Abstract
Iron plays a fundamental role in multiple brain disorders. However, the genetic underpinnings of brain iron and its implications for these disorders are still lacking. Here, we conduct an exome-wide association analysis of brain iron, measured by quantitative susceptibility mapping technique, across 26 brain regions among 26,789 UK Biobank participants. We find 36 genes linked to brain iron, with 29 not being previously reported, and 16 of them can be replicated in an independent dataset with 3,039 subjects. Many of these genes are involved in iron transport and homeostasis, such as FTH1 and MLX. Several genes, while not previously connected to brain iron, are associated with iron-related brain disorders like Parkinson’s (STAB1, KCNA10), Alzheimer’s (SHANK1), and depression (GFAP). Mendelian randomization analysis reveals six causal relationships from regional brain iron to brain disorders, such as from the hippocampus to depression and from the substantia nigra to Parkinson’s. These insights advance our understanding of the genetic architecture of brain iron and offer potential therapeutic targets for brain disorders.
Subject terms: Genetics of the nervous system, Diseases of the nervous system, Genetic association study
Brain iron accumulation has been associated with multiple brain disorders, but the mechanism is not well understood. Here, the authors investigate the genetic determinants of brain iron accumulation and their implications for neuropsychiatric disorders.
Introduction
Iron is crucial for numerous physiological processes, including neurotransmitter synthesis, myelin formation, DNA synthesis and mitochondrial functions, and it profoundly influences neurodevelopment, cognition, and brain outcomes1,2. Our brain maintains a precise regulation of iron homeostasis. Any disturbance in this intricate balance, whether through iron overload or deficiency, may result in the emergence of brain disorders3. For example, iron accumulation might facilitate neuronal cell death in some neurodegenerative diseases, such as Alzheimer’s disease (AD)4. In addition, the substantia nigra often has excess iron in Parkinson’s disease (PD), which possibly promotes oxidative stress and neuronal damage5. Moreover, cerebral iron deficiency, linked to alterations in hippocampal glucocorticoid receptor signaling, has been implicated in inducing depression6. Given iron’s important role in brain development and its connection to multiple brain disorders, understanding the genetic architecture of brain iron accumulation can provide insights into brain development and the underlying mechanisms of these disorders, allowing for designing better diagnostic and therapeutic strategies.
Quantitative susceptibility mapping (QSM) is an emerging technique that enables the non-invasive measurement of brain iron levels with high spatial resolution and sensitivity7. Built upon susceptibility-weighted MRI (swMRI), QSM has been demonstrated to be more sensitive to reflect tissue iron contents both phenotypically and genetically than other swMRI-derived measures, such as T2*, and has shown higher robustness to acquisition noises and increased reproducibility8. A high positive correlation between brain iron level and QSM has been established from postmortem studies9. Previous studies also showed that brain iron has a high heritability in multiple regions, such as the putamen, substantia nigra, and pallidum8. While genome-wide association studies (GWAS) have found several loci associated with brain iron, these findings remain constrained to a few brain regions and common genetic variations (minor allele frequency > 1%)8. Additionally, many loci identified by GWAS map to noncoding regions of the genome, posing challenges in exploring the underlying mechanism. To overcome these limitations, a powerful technique, whole-exome sequencing (WES)10, can be used to identify protein-coding variants that are associated with brain iron. A large-scale exome-wide association analysis on multiple brain regions can uncover the intricate genetic architecture of brain iron accumulation and potentially highlight neural pathways crucial to iron-related brain disorders.
In this study, we conducted the most extensive exome-wide association study (EWAS) of brain iron accumulation to date. Leveraging genetic, brain imaging and phenotypic data from 26,789 subjects in the UK Biobank dataset, we systemically identified protein-coding variants associated with brain iron and studied the relationships between iron-related genes and brain disorders and phenotypes. Specifically, this study has four major goals. Firstly, we will identify rare and common genes that are associated with brain iron accumulation across multiple brain regions covering subcortical and cerebellar structures. Secondly, we aim to explore the biological functions of the identified genes, such as the biological pathways in which they are enriched in. Thirdly, we aim to explore the relationships between brain iron-related genes and disorders, including whether regional brain iron accumulation has causal relationships to multiple brain disorders. Finally, we used phenome-wide association study (PheWAS) to identify genetic associations of brain iron-related genes with a broad set of phenotypic variables.
Results
An overview of data and analysis pipeline
Our study primarily used brain imaging and phenotypic and genetic data from the UK Biobank, including 26 regional QSM features extracted from the swMRI data, exome-sequencing data, and diverse phenotypes for phenome-wide association studies. In the primary analysis of EWAS, we included a total of 29,828 individuals of white British ethnicity without illness conditions of brain cancer, stroke, or dementia, aged between 40 and 69, with ~52% of them being females. Among them, 26,789 of them are in the discovery set and the remaining 3039 are in the replication set (see the “Methods” section). A summary of the demographic information is provided in Table 1. A total of 18,800 rare genes and 41,790 common variants were analyzed in this study (see the “Methods” section).
Table 1.
Demographic information of participants in this study
| Discovery set | Replication set | |
|---|---|---|
| N | 26,789 | 3039 |
| Age (years; mean ± sd) | 55.22 (7.43) | 52.92(7.34) |
| Sex (female; percent) | 14,041 (52.4) | 1621 (53.3) |
| BMI | 26.57 (4.17) | 26.34 (4.14) |
| Educational qualification (%) | ||
| A levels | 1520 (5.7) | 172 (5.7) |
| College | 12,061 (45.2) | 1404 (46.3) |
| CSE | 726 (2.7) | 88 (2.9) |
| None of above | 1830 (6.9) | 138 (4.6) |
| NVQ | 4142 (15.5) | 529 (17.5) |
| O levels | 2966 (11.1) | 332 (11.0) |
| Other | 3451 (12.9) | 368 (12.1) |
| Smoking status (%) | ||
| Never | 16,335 (61.1) | 1938 (63.9) |
| Previous | 8863 (33.1) | 932 (30.7) |
| Current | 1543 (5.8) | 164 (5.4) |
| Drinking status (%) | ||
| Never | 519 (1.9) | 58 (1.9) |
| Previous | 552 (2.1) | 46 (1.5) |
| Current | 25,712 (96.0) | 2935 (96.6) |
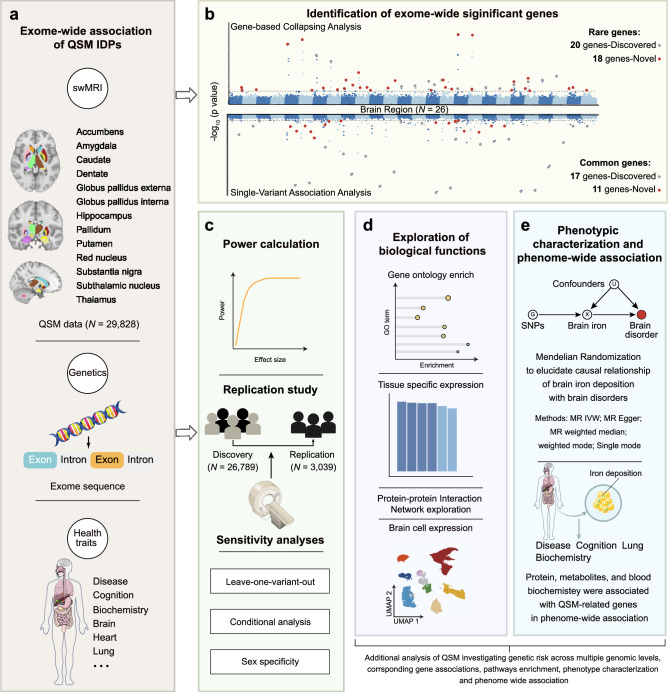
A comprehensive visualization of our analysis pipeline is provided in Fig. 1. The study was initiated with the discovery of EWAS (N = 26,789) to identify genetic variants correlated with brain iron as measured by QSM techniques. (Fig. 1a and b). Validation and replication studies (N = 3039) were conducted to verify the robustness of our findings (Fig. 1c). Further, we examined the functionality and organization of the identified genes (Fig. 1d) and performed Mendelian randomization (MR) analysis to investigate the potential causal relationship between brain iron and multiple brain disorders (Fig. 1e). Lastly, a PheWAS was conducted to explore a broad set of brain iron-phenotype associations (Fig. 1e).
