Abstract
Human cytomegalovirus (CMV) is the leading cause of prenatal viral infection. Affected infants may suffer intrauterine growth retardation and serious neurologic impairment. Analysis of spontaneously aborted conceptuses shows that CMV infects the placenta before the embryo or fetus. In the human hemochorial placenta, maternal blood directly contacts syncytiotrophoblasts that cover chorionic villi and cytotrophoblasts that invade uterine vessels, suggesting possible routes for CMV transmission. To test this hypothesis, we exposed first-trimester chorionic villi and isolated cytotrophoblasts to CMV in vitro. In chorionic villi, syncytiotrophoblasts did not become infected, although clusters of underlying cytotrophoblasts expressed viral proteins. In chorionic villi that were infected with CMV in utero, syncytiotrophoblasts were often spared, whereas cytotrophoblasts and other cells of the villous core expressed viral proteins. Isolated cytotrophoblasts were also permissive for CMV replication in vitro; significantly, infection subsequently impaired the cytotrophoblasts' ability to differentiate and invade. These results suggest two possible routes of CMV transmission to the fetus: (i) across syncytiotrophoblasts with subsequent infection of the underlying cytotrophoblasts and (ii) via invasive cytotrophoblasts within the uterine wall. Furthermore, the observation that CMV infection impairs critical aspects of cytotrophoblast function offers testable hypotheses for explaining the deleterious effects of this virus on pregnancy outcome.
Human cytomegalovirus (CMV) infection, which usually has a benign course in immunocompetent individuals, can have catastrophic consequences during pregnancy (3). Primary CMV infection during gestation poses a 30 to 40% risk of intrauterine transmission and clinical disease (58, 59). Reactivated infection is associated with at least a 10-fold-lower rate of transmission. Congenital CMV infection is a relatively common occurrence, as approximately 1 to 4% of newborns in the United States and Europe are infected with CMV (3), and transmission could be higher in developing countries (13). Many infected infants show no clinical manifestations of the congenital CMV syndrome. Symptomatic infants often succumb in the neonatal period (12%), and most survivors have permanent debilitating sequelae, including mental retardation, vision loss, and sensorineural deafness. Since CMV establishes latent infections in granulocyte-dendritic progenitors (25, 34, 56), the fetus may also become infected after reactivation of maternal infection, a scenario that is usually associated with less severe clinical disease in the offspring (18, 59). CMV seroconversion rates and restriction endonuclease analyses of virus strains indicate that heterosexual activity (5, 6, 17, 27) and contact with young children (30, 47) are the major modes of virus dissemination in women of childbearing age.
Despite the morbidity and mortality associated with prenatal CMV infection, little is known about how the virus infects the conceptus. Approximately 15% of women with primary infections during early pregnancy abort spontaneously (24). In this case the placenta, but not the fetus, shows evidence of infection, which suggests that placental involvement is important in its own right and precedes virus transmission to the fetus (1, 28, 44). Later in pregnancy CMV infection causes premature delivery and, in 25% of affected infants, intrauterine growth retardation (31), outcomes that are often associated with placental pathology. Numerous reports indicate that placentas from these births also contain viral proteins (44, 45), suggesting that placental infection and virus transmission to the infant are related causally.
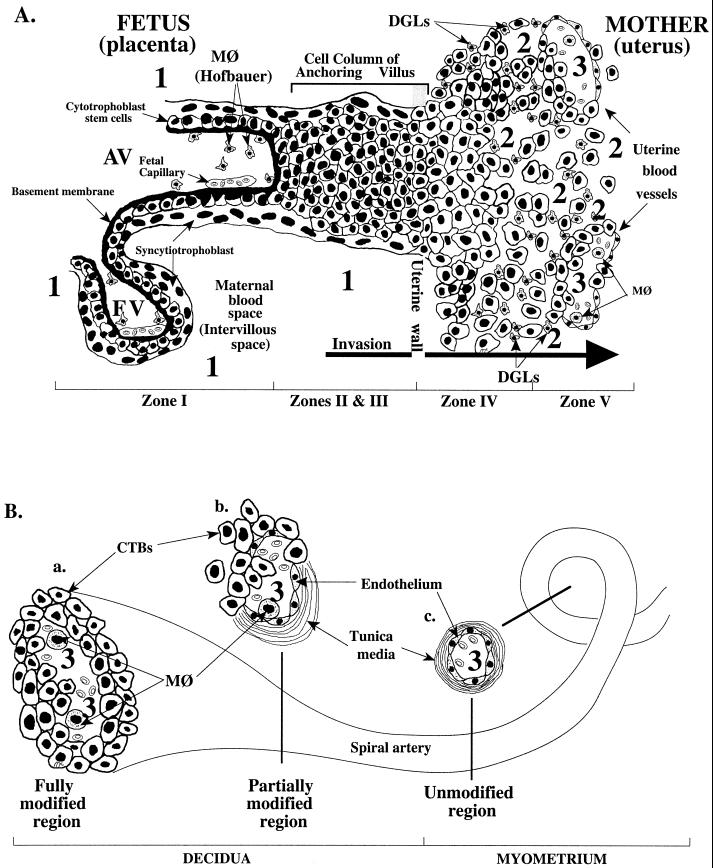
An important role for the placenta in CMV transmission to the fetus is also suggested by the unusual anatomy of the maternal-fetal interface (Fig. 1), which is determined in large part by placental development (reviewed in references 9 and 10). Placentation is a stepwise process that entails differentiation of the organ's specialized epithelial stem cells, termed cytotrophoblasts. Two pathways give rise to the differentiated trophoblast cells that are found in floating and anchoring chorionic villi. In the pathway that gives rise to floating villi, cytotrophoblasts differentiate by fusing into multinucleate syncytiotrophoblasts that cover the villous surface, where they are in direct contact with maternal blood. This trophoblast population is specially adapted for transporting a wide variety of substances to and from the embryo or fetus. In the pathway that gives rise to anchoring villi, cytotrophoblasts remain as single cells that aggregate into columns and invade the endometrium and the first third of the myometrium (interstitial invasion). They also breach the portions of maternal arterioles that span these regions (endovascular invasion). By midgestation, the latter population of cells completely replaces the endothelial lining and much of the smooth muscle wall of these vessels. The result is a hybrid vasculature composed of fetal and maternal cells.
FIG. 1.
(A) Diagram of a longitudinal section that includes a floating and an anchoring chorionic villus at the fetal-maternal interface near the end of the first trimester of human pregnancy (10 weeks of gestational age) (modified from references 10 and 66). The anchoring villus (AV) functions as a bridge between the fetal and maternal compartments, whereas the floating villus (FV), containing macrophages (MØ, Hofbauer cells) and fetal blood vessels, is bathed by maternal blood. Cytotrophoblasts in AV (zone I) form cell columns that attach to the uterine wall (zones II and III). Cytotrophoblasts then invade the uterine interstitium (decidua and first third of the myometrium; zone IV) and maternal vasculature (zone V), thereby anchoring the fetus to the mother and accessing the maternal circulation. Zone designations mark areas in which cytotrophoblasts have distinct patterns of stage-specific antigen expression, including integrins and HLA-G. Decidual granular leukocytes (DGLs) and macrophages (MØ) in maternal blood and fetal capillaries in villous cores are indicated in panels A and B. Areas proposed as sites of natural CMV transmission to the placenta in utero are numbered 1, 2, and 3. (B) Diagram of a uterine (spiral) artery in which endovascular invasion is in progress (10 to 20 weeks of gestation). Endometrial and then myometrial segments of spiral arteries are modified progressively. In fully modified regions (a), the vessel diameter is large. Cytotrophoblasts (CTBs) are present in the lumen and occupy the entire surface of the vessel wall. A discrete muscular layer (tunica media) is not evident. (b) Partially modified vessel segments. Cytotrophoblasts and maternal endothelium occupy discrete regions of the vessel wall. In areas of intersection, cytotrophoblasts appear to lie deep in the endothelium and in contact with the vessel wall. (c) Unmodified vessel segments in the myometrium. Vessel segments in the superficial third of the myometrium will become modified when endovascular invasion reaches its fullest extent (about midgestation), while deeper segments of the same artery will retain their normal structure.
