Highlights
-
•
Patients with degenerative spinal disease often have underlying osteoporosis.
-
•
Osteoporosis is an important risk factor for mechanical complications after lumbar fusion.
-
•
Routine preoperative screening allows interventions that can reduce complication risk.
-
•
Alternative screening recommendations may be necessary for surgical spine patients.
-
•
Standardized methods of bone health evaluation and complication reporting are needed.
Keywords: Osteoporosis, Bone mineral density, Lumbar fusion, Instrumentation failure, Degenerative spine disease, Hounsfield units, Opportunistic screening, Spine surgery, Complications
Abstract
Background
Adults undergoing spine surgery often have underlying osteoporosis, which may be a risk factor for postoperative complications. Although these associations have been described, osteoporosis remains profoundly underdiagnosed and undertreated in the spine surgery population. A thorough, comprehensive systematic review summarizing the relationships between bone mineral density (BMD) and specific complications of lumbar fusion surgery could be a valuable resource for raising awareness and supporting clinical practice changes.
Methods
PubMed, Embase, and Web of Science databases were searched for original clinical research articles reporting on BMD, or surrogate measure, as a predictor of complications in adults undergoing elective lumbar fusion for degenerative disease or deformity. Endpoints included cage subsidence, screw loosening, pseudarthrosis, vertebral fracture, junctional complications, and reoperation.
Results
A total of 71 studies comprising 12,278 patients were included. Overall, considerable heterogeneity in study populations, methods of bone health assessment, and definition and evaluation of clinical endpoints precluded meta-analysis. Nevertheless, low BMD was associated with higher rates of implant failures like cage subsidence and screw loosening, which were often diagnosed with concomitant pseudarthrosis. Osteoporosis was also a significant risk factor for proximal junctional kyphosis, particularly due to fracture. Many studies found surgical site-specific BMD to best predict focal complications. Functional outcomes were inconsistently addressed.
Conclusions
Our findings suggest osteoporosis is a significant risk factor for mechanical complications of lumbar fusion. These results emphasize the importance of preoperative osteoporosis screening, which allows for medical and surgical optimization of high-risk patients. This review also highlights current practical challenges facing bone health evaluation in patients undergoing elective surgery. Future prospective studies using standardized methods are necessary to strengthen existing evidence, identify optimal predictive thresholds, and establish specialty-specific practice guidelines. In the meantime, an awareness of the surgical implications of osteoporosis and utility of preoperative screening can provide for more informed, effective patient care.
Background
Osteoporosis is a highly prevalent, age-related skeletal disorder characterized by a progressive loss of bone mass and increased susceptibility to fractures [1]. As the global population ages, the prevalence and significance of osteoporosis and other age-related degenerative conditions will continue to rise [1,2]. This demographic shift is of particular significance to the spine surgeon, who will be increasingly faced with the challenge of treating patients with degenerative spinal pathologies, poor bone quality, and sequelae of osteoporosis. These conditions are frequently comorbid, with one recent systematic review estimating 79% of spine surgery patients over the age of 50 have osteoporosis or low bone mass [3]. Previous studies have reported higher rates of complications, longer hospitalizations, more frequent readmissions and reoperations, and increased total healthcare costs in osteoporotic patients following spine surgery [[4], [5], [6]]. Osteoporosis has also been suggested to be an independent risk factor for mechanical complications like cage subsidence (CS), pedicle screw loosening (SL), pseudarthrosis, vertebral compression fracture (VCF), and proximal junctional kyphosis (PJK) or failure (PJF) [[7], [8], [9], [10], [11]].
However, despite this prevalence and association with poor outcomes, osteoporosis remains profoundly underdiagnosed and undertreated in the spine surgery population [12,13]. Although many surgeons anecdotally recognize the challenges of instrumenting osteoporotic bone and may modify their surgical plan in the setting of this diagnosis, dedicated bone health assessments are infrequently performed preoperatively [[14], [15], [16]]. Underutilization of osteoporosis screening may be related to a variety of issues including logistical difficulties, concerns about the accuracy of lumbar T-scores in the degenerative spine, or lack of consensus regarding the implications of low bone density for surgical management. In the absence of clear specialty-specific guidelines addressing bone density in elective lumbar fusion, many surgeons may feel uncomfortable assuming the responsibility for osteoporosis screening and treatment [17,18]. Inadequate insurance coverage and reimbursement practices can also discourage providers from ordering diagnostic testing or prescribing pharmacologic therapies, and may make patient adherence to treatment cost-prohibitive [19,20].
Consequently, while an association between osteoporosis and surgical complications may seem intuitive, it is not reflected in current practices, specialty guidelines, or healthcare policies. Addressing this gap will require engagement of patients, surgeons, and policymakers regarding the importance of bone health in spine surgery and the utility of preoperative screening for preventing complications. The purpose of this manuscript is therefore to summarize existing literature on osteoporosis in lumbar fusion, focusing on mechanical complications that can be attributed to poor bone health. Rather than concentrating on a single outcome in isolation, the authors felt a comprehensive review that encompasses the spectrum of osteoporosis-related complications is necessary to put the significance of this condition into perspective and advocate for changes in the standard of care. Moving forward, these findings can serve as a reference to inform current practices, identify areas in need of further study, and ultimately provide for more consistent, effective, and evidence-based patient care.
Methods
Literature search strategy
PubMed, Embase, and Web of Science databases were searched for original research articles reporting on the risk of specific mechanical complications of lumbar fusion surgery in relation to bone mineral density (BMD), or surrogate measurement. Details on individual search strategies are provided in Supplementary File, Table S1. Articles were independently screened for eligibility by 2 reviewers (A.F. + A.B.) using the criteria in Table 1.
Table 1.
