Abstract
Stressors can initiate a cascade of central and peripheral changes that modulate mesocorticolimbic dopaminergic circuits and, ultimately, behavioral response to rewards. Driven by the absence of conclusive evidence on this topic and the Research Domain Criteria framework, random-effects meta-analyses were adopted to quantify the effects of acute stressors on reward responsiveness, valuation, and learning in rodent and human subjects.
In rodents, acute stress reduced reward responsiveness (g = −1.43) and valuation (g = −0.32), while amplifying reward learning (g = 1.17). In humans, acute stress had marginal effects on valuation (g = 0.25), without affecting responsiveness and learning. Moderation analyses suggest that acute stress neither has unitary effects on reward processing in rodents nor in humans and that the duration of the stressor and specificity of reward experience (i.e., food vs drugs) may produce qualitatively and quantitatively different behavioral endpoints.
Subgroup analyses failed to reduce heterogeneity, which, together with the presence of publication bias, pose caution on the conclusions that can be drawn and point to the need of guidelines for the conduction of future studies in the field.
Keywords: Meta-analysis, Acute stress, RDoC, Reward learning, Reward responsiveness, Reward valuation
1. Introduction
Appropriate responses to stress are essential to cope with life threats. Conversely, maladaptive stress-coping strategies are often associated with mental health problems and exposure to aversive and stressful experience has been shown to increase risk of initiation, maintenance and relapse of a variety of psychiatric conditions (Hammen, 2015; McEwen, 2004; McEwen and Akil, 2020; Slavich, 2016). One of the pathways through which stressful events are hypothesized to exert their pathogenic influence is by disrupting reward processing, which is indeed implicated in psychopathological conditions such as for example unipolar and bipolar depression and substance use disorder (e.g., Oltean et al., 2023; Stanton et al., 2019).
While it is widely recognized that sustained and chronic stressors blunt reward processing, for example leading to depressive symptoms in vulnerable individuals, the effects of acute stress on reward processing are less established (e.g., Ironside et al., 2018). Longitudinal evidence, however, exists suggesting that acute stressors are stronger predictors of major depression when compared to chronic stressor (e.g., Burani et al., 2023; Hammen et al., 2009). Unfortunately, despite the clinical relevance of the effects of acute stressors on reward-related behavior, existing literature findings are inconsistent and often contradictory.
Among the first observations of stress-induced modulation of the reward system, acute emotional (but not physical) stressors were found to increase the rewarding properties of cocaine (Ramsey and Van Ree, 1993), while reducing those of amphetamine and sucrose (Zurita et al., 1996), suggesting a possible distinctive effect of a single aversive event depending on the specific reward experience. Since then, the effects of aversive and stressful exposure on reward processing have been thoroughly investigated in several rodent models of acute stress, providing mixed results. More specifically, adopting behavioral tests targeting more fine-grained reward processes in rodents, it has been shown that different reward-related behavioral responses can be decreased (e.g., Bryce and Floresco, 2016; Wanat et al., 2013), increased (Antelman and Szechtman, 1975; Wada et al., 2020; Zacharko et al., 1983) or left unchanged (Shafiei et al., 2012) by exposure to acute stressors. For example, 1-h restraint stress was found to reduce the willingness to exert effort for reward (Bryce and Floresco, 2016), while increasing the attractiveness of food-related cues (i.e., incentive salience attribution) (Fuentes et al., 2018).
From a neurobiological perspective, reward processing is primarily -although not exclusively-mediated by dopamine release in the Nucleus Accumbens (NAc) or ventral striatum (Wise and Bozarth, 1985). Increased dopamine release within the NAc is observable in rodents exposed to natural and conditioned rewards, and experimental manipulations capable of preventing/reducing this response interfere with reward-related behavior, such as feeding or learned responses to stimuli that have been paired with rewards (e.g., Bassareo and Di Chiara, 1999; Saunders and Robinson, 2012; Steidl et al., 2017; Berridge, 2018).
Enhanced dopamine availability within the NAc in response to reward has also been seen in humans using positron emission tomography (Schott et al., 2008; Volkow et al., 2009; Hahn et al., 2021). Of note, exposure to single aversive experiences has been observed to enhance dopamine transmission in the NAc in both rodents (Ventura et al., 2007; Cabib and Puglisi-Allegra, 2012; Tye et al., 2013; Wenzel et al., 2018; de Jong et al., 2022) and humans (Scott et al., 2006; Bloomfield et al., 2019; Saraf et al., 2021), revealing a partially overlapping brain circuitry involved in both reward and stress responding. This overlap has driven the hypothesis of a cross-sensitization between stress- and reward-related responses, especially for drug rewards (Kalivas and Stewart, 1991; Robinson and Berridge 1993; Leyton and Vezina, 2014).
Critically, the heterogeneity of the experimental designs, the specificity of the rodent species and strains investigated, and the different types of stressors implemented as well as the individual differences in response to stressors may explain the divergent behavioral results, making it difficult to inform human studies on the neurobiological underpinnings of these effects. Unfortunately, such heterogeneity in experimental approaches and inconsistency of results is mirrored in human investigations of the effects of acute stressors on reward processing, where a brief exposure to a physical and/or psychological stressor has resulted in blunted (e.g., Carvalheiro et al., 2021a, Carvalheiro et al., 2021b), augmented (e.g., Boyle et al., 2020) or unchanged performance (e.g., Steins-Loeber et al., 2020) on tasks measuring reward-related behavior.
A possible explanation of the above-mentioned mixed findings in both rodents and humans is that the effects of acute stressors on reward processing may vary depending on the specific subprocess under examination. Indeed, all existing conceptualizations agree that reward processing may be parsed into different components (e.g., Der-Avakian et al., 2015; Porcelli and Delgado, 2017; Salamone and Correa, 2012), with the Research Domain Criteria (RDoC; Insel et al., 2010) being the most comprehensive translational framework to understand the biobehavioral processes involved in basic dimensions of functioning, including reward processing. For this reason and considering that parceling reward processing into specific components improves clinical reliability in understanding motivational disturbances (e.g., Der Avakian and Markou, 2012), we hereby adopted the Positive Valence Systems classification of the RDoC. In the present study, we considered reward-related behavior as bared into the following components (PVS Work Group, 2011):
-
1)
reward responsiveness, the processes that govern an organism's hedonic response to the impending reward, the receipt of reward and following repeated receipt of reward;
-
2)
reward valuation, the processes by which the probability and benefits of a prospective outcome are computed by reference to external information and/or prior experience;
-
3)
reward learning, the processes by which organisms acquire information about stimuli, actions, and contexts predicting positive outcomes.
These subcomponents of reward processing possibly engage dissociable circuits and neural mechanisms that are distinctively influenced by the impact of acute stressors (Hollon et al., 2015; Zalachoras et al., 2022). Thus, considering the presence of inconsistent and contradictory findings in both rodent and human investigations on the topic and the need to clarify the cross-sectional mechanisms through which acute stressors affects reward processing, the present study aims to quantify the effects of acute stress exposure on behavioral measures targeting reward responsiveness, reward valuation, and reward learning. To this aim, we performed a series of meta-analyses for each subcomponent of reward processing separately in rodents and humans, using the RDoC as the underlying theoretical framework. The moderating role of relevant sources of heterogeneity was examined, namely subject-related (e.g., age, sex, as well as species and strain for the rodent studies), stressor-related (e.g., duration, timing, and nature of the stressor), and reward-related (e.g., type of reward or of behavioral task) factors.
2. Methods
2.1. Information sources and search strategy
The protocol for this study was pre-registered on PROSPERO (CRD42022309786). For transparency, it is important to note that the pre-registered protocol also intended to evaluate dopamine changes following an acute stressor, thus encompassing a broader scope of dopamine-dependent motivated behavior. However, due to insufficient studies including dopamine assessment, we have narrowed our investigation to focus on reward processing as classified by the Positive Valence Systems within the RDoC framework. MEDLINE/PubMed and Scopus were searched to identify rodent and human experimental studies on the effects of acute stress on reward processing, with no publication date restrictions applied.