These unusual cell-cell interactions are the result of an equally unusual molecular differentiation program. For example, syncytiotrophoblasts that cover floating villi upregulate expression of the neonatal immunoglobulin G (IgG) Fc receptor (hFcRn), which binds and transports maternal IgG to the fetus (38, 53, 62). This important process establishes passive immunity to certain infectious agents. Invading cytotrophoblasts that are components of anchoring villi switch on the expression of adhesion molecules (e.g., integrin α1β1) and proteinases (e.g., matrix metalloproteinase-9) that are needed for invasion, as well as molecules that elicit maternal immune tolerance (e.g., the nonclassical major histocompatibility complex [MHC] class Ib molecule HLA-G [35, 43]) and the cytokine interleukin-10 [51]). In a process termed pseudovasculogenesis, invading cells also transform their adhesion receptor phenotype to resemble that of the endothelial cells that they replace. For example, they express αvβ3 integrin, a marker of angiogenic endothelium, and vascular endothelial cadherin (10). Both cytotrophoblast invasion and pseudovasculogenesis are essential for normal pregnancy, as serious complications (e.g., preeclampsia) can occur when this process fails (41, 48, 64, 65).
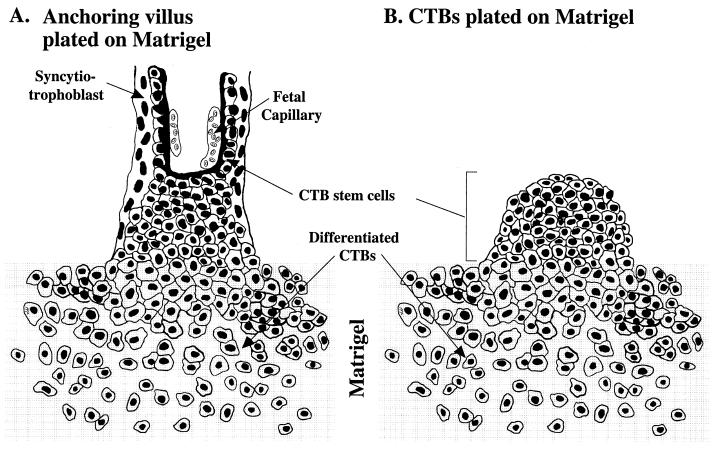
A great deal of information about the human placenta, largely inaccessible for study in utero, has been obtained by studying culture models of the trophoblast populations that lie at the maternal-fetal interface. The two most commonly used models are villous explants and isolated cytotrophoblasts (15, 16, 20, 40). Explants (Fig. 2A) are essentially organ cultures of anchoring villi in which cell columns attach to, and subsequently invade, an extracellular matrix substrate. When isolated cells are plated on extracellular matrixes (Fig. 2B), they rapidly differentiate along the invasive pathway, acquiring the specialized properties of the cytotrophoblast subpopulation that is found within the uterine wall. Both models have been integral to recent progress made in understanding the factors that govern assembly of the human maternal-fetal interface in normal pregnancy and how this process goes awry in pregnancy complications such as preeclampsia (10).
FIG. 2.
Culture models for studying CMV infection of anchoring villus explants and differentiating cytotrophoblasts (CTBs). (A) Diagram of an anchoring villus explant attached to a Matrigel substrate via cytotrophoblasts that migrate from the cell columns. (B) Diagram of purified cytotrophoblasts cultured on Matrigel. The cytotrophoblast stem cells aggregate, invade the matrix, and express stage-specific molecules, including integrins and HLA-G. Cultured cytotrophoblasts mimic the differentiation phenotype and morphology of cell columns formed in placentas in utero. For infection, CMV is added to the medium bathing the explants and cytotrophoblasts.
Here we used these culture models to study CMV infection of human placental cells in vitro. During the course of these experiments, we discovered that a significant number of placentas had already been infected with CMV in utero, allowing a rare glimpse into the natural process. This also gave us an interesting opportunity to compare the populations of placental cells that expressed viral proteins in the two situations. We also investigated the consequences of infection in vitro on the ability of isolated cytotrophoblasts to differentiate along the invasive pathway. We found that the placenta is not an effective barrier to CMV transmission. Rather, cytotrophoblasts in several locations become infected, suggesting specific routes by which the virus reaches the fetus in utero. Furthermore, cytotrophoblasts are not a passive conduit: CMV infection resulted in significant deficits in their ability to differentiate and invade. Together, the results of these experiments suggest an explanation for the association between CMV infection of the fetus and intrauterine growth retardation, as well as strategies for blocking the routes of transmission that we identified.
MATERIALS AND METHODS
Chorionic villus isolation and explant culture.
Filters (12-mm diameter) with 0.4-μm pores (Millipore Products Division, Bedford, Mass.) were coated with 100 μl of Matrigel (Collaborative Research, Bedford, Mass.) as previously described (20). Six- to eight-week human placentas were obtained from donors who had normal pregnancies prior to termination. Approval for this project was obtained from the Institutional Review Board at the University of California San Francisco. Anchoring villi were dissected from placentas and transferred to the coated filters. Cultures established from 13 placentas were used in this study. Initially, 22 fragments containing tree-like anchoring villi were dissected from the entire surface of each placenta. Ten were immediately fixed and processed for immunolocalization studies as previously described (11). The remaining 12 were cultured on Matrigel substrates in Dulbecco's modified Eagle's medium-F12 medium (DMEM-F12; 1:1, vol/vol; GIBCO, Rockville, Md.) supplemented with 10% fetal calf serum. After 12 h, six were infected with CMV as described below. Explants were maintained for up to 96 h. This model system is diagrammed in Fig. 2A.
Isolation and culture of purified cytotrophoblasts.
Highly purified cytotrophoblasts were isolated from 10- to 16-week placentas as previously described (40). A small fraction of the cells (5 × 104) were immobilized on slides by centrifugation (Cytospin Cell Preparation System; Shandon Inc., Pittsburgh, Pa.) and then fixed and stained with monoclonal antibody (MAb) CH160-5 to CMV immediate-early proteins 1 and 2 (IE1/2) (14). The results showed whether cytotrophoblasts were infected in utero. The remainder were resuspended in DMEM containing 2% Nutridoma (Boehringer Mannheim Corp., Indianapolis, Ind.). Transwell filters (6.5 mm in diameter, 5-μm pore size; Costar, Corning, N.Y.) were coated with 10 μl of Matrigel, and then 2.5 × 105 to 5.0 × 105 cells were plated on each. After 12 h, half the cultures were infected with virus as described below. Cultures were maintained for up to 96 h. This model system is diagrammed in Fig. 2B.
CMV stock viruses, infection, and titration.
The construction of CMV(AD169) mutants RV798 and RV670 with deletions in genes that downregulate expression of classical MHC class I molecules has been published (33). Stock viruses were prepared in human foreskin fibroblasts (HFF) grown in roller bottles, and the infectivity titers were determined by immunofluorescence using a rapid infectivity assay (46). At 12 h after plating, villous explants and purified cytotrophoblasts were infected with 106 PFU per filter. To count CMV progeny virions, cytotrophoblasts (2.5 × 105 to 5.0 × 105) and HFF (0.5 × 105 to 1.0 × 105) were plated on Matrigel-coated filters (0.4-μm pores) and infected with CMV(AD169) at 10 and 1 PFU/cell, respectively. At 24-h intervals, cells were harvested, sonicated to release intracellular virus, and centrifuged at low speed to remove cell debris. Released virions in the culture medium were counted separately.
Antibodies.
A mouse MAb, CH160-5, to the CMV IE1/2 proteins was produced in the Pereira laboratory (14) and obtained as purified IgG from the Goodwin Institute (Plantation, Fla.). Guinea pig antiserum to CMV gB (UL55) was a generous gift from Chiron Corporation (Emeryville, Calif.). The following antitrophoblast antibodies were produced in the Fisher laboratory unless otherwise noted: a rat MAb, 7D3, to cytokeratin (11); a mouse MAb, 4H84, to a synthetic peptide of the α1 domain of HLA-G (42); a mouse MAb, BIIG2, to integrin α5; and anti-VLA-1 to integrin α1 (T-Cell Sciences, Cambridge, Mass.). The specificities of the secondary antibodies, all of which were obtained from Jackson ImmunoResearch Laboratories Inc. (West Grove, Pa.), were as follows: goat anti-mouse IgG labeled with fluorescein isothiocyanate (FITC) or rhodamine, goat anti-rat IgG labeled with rhodamine, and goat anti-guinea pig IgG labeled with FITC. Antibodies were used at the following dilutions: 1:500, anti-gB; 1:100, anti-CMV IE1/2; 1:50, anti-integrin α5; 1:50, anti-integrin α1; and 1:20, anti-HLA-G.
