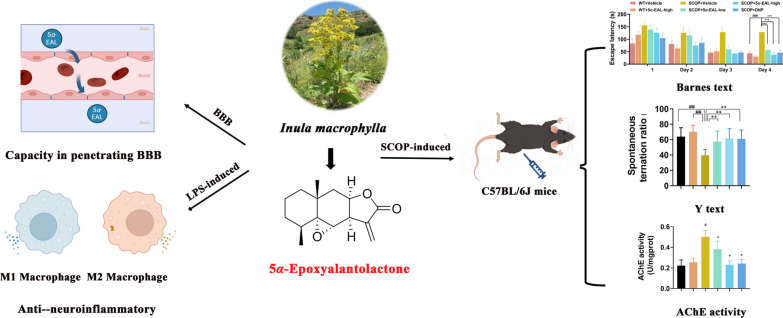
Abstract
Alzheimer’s disease (AD) is a complex neurodegenerative condition. 5α-epoxyalantolactone (5α-EAL), a eudesmane-type sesquiterpene isolated from the herb of Inula macrophylla, has various pharmacological effects. This work supposed to investigate the improved impact of 5α-EAL on cognitive impairment. 5α-EAL inhibited the generation of nitric oxide (NO) in BV-2 cells stimulated with lipopolysaccharide (LPS) with an EC50 of 6.2 μM. 5α-EAL significantly reduced the production of prostaglandin E2 (PGE2) and tumor necrosis factor-α (TNF-α), while also inhibiting the production of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) proteins. The ability of 5α-EAL to penetrate the blood–brain barrier (BBB) was confirmed via a parallel artificial membrane permeation assay. Scopolamine (SCOP)-induced AD mice model was employed to assess the improved impacts of 5α-EAL on cognitive impairment in vivo. After the mice were pretreated with 5α-EAL (10 and 30 mg/kg per day, i.p.) for 21 days, the behavioral experiments indicated that the administration of the 5α-EAL could alleviate the cognitive and memory impairments. 5α-EAL significantly reduced the AChE activity in the brain of SCOP-induced AD mice. In summary, these findings highlight the beneficial effects of the natural product 5α-EAL as a potential bioactive compound for attenuating cognitive deficits in AD due to its pharmacological profile.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13659-024-00462-y.
Keywords: Alzheimer’s disease, 5α-Epoxyalantolactone (5α-EAL), Anti-neuroinflammation, Attenuates cognitive deficits
Introduction
Alzheimer’s disease (AD) is a multifactorial neurodegenerative condition and the most common type of dementia, that manifests itself as memory impairment and cognitive dysfunction. AD is characterized by a disability in performing daily living activities, and is often accompanied by a range of mental symptoms and behavioral disorders [1, 2]. As the worldwide population continues to grow older, AD patients are expected to reach 152 million by 2050. The economic impact of AD is expected to cost the global economy over 1 trillion dollars [3]. Improvement of subjective cognitive deficits might be a valuable therapeutic strategy in the management of dementia, which delays or prevents the progression of cognitive impairment to AD [4].
The diversity of scaffolds and the structural complexity found in natural products have made a crucial contribution throughout the history of the evolution of drugs [5], which has proved to be a treasure of drug treatment for AD. For example, galantamine and huperzine A, natural alkaloids cholinesterase inhibitors, were extensively utilized in the treatment of AD [6, 7]. The genus Inula, from the Asteraceae family, has been commonly employed in traditional medicine and has recently attracted increasing attention due to its application in neurodegenerative conditions [8–11]. Eudesmane-type sesquiterpene lactones (SLs) containing an α-methylene-γ-lactone moiety as the representative genus Inula secondary metabolites, have attracted increasing attention for their pharmacological activities against central nervous system-related disorders [6, 12, 13]. For example, the alantolactone and isoalantolactone, eudesmane-type SLs isolated from Inula helenium, which has neuroprotective effects by significantly attenuating Aβ25–35 (10 μM) demonstrated cytotoxicity in neuronal cells derived from the mice brain and preventing ROS-mediated cognitive impairment through the Nrf2 signaling pathway [14]. Hence, the eudesmane-type SLs in genus Inula exhibit significant potential as lead compounds for further exploration of preventative and therapeutic agents of AD.
Inula macrophylla is mainly restricted to northern temperate regions. In Central Asia, Inula macrophylla is traditionally used as a remedy for lung diseases, gastric ulcers, bronchitis, diabetes, and intestinal ulcers in traditional medicine [14, 15]. Nevertheless, the effects of Inula macrophylla on AD therapy have been less explored currently. Our previous research screened secondary metabolites isolated from Inula macrophylla by an anti-neuroinflammatory cell model. 5α-Epoxyalantolactone (5α-EAL) was identified as an eudesmane-type SLs that exhibited excellent inhibition of NO production in LPS-stimulated BV-2 cells [16]. In this work, the effects of 5α-EAL on the production of nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), and interleukin 10 (IL-10), as well as the protein expression of cyclooxygenase-2 (COX-2) and nitric oxide synthase (iNOS) were thoroughly investigated using LPS-stimulated BV-2 microglial cell model. Furthermore, the potential improvement in cognitive deficits in SCOP-induced AD mice resulting from 5α-EAL was also evaluated.
Results and discussion
Chemistry
The ethanol extract of the whole of Inula macrophylla was separated and purified through silica gel column chromatography and preparative HPLC to yield 5α-EAL (Fig. 1, ~ 0.07%). Its structure was elucidated by a comprehensive analysis of spectroscopic data (HR-ESI–MS, 1H NMR, and 13C-NMR) and verified as reported in the literature [17]. The structural difference between alantolactone and 5α-EAL is confined to the C-5 and C-6 epoxide ring. Then 5α-EAL could be synthesized from alantolactone through epoxidation via the Prilezhaev Reaction (Scheme 1), which is beneficial for the subsequent animal experiments. The structure of synthesized 5α-EAL was further confirmed by comparison of 1D NMR with isolated 5α-EAL (Fig. S3). The purity of 5α-EAL was determined to be 98.1% by using HPLC (Fig. S5).
Fig. 1.
Chemical structures of natural product 5α-EAL
Scheme 1.