Details of article selection criteria
| Inclusion criteria |
|---|
| 1) Original research articles with a minimum study size of 10 patients |
| 2) Study population must be adult patients undergoing elective instrumented lumbar fusion surgery (includes standalone interbody fusions) |
| 3) Measured primary outcome(s) of the incidence of specific radiographic surgical complication(s) or need for revision surgery |
| 4) Comparison between osteoporosis or low bone mass group and normal bone density group* or risk stratification based on bone density (or surrogate measure) |
| *Studies must clearly specify the diagnostic criteria for osteoporosis (ex., T-score ≤ -2.5 or history of fragility fracture) |
| 5) Publication date from 2002 onward |
| Exclusion criteria |
| 1) Reviews, case reports, biomechanical studies, cadaveric research |
| 2) Surgical interventions including non-instrumented procedures (ex., decompression alone, vertebroplasty) or those performed for indications other than degenerative disease (ex., infection, trauma, malignancy) |
| 3) Investigations of only osteoporotic patients (no internal control group) |
| 4) Failure to specify the incidence of specific complications (i.e., reporting generalized results for “all complications”) |
| 5) Studies reporting on mixed populations without stratifying results based on osteoporosis assessment |
| 6) Studies that did not perform a baseline evaluation of bone health for all eligible participants |
Data extraction and outcome measures
Data was extracted by the first author (A.F.) and validated by 2 additional authors (K.H. + J.R.). Variables included information related to (1) study design and setting; (2) patient demographics; (3) treatment characteristics: surgical procedures and perioperative anti-osteoporosis therapies; (4) prognostic factor assessment: imaging modality, anatomical site(s), and cutoff thresholds or diagnostic criteria used (if applicable); and (5) primary outcomes evaluated: imaging modality, timing, and diagnostic criteria. Mechanical complications reported by at least 2 studies, with relevant statistics, were considered for analysis (Table 2). Missing data were sought out through contact with corresponding authors.
Table 2.
Primary outcomes included
| Interbody cage subsidence Screw loosening Pseudarthrosis New vertebral fracture Junctional pathologies (adjacent segment disease, proximal junctional kyphosis or failure) |
| Revision surgery |
For each primary outcome we presented studies’ findings of prognostic effect, including estimated odds ratio (OR) for binary outcomes and mean differences or unit odds ratio (UOR) for continuous outcomes. Event rates for dichotomous data were used to generate forest plots for each outcome. Overall, data were summarized but not pooled due to substantial variability in study methodologies.
Methodological quality assessment
Evidence quality was evaluated using the system proposed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group [21]. Using major GRADE criteria, articles were evaluated in the context of methodological domains thought to be highly important for studies of prognostic factors, including patient selection and comparability of subjects, prognostic factor assessment, appropriateness of clinical endpoints, data collection and analysis practices, and disclosure of funding [[22], [23], [24]]. Assessments were performed independently by 2 reviewers (A.F. + A.R.), with discrepancies reconciled in discussion.
Results
Study selection
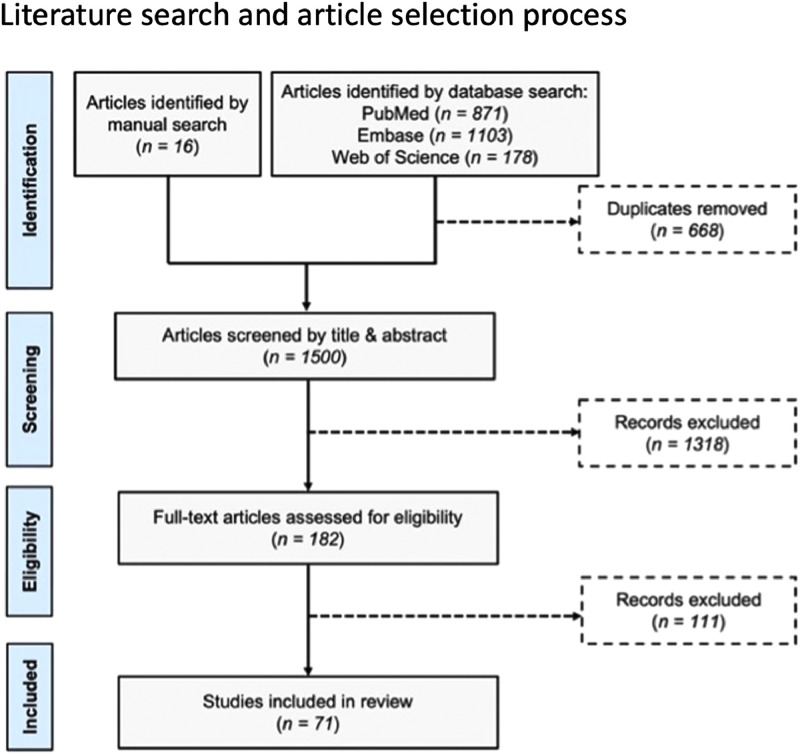
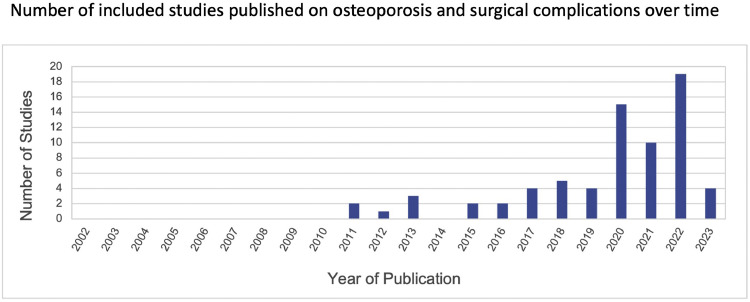
The article selection process is detailed in Figure 1. An initial database search returned 2,112 citations, 1,500 after removal of duplicates. An additional 16 articles were identified manually. Screening by title and abstract left 182 references for full-text review. Ultimately, 71 studies satisfied our inclusion and exclusion criteria. Notably, 48 of these (67.6%) were published since the year 2020 and nearly one-third since 2022 (Figure 2).
Fig. 1.
Flow diagram depicting literature search and article selection process.
Fig. 2.
Number of included studies published on osteoporosis and surgical complications over time
Among 71 total studies. The electronic search included the years 2002 to 2023.
Study characteristics and quality assessment
Main characteristics of the included studies are presented in Table 3. In total, 12,278 patients (63% female) were included, with mean ages ranging between 44.9 and 72.5 years. All patients underwent primary or revision lumbar fusion for degenerative disease or deformity.
Table 3.
