Abstract
Recombinant human parainfluenza virus type 3 (PIV3) was used as a vector to express the major protective antigen of measles virus, the hemagglutinin (HA) glycoprotein, in order to create a bivalent PIV3-measles virus that can be administered intranasally. The measles virus HA open reading frame (ORF) was inserted as an additional transcriptional unit into the N-P, P-M, or HA-neuraminidase (HN)-L gene junction of wild-type PIV3 or into the N-P or P-M gene junction of an attenuated derivative of PIV3, termed rcp45L. The recombinant PIV3 (rPIV3) viruses bearing the HA inserts replicated more slowly in vitro than their parental viruses but reached comparable peak titers of ≥107.5 50% tissue culture infective doses per ml. Each of the wild-type or cold-passaged 45L (cp45L) PIV3(HA) chimeric viruses replicated 5- to 10-fold less well than its respective parent virus in the upper respiratory tract of hamsters. Thus, insertion of the ∼2-kb ORF itself conferred attenuation, and this attenuation was additive to that conferred by the cp45L mutations. The attenuated cp45L PIV3(HA) recombinants induced a high level of resistance to replication of PIV3 challenge virus in hamsters and induced very high levels of measles virus neutralizing antibodies (>1:8,000) that are well in excess of those known to be protective in humans. rPIV3s expressing the HA gene in the N-P or P-M junction induced about 400-fold more measles virus-neutralizing antibody than did the rPIV3 with the HA gene in the HN-L junction, indicating that the N-P or P-M junction appears to be the preferred insertion site. Previous studies indicated that the PIV3 cp45 virus, a more attenuated version of rcp45L, replicates efficiently in the respiratory tract of monkeys and is immunogenic and protective even when administered in the presence of very high titers of passively transferred PIV3 antibodies (A. P. Durbin, C. J. Cho, W. R. Elkins, L. S. Wyatt, B. Moss, and B. R. Murphy, J. Infect. Dis. 179:1345–1351, 1999). This suggests that this intranasally administered PIV3(HA) chimeric virus can be used to immunize infants with maternally acquired measles virus antibodies in whom the current parenterally administered live measles virus vaccine is ineffective.
Measles virus is a member of the Morbillivirus genus of the Paramyxoviridae family (23). It is one of the most contagious infectious agents known to humans and is transmitted from person to person via the respiratory route (23). The virus has a complex pathogenesis, involving replication in both the respiratory tract and various systemic sites (23). Although both mucosal immunoglobulin A (IgA) and serum IgG measles virus-specific antibodies can participate in the control of measles virus infection, the absence of measles virus disease in very young infants possessing only maternally acquired measles virus-specific IgG antibodies identifies serum antibodies as the major mediator of resistance to disease (23). This conclusion is supported by the high level of efficiency of measles virus-specific IgG antibodies in preventing measles when given early after exposure (30, 36). Like other paramyxoviruses, the measles virus hemagglutinin (HA) and fusion (F) glycoproteins are the major neutralization and protective antigens (23). A vaccine has been available for more than three decades and has been successful in eradicating indigenous measles disease from the United States, but the World Health Organization estimates that more than 45 million cases of measles still occur annually, killing more than 2,000 young children per day, mostly in the developing world (22). In 1996 the World Health Organization, the Pan American Health Organization, and the Centers for Disease Control and Prevention established the goal of global measles virus eradication by the years 2005 to 2010 (5). Although progress toward measles virus control has been made, measles still accounts for 10% of global mortality among children aged 5 years or less (67).
The currently available live attenuated measles virus vaccine is administered by a parenteral route (23). Both wild-type measles virus and the vaccine virus are very readily neutralized by antibodies, and the measles virus vaccine is rendered noninfectious by even very low levels of maternally acquired measles virus-specific neutralizing antibodies (1, 26, 43). For this reason, the vaccine is not given until the passively acquired maternal antibodies have decreased to undetectable levels. In the United States, the measles virus vaccine is not given until 12 to 15 months of age, a time when it can readily infect almost all children. In the developing world, measles virus continues to have a high mortality rate, especially in children within the latter half of the first year of life (22, 59). This occurs because the measles virus, which is highly prevalent in these regions, is able to infect that subset of infants in whom maternally acquired measles virus-specific antibody has decayed to a nonprotective level. Therefore, there is a need for a measles virus vaccine that is able to induce a protective immune response even in the presence of maternally derived measles virus-neutralizing antibodies. The goal of such a vaccine would be the elimination of measles disease that occurs within the first year of life as well as that which occurs thereafter. Given this need, there have been numerous attempts to develop an immunization strategy to protect infants in the latter half of the first year of life against measles virus, but an immunization strategy to protect the 6- to 12-month-old infant has not emerged (2, 12, 19, 20, 35, 38, 42, 47, 51, 58, 64, 65) (see Discussion).
The ability to recover infectious wild-type parainfluenza virus type 3 (PIV3) from cDNA by using recombinant DNA technology (14) has allowed us to create attenuated chimeric PIV3s expressing the HA protein of measles virus. Such chimeric viruses exhibit properties that should overcome the difficulties experienced to date in the immunization of infants against measles virus. PIV3 is a member of the Respirovirus genus of the Paramyxoviridae family in the order Mononegavirales. PIV3 is a common cause of serious lower respiratory tract infection in infants and children less than 1 year of age and is the second leading cause of hospitalization for viral lower respiratory tract disease in this age group, surpassed only by respiratory syncytial virus (RSV) (8, 39), indicating that there is a need for a PIV3 vaccine. A live attenuated cold-passaged PIV3 vaccine, cp45, is a very promising vaccine candidate for use in infants and children (33). Importantly, when administered in the presence of very high titers of passively acquired PIV3 antibodies, cp45 was found to protect rhesus monkeys against challenge with wild-type PIV3 (13). This ability to infect and induce a protective immune response in passively immunized animals suggested that this attenuated virus could be useful as a vector for the measles virus HA protein that might induce immunity to measles virus during the first year of life.
By using recombinant DNA technology, wild-type PIV3 has been recovered from DNA and the mutations present in the cp45 virus which determine its temperature-sensitive (ts), cold-adapted, and attenuation (att) phenotypes have been identified (14, 27, 52, 53). We found that the three mutations at amino acid (aa) positions 942, 992, and 1558 in the L protein confer the majority of the ts and att phenotypes of cp45. In this paper, we describe the recovery of five chimeric PIV3 viruses expressing the HA protein of measles virus: three in the wild-type backbone of PIV3 and two in the att derivative cp45L which bears the three attenuating ts mutations in L. The wild-type and att PIV3 viruses expressing the measles virus HA protein were highly immunogenic in hamsters. A possible role of such PIV3(HA) recombinants in control and eradication of measles disease in humans is suggested.
MATERIALS AND METHODS
Cells and viruses.
HEp-2, Vero, and LLC-MK2 monolayer cell cultures were maintained in either Eagle minimum essential medium (EMEM) (Life Technologies, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS), gentamicin sulfate (50 μg/ml), and 4 mM glutamine or VP-SFM (Life Technologies) supplemented with gentamicin sulfate (50 μg/ml) and 4 mM glutamine. The modified vaccinia virus strain Ankara (MVA) recombinant that expresses bacteriophage T7 RNA polymerase was generously provided by L. Wyatt and B. Moss (68). The JS wild-type strain of PIV3, its recombinant derivative rJS, and its recombinant attenuated ts derivative, rcp45L, were propagated in LLC-MK2 cells as described previously (14, 25, 52). The measles Edmonston wild-type virus and the live attenuated Moraten strain of measles virus were generously provided by W. Bellini and were propagated in Vero cell monolayers in EMEM supplemented with 10% FBS, 4 mM glutamine, and 50 μg of gentamicin per ml at 32°C in 5% CO2.
cDNAs.
We previously described the cDNA clone p3/7(131)2G, which encodes the complete 15,462-nucleotide (nt) antigenome of the JS wild-type strain of PIV3, and pFLCcp45L, which encodes the antigenome of the ts derivative of wild-type JS containing the three ts mutations in the L open reading frame (ORF) of PIV3 (14, 52). These clones were used as templates for the insertion of the HA gene of measles virus to create both wild-type and att PIV3 derivatives which express the HA protein. The size of each insert containing the HA gene of measles was designed to be a multiple of six such that the virus recovered from the cDNA would conform to the rule of six (16).
Construction of full-length PIV3 cDNAs encoding the HA protein of measles in the HN-L junction.