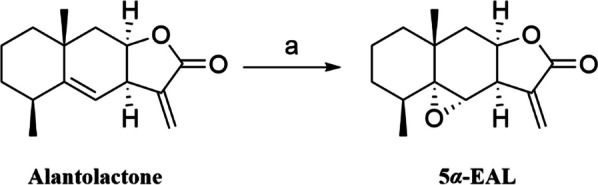
Synthesis of 5α-EAL by epoxidation of alantolactone. Reagents and condition: (a) m-CPBA, CH2Cl2, rt, 87%
Preliminary screening of NO inhibition in LPS-induced BV-2 cells
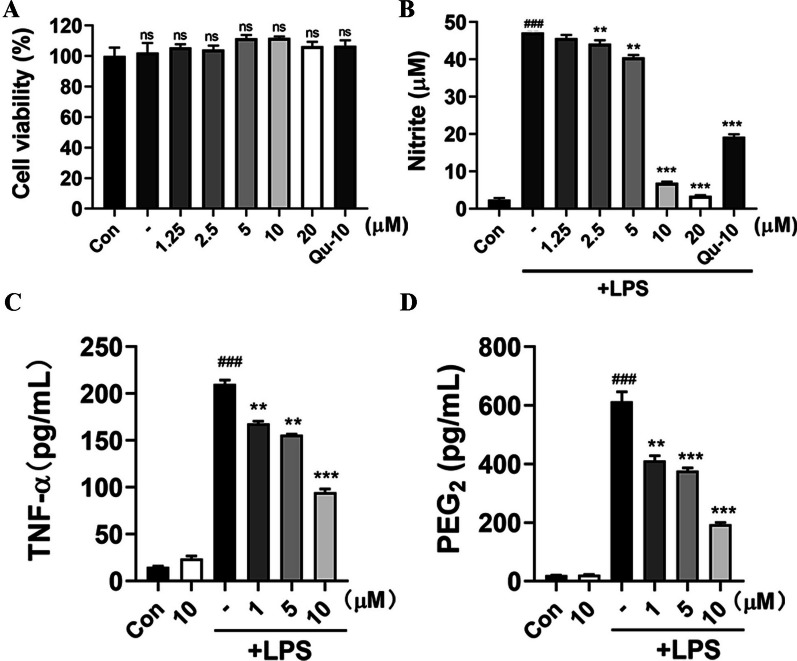
Utilizing LPS-induced BV-2 cells as a model for neuroinflammatory cells, our previous work screened secondary metabolites isolated from Inula macrophylla at a 20 μM concentration, in which 5α-EAL exhibited a potential NO inhibition effect [16]. This study aims to further assess the level of NO production in microglia BV-2 induced by LPS, to estimate the anti-neuroinflammatory activity of 5α-EAL. First, the viability of BV-2 cells was assessed after treatment with different concentrations of the 5α-EAL (1.25, 2.5, 5, 10, and 20 μM). The findings indicated that 5α-EAL had no cytotoxicity towards BV-2 cells within the concentration ranging from 1.25 to 20 μM (Fig. 2A). The impact of 5α-EAL on NO production in BV-2 microglial cells was investigated in cells pre-treated and activated with LPS (1 μg/mL). In comparison to the control group (2.43 ± 1.08 pg/mL), stimulation of BV-2 cells with LPS led to a substantial increase in NO production (47.21 ± 1.97 pg/mL), and the treatment of 5α-EAL at different concentrations dose-dependently inhibited NO production (Fig. 2B). The NO levels were dramatically decreased to 6.93 ± 0.87 and 3.42 ± 0.53 pg/mL at 10 and 20 μM of 5α-EAL, respectively, which showed stronger inhibitory effect than that of positive control group (Quercetin, Qu) at 10 μM (19.26 ± 1.90 pg/mL). The EC50 value of 5α-EAL was calculated and determined to be 6.2 μM.
Fig. 2.
Effects of 5α-EAL on A BV-2 cell viability, B NO, C TNF-α, and D PGE2 production in BV-2 microglial cells induced by LPS. Cells were stimulated with or without 1 mg/mL LPS and treated with increasing concentration of 5α-EAL for 24 h. All values are the mean ± SEM of three independent experiments. ###p < 0.001 significantly different from the control group and **p < 0.01 and ***p < 0.001 vs LPS-treated control group. ns: not significant
5α-EAL inhibits the production of TNF-α and PGE2 in LPS-stimulated BV-2 cells
Microglia serve as the primary defense mechanism for the central nervous system (CNS), functioning for resident macrophages within the brain, they exhibit two different phenotypes: pro-inflammatory M1 and anti-inflammatory M2 [18]. TNF-α and PGE2 are typical neuroinflammatory factors produced by M1 phenotypes of Microglia, which can cause neuroinflammation in the brain, and the production of TNF-α and PGE2 is essential for the synergistic generation of NO in LPS-stimulated BV-2 cells [19]. The effects of 5α-EAL treatments on TNF-α and PGE2 levels in LPS-stimulated BV-2 cells were evaluated. As depicted in Fig. 2C and D, at various concentrations (1, 5 and 10 μM), the production of TNF-α and PGE2 in LPS-stimulated BV-2 cells was significantly suppressed by 5α-EAL in a dose-dependent manner, and exhibited the highest inhibition effect at 10 μM among tested concentrations with the levels of TNF-α and PGE2 decreased to 94.77 ± 4.82 and 194.33 ± 8.39 pg/mL, respectively. The above result indicated that 5α-EAL can effectively inhibit the expression of proinflammatory cytokines TNF-α and PGE2.