Characteristics of included studies
| Author | Year | Study Design | No. patients |
Age, years Mean ± SD (range) |
Bone Health Assessment |
Osteoporosis treatment | Surgical Intervention | Clinical Follow-up |
Primary Outcomes | Radiographic assessment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic modality | Measurement location | Mean ± SD (range) | Min | |||||||||
| Alan [31] | 2022 | Retrospective review | 55 | 63.6 ± 10.1 (18+) | CT (HU) DXA |
LIF segment NR |
3.6% (2/55) | Single or multilevel LLIF | 13.3 ± 8.5 mo | 1 y | Cage subsidence | X-ray ± CT |
| Amorim-Barbosa [26] | 2022 | Retrospective review | 165 | CS: 52 ± 16, No CS: 49 ± 12 | CT (HU) DXA |
Global lumbar Lumbar |
NR | Single or multilevel TLIF/PLIF | NR | 6 mo | Cage subsidence | X-ray |
| Barton [118] | 2017 | Retrospective review | 94* | 58.6 ± 12.7 (23-82) | DXA or ultrasound | NR | NR | Multilevel posterior or AP fusion with osteotomy | 30 mo | 2 y | Junctional disease | NR |
| Bokov [119] | 2018 | Retrospective review | 250 | 52 ± 12.1 (28-74) | CT (HU) | L3 | NR | Short-segment lumbar fusion ± LIF | NR | 1.5 y | Screw loosening | CT |
| Chen [60] | 2011 | Retrospective review | 109 | 53.4 (28-72) | DXA | Lumbar | NR | L4/5 PLIF | 39.3 mo (24-52 mo) | 2 y | Junctional disease | X-ray |
| Chen [37] | 2023 | Retrospective review | 174 | 63.5 ± 7.8 (50+) | MRI (VBQ) DXA |
Global lumbar Lowest T-score |
NR | Short-segment lumbar fusion with PLIF | 14.6 mo (12-37 mo) | 1 y | Screw Loosening | X-ray |
| Cho [34] | 2018 | Retrospective review | 86 | Osteoporosis: 66.1 ± 8.0, Normal BMD: 65.8 ± 7.8 | DXA | Lumbar | 19.7% (17/86) | Single-level PLIF | NR | 2 y | Cage Subsidence Screw loosening Pseudarthrosis |
CT X-ray or CT X-ray and CT |
| Choi [44] | 2023 | Retrospective review | 79 | Osteoporosis: 69.9 ± 6.9, Low BMD: 62.6 ± 7.8, Normal BMD: 56.6 ± 7.7 | CT (HU) Partial (38%) DXA |
L1 NR |
44.3% (38/79) | Single-level TLIF | 40.3 mo | 2 y | Pseudarthrosis | CT |
| Duan [53] | 2020 | Retrospective review | 54 | 64.9 ± 7.6 (50+) | CT (HU) | UIV, UIV+1, UIV+2 | NR | Long posterior fusion | 3.19 ± 1.14 y | 2 y | Junctional disease | X-ray |
| Ehresman [66] | 2020 | Case-control | 90 | Case: 63.5 ± 8.2, Control: 63.1 ± 10.6 (18+) | MRI (VBQ) Partial (39%) DXA |
Global lumbar FN |
NR | Multilevel lumbar fusion | Case: 6.1 ± 4.1 y, Control: 3.5 ± 1.1 y | 2 y | Reoperation | N/A |
| Guha [27] | 2022 | Retrospective review | 89 | 61.6 ± 10.5 (18+) | CT (HU) DXA |
LIF segment FN |
NR | Single or multilevel LLIF | 19.9 ± 13.9 mo | 6 mo | Cage subsidence Reoperation |
X-ray |
| Ha [45] | 2019 | Retrospective review | 157 | 68.0 ± 6.3 (60+) | DXA | Lowest T-score | NR | Long posterior fusion | 53.2 ± 34.3 mo (24–152) | 2 y | Junctional disease | X-ray |
| Hiyama [120] | 2022 | Retrospective review | 59 | 68.9 ± 10.6 (25-89) | CT (HU) | LIF segment (EP) | NR | Single-level LLIF | NR | 1 y | Cage subsidence | X-ray and CT |
| Hiyama [54] | 2022 | Retrospective review | 52 | 70.2 ± 9.2 (20+) | CT (HU) | UIV, UIV+1, UIV+2 | NR | Staged multilevel LLIF and long posterior fusion | 17.7 ± 9.5 mo | 1 y | Junctional disease | X-ray |
| Hu 39(p) | 2022 | Retrospective review | 242 | 60.5 ± 13.3 (18+) | MRI (VBQ) Partial (21%) DXA |
Global lumbar TH, FN, lumbar |
6.6% (16/242) | Single-level TLIF | 35.77 ± 16.33 mo | 2 y | Cage subsidence | X-ray |
| Hyun [121] | 2016 | Retrospective review | 44 | PJK: 64.7 ± 7.3, No PJK: 63.4 ± 7.3 (20+) | DXA | NR | NR | Long posterior or AP fusion | NR | 2 y | Junctional disease | X-ray |
| Jones [122] | 2021 | Retrospective review | 347 | 61.7 ± 11.1 (18+) | QCT (vBMD) | Mean L1/2; LIF segment (EP) | NR | Single or multilevel LLIF | NR | 5 mo | Cage subsidence | X-ray or CT |
| Jones [123] | 2022 | Retrospective review | 89 | 65.94 ± 10.44 (18+) | MRI (VBQ, EBQ) QCT (vBMD) |
Global lumbar; LIF segment (EP) Mean L1/2 |
NR | Single or multilevel standalone LLIF | NR | 5 mo | Cage subsidence | X-ray or CT |
| Jung [32] | 2019 | Retrospective review | 84 | Osteopenia: 65.3 ± 7.2, Normal BMD: 64.2 ± 10.2 | DXA | FN | NR | Single-level D-LIF | Osteopenia: 44.3 ± 14.3 mo, Normal BMD: 43.2 ± 12.2 mo | 2 y | Cage subsidence Pseudarthrosis |
X-ray X-ray and CT |
| Kim, MC [124] | 2013 | Retrospective review | 104 | 61.3 ± 9.8 (38-79) | DXA | NR | NR | 1 or 2-level MI-TLIF | 31.3 ±10.8 mo (24-45 mo) | 2 y | Cage subsidence | X-ray |
| Kim, HJ [33] | 2013 | Retrospective review | 364 | PJK: 53.3 ± 14.5, No PJK: 48.9 ± 15.0 (18+) | DXA | NR | NR | Long posterior or AP fusion | 3.5 y (2-6 y) | 2 y | Junctional disease | X-ray |
| Kim, DK [125] | 2017 | Retrospective review | 49 | PJK: 62.5 (56-69), No PJK: 61.9 (54-69) | DXA | NR | NR | Long posterior or AP fusion | PJK: 47.7 ± 23.4 mo, No PJK: 45.6 ± 25.6 mo | 2 y | Junctional disease | X-ray |
| Kim, KH [38] | 2022 | Retrospective review | 113 | 65.2 ± 10.8 | CT (HU) Partial (73%) DXA |
Global lumbar Lumbar |
NR | Single or multilevel lumbosacral fusion | NR | 6 mo | Screw Loosening | X-ray |
