and tissues. Therefore, we cannot rule out the possibility that a substantial proportion of the Vδ2 cells detected in the colon tissues may in fact originate from the microvasculature, while Vδ1 and non-Vδ1 Vδ2 cells might more likely represent tissue-resident cells, as previously documented in lung and ovarian cancer [35, 36].
Both Vδ1 and Vδ2 cells have been attributed potent anti-tumour effects, while the effect of other γδ T cells in the tumour microenvironment is more elusive [37]. Vδ1 cells possess potent cytotoxic activity towards cancer cells in vitro and a high expression of cytotoxic effector proteins, such as Granzyme B [5, 38]. Previous detailed transcriptional analyses of tumour-infiltrating γδ T cells revealed distinct clusters based on the transcriptional profiles of Vδ1 and Vδ2 cells that exhibited similar expression of cytotoxic markers as the clusters of CD8+ T cells and NK cells [28]. In our study, we identified several clusters of both Vδ1 and Vδ2 cells with both overlapping and unique features. A distinct feature of Vδ1 and Vδ2 cells from both cell surface staining and mRNA quantification was a strong cytotoxic profile comprising both NK cell receptors and cytotoxic effector molecules. Still, cytotoxic molecules and NK cell receptors were partly differentially expressed, as previously described [28, 39]. Using a mass cytometry panel, all non-Vδ1Vδ2 γδ T cells formed a single and relatively small cluster. This is somewhat different to the flow cytometry results and may be explained by the less distinct signals in mass compared to flow cytometry. The non-Vδ1Vδ2 cells were characterized by a low surface expression of NK cell receptors and also appeared to be more activated, while they showed little sign of exhaustion. Non-Vδ1Vδ2 cells also had higher mRNA expression of neutrophil-recruiting chemokines, a tumour-promoting factor [7]. Furthermore, one of the genes we identified as more highly expressed by non-Vδ1Vδ2 cells was TGFBI. TGFBI has been implicated in tumour progression, and elevated levels have been associated with a poor clinical outcome, as it promotes angiogenesis and tumour cell migration, not least in CRC [40], and also reduces T cell activation [41, 42]. The expression of Galectin-3 in non-Vδ1Vδ2 cells is also interesting, as recent studies link Galectin-3 production to a poor patient outcome in CRC, increased metastatic potential, and to a γδ17 phenotype, both in healthy tissues and tumours [43, 44]. A cluster of expanded γδ T cells with high expression of Galectin-3 and other IL-17 associated genes was also recently found in human CRC tumours using single-cell RNA sequencing [26].
Functional analyses of the non-Vδ1Vδ2 cells revealed that they had a much lower expression of IFN-γ and TNF than Vδ2 cells, suggesting a lower capacity to support anti-tumour immunity. Additionally, non-Vδ1Vδ2 cells were the main source of IL-17A among γδ T cells, even though the production was limited compared to other cytokines. This is consistent with a study by Harman et al. [11], who also found IL-17-producing γδ T cells among Vδ3 cells. The restriction of IL-17 to non-Vδ1Vδ2 cells is also in line with murine studies, where distinct γδ T cell subsets provide IL-17 in the tumour microenvironment [26, 45]. Generally, intratumoural IL-17 production has been associated with a poor prognosis [8, 12], but the source of intratumoural IL-17 is not yet fully resolved [27]. Based on our current results and previous literature, it is likely that a major part of IL-17 produced in the tumour microenvironment is provided by CD4+ Th17 cells, rather than γδ17 cells [35, 46]. Likewise, TNF production from γδ T cells may not be crucial for the overall cytokine balance in colon tumours, while γδ T cells produce IFN-γ to an extent comparable to or higher than conventional αβ T cells.
This is a single-centre study with a well-defined patient cohort. However, one limitation of the study is the relatively small number of patients included, and the varying number of patients used for different analyses. The latter was caused by several samples containing quite few γδ T cells, and we thus had to prioritize between assays. With a larger cohort, we might have been able to detect correlations between γδ T cell subsets or functions and patient outcome.