Immunochemistry.
Samples were processed for double indirect immunofluorescence localization as described previously (11, 15, 20). Briefly, the explants and filters were rinsed in phosphate-buffered saline, fixed in 3% paraformaldehyde overnight, and infiltrated with 5 to 15% sucrose followed by embedding in optimal-cutting-temperature compound. Before the final embedding step, the explants and Matrigel were removed from the inserts; after embedding was completed, they were frozen in liquid nitrogen. Sections (5 to 7 μm) were cut on a Hacker-Slee cryostat and collected on slides. Isolated cytotrophoblasts plated on Matrigel-coated filters were fixed in 3% paraformaldehyde for 20 min, washed, and permeabilized for 5 min with cold methanol. In some experiments fixed tissue sections or cells were stained for 1 h with a mixture of rat anti-human cytokeratin (to identify trophoblasts) and anti-CMV antibodies. In other experiments the mixture contained anti-gB and an antibody that recognized either an integrin or HLA-G. The samples were then washed and incubated with the appropriate secondary antibodies conjugated to FITC or rhodamine. Samples were viewed with a Zeiss Axiophot epifluorescence microscope equipped with filters to selectively view the rhodamine and fluorescein images.
Invasion assay.
Cytotrophoblast invasiveness was quantified in an in vitro invasion assay (diagrammed in Fig. 8) as previously described (40). Briefly, the cells were isolated and plated on Matrigel-coated filters (six total per experiment), and half of the cultures were infected with CMV as described above. After 48 h, the filter inserts, together with the cultured cells, were excised with a scalpel blade. The samples were stained with a mixture of antibodies that recognized cytokeratin (7D3) and CMV IE1/2 proteins (CH160-5). Afterwards the filters were mounted on slides. CMV infection was evaluated by assessing IE1/2 and cytokeratin expression on the top surface of the filter. Invasion was quantified by counting cytokeratin-positive cell processes that penetrated the Matrigel and appeared on the underside of the filter. The entire experiment was repeated three times.
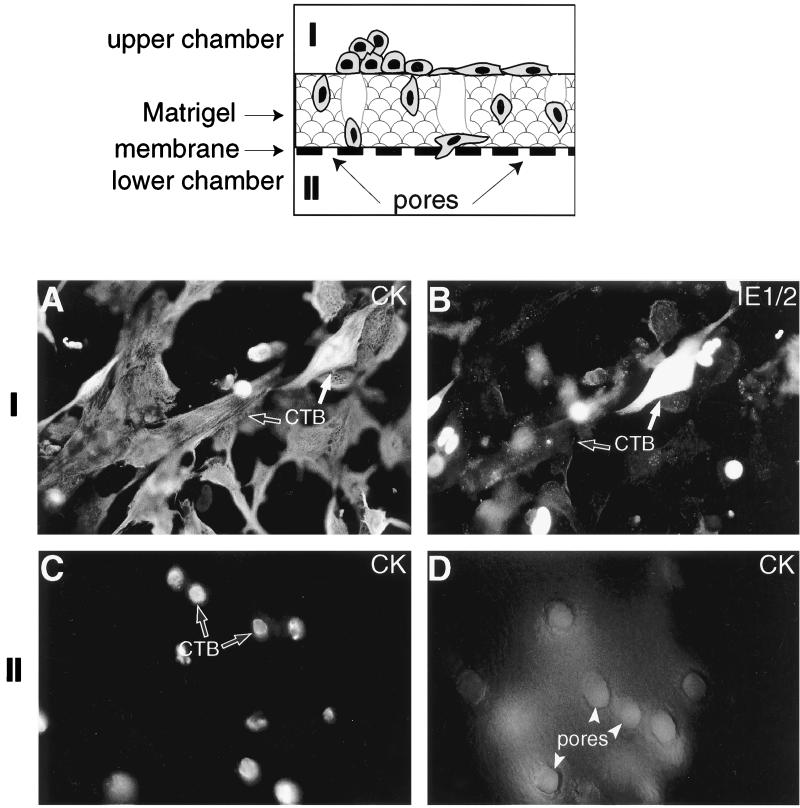
FIG. 8.
CMV infection impairs cytotrophoblast invasion in vitro. (Upper panel) Diagram of the assay that assesses the ability of cells to penetrate the Matrigel substrate, migrate through pores in the underlying filter, and emerge on the underside. Purified cytotrophoblasts (CTB) were cultured on Matrigel-coated filters and infected with CMV 12 h later. Staining of the upper surface of the filter (I) for (A) cytokeratin (CK) and (B) IE1/2 proteins expression showed that ∼30% of the cells were infected 48 h after plating. Invasion was quantified by determining the number of cytokeratin-positive cell processes that penetrated the Matrigel and appeared in the pores (marked with arrowheads) that open on the underside of the filter (II). In control cultures (C) many processes were visible, whereas in CMV-infected samples (D) only the pores were visible, indicating a significant reduction in invasion.
RESULTS
CMV proteins are expressed in distinct patterns in placental cells in chorionic villi after infection either in vitro or in utero.
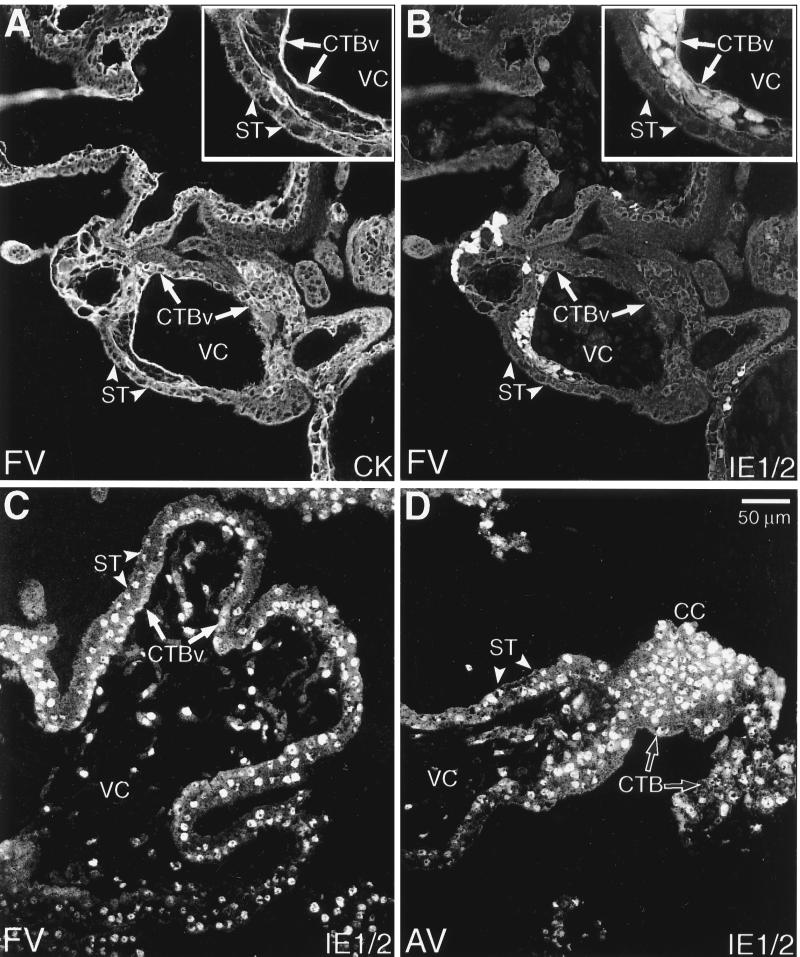
First, we investigated CMV infection of chorionic villi in vitro using the culture model illustrated in Fig. 2A. As described in Materials and Methods, an important part of the experimental design was to show that the placentas from which the chorionic villi were dissected had not been infected in utero. Figure 3 shows tissue sections of villous explants that were incubated for 4 days after infection with CMV. The sections were double stained with anticytokeratin to identify trophoblast cells (Fig. 3A and C) and an MAb to CMV IE1/2 proteins to identify infected cells (Fig. 3B and D). Routinely, syncytiotrophoblasts that cover the villous surface were not infected and failed to stain with the MAb to CMV IE1/2 proteins. Unexpectedly, we observed nuclear staining of isolated clusters of underlying cytotrophoblast stem cells (Fig. 3B). The pattern was distinctive; in each section, groups of ≤10 adjacent cells reacted with the antibody. We observed this staining pattern in villous explants from seven different placentas that were infected with CMV in vitro. In some explants CMV IE1/2 protein expression was also detected in cytotrophoblasts found in the cell columns of anchoring villi (Fig. 3D). In one instance, we found that the majority of cytotrophoblast stem cells expressed CMV IE1/2 proteins (data not shown). Explants from five other healthy placentas failed to develop infection at 5 days after culture with CMV.