| Kotheeranurak [126] | 2021 | Retrospective review | 107 | 67.4 | DXA | NR | NR | Single or multilevel OLIF | 34.2 mo (24–72 mo) | 2 y | Cage subsidence | X-ray and CT |
| Kuo[59] | 2023 | Retrospective review | 116 | 64.1 ± 6.8 (50+) | MRI (VBQ) Partial (61%) DXA |
Global lumbar Lumbar, TH, FN |
NR | Thoracolumbar fusion | PJK/PJF: 27.6 ± 15.4 mo, No PJK/PJF: 24.7 ± 12.0 mo | 1 y | Junctional disease | X-ray |
| Kurra [47] | 2022 | Retrospective review | 92 | 64 (42-81) | CT (HU) Partial (52%) DXA |
UIV-1, UIV, UIV+1 NR |
NR | Long posterior fusion | 1.5 y (0.2-4 y) | NR | New VCF Junctional disease |
CT X-ray |
| Lee [127] | 2020 | Retrospective review | 59 | 69.6 ± 5.9 (60+) | DXA | NR | NR | Long posterior fusion | 87.4 ± 37.5 mo | 2 y | Pseudarthrosis | CT |
| Li [42] | 2023 | Retrospective review | 56 | 56.6 ± 11.96 | CT (HU) | LIF segment, screw insertion point | NR | L4/5 OLIF | 12.2 mo (11-13.5 mo) | 1 y | Screw loosening | CT |
| Liu [84] | 2020 | Prospective cohort | 105 | 58.5 (43-71) | Micro CT (BS/TV) DXA (BMD) |
Spinous process specimen (ex-vivo) Lumbar, FN |
NR | Single-level PLIF | NR | 2 y | Pseudarthrosis | CT |
| Löffler [128] | 2021 | Case-control | 46 | 69.9 ± 9.1 (48-85) | CT (vBMD) | Global, segmental (L1-4) | NR | Short-segment lumbar fusion | Median 365 d (71-2225 d) | 6 mo | Screw loosening | X-ray or CT |
| Luo [46] | 2020 | Retrospective review | 669 | 59.92 ± 7.41 | DXA | Lumbar | NR | Short-segment fusion with PLIF | 2.7 ± 1.1 y (2–4 y) | 2 y | New VCF | X-ray |
| Matsukawa [39] | 2018 | Retrospective review | 92 | 63.4 ± 14.8 (31-88) | CT (HU sum x1000) DXA |
Screw trajectory Lumbar, FN |
NR | Single-level PLIF, pedicle screw fixation using CBT | 25.6 ± 10.2 mo | 1 y | Screw loosening | CT |
| Meredith [48] | 2013 | Case-control | 40 | Case: 66 (49-88), Control: 62 (49-80) | CT (HU) | Global thoracolumbar, fracture level | 17.5% (7/40) | Multilevel posterior or AP fusion | NR | 6 mo | New VCF | X-ray, CT, MRI, or bone scan |
| Mi [129] | 2017 | Case-control | 36 | Case: 53 (23-73), Control: 54 (25-71) | CT (HU) | Global lumbar, LIF segment | 0 | Single-level TLIF with unilateral fixation | NR | 6 mo | Cage subsidence | CT |
| Mikula [55] | 2021 | Retrospective review | 150 | 66 ± 7.4 (50+) | CT (HU) Partial (55%) DXA |
Mean L3/4, UIV/UIV+1 FN, TH, lumbar |
55% (83/150) | Long instrumented fusion | 31.8 ± 20.2 mo | 1 y | Junctional disease | X-ray |
| Mikula [56] | 2022 | Retrospective review | 81 | 66 ± 6.9 (50+) | CT (HU) Partial (70%) DXA |
Mean L3/4, UIV/UIV+1 FN, TH, lumbar |
56% (45/81) pre-op 22.2% (18/81) post-op |
Long instrumented fusion | 38 ± 25 mo | 1 y | Junctional disease | X-ray |
| Mugge [64] | 2022 | Retrospective review | 532 | Osteoporosis: 69 ± 11, No osteoporosis: 59 ± 19 (18+) | DXA | Femoral head | 27% (144/532) | Long thoracolumbar fusion | 18.5 ± 68.7 mo | NR | Reoperation | N/A |
| Nguyen [130] | 2015 | Case-control | 20 | Case: 44.4 ± 12.14, Control: 45.4 ± 10.65 | CT (HU) | Global lumbar (L1-3), UIV/UIV-1 | NR | L4-S1 posterolateral fusion | NR | 1 y | Pseudarthrosis | Intraoperative or radiographic |
| Oh [131] | 2015 | Retrospective review | 102 | 65.17 ± 8.59 (37-86) | DXA | Lumbar (LIF segment) | NR | Single or multilevel PLIF | 4.1 y (1.4-7.7 y) | 1 y | Cage subsidence | CT |
| Okano [132] | 2020 | Retrospective review | 96 | Median 68 [IQR 62.2-74.3] | QCT (vBMD) | Mean L1/2, LIF segment (Tb, EP) | NR | Single or multilevel standalone LLIF | Median 26 mo [IQR 8-102 mo] | 6 mo | Cage subsidence | X-ray or CT |
| Otsuki [133] | 2021 | Retrospective review | 85 | Nonunion: 72.1 ± 6.9, Union: 68.2 ± 8.4 (50+) | CT (HU) | LIF segment | NR | L4/5 TLIF | NR | 1 y | Pseudarthrosis | X-ray and CT |
| Park, MK [66] | 2019 | Prospective cohort | 784 | 63.3 (20-85) | DXA† | Lumbar (LIF segment) | NR | Single or multilevel TLIF | NR | 1.5 y | Cage subsidence | X-ray and CT |
| Park81, SJ [52] | 2020 | Retrospective review | 63 | 67.2 ± 6.3 (50+) | DXA | NR | NR | Long posterior or AP fusion | 51.7 ± 33.1 mo | 2 y | Junctional disease | X-ray |
| Pisano [134] | 2020 | Retrospective review | 89 | 59.9 (50+) | CT (HU) | L1 | NR | Single or multilevel TLIF | 27 mo | 1 y | Cage subsidence | CT |
| Pu [30] | 2022 | Retrospective review | 71 | 59.6 ± 10.1 | CT (HU) DXA |
Global lumbar, LIF segment Lumbar, forearm |
NR | L4/5 PLIF | 13.6 ± 5.1 mo | 1y | Cage subsidence | CT |
| Ran [135] | 2022 | Retrospective review | 70 | 59 ± 10.4 | CT (HU) | Global lumbar, LIF segment (Tb, EP) | 8.57% (6/70) | L4/5 OLIF | 15.4 ± 6 mo (12-40) | 1 y | Cage subsidence | CT |
| Rentenberger [65] | 2020 | Retrospective review | 133 | Revision: 68.6 ± 10.6, No revision: 66.3 ± 10.6 (18+) | QCT (vBMD) | Mean L1/2 | NR | Single or multilevel standalone LLIF | NR | 1 y | Cage subsidence Reoperation |
X-ray or CT |
| Sakai [40] | 2018 | Retrospective review | 52 | 68.2 ± 10.1 (44-83) | CT (HU) DXA |
Screw trajectory Lumbar |