5α-EAL inhibited the expression of iNOS and COX-2 in the LPS-stimulated BV-2 cells
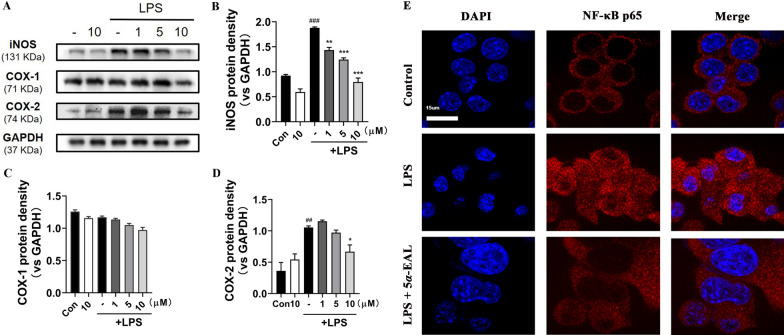
iNOS is an important enzyme for controlling the production of NO in LPS-simulated BV-2 cells. Increased production of NO through activation of iNOS expression leads to neuronal degeneration [20]. Based on the significant decrease in NO levels after the administration of 5α-EAL, the expression of iNOS was further studied by Western blot analysis (Fig. 3A and B). The iNOS expression level in LPS-simulated cells was significantly higher than control and 5α-EAL at 10 μM group (p < 0.001) without LPS treatment. 5α-EAL pretreatment at the different concentrations (1, 5 and 10 μM) remarkably inhibited iNOS expression in a concentration-dependent manner. 5α-EAL exhibited the highest inhibition effect (0.79 ± 0.11, p < 0.001) at 10 μM among tested concentrations and still exhibited a statistically significant disparity in comparison to the LPS-treated group (1.43 ± 0.08, p < 0.01) at 1.0 μM. The protein expressions of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) which are responsible for the production of PGE2 were further investigated (Fig. 3A, C, and D). 5α-EAL dose-dependently reduces the COX-2 expression level while almost having no effect on the expression of COX-1 within the range of test concentrations. Meanwhile, the expression level of COX-2 in BV-2 cells without LPS treatment was significantly lower than that of COX-1. These data demonstrated that 5α-EAL could regulate NO and PGE2 production by inhibiting the expression of iNOS and COX-2. Nuclear factor-kappa-B (NF-κB) is one of a key factor in the expression of iNOS and COX-2, which is mediated by LPS-stimulated inflammatory mediators [21]. To confirm the blockade effect of 5α-EAL on NF-κB p65 activity, the nuclear translocation of NF-κB p65 was evaluated by using immunofluorescence assay. The results revealed that 10 μM of 5α-EAL treatment effectively reduced LPS-induced nuclear translocation of NF-κB p65 (Fig. 3E). This result suggests that the inhibition of NF-κB activation by 5α-EAL may be the key signaling pathway responsible for suppressing iNOS, COX-2, and other proinflammatory cytokines in LPS-stimulated BV-2 cells.
Fig. 3.
Inhibitory effects of 5α-EAL on iNOS, COX-1, and COX-2 production induced by LPS in BV-2 microglial cells. Cells were stimulated with or without 1 mg/mL LPS and treated with increasing concentrations of 5α-EAL for 24 h. A The iNOS, COX-1, and COX-2 protein expressions in BV-2 cells were determined by western blotting. Densitometric analyses of the B iNOS, C COX-1, and D COX-2. All values are the mean ± SEM of three independent experiments. ##p < 0.01, ###p < 0.001 significantly different from the control group and *p < 0.05 and **p < 0.01 and ***p < 0.001vs LPS-treated control group. E Effects of 5α-EAL at 10 μM on the translocation of NF-κB p65 in LPS-stimulated BV-2 cells. NF-κB p65 (red), DAPI (blue), Scale bar = 15 μM. Images were captured by confocal fluorescence microscope with ×100 objective
5α-EAL inhibited the production of IL-10 in LPS-stimulated BV-2 cells
In contrast to the M1 phenotype, the anti-inflammatory M2 phenotype of microglia can inhibit the inflammatory response, and facilitate the transition of macrophage polarization from the M1 phenotype to the M2 phenotype, which helps exert a neuroprotection effect in the treatment of CNS-related disease [18]. To investigate whether 5α-EAL affects the BV-2 cell polarization, the representative M2 phenotype markers, IL-10, were evaluated. As shown in Fig. 4, after treatment with LPS, the M2 pro-inflammatory cytokine IL-10 level did not show significant changes in BV-2 cells. Furthermore, 5α-EAL significantly increased the production of IL-10 (93.67 ± 3.54 pg/mL, p < 0.01) at a concentration of 10 μM. The above results indicated that 5α-EAL can facilitate the conversion process of microglia phenotype from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype.
Fig. 4.
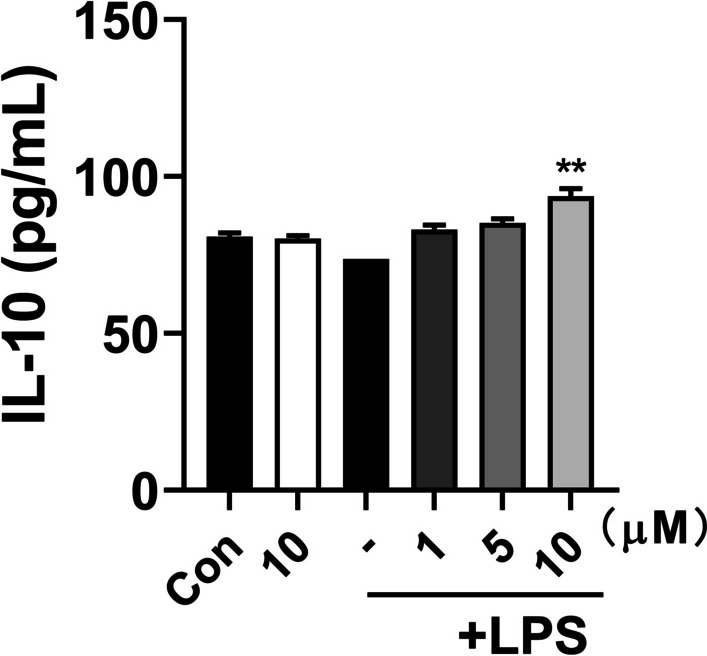
Inhibitory effects of 5α-EAL on IL-10 production induced by LPS in BV-2 microglial cells. Cells were stimulated with or without 1 mg/mL LPS and treated with increasing concentrations of 5α-EAL for 24 h. All values are the mean ± SEM of three independent experiments. **p < 0.01 vs LPS-treated control group
Capacity of 5α-EAL in penetrating blood–brain barriers (BBB)
In the process of developing CNS drugs, it is essential to assess and predict the BBB permeability of candidate compounds. The parallel artificial membrane permeability assay (PAMPA) is a high-throughput technique that has been extensively used for predicting BBB permeability [22]. The permeability (Pe) of 5α-EAL and positive drugs (carbamazepine and hydrocortisone) was quantified, following the previously reported method [23]. The results presented in Table 1 demonstrate that the Pe values of the two positive drugs are in line with the previously reported findings, indicating the reliability of the assay employed in this study. According to these ranges, it was found that 5α-EAL is capable of crossing the BBB (Pe (10−6 cm/s) = 6.45), which is beneficial for the management of neurodegenerative diseases.