Fig. 1. A summary of the analysis pipeline of the current study.
a The UK Biobank data used in the current study, including quantitative susceptibility mapping (QSM) features derived from susceptibility-weighted MRI, whole-exome sequencing data and phenotypic data. b Exome-wide association study (EWAS) of brain iron with rare and common variants. c Validation study and replication analysis of EWAS. d Post EWAS analysis, including gene set enrichment analysis, protein–protein interaction, tissue and single-cell expression, and e phenotype-wide association study and Mendelian randomization analysis. swMRI susceptibility-weighted MRI, QSM quantitative susceptibility mapping, MR Mendelian randomization, IVW inverse variance weighted. This figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 4.0 unported license.
Rare protein-coding variants associated with brain iron
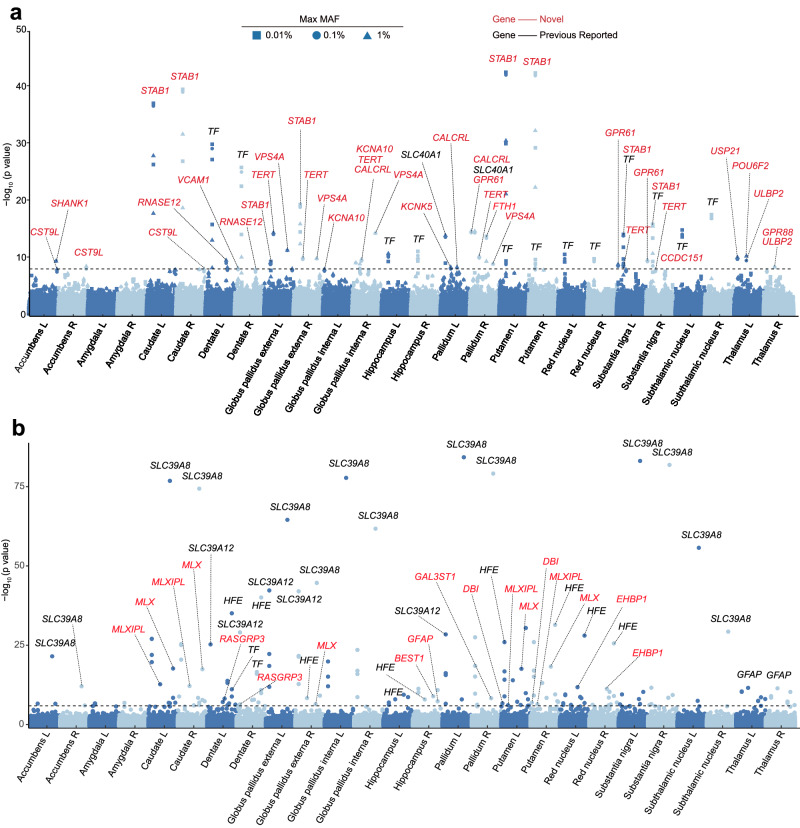
We conducted an exome-wide association study on 26,789 subjects to identify rare variants associated with brain iron levels across 26 brain regions. Out of the resulting associations, a total of 207 reached exome-wide significance (Bonferroni corrected , as detailed in the “Methods” section), covering 24 out of the 26 investigated brain regions (no associations were found for the left and right amygdala) (Figs. 2a and 3, Supplementary Data 1). These identified variants were mapped to 20 different protein-coding genes, with 18 of them not being previously reported in GWAS, while 2 overlapped with findings from a previous study8. The Manhattan plots and Q–Q plots for each brain region can be found in Supplementary Fig. S1. We further performed a replication study using 3039 subjects (see the “Methods” section). From the previously mentioned 20 genes, 4 are significant in both sets (p < 0.05), encompassing 24 gene-based associations (Supplementary Data 2). This was notable considering the number of genes to replicate is just one under the null hypothesis of no associations.
Fig. 2. Exome-wide association analysis of rare and common protein-coding genes with brain iron across 26 regions.
a Significance levels of genes that are mapped from rare variants. b Significance levels of genes that are mapped from common variants. The p-values reported are two-sided and unadjusted. The x-axis represents the brain regions analyzed in the current study (L and R represent left and right brain regions), and the y-axis represents the p-values of each gene. The gray dashed line is the exome-wide significance threshold, based on the Bonferroni-corrected p < 0.05. Genes in red are discovered by the current study, and in black are previously reported. MAF minor allele frequency, R right, L left.
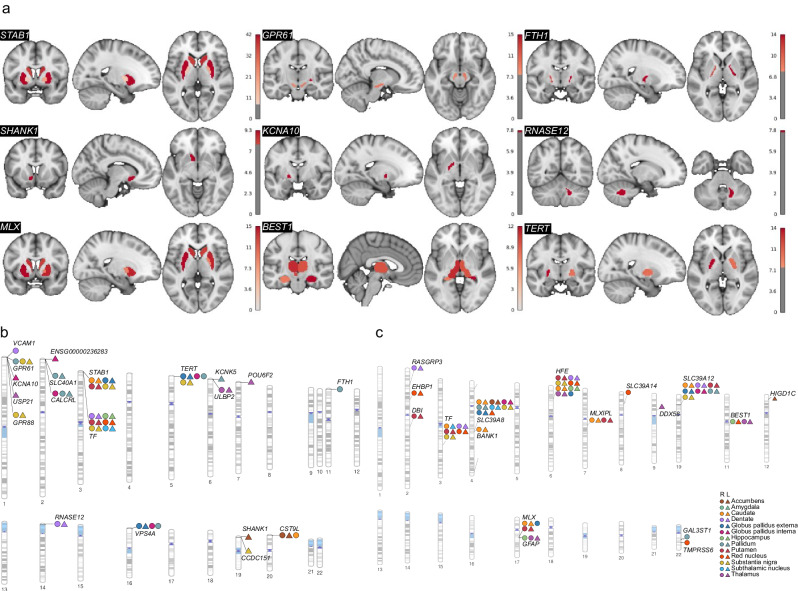
Fig. 3. Brain-wide and chromosome-wide mapping of brain iron-associated genes.
a Brain-wide association map of 16 example genes that are associated with brain iron. b Ideogram of rare genes that influence brain iron. c Ideogram of common genes that influence brain iron. R right, L left.
We performed a power analysis of our WES analysis based on the method of a previous study11. The range of variants’ effect sizes is from 0.03 to 0.08 based on an analysis of our WES results (the regression coefficients and standard error)11. Therefore, given a sample size of N = 29,828, the estimated power is from 39.7% to 100% for the smallest effect to the largest to reach the significance level of uncorrected (Supplementary Data 3).
Furthermore, leave-one-variants out (LOVO) analysis was conducted for each of the significant associations to assess the sensitivity of our findings to analytical approaches (see the “Methods” section). The results showed that over 99.4% of the significant associations involving the above 20 significant genes remained significant in the LOVO analysis. This suggests that most significant associations arise from multiple contributing rare variants. There were a few exceptions. For instance, the association between the thalamus and ULBP2 was influenced by single variants. The LOVO findings are cataloged in Supplementary Data 4. In addition, we conducted a conditional analysis to evaluate whether the identified rare variant signals were independent of nearby common variants (see the “Methods” section). As a result, all variants are significant, indicating that the rare variants signals are independent of the nearby common variants (Supplementary Data 5). Additionally, sex-specific EWAS were executed. We found that 12 of the 20 rare genes are significant for males, and for females, 9 of the 20 rare genes are significant (). (Supplementary Data 6).