For the meta-analysis of rodent studies, the search was conducted on July 4, 2022. Research strategy contained the keywords [(acute OR single) AND stress*] AND (reward* OR motivat*) included in the title and/or abstract. For the meta-analysis of human studies, the search was conducted on February 10, 2022. Research strategy contained the keywords (stress* AND reward*), included in the title and/or abstract. The decision to use a more specific string for the meta-analysis of rodent studies was driven by two main factors: i) the intention to filter out studies utilizing chronic stressor protocols, which are prevalent in this field, and ii) the observation that relying solely on the term “reward” might not capture studies employing related terms such as “reinforcer,” “appetitive,” “incentive,” “hedonic,” and so on. In both cases, the search was limited to English-language manuscript published in international peer-reviewed journals. The reference lists of previous systematic reviews and the citations of the included studies were searched as further information sources.
2.2. Inclusion and exclusion criteria
Inclusion criteria for all the performed meta-analyses were as follows: a) within- or between-subjects controlled studies involving healthy rodents (older than 10 weeks) and humans (older than 18 years); b) presence of an acute stress induction (pharmacological, social, physical, emotional, cognitive); c) presence of a behavioral outcome. A priori reasons for exclusion were a) review article and meta-analysis); b) correlational studies; c) studies whose design were unsuitable for calculating one or more effect size(s); d) task involving risky options that encompassed potential punishments (see Starcke and Brand, 2016 for a meta-analysis on the effects of stress on decisions under risk/uncertainty in humans). Following a thorough examination of the full texts, certain studies were excluded based on additional specific criteria that emerged for both rodents and humans (see Fig. 1, Fig. 2 for the PRISMA flowcharts). Each rodent/participant was included only once in each meta-analysis (Cooper and Patall, 2009).
Fig. 1.
PRISMA flowchart showing study selection for the meta-analyses of rodent studies.
Fig. 2.
PRISMA flowchart showing study selection for the meta-analyses of human studies.
2.3. Selection and coding
For both the meta-analyses of rodent and human studies, four of the authors (LC, COr, SC, VT, and MS, PC, VG, VT, respectively) participated in the selection and data coding of relevant articles. Duplicate identification and removal were performed by using a reference management software (Endnote version 9), separately for rodent and human studies. A total of 1180 (52 included in the analyses) and 7045 (53 included in the analyses) studies were retrieved for the search on rodent and human studies, respectively (Fig. 1, Fig. 2 for PRISMA flowcharts). Additional data not published in the reviewed article but needed to calculate effect sizes or to run moderator analyses were received from the authors for 13 rodent studies and for 12 human studies (in the absence of response, where possible, data were digitally extracted from figures using WebPlotDigitizer (Rohatgi, 2022)).
In order to calculate the percentage of intercoder reliability, a subsample of articles (20 %) was read and coded by at least two different members of the research team. Intercoder agreement was 87.9.%. Disagreements in the selection and coding were resolved through research team discussion.
For the meta-analysis of studies conducted in rodents (Appendix A), the following items were coded: total and subgroup sample sizes, mean age, sex (% of females), species, strain, food restricted (yes/no), testing condition (light/dark), type of stressor (psychogenic vs systemic; Appendix B), stress duration, stressor-task time lag (minutes between stressor onset and the beginning of the task), reward type delivered (natural, pharmacological), Positive Valence System investigated, reward task administered (self-administration vs consumption; Appendix C), outcome, means and standard deviations for the outcome.
Where studies reported multiple stress paradigms, we opted for the most stressful (as indicated by objective or subjective measures) or the most common conditions among other studies. Studies reporting on psychopathological samples were included only if it was possible to obtain, either from the article or by contacting the authors, the relevant data related to the healthy controls.
The following coded variables could not be considered as moderators in the analyses i) age, because most studies failed to report the mean age of the sample(s) and only reported the age range; ii) sex, due to an insufficient number of female subjects.
For the meta-analysis of studies conducted in humans (Appendix D), the following items were coded: Positive Valence Systems investigated, type of stressor (systemic, psychogenic, and social; Appendix E), reward task administered (Appendix F), outcome, means and standard deviations for the outcome, total and subgroup sample sizes, mean age (years), sex (% of females), ethnicity (% of Caucasians), stress duration, stressor-task time lag (minutes between stressor onset and the beginning of the task; recoded in simultaneous versus delayed stressors), and assessment of cortisol and/or alpha-amylase increases to support the efficacy of the stress induction (yes vs no). The latter was included considering that acute stress sets off two biological systems simultaneously: the rapid-response neural pathway, also termed the sympathetic adreno-medullary system (Cannon, 1914), and the slower-acting hypothalamus–pituitary–adrenal axis (HPA axis; Selye, 1956). The first initiates swiftly, evidenced by increased alpha-amylase levels in saliva or blood, beginning promptly upon exposure to an acute stressor and typically returning to baseline around 10 min after its cessation. Conversely, HPA axis activation prompts the release of cortisol/corticosterone, peaking approximately 21–40 min following stress onset.
The following coded variables could not be considered as moderators in the analyses: i) ethnicity, because this variable was not reported in most of the examined studies; ii) type of stressor, due to the prevalence of psychogenic stressors such as the Trier Social Stress Test, threat of electric shock or distressing movie clips with only a few studies employing systemic stressors such as pharmacological induction of a physiological stress response; iii) reward task administered, due to an extremely high heterogeneity (e.g., real time smoking, Pavlovian-Instrumental transfer, reinforcing value of food, etc.).
2.4. Synthesis methods
Relevant behavioral outcomes from each study were categorized as measuring reward responsiveness, reward valuation and reward learning to conduct independent meta-analyses both in rodents and humans.
Hedges’ g effect size was computed for each included study. The g coefficient represented the within-subject difference between responding to an acute stress induction and a control/baseline condition or the difference between the experimental (stressed) group and the control group (in the case of a between-subject design), divided by the pooled standard deviation (Hedges and Olkin, 2014). Based on conventional standards, effect sizes of g equal to 0.20, 0.50, and 0.80 were considered small, medium, and large, respectively.
In the meta-analysis of rodent studies, the positive sign of the effect size indicates improved performance in task measuring reward-related behavior induced by the stress condition compared with a control/baseline condition and/or group. For reward responsiveness, this is indexed by a larger i) consumption of palatable food, sucrose solution, or alcohol, ii) intracranial self-stimulation, or iii) lever pressing for rewards (drug or food) on the first session of instrumental training (i.e., self-administration). For reward valuation, this is indexed by reduced latency to obtain a reward or increased i) effort to obtain the reward, ii) lever pressing after devaluation of reward, iii) Pavlovian-Instrumental transfer effect, iv) willingness to wait for a more valuable reward, or v) time spent in the conditioning environment in Conditioned Place Preference studies, when acute stress was induced after the associative learning. For reward learning, this is indexed by increased i) rate of acquisition of self-administration, or ii) time spent in the conditioning environment in CPP experiments when acute stress was induced before the associative learning.
In the meta-analysis of human studies, a positive sign of the effect size indicates improved performance in tasks measuring reward-related behavior in the stress condition(s) compared with a control/baseline condition and/or group. For reward responsiveness, this is indexed by i) faster reaction times to incentivized (vs neutral) reward cues, or ii) a greater percentage of food/alcohol consumption. For reward valuation, this is indexed by enhanced i) Pavlovian-Instrumental transfer effect, ii) willingness to exert greater efforts for higher monetary rewards, or iii) response accuracy to high-probability reward choices when acute stress was induced after the acquisition of reward learning contingencies. For reward learning, this is indexed by a greater i) reward bias, ii) learning rate, iii) habitual response style, or iv) response accuracy to high-probability reward choices when acute stress was induced before the acquisition of reward learning contingencies.