Table 1.
Permeability results of 5α-EAL and control drugs from the PAMPA-BBB assay
| Compounds | Pe (× 10–6 cm/s)a | CNS prediction |
|---|---|---|
| 5α-EAL | 6.45 | + |
| Carbamazepine | 10.8 | + |
| Hydrocortisone | 1.9 | − |
aCompound was dissolved in DMSO at 8 mg/mL, then diluted with PBS. Compounds with permeabilities Pe > 4.7 × 10–6 cm s−1 could cross the BBB by passive diffusion. Pe (10−6 cm/s) > 4.0, “CNS+” (high BBB permeation); Pe (10−6 cm/s) < 2.0, “CNS−” (low BBB permeation)
5α-EAL ameliorates the cognitive dysfunction of mice induced by scopolamine in the behavioral tests
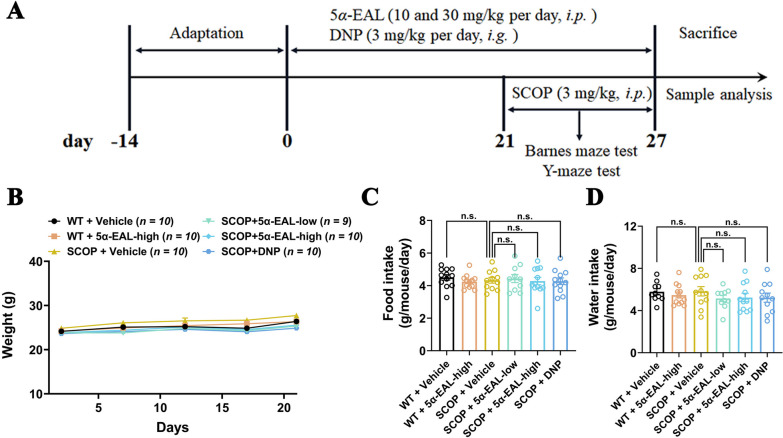
The muscarinic antagonist scopolamine (SCOP)-induced amnesia model is a widely used animal model to induce amnesia in mammals [24]. Spatial learning and memory are known to be impacted by the cognitive deficits which associated with the progression of AD. The activity of 5α-EAL on the ameliorative effects against cognitive dysfunction in the SCOP-induced AD mice model was further investigated, and donepezil (DNP) served as a positive control drug. The trial schedule is detailed in Fig. 5A. The body weight, food, and water intake were evaluated in WT and SCOP-induced model mice that received either vehicle or 5α-EAL treatment to examine the potentially toxic effects of 5α-EAL on the mice. As shown in Fig. 5B–D, the body weight of mice after treatment with 5α-EAL did not differ from that of the WT group (WT + Vehicle, 25.15 ± 1.79 g; WT + 5α-EAL-high, 25.38 ± 1.27 g; SCOP + Vehicle, 26.35 ± 1.86 g; SCOP + 5α-EAL-low, 24.42 ± 1.81 g; SCOP + 5α-EAL-high, 24.88 ± 1.78 g; SCOP + DNP, 24.26 ± 1.21 g). Furthermore, there were no significant differences in dietary and water intake observed among these groups. The results suggest that the administration of 5α-EAL is not toxic to mice.
Fig. 5.
Timeline and the effects of 5α-EAL supplementation on mice’s body weight, food intake, and water intake. A Overall experimental schedule. B Change of the body weight of mice with time. There is no significant difference in the weight of all mice in the period. The food (C) and water (D) intakes of mice in each group, respectively. No significant difference is among all groups. Data are means ± SEM, n.s., not significant; one-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test
5α-EAL ameliorates the spatial memory impairment in the Barnes maze task
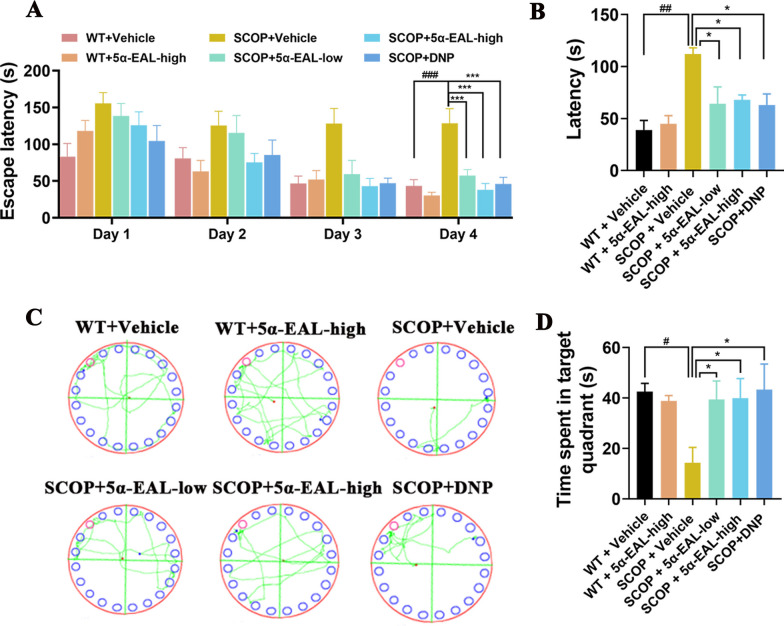
The Barnes maze was designed to evaluate the visuospatial learning and memory of SCOP-induced mice in behavioral tests. The escape latency has no obvious differences on days 1 and 2 but exhibited significant differences on days 3 and 4. At day 4, the escape latency of the mice induced by SCOP with 5α-EAL treatment (SCOP + 5α-EAL-low, 57.35 ± 23.43 s; SCOP + 5α-EAL-high, 38.04 ± 26.18 s) was significantly reduced compared to the SCOP + Vehicle group (128.49 ± 56.45 s) (Fig. 6A). Moreover, the SCOP + Vehicle group exhibited a longer escape latency compared to the group of WT + Vehicle (43.36 ± 25.56 s), demonstrating that mice induced by SCOP caused cognitive impairments and spatial memory loss. In the spatial exploration experiment, the escape latency and characteristic traces on day 5 of mice are shown in Fig. 6B and C. The group of SCOP + 5α-EAL (SCOP + 5α-EAL-low, 64.31 ± 45.74 s; SCOP + 5α-EAL-high, 67.96 ± 12.47 s) spent a shorter period in the target quadrant in comparison to the SCOP + Vehicle group (112.03 ± 15.95 s), which exhibited similar performances in the SCOP + DON group (63.09 ± 30.12 s). As shown in Fig. 6D, the time proportion in the group of SCOP + Vehicle (14.37 ± 17.09 s) was considerably below that in the WT + Vehicle group (42.53 ± 10.43 s), after the treatment of 5α-EAL in SCOP-induce mice, the time proportion was significantly increased (SCOP + 5α-EAL-low, 39.49 ± 21.79 s; SCOP + 5α-EAL-high, 39.98 ± 21.96 s). These results indicate that 5α-EAL can ameliorate cognitive impairment in AD mice.