In summary, this study demonstrates a large variation in γδ T cell composition between individual tumours with regard to phenotypic markers, functional potential, and TCR usage. Recent studies clearly demonstrate both antitumour and tumour-promoting functions of tumour-infiltrating γδ T cell subsets, which were distinguished based on TCR usage [11, 26]. Our results show substantial infiltration of non-Vδ1Vδ2 cells, primarily using Vδ3, in colon tumours and based on their low expression of cytotoxic molecules combined with higher expression of some tumour-promoting mediators, we suggest that they contribute mainly to a tumour-promoting immune response.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all patients for their participation in this study and Angelica Wingård and Zunash Malik at the Surgical Oncology Laboratory at Sahlgrenska University Hospital for their valuable assistance with collection of clinical samples. This work was supported by the Swedish Research Council (2021-01361 and 2021-01008), the Swedish Cancer Foundation (21-1646 and 22-2080), grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (144381 and 965065), Region Västra Götaland, the Sjöberg Foundation, and Assar Gabrielsson Foundation.
Abbreviations
- CDR3
Complementarity-determining region 3
- CRC
Colorectal cancer
- MSI
Microsatellite instable
- MSS
Microsatellite stable
- PBMC
Peripheral blood mononuclear cells
- PMA
Phorbol 12-myristate 13-acetate
- TCR
T cell receptor
- TGFBI
Transforming growth factor-beta induced
Author contribution
MQJ conceptualized the study; WR, LS, TR, FTK, TÖ, PS, and SH helped in methodology; WR, LS, TR, FTK, TÖ, and MQJ contributed to formal analysis and investigation; AS, EBL, and MQJ were involved in funding acquisition; AC, AS, EBL, and MQJ supervised the study; YW, AC, AS, and EBL helped in resources; WR and MQJ were involved in writing—original draft preparation; all authors helped in writing—review and editing.
Funding
Open access funding provided by University of Gothenburg. This work was supported by the Swedish Research Council (55X-13428 and 2021–01008), the Swedish Cancer Foundation (130593 and 22–2080), grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (144381 and 965065), Region Västra Götaland, the Sjöberg Foundation, and Assar Gabrielsson Foundation.
Data availability
TCR sequencing raw data in fastq format have been deposited to the NCBI short read archive (SRA; https://www.ncbi.nlm.nih.gov/sra) with accession number PRJNA1107040. Normalized Nanostring data have been deposited to Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) with accession number GSE266504.
Declarations
Conflict of interest
AS declares stock ownership and is a board member in Tulebovaasta, Iscaff Pharma, and SiMSen Diagnostics. AS is co-inventor of the SiMSen-Seq technology that is patent protected (US Serial No.:15/552,618). MQJ has received consultancy fees from Biomunex Pharmaceuticals.
Ethical approval
This study was performed according to the Declaration of Helsinki and approved by the Regional Board of Ethics in Medical Research in west Sweden (249–15, approved 06/03/2015).