17.3% (9/52) | Single-level PLIF | NR | 3 mo | Screw loosening | CT |
| Salzmann [136] | 2019 | Case-control | 63 | Case: 66.4 ± 8.5, Control: 65.3 ± 7.9 | QCT (vBMD) | Mean L1/2, S1, sacral ala | NR | Multilevel posterior fusion to S1 | NR | 6 mo | New VCF | NR |
| Shin [137] | 2022 | Retrospective review | 478 | 65.0 ± 10.6 (22-88) | CT (HU) | L4 | NR | Short-segment lumbar fusion with PLIF | 43.2 ± 27.25 mo (12-113) | 1 y | Screw loosening | X-ray and CT |
| Wang, H [57] | 2016 | Retrospective review | 98 |
PJK: 62.3 ± 6.8 No PJK: 62.5 ± 7.5 (50+) |
DXA | NR | NR | Long posterior fusion | 2.8 y (2-6) | 2 y | Junctional disease | X-ray |
| Wang, H [61] | 2017 | Retrospective review | 237 | 53.2 ± 10.8 (37-69) | DXA | NR | NR | 1 or 2-level TLIF or PLIF | Adjacent disease: 2.6 ± 0.2 y, No adjacent disease: 2.5 ± 0.3 y | 2 y | Junctional disease | X-ray |
| Wang, Q [138] | 2020 | Retrospective review | 104 | 63.2 (49-80) | CT (HU) | L1 | 0% (0/104) | Long instrumented fusion | 35.7 mo | 2 y | Junctional disease | X-ray |
| Wang, SK [63] | 2022 | Retrospective review | 821 | Early revision: 68.1 ± 11.6, Late revision: 66.9 ± 9.5, No revision: 64.9 ± 11.1 (18+) | DXA | NR | 4.5% (37/821) | Short-segment lumbar fusion with TLIF | NR | 2 y | Reoperation | |
| Xi [139] | 2020 | Retrospective review | 68 | 61.1 ± 13.3 (18+) | CT (HU) | Global lumbar, LIF segment | Criteria for exclusion | Single-level LLIF | 25.3 ± 10.4 mo | 1 y | Cage subsidence | X-ray |
| Xie [28] | 2022 | Retrospective review | 279 | 50.9 ± 8.8 (18+) | CT (HU) Partial (24%) DXA |
Global lumbar, segmental (L1-4) FN and/or lumbar |
NR | Single-level TLIF | Median 18 mo [12-40] | 1 y | Cage subsidence | NR |
| Xu [43] | 2020 | Retrospective review | 143 | SL: 62.0 ± 6.7, No SL: 62.0 ± 6.4 (50+) | CT (HU) | L3 (vertebral body and pedicle) | NR | L3-5 posterolateral fusion | NR | 1 y | Screw loosening | X-ray |
| Xu [35] | 2022 | Retrospective review | 78 | 63 (45-80) | DXA | Lumbar, TH | NR | Long posterior fusion | NR | 2 y | Screw loosening | X-ray ± CT |
| Yagi, [140] | 2011 | Retrospective review | 157 | 46.9 (22-81) | DXA | FN | NR | Long posterior, anterior, or AP fusion | 4.3 y (2-12) | 2 y | Junctional disease | X-ray |
| Yagi [141] | 2012 | Retrospective review | 76 | 48.8 (23-75) | DXA | FN | NR | Long posterior, anterior, or AP fusion | 7.3 y (5-14) | 5 y | Junctional disease | X-ray |
| Yagi [142] | 2018 | Retrospective review | 113 | 62.2 (20+) | DXA | FN | NR | Long thoracolumbar fusion | NR | 2 y | Junctional disease | X-ray |
| Yao [143] | 2020 | Retrospective review | 93 | 66.5 ± 12.2 (18+) | DXA | NR | NR | 1-2 level MI-TLIF | 36.9 ± 5.7 mo (24-46) | 2 y | Cage subsidence | X-ray |
| Yao [49] | 2021 | Retrospective review | 63 | 58.4 ± 14.9 (18+) | CT (HU) | Mean UIV/UIV+1 | NR | Long posterior fusion | 13.1 mo | 1 y | Junctional disease | X-ray |
| Ye [62] | 2021 | Case-control | 1258 | 56.4 ± 12.4 (20-87) | DXA | NR | NR | TLIF | 35.0 ± 17.8 mo (24-123) | 2 y | Junctional disease | NR |
| Yuan [36] | 2021 | Retrospective review | 130 | 62.89 ± 7.08 (40-79) | DXA | NR | NR | Long posterior fusion | 34.4 mo (12-98) | 1 y | Screw loosening | X-ray ± CT |
| Yuan [51] | 2021 | Retrospective review | 84 | PJK: 63.53 ± 7.33, No PJK: 62.69 ± 6.4 (40+) | DXA | NR | NR | Long posterior fusion | 40.83 mo | 2 y | Junctional disease | X-ray |
| Zhang [58] | 2022 | Retrospective review | 333 | PJK 74 ± 6, No PJK 70.6 ± 4.2 (65+) | CT (HU) | UIV | NR | Multilevel posterior fusion | 24.2 mo (18-46) | 1.5 y | Junctional disease | X-ray |
| Zhao [144] | 2022 | Retrospective review | 242 | Severe CS: 69.1 ± 9.9, Mild CS: 66.3 ± 10.7, No CS: 64.5 ± 9.1 | DXA | Lowest (hip) | NR | L4/5 OLIF | NR | 1 y | Cage subsidence | X-ray |
| Zhou [29] | 2021 | Retrospective review | 76 | 56.1 ± 10.4 (29-81) | CT (HU) DXA |
Global lumbar, L1, LIF segment Lowest T-score |
NR | Single or multilevel standalone OLIF | 28.2m ± 9.3m | 6m | Cage subsidence | X-ray ± CT |
| Zou [145] | 2020 | Retrospective review | 503 | 61.2 ± 6.7 (50–83) | CT (HU) | Global and segmental (L1-4) | NR | Short-segment lumbar fusion ± PLIF | NR | 1y | Screw loosening | X-ray |
| Zou [41] | 2020 | Retrospective review | 252 | 62.4 ± 6.7 (50-83) | CT (HU) DXA |
Global lumbar Lumbar, lowest T-score |
NR | Short-segment lumbar fusion ± PLIF | NR | 1y | Screw loosening | X-ray |
Data presented describe entire study population unless otherwise specified as cohort statistics. Surgery types include long (5+ levels) posterior, anterior, or combined (AP) fusion, short-segment lumbar fusion, and lumbar interbody fusion (LIF); all LIF performed with supplemental screw fixation unless indicated to be a standalone procedure. LIF types include: anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (LLIF), direct lateral interbody fusion (D-LIF), oblique lateral interbody fusion (OLIF), posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), and minimally invasive TLIF (MI-TLIF).