Fig. 6.
Effects of 5α-EAL on scopolamine-induced cognitive dysfunction based on the Barens maze test results in scopolamine-induced AD mice. A The escape latency of four consecutive days test. B The escape latency of day 5. C Barens maze path of day 5 and D time spent in the target quadrant were recorded in the Barens maze test. Data are expressed as the mean ± SEM (n = 9–10). ##p < 0.01and #p < 0.05 vs. the WT + Vehicle group. *p < 0.05 and ***p < 0.001vs. the SCOP + Vehicle. WT + Vehicle: Control group; SCOP + Vehicle: Scopolamine group; SCOP + 5α-EAL-low: Scopolamine + 10 mg/kg 5α-EAL group; SCOP + 5α-EAL-high: Scopolamine + 30 mg/kg 5α-EAL group; SCOP + DNP: Scopolamine + Donepezil group
5α-EAL ameliorates the working memory impairment in the Y-maze task
The Y-maze was used to examine the effect of 5α-EAL on spontaneous alternation behavior. The findings indicated that the group of SCOP + Vehicle (39.57 ± 7.23 s) exhibited a significantly lower percentage of spontaneous alterations in comparison with the WT + Vehicle group (63.91 ± 11.56 s) (Fig. 7). On the contrary, treatment with 5α-EAL (SCOP + 5α-EAL-low, 57.42 ± 13.63 s; SCOP + 5α-EAL-high, 61.55 ± 12.75 s) significantly increase the percentage of spontaneous alternation in comparison to the group of SCOP + Vehicle, indicating that the administration of 5α-EAL could mitigate the working memory impairment of mice after treated by SCOP.
Fig. 7.
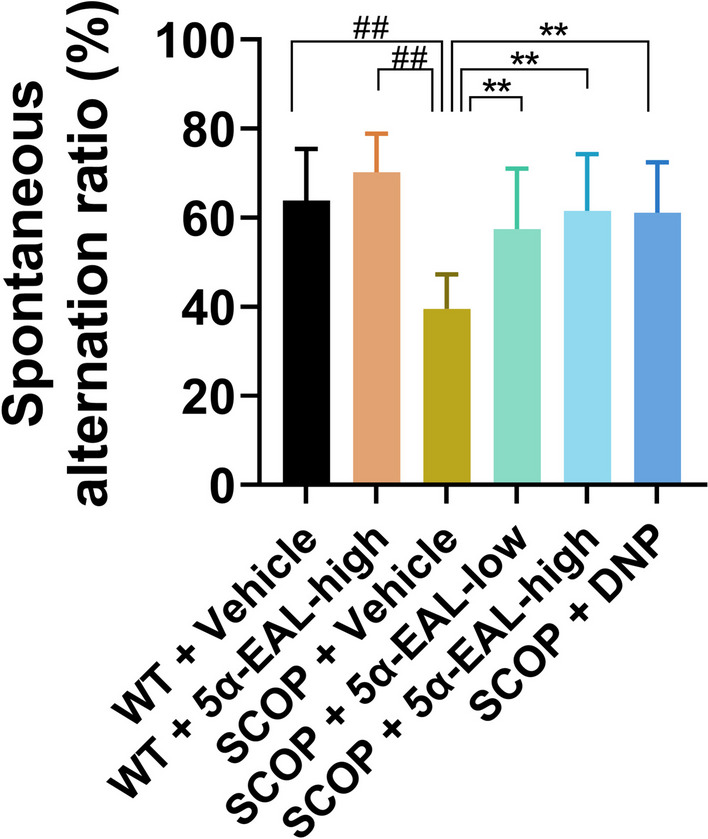
Effects of 5α-EAL on scopolamine-induced cognitive dysfunction based on spontaneous alternation ratio in a Y-maze test. Data are expressed as the mean ± SEM (n = 9–10). ##p < 0.01 vs. the WT + Vehicle group. **p < 0.01 vs. the SCOP + Vehicle
Effect of 5α-EAL on AChE activity in the hippocampus and cortex
AChE is a cholinergic enzyme in charge of breaking down acetylcholine [25], which is a key enzyme in the cholinergic system. The AChE activity in cortex and hippocampus after 5α-EAL treatment was investigated and the results was depicted in Fig. 8. The activity of AChE was substantially elevated in the SCOP + Vehicle group (0.50 ± 0.08 U/mg prot) in comparison with the WT + Vehicle group (0.22 ± 0.15 U/mg port). Whereas the group of SCOP + 5α-EAL-high group (0.23 ± 0.05 U/mg prot) could significantly decrease the activity of AChE in comparison with the SCOP + Vehicle group (0.50 ± 0.10 U/mg prot). Briefly, 5α-EAL has the potential to mitigate the cholinergic system imbalance in SCOP-induced mice.
Fig. 8.

Effect of 5α-EAL on AChE activity in the hippocampus and cortex of the scopolamine-induced mice. The activities of AChE in the brain were measured by assay kits. Data are expressed as the mean ± SEM (n = 9–10). #p < 0.05 vs. the WT + Vehicle group and *p < 0.05 vs. the SCOP + Vehicle group
Conclusion
In summary, it was found that 5α-EAL could significantly reduce NO, TNF-α, and PGE2 production by inhibiting iNOS and COX-2 expressions in LPS-stimulated BV-2 cells. 5α-EAL modulates the transformation of M1 phenotype macrophages to M2 phenotype macrophages after LPS stimulation in vitro, as well as penetrating the BBB. Additionally, 5α-EAL (10 and 30 mg/kg per day, i.p.) significantly improves cognitive impairments of AD mice in behavioral tests including Barnes and Y-maze tests, and 5α-EAL could reduce the activity of AChE in the hippocampus and cortex of AD mice. These findings confirmed that the 5α-EAL may be a promising natural product worthy of further exploitation for AD therapy.