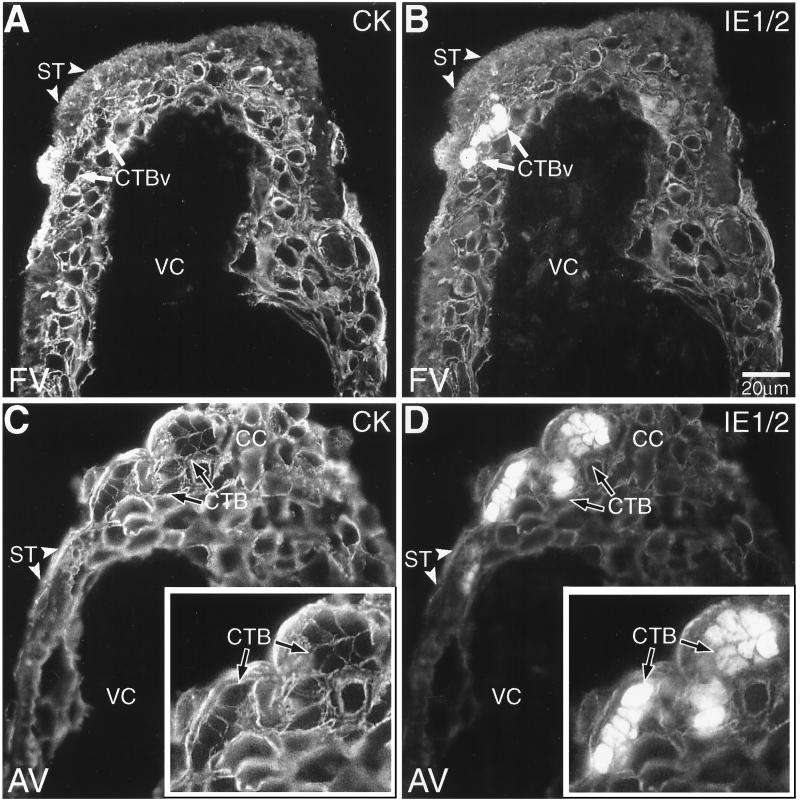
FIG. 3.
Cytotrophoblasts (CTBs) in villous explants were infected with CMV in vitro. Tissue sections prepared from both floating villi (FV; A and B) and anchoring villi (AV; C and D) were analyzed by double staining with anticytokeratin and anti-CMV IE1/2. (A) Cytokeratin (CK) staining of floating villi showed the multinucleate syncytiotrophoblasts (ST; arrowheads) that cover the villous surface and the underlying villous cytotrophoblast stem cells (CTBv; arrows). (B) CMV IE1/2 proteins were expressed by underlying clusters of infected villous cytotrophoblast stem cells. The inner stromal villous cores (VC) were consistently negative for anti-IE1/2 antibody staining. (C and D) IE1/2 protein expression was also detected in cytotrophoblasts found in the cell columns (CC) of anchoring villi. Insets show infected cytotrophoblasts at higher magnification. As a control, staining was performed as described for the experimental situation except that the primary or secondary antibody was omitted. No staining was detected (data not shown).
Because we were screening placentas for CMV IE1/2 protein expression prior to culture, we obtained five specimens that had already been infected in utero. The staining patterns that we saw had remarkable similarities to and differences from those we observed after CMV infection in vitro. With regard to similarities, in three specimens we observed areas in which CMV IE1/2 protein staining patterns were virtually indistinguishable from those observed after infection in vitro; isolated clusters of cytotrophoblasts underlying the syncytium were the only CMV-infected cells (Fig. 4B). In one placenta, we found that nearly all the cytotrophoblast stem cells (Fig. 4C), as well as those found within columns, expressed CMV IE1/2 proteins (Fig. 4D). Comparatively fewer syncytial nuclei stained, but numerous cells within the villous cores expressed these CMV proteins (Fig. 4C). Based on morphological criteria, these included fibroblasts, macrophages, and endothelial cells. In a different placenta we detected yet another staining pattern. Nearly all the fibroblasts in the villous core expressed CMV antigens. Comparatively fewer cytotrophoblasts were stained, primarily clusters of villous stem cells. About 50% of syncytiotrophoblasts were also stained.
FIG. 4.
Cytotrophoblasts (CTBs) and other cells show evidence of natural infection of chorionic villi with CMV in utero. Both floating villi (FV; A to C) and anchoring villi (AV; D) were studied. Tissues were analyzed by using immunolocalization techniques for expression of (A) cytokeratin (CK) and (B to D) CMV IE1/2 proteins. (B) In some cases, clusters of CMV-infected villous cytotrophoblast stem cells (CTBv; arrows) underlying the syncytium (ST, arrowheads) were the only sites of antibody reactivity. More often, numerous cells throughout the villi stained with anti-IE1/2 antibody. (C) In floating villi, nuclei of syncytiotrophoblasts, villous cytotrophoblasts, and stromal components expressed IE1/2 proteins. (D) The same pattern of immunoreactivity was seen in infected anchoring villi. Additionally, cytotrophoblasts in cell columns (CC) stained brightly. As a control, staining was performed as described for the experimental situation except that the primary or secondary antibody was omitted. No staining was detected (data not shown).
CMV replicates and virions are released from differentiating cytotrophoblasts infected in vitro.
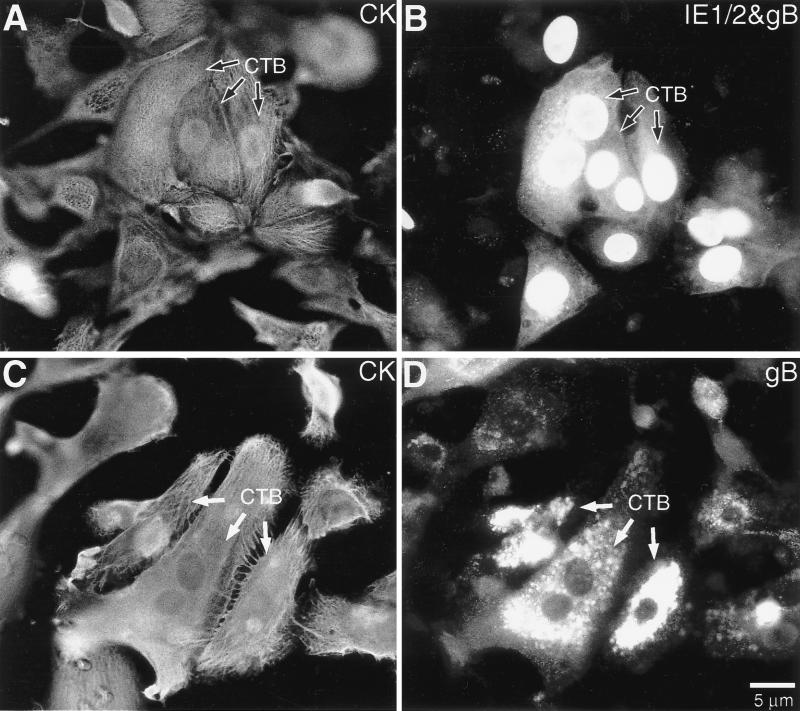
We further investigated CMV replication in human placental cells by using a second in vitro model (illustrated in Fig. 2B). In this model, cytotrophoblast stem cells isolated from chorionic villi are plated as a monolayer on Matrigel. Under these culture conditions, the cells form aggregates, analogous to columns, and differentiate along the invasive pathway.
In these experiments cytotrophoblasts were plated and then infected with CMV. Replication was assessed by immunolocalizing CMV IE1/2 proteins and a virion envelope glycoprotein, gB, either immediately after isolation (control) or at various intervals postinfection (experimental). IE1/2 protein expression was detected, in a nuclear pattern, from 24 h onward (data not shown). From 72 h onward, staining for both IE1/2 (nuclear) and gB (cytoplasmic) proteins was detected (Fig. 5B). At 96 h postinfection, the accumulation of gB in cytoplasmic vesicles was particularly striking (Fig. 5D). In 10 separate experiments, 20 to 40% of the cells showed the latter staining pattern at the end of the culture period.