Abbreviations: not recorded (NR), dual-energy x-ray absorptiometry (DXA), computed tomography (CT) quantitative CT (QCT), Hounsfield Units (HU), bone mineral density (BMD), volumetric BMD (vBMD), magnetic resonance imaging (MRI), vertebral bone quality (VBQ), endplate bone quality (EBQ), endplate (EP), trabecular (Tb), femoral neck (FN), total hip (TH), upper instrumented vertebra (UIV), cage subsidence (CS), screw loosening (SL), vertebral compression fracture (VCF), proximal junctional kyphosis (PJK), proximal junctional failure (PJF), inter-quartile range (IQR)
Ninety-four operations in 88 patients
Preoperative DXA obtained in 84.3% (661/784) of study patients according to criteria: 1) all age 60 or older (n=598) or 2) age younger than 60 with comorbidity or chronic medication with potential to cause osteoporosis (n=63)
In terms of prognostic factor assessment, 38 studies used BMD measured by dual-energy X-ray absorptiometry (DXA), the current gold-standard for diagnosing osteoporosis [25]. Only 22 studies specified the anatomic site(s) of DXA scans, typically either the proximal femur or lumbar spine for all participants. Five studies used each patient's lowest T-score, noting severe degeneration, scoliosis, or instrumentation to variably preclude certain measurements. Citing concerns regarding the availability or accuracy of DXA, 42 studies investigated alternative techniques, most commonly opportunistic measurement of Hounsfield Units (HU) from preoperative CT scans (Table 4).
Table 4.
Overview of methods used for bone health assessment, and their proportions
| Tool | Metric measured variable (units) | Measurement location Including standardized protocol and experimental sites | Diagnostic criteria Thresholds for identifying poor bone health | No. studies with complete population data |
|---|---|---|---|---|
| Gold-standard | ||||
| DXA | BMD (g/cm2) T-score | Femoral neck/total femur region, lumbar spine, distal radius* | Normal: T-score ≥ -1 Osteopenia: -2.5 < T-score < -1 Osteoporosis: T-score ≤ -2.5† |
38 ‡ |
| Alternatives investigated | ||||
| QCT | vBMD (mg/cm3) | L1-2 Experimental sites: fusion-level vertebral endplates, sacral ala |
Normal: vBMD ≥ 120 mg/cm3 Low bone mass: 80 mg/cm3 < vBMD < 120 mg/cm3 Osteoporosis: vBMD ≤ 80 mg/cm3§ |
5 |
| CT | Hus | Variable Experimental sites: thoracolumbar spine (mean global, segmental, individual levels) including intra-vertebral sites (endplates, pedicles, screw trajectory) |
Normal: HU > 120; 135 Low bone mass: 90; 110 < HU < 120; 135 Osteoporosis: HU < 90; 110 ǁ |
33 |
| MRI | VBQ score | T1-weighted sagittal scans: median L1-4 signal standardized against CSF at L3 Experimental sites: fusion-level vertebral endplates |
Research method | 5 |
| Micro-CT | BS/TV, BS/BV, Tb.Th, Tb.N, Tb.Sp | Spinous process specimen obtained from index surgery (ex-vivo) | Research method | 1 |
According to International Society for Clinical Densitometry (ISCD) guidelines [146] routine BMD screening is indicated for all females over age 65 and males over age 70, as well as younger patients with risk factors for low bone mass. The current best-established standard for diagnosing osteoporosis or osteopenia relies on T-scores derived from areal BMD (g/cm2) measured by DXA, ideally of the femoral neck or lumbar spine. These thresholds can be applied in postmenopausal women and men over age 50. Alternatively, volumetric BMD (mg/cm3) can be directly measured by QCT. Of the 2 methods, central DXA is generally preferred for making therapeutic decisions and limiting radiation exposure, however, QCT may be considered superior to DXA in settings of severe degenerative disease or scoliosis [147]. A number of studies have suggested that fracture risk can also be assessed with CT attenuation in Hounsfield units (HU), which can be measured from CT scans obtained for other purposes that include the lumbar spine (opportunistic bone density measurement). In the absence of established protocols, methodologies for HU measurement varied widely and included standardized (mean or segmental) and patient-specific (ex., junctional vertebrae, screw trajectory, fusion-level endplates) sites. MRI and micro-CT are other techniques used to assess bone quality; as purely research methods, both follow standardized protocols but do not have established clinical correlates or guidelines for identifying at-risk patients. Additionally, while MRI metrics can be obtained via opportunistic measurement, micro-CT is an ex-vivo study and therefore cannot be used for screening preoperatively.