Experimental section
Chemistry
Isolation and identification of 5α-EAL from Inula macrophylla
Whole plant collections are from Uzbekistan, in 2021 and were recognized as Inula macrophylla by Dr. K. A. Eshbakova. The specimen of the voucher was archived at the Academy of Sciences of the Republic of Uzbekistan.
10.0 kg of the Inula macrophylla were air-dried, powdered, and extracted with 90% ethanol. After evaporation, the residue was suspended in H2O and successively partitioned with EtOAc. The EtOAc layer (308 g) was fractionated into five fractions (A‒E) via silica gel column chromatography eluting with CHCl3–MeOH (from 60:1 to 3:1, v/v). Fr. B (18 g) was fractionated using medium-pressure liquid chromatography with a MeOH–H2O mobile phase (from 10:90 to 90:10, v/v) over a period of 50 min, resulting in the isolation of five fractions. Fr. B2 was purified by preparative HPLC (MeCN–H2O from 40:60 to 60:40, v/v, 60 min) to obtain 5α-EAL. The 1D NMR spectra were detected via a Bruker Avance III 400 instrument (Figs. S1 and S2).
In vitro activity
Cell viability assay in BV-2 cells
Murine microglial BV-2 cells were plated at a concentration of 8 × 103 cells/well in 96-well plates and cultured overnight. After treatment with different levels of 5α-EAL (1.25, 2.5, 5, 10 and 20 µM) for 24 h, 100 µL of MTT solution (5 mg/mL) from Solarbio in China was pipetted into each well. The plates were then incubated at 37 °C for 2 h. The blue formazan products in the cells were diluted in DMSO (200 µL/well) and mixed thoroughly. The optical density of the reaction medium was read at a wavelength of 570 nm using a microplate reader.
Inhibition of nitric oxide production in BV-2 cells
NO production inhibitory was measured according to the previous method [8]. Briefly, 96-well plates were plated with murine microglial BV-2 cells (2 × 105/well) and treated for 24 h with or without 1 μg/mL LPS (Escherichia coli 0111: B4, Sigma, U.S.) by adding 5α-EAL. The NO concentration in the supernatant was determined with an NO content assay kit (Solarbio, China).
ELISA assay
After treatment of LPS-stimulated BV-2 cells with 5α-EAL for 24 h, pro-inflammatory (TNF-α, PGE2) and anti-inflammatory (IL-10) mediator levels in culture supernatants were measured using ELISA kits according to the manufacturer’s method. (TNF-α: SU-BN20852, IL-10: SU-BN20162, R&D, USA; PGE2: KA0326, Abnova, China, Arg-1: ab269541, Abcam, USA).
Western blotting assay
The western blotting assays were measured according to the previous method [26]. In short, BV-2 cells were either exposed to 1 μg/mL LPS or left untreated, followed by treatment with DMSO or 5α-EAL at various concentrations. After 24 h, the cells were rinsed with chilled PBS (Solarbio). The chilled lysis buffer containing protease inhibitor and phosphatase inhibitor mixture (AbMole BioScience, China) was added to the cells and then gently scratched off. After centrifuging at 12,000 rpm for 10 min at 4 °C, Protein concentrations were assessed utilizing a bicinchoninic acid (BCA) assay kit from TIANGEN, China. Equivalent quantities of protein were subjected to electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels from EpiZyme, China, and subsequently transferred to NC membranes manufactured by GVS, USA. Following this, the membranes underwent blocking with 5% nonfat milk from BD for 1 h before being incubated overnight at 4 °C with primary antibody diluted appropriately. Subsequent to washing, the membranes were exposed to a specific secondary antibody for a duration of 2 h. The chemiluminescence western blotting detection system (Thermo Fisher Scientific, USA) was used to visualize the immunoreactive bands. ImageJ software was then used for band analysis. The main primary antibodies used in this study were rabbit monoclonal anti-iNOS (1:1000, Abcam, USA), rabbit monoclonal anti-COX-1 (1:1000, CST, USA), rabbit monoclonal anti-COX-2 (1:1000, CST, USA) and rabbit monoclonal anti-GAPDH (1:1000, CST). The secondary HRP-conjugated antibody was goat anti-rabbit IgG (1:5000, SAB).
Immunofluorescence for NF-κB translocation assay
Immunofluorescence experiment was conducted according to the previous method to evaluate the nuclear translocation of NF-κB p65 [26]. In brief, the BV-2 cells were plated in 8-well plates and incubated for 24 h. The cells were pretreated with 5α-EAL (10 μM) for 1 h and then activated by LPS (1 μg/mL). Then, the fixed cells were permeabilized with 0.2% (v/v) Triton X-100 for 10 min in 4 °C and washed three times with PBS containing 5% BSA and blocked with 5% bovine serum albumin (BSA in PBS) for 1 h at room temperature. The permeabilized cells were incubated with primary antibody (anti-p-NF-κB p65) overnight at 4 °C. After removing the primary antibody, the cells were incubated with Anti-rabbit IgG (H+L), F (ab′)2 Fragment (Alexa Fluor® 647 Conjugate) for 1 h at room temperature. After removing the secondary antibody, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI, 5 μg/mL in PBS) for 10 min. All images were obtained using a fluorescence microscope (Leica German).
Blood–brain barrier permeability assay
The BBB permeability of 5α-EAL was assessed using the parallel artificial membrane permeation assay (PAMPA) method, as previously described. The pre-coated PAMPA plate system (Cat. No. 353015) from Coning, U.S., was used to perform the permeability assays. Carbamazepine and hydrocortisone were supplied by Sigma. 5α-EAL was diluted in DMSO at a concentration of 8 mg/mL to form a stock solution, which was then diluted in PBS to a final concentration of 400 μg/mL. The solution was filtered through 0.22 μM syringe filters and pipetted into the donor wells (300 μL/well), while PBS was added to the wells of the recipient plate (200 μL/well). The filter plate was then assembled with the acceptor plate, and the entire assembly was incubated at room temperature for 16 h without agitation. At the end of the incubation, 150 μL of supernatant was removed from the donor and acceptor well and quantified by HPLC with a UV screening detector at 210 nm.