The HA ORF of measles virus was cloned into the HA-neuraminidase (HN)-L noncoding region of PIV3 in four steps (see Fig. 1A). First, a StuI site was introduced at nt 8600 of the full-length antigenomic clone p3/7(131)2G by using Kunkel mutagenesis (37), yielding the plasmid p3/7(131)2G-Stu. The ORF of the measles HA gene flanked by PIV3 noncoding sequence was then amplified in three different PCRs by using the Advantage PCR kit (Clontech, Palo Alto, Calif.). The first PCR used the forward primer 5′GACAATAGGCCTAAAAGGGAAATATAAAAAACTTAGGAGTAAAGTTACGCAATCC3′, which contains a StuI site (italicized) in the PIV3 HN-L noncoding region, and the reverse primer 5′GTAGAACGCGTTTATCCGGTCTCG T TGTGGTGACATCTCGAAT T TGGAT T TGTCTATTGGGTCCT TCC3′, which contains the beginning of the measles virus HA ORF (boldface type) followed by a silently introduced MluI site (italicized). This fragment, designated PCR fragment 1, is flanked by a StuI site at the 5′ end and an MluI site at the 3′ end and contains the first 36 nt of the measles HA ORF downstream of PIV3 HN 5′ noncoding sequence. The second PCR used the forward primer 5′CAGTCACCCGGGAAGATGGAACCAATCGCAGATAGTCATAATTAACCATAATATGCATCAATCTATCTATAATACAA3′ (sequence in boldface type represents the downstream end of the measles virus HA ORF, italicized sequence is the naturally occurring restriction enzyme site XmaI, and sequence in normal type represents PIV3 HN 3′ noncoding sequence) and the reverse primer 5′CCATGTAATTGAATCCCCCAACACTAGC3′, which spans nt 11448 to 11475 within the L gene of the full-length PIV3 antigenome. PCR fragment 2, which resulted from this reaction, contains the last 35 nt of the measles HA ORF and approximately 2,800 nt of the L ORF of PIV3 and is flanked by an XmaI site and an SphI site. The full-length antigenomic cDNA of PIV3, p3/7(131)2G-Stu, was used as the PCR template in reactions 1 and 2.
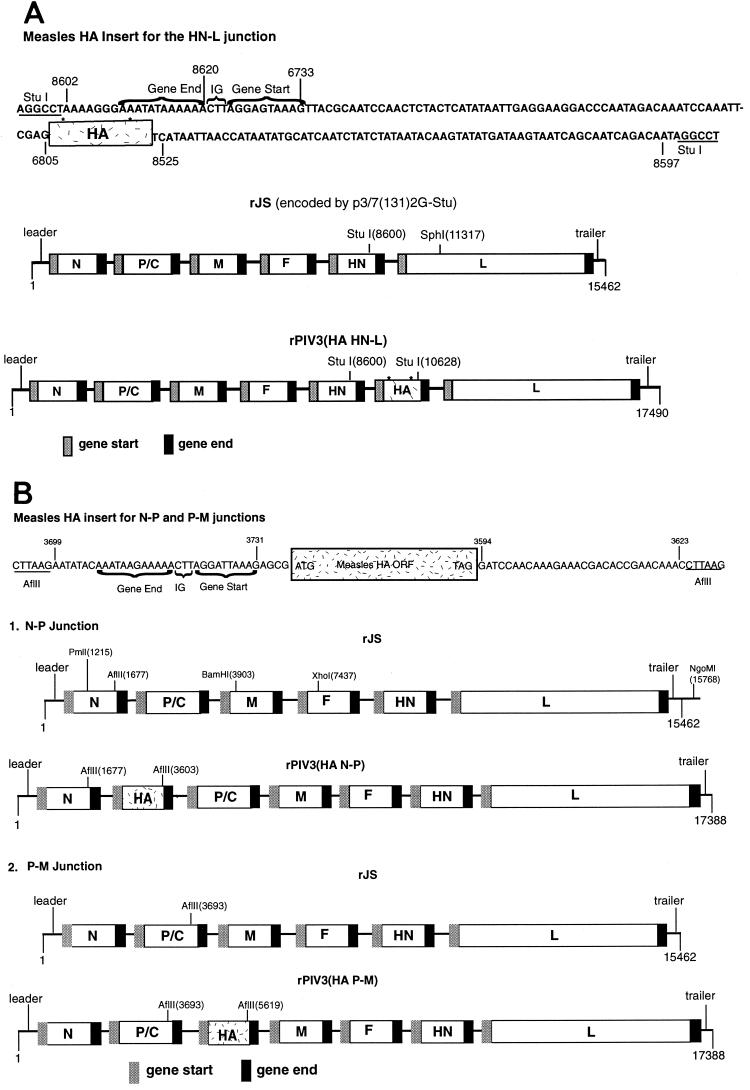
FIG. 1.
Insertion of the HA ORF of measles virus into the genome of recombinant PIV3. (A) Diagram (not to scale) of the 2,028-nt insert containing the complete ORF of the HA gene of measles virus. The insert contains, in 5′ to 3′ order, the following: a StuI site; nt 8602 to 8620 from the PIV3 antigenome, which consists of downstream noncoding sequence from the HN gene and its gene end signal; the conserved PIV3 intergenic (IG) trinucleotide; nt 6733 to 6805 from the PIV3 antigenome, which contains the HN gene start and upstream noncoding region; the measles virus HA ORF; PIV3 nt 8525 to 8597, which are downstream noncoding sequences from the HN gene; and a second StuI site. The construction is designed to, upon insertion, regenerate the PIV3 HN gene containing the StuI site and to place the measles virus ORF directly thereafter, flanked by the transcription signals and noncoding region of the PIV3 HN gene. The complete antigenome of PIV3 wild-type JS (rJS) with the introduced StuI site at nt 8600 in the 3′ noncoding region of the HN gene is illustrated in the middle diagram. The bottom diagram is the antigenome of PIV3 expressing the measles HA protein inserted into the StuI site. The HA cDNA used for this insertion came from a cDNA clone that had two amino acid differences from the wild-type Edmonston HA protein, indicated by the asterisks. (B) Diagram (not to scale) of the 1,926-nt insert containing the complete ORF of the measles virus HA gene, with a sequence confirmed to be identical to that of the Edmonston wild-type strain. The insert contains, in 5′ to 3′ order, the following: an AflII site; nt 3699 to 3731 from the PIV3 antigenome, which contains the P-M gene junction including the downstream noncoding sequence for the P gene, its gene end signal, the intergenic region, and the M gene start signal; three additional nonviral nucleotides (GCG); the complete HA ORF; PIV3 nt 3594 to 3623 from the downstream noncoding region of the P gene; and a second AflII site. Panel 1 illustrates the complete antigenome of the wild-type JS strain of PIV3 with the introduced AflII site in the 3′ noncoding region of the N gene before (top) and after (bottom) insertion of the measles HA ORF. Panel 2 illustrates the antigenome of the wild-type JS strain of PIV3 with the introduced AflII site in the 3′ noncoding region of the P gene before (top) and after (bottom) insertion of the measles HA ORF. Versions in the cp45L backbone differ only in the amino acid substitutions at positions 942, 992, and 1558 in the L protein and accompanying silent restriction enzyme markers (52, 53).
The third PCR amplified the largest portion of the measles HA ORF from the template cDNA pTM-7, a plasmid generously provided by S. Rosenblatt and B. Moss which contains the HA ORF of the Edmonston strain of measles virus supplied by the American Type Culture Collection. The forward primer 5′CGGATAAACGCGTTCTACAAAGATAACC3′ (MluI site italicized) and reverse primer 5′CCATCTTCCCGGGTGACTGTGCAGC3′ (XmaI site italicized) amplified PCR fragment 3 which contained nt 19 to 1838 of the measles HA ORF. PCR product 1 was digested with StuI and MluI, while PCR fragment 3 was digested with MluI and XmaI. These two digested fragments were then cloned by triple ligation into the StuI-XmaI window of pUC118 which had been modified to include a StuI site in its multiple cloning region. The resultant plasmid, pUC118(HA 1+3), was digested with StuI and XmaI, while PCR product 2 was digested with XmaI and SphI. The two digested products were then cloned into p3/7(131)2G-Stu digested with StuI and SphI, yielding the plasmid pFLC(HA HN-L). The StuI-SphI fragment, including the entire measles HA ORF, was then sequenced by using dRhodamine Terminator Cycle Sequencing Ready Reaction (ABI prism; PE Applied Biosystems, Foster City, Calif.). It was found upon sequencing pTM-7 and pFLC(HA HN-L) that, in comparison to the published sequence of Edmonston wild-type measles virus (GenBank accession number U03669), two mutations existed in the measles HA ORF of both plasmids, a serine-to-phenylalanine change at aa 46 and asparagine-to-tyrosine change at aa 481. Although these mutations are not found in the Edmonston wild-type virus, they are present in the published sequence of the HA protein of the measles Moraten strain vaccine virus (GenBank accession number M81899) which is known to induce protective immunity in young children.
Construction of full-length PIV3 cDNAs encoding the HA protein of measles virus in the N-P or P-M junction.
The PmlI-BamHI fragment of PIV3 antigenomic cDNA p3/7(131)2G (nt 1215 to 3903 of the PIV3 antigenome) was subcloned into the plasmid pUC119 [pUC119(PmlI-BamHI)] which had been modified to include a PmlI restriction site in the multiple cloning region (see Fig. 1B). Two single-stranded mutagenesis reactions were performed on pUC119(PmlI-BamHI) using Kunkel's method (37). First, an AflII site was introduced into the 3′ noncoding region of the N gene by mutagenizing the nucleotide sequence CTAAAT (PIV3 nt 1677 to 1682) to CTTAAG to give p(AflII N-P). Second, an AflII site was introduced into the 3′ noncoding region of the P gene by mutagenizing the nucleotide sequence TCAATC (PIV3 nt 3693 to 3698) to yield p(AflII P-M).