Study acronyms are explained in the first footnote to Table 3. Abbreviations: CSF, cerebrospinal fluid; BS/TV, bone surface / total volume; BS/BV, bone surface / bone volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation.
Hip and spine measurements preferred; distal radius recommended only when hip and spine cannot be obtained.
According to the World Health Organization (WHO), gold-standard T-score thresholds used for the diagnosis of osteoporosis and osteopenia [25]
Includes one prospective observational study in which all patients over the age of 60 years (n=598), as well as all younger patients with risk factors for osteoporosis (n=63), underwent a preoperative lumbar DXA. In total, this amounted to 84.7% (661/784) of participants [81].
According to American College of Radiology (ACR) practice guidelines, vBMD (mean L1/2) thresholds for osteoporosis and low bone mass [147]
Although there has been no established consensus regarding HU thresholds for diagnosing osteoporosis or osteopenia, several large-scale studies utilizing L1 HU have suggested values of 90 or 110 for osteoporosis and 135 or 120 for low bone mass [148,149]. A recent systematic review of studies reporting on the correlation between lumbar HU and DXA T-scores identified 16 studies describing a cutoff for identifying osteoporosis (thresholds ranged 49.4–160), with a medium HU value of 114.8 (95% CI 90.9–138.7, p<.001). Notably, there was significant heterogeneity (I2=94.94%) among studies, including patient populations and location of HU measurement [150]. Another meta-analysis of studies evaluating the accuracy of osteoporosis diagnosis using CT HU compared to DXA suggested a threshold of 135 to diagnose osteoporosis; the authors similarly noted that their conclusions were significantly limited by study heterogeneity [151].
Evidence quality was designated as high, moderate, low, or very low based on GRADE criteria (Supplementary File, Table S2). Studies were frequently downgraded for risk of bias or indirectness in prognostic factor assessment, either by employing imaging-based selection criteria or lacking a gold-standard comparison. Insufficient accounting for confounders was another common reason for downgrading.
Primary outcomes
An overview of findings for each primary outcome is shown in Table 5. Ultimately, a meta-analysis was not possible due to different non-comparable methods of prognostic factor assessment, variable definitions and timing of clinical endpoints, and the use of different cut point values for statistical analysis.
Table 5.
Summary of findings
| Outcome timing of radiographic follow-up | No. participants (studies) | Reported complication rates | Reported risk factors |
|
|---|---|---|---|---|
| Bone quality | Other independent risk factors | |||
| Cage subsidence, Measured in millimeters or relative loss of disc height on X-ray and/or CT Minimum follow-up: range 5 to 24 months |
3,555 patients, 4,439 levels (24 studies: 22 RR, 1 PC, 1 CC) |
8.25%–59% of levels* | Twenty-one studies found poor bone health associated with CS, and 3 did not.† DXA-diagnosed osteoporosis was a significant risk factor in 6 studies. Lumbar HU also predicted CS in 11 of 12 studies; outcomes were presented as an odds ratio (OR), often using a calculated optimal cutoff (ranged 104.2-135), or odds ratios per unit change (UOR) in HU. Among five studies comparing HU and T-scores, 4 found HU to best predict CS and one found nondominant forearm T-scores to be superior to both lumbar T-scores and mean HU. Four studies reported lower endplate density or quality in patients with CS. Several studies found a linear correlation between the amount of CS and either DXA T-scores (2 studies) or VBQ score (one study). | Age [126], BMI [143], paraspinal muscle atrophy [126], disc morphology [81,144], cage height [126,134,143] or shape [26,33, 39,81, 143], cage position disc overdistraction [144], intraoperative endplate injury [81,144], SA fusions [27,122], L5/S1 level [33], absence of endplate sclerosis [144] |
| Screw Loosening Defined by peri-screw lucency on X-ray and/or CT Minimum follow up: range 3 to 24 months |
2,454 patients (14 studies: 13 RR, 1 CC) |
13%–54.6% of patients | All studies found an association between bone health and SL. DXA-diagnosed osteoporosis was an independent risk factor in 2 studies. Bone density was most commonly assessed using CT or QCT (10 studies). Eight studies found lumbar HU to be an independent risk factor for SL; calculated cutoffs ranged 104-130,‡ though results were frequently presented using a UOR. Four studies measured regional bone density from the pedicle or screw trajectory, which was highly predictive of SL. All studies with data for comparison found DXA to be less predictive than alternatives. | Age [35], male sex [40,145], BMI [43], pedicle diameter [43], vertebral subluxation [36,119], cage type [119], bilateral facetectomy [119], laminectomy without interbody fusion [119], postoperative SVA [36,137] or TLK [36], number of fused levels [41,119,137,145], fusion to the sacrum [37,36,145] |
| Pseudarthrosis Variable criteria, commonly included 1) dynamic motion, 2) lack of bridging bone, or 3) implant loosening on x-ray and/or CT§ Minimum follow up: range 12 to 24 months |
518 patients (7 studies: 5 RR, 1 PC, 1 CC) |
5.95%–38.98% of patients | None of the studies utilizing DXA found an association between osteoporosis and fusion outcomes. Two of 3 studies using HU found a relationship with fusion, one of which showed that patients with osteoporosis (defined by L1 HU) had significantly longer mean times to fusion. § One study showed that bone quality of surgical specimens (assessed using ex-vivo micro CT) was a significant predictor of fusion status and functional outcomes.ǁ | Other instrumentation failures (SL [34,41,81,137,145], CS [81,120,135,144]); age [133], PEEK cages [127], lack of pelvic fixation [127], larger filling index [133] |
| New VCF See footnotes¶ Minimum follow up: range 2.4 to 24 months |
1,084 patients (6 studies: 4 RR, 2 CC) |
3.86%–11.95% of patients | All 5 studies evaluating proximal VCF found an association between bone density and fracture risk. Two of these were PJK subgroup analyses, in which T-scores and mean junctional HU independently predicted failure. Two other studies observed fracture patients to have lower HU both globally and at junctional or fracture levels. In the only study of sacral fractures, vBMD was not a risk factor. | Age [46], postoperative PJA [45], change in LL [46] Obesity (sacral fractures) [136] |