Common protein-coding variants associated with brain iron
Expanding upon the above analyses, we performed an EWAS to identify common protein-coding variants associated with brain iron (see the “Methods” section). In summary, 105 associations were discovered that pass the genome-wide significance threshold (Bonferroni-corrected , as detailed in the “Methods” section) (Figs. 2b, 3, Supplementary Data 7). The identified variants map to 17 genes, with 11 of them not being previously reported8. It is worth noting that, as a comparison to ref. 8, our analyses specifically focused on variants on the exome regions and used a broader array of brain regions of interest. Manhattan plots and Q–Q plots for each brain region are shown in Supplementary Fig. S2.
As in the rare variants analysis, we performed a replication study. Among the above 17 identified genes, 13 of them can still be found significant (Supplementary Data 8, uncorrected p < 0.05). In expectation, the number of genes that can be replicated is one.
Brain iron-associated genes are significant in existing GWAS of brain-related traits and diseases
We performed a literature search to explore how the 36 brain iron-associated genes in our analysis overlap with existing GWAS findings. We found that 3 genes are significant in AD GWAS, 4 genes are associated with cognitive-related traits, 2 genes are associated with depression-related traits, 1 gene is associated with PD, and 7 genes are reported in bipolar disorder and schizophrenia GWAS. The full list of our literature search results is shown in Supplementary Data 9.
Functional enrichment and biological validation of brain iron-associated genes
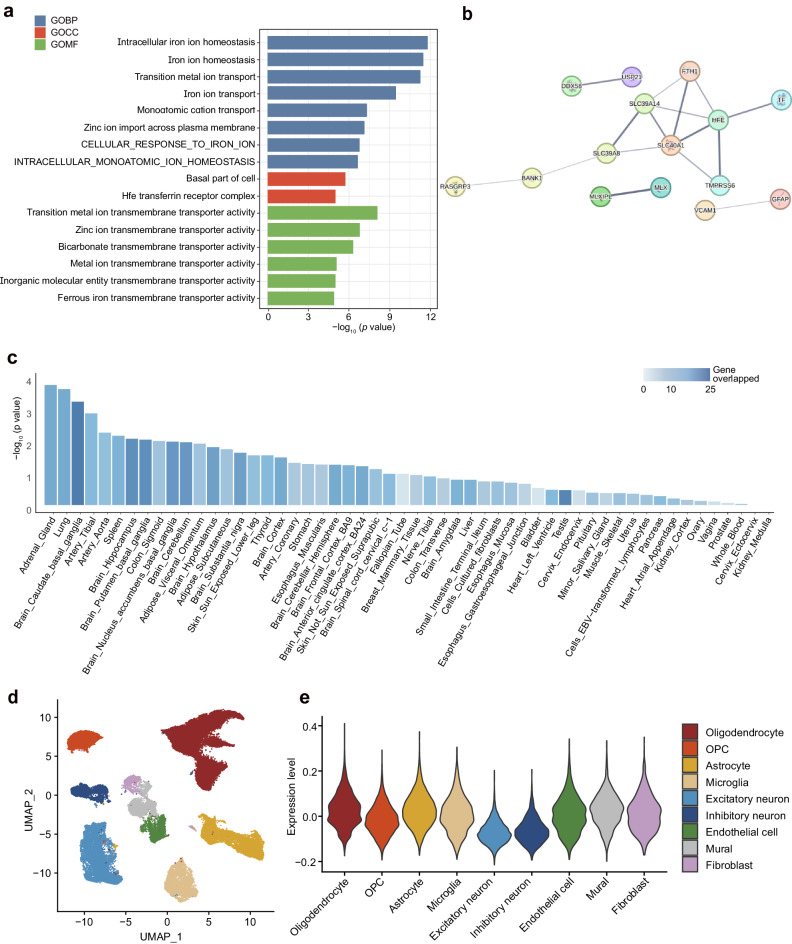
To dive deeper into and verify the biological attributes of the identified genes, we conducted a functional enrichment analysis. Results demonstrated that brain iron-related genes are robustly enriched in iron-related functions. Notably, the pathway of intracellular iron ion homeostasis showed the highest statistical significance () followed by iron ion homeostasis () and transition metal ion transport () (Fig. 4a, Supplementary Data 10). Furthermore, the protein–protein map of these genes forms a dense network associated with iron, including key genes in the SLC families and FTH1 (visible as brown clusters in Fig.4b, Supplementary Data 11). We then tested whether the identified genes were differentially expressed across various tissues, using the GTEx database (12). Notably, our genes exhibited significant differential expression in brain tissues compared to others (Fig. 4c). Notably, these included regions in our analysis, such as the substantia nigra, putamen, and cerebellum () (Fig. 4c, Supplementary Data 12). Leveraging single-cell RNA sequencing data of the human brain, we found that brain iron-associated genes showed higher expression levels in both excitatory and inhibitory neurons (Fig. 4d). Removing the top associated genes still kept most of the enriched pathways and differential expressed genes significantly (Supplementary Fig. S3, Supplementary Data 13 and 14). These analyses verify the biological relevance of our findings across both rare and common protein-coding variants.
Fig. 4. Biological functions of genes associated with brain iron.
a Gene set enrichment analysis of significant genes identified in the exome-wide association analysis, using Gene Ontology and KEGG Ontology database. The p-values reported are two-sided and unadjusted. b Protein–protein interaction network of significant brain iron-related genes. c Tissue-wide differential expression analysis of significant brain iron-related genes using GTEx dataset. The p-values reported are two-sided and unadjusted. d Uniform manifold approximation and projection (UMAP) visualization of human brain single-cell sequencing data. e Expression levels of significant brain iron-related genes in human brain single-cell sequencing data. The colors indicate the tissue types. GOBP gene ontology biological process, GOCC gene ontology cellular component, GOMF gene ontology molecular function, OPC oligodendrocyte progenitor cell.
Mendelian randomization analysis of regional brain iron and brain disorders
Brain iron plays a crucial role in various brain disorders. To investigate whether regional brain iron has causal relationships with brain disorders, we conducted a two-sample Mendelian randomization (MR) analysis. Four brain disorders were selected for this study: depression, bipolar disorder, PD, and AD. Our primary focus was to understand the influence of brain iron on these disorders. Therefore, regional brain iron features were used as exposures, while the brain disorders were used as outcomes in the MR analysis (see the “Methods” section).
Our investigation revealed 11 significant causal relationships for the four candidate brain disorders (Table 2, Supplementary Data 15 and 16): (1) from the subthalamic nucleus, accumbens and thalamus to bipolar disorder (top , FDR = ); (2) from the caudate, substantia nigra, dentate and putamen to PD (top , FDR = 0.0028); (3) from the hippocampus and subthalamic nucleus to depression (top , FDR = 0.046). Additionally, we also conducted a reverse MR analysis (from disease to regional brain iron), revealing that the above significant associations are not significant in this analysis (Supplementary Data 17), indicating that the direction of causality was not biased by reverse causation. It is worth noting that while previous studies have documented statistical associations12–14, our results uncovered potential causal linkages from the brain to diseases.
Table 2.
Causal relationships identified by Mendelian randomization analysis
| Brain iron (exposure) | Diseases (outcome) | Beta | p value | FDR |
|---|---|---|---|---|
| Subthalamic nucleus (right) | Bipolar | 0.011 | 3.59E−07 | 2.15E−05 |
| Accumbens (left) | Bipolar | 0.023 | 3.11E−05 | 9.33E−04 |
| Substantia nigra (right) | PD | 0.010 | 1.45E−04 | 2.90E−03 |
| Substantia nigra (left) | PD | 0.011 | 1.33E−03 | 1.92E−02 |
| Caudate (right) | PD | 0.013 | 1.60E−03 | 1.92E−02 |
| Dentate (right) | PD | 0.006 | 4.25E−03 | 4.25E−02 |
| Putamen (left) | PD | 0.010 | 5.11E−03 | 4.34E−02 |
| Thalamus (left) | Bipolar | 0.024 | 5.79E−03 | 4.34E−02 |
| Putamen (right) | PD | 0.010 | 6.71E−03 | 4.47E−02 |
| Subthalamic nucleus (right) | Depression | 0.003 | 7.67E−03 | 4.60E−02 |
| Hippocampus (right) | Depression | 0.017 | 8.49E−03 | 4.63E−02 |
The iron in brain regions are the exposure variables and the brain disorders are the outcome variables.