Random-effects models were used in all the analyses as they account for the amount of variance caused by differences between associations and individuals. 95% confidence intervals (CI) around the point estimate of an effect size were computed and the Q and I2 statistics were used to assess heterogeneity among studies. The problem of publication bias was estimated informally by using a funnel plot of effect size against standard error for asymmetry and formally by using Begg and Mazumdar's rank correlations, and Egger's regression intercept test. Moderator analysis was performed first including the entire set of studies and then without outliers using random-effects categorical or meta-regression models. Studies were considered outliers if they had statistically significant standardized residuals (Ellis, 2010). ProMeta Version 2.0 (Internovi) was used for the analysis. Statistics reported in this meta-analysis conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Page et al., 2021) statement (see Appendix G for the PRISMA checklist).
3. Results
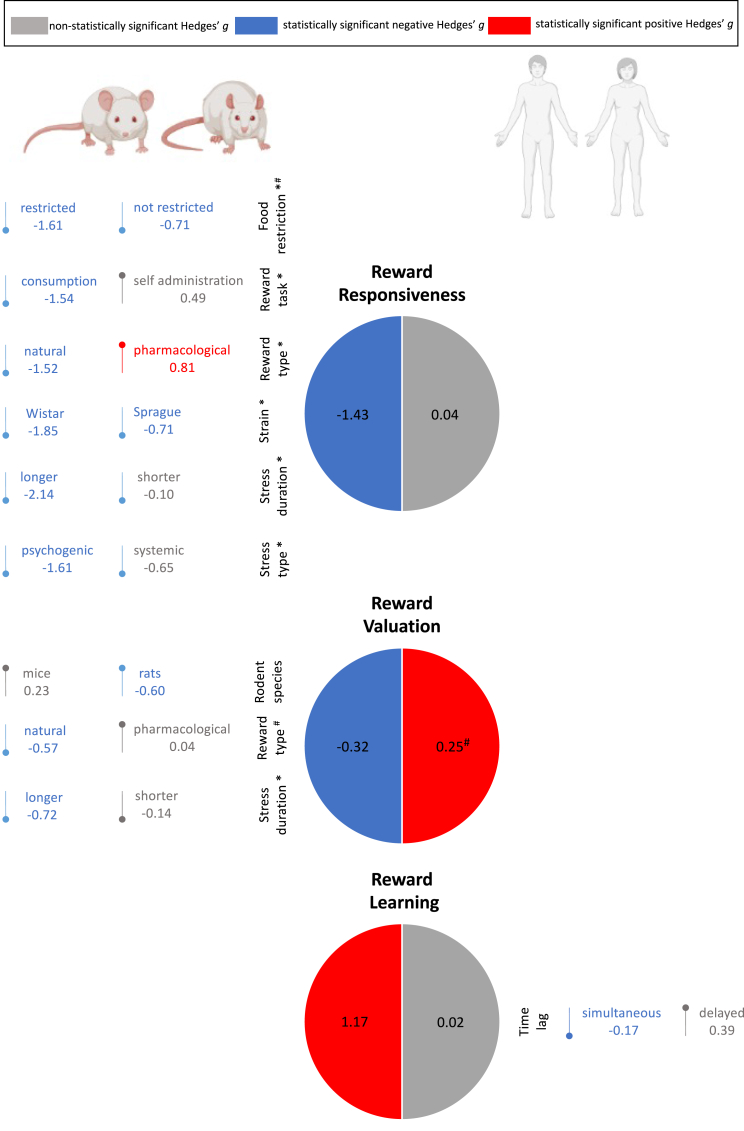
Fig. 3 shows a schematic summary of the results of the present series of meta-analyses.
Fig. 3.
Schematic summary of the effects of acute stress on reward processing.
Note. Pie charts quantifying the effect of acute stress (Hedges' g) on Reward Responsiveness, Reward Valuation and Reward Learning, separately for rodents (left side) and humans (right side). The direction of the oval arrow represents the sign of the effect, indicating negative (down) or positive (up) effects. Colors denote statistical significance (blue = negative and statistically significant effect; red = positive and statistically significant effect; grey = non statistically significant effect). # = marginally significant effect; * after extreme outliers' removal. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.1. Reward responsiveness in rodents and humans
3.1.1. In rodents
In rodent studies, a significant negative effect of acute stress on reward responsiveness emerged (k = 43, n = 789, g = −1.43, 95% CI [−1.93, −0.92], p < 0.0001; Fig. 4). Heterogeneity across studies was extremely high, as shown by the Q (42) = 440.40, p < 0.0001 and I2 = 90.46 statistics.
Fig. 4.
Forest plots for meta-analyses on acute stress effects on reward responsiveness in rodents. Note: Letters after the year refer to studies conducted on different samples within the same publication.
Visual inspection of the funnel plot (Appendix H) indicated the presence of publication bias, a result formally supported by Egger's linear regression test (intercept = −5.45; t = −5.14; p < 0.0001), and Begg's test (Z = −4.85; p < 0.0001).
Exclusion of extreme outliers (Hotta et al., 1999 study a and b; Sekino et al., 2004 study c) did not significantly reduce the effect size (k = 40, n = 747, g = −1.17, 95% CI [−1.64, −0.70], p < 0.0001), or heterogeneity Q (39) = 366.63, p < 0.0001; I2 = 89.36. Publication bias remained statistically significant after exclusion of outliers (Egger's intercept = −5.39; t = −3.78; p = 0.001; Begg's test Z = −4.08; p < 0.0001).
Given the effect size of g > 12 that characterized three extreme outliers (Hotta et al., 1999 study a and b; Sekino et al., 2004 study c), moderation analysis was only performed without these studies.
Food restriction was a marginally significant moderator of the negative association between acute stress and reward responsiveness, Q (1) = 3.79, p = 00.051, with larger effects when rodents were food restricted (g = −1.61, k = 19, n = 358 vs g = −0.71, k = 19, n = 322). Subgroup analysis was not effective in reducing heterogeneity which remained significant, p < 00.0001; I2 > 84.55.
Meta-regression analysis yielded significant effects of stress duration as a continuous moderator (Y = −0.41, slope = −0.01, p = 0.02). To better understand this effect, while keeping a comparable number of studies in each subgroup to reliably detect moderators’ effects, stress duration was recoded as longer vs shorter than 30 min. This analysis confirmed a significant difference between subgroups (Q (1) = 20.78, p < 00.0001), with only studies with stressors lasting longer than 30 min being characterized by a significant and negative effect of acute stress on reward responsiveness (g = −2.14, 95% CI [−2.85, −1.42], k = 15, n = 302) compared to studies with stressors of shorter durations (g = −0.10, 95% CI [−0.60, 0.41], k = 18, n = 303). Both sets of studies were characterized by significant heterogeneity (p < 0.001; I2 > 82.88).
The comparison of studies investigating the effect of acute stress on reward responsiveness using consumption tasks vs. self-administration tasks, yielded a significant difference, Q (1) = 23.06, p < 00.0001. Acute stress significantly reduced reward responsiveness in studies using consumption tasks (g = −1.54, k = 33, n = 590), whereas an increase in reward responsiveness, although non-significant (g = 0.49, k = 7, n = 132), appeared after acute stress in studies using self-administration tasks. Both sets of studies were heterogeneous (p < 00.0001; I2 > 75.85).
Reward type played a role as a significant moderator, Q (1) = 32.15, p < 00.0001, with reward responsiveness decreasing in studies adopting natural rewards (g = −1.52, k = 33, n = 615) and increasing in studies using a pharmacological reward (mostly drugs) (g = 0.81, k = 4, n = 91) following an acute stressor. Only studies employing natural rewards presented substantial heterogeneity, Q (32) = 261.46, p < 00.0001; I2 = 87.76.
Strain significantly moderated the effects of acute stressors on reward responsiveness, Q (1) = 4.90, p = 00.027, with larger effects on Wistar (g = −1.85, k = 16, n = 292) versus Sprague-Dawley (g = −0.71, k = 13, n = 243) rats. This subgroup analysis was not effective in reducing heterogeneity (ps < 0.0001; I2 > 82.6).