In vivo activity
Animals and ethical statement
Male C57BL/6J mice were obtained from the Fourth Military Medical University, Shaanxi, China (n = 10/group) at 6–8 weeks of age and 25–30 g in weight. Animals were placed in a dedicated germ-free animal room under conditions of 12/12 light/dark cyclicity. They were provided with standardized nutrition, which consisted of pure water and AIN-93M. All animal procedures were performed under the principles and guidelines outlined in the Care and Use of Laboratory Animals.
In this study, all mice were randomized into six groups (n = 10/group) as shown below: group I, control (WT + Vehicle); group II, control + high dose 5α-EAL (30 mg/kg per day, WT + 5α-EAL-high); group III, SCOP (SCOP + Vehicle); group IV, SCOP + low-dose 5α-EAL (10 mg/kg per day, SCOP + 5α-EAL-low); group V, SCOP + high-dose 5α-EAL (30 mg/kg per day, SCOP + 5α-EAL-high); group VI, SCOP + DNP (3 mg/kg per day, SCOP + DNP). 5α-EAL (10 and 30 mg/kg per day) was dissolved in a solution of water containing 10% Tween 80, 10% ethanol, and 80% distilled water (0.1% w/vol). Donepezil Hydrochloride was dissolved in saline. The aforementioned groups were administered to 8-month-old WT mice for a period of 3 weeks. The WT + Vehicle, WT + 5α-EAL-high, SCOP + 5α-EAL-low, and SCOP + 5α-EAL-high groups received treatment via intraperitoneal (i.p.) injection of 5α-EAL, while the DNP group was treated with an oral gavage (i.g.) of DNP.
Barnes maze task
The Barnes Maze is the most widely used test in behavioral neuroscience to study spatial learning and memory in rodents. Briefly, mice were acclimated to the experimental room for 30 min before each daily experiment. Subsequently, they were placed in the center of the Barnes Maze in a starting box for 20 s. The start box was taken away and the mice were permitted to explore the Barnes maze for 3 min. If the mice did not locate the destination box on the first day, they were guided to find the hole and then remain in the box for 1 min. There was no guidance provided on days 2–4. Mice were under study for 4 consecutive days. On the fifth day, the dark box of the target hole was withdrawn, and the latency time, residence time in the destination quadrant, number of times of exploring the target hole, and the movement trajectory of the mice were recorded in the 90 s period. The table was wiped with 75% disinfectant alcohol before and after the test to remove odor interference.
Y-maze task
The Y-maze experiment is a common experimental tool for assessing working memory. The Y-maze comprises three equal arms (40 cm × 15 cm × 9 cm). Mice were placed in the center of the Y-maze and given 5 min to explore the three arms. Their motion was automatically recorded with a video tracker.
Measurement of AChE activity
After the behavior tests on day 28, Mice were executed by cervical dislocation. The mouse cortex and hippocampus were collected, washed with cold sterile saline, and stored at − 80 °C prior to use. The tissues of cortices and hippocampi were weighed, homogenized, and centrifuged at 4 °C 4000 rpm for 5 min. After 10 min collect the supernatant. The measurement of AChE activity was conducted following the instruction of acetylcholinesterase assay kit. This assay was conducted in a 2 mL centrifuge tube, adding 30 µL of 10% tissue solution, 500 µL of substrate buffer, and 500 µL of coloring liquid for each group. Incubate at 37 °C for 6 min, then add 30 µL of inhibitor and 100 µL of transparent agent, respectively. After incubation for 15 min, the OD value was measured at 412 nm using a microplate reader, and then activity was calculated.
Statistical analysis
GraphPad 8.0 software was used for statistical analysis. The experimental data were presented as mean ± SEM for each group. Student’s t-test or two-way analysis of variance (ANOVA) followed by Tukey post hoc test was used for all statistical analyses. *p < 0.05, **p < 0.01, and ***p < 0.001 were regarded as statistically significant for between-group differences.
Supplementary Information
Supplementary Material 1. Synthesis of 5α-EAL by epoxidation of alantolactone; NMR spectrum, HR-ESI–MS spectrum, and HPLC spectra for 5α-EAL.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2021YFE0103700), the National Natural Science Foundation of China (U21A20407, 22274163, and 22374160) and Shaanxi Fundamental Science Research Project for Chemistry and Biology (No. 23JHQ054).
Author contributions
RM: writing—original draft, data curation, conceptualization. XY F: software, methodology, visualization. JJ T: methodology, writing—review and editing, formal analysis, conceptualization. WH: writing—review and editing, funding acquisition, resources, project administration. YP S: funding acquisition, data curation, project administration, supervision, resources.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui Ma and Xu-Yao Feng contributed equally to this work.
Contributor Information
Wei Ha, Email: hawei2012@licp.cas.cn.
Yan-Ping Shi, Email: shiyp@licp.cas.cn.