FIG. 5.
Purified cytotrophoblasts (CTBs) could be infected with CMV as they differentiated along the invasive pathway in vitro. At 72 h, the cells were stained for expression of (A) cytokeratin (CK) and (B) gB (the major structural glycoprotein in the virion envelope) and IE1/2 proteins. Anti-IE1/2 antibody reacted with the nuclei, and anti-gB antibody showed diffuse cytoplasmic staining. At 96 h, the cells were stained for expression of (C) cytokeratin and (D) gB, which was detected in granules in the cytoplasm.
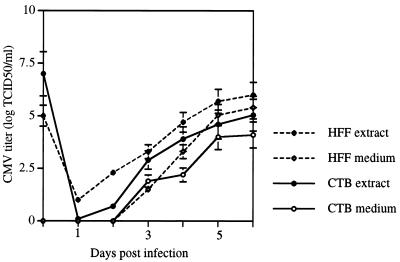
Next, we compared titers of infectious progeny made in CMV-infected cytotrophoblasts and HFF during a 6-day period. To do so, we monitored virus levels in the intracellular and extracellular compartments daily (Fig. 6). Cytotrophoblasts were plated at fivefold-higher numbers than HFF to account for the fact that cells in the middle of aggregates are sequestered from virus (see Fig. 2B). For the same reason, cytotrophoblasts were infected with a 10-fold-higher virus titer than was used to infect HFF. Although yields were higher in HFF, the placental cytotrophoblasts produced and released into the medium substantial amounts of virus. The results of these experiments indicated that differentiating or invading cytotrophoblasts were fully permissive for CMV replication.
FIG. 6.
Purified cytotrophoblasts (CTBs) were fully permissive for CMV infection in vitro. Quantitation and comparison of CMV progeny virions produced in cell extracts (intracellular virus) and culture medium (released virions) of purified cytotrophoblasts and human foreskin fibroblasts (HFF) infected in vitro. TCID50, 50% tissue culture infective dose.
CMV infection in vitro downregulates α1β1 integrin expression and impairs cytotrophoblast invasion.
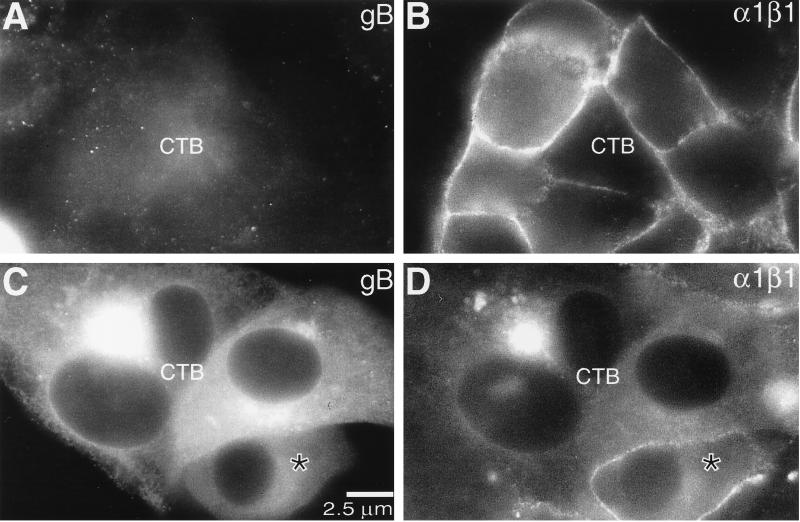
The association of CMV infection with pregnancy complications thought to involve the placenta prompted us to examine the effects of CMV infection on the expression of the laminin/collagen receptor integrin α1β1. We studied this extracellular matrix receptor both as a stage-specific antigen whose expression is preferentially associated with cytotrophoblasts inside the uterine wall (11) and as an adhesion molecule that mediates invasion in vitro (12). First, we colocalized CMV gB (Fig. 7A and C) and integrin α1β1 expression (Fig. 7B and D) in cytotrophoblast cultures that were infected with CMV for 96 h in vitro. As expected, the cells that did not stain for gB (Fig. 7A) expressed integrin α1 in a plasma membrane pattern (Fig. 7B). Diffuse cytoplasmic staining for gB was also correlated with integrin α1 expression (see cell marked with a * in C and D), but accumulation of gB in vesicles (Fig. 7C) was associated with the absence of staining for integrin α1 (Fig. 7D). In contrast, immunostaining for another integrin whose expression is upregulated as the cells invade, the fibronectin receptor α5β1, was not affected (data not shown). In this context it is interesting to consider that α5β1 functions to inhibit invasion, thereby counterbalancing the activity of integrin α1β1 (12).
FIG. 7.
CMV infection in vitro eventually downregulates cytotrophoblast (CTB) expression of integrin α1. Purified cytotrophoblasts were infected with CMV in vitro as described in Materials and Methods. At 72 h after infection, the cells were fixed and stained for expression of gB and integrin α1. Cytotrophoblasts that did not express gB (A) displayed prominent staining for integrin α1 in a plasma membrane-associated pattern (B). Likewise, cells that stained in a diffuse cytoplasmic pattern for gB (C) also reacted with the anti-integrin antibody (D, cell marked with *). However, when gB was localized in a vesicular pattern, integrin staining was not detected (D). The intense intracellular staining was bleedthrough from anti-gB signal.
Next, we evaluated the impact of CMV infection in vitro on cytotrophoblast invasion, using the assay illustrated in Fig. 8. Isolated cytotrophoblasts were plated on the upper surfaces of Transwell filters coated with Matrigel to an approximate depth of 100 μm. The assay tests a cell's ability to penetrate the Matrigel, pass through pores in the underlying filter, and emerge on the lower surface of the membrane (12, 40). Invasion is quantified by determining the number of cytokeratin-positive cell processes that emerge through the filter pores. In three separate experiments we found that CMV infection in vitro dramatically impaired invasion, which was reduced to 16% ± 3.2% (mean ± standard error of the mean) of that observed in control uninfected cells. We noted that the effect on invasion was greater than could be accounted for in terms of the number of CMV-infected cells (e.g., 20 to 40%). This result suggests that the presence of infected cells in the invading aggregates (see Fig. 2B) influences the behavior of the population as a whole.
Figures 8A to D are micrographs showing typical filters. Two days postinfection, many of the cytokeratin-positive cytotrophoblasts in the upper chamber (Fig. 8A) had become infected with CMV in vitro, as shown by immunolocalization of CMV IE1/2 proteins to the nuclei (Fig. 8B). This was in contrast to control cytotrophoblasts maintained under the same culture conditions in the absence of virus, which failed to demonstrate any immunoreactivity (data not shown). Examination of the filter underside from control cultures showed that most of the pores contained cytokeratin-positive processes of cytotrophoblasts that were emerging on the filter underside (Fig. 8C). In contrast, the processes of CMV-infected cells showed diffuse immunofluorescence because they had not yet penetrated the Matrigel to reach the filter pores (Fig. 8D).
CMV infection in vitro downregulates cytotrophoblast expression of the nonclassical MHC class Ib molecule HLA-G.
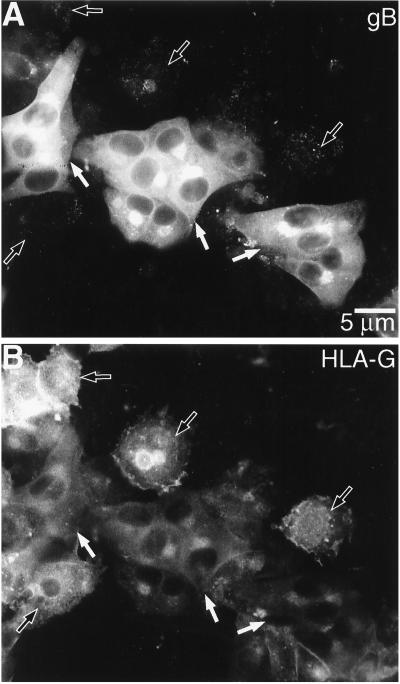
Multiple loci in the CMV genome downregulate expression of MHC class Ia molecules from the surface of infected cells (32). Thus, we investigated whether CMV infection in vitro affects expression of the cytotrophoblast MHC class Ib molecule HLA-G. Immunolocalization experiments showed that at late times after infection, when high levels of CMV gB were detected (Fig. 9A), staining for HLA-G was either greatly reduced or lost (Fig. 9B). This was in contrast to cells in the same microscope field (e.g., internal controls) that were not infected with CMV and that stained with anti-HLA-G. To identify the relevant CMV glycoproteins responsible, we infected cytotrophoblasts with two CMV mutants, RV798 and RV670, in which all of the genes known to downregulate cell surface expression of classical MHC class Ia molecules have been deleted (33). The results of five separate experiments showed that both mutants RV798 and RV670 downregulated HLA-G expression in infected cytotrophoblasts from different placentas (data not shown). Therefore, the mechanism of HLA-G downregulation does not involve glycoproteins that alter class Ia expression and is most likely novel.