Because of the two coding changes in the HA cDNA described above, this sequence was recloned from measles virus RNA obtained from the Edmonston wild-type virus (GenBank accession number U03669). Measles virus RNA was purified from clarified medium using Trizol-LS (Life Technologies) following the manufacturer's recommended procedure. Reverse transcription-PCR was performed with the Advantage RT-for-PCR and Advantage-HF PCR kits (Clontech) following the recommended protocols. Primers were used to generate a PCR fragment spanning the entire ORF of the measles virus HA gene flanked by PIV3 noncoding sequence and an AflII restriction enzyme site (Fig. 1B). The forward primer 5′TTAATCTTAAGAATATACAAATAAGAAAAACTTAGGATTAAAGAGCGATGTCACCACAACGAGACCGGATAAATGCCTTCTAC3′ encodes an AflII restriction site (italicized) upstream of PIV3 gene junction and noncoding sequence (underlined) and the beginning of the measles HA ORF (boldface type). The reverse primer 5′ATTATTGCTTAAGGTTTGTTCGGTGTCGTTTCTTTGTTGGATCCTATCTGCGATTGGTTCCATCTTC3′ encodes an AflII restriction site (italicized) downstream of the PIV3 noncoding sequence (underlined) and the end of the measles HA ORF (boldface type). The resultant PCR fragment was then digested with AflII and cloned into p(AflII N-P) and p(AflII P-M) to create pUC119(HA N-P) and pUC119(HA P-M), respectively. pUC119(HA N-P) and pUC119(HA P-M) were sequenced over the entire AflII insert by using dRhodamine Terminator Cycle Sequencing Ready Reaction (ABI prism; PE Applied Biosystems), and the correct sequence was confirmed.
The PmlI-BamHI fragments of pUC119(HA N-P) and pUC119(HA P-M) were separately cloned into the full-length antigenome cDNA plasmid p3/7(131)2G as previously described (14) to create pFLC(HA N-P) and pFLC(HA P-M) (Fig. 1B). The XhoI-NgoMI fragment (nt 7437 to 15929) of pFLCcp45L was then cloned into both pFLC(HA N-P) and pFLC(HA P-M), after digestion with XhoI and NgoMI, to create pcp45L(HA N-P) and pcp45L(HA P-M). pFLCcp45L encodes the three amino acid changes in the L protein of PIV3 (aa 942, 992, and 1558) which confer most of the temperature sensitivity and attenuation of the cp45 vaccine candidate virus (52).
Recovery of recombinant wild-type PIV3 and cp45L expressing the HA protein of measles virus from different intergenic junctions.
The five full-length cDNAs bearing the measles HA ORF were separately transfected into HEp-2 cells on six-well plates (Costar, Cambridge, Mass.) together with the support plasmids [pTM(N), pTM(P no C), and pTM(L)] and LipofectACE (Life Technologies) and were infected with MVA-T7 as previously described (14–16). After incubation at 32°C for 3 days, the transfection harvest was passaged onto a fresh monolayer of Vero or LLC-MK2 cells in a T25 flask and was incubated for 5 days at 32°C (referred to as passage 1).
The rPIV3(HA HN-L) virus present in the supernatant of passage 1 harvest was plaque purified three times on LLC-MK2 cell monolayers as previously described (25). rPIV3(HA N-P), rcp45L(HA N-P), rPIV3(HA P-M), and rcp45L(HA P-M) were biologically cloned by terminal dilution by using serial twofold dilutions on 96-well plates (12 wells per dilution) of Vero cells as previously described (15). The biologically cloned recombinant viruses from the third round of plaque purification or from the third round of terminal dilution were then amplified twice in LLC-MK2 cells [rPIV3(HA HN-L) or Vero cells [rPIV3(HA N-P), rcp45L(HA N-P), rPIV3(HA P-M), or rcp45L(HA P-M)] and incubated at 32°C to produce virus for further characterization.
Protein expression analysis by immunoprecipitation and neutralization assay.
Monolayers of LLC-MK2 cells in T25 flasks were infected at a multiplicity of infection (MOI) of 5 with either rcp45L(HA N-P), rcp45L(HA P-M), or rJS or were mock infected. Monolayers of Vero cells in T25 flasks were infected with the Edmonston wild-type strain of measles virus at an MOI of 5. Vero cell monolayers were chosen for the measles Edmonston virus infection because measles virus does not grow well in LLC-MK2 cells. At 24 h postinfection, the monolayers were washed with methionine-negative Dulbecco's modified Eagle medium (DMEM) (Life Technologies), and 1 ml of methionine-negative DMEM was added to each flask. After incubation for 1 h at 32°C, the monolayers were again washed with methionine-negative DMEM. [35S]methionine was added to DMEM-negative medium at a concentration of 10 μCi/ml, and 1 ml was added to each flask which was then incubated at 32°C for 6 h. The cells were harvested and washed three times in phosphate-buffered saline. The cell pellets were resuspended in 1 ml of radioimmunoprecipitation assay (RIPA) buffer (1% [wt/vol] sodium deoxycholate, 1% [vol/vol] Triton X-100 [Sigma], 0.2% [wt/vol] sodium dodecyl sulfate, 150 mM NaCl, 50 mM Tris-HCl [pH 7.4]), were freeze-thawed, and were pelleted at 6,500 × g. The cell extract was transferred to a fresh Eppendorf tube, and a mixture of monoclonal antibodies which recognizes the HA glycoprotein of measles virus (79-XV-V17, 80-III-B2, and 81-1-366, generously provided by Steven Jacobson, National Institute of Neurological Disorders and Stroke, National Institutes of Health) (29, 48) or which recognizes the HN protein of PIV3 (101/1, 403/7, and 166/11) (61) was added to each sample and incubated with constant mixing for 2 h at 4°C. Immune complexes were precipitated by adding 200 μl of a 10% suspension of protein A Sepharose beads (Sigma, St. Louis, Mo.) to each sample followed by constant mixing at 4°C overnight. Each sample was suspended in 90 μl of 1× loading buffer, and 10 μl of reducing agent (Novex, San Diego, Calif.) was added. After heating at 70°C for 10 min, 20 μl of each sample was loaded onto a 4 to 12% polyacrylamide gel (NuPAGE; Novex) per the manufacturer's recommendations. The gel was dried and autoradiographed.
The ability of the rPIV3(HA) chimeric viruses to be neutralized by measles virus antisera was evaluated by a complement-enhanced 60% plaque reduction neutralization titer (PRNT) as previously described (7). Four cotton rats were infected with either 106 PFU of HPIV3 (wild-type JS) intranasally or 105 PFU of measles virus (Moraten) intramuscularly, and serum was harvested on days 0 and 28. The sera from the four animals infected with PIV3 or measles virus were combined to make a PIV3 antiserum (geometric mean PRNT against wild-type JS, >1:2,560) and a measles virus antiserum (PRNT against wild-type measles virus Edmonston, 1:2,290).
Multicycle replication of rPIV3s.
Monolayers of LLC-MK2 cells in six-well plates were infected with each virus at an MOI of 0.01 and were incubated at 32°C in 5% CO2. Six replicate cultures were sampled each day for each virus. Samples (500 μl) were removed from each culture at 24-h intervals for 7 consecutive days and were flash frozen. An equivalent volume of fresh medium was replaced at each time point. Each sample was titered on LLC-MK2 cell monolayers in 96-well plates incubated for 7 days at 32°C. Virus was detected by hemadsorption and was reported as mean log10 50% tissue culture infective dose (TCID50)/ml.
Replication of recombinant chimeric PIV3(HA) viruses at various temperatures.
Serial 10-fold dilutions of virus were prepared in L-15 medium (Quality Biological, Gaithersburg, Md.) supplemented with 5% FBS, 4 mM glutamine, and 50 μg of gentamicin per ml. Diluted viruses were used to infect LLC-MK2 cell monolayers in 96-well plates, and infected plates were incubated at various temperatures for 7 days as described (25). Virus titers were determined as described above.
Animal studies. (i) Determination of level of replication.
Groups of six to eight golden Syrian hamsters (4 to 6 weeks old) were inoculated intranasally with 0.1 ml of EMEM (Life Technologies) containing 106 TCID50 or 106 PFU of virus. The lungs and nasal turbinates were harvested on day 4 postinoculation as previously described (14), and virus titers were determined as described above. Virus titers were expressed as the mean log10 TCID50 per gram.
(ii) Determination of immunogenicity.
Groups of six to nine golden Syrian hamsters (age 4 to 6 weeks) were infected intranasally with 106.0 PFU of rPIV3 or control virus on day 0. Serum was collected from each hamster on day −1 preinoculation and on day 25 or 30 postinoculation. Serum antibody response to PIV3 was evaluated by hemagglutination inhibition (HAI) assay as previously described (62), and serum antibody response to measles virus was evaluated by a complement-enhanced 60% plaque reduction assay as previously described (7). Immunized animals were challenged intranasally with 106.0 PFU of wild-type JS PIV3 28 days after postimmunization, and lungs and nasal turbinates were harvested 4 days later for virus titration as described above.
RESULTS
Recovery of recombinant wild-type and attenuated PIV3s expressing the HA protein of measles virus.
The ORF encoding the measles virus HA protein was placed under the control of PIV3 gene start and gene end transcription signals and inserted into a wild-type PIV3 antigenomic cDNA between the N and P genes, the P and M genes, or the HN and L genes, yielding, respectively, pFLC(HA N-P), pFLC(HA P-M), and pFLC(HA HN-L). In addition, the N-P and P-M insertions also were made by using the attenuated cp45L backbone (see the Introduction), yielding pFLCcp45L(HA N-P) and pFLCcp45L(HA P-M).