Consent to participate
All patients gave a written informed consent before participation in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kazen AR, Adams EJ (2011) Evolution of the V, D, and J gene segments used in the primate gammadelta T-cell receptor reveals a dichotomy of conservation and diversity. Proc Natl Acad Sci U S A 108:E332–E340. 10.1073/pnas.1105105108 10.1073/pnas.1105105108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulou M, Sanchez Sanchez G, Vermijlen D (2020) Innate and adaptive gammadelta T cells: How, when, and why. Immunol Rev 298:99–116. 10.1111/imr.12926 10.1111/imr.12926 [DOI] [PubMed] [Google Scholar]
- 3.Clark BL, Thomas PG (2020) A Cell for the ages: human gammadelta T cells across the lifespan. Int J Mol Sci. 10.3390/ijms21238903 10.3390/ijms21238903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simoes AE, Di Lorenzo B, Silva-Santos B (2018) Molecular Determinants of Target Cell Recognition by Human gammadelta T Cells. Front Immunol 9:929. 10.3389/fimmu.2018.00929 10.3389/fimmu.2018.00929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikulak J, Oriolo F, Bruni E et al (2019) NKp46-expressing human gut-resident intraepithelial Vdelta1 T cell subpopulation exhibits high antitumor activity against colorectal cancer. JCI Insight. 10.1172/jci.insight.125884 10.1172/jci.insight.125884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JH, Lee HK (2021) Function of gammadelta T cells in tumor immunology and their application to cancer therapy. Exp Mol Med 53:318–327. 10.1038/s12276-021-00576-0 10.1038/s12276-021-00576-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentles AJ, Newman AM, Liu CL et al (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21:938–945. 10.1038/nm.3909 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meraviglia S, Lo Presti E, Tosolini M et al (2017) Distinctive features of tumor-infiltrating gammadelta T lymphocytes in human colorectal cancer. Oncoimmunology 6:e1347742. 10.1080/2162402X.2017.1347742 10.1080/2162402X.2017.1347742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorsson V, Gibbs DL, Brown SD et al (2018) The Immune Landscape of Cancer. Immunity 48(812–30):e14. 10.1016/j.immuni.2018.03.023 10.1016/j.immuni.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Wang Z, Hu Y, Wang Y, Lu N, Zhang C (2023) Tumor-infiltrating gamma delta T-cells reveal exhausted subsets with remarkable heterogeneity in colorectal cancer. Int J Cancer 153:1684–1697. 10.1002/ijc.34669 10.1002/ijc.34669 [DOI] [PubMed] [Google Scholar]
- 11.Harmon C, Zaborowski A, Moore H et al (2023) gammadelta T cell dichotomy with opposing cytotoxic and wound healing functions in human solid tumors. Nat Cancer 4:1122–1137. 10.1038/s43018-023-00589-w 10.1038/s43018-023-00589-w [DOI] [PubMed] [Google Scholar]
- 12.Tosolini M, Kirilovsky A, Mlecnik B et al (2011) Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 71:1263–1271. 10.1158/0008-5472.CAN-10-2907 10.1158/0008-5472.CAN-10-2907 [DOI] [PubMed] [Google Scholar]
- 13.Bindea G, Mlecnik B, Tosolini M et al (2013) Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39:782–795. 10.1016/j.immuni.2013.10.003 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 14.Ma S, Cheng Q, Cai Y et al (2014) IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res 74:1969–1982. 10.1158/0008-5472.CAN-13-2534 10.1158/0008-5472.CAN-13-2534 [DOI] [PubMed] [Google Scholar]
- 15.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331–341. 10.1016/j.immuni.2009.08.001 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 16.Wu D, Wu P, Qiu F, Wei Q, Huang J (2017) Human gammadeltaT-cell subsets and their involvement in tumor immunity. Cell Mol Immunol 14:245–253. 10.1038/cmi.2016.55 10.1038/cmi.2016.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundstrom P, Szeponik L, Ahlmanner F, Sundquist M, Wong JSB, Lindskog EB, Gustafsson B, Quiding-Jarbrink M (2019) Tumor-infiltrating mucosal-associated invariant T (MAIT) cells retain expression of cytotoxic effector molecules. Oncotarget 10:2810–2823. 