FIG. 9.
CMV infection impairs cytotrophoblast expression of HLA-G in vitro. Purified cytotrophoblasts were isolated and infected with CMV as described in Materials and Methods. At 72 h after infection, the cells were stained for gB (A) and HLA-G (B) expression. Cells that did not express gB (black arrows) expressed HLA-G. In contrast, staining for gB (white arrows) was associated with a marked reduction in HLA-G expression.
DISCUSSION
The impetus for this study was the long-standing hypothesis that CMV infection of the placenta precedes that of the embryo or fetus, suggesting that the extraembryonic membranes play a critical role in pathogenesis. Given the difficulties inherent in studying the infection process during human pregnancy, much of the direct experimental evidence in support of this hypothesis comes from animal models. In this context it is important to consider the tremendous diversity in placental structure among animals—even close genetic relatives. For this reason studies in the guinea pig, which, like the human, has a hemomonochorial placenta in which a single trophoblast layer separates the fetal from the maternal circulation, are of particular interest (23, 39). Dams inoculated in the axilla with guinea pig CMV at midgestation show hematogenous dissemination of infection to the placenta, where viral nucleocapsids are present in nuclei of syncytiotrophoblasts and viral proteins are expressed in the transitional zone between the capillarized trophoblast labyrinth and the noncapillarized interlobium (23). Furthermore, CMV, which replicates in the presence of maternal antiviral antibodies, is detected in placental tissues long after virus is cleared from blood. Whenever infection of the fetus occurred, virus was isolated from the associated placenta. Conversely, when CMV infected the placentas, only 27% of fetuses contained virus, suggesting that the guinea pig placenta serves as a reservoir in which virus replicates prior to reaching the embryo or fetus.
Our results suggest that this is also the likely scenario in human pregnancy and that CMV-infected cytotrophoblasts play a central role in virus transmission to the fetus. We found evidence of CMV replication in the trophoblast populations that lie at the maternal-fetal interface, either in vitro or in utero. Specifically, trophoblast cells in several locations expressed CMV proteins after infection. Given the 3- to 4-day CMV replication cycle, the in vitro studies, in which we detected infection of cytotrophoblast stem cells as well as of the invasive subpopulation, likely model the initial steps in virus transmission. In contrast, the tissues infected in utero show how the virus is transmitted from trophoblasts to other types of cells within the villous core.
These findings offer important clues about how transmission occurs in utero. In reconstructing possible routes, we considered immunohistochemical analyses of CMV-infected placentas (44, 45, 55) and recent data showing that CMV persistently or latently infects many of the cell types that trophoblasts encounter in the uterus. Specifically, CMV establishes latent infection in and reactivates from granulocyte-dendritic progenitors (25, 56). Macrophages disseminate virus by contact with endothelial cells that line blood vessels and tissues of solid organs (63). CMV also directly infects endothelial cells in vivo, which have subsequently been found circulating in blood (22). Uterine tissues may become infected via a hematogenous route or by sexual contact; currently there is no evidence that strongly supports either mechanism. Circumstantial evidence for sexual transmission includes high rates of CMV infection in sexually active adolescents who are likely to become pregnant (7, 57). Consequently, CMV infection would spread in an ascending manner from the cervix to the uterus in cases of primary or reactivated infections. In support of this hypothesis, CMV is shed from the cervix in young nonpregnant women with multiple new sex partners (5–7, 57), and this rate increases during pregnancy (35%) (4, 60). High levels of CMV DNA are detected in cervical smears and uterine tissues (50% positive) compared with lung, liver, kidney, and blood vessels (15% positive), and viral proteins are detected in uterine glandular epithelial cells, endothelial cells, and interstitial leukocytes (19). Together, these data indicate that CMV productively replicates in and is shed from uterine tissues of sexually active women.
These data also suggest possible routes by which CMV infection spreads from the uterine tissues, first to the placenta and then to the embryo or fetus. One likely site of transmission is via the syncytiotrophoblast layer that covers floating chorionic villi (Fig. 1, site 1). These placental cells are also in direct contact with maternal blood. Our data suggest that initially the syncytium may function by allowing passage of CMV to the underlying layer of cytotrophoblast stem cells, which are capable of supporting viral replication. Later in the infection process syncytiotrophoblasts may also become infected. Another likely site of transmission is within the uterine wall (Fig. 1, sites 2 and 3). Cytotrophoblasts involved in interstitial invasion could encounter infected uterine glands, decidual granular leukocytes, and muscle cells. Cytotrophoblasts involved in endovascular invasion could encounter infected endothelial and vascular smooth muscle cells as well as maternal blood. Once cytotrophoblasts within the uterine wall become infected, CMV could spread in a retrograde manner through the cell columns to the anchoring chorionic villi. We also saw, in a number of samples infected in utero, extensive expression of CMV IE1/2 proteins throughout the villous stromal cores. This unexpected result suggests that virus is often transmitted from infected trophoblasts to fibroblasts, fetal macrophages (Hofbauer cells), and possibly endothelial cells that line chorionic vessels—patterns of CMV infection in the placenta and other tissues (45, 54). Infected macrophages and sloughed endothelial cells seem likely candidates for entering the venous circulation of the placenta and subsequently carrying the infection via the placental circulation to the fetus.
It is equally interesting to consider the possible molecular cascade that results in CMV transmission via the placenta to the fetus. With regard to transmission within the uterine wall, the best analogy may be reactivation of CMV in transplant patients whose immune systems have been pharmacologically suppressed. Likewise, the placenta, which is often described as a hemiallograft, probably induces a state of local immunosuppression in the uterus. For example, the invasive cytotrophoblast subpopulation secretes high levels of interleukin-10 (50). We speculate that this specialized immunologic milieu could support reactivation of latent virus. With regard to transmission in the intervillous space, several possible mechanisms exist. For example, human syncytiotrophoblasts express the neonatal Fc receptor hFcRn, which transcytoses IgG from maternal blood to the fetus (53). The abundance of nonneutralizing antiviral antibodies with low avidity in women with primary CMV infection who transmit virus to the embryo or fetus (2, 37) may enhance virion transcytosis across syncytiotrophoblasts to cytotrophoblast stem cells. This finding is in accord with our observation that, in floating villi infected with CMV in vitro, syncytiotrophoblasts failed to stain with antibodies to viral proteins, whereas clusters of underlying cytotrophoblasts did. Currently we are investigating whether hFcRn expressed at the apical surface of syncytiotrophoblasts binds and transports both maternal IgG, which we and others (36, 53) have localized to submembrane vesicles in these cells, and antibody-coated CMV virions. Interestingly, virus transmission to the embryo or fetus by transcytosis in syncytiotrophoblasts would explain the phenomenon of efficient intrauterine infection in the presence of high antibody titers to CMV gB (2).
Others have reported that CMV replicates in trophoblasts from first-trimester and full-term placentas infected in vitro (26, 29). CMV virions were found in trophoblast culture medium, and infection kinetics varied among laboratory strains and virus isolates. These studies did not address the consequences of infection on cytotrophoblast function. Our data suggest that CMV impairs cytotrophoblast differentiation/invasion in vitro. Experiments currently in progress are addressing the critical question of whether these same changes are seen as a consequence of infection in utero. Data gathered thus far suggest that this is the case. To date we have isolated cytotrophoblasts from two second-trimester placentas that had been naturally infected with CMV in utero, as demonstrated by their nuclear expression of IE1/2 proteins and cytoplasmic expression of gB before culture. After 3 days, they continued to express gB and expression of both α1β1 integrin and HLA-G was downregulated. In both cases invasiveness after 2 days in culture, quantified by using the assay depicted in Fig. 8, was only ∼5% of the levels commonly observed in cultures of gestation-matched uninfected cells.