Stressor type also emerged as a significant moderator, Q (1) = 4.08, p = 00.043. In particular, only studies adopting psychogenic stressors (g = −1.61, k = 22, n = 426) yielded a significant and negative effect of acute stressors on reward responsiveness compared to those employing systemic stressors (g = −0.65, k = 18, n = 321). Both subgroups were characterized by substantial heterogeneity (p < 00.0001; I2 > 88.11).
Circadian phase (day/night) was not a significant moderator of the effects of acute stress on reward responsiveness. The moderating role of stressor-task time lag could not be examined as most studies (k = 27) had a latency of 0 (i.e., simultaneous occurrence of the stressor and the reward). Similarly, moderation analysis examining the role of species could not be performed due to an insufficient number of studies with mice (k = 3).
3.1.2. In humans
In human studies, acute stress did not have a significant effect on reward responsiveness (k = 14, 525 participants, g = 0.04, 95% CI [−0.16–0.24], p = 0.71; Fig. 5). Heterogeneity across studies was high, as shown by the Q (13) = 33.38, p = 0.0001 and I2 = 61.05 statistics.
Fig. 5.
Forest plots for meta-analyses on acute stress effects on reward responsiveness humans.
Visual inspection of the funnel plot (Appendix I) indicated the absence of publication bias, a result formally supported by Egger's linear regression test (p = 0.99) and Begg's test (p = 0.87).
The exclusion of one outlier (Gaillard et al., 2020) did not influence the results but significantly reduced heterogeneity Q (12) = 17.88, p = 0.21, I2 = 32.88.
Regardless the exclusion of the outlier, no significant moderators emerged among % of women, cortisol/alpha amylase assessment, other physiological variables assessment, duration of stress manipulation, mean age, and stressor-task time lag.
3.2. Reward valuation in rodents and humans
3.2.1. In rodents
In rodents, acute stress decreased reward valuation, g = −0.32, 95% CI [−0.62, −0.02], p = 0.037; k = 37, n = 614 (Fig. 6) in a set of heterogeneous studies, Q (37) = 174.28, p < 0.0001; I2 = 79.34.
Fig. 6.
Forest plots for meta-analyses on acute stress effects on reward valuation in rodents. Note: Letters after the year refer to studies conducted on different samples within the same publication.
Publication bias was not detected by the funnel plot (Appendix J), Egger's test (p = 0.92), or Kendall's tau (p = 0.90).
Exclusion of extreme outliers (Braun and Hauber, 2013 study a; Chu et al., 2021 study a and b; Matthews et al., 2008 study a; Wada et al., 2020) increased the effect size (k = 32, n = 526, g = −0.48, 95% CI [−0.71, −0.24], p < 0.0001), without reducing heterogeneity, Q (32) = 86.11, p < 0.0001; I2 = 64 and influencing publication bias.
Species was a significant moderator, Q (1) = 5.72, p = 00.017, with studies on rats finding a negative effect of acute stress on reward valuation (g = −0.60, k = 24, n = 411), and studies on mice finding non-significant effects (g = 0.23, k = 13, n = 203). Heterogeneity was high in both subgroups (p < 0.001; I2 > 71.58).
Reward type was marginally significant as a moderator, Q (1) = 3.50, p = 00.06, with a negative effect of acute stress on reward valuation in studies adopting natural rewards (g = −0.57, k = 21, n = 362) and a non-significant effect in studies adopting pharmacological rewards (g = 0.04, k = 16, n = 252). Both subsets of studies were characterized by significant heterogeneity (p < 0.001; I2 > 71.01).
No other significant moderators (circadian phase, strain, food restriction, reward task, stress duration, stressor-task time lag, and type of stressor) of the effects of acute stress on reward valuation in rodents emerged.
After outliers’ exclusion, stress duration became a significant moderator of the effects of acute stressors on reward valuation, Q (1) = 5.95, p = 00.015. Only studies using stressors equal to or longer than 30 min yielded significant and negative effects (g = −0.72, 95% CI [−1.02, −0.43], k = 17, n = 269), compared with studies adopting stressors of shorter duration (g = −0.14, 95% CI [−0.50, 0.22], k = 14, n = 253). Both sets of studies were characterized by significant heterogeneity (p < 0.008; I2 > 53.96).
3.2.2. In humans
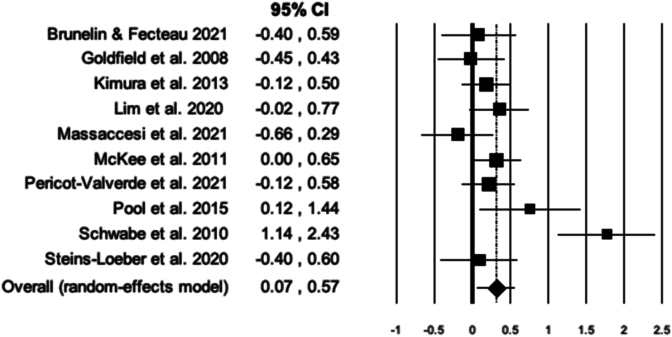
In humans, the overall combined effect size for the total set of 11 studies (406 participants) was only marginally significant, g = 0.25, 95% CI [−0.02, 0.53], p = 0.073 (Fig. 7) in a heterogeneous set of studies, Q (9) = 39.57, p < 0.0001; I2 = 74.73.
Fig. 7.
Forest plots for meta-analyses on acute stress effects on reward valuation in humans.
No evidence of publication bias was detected, as shown by the symmetrical funnel plot (Appendix K), Begg's rank test (p = 0.82), and Egger's regression test (p = 0.83).
In this meta-analysis, a sufficient number of studies was available to examine the role of % of women, duration of stress manipulation, age, reward task (recoded as delay vs others), and stressor-task time lag as moderators, with no significant results.
Exclusion of an extreme outlier (Schwabe et al., 2010) reduced the effect size (g = 0.14, 95% CI [−0.04, 0.33], p = 0.13) and was effective in removing heterogeneity, Q (9) = 16.32; p = 0.06; I2 = 44.86. Publication bias remained absent.
3.3. Reward learning in rodents and humans
3.3.1. In rodents
This meta-analysis showed significant effects of acute stress on reward learning in rodents (14 studies; n = 247; g = 1.17, 95% CI [0.57, 1.77], p < 0.001), which was large in size. Fig. 8 illustrates the forest plot. Significant heterogeneity was shown by the Q (13) = 60.74, p < 0.0001 and I2 = 78.60 statistics. Kendall's tau detected the presence of publication bias (Z = 2.14; p = 00.03), which was confirmed by Begg and Mazumdar's rank correlation test (intercept = 5.26, t = 2.35, p = 00.04) and visually by the funnel plot (Appendix L). No outliers were identified in this meta-analysis.
Fig. 8.
Forest plots for meta-analyses on acute stress effects on reward learning in rodents. Note: Letters after the year refer to studies conducted on different samples within the same publication.
No moderation effects of stressor-task time lag, stress duration, type of stressor or type of reward emerged.
3.3.2. In humans
In humans, analysis of 20 studies (911 individuals) showed no significant effect of acute stress on reward learning (g = 0.02, 95% CI [−0.18, 0.22], p = 0.87), in a heterogeneous set of studies (Q (19) = 99.38, p < 0.0001; I2 = 80.88). Fig. 9 shows the forest plot.
Fig. 9.
Forest plots for meta-analyses on acute stress effects on reward learning in humans.
We did find evidence of publication bias using the funnel plot (Appendix M), Begg's rank test (Z = 3.24; p = 0.001), and Egger's regression test (intercept = 5.02, t = 4.31, p < 0.0001).
The comparison of studies using simultaneous versus delayed stressors yielded a significant difference Q (1) = 5.90, p = 0.01, with only the formers showing significant and negative effects on reward learning (g = −0.17, k = 10, n = 479 vs g = 0.39, k = 9, n = 379). Both sets of studies presented significant heterogeneity (p < 0.01; I2 > 60.44).
Exclusion of an extreme outlier (Schwabe et al., 2012) did not influence effect size, heterogeneity, publication bias, and moderation analysis.