Several sensitivity analyses were also conducted to assess the robustness of our results. MR results from alternative approaches are shown in Supplementary Data 16 and 18. The MR-Egger intercept was close to zero, and the pleiotropy test was not significant (Supplementary Data 15 and 17), suggesting that there was no directional pleiotropy in our analysis. In addition, we performed multivariate MR to assess the independence of causal effects (see the “Methods” section). The results revealed that the causal association between iron levels in 26 brain regions and brain disorders was not significant (Supplementary Data 19). This observation suggests that the causal relationship between brain iron levels and brain disorders may not be entirely independent across different brain regions.
PheWAS of brain iron-associated genes
Brain iron plays a pivotal role in numerous fundamental biological processes. Conducting a PheWAS enables us to attain a comprehensive understanding of the diverse impacts of brain iron across the phenotypic landscape and enhances the validity of the findings of the current study15–17.
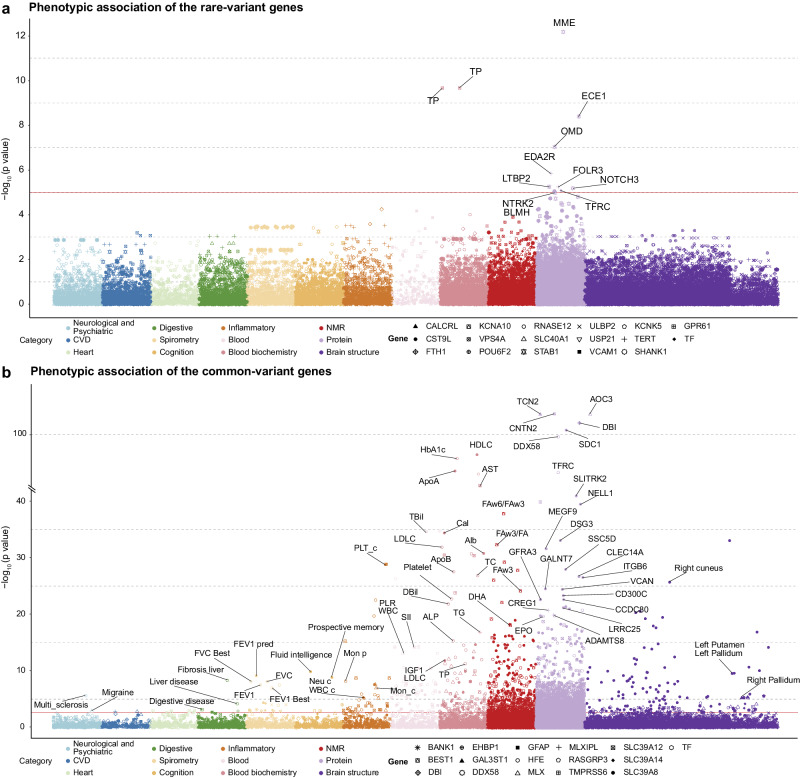
The phenotypes considered in this analysis encompass cognition metrics, neurological and psychiatric conditions, blood chemistry measures, neuroimaging phenotypes, and plasma protein levels. Among the assessed phenotypes, the most robust associations were observed between brain iron-related genes and plasma proteins. STAB1 is associated with multiple plasma protein, including, e.g. MME (), ECE1 (), OMD (), LTBP2 (). TERT is associated with proteins EDA2R () and FOLR3 (). SHANK1 is associated with protein NTRK2 () (Fig. 5a). Genes mapped to common variants have more significant levels of associations, as shown in Fig. 5b.
Fig. 5. Phenome-wide association analysis of brain iron-associated genes.
a Phenome-wide associations of genes mapped from rare variants. b Phenome-wide associations of genes mapped from common variants. Scatterplot showing associations between brain iron-related genes and a wide range of phenotypes, including 12 categories, listed at the bottom left of each figure. The y-axis indicates the of the p-value for each association, and the x-axis represents different phenotype categories. The p-values shown are two-sided and unadjusted for multiple testing. Linear regression models and SKAT-O tests were used for gene-based analysis, and the model adjusted for age, gender, and top 10 ancestral principal components. Red line in each figure is the FDR 0.05 correction threshold. CVD cardiovascular disease, FEV1 forced expiratory volume in 1 s, FEV1_Best best measure of FEV1, FEV1_predperc predicted percentage of FEV1TP, total protein, HDLC high-density lipoprotein cholesterol, LDLC low-density lipoprotein cholesterol, WBC white blood cell count, Neu_c neutrophil count, PLT platelet count, HBA1C glycated hemoglobin, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammation index.
Our exploration of cognitive metrics and disease phenotypes revealed five significant gene-phenotype associations, all mapped to common variants (FDR-corrected p < 0.05): SLC39A8 is associated with fluid intelligence () and prospective memory (); HFE is associated with multiple sclerosis (), fibrosis liver () and broader liver disease (); EHBP1 is associated with the fluid intelligence (); MLX is associated with migraine () (Fig. 5b). These associations were especially noteworthy as these genes ranked among the most significantly associated in our primary association analyses (Fig. 2b).
In other phenotypic variables that we analyzed, for genes mapped to rare variants, RNASE12 is associated with the insulin-like growth factor 1 (IGF-1, ). Common variants show stronger phenotype-wide associations (Fig. 5b). For instance, the gene BEST1 is associated with the ratio of omega-6 to omega-3 fatty acids (), as determined by nuclear magnetic resonance (NMR) spectroscopy.
Discussion
In our large-scale EWAS of brain iron, we uncovered the genetic underpinnings of brain iron accumulation using high-quality genetic data and QSM from swMRI scans of 29,828 UK Biobank participants. Our discovery of EWAS revealed 36 genes (N = 26,789), mapped from either rare or common protein-coding variants, associated with brain iron accumulation. Remarkably, 29 of these genes were not previously reported. 16 of them can be replicated in an independent dataset with 3039 individuals. Our functional enrichment analysis revealed that the identified genes are enriched in biological pathways involving ion transport and homeostasis. MR analysis identified several interesting causal relationships between regional brain iron accumulation to brain disorders, such as from the substantia nigra to PD, and from the accumbens nucleus to AD, and from the hippocampus to depression. Furthermore, our phenotype-wide association study highlighted genes like SLC39A8 and EHBP1’s associations with fluid intelligence and HFEs linked to multiple sclerosis and liver-related diseases.
Our analyses revealed multiple rare genes that have been previously proposed as important contributors to the development of brain disorders or related to iron transport and ferritin (Fig. 3a, b). For example, the gene STAB1 has the overall highest significant level in our EWAS and displayed replicated associations with multiple brain regions, including those of the putamen, caudate, globus pallidus externa and substantia nigra. It has previously been reported as a candidate gene for bipolar disorder18 and cerebrovascular diseases19. FTH1 is associated with the pallidum in our EWAS. This gene was closely linked to various brain disorders, as it is responsible for encoding the heavy chain of ferritin20. Our rare variants analysis also identified the transferrin gene (TF) that is associated with the hippocampus. This gene was known to participate in iron transport. It exhibited an interesting convergence of rare and common variant evidence. KCNA10 is associated with globus pallidus interna, whose changes in expression level were observed in PD patients21.