Therefore, it seems likely that the extensive infection of the trophoblast populations that we detected in first-trimester chorionic villi infected in utero could adversely affect placental development and consequently the outcome of the pregnancy. The downstream consequences are likely to vary depending on the gestational age. Infection of trophoblasts soon after implantation might compromise the ability of the human embryo to carry out interstitial implantation, which buries the conceptus deep within the uterine wall. This could explain the early pregnancy loss that often occurs in women with primary infection. Infection at a slightly later stage could impair the formation of both floating and anchoring villi. In the former case, placental structure may remain relatively undeveloped, perhaps exhibiting the reduction in surface area of the villous tree that has been noted in intrauterine growth retardation. In the latter case, a constellation of critical events could be affected, including the attachment of cell columns to the uterus and both interstitial and endovascular invasion—placental pathologies that are associated with preeclampsia and a subset of pregnancies that are complicated by idiopathic preterm labor (49). Although the consequences of CMV infection of the developing trophoblast in early pregnancy are not known, the effects that we propose could explain why CMV infection later in pregnancy is frequently associated with both intrauterine growth retardation and preterm labor (31).
Our results also indicate that CMV infection impairs cytotrophoblast expression of HLA-G, likely an important component of the mechanism that protects these fetal cells from removal by maternal immune cells that are abundant in the decidua, particularly during the first trimester of pregnancy. This is in accord with the previously reported effects of CMV infection on HLA-G expression by human choriocarcinoma cells (52). Furthermore, the mechanism of HLA-G downregulation does not involve CMV genes that alter class Ia expression and is most likely novel. This finding is in accord with the fact that expression of HLA-G, which lacks an interferon response element in its promoter, is regulated in a manner distinct from that of class Ia molecules (8). One consequence of downregulating HLA-G expression could be activation of the maternal immune response against the subpopulation of cytotrophoblasts that express this molecule—namely, those that carry out interstitial and endovascular invasion. Thus, it is possible that infected cytotrophoblasts become targets of the unusual natural killer (NK) cell population that dominates the granular leukocyte population in the uterine decidua (61). As noted above, the timing of infection would determine the effect on pregnancy outcome.
The data presented in this study also raise several interesting questions that we cannot yet answer. For example, it is possible, even probable, that the widespread expression of CMV IE1/2 proteins in first-trimester chorionic villi that were infected in utero could be evidence of other underlying pathologies, including those involving infectious organisms other than CMV. Given the complex interplay between viruses, bacteria, and host cells that takes place in the uterine environment, this scenario seems very likely (21). Thus, it will be important to place our findings in the larger context of the microbial ecology of the female reproductive tissues. Finally, CMV infection may be indicative of abnormal cross talk between the fetal and maternal cells that orchestrate the complex immune interactions required for human pregnancy to proceed normally. Imbalances in trophoblast differentiation, decidualization, and/or decidual granular leukocyte infiltration could be related to the phenomena that we observed.
In summary, our findings open the door to testing a variety of hypotheses regarding CMV infection of placental tissues. It is hoped that these studies will resolve the serious dichotomy between our understanding of the devastating consequences of congenital CMV infection and our lack of knowledge, at the molecular level, of the mechanisms involved. Understanding how CMV transmission occurs is the crucial first step toward the rational design of therapies to prevent prenatal infection. These treatments could either enhance the normal barrier function of the placenta or subvert the ability of maternal cells to transmit CMV to cytotrophoblasts—fetal placental cells that are the likely conduit for CMV infection of the embryo or fetus.
ACKNOWLEDGMENTS
All authors contributed equally to this paper.
We thank Thomas Jones for CMV deletion mutant viruses. We also thank Edward Mocarski and members of the Fisher and Pereira labs for thoughtful discussions. We are grateful to Zoya Kharitonov for excellent laboratory expertise and Evangeline Leash for editing the manuscript.
This work was supported by Public Health Service grants HD30367 (S.F.), EY10138 (L.P.), and AI46657 (L.P. and S.F.) from the National Institutes of Health.
REFERENCES
- 1.Benirschke K, Mendoza G R, Bazeley P L. Placental and fetal manifestations of cytomegalovirus infection. Virchows Arch B Cell Pathol. 1974;16:121–139. doi: 10.1007/BF02894070. [DOI] [PubMed] [Google Scholar]
- 2.Boppana S B, Britt W J. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis. 1995;171:1115–1121. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- 3.Britt W J. Congenital cytomegalovirus infection. In: Hitchcock P J, MacKay H T, Wasserheit J N, editors. Sexually transmitted diseases and adverse outcomes of pregnancy. Washington, D.C.: ASM Press; 1999. pp. 269–281. [Google Scholar]
- 4.Chandler S H, Alexander E R, Holmes K K. Epidemiology of cytomegaloviral infection in a heterogeneous population of pregnant women. J Infect Dis. 1985;152:249–256. doi: 10.1093/infdis/152.2.249. [DOI] [PubMed] [Google Scholar]
- 5.Chandler S H, Handsfield H H, McDougall J K. Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J Infect Dis. 1987;155:655–660. doi: 10.1093/infdis/155.4.655. [DOI] [PubMed] [Google Scholar]
- 6.Chandler S H, Holmes K K, Wentworth B B, Gutman L T, Wiesner P J, Alexander E R, Handsfield H H. The epidemiology of cytomegaloviral infection in women attending a sexually transmitted disease clinic. J Infect Dis. 1985;152:597–605. doi: 10.1093/infdis/152.3.597. [DOI] [PubMed] [Google Scholar]
- 7.Coonrod D, Collier A C, Ashley R, DeRouen T, Corey L. Association between cytomegalovirus seroconversion and upper genital tract infection among women attending a sexually transmitted disease clinic: a prospective study. J Infect Dis. 1998;177:1188–1193. doi: 10.1086/515292. [DOI] [PubMed] [Google Scholar]
- 8.Cross J C, Lam S, Yagel S, Werb Z. Defective induction of the transcription factor interferon-stimulated gene factor-3 and interferon alpha insensitivity in human trophoblast cells. Biol Reprod. 1999;60:312–321. doi: 10.1095/biolreprod60.2.312. [DOI] [PubMed] [Google Scholar]
- 9.Cross J C, Werb Z, Fisher S J. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 10.Damsky C H, Fisher S J. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10:660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- 11.Damsky C H, Fitzgerald M L, Fisher S J. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Investig. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damsky C H, Librach C, Lim K H, Fitzgerald M L, McMaster M T, Janatpour M, Zhou Y, Logan S K, Fisher S J. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 13.de Jong M D, Galasso G J, Gazzard B, Griffiths P D, Jabs D A, Kern E R, Spector S A. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res. 1998;39:141–162. doi: 10.1016/s0166-3542(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 14.Dondero D V, Pereira L. Monoclonal antibody production. In: Emmons R, Schmidt N, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington, D.C.: American Public Health Association; 1990. pp. 101–124. [Google Scholar]
- 15.Fisher S J, Cui T Y, Zhang L, Hartman L, Grahl K, Zhang G Y, Tarpey J, Damsky C H. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989;109:891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher S J, Leitch M S, Kantor M S, Basbaum C B, Kramer R H. Degradation of extracellular matrix by the trophoblastic cells of first-trimester human placentas. J Cell Biochem. 1985;27:31–41. doi: 10.1002/jcb.240270105. [DOI] [PubMed] [Google Scholar]
- 17.Fowler K B, Pass R F. Sexually transmitted diseases in mothers of neonates with congenital cytomegalovirus infection. J Infect Dis. 1991;164:259–264. doi: 10.1093/infdis/164.2.259. [DOI] [PubMed] [Google Scholar]
- 18.Fowler K B, Stagno S, Pass R F, Britt W J, Boll T J, Alford C A. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa T, Jisaki F, Sakamuro D, Takegami T, Murayama T. Detection of human cytomegalovirus genome in uterus tissue. Arch Virol. 1994;135:265–277. doi: 10.1007/BF01310013. [DOI] [PubMed] [Google Scholar]