4. Discussion
In a series of meta-analyses of rodent and human studies, we investigated the effects of acute stressors on reward-related behavior, parcellated according to the Positive Valence Systems subconstructs of the RDoC. The rationale for this work comes from the presence of inconsistent and contradictory findings in both rodent and human investigations on the topic and the need to clarify the cross-sectional mechanisms through which stress affects reward processing.
Given the existing knowledge on the detrimental effects of chronic stress on the one hand and traumatic events on the other, it would be essential to understand the extent to which acute stressors (e.g., daily hassles) impact reward-related behavior and which factors may modulate such effects. This would be clinically relevant in light of the role played by impairment in reward processing in the onset, maintenance, and recurrence of several psychopathological conditions, such as depression (e.g., Pizzagalli, 2022) and substance use disorder (e.g., Volkow et al., 2019).
Overall, a general effect of acute stress on reward-related behaviors could be ruled out by the evidence of variable (and sometimes opposite) effects depending on the examined RDoC construct, the duration of the stressful manipulation, and the testing protocols. Indeed, large to extreme variability characterized the data collected by the selected studies. With these limitations in mind, current meta-analytic findings first suggest that acute stress strongly reduces reward responsiveness -the organism's hedonic response to reward cues or anticipation or receipt of reward- in rodents, without any significant effect in humans. Second, acute stress significantly decreases reward valuation -the process by which the probability and benefits of a prospective outcome are computed by reference to external information, social context, and/or prior experience- in rodents and only marginally increases it in humans. Last, acute stress strongly increases reward learning -the process of adapting behavior based on the (past and expected) rewards in the environment- in rodents, with no effects in humans.
The divergence of findings between rodents and humans may be due to the fact that the experimental stressors used to challenge human participants must be non-pathogenic; therefore, they are temporary and relatively short-lasting mild aversive experiences. Conversely, studies in rodents generally aim to identify the psychobiological determinants of human behavioral dysfunctions; for example, prolonged (>30 min) inescapable acute stressors are used in animal studies to reproduce symptoms of post-traumatic stress disorder (Pooley et al., 2018), which include disturbances of reward processing (Vinograd et al., 2022). In line with this view, moderation analysis showed that acute stressful experiences longer than 30 min reduce reward responsiveness and valuation in rodents.
Stressors adopted in animal models are both inescapable and uncontrollable as this condition is necessary for inducing negative psychogenic effects (Maier and Seligman, 2016). The initial response to an acute encounter with these stressors is active coping (escape attempts), supported by an increase in dopamine outflow in the NAc Shell (NAcSh). However, repeated failures to escape or control the stressor lead to a progressive decrease of NAcSh dopamine levels below baseline, accompanied by motivational blunting (helplessness) (Cabib and Puglisi-Allegra, 2012). Inhibition of NAcSh dopamine transmission following prolonged exposure to acute, inescapable/uncontrollable stressors elucidates their negative impact on reward response and valuation in animal models, as DA transmission within this brain area is crucial for reward-motivated behaviors (Faure et al., 2008).
Regarding stress effects on reward learning, the acquisition of Pavlovian conditioned approach behavior and reinforcement learning requires dopamine transmission in the core region of the NAc, rather than in the NAcSh (Flagel et al., 2011; Aitken et al., 2016; Grima et al., 2022), while habit learning (the other subconstruct of reward learning) engages the dorsolateral striatum and involves the inhibition of dopamine transmission in the NAcSh (Everitt et al., 2008). Therefore, the divergent effects of acute stress on behaviors encompassed within the constructs of reward responsiveness and valuation, on one hand, and reward learning, on the other, may depend on the engagement of different neurobiological mechanisms.
Notably, the effect of acute stress on reward learning becomes significant in humans (albeit in a direction opposite to that observed in rodents, i.e., negative) only when there is no delay between the cessation of the stressor and the commencement of the task involving rewards. Numerous studies corroborate the notion that during the fight or flight response, the brain adopts a precautionary stance, prioritizing negative stimuli at the expense of positive ones (e.g., Van den Bergh et al., 2021; van Oort et al., 2017). Consistent with this perspective, research indicates that when acute stress coincides with the task involving rewards, it impairs behavioral responses to monetary gains but not losses and such impairment has been computationally linked to decreased learning rate for positive prediction errors (Berghorst et al., 2013; Carvalheiro et al., 2021a, Carvalheiro et al., 2021b). The stressor-to-task time lag is critical, as the rapid release of catecholamines early on and the delayed actions of glucocorticoids may yield varying effects on brain function (Hermans et al., 2014).
The present findings also highlight the intensity of the stressor as a critical variable in the examined phenomenon, playing a pivotal role in elucidating divergent outcomes between rodents and humans; however, very few studies assessed and reported the physiological correlates of the stress-response (e.g., corticosterone levels in rodents and cortisol or alpha amylase levels in humans), to allow a quantification of the entity of such response. For this reason, despite our best attempt to include this variable as a moderator, the intensity of the stressful experience could be assessed neither from a physiological nor a subjective (in human studies only) point of view.
Our analysis suggested that the type of reward rodents receive moderates stress effects. More than one factor may concur to explain why acute stress increases the responsiveness to drugs of abuse while it decreases that to food in rodent paradigms. First, the neural substrates encoding non-drug and drug rewards are common but not completely overlapping (reviewed in Nall et al., 2021; Amaral et al., 2022). Second, the behavioral tasks used to measure reward intake are highly dissimilar and distinctively influenced by stress-induced psychomotor activation. Responsiveness to drugs is measured by self-administration whereas lever pressing for drugs is an instrumental behavior which is learned after a long training always in the same environment. Across such training, various stimuli (either programmed or unprogrammed) are temporally contingent with the drug reward becoming associated to stimuli capable of instigating the lever pressing by acting as incentive conditioned stimuli, occasion setters or triggers for habitual instrumental response. Moreover, after lever pressing is initiated, the drug's psychoactive effect adds up further, favoring the expression of the instrumental behavior (Ramsey and Van Ree, 1993; Makhijani et al., 2021). By contrast, responsiveness to food is measured by spontaneous eating behavior often in a relatively novel environment (e.g., Calvez et al., 2011; Cifani et al., 2013). In this case, stress-induced psychomotor activation does not favor food consumption, because eating in a potentially unsafe environment is not functional to the survival of collector species such as rodents. This interpretation is supported by the significant role of type of task as a mediator of the effects of acute stress on reward responsiveness, according to which responsiveness was reduced in studies adopting consumption tasks and enhanced in those employing self-administration tasks.
A similar pattern of results emerged for the moderation analyses of rodent studies with reward valuation as outcome, with larger effects for stressors of longer durations and significant and negative effects only for natural (and not pharmacological) rewards. The difference mentioned above between consumption and self-administration tasks may also help explaining the opposite (i.e., positive, although only marginally significant) effect of acute stress on reward valuation found in humans compared to rodents. Indeed, the tasks adopted in human studies that assessed reward valuation are more similar to self-administration tasks, as they mostly imply learning an instrumental behavior (e.g., Goldfield et al., 2008; Schwabe et al., 2010).
In this regard, it is worth pointing out that acute stress in human subjects and animal models triggers the reconfiguration of large-scale neural networks, fostering a shift from goal-directed to ‘habitual’ learning (Schwabe and Wolf, 2012; Schwabe, 2017). The latter is a subconstruct of reward learning characterized by inflexible patterns of instrumental responding insensitive to changes in reward value. The shift from goal-directed to ‘habitual’ responses is determined by the control over an acquired instrumental response passing from mainly limbic to mainly motor cortical-striatal-thalamic-cortical loops due to overtraining, stress hormones, or prolonged experience of addictive drugs (Coutureau and Killcross, 2003; Killcross and Coutureau, 2003; Everitt and Robbins, 2016; Schwabe, 2017). Habitual responses are demonstrated when devaluation procedures, such as satiety after overeating, reduce reward consumption but not operant responding (Dickinson and Balleine, 1994). Thus, also the selective adverse effects of acute stress on reward responsiveness by rodents that consumed an available reward, but not in those that obtained the reward by an instrumental response can be due to the response becoming habitual. Importantly, chronic stress has been shown to foster habit-like responses (Dias-Ferreira et al., 2009) and restricted feeding, often used as a chronic stressful condition when repeated (Cabib et al., 2000; Stamp et al., 2008; Campus et al., 2017), significantly moderated the negative association between acute stress challenge and reward responsiveness in the present meta-analysis of rodent studies.