A minor detail of the construction of one of the cDNAs, pFLC(HA HN-L), should be noted. First, in this insert, the HA ORF was designed to be flanked by the relatively long nontranslated regions of the PIV3 HN gene (Fig. 1A), whereas the insert used in the other constructs has shorter nontranslated regions (Fig. 1B). Second, this ORF had been obtained from an existing cDNA clone, and subsequent analysis showed that aa 46 and 481 had assignments (F and Y, respectively) that matched those of the Moraten vaccine strain rather than the Edmonston wild-type strain (which has assignments S and N, respectively). All of the other constructs were made by using a recloned HA cDNA whose sequence exactly matched that of the Edmonston wild type. In addition, this ORF was designed to be flanked by shorter nontranslated regions (Fig. 1B).
The three wild-type-based cDNAs, pFLC(HA N-P), pFLC(HA P-M), and pFLC(HA HN-L), and the two cp45L-based cDNAs, pcp45L(HA N-P) and pcp45L(HA P-M), were respectively transfected into HEp-2 cells along with the three PIV3 support plasmids [pTM(N), pTM(P no C), and pTM(L)] and recombinant virus, respectively designated rPIV3(HA N-P), rPIV3(HA P-M), rPIV3(HA HN-L), rcp45L(HA N-P), and rcp45L(HA P-M), was recovered following transfection (data not shown). Each rPIV3(HA) was characterized regarding the location and size of the HA insert and the presence of the cp45L mutations by restriction enzyme analysis of reverse transcription-PCR products generated from vRNA as previously described (52). Each virus was found to be as designed (data not shown). Such PCR fragments were generated from both the passage 1 harvest as well as the final cloned pool of each recombinant chimeric virus, demonstrating that the insert was stable over at least eight passages. The generation of each PCR product was dependent upon the inclusion of RT, indicating that each was derived from RNA and not from contaminating cDNA.
To confirm that the introduced measles virus HA ORF was expressed, RIPAs were carried out by using lysates from cells infected with rcp45L(HA N-P) and rcp45L(HA P-M) (Fig. 2). Lysates from cells infected with wild-type rJS virus and Edmonston wild-type measles virus were analyzed in parallel. rcp45L(HA N-P) and rcp45L(HA P-M) each encoded a protein which was the same size as the measles HA protein and which was precipitated by the measles virus HA monoclonal antibodies. Both viruses were confirmed to be PIV3 by the expression of PIV3 HN protein. rPIV3(HA) viruses not tested by RIPA were found to express HA protein by their immunogenicity in animals (see below).
FIG. 2.
Expression of the HA protein of measles virus by rPIV3-measles virus-HA chimeric viruses in LLC-MK2 cells. Cells were infected with rcp45L(HA P-M), rcp45L(HA N-P), the Edmonston wild-type strain of measles virus, wild-type rJS PIV3. Following labeling with [35S]methionine, lysates were prepared and immunoprecipitated by a mixture of three monoclonal antibodies specific to the PIV3 HN protein (lanes a). The 64-kDa band corresponding to the HN protein (open arrow) is present in each of the three PIV3-infected cell lysates (lanes 3, 5, and 7), but not in the measles virus-infected cell lysates (lane 9). Lanes b, the 76-kDa band corresponding to the measles virus HA protein (closed arrow) is immunoprecipitated from lysates from cells infected with the rcp45(HA) chimeric viruses (lanes 6 and 8) and the measles virus (lane 10), but not in the lysates from rJS-infected cells (lane 4).
To indirectly assess whether the HA protein expressed by the rPIV3(HA) chimeric viruses was incorporated into the virion envelope, the sensitivity of rcp45L(HA N-P) and rcp45L(HA P-M) to neutralization by a measles virus-specific cotton rat antiserum was determined. A control PIV3-specific antiserum readily neutralized rcp45L(HA N-P) and rcp45L(HA P-M) with a geometric mean PRNT of 1:3,691 (control serum lacking PIV3 antibody was <1:40). As expected, wild-type measles virus Edmonston was not neutralized with the anti-PIV3 sera (PRNT, <1:40). The measles virus antisera had no demonstrable neutralizing activity (PRNT, <1:40) against the rcp45L(HA) chimeric viruses or against rJS but had a PRNT against measles virus of 1:2,352. These data suggest that the measles HA protein expressed by the rPIV3(HA) viruses is not incorporated into the viral envelope in significant quantities or that, if incorporated, it does not render the virus susceptible to neutralization by measles virus antiserum.
Replication in cell culture of recombinant PIV3 expressing the HA protein of measles virus.
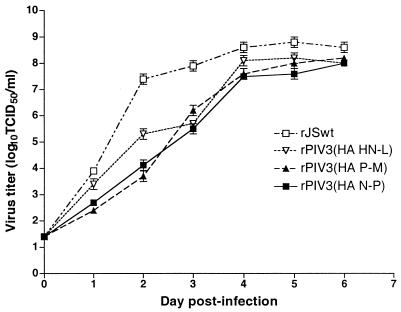
LLC-MK2 cell monolayers were infected with rPIV3(HA N-P), rPIV3(HA P-M), rPIV3(HA HN-L), or rJS at an MOI of 0.01 and were incubated at 32°C for 7 days (Fig. 3). Each of the rPIV3(HA) viruses attained a peak titer comparable to that of wild-type rJS; however, their rate of virus replication was delayed compared to rJS, indicating that insertion of the HA gene modified replication in vitro.
FIG. 3.
Multicycle replication of rPIV3(HA N-P), rPIV3(HA P-M), or rPIV3(HA HN-L) compared with the parent virus rJS. The virus titers are shown as log10 TCID50 per milliliter and are the averages of six samples. The lower limit of detection of this assay is 50 TCID50/ml.
Evaluation of the level of temperature sensitivity of replication of rPIV3(HA)s in cell culture.
Recombinant PIV3 expressing the measles virus HA protein in both the wild-type [rPIV3(HA N-P), rPIV3(HA P-M), and rPIV3(HA HN-L)] and attenuated backbones [rcp45L(HA N-P) and rcp45L(HA P-M)] were evaluated for their replicative ability at permissive temperature (32°C) and elevated temperature (36, 37, 38, 39, or 40°C) and were compared with their parental viruses, rcp45L or wild-type rJS. Interestingly, all three chimeric viruses in the wild-type backbone acquired a ts phenotype with a shutoff temperature of 38°C [rPIV3(HA HN-L)] or 39°C [rPIV3(HA N-P) and rPIV3(HA P-M)] (Table 1). The ts phenotype of the recombinant chimeric viruses in the rcp45L background was maintained, with rcp45L(HA N-P) exhibiting a shutoff temperature of 38°C, the same as that of rcp45L, whereas rcp45L(HA P-M) had a shutoff temperature 1° lower, at 37°C.
TABLE 1.
Replication at permissive and elevated temperatures of recombinant PIV3s expressing the HA protein of measles virus as an extra gene in the N-P, P-M, or HN-L junction
| Virus | Virus titer (log10 TCID50/ml) at indicated temperature (°C)
|
|||||
|---|---|---|---|---|---|---|
| 32a | 36 | 37 | 38 | 39 | 40 | |
| rJSb | 8.7 | 9.0 | 9.0 | 8.4 | 8.2 | 9.0 |
| rPIV3(HA N-P)c | 7.5 | NTf | NT | 6.7 | 4.2g | 3.7 |
| rPIV3(HA P-M)c | 7.7 | NT | NT | 6.5 | 4.7 | 5.2 |
| rPIV3(HA HN-L)c | 8.0 | NT | NT | 6.0 | 5.0 | 4.2 |
| rcp45Ld | 8.2 | 8.2 | 7.2 | 5.2 | 3.4 | 3.0 |
| rcp45L(HA N-P)e | 7.4 | 7.2 | 5.7 | 4.2 | 2.2 | <1.2 |
| rcp45L(HA P-M)e | 7.4 | 6.7 | 5.2 | 4.2 | 1.4 | 1.4 |
Permissive temperature.
Recombinant wild-type JS PIV3.
Recombinant wild-type JS PIV3 expressing the HA protein of measles virus.
Recombinant ts derivative of the wild-type JS PIV3, bearing three attenuating amino acid substitutions derived from cp45.
Recombinant attenuated ts derivative of wild-type JS PIV3 expressing the HA protein of measles virus.
NT, not tested at indicated temperature.
Underlined titers represent the lowest restrictive temperature at which a 100-fold or greater reduction in titer from that at 32°C is seen and defines the shutoff temperature of the virus.
Replication of wild-type and attenuated rPIV3(HA) in hamsters.
Replication of the wild-type rPIV3(HA) viruses was evaluated first (Table 2). Replication of each of the three measles HA chimeric viruses in the wild-type PIV3 background was somewhat reduced (5- to 10-fold), compared with wild-type rJS, in the upper respiratory tract of the hamsters, demonstrating that the insertion of the HA ORF itself conferred a very modest degree of attenuation in the upper respiratory tract. rPIV3(HA N-P) was the most attenuated at this site. Replication of rPIV3(HA P-M) was comparable to that of rJS in the lower respiratory tract of the hamsters, whereas replication of both rPIV3(HA N-P) and rPIV3(HA HN-L) was slightly reduced at this site compared with wild-type rJS. As expected, replication of rcp45L, the recombinant attenuated virus bearing the three ts mutations in the L protein, was significantly more attenuated than any of the wild-type rPIV3(HA) viruses in both the upper and lower respiratory tracts of the hamsters.