10.18632/oncotarget.26866 10.18632/oncotarget.26866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren A, Stromberg E, Sjoling A et al (2005) Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun 73:523–531. 10.1128/IAI.73.1.523-531.2005 10.1128/IAI.73.1.523-531.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang F, Rezapour A, Falk P, Angenete E, Yrlid U (2021) Cryopreservation of whole tumor biopsies from rectal cancer patients enable phenotypic and in vitro functional evaluation of tumor-infiltrating T cells. Cancers (Basel). 10.3390/cancers13102428 10.3390/cancers13102428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson G, Kaltak M, Rimniceanu C et al (2020) Ultrasensitive DNA Immune Repertoire Sequencing Using Unique Molecular Identifiers. Clin Chem 66:1228–1237. 10.1093/clinchem/hvaa159 10.1093/clinchem/hvaa159 [DOI] [PubMed] [Google Scholar]
- 21.Shugay M, Britanova OV, Merzlyak EM et al (2014) Towards error-free profiling of immune repertoires. Nat Methods 11:653–655. 10.1038/nmeth.2960 10.1038/nmeth.2960 [DOI] [PubMed] [Google Scholar]
- 22.Szeponik L, Ahlmanner F, Sundstrom P, Rodin W, Gustavsson B, Bexe Lindskog E, Wettergren Y, Quiding-Jarbrink M (2021) Intratumoral regulatory T cells from colon cancer patients comprise several activated effector populations. BMC Immunol 22:58. 10.1186/s12865-021-00449-1 10.1186/s12865-021-00449-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei HE, Leipold MD, Schulz AR, Chester C, Maecker HT (2015) Barcoding of live human peripheral blood mononuclear cells for multiplexed mass cytometry. J Immunol 194:2022–2031. 10.4049/jimmunol.1402661 10.4049/jimmunol.1402661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davey MS, Willcox CR, Hunter S et al (2018) The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(-) subsets. Nat Commun 9:1760. 10.1038/s41467-018-04076-0 10.1038/s41467-018-04076-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odaira K, Kimura SN, Fujieda N, Kobayashi Y, Kambara K, Takahashi T, Izumi T, Matsushita H, Kakimi K (2016) CD27(-)CD45(+) gammadelta T cells can be divided into two populations, CD27(-)CD45(int) and CD27(-)CD45(hi) with little proliferation potential. Biochem Biophys Res Commun 478:1298–1303. 10.1016/j.bbrc.2016.08.115 10.1016/j.bbrc.2016.08.115 [DOI] [PubMed] [Google Scholar]
- 26.Reis BS, Darcy PW, Khan IZ et al (2022) TCR-Vgammadelta usage distinguishes protumor from antitumor intestinal gammadelta T cell subsets. Science 377:276–284. 10.1126/science.abj8695 10.1126/science.abj8695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu P, Wu D, Ni C et al (2014) gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40:785–800. 10.1016/j.immuni.2014.03.013 10.1016/j.immuni.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizzolato G, Kaminski H, Tosolini M et al (2019) Single-cell RNA sequencing unveils the shared and the distinct cytotoxic hallmarks of human TCRVdelta1 and TCRVdelta2 gammadelta T lymphocytes. Proc Natl Acad Sci U S A 116:11906–11915. 10.1073/pnas.1818488116 10.1073/pnas.1818488116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marlin R, Pappalardo A, Kaminski H et al (2017) Sensing of cell stress by human gammadelta TCR-dependent recognition of annexin A2. Proc Natl Acad Sci U S A 114:3163–3168. 10.1073/pnas.1621052114 10.1073/pnas.1621052114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangan BA, Dunne MR, O’Reilly VP, Dunne PJ, Exley MA, O’Shea D, Scotet E, Hogan AE, Doherty DG (2013) Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vdelta3 T cells. J Immunol 191:30–34. 10.4049/jimmunol.1300121 10.4049/jimmunol.1300121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice MT, von Borstel A, Chevour P et al (2021) Recognition of the antigen-presenting molecule MR1 by a Vdelta3(+) gammadelta T cell receptor. Proc Natl Acad Sci U S A. 10.1073/pnas.2110288118 10.1073/pnas.2110288118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedard M, Shrestha D, Priestman DA et al (2019) Sterile activation of invariant natural killer T cells by ER-stressed antigen-presenting cells. Proc Natl Acad Sci U S A 116:23671–23681. 