Several limitations need to be acknowledged. Although random-effects models were used to address heterogeneity, the present work is limited by the marked heterogeneity across studies, which remained significant even after subgroup analysis. It is of course plausible that other moderators, rather than those considered in the present work, are capable of explaining such heterogeneity (pre-existing individual differences as shown, for example, by Zalachoras et al., 2022). The second major limitation is the publication bias found in all the studies conducted in rodents, likely due to the tendency of the authors and scientific journals to publish only animal studies with significant findings. With this regard, the present series of meta-analyses did not include the so-called grey literature. This methodological choice was motivated by a drive for methodological rigor, given that grey literature might not employ peer review; however, the presence of publication bias suggests that this may have inflated the size of the effects (Rothstein and Hopewell, 2009).
The observed differences in outcomes between rodents and humans raise questions about the translational applicability of rodent models in stress-related psychopathology. Nonetheless, the inconsistency in results seems to stem from variations in the duration (and likely the intensity) of the stressor and the experimental paradigm utilized. Future investigations aimed at elucidating this issue through direct comparisons of instrumental and non-instrumental paradigms, utilizing identical stressors of different duration and/or intensity and the same rewarding stimuli, are warranted. Moreover, future studies in humans should try to always incorporate an assessment of the physiological correlates of the stress response, to test whether the direction of the effect changes based on the intensity of the stressor. This is not unplausible if we consider the effect of stress load on other brain functions such as memory, where smaller stress-induced responses facilitate encoding and greater responses impair it (Sandi, 2013). Intriguingly, also in the case of memory chronic stress consistently impairs encoding, whereas acute stress has different effects depending on the stress load and the time of occurrence (reviewed in Lindau et al., 2016). To conclude, the present work points to the need to develop translationally valid and replicable studies that directly address the factors underlying the different and inconsistent effects of acute stress on reward responsiveness, learning and valuation in rodents and humans, as it has been done in the field of memory functions.
Funding
This work was supported by Sapienza University of Rome [grant number RM12117A8A578DB4].
CRediT authorship contribution statement
Martino Schettino: Writing – original draft, Methodology, Data curation, Conceptualization. Valeria Tarmati: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Paola Castellano: Writing – review & editing, Methodology, Investigation. Valeria Gigli: Methodology, Investigation, Data curation. Luca Carnevali: Writing – review & editing, Methodology, Investigation, Data curation. Simona Cabib: Writing – original draft, Supervision, Methodology, Investigation, Data curation. Cristina Ottaviani: Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization. Cristina Orsini: Writing – original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Rongjun Yu
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2024.100647.
Contributor Information
Cristina Ottaviani, Email: cristina.ottaviani@uniroma1.it.
Cristina Orsini, Email: cristina.orsini@uniroma1.it.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- References marked with an asterisk indicate studies included in the meta-analyses.
- Aitken T.J., Greenfield V.Y., Wassum K.M. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J. Neurochem. 2016;136(5):1026–1036. doi: 10.1111/jnc.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral I.M., Scheffauer L., Hofer A., El Rawas R. Protein kinases in natural versus drug reward. Pharmacol. Biochem. Behav. 2022;221 doi: 10.1016/j.pbb.2022.173472. [DOI] [PubMed] [Google Scholar]
- Antelman S.M., Szechtman H. Tail pinch induces eating in sated rats which appears to depend on nigrostriatal dopamine. Science (New York, N.Y.) 1975;189(4204):731–733. doi: 10.1126/science.1154024. [DOI] [PubMed] [Google Scholar]
- Bassareo V., Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 1999;11(12):4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Berghorst L.H., Bogdan R., Frank M.J., Pizzagalli D.A. Acute stress selectively reduces reward sensitivity. Front. Hum. Neurosci. 2013;7:133. doi: 10.3389/fnhum.2013.00133. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. Evolving Concepts of emotion and motivation. Front. Psychol. 2018;9:1647. doi: 10.3389/fpsyg.2018.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield M.A., McCutcheon R.A., Kempton M., Freeman T.P., Howes O. The effects of psychosocial stress on dopaminergic function and the acute stress response. Elife. 2019;8 doi: 10.7554/eLife.46797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle C.C., Stanton A.L., Eisenberger N.I., Seeman T.E., Bower J.E. Effects of stress-induced inflammation on reward processing in healthy young women. Brain Behav. Immun. 2020;83:126–134. doi: 10.1016/j.bbi.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S., Hauber W. Acute stressor effects on goal-directed action in rats. Learn. Mem. 2013;20(12):700–709. doi: 10.1101/lm.032987.113. ∗. [DOI] [PubMed] [Google Scholar]
- Bryce C.A., Floresco S.B. Perturbations in effort-related decision-making driven by acute stress and corticotropin-releasing factor. Neuropsychopharmacology : Off. Publ. Am. College of Neuropsychopharmacol. 2016;41(8):2147–2159. doi: 10.1038/npp.2016.15. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burani K., Brush C.J., Shields G.S., Klein D.N., Nelson B., Slavich G.M., Hajcak G. Cumulative lifetime acute stressor exposure interacts with reward responsiveness to predict longitudinal increases in depression severity in adolescence. Psychol. Med. 2023;53(10):4507–4516. doi: 10.1017/S0033291722001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S., Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012;36(1):79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Cabib S., Orsini C., Le Moal M., Piazza P.V. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science (New York, N.Y.) 2000;289(5478):463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Calvez J., Fromentin G., Nadkarni N., Darcel N., Even P., Tomé D., Ballet N., Chaumontet C. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol. Behav. 2011;104(5):675–683. doi: 10.1016/j.physbeh.2011.07.012. ∗. [DOI] [PubMed] [Google Scholar]
- Campus P., Canterini S., Orsini C., Fiorenza M.T., Puglisi-Allegra S., Cabib S. Stress-induced reduction of dorsal striatal D2 dopamine receptors prevents retention of a newly acquired adaptive coping strategy. Front. Pharmacol. 2017;8:621. doi: 10.3389/fphar.2017.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon W.B. The interrelations of emotions as suggested by recent physiological researches. Am. J. Psychol. 1914;25(2):256–282. doi: 10.2307/1413414. [DOI] [Google Scholar]
- Carvalheiro J., Conceição V.A., Mesquita A., Seara-Cardoso A. Acute stress blunts prediction error signals in the dorsal striatum during reinforcement learning. Neurobiol. Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100412. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheiro J., Conceição V.A., Mesquita A., Seara-Cardoso A. Acute stress impairs reward learning in men. Brain Cognit. 2021;147 doi: 10.1016/j.bandc.2020.105657. ∗. [DOI] [PubMed] [Google Scholar]
- Chu J., Deyama S., Li X., Motono M., Otoda A., Saito A., Esaki H., Nishitani N., Kaneda K. Role of 5-HT1A receptor-mediated serotonergic transmission in the medial prefrontal cortex in acute restraint stress-induced augmentation of rewarding memory of cocaine in mice. Neurosci. Lett. 2021;743 doi: 10.1016/j.neulet.2020.135555. ∗. [DOI] [PubMed] [Google Scholar]
- Cifani C., Di Bonaventura M.V.M., Ciccocioppo R., Massi M. In: Animal Models of Eating Disorders. Avena N.M., editor. Humana Press; 2013. Binge eating in female rats induced by yo-yo dieting and stress; pp. 27–49. ∗. [DOI] [Google Scholar]