For common genes, owing to the involvement of a larger number of candidate brain regions than the previous study8, we identified several gene–brain associations(Fig. 3a, c). For example, HFE was associated with the dentate nucleus and red nucleus, which has been previously reported to lead to an increased risk of developing movement disorders22. The gene MLX is associated with putamen. This gene controls the transport and storage of ferrous iron23.
Importantly, our EWAS identified eight genes linked to the brain iron levels of substantia nigra (SN): STAB1, TF, GPR61, TERT, SLC39A8, SLC39A12 and HFE. These genes are linked to PD’s underlying mechanisms. For instance, previous research has suggested that dysregulated STAB1 expression in microglia might play a role in the pathogenesis of PD24. The gene SLC39A8, encoding a metal ion transporter, has been linked to various conditions, including PD25. Interestingly, our MR analyses further revealed significant causal relationships between brain iron accumulation in the SN to PD. PD is marked by motor impairments, stemming largely from the loss of dopamine-producing neurons in the SN26. Dopamine, a pivotal neurotransmitter, governs our motor functions and coordination. A deficit in dopamine results in multiple movement challenges in PD patients, such as tremors, stiffness, bradykinesia, and balance issues. Notably, increased iron accumulation within the substantia nigra has been observed in PD patients, complementing our MR results and indicating a potential causal link between the SN and PD. Diving deeper into these biological pathways could enrich our understanding of PD, potentially leading to more targeted and effective therapeutic interventions27.
We identified four genes associated with the hippocampal iron level: BEST1, HFE, TF, and GFAP. These genes may provide insights into the connection between the hippocampus and depression. For example, Astrocytes, characterized by their expression of GFAP, play an important role in the central nervous system. They are abundant in the hippocampus, a central component of the limbic system that has long been theorized to play a role in depression’s neuropathology28. Post-mortem studies have highlighted the involvement of cerebral astrocytes immunoreactive to GFAP in the pathogenesis of depression29,30. In addition, the HFE gene is primarily known for its role in hereditary hemochromatosis, a genetic disorder that causes the body to absorb too much iron. Mutations in the HFE gene can lead to excessive iron accumulation. Notably, the brain’s uptake of blood iron is important for the optimal synthesis of neurotransmitters such as serotonin, dopamine, and noradrenaline. These neurotransmitters, involved in regulating emotional behaviors, rely on neuron aromatic hydroxylase, with iron acting as an important cofactor. Notably, noradrenaline affects neuroplasticity through the brain-derived neurotrophic factor, which is important for the functioning of prefrontal and hippocampal neurons implicated in depression31. Moreover, research has also established a correlation between severe depression symptoms and high body iron levels32,33 as well as increased brain iron levels14. Our MR analysis revealed a significant causal linkage between the hippocampus and depression. In studies using mouse models, cerebral iron deficiency, leading to the suppression of the hippocampal glucocorticoid receptor signaling pathway, has been implicated in inducing depression6. A consistent observation among depressed individuals is reduced hippocampal volume, correlating with the length and recurrence of depressive episodes34,35. Furthermore, the hippocampus closely interacts with other brain regions responsible for emotional and mood regulation, such as the amygdala and prefrontal cortex. Any malfunction within these networks might hamper emotional processing, thus amplifying the mood-related symptoms synonymous with depression. Iron potentially holds essential significance in the survival of hippocampus neurons, thereby influencing the progression of depression.
Our findings also reveal several insights into the relationships between brain iron accumulation and AD. We identified five genes associated with the brain iron levels of the accumbens nucleus: SHANK1, CST9L, HIGD1C and SLC39A8. Notably, the cognitive deficits typical of AD often coincide with synaptic loss attributed to disruptions in the postsynaptic density. Such disruptions are evidenced by a marked reduction in SHANK1 protein levels36. Given the accumbens nucleus’s role in dopamine regulation37, the research highlighted the potential consequence of perturbed dopaminergic signaling in the context of AD38. Furthermore, excess iron levels in neural tissues can induce oxidative stress, known to adversely affect neural integrity and functionality39. Such oxidative stress has been linked to disturbing neurotransmission, particularly of dopamine, which in turn influences the function of the accumbens nucleus40. In PheWAS, we also find that SLC39A8 is associated with the cognition test scores (i.e., fluid intelligence and prospective memory), which aligns with the known characteristics of AD as a progressive neurodegenerative disorder characterized by memory loss and cognitive deficits.
A major strength of our research lies in its pioneering use of the most extensive WES analyses for brain iron accumulation to date. This approach has led us to identify multiple genes not previously reported, further enriching our understanding of the genetic architecture behind brain iron accumulation. The current study also has some potential limitations. Our analysis predominantly encompasses participants of European genetic ancestries and is constrained by the available sample size for brain imaging data. Incorporating cortical brain regions in our study might have enhanced our findings, offering a more comprehensive understanding of the genetic structure and associations concerning regional brain iron levels. In addition, a large external validation dataset would be beneficial to verify the solidity of our findings.
In conclusion, our investigation identified 36 genes associated with brain iron accumulation across multiple brain regions. Many of these genes are enriched in pathways related to iron transport and homeostasis and are linked to iron-related brain disorders. Furthermore, our study also revealed several causal pathways from regional brain iron accumulation to disorders such as PD, AD and depression. These findings provided insights into the genetic architecture of brain iron accumulation and uncovered the important relationships of brain iron with several brain disorders and behavioral traits. We anticipate that our findings will serve as a groundwork for future research, aiding in the elucidation of how these genes impact brain iron levels and contribute to the onset and progression of brain disorders.
Methods
Study population
The UK Biobank (UKB) (https://www.ukbiobank.ac.uk/) is a population-based prospective cohort of ~500,000 participants aged from 40 to 69 years old at enrollment between 2006 and 201041, among which whole-exome sequencing data are available for 454,787 participants. UKB received ethical approval from the National Health Service National Research Ethics Service (reference: 11/NW/0382) and all participants provided written informed consent. This study was conducted under application number 19542.
Brain imaging-derived phenotypes
We used an automated quantitative susceptibility mapping (QSM) pipeline8, based on the susceptibility-weighted MRI (swMRI) data, to measure the brain iron. We used swMRI data from 37,213 subjects in the UK Biobank (release 2022). The detailed MRI data acquisition, quality control, and QSM processing pipelines have been reported in the original paper8. The individual space voxel-wise QSM maps are downloaded and warped to the MNI152 standard space based on the warp field maps. We extracted the median QSM values from 26 subcortical and cerebellum structures as shown in Fig. 1. Among them, 16 of the 26 subcortical structures were defined based on the original paper (the accumbens, amygdala, caudate, hippocampus, pallidum, putamen, substantia nigra and thalamus, both left and right)8. The corresponding field IDs for these imaging-derived phenotypes are listed in Supplementary Data 20. We further selected 10 additional regions of interest (the red nucleus, subthalamic nucleus, globus pallidus externa, globus pallidus interna and dentate, both left and right), based on the segmentation masks of the multi-contrast PD25 atlas42 and the deep cerebellar nuclei probabilistic atlas43,44.
Exome sequencing and quality control
Whole-exome sequencing was conducted on 454,787 participants in the Regeneron Genetics Center (RGC) and protocols were described in detail elsewhere10. The OQFE WES pVCF files in GRCh38 human reference genome build45 were utilized in this study, and we performed additional quality control similar to the previous study46. First, multi-allelic sites were split into bi-allelic sites and all calls that have a low genotype quality and extremely low/high genotype depth were set to no-call. Variants with call rate <90%, Hardy–Weinberg P-value < 10−15, and in Ensembl low-complexity regions were excluded. Samples withdrawn from the study, duplicates, with discordance between self-reported and genetically inferred sex, samples whose Ti/Tv, Het/Hom, SNV/indel, and number of singletons exceed 8 standard deviations from the mean were removed. We used King software to calculate the kinship coefficient using the high-quality variants (MAF > 0.1%, missingness <1%, HWE P > 10−6 and two rounds of pruning using --indep-pairwise 200 100 0.1 and --indep-pairwise 200 100 0.05). Unrelated samples were defined using the kinship coefficient threshold at 0.0884, indicating the 2nd relatedness. To maximize the sample size, participants related to multiple other individuals were first iteratively removed until none remained. Then, one of the remaining kinship pairs was removed at random. In this study, samples were mainly restricted to White British (filed 22006) and ancestry-specific principal components were calculated, which were used in the following analysis.