TABLE 2.
Replication of wild-type rPIV3(HA) chimeric viruses in the upper and lower respiratory tract of hamsters
| Virusa | Virus titer (log10 TCID50/g ± SE) (Tukey-Kramer grouping)b
|
|
|---|---|---|
| Nasal turbinates | Lungs | |
| rJS | 6.5 ± 0.1 (A) | 6.6 ± 0.2 (A) |
| rPIV3(HA N-P) | 5.1 ± 0.1 (B) | 5.9 ± 0.1 (B) |
| rPIV3(HA P-M) | 5.9 ± 0.1 (C) | 6.7 ± 0.2 (A) |
| rPIV3(HA HN-L) | 5.9 ± 0.2 (C) | 5.8 ± 0.1 (B) |
| rcp45L | 4.0 ± 0.1 (D) | 1.5 ± 0.1 (C) |
Animals received 106 TCID50 of the indicated virus intranasally in a 0.1-ml inoculum, and the lungs and nasal turbinates were harvested 4 days later. Each group contained eight animals.
Mean virus titers were assigned to statistically similar groups (A to D) by the Tukey-Kramer test. Therefore, means in each column with different letters are significantly different (α = 0.05), and those with the same letter are not significantly different.
The replication of the rPIV3(HA) viruses in the attenuated cp45L background was next compared with that of rJS and rcp45L. Replication of both rcp45L(HA N-P) and rcp45L(HA P-M) was reduced more than 100-fold in both the upper and lower respiratory tracts of hamsters compared with wild-type virus (Table 3). The rcp45L(HA) viruses were approximately 10-fold more attenuated than rcp45L in the upper respiratory tract of hamsters, indicating that the attenuating effect of the measles HA ORF insert is additive to the attenuating effect of the cp45L mutations for the upper respiratory tract. rcp45L and the two rcp45L(HA) viruses were equally attenuated in the lower respiratory tract of hamsters. The latter observation suggests that the cp45L ts mutations are dominant in the lower respiratory tract and that the attenuating effect of the HA insert is masked by that of the cp45L mutations.
TABLE 3.
Replication of the attenuated rcp45L(HA) viruses in the upper and lower respiratory tract of hamsters
| Virusa | No. of animals | Virus titer (log10 TCID50/g ± SE) (Tukey-Kramer grouping)b
|
|
|---|---|---|---|
| Nasal turbinates | Lungs | ||
| rcp45L | 6 | 4.7 ± 0.2 (A) | 2.9 ± 0.1 (A) |
| rcp45L(HA N-P) | 6 | 3.7 ± 0.2 (B) | 2.9 ± 0.1 (A) |
| rcp45L(HA P-M) | 7 | 3.7 ± 0.1 (B) | 2.9 ± 0.2 (A) |
| rJS | 7 | 6.5 ± 0.1 (C) | 5.6 ± 0.2 (B) |
Animals received 106 PFU of the indicated virus intranasally in a 0.1-ml inoculum, and the lungs and nasal turbinates were harvested 4 days later.
Mean virus titers were assigned to statistically similar groups (A to C) by the Tukey-Kramer test. Therefore, means in each column with different letters are significantly different (α = 0.05), and those with the same letter are not significantly different.
Immunogenicity of wild-type and attenuated rPIV3(HA) viruses.
The immunogenicity of the rPIV3(HA) viruses in the wild-type background was evaluated first (Table 4). Each of the rPIV3(HA) chimeric viruses in the wild-type background induced a moderate-to-high level of serum neutralizing antibodies against measles virus as well as a high level of antibodies against PIV3. Interestingly, the rPIV3(HA HN-L) chimeric virus elicited significantly less serum neutralizing antibody against measles virus than did the chimeric viruses with the measles HA ORF inserted upstream in either the N-P or P-M noncoding region. It is likely that the greater immunogenicity of rPIV3(HA N-P) and rPIV3(HA P-M) versus rPIV3(HA HN-L) (Table 4) resulted from their more 3′-proximal position in the chimeric virus genome. However, the former two viruses also differed in the sequence of the HA gene and in the length of the 3′ noncoding region of the HA gene from that in rPIV3(HA HN-L), as detailed above, which might have affected the level of protein expression or immunogenicity and contributed to the observed differences in the magnitude of the antibody response.
TABLE 4.
rPIV3(HA) viruses elicit moderate to high levels of serum antibodies to both measles virus and PIV3
| Virusa | Serum antibody response to measles virus (60% PRNT, mean reciprocal log2 ± SE) (Tukey-Kramer grouping)b
|
Serum antibody response to human PIV3 (HAI titer, mean reciprocal log2 ± SE) (Tukey-Kramer grouping)b
|
||
|---|---|---|---|---|
| Day −1 | Day 30 | Day −1 | Day 30 | |
| rPIV3(HA N-P) | ≤3.3 ± 0 (A) | 14.8 ± 0.3 (A) | ≤2.0 ± 0 (A) | 9.0 ± 0.4 (A) |
| rPIV3(HA P-M) | ≤3.3 ± 0 (A) | 15.3 ± 0.3 (A) | ≤2.0 ± 0 (A) | 9.0 ± 0.0 (A) |
| rPIV3(HA HN-L) | ≤3.3 ± 0 (A) | 6.1 ± 0.7 (B) | ≤2.0 ± 0 (A) | 9.4 ± 0.3 (A) |
Virus was administered at a dose of 106.0 PFU to hamsters in a 0.1-ml inoculum intranasally on day 0. Each group contained eight animals.
Mean HAI and neutralizing antibody titers were assigned to statistically similar groups (A or B) by the Tukey-Kramer test. Therefore, means in each column with different letters are significantly different (α = 0.05), and means with the same letter are not significantly different.
We next compared the immunogenicity of an attenuated chimera, rcp45L(HA P-M), with its wild-type counterpart, rPIV3(HA P-M), and with rcp45L(HA N-P). The serum antibody response of hamsters infected with rcp45L(HA N-P), rcp45L(HA P-M), or rPIV3(HA P-M) was compared with that from an additional control group which consisted of cotton rats that received 105.0 PFU of the live attenuated measles virus vaccine (Moraten strain) administered intramuscularly on day 0. Cotton rats, rather than hamsters, were used in this group because measles virus has low infectivity for hamsters. Each of the rcp45L(HA) chimeric viruses induced a high level of serum neutralizing antibodies against measles virus (Table 5). There was no significant difference between the amount of serum neutralizing antibody induced by the attenuated derivative rcp45L(HA P-M) compared to its counterpart constructed in the wild-type background, rPIV3(HA P-M). Furthermore, the level of measles virus-neutralizing serum antibodies induced by the rPIV3(HA) viruses were, on average, fivefold greater than that achieved by intramuscular immunization with the licensed live attenuated measles virus vaccine. In addition, the PIV3-specific serum antibody response produced by all the chimeric viruses was also robust and comparable to that induced by infection with wild-type rJS.
TABLE 5.
rcp45L(HA) viruses elicit high levels of serum antibodies to both measles virus and PIV3
| Virusa | No. of animals | Serum antibody response to measles virus (60% PRNT, mean reciprocal log2 ± SE) (Tukey-Kramer grouping)b
|
Serum antibody response to PIV3 (HAI titer, mean reciprocal log2 ± SE) (Tukey-Kramer grouping)b
|
||
|---|---|---|---|---|---|
| Day −1 | Day 25 | Day −1 | Day 25 | ||
| rcp45L(HA P-M) | 24 | ≤3.3 ± 0 (A) | 12.8 ± 0.1 (A) | ≤2.0 ± 0 (A) | 9.2 ± 0.2 (B) |
| rcp45L(HA N-P) | 6 | ≤3.3 ± 0 (A) | 13.4 ± 0.4 (A) | ≤2.0 ± 0 (A) | 10.8 ± 0.3 (A) |
| rPIV3(HA P-M) | 6 | ≤3.3 ± 0 (A) | 13.3 ± 0.3 (A) | ≤2.0 ± 0 (A) | 10.3 ± 0.2 (A) |
| rcp45L | 18 | ≤3.3 ± 0 (A) | ≤3.3 ± 0 (B) | ≤2.0 ± 0 (A) | 10.3 ± 0.2 (A) |
| rJS | 6 | ≤3.3 ± 0 (A) | ≤3.3 ± 0 (B) | ≤2.0 ± 0 (A) | 10.7 ± 0.2 (A) |
| Measles virus (Moraten)c | 4 | ≤3.3 ± 0 (A) | 10.8 ± 0.2 (C) | ≤2.0 ± 0 (A) | ≤2.0 ± 0.0 (C) |
Virus was administered at a dose of 106.0 PFU to the indicated number of hamsters in a 0.1-ml inoculum intranasally on day 0 to all animals with the exception of those in the measles virus (Moraten) group.
Mean HAI and neutralization antibody titers were assigned to statistically similar groups (A to C) by the Tukey-Kramer test. Therefore, means in each column with different letters are significantly different (α = 0.05), and means with the same letter are not significantly different.
The live attenuated measles vaccine virus, Moraten strain, was administered intramuscularly at a dose of 105 PFU in a 0.1-ml inoculum to four cotton rats in a separate study and is presented for reference value only.