- 20.Genbacev O, Schubach S A, Miller R K. Villous culture of first trimester human placenta—model to study extravillous trophoblast (EVT) differentiation. Placenta. 1992;13:439–461. doi: 10.1016/0143-4004(92)90051-t. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg R L, Andrews W W, Yuan A, MacKay H T, St. Louis M. Pregnancy outcomes related to sexually transmitted diseases. In: Hitchcock P J, MacKay H T, Wasserheit J N, editors. Sexually transmitted diseases and adverse outcomes of pregnancy. Washington, D.C.: ASM Press; 1999. pp. 1–24. [PubMed] [Google Scholar]
- 22.Grefte A, van der Giessen M, van Son W, The T H. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J Infect Dis. 1993;167:270–277. doi: 10.1093/infdis/167.2.270. [DOI] [PubMed] [Google Scholar]
- 23.Griffith B P, McCormick S R, Fong C K, Lavallee J T, Lucia H L, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. J Virol. 1985;55:402–409. doi: 10.1128/jvi.55.2.402-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffiths P D, Baboonian C. A prospective study of primary cytomegalovirus infection during pregnancy: final report. Br J Obstet Gynaecol. 1984;91:307–315. doi: 10.1111/j.1471-0528.1984.tb05915.x. [DOI] [PubMed] [Google Scholar]
- 25.Hahn G, Jores R, Mocarski E S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halwachs-Baumann G, Wilders-Truschnig M, Desoye G, Hahn T, Kiesel L, Klingel K, Rieger P, Jahn G, Sinzger C. Human trophoblast cells are permissive to the complete replicative cycle of human cytomegalovirus. J Virol. 1998;72:7598–7602. doi: 10.1128/jvi.72.9.7598-7602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handsfield H H, Chandler S H, Caine V A, Meyers J D, Corey L, Medeiros E, McDougall J K. Cytomegalovirus infection in sex partners: evidence for sexual transmission. J Infect Dis. 1985;151:344–348. doi: 10.1093/infdis/151.2.344. [DOI] [PubMed] [Google Scholar]
- 28.Hayes K, Gibas H. Placental cytomegalovirus infection without fetal involvement following primary infection in pregnancy. J Pediatr. 1971;79:401–405. doi: 10.1016/s0022-3476(71)80147-6. [DOI] [PubMed] [Google Scholar]
- 29.Hemmings D G, Kilani R, Nykiforuk C, Preiksaitis J, Guilbert L J. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J Virol. 1998;72:4970–4979. doi: 10.1128/jvi.72.6.4970-4979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutto C, Ricks R, Garvie M, Pass R F. Epidemiology of cytomegalovirus infections in young children: day care vs. home care. Pediatr Infect Dis. 1985;4:149–152. doi: 10.1097/00006454-198503000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Istas A S, Demmler G J, Dobbins J G, Stewart J A. Surveillance for congenital cytomegalovirus disease: a report from the National Congenital Cytomegalovirus Disease Registry. Clin Infect Dis. 1995;20:665–670. doi: 10.1093/clinids/20.3.665. [DOI] [PubMed] [Google Scholar]
- 32.Jones T R, Hanson L K, Sun L, Slater J S, Stenberg R M, Campbell A E. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J Virol. 1995;69:4830–4841. doi: 10.1128/jvi.69.8.4830-4841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones T R, Muzithras V P. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J Virol. 1992;66:2541–2546. doi: 10.1128/jvi.66.4.2541-2546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo K, Kaneshima H, Mocarski E S. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci USA. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovats S, Main E K, Librach C, Stubblebine M, Fisher S J, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 36.Kristoffersen E K, Matre R. Co-localization of the neonatal Fc gamma receptor and IgG in human placental term syncytiotrophoblasts. Eur J Immunol. 1996;26:1668–1671. doi: 10.1002/eji.1830260741. [DOI] [PubMed] [Google Scholar]
- 37.Lazzarotto T, Spezzacatena P, Pradelli P, Abate D A, Varani S, Landini M P. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin Diagn Lab Immunol. 1997;4:469–473. doi: 10.1128/cdli.4.4.469-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leach J L, Sedmak D D, Osborne J M, Rahill B, Lairmore M D, Anderson C L. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- 39.Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol. 1994;102:122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- 40.Librach C L, Werb Z, Fitzgerald M L, Chiu K, Corwin N M, Esteves R A, Grobelny D, Galardy R, Damsky C H, Fisher S J. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim K H, Zhou Y, Janatpour M, McMaster M, Bass K, Chun S H, Fisher S J. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol. 1997;151:1809–1818. [PMC free article] [PubMed] [Google Scholar]
- 42.McMaster M, Zhou Y, Shorter S, Kapasi K, Geraghty D, Lim K H, Fisher S. HLA-G isoforms produced by placental cytotrophoblasts and found in amniotic fluid are due to unusual glycosylation. J Immunol. 1998;160:5922–5928. [PubMed] [Google Scholar]
- 43.McMaster M T, Librach C L, Zhou Y, Lim K H, Janatpour M J, DeMars R, Kovats S, Damsky C, Fisher S J. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154:3771–3778. [PubMed] [Google Scholar]
- 44.Mostoufi-zadeh M, Driscoll S G, Biano S A, Kundsin R B. Placental evidence of cytomegalovirus infection of the fetus and neonate. Arch Pathol Lab Med. 1984;108:403–406. [PubMed] [Google Scholar]
- 45.Mühlemann K, Miller R K, Metlay L, Menegus M A. Cytomegalovirus infection of the human placenta: an immunocytochemical study. Hum Pathol. 1992;23:1234–1237. doi: 10.1016/0046-8177(92)90290-j. [DOI] [PubMed] [Google Scholar]
- 46.Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 47.Pass R F, Little E A, Stagno S, Britt W J, Alford C A. Young children as a probable source of maternal and congenital cytomegalovirus infection. N Engl J Med. 1987;316:1366–1370. doi: 10.1056/NEJM198705283162203. [DOI] [PubMed] [Google Scholar]
- 48.Robertson W B, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976;5:115–127. [PubMed] [Google Scholar]
- 49.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon B H. The preterm labor syndrome. In: Elder M, Lamont R F, Romero R, editors. Preterm labor. New York, N.Y: Churchill Livingstone; 1997. pp. 29–49. [Google Scholar]
- 50.Roth I, Corry D B, Locksley R M, Abrams J S, Litton M J, Fisher S J. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth I, Fisher S J. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev Biol. 1999;205:194–204. doi: 10.1006/dbio.1998.9122. [DOI] [PubMed] [Google Scholar]
- 52.Schust D J, Tortorella D, Seebach J, Phan C, Ploegh H L. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J Exp Med. 1998;188:497–503. doi: 10.1084/jem.188.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simister N E, Story C M, Chen H L, Hunt J S. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 54.Sinzger C, Grefte A, Plachter B, Gouw A S, The T H, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76:741–750. doi: 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 55.Sinzger C, Müntefering H, Löning T, Stöss H, Plachter B, Jahn G. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch A Pathol Anat Histopathol. 1993;423:249–256. doi: 10.1007/BF01606887. [DOI] [PubMed] [Google Scholar]
- 56.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 57.Sohn Y M, Oh M K, Balcarek K B, Cloud G A, Pass R F. Cytomegalovirus infection in sexually active adolescents. J Infect Dis. 1991;163:460–463. doi: 10.1093/infdis/163.3.460. [DOI] [PubMed] [Google Scholar]
- 58.Stagno S, Pass R F, Cloud G, Britt W J, Henderson R E, Walton P D, Veren D A, Page F, Alford C A. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904–1908. [PubMed] [Google Scholar]
- 59.Stagno S, Pass R F, Dworsky M E, Henderson R E, Moore E G, Walton P D, Alford C A. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N Engl J Med. 1982;306:945–949. doi: 10.1056/NEJM198204223061601. [DOI] [PubMed] [Google Scholar]
- 60.Stagno S, Reynolds D, Tsiantos A, Fuccillo D A, Smith R, Tiller M, Alford C A., Jr Cervical cytomegalovirus excretion in pregnant and nonpregnant women: suppression in early gestation. J Infect Dis. 1975;131:522–527. doi: 10.1093/infdis/131.5.522. [DOI] [PubMed] [Google Scholar]
- 61.Starkey P M, Sargent I L, Redman C W. Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology. 1988;65:129–134. [PMC free article] [PubMed] [Google Scholar]
- 62.Story C M, Mikulska J E, Simister N E. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waldman W J, Knight D A, Huang E H, Sedmak D D. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J Infect Dis. 1995;171:263–272. doi: 10.1093/infdis/171.2.263. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, Damsky C H, Chiu K, Roberts J M, Fisher S J. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Investig. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y, Damsky C H, Fisher S J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype: one cause of defective endovascular invasion in this syndrome? J Clin Investig. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, Fisher S J, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky C H. Human cytotrophoblasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J Clin Investig. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]