Variant annotation
SnpEff47 was used to annotate rare variants (MAF < 1%), and for those annotated with multiple consequences, the most severe consequence was kept for each gene transcript. Loss of function (LOF) was defined for variants annotated as stop gained, start lost, splice donor, splice acceptor, stop lost, or frameshift. Likely deleterious missense was determined if variants were consistently predicted as deleteriousness in SIFT48; PolyPhen2 HDIV and PolyPhen2 HVAR49; LRT50; and MutationTaster51. As for common variants (MAF ≥ 1%), ANNOVAR52 was utilized to annotate variants using refGene as a reference panel, and those annotated as exonic, UTR3, or UTR5 were kept in the following single-variant EWAS analysis.
Data partition
In this study, we partitioned the entire dataset into two subsets: a discovery set and a replication set. This partitioning was based on the availability of longitudinal swMRI scans for individual subjects. Two independent association tests were conducted as follows: Discovery set: This subset comprised baseline imaging data from 26,789 subjects who did not have longitudinal swMRI scans available. Statistical significance in the discovery set was determined using the genome-wide significance threshold. Replication set: This subset consisted of repeated scan brain imaging data from 3039 subjects. Statistical significance in the replication set was set at an uncorrected p-value threshold of <0.05, following the approach utilized in a previous study53.
Exome-wide association analysis
EWAS was performed in the discovery and replication sets using unrelated British with both whole exome sequence (WES) and swMRI brain imaging data available using a generalized mixed model implemented in SAIGE-GENE+ 54 A total of 18,800 variants were analyzed. Rare pLOF and likely deleterious missense variants with MAF < 0.01 were collapsed in each gene, and the EWAS was calculated using the Burden test, SKAT, and SKAT-O, adjusting for age, sex, and the first ten principal components. We constructed six frequency-function collapsing masks for each gene in the gene-based collapsing test: for the frequency of variants, including MAF < 0.01, <0.001, and <0.0001; for the function of variants, pLOF and pLOF+ likely deleterious missense variants. For common variants, a single-variant association analysis was performed, adjusting for the same covariates as in the gene-based collapsing test. A sparse genetic relationship matrix was constructed using the high-quality variants with the recommended relative coefficient cutoff of 0.05. Bonferroni correction was applied and the significance threshold was set to . This is computed as 0.05/(18,800 × 3 × 2 × 26), where 18,800 is the number of candidate rare genes, 3 is the number of minor allele frequency cutoffs, 2 is the number of variants annotation groups, and 26 is the number of brain regions.
Robustness of EWAS
To assess the robustness of the results and detect the variants driving the association in the collapsing test, we further performed leave-one-variant-out analysis. The variant maximized the p-value after removing the collapsing test was identified as the driving variant. For the significant gene–phenotype associations found in the gene-based EWAS, conditional analyses were further performed to evaluate the influence of the nearby common loci (defined as independent index variants after clumping (--clump-p1 1 × 10−5 --clump-r2 0.01), ±500 kb of the identified gene, MAF > 0.5% in the UKB imputed data). For the significant gene–phenotype associations, gene-based collapsing tests were reperformed to condition on nearby common variants signals. For single variant EWAS, independent significant SNPs were further identified ( and r2 ≤ 0.6 within a 1 Mb window). Independent significant SNPs were then clumped to obtain lead SNPs (r2 ≤ 0.1 within a 1 Mb window). Genomic loci were defined by merging lead SNPs within 250 kb. In addition, we performed subgroup analysis based on sex with the same covariates adjusted except for the subgroup factor.
Common variants association analysis
For common exonic variants (MAF 1%), a single variant association analysis was conducted among the unrelated Caucasian cohort using SAIGE-GENE+ 54, adjusting age, gender, and ten principal components. Lead SNPs were identified as independent significant SNPs which meet significant thresholds and are independent of other significant SNPs with r2 < 0.1 within a 1 Mb window. The significance threshold was set to (0.05/(41,790 × 26), Bonferroni correction for 41,790 exonic coding SNPs in 26 brain regions).
Tissue and pathway enrichment analysis
Tissue enrichment analysis was performed by the GENE2FUNC function in FUMA55 with all mapped genes in EWAS as input. Briefly, Differentially Expressed Gene (DEG) sets were pre-calculated by performing a two-sided t-test for any one type of tissue against all other tissues of 54 tissue types based on data from the GTEx database56. Then hypergeometric tests were used to test brain iron-associated genes against each of the DEG sets. For pathway enrichment analysis, hypergeometric tests were also performed to test if brain iron-associated genes are overrepresented in any of the pre-defined gene sets, covering Gene Ontology, Reactome, GWAScatalog, and Immunologic signatures.
Single-nucleus RNA sequencing data source and analyses
We used single-nucleus RNA sequencing (snRNA-seq) data of human brain vasculature obtained from a recent study conducted by Garcia et al. on the Gene Expression Omnibus database with the accession ID: GSE17373157. The clustering and annotation of the cell types were conducted via the metadata file provided by the authors. We also computed the gene set score of the brain iron-associated genes using results from both single and gene-based EWAS, using AddModuleScore function. The primary analysis and subsequent visualization were conducted using the R package Seurat.
Protein–protein interaction network
Protein–protein interaction based on the significant gene set derived from the single variant and gene-based EWAS results were investigated using the human STRING database58. Interactions with a confidence score of at least medium confidence were extracted and subsequently visualized in Cytoscape59 (version 3.9.0). Proteins were further clustered using the Markov clustering (MCL) algorithm to investigate the functional clusters using the default settings60.
Phenome-wide association study
To explore broader phenome-wide associations and underlining mechanisms for the brain iron-related genes or variants derived from single and gene-based EWAS results, we investigated their associations with additional phenotypes. We mainly focused on brain structures (N = 220: thickness, surface area, volume for 68 cortical regions and volume for 16 subcortical structures), biochemistry (N = 30), inflammatory (N = 11) markers, metabolomics (N = 249), and proteomics (N = 1463). As for rare variants, gene-based linear mixed models in SAIGE-GENE+ were employed. And for common variants, single-variant association analysis was performed using linear regression by PLINK v2. Both models were adjusted for age, sex, and the first ten genetic principal components.
Standard Siemens Skyra 3T running VD13A SP4 with a 32-channel head coil was used to acquire the T1-weighted neuroimaging data with a resolution of 1 × 1 × 1 mm (Field 20252) (detailed acquisition protocol can be found at https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/brain_mri.pdf). The cortical surface areas, volumes, and mean thickness for 68 cortical regions were extracted based on FreeSurfer’s surface templates using aparc atlas61.the volume for 16 subcortical regions was estimated via FreeSurfer’s aseg tool62.
Biochemistry or inflammatory markers were obtained from blood count data (Category 100081) and blood biochemistry data (Category 17518) based on UK Biobank blood samples (detailed protocol can be found at https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100080). Four blood cell count ratios were additionally calculated for downstream analysis, including the neutrophils to lymphocytes ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and the systemic immune-inflammation index (SII).
Nuclear magnetic resonance (NMR) metabolomics data (Category 220) were acquired from randomly selected EDTA plasma samples using a high-throughput NMR-based metabolic biomarker profiling platform. This platform covers 249 metabolic spanning multiple metabolic pathways, including lipoprotein lipids, fatty acids, fatty acid compositions, and various low-molecular-weight metabolites (detailed protocol can be found at https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=220).
Proteomics data (Category 1838) of 1463 proteins in plasma were measured by Olink Explore platform, using Proximity Extension Assay (PEA) (detailed information on sample collection, processing, normalization, and quality control procedures can be found at https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=1839).