The efficacy of rcp45L(HA N-P) and rcp45L(HA P-M) in providing protection against challenge with wild-type PIV3 was next examined (Table 6). Hamsters infected with the chimeric viruses, whether in the attenuated or wild-type background, were significantly resistant to replication of wild-type PIV3 challenge virus in both the upper and lower respiratory tract. Thus, despite the attenuating effect on replication of the rcp45L(HA) antigenic chimeric viruses produced by the acquisition of the measles virus HA gene, infection with either rcp45L(HA P-M) or rcp45L(HA N-P) induced a high level of protection against PIV3 as indicated by approximately a 100- to 1,000-fold reduction of its replication in the upper or lower respiratory tracts of hamsters. Unfortunately, wild-type measles virus does not replicate efficiently in hamsters, precluding a measles virus challenge. However, it is reasonable to infer that the attenuated antigenic chimeric rcp45L(HA) vaccine candidates would be efficacious against measles virus since high levels of neutralizing antibody were induced. Such levels of measles virus antibodies are associated with strong resistance to measles virus disease in humans (6).
TABLE 6.
Attenuated and wild-type PIV3-measles HA chimeric viruses are highly protective against replication of wild-type PIV3 challenge virus in the upper and lower respiratory tracts of hamstersa
| Virus used to immunize animals | Virus titer (log10 TCID50/g ± SE) (Tukey-Kramer grouping)b
|
Reduction in titer (log10)
|
||
|---|---|---|---|---|
| Nasal turbinates | Lungs | Nasal turbinates | Lungs | |
| RSVc | 7.0 ± 0.3 (A) | 5.7 ± 0.4 (A) | NAd | NA |
| rcp45L(HA P-M) | 3.4 ± 0.3 (B) | 2.9 ± 0.0 (B) | 3.6 | 2.8 |
| rcp45L(HA N-P) | 2.6 ± 0.3 (B) | 3.4 ± 0.2 (B) | 4.4 | 2.3 |
| rPIV3(HA P-M) | 2.0 ± 0.3 (B) | 3.2 ± 0.1 (B) | 5.0 | 2.5 |
| rcp45L | 1.9 ± 0.2 (B,C) | 3.6 ± 0.1 (B) | 5.1 | 2.1 |
| rJS | <1.4 ± 0.0 (C) | 2.9 ± 0.2 (B) | >5.7 | 2.8 |
All groups were challenged with 106 PFU of biologically derived wild-type JS PIV3 in a 0.1-ml inoculum given intranasally on day 28 after immunization, and lungs and nasal turbinates were harvested 4 days later. Each group contained six animals.
Mean virus titers were assigned to statistically similar groups (A to C) by the Tukey-Kramer test. Therefore, means in each column with different letters are significantly different (α = 0.05), and means with the same letter are not significantly different.
RSV is a negative control.
NA, not applicable.
DISCUSSION
Successful immunization of young infants with the live attenuated measles virus vaccine has been very difficult to achieve because the parenterally administered vaccine virus is readily neutralized by maternally derived serum antibody. Several immunization strategies to overcome this obstacle have been developed, but each has significant flaws. The first strategy involved administration of one of the licensed live attenuated measles virus vaccines intranasally by drops (2, 35, 51) or into the lower respiratory tract by aerosolization (10, 46). Intranasal administration did not consistently infect vaccinees. Aerosol administration infected a majority of vaccinees in highly controlled experimental studies, but it has been difficult to reproducibly deliver a live attenuated measles virus to young infants in a field setting using this methodology (10). By using another approach, the measles vaccine virus was administered parenterally at a 10- to 100-fold-increased dose (38). This improved seroconversion in infants 4 to 6 months of age, but there was an associated increase in mortality in the high-titer vaccine recipients later in infancy (22, 28, 38).
A second strategy involved the use of an inactivated whole virus vaccine or a subunit virus vaccine. However, formalin-treated measles virus and respiratory syncytial virus both potentiated rather than prevented their respective diseases (20, 34, 42). Because of this experience with nonliving measles virus vaccines and also because the immunogenicity of such parenterally administered vaccines can be decreased by passively transferred antibodies (41), there has been considerable reluctance to evaluate such vaccines in human infants.
A third strategy involves the use of virus vectors to express a measles virus antigen. A variety of vectors, either replication competent or replication defective, including poxviruses, rhabdoviruses, and adenoviruses (12, 19, 48, 58, 64, 65), has been explored. Recombinants expressing the F or HA glycoprotein of measles virus are highly immunogenic when given parenterally. However, their immunogenicity is decreased in a host with passively acquired measles virus antibodies (21, 43, 49, 50, 60), and this has also been observed with other paramyxoviruses (17, 41). Replication-competent vaccinia virus recombinants are not sufficiently attenuated for use in immunocompromised hosts, and therefore, they are no longer being pursued as vectors for use in humans (18, 45). Current poxvirus research employs safe, replication-defective vectors such as the MVA vector (3, 4, 11, 40, 55, 56, 63), but the immunogenicity and efficacy of MVAs expressing the PIV3 protective antigens were abrogated in passively immunized rhesus monkeys whether delivered by a parenteral or mucosal route (13). It is possible that an intranasally administered recombinant vesicular stomatitis virus (VSV) expressing a measles virus antigen could replicate and be immunogenic in a host with passively acquired maternal measles virus-specific antibodies, but there is no experience in immunizing humans with this virus. The immunogenicity of DNA vaccines expressing measles virus protective antigens delivered parenterally was also decreased in passively immunized hosts (50). Based on these observations, it appears unlikely that a parenterally administered, replication-competent or replication-defective virus vector or a DNA vaccine expressing a measles virus protective antigen will be satisfactorily immunogenic or efficacious in infants possessing passively acquired maternal measles virus-specific antibodies. The rPIV3(HA) chimeras described in this paper should overcome some of the deficiencies of these previous attempts.
The PIV3(HA) chimeras offer six advantages over previous attempts to immunize the young infant against measles virus. First, the PIV3(HA) chimeras are highly immunogenic with respect to measles virus, inducing a level of neutralizing antibodies far in excess of that required for protection against measles virus disease in humans (6). Chimeras with the HA ORF inserted in the N-P or P-M junction induced 400-fold more measles virus-neutralizing antibodies than did the chimera with the HA ORF inserted in the HN-L junction, identifying these 3′-proximal positions as preferred sites for expression of a foreign antigen.
Second, the rPIV3 backbone carrying the HA gene of measles virus (or other protective antigen of another microbial pathogen) will induce a dual protective immune response against both PIV3, for which there is a compelling independent need for a vaccine, and measles virus. This is in contrast to the VSV-measles virus HA recombinant which will induce immunity to only one human pathogen, i.e., the measles virus, and in which the immune response to the vector itself is irrelevant. Use of a backbone from a human pathogen for which immunization is needed will favor the introduction of such a dual purpose live attenuated virus vector into an already crowded early childhood immunization schedule.
Third, the recombinant PIV3 backbone expressing the measles virus antigen is a highly characterized attenuated virus bearing identified mutations, the three amino acid substitutions in the L protein, that are known to provide most of the attenuation of the cp45 vaccine candidate, a virus known to be safe, immunogenic, and phenotypically stable in seronegative human infants (24, 33, 52, 53). However, each rcp45L(HA) virus contains only three of the 15 mutations found in cp45. An unexpected finding in our study was that the addition of the measles HA ORF, regardless of the site of insertion, attenuated the PIV3(HA) chimeric viruses for the upper respiratory tract of hamsters. This level of additional attenuation is equal to or greater than that specified by the other 12 mutations present in cp45 (52). The additional attenuation provided by the HA insertion should add to the phenotypic stability provided by the cp45L backbone. The extensive history of prior clinical evaluation of the cp45 parent virus should facilitate evaluation, in the very young human infant as well as adults, of recombinant derivatives of this virus bearing foreign antigens. This, again, is in contrast to a VSV backbone which would have to be attenuated for use in humans and which would require a significant amount of clinical research involving human infants to identify a recombinant VSV that has achieved a satisfactory balance between attenuation and immunogenicity.
Fourth, immunization via the mucosal surface of the respiratory tract offers additional advantages. cp45 was shown to replicate in the respiratory tract of rhesus monkeys and to induce a protective immune response against challenge with wild-type PIV3 when given in the presence of a high titer of passively derived PIV3 antibodies (13), and this was also true for an RSV vaccine candidate in chimpanzees (9). In humans, a live attenuated PIV3 vaccine candidate readily initiated infection and replicated to a moderate level in the upper respiratory tract of very young infants who possessed maternally acquired PIV3 antibodies (31–33), a finding in contrast to the currently licensed measles virus vaccine which is poorly infectious when administered to the upper respiratory tract of infants and young children (2, 35, 51). Based on the above experience with the live attenuated PIV3 vaccine candidate and the finding that the rPIV3(HA) viruses are not neutralized by antibody to measles virus, we would expect these chimeric viruses to induce an immune response in young infants possessing maternal IgG directed against measles. Replication of the PIV3 vector in the respiratory tract will stimulate the production of both mucosal IgA and systemic IgG to both PIV3 and measles virus. Upon subsequent natural exposure to measles virus, the existence of vaccine-induced local and systemic immunity should serve to restrict its replication at both its portal of entry, i.e., the respiratory tract, as well as at systemic sites of replication.
A fifth advantage of the PIV3(HA) chimeras is their ability to replicate to a titer greater than 107.5 TCID50/ml in vitro. PIV3 cp45 viruses are known to infect almost all seronegative humans at a dose of 105.0 (33). This demonstrates the feasibility for large-scale production of the PIV3(HA) chimeras for vaccine use.