References
- 1.Alzheimer’s Association Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;2015(11):332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, Ames D, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balakrishnan R, Kim Y-S, Kim G-W, Hong S-M, Kim C-G, et al. Standardized extract of Glehnia Littoralis abrogates memory impairment and neuroinflammation by regulation of CREB/BDNF and NF-κB/MAPK signaling in scopolamine-induced amnesic mice model. Biomed Pharmacother. 2023;165:115106. doi: 10.1016/j.biopha.2023.115106. [DOI] [PubMed] [Google Scholar]
- 5.Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talebi M, Khoramjouy M, Feizi A, Ali Z, Khan IA, Ayatollahi NA, et al. Novel multi-target therapeutic potential of the genus Inula: advances and opportunities for neuroprotection. Pharmacol Res Modern Chin Med. 2023;7:100263. doi: 10.1016/j.prmcm.2023.100263. [DOI] [Google Scholar]
- 7.Tseng P-T, Zeng B-S, Suen M-W, Wu Y-C, Correll CU, Zeng B-Y, et al. Efficacy and acceptability of anti-inflammatory eicosapentaenoic acid for cognitive function in Alzheimer’s dementia: a network meta-analysis of randomized, placebo-controlled trials with omega-3 fatty acids and FDA-approved pharmacotherapy. Brain Behav Immun. 2023;111:352–364. doi: 10.1016/j.bbi.2023.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Tang J-J, Huang L-F, Deng J-L, Wang Y-M, Guo C, Peng X-N, et al. Cognitive enhancement and neuroprotective effects of 1,6-O, O-diacetylbritannilactone, a sesquiterpene lactone in 5xFAD Alzheimer’s disease mice model. Redox Biol. 2022;50:102229. doi: 10.1016/j.redox.2022.102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceylan R, Zengin G, Mahomoodally MF, Sinan KI, Ak G, Jugreet S, et al. Enzyme inhibition and antioxidant functionality of eleven Inula species based on chemical components and chemometric insights. Biochem Syst Ecol. 2021;95:104225. doi: 10.1016/j.bse.2021.104225. [DOI] [Google Scholar]
- 10.Liu F, Dong B, Yang X, Yang Y, Zhang J, Jin D-Q, et al. NO inhibitors function as potential anti-neuroinflammatory agents for AD from the flowers of Inula japonica. Bioorg Chem. 2018;77:168–175. doi: 10.1016/j.bioorg.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Ma R, Ha W, Zhang H-X, Shi Y-P. An MnO2-ZIF-67 immobilized acetylcholinesterase method for acetylcholinesterase activity assay and inhibitor screening from Inula macrophylla based on capillary electrophoresis. Talanta. 2023;253:124025. doi: 10.1016/j.talanta.2022.124025. [DOI] [Google Scholar]
- 12.Shi Y, Dong T, Zeng B, Yao M, Wang Y, Xie Z, et al. Production of plant sesquiterpene lactone parthenolide in the yeast cell factory. ACS Synth Biol. 2022;11:2473–2483. doi: 10.1021/acssynbio.2c00132. [DOI] [PubMed] [Google Scholar]
- 13.Zhi X-Y, Zhang Y, Li Y-F, Liu Y, Niu W-P, Li Y, et al. Discovery of natural sesquiterpene lactone 1-O-acetylbritannilactone analogues bearing oxadiazole, triazole, or imidazole scaffolds for the development of new fungicidal candidates. J Agric Food Chem. 2023;71:11680–11691. doi: 10.1021/acs.jafc.3c02497. [DOI] [PubMed] [Google Scholar]
- 14.Fu B, Su B-N, Takaishi Y, Honda, Ito M, Takeda Y, et al. A bis-sesquiterpene and sesquiterpenolides from Inula macrophylla. Phytochemistry. 2001;58:1121–1128. doi: 10.1016/S0031-9422(01)00334-X. [DOI] [PubMed] [Google Scholar]
- 15.Su B-N, Takaishi Y, Yabuuchi T, Kusumi T, Tori M, Takaoka S, et al. Sesquiterpenes and monoterpenes from the bark of Inula macrophylla. J Nat Prod. 2001;64:466–471. doi: 10.1021/np000211h. [DOI] [PubMed] [Google Scholar]
- 16.Ma R, Komilov B, Wang C-B, Eshbakova KA, Yang J-L, Ha W, et al. Sesquiterpenes from Inula macrophylla and their anti-neuroinflammatory activity. Phytochem Lett. 2024;60:257–263. doi: 10.1016/j.phytol.2024.02.003. [DOI] [Google Scholar]
- 17.Cantrell CL, Abate L, Fronczek FR, Franzblau SG, Fischer NH. Antimycobacterial eudesmanolides from Inula helenium and Rudbeckia subtomentosa. Planta Med. 1999;65:351–355. doi: 10.1055/s-1999-14001. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Jia H, Si M, Li X, Ma Z, Zhu Y, et al. Loureirin B protects against cerebral ischemia/reperfusion injury through modulating M1/M2 microglial polarization via STAT6/NF-kappaB signaling pathway. Eur J Pharmacol. 2023;953:175860. doi: 10.1016/j.ejphar.2023.175860. [DOI] [PubMed] [Google Scholar]
- 19.Dogan Z, Telli G, Tel BC, Saracoglu I. Scutellaria brevibracteata Stapf and active principles with anti-inflammatory effects through regulation of NF-κB/COX-2/iNOS pathways. Fitoterapia. 2022;158:105159. doi: 10.1016/j.fitote.2022.105159. [DOI] [PubMed] [Google Scholar]
- 20.Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Rev. 2005;48:251–256. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Nair S, Sobotka KS, Joshi P, Gressens P, Fleiss B, Thornton C, Mallard C, Hagberg H. Lipopolysaccharide-induced alteration of mitochondrial morphology induces a metabolic shift in microglia modulating the inflammatory response in vitro and in vivo. Glia. 2019;67:1047–1061. doi: 10.1002/GLIA.23587. [DOI] [PubMed] [Google Scholar]
- 22.Konczol A, Muller J, Foldes E, Beni Z, Vegh K, Kery A, et al. Applicability of a blood–brain barrier specific artificial membrane permeability assay at the early stage of natural product-based CNS drug discovery. J Nat Prod. 2013;76:655–663. doi: 10.1021/np300882f. [DOI] [PubMed] [Google Scholar]
- 23.Di L, Kerns EH, Fan K, McConnell OJ, Carter GT. High throughput artificial membrane permeability assay for blood–brain barrier. Eur J Med Chem. 2003;38:223–232. doi: 10.1016/S0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland T, Tariot PN, Weingartner H, Murphy DL, Newhouse PA, Mueller EA, et al. Pharmacologic modelling of Alzheimer’s disease. Progr Neuro-Psychopharmacol Biol Psychiatry. 1986;10:599–610. doi: 10.1016/0278-5846(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 25.Sandeep Ganesh G, Konduri P, Kolusu AS, Namburi SV, Chunduru BT, Nemmani KV, et al. Neuroprotective effect of saroglitazar on scopolamine-induced Alzheimer’s in rats: insights into the underlying mechanisms. ACS Chem Neurosci. 2023;14:3444–3459. doi: 10.1021/acschemneuro.3c00320. [DOI] [PubMed] [Google Scholar]
- 26.Tang J-J, Wang M-R, Dong S, Huang L-F, He Q-R, Gao J-M. 1,10-Seco-eudesmane sesquiterpenoids as a new type of anti-neuroinflammatory agents by suppressing TLR4/NF-κB/MAPK pathways. Eur J Med Chem. 2021;224:113713. doi: 10.1016/j.ejmech.2021.113713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Synthesis of 5α-EAL by epoxidation of alantolactone; NMR spectrum, HR-ESI–MS spectrum, and HPLC spectra for 5α-EAL.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.