Causal relationship between regional brain iron and brain disorders
To investigate the causal relationships between QSM and multiple brain disorders, we first performed a Genome-Wide Association Study (GWAS) using imputed SNP (to the Haplotype Reference Consortium) genotype data obtained from the UK Biobank resource63. A total of 8,445,740 SNPs were included in the GWAS. Samples were mainly restricted to white British and those who were used in computing the principal components. Individuals with a missing genotype rate > 0.05, with mismatch self-reported (Data field 31) and genetic sex (Data field 22001), with abnormal sex chromosome aneuploidy, and have more than 10 putative third-degree relatives been further removed. We also excluded variants with call rate < 0.95, MAF < 0.01, Hardy–Weinberg P-value < 10−6, or imputation quality score < 0.5. GWAS were independently performed for each phenotype using linear regression models implemented in PLINK264. Covariates included age, sex, and the first ten genetic principal components. A total of 26,776 to 28,129 participants with phenotype and covariates available for 26 subcortical and cerebellum structures were included in the final GWAS.
Our primary two-sample MR analyses were conducted using regional brain iron as exposure, and diseases as outcomes. For diseases, we leveraged GWAS summary data of iron-related brain disorders, including Parkinson’s disease (Ncase = 33,674, Ncontrol = 449,056)65, Depression (excluding 23andMe and UK Biobank: Ncase = 45,591, Ncontrol = 97,674)66, Bipolar (Ncase = 20,352, Ncontrol = 31,358)67, and Alzheimer’s disease (Ncase = 21,982, Ncontrol = 41,944)68. Instrumental variables (IV) were selected based on a significant level () and followed by LD clumping (R2 > 0.001). Then IV from the exposure and outcome data were harmonized to the same effect alleles. F-statistics were computed to assess the strength of the instruments. When only a single SNP was available, the Wald ratio was used to estimate the causality of exposure to outcome. When more than one SNP was available, the inverse-variance weighted (IVW) with multiplicative random effects method was employed69. MR-PRESSO test70 was used to detect outliers, and if an outlier was detected, the original p-value was replaced by the outlier-corrected p-value. The q-value FDR approach was used to correct for multiple comparisons across brain regions and diseases71.
To assess the robustness of our analysis, several sensitivity analyses were conducted. This involved using different MR methods, including MR Egger72, Wald ratio73, and Weighted median74. The intercept of MR Egger was used to identify the presence of directional pleiotropy. Considering the similarity of genetic architecture between different brain regions, LASSO feature selection (mv_lasso_feature_selection () function in “TwoSampleMR”) followed by a Multivariable MR (MVMR) was performed to further assess whether the causal effects were independent75. In addition, we also performed a reverse MR (from neurologic and psychiatric disorders to regional brain iron) to infer the direction of causality. The MR analysis was performed using the “TwoSampleMR” version 0.5.6 in R version 4.2.
Power analysis
We simulated 1000 datasets for each combination of the estimated effect sizes (i.e., based on the regression coefficient and its estimated errors) and the cMAC per gene of our analysis, based on the method in ref. 11. Carrier status was randomized across N = 29,828 (sample size for our collapsing tests) participants. A linear regression model was used to test for the association with a threshold of (corresponding to the significance threshold in our collapsing tests).
Statistics and reproducibility
The code used in the paper is made publicly available for reproducibility purposes. Statistical analyses are given as well. There is no randomness for all results presented in this study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We gratefully thank all UK Biobank participants for their time and UK Biobank team members for collating the data. J.-T. Yu was supported by grants from the Science and Technology Innovation 2030 Major Projects (2022ZD0211600), National Natural Science Foundation of China (82071201, 81971032, 92249305), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), Research Start-up Fund of Huashan Hospital (2022QD002), Excellence 2025 Talent Cultivation Program at Fudan University (3030277001), Shanghai Talent Development Funding for The Project (2019074), and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University. W. Cheng was supported by grants from the National Natural Sciences Foundation of China (no. 82071997) and the Shanghai Rising-Star Program (no. 21QA1408700). J.F. Feng was supported by the National Key R&D Program of China (No.2018YFC1312904 and No. 2019YFA0709502), the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), the 111 Project (No. B18015), Shanghai Center for Brain Science and Brain-Inspired Technology and Zhangjiang Lab. Figure 1 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 4.0 unported license.
Author contributions
W.K. Gong, W. Cheng, J.-T. Yu and J.F. Feng designed the study. W.K. Gong, Y. Fu, B.-S. Wu, and J.N. Du conducted the main analyses and drafted the manuscript. W.K. Gong, Y. Fu, B.-S. Wu, J.N. Du, L. Yang, Y.-R. Zhang, S.-D. Chen, J.J. Kang contributed to data collection and analyses. W.K. Gong, J.-T. Yu, W. Cheng, Y. Mao, Q. Dong, L. Tan, and J.F. Feng critically revised the manuscript. All authors reviewed and approved the final version.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The main data, including the individual-level phenotypic and genetic data used in this study, were accessed from the UK Biobank under application number 19542 and were available through UKB. The EWAS summary statistics are available at 10.5281/zenodo.1117006476.
Code availability
All software and R packages used to perform the analyses in this work are freely available online: SnpEff, https://pcingola.github.io/SnpEff/; SAIGE-GENE+, https://github.com/saigegit/SAIGE; R, https://www.r-project.org; PLINK2, https://www.cog-genomics.org/plink/2.0/; FUMA, https://fuma.ctglab.nl; MAGMA, https://ctg.cncr.nl/software/magma/; STRING, https://www.string-db.org/.The scripts76 used to conduct the main analyses are available at https://github.com/weikanggong/BrainIronWES.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Weikang Gong, Yan Fu, Bang-Sheng Wu, Jingnan Du.
Contributor Information
Weikang Gong, Email: weikanggong@fudan.edu.cn.
Wei Cheng, Email: wcheng@fudan.edu.cn.
Jin-Tai Yu, Email: jintai_yu@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-49702-2.
References
- 1.Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat. Rev. Neurosci. 2013;14:551–564. doi: 10.1038/nrn3453. [DOI] [PubMed] [Google Scholar]
- 2.Hare D, Ayton S, Bush A, et al. A delicate balance: Iron metabolism and diseases of the brain[J]. Frontiers in aging neuroscience, 2013, 5: 34. [DOI] [PMC free article] [PubMed]
- 3.Ndayisaba A, Kaindlstorfer C, Wenning GK. Iron in neurodegeneration–cause or consequence? Front. Neurosci. 2019;13:180. doi: 10.3389/fnins.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei P, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, et al. Cerebral iron deficiency may induce depression through downregulation of the hippocampal glucocorticoid-glucocorticoid receptor signaling pathway. J. Affect. Disord. 2023;332:125–135. doi: 10.1016/j.jad.2023.03.085. [DOI] [PubMed] [Google Scholar]
- 7.van der Weijden CW, et al. Quantitative myelin imaging with MRI and PET: an overview of techniques and their validation status. Brain. 2023;146:1243–1266. doi: 10.1093/brain/awac436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, et al. Phenotypic and genetic associations of quantitative magnetic susceptibility in UK Biobank brain imaging. Nat. Neurosci. 2022;25:818–831. doi: 10.1038/s41593-022-01074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langkammer C, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012;62:1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Hout CV, et al. Exome sequencing and characterization of 49,960 individuals in the UK Biobank. Nature. 2020;586:749–756. doi: 10.1038/s41586-020-2853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holstege H, et al. Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer’s disease. Nat. Genet. 2022;54:1786–1794. doi: 10.1038/s41588-022-01208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du G, et al. Dynamics of nigral iron accumulation in Parkinson’s disease: from diagnosis to late stage. Mov. Disord. 2022;37:1654–1662. doi: 10.1002/mds.29062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, et al. Iron deposition in Parkinson’s disease by quantitative susceptibility mapping. BMC Neurosci. 2019;20:1–8. doi: 10.1186/s12868-019-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan X, et al. Quantitative susceptibility mapping of brain iron deposition in patients with recurrent depression. Psychiatry Investig. 2022;19:668. doi: 10.30773/pi.2022.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diogo D, et al. Phenome-wide association studies across large population cohorts support drug target validation. Nat. Commun. 2018;9:4285. doi: 10.1038/s41467-018-06540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu JX, Thomas CE, Brunak S. Network biology concepts in complex disease comorbidities. Nat. Rev. Genet. 2016;17:615–629. doi: 10.1038/nrg.2016.87. [DOI] [PubMed] [Google Scholar]
- 17.Shen X, et al. A phenome-wide association and Mendelian Randomisation study of polygenic risk for depression in UK Biobank. Nat. Commun. 2020;11:2301. doi: 10.1038/s41467-020-16022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witt SH, et al. Investigation of manic and euthymic episodes identifies state-and trait-specific gene expression and STAB1 as a new candidate gene for bipolar disorder. Transl. Psychiatry. 2014;4:e426–e426. doi: 10.1038/tp.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan, Z. -L. & Chen, C. -Y. Analysis of multi-tissue transcriptomes reveals candidate genes and pathways influenced by cerebrovascular diseases. Preprint at bioRxiv10.1101/8068933 (2019).