- Cooper H., Patall E.A. The relative benefits of meta-analysis conducted with individual participant data versus aggregated data. Psychol. Methods. 2009;14(2):165–176. doi: 10.1037/a0015565. [DOI] [PubMed] [Google Scholar]
- Coutureau E., Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav. Brain Res. 2003;146(1–2):167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- de Jong J.W., Fraser K.M., Lammel S. Mesoaccumbal dopamine heterogeneity: what do dopamine firing and release have to do with it? Annu. Rev. Neurosci. 2022;45:109–129. doi: 10.1146/annurev-neuro-110920-011929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A., Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A., Barnes S.A., Markou A., Pizzagalli D.A. In: Robbins T.W., Sahakian B.J., editors. Vol. 28. Springer Publisher; Cham: 2015. Translational assessment of reward and motivational deficits in psychiatric disorders. (Translational Neuropsychopharmacology. Current Topics in Behavioral Neurosciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E., Sousa J.C., Melo I., Morgado P., Mesquita A.R., Cerqueira J.J., Costa R.M., Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science (New York, N.Y.) 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dickinson A., Balleine B. Motivational control of goal-directed action. Anim. Learn. Behav. 1994;22:1–18. doi: 10.3758/BF03199951. [DOI] [Google Scholar]
- Ellis P.D. Cambridge university press; 2010. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results. [Google Scholar]
- Everitt B.J., Belin D., Economidou D., Pelloux Y., Dalley J.W., Robbins T.W. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Faure A., Reynolds S.M., Richard J.M., Berridge K.C. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J. Neurosci. : Off. J. Soc. Neurosci. 2008;28(28):7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel S.B., Clark J.J., Robinson T.E., Mayo L., Czuj A., Willuhn I., Akers C.A., Clinton S.M., Phillips P.E., Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S., Carrasco J., Hatto A., Navarro J., Armario A., Monsonet M., Ortiz J., Nadal R. Sex-dependent impact of early-life stress and adult immobilization in the attribution of incentive salience in rats. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190044. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard C., Guillod M., Ernst M., Federspiel A., Schoebi D., Recabarren R.E., Ouyang X., Mueller-Pfeiffer C., Horsch A., Homan P., Wiest R., Hasler G., Martin-Soelch C. Striatal reactivity to reward under threat-of-shock and working memory load in adults at increased familial risk for major depression: a preliminary study. NeuroImage. Clinical. 2020;26 doi: 10.1016/j.nicl.2020.102193. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfield G.S., Adamo K.B., Rutherford J., Legg C. Stress and the relative reinforcing value of food in female binge eaters. Physiol. Behav. 2008;93(3):579–587. doi: 10.1016/j.physbeh.2007.10.022. ∗. [DOI] [PubMed] [Google Scholar]
- Grima L.L., Panayi M.C., Härmson O., Syed E.C.J., Manohar S.G., Husain M., Walton M.E. Nucleus accumbens D1-receptors regulate and focus transitions to reward-seeking action. Neuropsychopharmacology : Off. Publ. Am. College of Neuropsychopharmacol. 2022;47(9):1721–1731. doi: 10.1038/s41386-022-01312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A., Reed M.B., Pichler V., Michenthaler P., Rischka L., Godbersen G.M., Wadsak W., Hacker M., Lanzenberger R. Functional dynamics of dopamine synthesis during monetary reward and punishment processing. J. Cerebr. Blood Flow Metabol. : Off. J. Int. Soc. Cerebral Blood Flow and Metabolism. 2021;41(11):2973–2985. doi: 10.1177/0271678X211019827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C.L. Stress and depression: old questions, new approaches. Curr. Opin. Psychol. 2015;4:80–85. doi: 10.1016/j.copsyc.2014.12.024. [DOI] [Google Scholar]
- Hammen C., Kim E.Y., Eberhart N.K., Brennan P.A. Chronic and acute stress and the prediction of major depression in women. Depress. Anxiety. 2009;26(8):718–723. doi: 10.1002/da.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L.V., Olkin I. Academic press; 2014. Statistical Methods for Meta-Analysis. [Google Scholar]
- Hermans E.J., Henckens M.J., Joëls M., Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37(6):304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Hollon N.G., Burgeno L.M., Phillips P.E.M. Stress effects on the neural substrates of motivated behavior. Nat. Neurosci. 2015;18(10):1405–1412. doi: 10.1038/nn.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta M., Shibasaki T., Arai K., Demura H. Corticotropin-releasing factor receptor type 1 mediates emotional stress-induced inhibition of food intake and behavioral changes in rats. Brain Res. 1999;823(1–2):221–225. doi: 10.1016/s0006-8993(99)01177-4. ∗. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ironside M., Kumar P., Kang M.S., Pizzagalli D.A. Brain mechanisms mediating effects of stress on reward sensitivity. Curr. Opin. Behav. Sci. 2018;22:106–113. doi: 10.1016/j.cobeha.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P.W., Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Brain Res. Rev. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Killcross S., Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebr. Cortex. 2003;13(4):400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Leyton M., Vezina P. Dopamine ups and downs in vulnerability to addictions: a neurodevelopmental model. Trends Pharmacol. Sci. 2014;35(6):268–276. doi: 10.1016/j.tips.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Almkvist O., Mohammed A.H. In: Stress: Concepts, Cognition, Emotion, and Behavior. Fink G., editor. Elsevier Academic Press; 2016. Effects of stress on learning and memory; pp. 153–160. [DOI] [Google Scholar]
- Maier S.F., Seligman M.E. Learned helplessness at fifty: insights from neuroscience. Psychol. Rev. 2016;123(4):349–367. doi: 10.1037/rev0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhijani V.H., Franklin J.P., Van Voorhies K., Fortino B., Besheer J. The synthetically produced predator odor 2,5-dihydro-2,4,5-trimethylthiazoline increases alcohol self-administration and alters basolateral amygdala response to alcohol in rats. Psychopharmacology. 2021;238(1):67–82. doi: 10.1007/s00213-020-05659-w. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.B., Morrow A.L., O'Buckley T., Flanigan T.J., Berry R.B., Cook M.N., Mittleman G., Goldowitz D., Tokunaga S., Silvers J.M. Acute mild footshock alters ethanol drinking and plasma corticosterone levels in C57BL/6J male mice, but not DBA/2J or A/J male mice. Alcohol (N. Y.) 2008;42(6):469–476. doi: 10.1016/j.alcohol.2008.05.001. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032(1):1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Akil H. Revisiting the stress concept: implications for affective disorders. J. Neurosci. 2020;40(1):12–21. doi: 10.1523/JNEUROSCI.0733-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall R.W., Heinsbroek J.A., Nentwig T.B., Kalivas P.W., Bobadilla A.C. Circuit selectivity in drug versus natural reward seeking behaviors. J. Neurochem. 2021;157(5):1450–1472. doi: 10.1111/jnc.15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltean L.E., Șoflău R., Miu A.C., Szentágotai-Tătar A. Childhood adversity and impaired reward processing: a meta-analysis. Child Abuse Negl. 2023;142(Pt 1) doi: 10.1016/j.chiabu.2022.105596. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed.) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A. Toward a better understanding of the mechanisms and pathophysiology of anhedonia: are we ready for translation? Am. J. Psychiatr. 2022;179(7):458–469. doi: 10.1176/appi.ajp.20220423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley A.E., Benjamin R.C., Sreedhar S., Eagle A.L., Robison A.J., Mazei-Robison M.S., Breedlove S.M., Jordan C.L. Sex differences in the traumatic stress response: PTSD symptoms in women recapitulated in female rats. Biol. Sex Differ. 2018;9(1):31. doi: 10.1186/s13293-018-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli A.J., Delgado M.R. Stress and decision making: effects on valuation, learning, and risk-taking. Curr. Opin. Behav. Sci. 2017;14:33–39. doi: 10.1016/j.cobeha.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PVS Work Group . 2011. Positive Valence Systems: Workshop Proceedings. [Google Scholar]