10.1073/pnas.1910097116 10.1073/pnas.1910097116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ussher JE, van Wilgenburg B, Hannaway RF et al (2016) TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells. Eur J Immunol 46:1600–1614. 10.1002/eji.201545969 10.1002/eji.201545969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter S, Willcox CR, Davey MS, Kasatskaya SA, Jeffery HC, Chudakov DM, Oo YH, Willcox BE (2018) Human liver infiltrating gammadelta T cells are composed of clonally expanded circulating and tissue-resident populations. J Hepatol 69:654–665. 10.1016/j.jhep.2018.05.007 10.1016/j.jhep.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Biswas D, Usaite I et al (2022) A local human Vdelta1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat Cancer 3:696–709. 10.1038/s43018-022-00376-z 10.1038/s43018-022-00376-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foord E, Arruda LCM, Gaballa A, Klynning C, Uhlin M (2021) Characterization of ascites- and tumor-infiltrating gammadelta T cells reveals distinct repertoires and a beneficial role in ovarian cancer. Sci Transl Med. 10.1126/scitranslmed.abb0192 10.1126/scitranslmed.abb0192 [DOI] [PubMed] [Google Scholar]
- 37.Willcox BE, Willcox CR (2019) gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol 20:121–128. 10.1038/s41590-018-0304-y 10.1038/s41590-018-0304-y [DOI] [PubMed] [Google Scholar]
- 38.Wu D, Wu P, Wu X et al (2015) Ex vivo expanded human circulating Vdelta1 gammadeltaT cells exhibit favorable therapeutic potential for colon cancer. Oncoimmunology 4:e992749. 10.4161/2162402X.2014.992749 10.4161/2162402X.2014.992749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cazzetta V, Bruni E, Terzoli S et al (2021) NKG2A expression identifies a subset of human Vdelta2 T cells exerting the highest antitumor effector functions. Cell Rep 37:109871. 10.1016/j.celrep.2021.109871 10.1016/j.celrep.2021.109871 [DOI] [PubMed] [Google Scholar]
- 40.Corona A, Blobe GC (2021) The role of the extracellular matrix protein TGFBI in cancer. Cell Signal 84:110028. 10.1016/j.cellsig.2021.110028 10.1016/j.cellsig.2021.110028 [DOI] [PubMed] [Google Scholar]
- 41.Patry M, Teinturier R, Goehrig D, Zetu C, Ripoche D, Kim IS, Bertolino P, Hennino A (2015) Betaig-h3 represses T-cell activation in type 1 diabetes. Diabetes 64:4212–4219. 10.2337/db15-0638 10.2337/db15-0638 [DOI] [PubMed] [Google Scholar]
- 42.Lecker LSM, Berlato C, Maniati E et al (2021) TGFBI production by macrophages contributes to an immunosuppressive microenvironment in ovarian cancer. Cancer Res 81:5706–5719. 10.1158/0008-5472.CAN-21-0536 10.1158/0008-5472.CAN-21-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu KL, Huang EY, Yeh WL, Hsiao CC, Kuo CM (2017) Synergistic interaction between galectin-3 and carcinoembryonic antigen promotes colorectal cancer metastasis. Oncotarget 8:61935–61943. 10.18632/oncotarget.18721 10.18632/oncotarget.18721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Zhou X, Ma L et al (2019) Galectin-3 may serve as a marker for poor prognosis in colorectal cancer: a meta-analysis. Pathol Res Pract 215:152612. 10.1016/j.prp.2019.152612 10.1016/j.prp.2019.152612 [DOI] [PubMed] [Google Scholar]
- 45.Szeponik L, Akeus P, Rodin W, Raghavan S, Quiding-Jarbrink M (2020) Regulatory T cells specifically suppress conventional CD8alphabeta T cells in intestinal tumors of APC(Min/+) mice. Cancer Immunol Immunother 69:1279–1292. 10.1007/s00262-020-02540-9 10.1007/s00262-020-02540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amicarella F, Muraro MG, Hirt C et al (2017) Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut 66:692–704. 10.1136/gutjnl-2015-310016 10.1136/gutjnl-2015-310016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
TCR sequencing raw data in fastq format have been deposited to the NCBI short read archive (SRA; https://www.ncbi.nlm.nih.gov/sra) with accession number PRJNA1107040. Normalized Nanostring data have been deposited to Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) with accession number GSE266504.