Sixth, the PIV1 and PIV2 antigenic serotypes of human PIV, for which there is an independent need for a vaccine, can also serve as a dual-vector vaccine, similar to the rPIV3(HA) chimeras described in this paper (54, 57). The presence of three antigenically distinct PIVs provides a unique opportunity to sequentially immunize the infant with antigenically distinct variants of human PIV, each bearing the same foreign protein, e.g., the HA protein of measles virus. In this manner, the sequential immunization will permit the development of a primary immune response to the measles virus HA which can be boosted during subsequent infections with the antigenically distinct human PIV also bearing the HA of measles virus. This PIV vector system offers considerable flexibility in formulating new strategies for immunization against multiple pathogens.
Although these initial results are quite promising, some questions remain to be answered. It is unclear if expression of the HA protein by these chimeric viruses could affect viral tropism or even predispose subjects to atypical measles. However, it has been demonstrated that both the F and HA proteins are required for cell-to-cell fusion and spread of measles virus (44, 66). Because rPIV3(HA) viruses express only HA of measles and not F and because it is unlikely that the HA protein is incorporated into their viral envelope, altered tropism of these viruses would not be expected to occur. In the past it was thought that atypical measles was due to an imbalance of the HA and F proteins because the F protein was not well preserved in the formalin-inactivated vaccine. Recently, however, it was demonstrated that atypical measles was not due to a failure to induce anti-F antibody but was most likely due to a short-lived immunity induced by the inactivated virus vaccine which rendered the host susceptible to measles infection and to an altered immune response to some component of the inactivated virus vaccine (44). For this reason, we would not expect infection by these chimeric vaccine viruses to predispose to atypical measles disease. To better answer these questions and to determine the immunogenicity and protective efficacy of these chimeric viruses when given in the presence of passive PIV3 and measles virus antibodies, we are planning to evaluate the PIV3(HA) viruses in rhesus monkeys, a primate species which develops disease following measles virus infection.
In summary, we have developed novel dual-purpose recombinant vaccine candidate viruses which induce a protective antibody response against both PIV3 and measles virus. We suggest that the intranasal route of immunization with a respiratory virus vector is a general method to induce local and systemic immunity and to reduce the inhibiting effect of preexisting serum antibodies on replication of vaccine virus. Given the considerable morbidity and mortality caused by measles virus in the world today, especially in children less than 1 year of age, an improved live attenuated vaccine candidate is greatly needed and could be a useful adjunct immunogen in the effort to eliminate the measles virus globally.
ACKNOWLEDGMENT
We thank Robert Chanock for careful review of the manuscript and for insightful comments.
REFERENCES
- 1.Albrecht P, Ennis F A, Saltzman E J, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 2.Black F L, Sheridan S R. Studies on an attenuated measles-virus vaccine. IV. Administration of vaccine by several routes. N Engl J Med. 1960;263:165–169. doi: 10.1056/NEJM196007282630404. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard T J, Alcami A, Andrea P, Smith G L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 4.Carroll M W, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Measles eradication: recommendations from a meeting cosponsored by the World Health Organization, the Pan American Health Organization, and CDC. Morbid Mortal Weekly Rep. 1997;46:1–21. [PubMed] [Google Scholar]
- 6.Chen R T, Markowitz L E, Albrecht P, Stewart J A, Mofenson L M, Preblud S R, Orenstein W A. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 7.Coates H V, Alling D W, Chanock R M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966;83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 8.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1313–1352. [Google Scholar]
- 9.Crowe J E, Jr, Bui P T, Siber G R, Elkins W R, Chanock R M, Murphy B R. Cold-passaged, temperature-sensitive mutants of human respiratory syncytial virus (RSV) are highly attenuated, immunogenic, and protective in seronegative chimpanzees, even when RSV antibodies are infused shortly before immunization. Vaccine. 1995;13:847–855. doi: 10.1016/0264-410x(94)00074-w. [DOI] [PubMed] [Google Scholar]
- 10.Cutts F T, Clements C J, Bennett J V. Alternative routes of measles immunization: a review. Biologicals. 1997;25:323–338. doi: 10.1006/biol.1997.0103. [DOI] [PubMed] [Google Scholar]
- 11.Drexler I, Heller K, Wahren B, Erfle V, Sutter G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol. 1998;79:347–352. doi: 10.1099/0022-1317-79-2-347. [DOI] [PubMed] [Google Scholar]
- 12.Drillien R, Spehner D, Kirn A, Giraudon P, Buckland R, Wild F, Lecocq J P. Protection of mice from fatal measles encephalitis by vaccination with vaccinia virus recombinants encoding either the hemagglutinin or the fusion protein. Proc Natl Acad Sci USA. 1988;85:1252–1256. doi: 10.1073/pnas.85.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durbin A P, Cho C J, Elkins W R, Wyatt L S, Moss B, Murphy B R. Comparison of the immunogenicity and efficacy of a replication-defective vaccinia virus expressing antigens of human parainfluenza virus type 3 (HPIV3) with those of a live attenuated HPIV3 vaccine candidate in rhesus monkeys passively immunized with PIV3 antibodies. J Infect Dis. 1999;179:1345–1351. doi: 10.1086/314769. [DOI] [PubMed] [Google Scholar]
- 14.Durbin A P, Hall S L, Siew J W, Whitehead S S, Collins P L, Murphy B R. Recovery of infectious human parainfluenza virus type 3 from cDNA. Virology. 1997;235:323–332. doi: 10.1006/viro.1997.8697. [DOI] [PubMed] [Google Scholar]
- 15.Durbin A P, McAuliffe J M, Collins P L, Murphy B R. Mutations in the C, D, and V open reading frames of human parainfluenza virus type 3 attenuate replication in rodents and primates. Virology. 1999;261:319–330. doi: 10.1006/viro.1999.9878. [DOI] [PubMed] [Google Scholar]
- 16.Durbin A P, Siew J W, Murphy B R, Collins P L. Minimum protein requirements for transcription and RNA replication of a minigenome of human parainfluenza virus type 3 and evaluation of the rule of six. Virology. 1997;234:74–83. doi: 10.1006/viro.1997.8633. [DOI] [PubMed] [Google Scholar]
- 17.Durbin A P, Wyatt L S, Siew J, Moss B, Murphy B R. The immunogenicity and efficacy of intranasally or parenterally administered replication-deficient vaccinia-parainfluenza virus type 3 recombinants in rhesus monkeys. Vaccine. 1998;16:1324–1330. doi: 10.1016/s0264-410x(98)00010-3. [DOI] [PubMed] [Google Scholar]
- 18.Fenner F, Henderson D A, Arita I, Kezek Z, Ladnyi I D. Smallpox and its eradication. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 19.Fooks A R, Schadeck E, Liebert U G, Dowsett A B, Rima B K, Steward M, Stephenson J R, Wilkinson G W. High-level expression of the measles virus nucleocapsid protein by using a replication-deficient adenovirus vector: induction of an MHC-1-restricted CTL response and protection in a murine model. Virology. 1995;210:456–465. doi: 10.1006/viro.1995.1362. [DOI] [PubMed] [Google Scholar]
- 20.Fulginiti V A, Eller J J, Downie A W, Kempe C H. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202:1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- 21.Galletti R, Beauverger P, Wild T F. Passively administered antibody suppresses the induction of measles virus antibodies by vaccinia-measles recombinant viruses. Vaccine. 1995;13:197–201. doi: 10.1016/0264-410x(95)93136-w. [DOI] [PubMed] [Google Scholar]
- 22.Gellin B G, Katz S L. Measles: state of the art and future directions. J Infect Dis. 1994;170:3–14. doi: 10.1093/infdis/170.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 23.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1267–1312. [Google Scholar]
- 24.Hall S L, Sarris C M, Tierney E L, London W T, Murphy B R. A cold-adapted mutant of parainfluenza virus type 3 is attenuated and protective in chimpanzees. J Infect Dis. 1993;167:958–962. doi: 10.1093/infdis/167.4.958. [DOI] [PubMed] [Google Scholar]
- 25.Hall S L, Stokes A, Tierney E L, London W T, Belshe R B, Newman F C, Murphy B R. Cold-passaged human parainfluenza type 3 viruses contain ts and non-ts mutations leading to attenuation in rhesus monkeys. Virus Res. 1992;22:173–184. doi: 10.1016/0168-1702(92)90049-f. [DOI] [PubMed] [Google Scholar]
- 26.Halsey N A, Boulos R, Mode F, Andre J, Bowman L, Yaeger R G, Toureau S, Rohde J, Boulos C. Response to measles vaccine in Haitian infants 6 to 12 months old. Influence of maternal antibodies, malnutrition, and concurrent illnesses. N Engl J Med. 1985;313:544–549. doi: 10.1056/NEJM198508293130904. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman M A, Banerjee A K. An infectious clone of human parainfluenza virus type 3. J Virol. 1997;71:4272–4277. doi: 10.1128/jvi.71.6.4272-4277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holt E A, Moulton L H, Siberry G K, Halsey N A. Differential mortality by measles vaccine titer and sex. J Infect Dis. 1993;168:1087–1096. doi: 10.1093/infdis/168.5.1087. [DOI] [PubMed] [Google Scholar]