- 20.Shieh, J. T. et al. Heterozygous nonsense variants in the ferritin heavy chain gene FTH1 cause a novel pediatric neuroferritinopathy. Preprint at medRxiv10.1101/2023.01.30.23285099 (2023). [DOI] [PMC free article] [PubMed]
- 21.Simunovic F, et al. Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson’s disease pathology. Brain. 2009;132:1795–1809. doi: 10.1093/brain/awn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loughnan R, et al. Association of Genetic Variant Linked to hemochromatosis with brain magnetic resonance imaging measures of iron and movement disorders. JAMA Neurol. 2022;79:919–928. doi: 10.1001/jamaneurol.2022.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo W, et al. Super-enhancer-driven MLX mediates redox balance maintenance via SLC7A11 in osteosarcoma. Cell Death Dis. 2023;14:439. doi: 10.1038/s41419-023-05966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corces MR, et al. Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer’s and Parkinson’s diseases. Nat. Genet. 2020;52:1158–1168. doi: 10.1038/s41588-020-00721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nebert DW, Liu Z. SLC39A8 gene encoding a metal ion transporter: discovery and bench to bedside. Hum. Genom. 2019;13:1–21. doi: 10.1186/s40246-019-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirtz D, et al. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 28.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 29.O’Leary LA, et al. Widespread decrease of cerebral vimentin-immunoreactive astrocytes in depressed suicides. Front. Psychiatry. 2021;12:640963. doi: 10.3389/fpsyt.2021.640963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim R, Healey KL, Sepulveda-Orengo MT, Reissner KJ. Astroglial correlates of neuropsychiatric disease: from astrocytopathy to astrogliosis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;87:126–146. doi: 10.1016/j.pnpbp.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berthou C, Iliou JP, Barba D. Iron, neuro‐bioavailability and depression. EJHaem. 2022;3:263–275. doi: 10.1002/jha2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson AC, et al. Higher body iron is associated with greater depression symptoms among young adult men but not women: observational data from the daily life study. Nutrients. 2015;7:6055–6072. doi: 10.3390/nu7085270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hidese S, Saito K, Asano S, Kunugi H. Association between iron‐deficiency anemia and depression: a web‐based Japanese investigation. Psychiatry Clin. Neurosci. 2018;72:513–521. doi: 10.1111/pcn.12656. [DOI] [PubMed] [Google Scholar]
- 34.Frodl T, et al. Hippocampal changes in patients with a first episode of major depression. Am. J. Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 35.Roddy DW, et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol. Psychiatry. 2019;85:487–497. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Grabrucker AM, et al. Amyloid beta protein-induced zinc sequestration leads to synaptic loss via dysregulation of the ProSAP2/Shank3 scaffold. Mol. Neurodegener. 2011;6:1–20. doi: 10.1186/1750-1326-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J. Neurosci. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan X, et al. Dopamine and dopamine receptors in Alzheimer’s disease: a systematic review and network meta-analysis. Front. Aging Neurosci. 2019;11:175. doi: 10.3389/fnagi.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L-B, et al. Iron exposure and the cellular mechanisms linked to neuron degeneration in adult mice. Cells. 2019;8:198. doi: 10.3390/cells8020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira A, Neves P, Gozzelino R. Multilevel impacts of iron in the brain: the cross talk between neurophysiological mechanisms, cognition, and social behavior. Pharmaceuticals. 2019;12:126. doi: 10.3390/ph12030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao Y, et al. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinson’s disease cohort. Data Brief. 2017;12:370–379. doi: 10.1016/j.dib.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 44.Diedrichsen J, et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage. 2011;54:1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Szustakowski JD, et al. Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat. Genet. 2021;53:942–948. doi: 10.1038/s41588-021-00885-0. [DOI] [PubMed] [Google Scholar]
- 46.Jurgens SJ, et al. Analysis of rare genetic variation underlying cardiometabolic diseases and traits among 200,000 individuals in the UK Biobank. Nat. Genet. 2022;54:240–250. doi: 10.1038/s41588-021-01011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaser R, Adusumalli S, Leng SN, Sikic M, Ng PC. SIFT missense predictions for genomes. Nat. Protoc. 2016;11:1–9. doi: 10.1038/nprot.2015.123. [DOI] [PubMed] [Google Scholar]
- 49.Adzhubei, I., Jordan, D. M. & Sunyaev, S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet.Chapter 7, Unit 7.20 (2013). [DOI] [PMC free article] [PubMed]
- 50.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19:1553–1561. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 52.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elliott LT, et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018;562:210–216. doi: 10.1038/s41586-018-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou W, et al. SAIGE-GENE+ improves the efficiency and accuracy of set-based rare variant association tests. Nat. Genet. 2022;54:1466–1469. doi: 10.1038/s41588-022-01178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017;8:1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Battle A, Brown CD, Engelhardt BE, Montgomery SB. Genetic effects on gene expression across human tissues. Nature. 2017;550:204–213. doi: 10.1038/nature24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia FJ, et al. Single-cell dissection of the human brain vasculature. Nature. 2022;603:893–899. doi: 10.1038/s41586-022-04521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szklarczyk D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–d613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30:1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 63.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang CC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nalls MA, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–1102. doi: 10.1016/S1474-4422(19)30320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wray NR, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stahl EA, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 2019;51:793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kunkle BW, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc. Natl Acad. Sci. USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife7, e34408 (2018). [DOI] [PMC free article] [PubMed]
- 74.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meeks KA, et al. Mendelian randomization analyses suggest a causal role for circulating GIP and IL-1RA levels in homeostatic model assessment-derived measures of β-cell function and insulin sensitivity in Africans without type 2 diabetes. Genome Med. 2023;15:108. doi: 10.1186/s13073-023-01263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gong, W. weikanggong/BrainIronWES: code for brain iron WES study. Zenodo10.5281/zenodo.11170064 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The main data, including the individual-level phenotypic and genetic data used in this study, were accessed from the UK Biobank under application number 19542 and were available through UKB. The EWAS summary statistics are available at 10.5281/zenodo.1117006476.
All software and R packages used to perform the analyses in this work are freely available online: SnpEff, https://pcingola.github.io/SnpEff/; SAIGE-GENE+, https://github.com/saigegit/SAIGE; R, https://www.r-project.org; PLINK2, https://www.cog-genomics.org/plink/2.0/; FUMA, https://fuma.ctglab.nl; MAGMA, https://ctg.cncr.nl/software/magma/; STRING, https://www.string-db.org/.The scripts76 used to conduct the main analyses are available at https://github.com/weikanggong/BrainIronWES.