- Ramsey N.F., Van Ree J.M. Emotional but not physical stress enhances intravenous cocaine self-administration in drug-naive rats. Brain Res. 1993;608(2):216–222. doi: 10.1016/0006-8993(93)91461-z. ∗. [DOI] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research. Brain Res. Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohatgi A. WebPlotDigitizer (v. 4.6) 2022. https://automeris.io/WebPlotDigitizer
- Rothstein H.R., Hopewell S. In: The Handbook of Research Synthesis. second ed. Cooper H., Hedges L.V., Valentine J., editors. Russell-Sage; New York, NY: 2009. The Grey literature. [Google Scholar]
- Salamone J.D., Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C. Stress and cognition. Wiley Interdiscipl. Rev. Cognitive Sci. 2013;4(3):245–261. doi: 10.1002/wcs.1222. [DOI] [PubMed] [Google Scholar]
- Saraf G., Pinto J.V., Cahn A., White A.G., Shahinfard E., Vafai N., Sossi V., Yatham L.N. Dopamine release during psychological stress in euthymic bipolar I disorder: a Positron Emission Tomography study with [11C]raclopride. J. Affect. Disord. 2021;295:724–732. doi: 10.1016/j.jad.2021.08.022. [DOI] [PubMed] [Google Scholar]
- Saunders B.T., Robinson T.E. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur. J. Neurosci. 2012;36(4):2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott B.H., Minuzzi L., Krebs R.M., Elmenhorst D., Lang M., Winz O.H., Seidenbecher C.I., Coenen H.H., Heinze H.J., Zilles K., Düzel E., Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. : Off. J. Soc. Neurosci. 2008;28(52):14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L. Memory under stress: from single systems to network changes. Eur. J. Neurosci. 2017;45(4):478–489. doi: 10.1111/ejn.13478. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Socially evaluated cold pressor stress after instrumental learning favors habits over goal-directed action. Psychoneuroendocrinology. 2010;35(7):977–986. doi: 10.1016/j.psyneuen.2009.12.010. ∗. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Stress modulates the engagement of multiple memory systems in classification learning. J. Neurosci. : Off. J. Soc. Neurosci. 2012;32(32):11042–11049. doi: 10.1523/JNEUROSCI.1484-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Tegenthoff M., Höffken O., Wolf O.T. Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. J. Neurosci. : Off. J. Soc. Neurosci. 2012;32(30):10146–10155. doi: 10.1523/JNEUROSCI.1304-12.2012. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D.J., Heitzeg M.M., Koeppe R.A., Stohler C.S., Zubieta J.K. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J. Neurosci. : Off. J. Soc. Neurosci. 2006;26(42):10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. McGraw-Hill; 1956. The Stress of Life. [Google Scholar]
- Sekino A., Ohata H., Mano-Otagiri A., Arai K., Shibasaki T. Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology. 2004;176(1):30–38. doi: 10.1007/s00213-004-1863-1. ∗. [DOI] [PubMed] [Google Scholar]
- Shafiei N., Gray M., Viau V., Floresco S.B. Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology : Off. Publ. Am. College of Neuropsychopharmacol. 2012;37(10):2194–2209. doi: 10.1038/npp.2012.69. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M. Life stress and health: a review of conceptual issues and recent findings. Teach. Psychol. 2016;43(4):346–355. doi: 10.1177/0098628316662768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp J.A., Mashoodh R., van Kampen J.M., Robertson H.A. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Stanton C.H., Holmes A.J., Chang S.W.C., Joormann J. From stress to anhedonia: molecular processes through functional circuits. Trends Neurosci. 2019;42(1):23–42. doi: 10.1016/j.tins.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K., Brand M. Effects of stress on decisions under uncertainty: a meta-analysis. Psychol. Bull. 2016;142(9):909–933. doi: 10.1037/bul0000060. [DOI] [PubMed] [Google Scholar]
- Steidl S., O'Sullivan S., Pilat D., Bubula N., Brown J., Vezina P. Operant responding for optogenetic excitation of LDTg inputs to the VTA requires D1 and D2 dopamine receptor activation in the NAcc. Behav. Brain Res. 2017;333:161–170. doi: 10.1016/j.bbr.2017.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steins-Loeber S., Lörsch F., van der Velde C., Müller A., Brand M., Duka T., Wolf O.T. Does acute stress influence the Pavlovian-to-instrumental transfer effect? Implications for substance use disorders. Psychopharmacology. 2020;237(8):2305–2316. doi: 10.1007/s00213-020-05534-8. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye K.M., Mirzabekov J.J., Warden M.R., Ferenczi E.A., Tsai H.C., Finkelstein J., Kim S.Y., Adhikari A., Thompson K.R., Andalman A.S., Gunaydin L.A., Witten I.B., Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh O., Brosschot J., Critchley H., Thayer J.F., Ottaviani C. Better safe than sorry: a common signature of general vulnerability for psychopathology. Perspect. Psychol. Sci.: J. Assoc. Psychol. Sci. 2021;16(2):225–246. doi: 10.1177/1745691620950690. [DOI] [PubMed] [Google Scholar]
- van Oort J., Tendolkar I., Hermans E.J., Mulders P.C., Beckmann C.F., Schene A.H., Fernández G., van Eijndhoven P.F. How the brain connects in response to acute stress: a review at the human brain systems level. Neurosci. Biobehav. Rev. 2017;83:281–297. doi: 10.1016/j.neubiorev.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Ventura R., Morrone C., Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc. Natl. Acad. Sci. U.S.A. 2007;104(12):5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd M., Stout D.M., Risbrough V.B. Anhedonia in posttraumatic stress disorder: prevalence, phenotypes, and neural circuitry. Curr. Topics in Behav. Neurosci. 2022;58:185–199. doi: 10.1007/7854_2021_292. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.J., Baler R., Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl. 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Michaelides M., Baler R. The neuroscience of drug reward and addiction. Physiol. Rev. 2019;99(4):2115–2140. doi: 10.1152/physrev.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S., Yanagida J., Sasase H., Zhang T., Li X., Kamii H., Domoto M., Deyama S., Hinoi E., Yamanaka A., Nishitani N., Nagayasu K., Kaneko S., Minami M., Kaneda K. Acute restraint stress augments the rewarding memory of cocaine through activation of α1 adrenoceptors in the medial prefrontal cortex of mice. Neuropharmacology. 2020;166 doi: 10.1016/j.neuropharm.2020.107968. ∗. [DOI] [PubMed] [Google Scholar]
- Wanat M.J., Bonci A., Phillips P.E. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16(4):383–385. doi: 10.1038/nn.3335. ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J.M., Oleson E.B., Gove W.N., Cole A.B., Gyawali U., Dantrassy H.M., Bluett R.J., Dryanovski D.I., Stuber G.D., Deisseroth K., Mathur B.N., Patel S., Lupica C.R., Cheer J.F. Phasic dopamine signals in the nucleus accumbens that cause active avoidance require endocannabinoid mobilization in the midbrain. Curr. Biol.: CB (Curr. Biol.) 2018;28(9):1392–1404.e5. doi: 10.1016/j.cub.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A., Bozarth M.A. Brain mechanisms of drug reward and euphoria. Psychiatr. Med. 1985;3(4):445–460. [PubMed] [Google Scholar]
- Zacharko R.M., Bowers W.J., Kokkinidis L., Anisman H. Region-specific reductions of intracranial self-stimulation after uncontrollable stress: possible effects on reward processes. Behav. Brain Res. 1983;9(2):129–141. doi: 10.1016/0166-4328(83)90123-7. [DOI] [PubMed] [Google Scholar]
- Zalachoras I., Astori S., Meijer M., Grosse J., Zanoletti O., de Suduiraut I.G., Deussing J.M., Sandi C. Opposite effects of stress on effortful motivation in high and low anxiety are mediated by CRHR1 in the VTA. Sci. Adv. 2022;8(12) doi: 10.1126/sciadv.abj9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita A., Murúa S., Molina V. An endogenous opiate mechanism seems to be involved in stress-induced anhedonia. Eur. J. Pharmacol. 1996;299(1–3):1–7. doi: 10.1016/0014-2999(95)00754-7. ∗. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.