- 29.Hummel K B, Bellini W J. Localization of monoclonal antibody epitopes and functional domains in the hemagglutinin protein of measles virus. J Virol. 1995;69:1913–1916. doi: 10.1128/jvi.69.3.1913-1916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janeway C A. Use of concentrated human serum gamma-globulin in the prevention and attenuation of measles. Bull N Y Acad Med. 1945;21:202–212. [PMC free article] [PubMed] [Google Scholar]
- 31.Karron R A, Makhene M, Gay K, Wilson M H, Clements M L, Murphy B R. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in two- to six-month-old infants. Pediatr Infect Dis J. 1996;15:650–654. doi: 10.1097/00006454-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Karron R A, Wright P F, Hall S L, Makhene M, Thompson J, Burns B A, Tollefson S, Steinhoff M C, Wilson M H, Harris D O, Clements M L, Murphy B R. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J Infect Dis. 1995;171:1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- 33.Karron R A, Wright P F, Newman F K, Makhene M, Thompson J, Samorodin R, Wilson M H, Anderson E L, Clements M L, Murphy B R, Belshe R B. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J Infect Dis. 1995;172:1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]
- 34.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 35.Kok P W, Kenya P R, Ensering H. Measles immunization with further attenuated heat-stable measles vaccine using five different methods of administration. Trans R Soc Trop Med Hyg. 1983;77:171–176. doi: 10.1016/0035-9203(83)90059-7. [DOI] [PubMed] [Google Scholar]
- 36.Krugman S. The clinical use of gamma globulin. N Engl J Med. 1963;269:195–201. doi: 10.1056/NEJM196307252690406. [DOI] [PubMed] [Google Scholar]
- 37.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 38.Markowitz L E, Sepulveda J, Diaz-Ortega J L, Valdespino J L, Albrecht P, Zell E R, Stewart J, Zarate M L, Bernier R H. Immunization of six-month-old infants with different doses of Edmonston-Zagreb and Schwarz measles vaccines. N Engl J Med. 1990;322:580–587. doi: 10.1056/NEJM199003013220903. . (Erratum, 332:863.) [DOI] [PubMed] [Google Scholar]
- 39.Marx A, Torok T J, Holman R C, Clarke M J, Anderson L J. Pediatric hospitalizations for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J Infect Dis. 1997;176:1423–1427. doi: 10.1086/514137. [DOI] [PubMed] [Google Scholar]
- 40.Mayr A, Stickl H, Muller H K, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentbl Bakteriol. 1978;167:375–390. . (In German.) [PubMed] [Google Scholar]
- 41.Murphy B R, Olmsted R A, Collins P L, Chanock R M, Prince G A. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol. 1988;62:3907–3910. doi: 10.1128/jvi.62.10.3907-3910.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nader P, Horwitz M, Rousseau J. Atypical exanthem following exposure to natural measles. Eleven cases in children previously inoculated with killed vaccine. J Pediatr. 1968;72:22–28. doi: 10.1016/j.jpeds.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Osterhaus A, van Amerongen G, van Binnendijk R. Vaccine strategies to overcome maternal antibody mediated inhibition of measles vaccine. Vaccine. 1998;16:1479–1481. doi: 10.1016/s0264-410x(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 44.Polack F P, Auwaerter P G, Lee S H, Nousari H C, Valsamakis A, Leiferman K M, Diwan A, Adams R J, Griffin D E. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat Med. 1999;5:629–634. doi: 10.1038/9473. [DOI] [PubMed] [Google Scholar]
- 45.Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 46.Sabin A B, Albrecht P, Takeda A K, Ribeiro E M, Veronesi R. High effectiveness of aerosolized chick embryo fibroblast measles vaccine in seven-month-old and older infants. J Infect Dis. 1985;152:1231–1237. doi: 10.1093/infdis/152.6.1231. [DOI] [PubMed] [Google Scholar]
- 47.Schnell M J, Buonocore L, Kretzschmar E, Johnson E, Rose J K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheshberadaran H, Norrby E, Rammohan K W. Monoclonal antibodies against five structural components of measles virus. II. Characterization of five cell lines persistently infected with measles virus. Arch Virol. 1985;83:251–268. doi: 10.1007/BF01309921. [DOI] [PubMed] [Google Scholar]
- 49.Siegrist C A, Cordova M, Brandt C, Barrios C, Berney M, Tougne C, Kovarik J, Lambert P H. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine. 1998;16:1409–1414. doi: 10.1016/s0264-410x(98)00100-5. [DOI] [PubMed] [Google Scholar]
- 50.Siegrist C A, Lambert P H. Maternal immunity and infant responses to immunization: factors influencing infant responses. Dev Biol Stand. 1998;95:133–139. [PubMed] [Google Scholar]
- 51.Simasathien S, Migasena S, Bellini W, Samakoses R, Pitisuttitham P, Bupodom W, Heath J, Anderson L, Bennett J. Measles vaccination of Thai infants by intranasal and subcutaneous routes: possible interference from respiratory infections. Vaccine. 1997;15:329–334. doi: 10.1016/s0264-410x(97)00104-7. [DOI] [PubMed] [Google Scholar]
- 52.Skiadopoulos M H, Durbin A P, Tatem J M, Wu S L, Paschalis M, Tao T, Collins P L, Murphy B R. Three amino acid substitutions in the L protein of the human parainfluenza virus type 3 cp45 live attenuated vaccine candidate contribute to its temperature-sensitive and attenuation phenotypes. J Virol. 1998;72:1762–1768. doi: 10.1128/jvi.72.3.1762-1768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skiadopoulos M H, Surman S, Tatem J M, Paschalis M, Wu S L, Udem S A, Durbin A P, Collins P L, Murphy B R. Identification of mutations contributing to the temperature-sensitive, cold-adapted, and attenuation phenotypes of the live-attenuated cold-passage 45 (cp45) human parainfluenza virus 3 candidate vaccine. J Virol. 1999;73:1374–1381. doi: 10.1128/jvi.73.2.1374-1381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skiadopoulos M H, Tao T, Surman S R, Collins P L, Murphy B R. Generation of a parainfluenza virus type 1 vaccine candidate by replacing the HN and F glycoproteins of the live-attenuated PIV3 cp45 vaccine virus with their PIV1 counterparts. Vaccine. 1999;18:503–510. doi: 10.1016/s0264-410x(99)00227-3. [DOI] [PubMed] [Google Scholar]
- 55.Stickl H, Hochstein-Mintzel V, Mayr A, Huber H C, Schafer H, Holzner A. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) Dtsch Med Wochenschr. 1974;99:2386–2392. doi: 10.1055/s-0028-1108143. . (In German.) [DOI] [PubMed] [Google Scholar]
- 56.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao T, Skiadopoulos M H, Durbin A P, Davoodi F, Collins P L, Murphy B R. A live attenuated chimeric recombinant parainfluenza virus (PIV) encoding the internal proteins of PIV type 3 and the surface glycoproteins of PIV type 1 induces complete resistance to PIV1 challenge and partial resistance to PIV3 challenge. Vaccine. 1999;17:1100–1108. doi: 10.1016/s0264-410x(98)00327-2. [DOI] [PubMed] [Google Scholar]
- 58.Taylor J, Weinberg R, Tartaglia J, Richardson C, Alkhatib G, Briedis D, Appel M, Norton E, Paoletti E. Nonreplicating viral vectors as potential vaccines: recombinant canarypox virus expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology. 1992;187:321–328. doi: 10.1016/0042-6822(92)90321-f. [DOI] [PubMed] [Google Scholar]
- 59.Taylor W R, Mambu R K, ma-Disu M, Weinman J M. Measles control efforts in urban Africa complicated by high incidence of measles in the first year of life. Am J Epidemiol. 1988;127:788–794. doi: 10.1093/oxfordjournals.aje.a114860. [DOI] [PubMed] [Google Scholar]
- 60.van Binnendijk R S, Poelen M C, van Amerongen G, de Vries P, Osterhaus A D. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J Infect Dis. 1997;175:524–532. doi: 10.1093/infdis/175.3.524. [DOI] [PubMed] [Google Scholar]
- 61.van Wyke Coelingh K L, Murphy B R, Collins P L, Lebacq-Verheyden A M, Battey J F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987;160:465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]
- 62.van Wyke Coelingh K L, Winter C, Murphy B R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985;143:569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]
- 63.Werner G T, Jentzsch U, Metzger E, Simon J. Studies on poxvirus infections in irradiated animals. Arch Virol. 1980;64:247–256. doi: 10.1007/BF01322704. [DOI] [PubMed] [Google Scholar]
- 64.Wild F, Giraudon P, Spehner D, Drillien R, Lecocq J P. Fowlpox virus recombinant encoding the measles virus fusion protein: protection of mice against fatal measles encephalitis. Vaccine. 1990;8:441–442. doi: 10.1016/0264-410x(90)90243-f. [DOI] [PubMed] [Google Scholar]
- 65.Wild T F, Bernard A, Spehner D, Drillien R. Construction of vaccinia virus recombinants expressing several measles virus proteins and analysis of their efficacy in vaccination of mice. J Gen Virol. 1992;73:359–367. doi: 10.1099/0022-1317-73-2-359. [DOI] [PubMed] [Google Scholar]
- 66.Wild T F, Malvoisin E, Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991;72:439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization. Expanded programme on immunization-accelerated measles strategies. Weekly Epidemiol Rec. 1994;69:229–234. [PubMed] [Google Scholar]
- 68.Wyatt L S, Moss B, Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology. 1995;210:202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]