| Junctional Disease Adjacent Segment Degeneration See footnotes♯ Minimum follow up: 24 mo Proximal junctional kyphosis (PJK) or failure (PJF) See footnotes†† Minimum follow up: range 2.4 to 60 mo |
1,604 patients (3 studies: 2 RR, 1 CC) |
6.3%–22.01% of patients | No study found BMD to predict adjacent segment degeneration, though 2 studies did show a trend towards significance for BMD in patients with symptomatic disease.⁎⁎ | BMI [61], hypertension [62], preoperative disc degeneration [61,62], superior facet violation [61] |
| 2,344 patients (20 studies: 20 RR) |
11.5%–53.7% of patients | All studies reporting on junctional deformity found a relationship with poor bone health. Seven studies showed DXA-based osteoporosis was an independent risk factor. All studies of junctional HU reported lower values in patients with PJK (optimal cutoffs ranging 104-159), which better predicted complications compared to HU measured at non-junctional levels.‡‡ Two studies, one of VBQ score and another of mean UIV/UIV+1 HU, found a direct linear relationship between poor bone health and proximal junctional angle (PJA) measurements. | Age [52], BMI [45,57], smoking [118], PJA ≥0°[52], preop TLK [45,51] and SS [51], paraspinal muscle atrophy, [51,58,121] primary vs revision [118], type of osteotomy [118], UIV level [57,138], degree of deformity correction [121], postoperative fall [118] | |
| Revision surgery See footnotes§§ Minimum follow up: range 6 to 24 months |
1,665 patients (5 studies: 4 RR, 1 CC) |
8.2%–22.4% of patients | Four studies found poor bone health to be a risk factor for reoperation. DXA-based osteoporosis was an independent risk factor in 2, and fusion-level HU in one other. Another case-control study found higher VBQ scores in case patients. One study did not find L1/2 vBMD, analyzed as a continuous or categorical variable, to predict revision within the first year after SA-LLIF. | Early revision: diabetes [63], foraminal stenosis [65] Late revision: multilevel (>2) fusion [63] Single-level LLIF: age, BMI, PEEK cage, SA-fusion [27] |
Study populations consisted of patients undergoing primary or revision instrumented lumbar fusion (specific indications and procedures varied) for degenerative disease. All studies assessed osteoporosis, or surrogate measure of bone health, as a risk factor for specific surgical complications including cage subsidence, screw loosening, pseudarthrosis, adjacent-level fractures, junctional disease, and revision surgery.
Study acronyms are explained in the first footnote to Table 3. Additional abbreviations: RR, retrospective review; PC, prospective cohort; CC, case-control; BMI, body mass index; PA, proximal junctional angle; TLK, thoracolumbar kyphosis; SS, sacral slope; LL, lumbar lordosis; SVA, sagittal vertical axis; OR, odds ratio; UOR, unit odds ratio; PEEK, Polyetheretherketone.
Explanations:
Two studies provided complication rates in terms of number of patients rather than number of cages
One study did not find DXA T-scores or lumbar HU to predict CS and discussed possible confounding factors including: (1) relatively low incidence of complications, (2) few patients with osteoporosis or low bone mass, all of whom were preoperatively referred for endocrinology evaluation and undergoing treatment at the time of surgery if indicated, and (3) patients with deficient BMD may be more likely to undergo supplemental pedicle screw fixation.
One study calculated different cutoffs for female (153.5, AUC 0.88) and male (186.5, AUC 0.635) patients; another showed optimal HU thresholds varied based on number of levels fused (1-4) and the degree of postoperative residual deformity
One study showed that fusion took significantly longer in patients with osteoporosis (defined by HU<90) compared to low (HU between 90 and 120) and normal (HU>120) BMD; this study also demonstrated that fusion rates significantly varied based on the diagnostic criteria used
One study found lower trabecular number and higher trabecular separation in spinous process specimens of patients with nonunion; bone quality was also shown to correlate with patient-reported postoperative outcomes of pain and disability.
No study provided a clear description of radiographic criteria used for diagnosis (ex., % loss of vertebral body height)
Adjacent segment degeneration after 1 to 2 level TLIF or PLIF. Two of 3 studies utilized flexion-extension X-rays, one of which reported specific diagnostic criteria. The third study specified including all symptomatic cases requiring revision.
One study presented T-scores of patients with and without adjacent segment degeneration (−1.23±0.23 vs. −1.12±0.19; p=.08), notably with very narrow SD and relatively better bone quality in both groups. The other study found higher rates of osteoporosis or severe osteoporosis in patients with progression of adjacent segment degeneration (30.7% vs. 17.5%; p=.069). Neither result reached statistical significance.
For outcomes of proximal junctional kyphosis (PJK), the most commonly utilized definition was that initially proposed by Glattes et al, [50] a proximal junctional angle (PJA), sagittal Cobb between the inferior endplate of the UIV and superior endplate of UIV+2, that is both >10° and at least 10° greater than the preoperative measurement. Proximal junctional failure (PJF) definitions varied more significantly and commonly included cases of PJK with additional signs of mechanical failure (fracture, spondylolisthesis, fixation failure) or any symptomatic PJK requiring revision.
In 2 studies investigating both L3/4 and UIV/UIV+1 HU measurements, junctional values were the only independent risk factor.
Surgical indications for revision were variably reported and included diagnoses related to hematoma, infection, pain or neurologic deficit, and construct failure. Follow-up timing was inconsistently reported. One study did not give a minimum follow-up time, instead providing a mean cohort follow-up of 18.5±68.7 months and mean time to reoperation of 32.2±64.1 months in osteoporotic patients and 24.2±36.6 months in those without osteoporosis
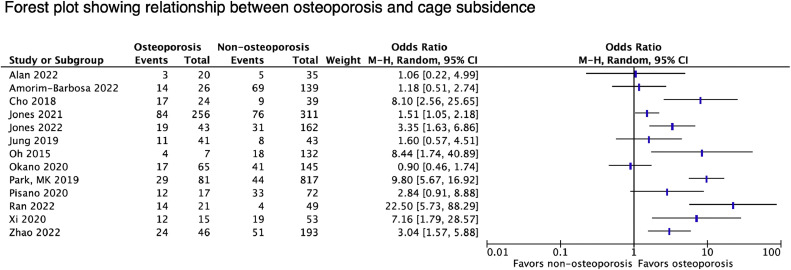
Cage subsidence
Cage subsidence was investigated by 24 studies in relation to BMD. Complication rates varied significantly (between 8.25% and 59% of levels), reflecting differences in surgical procedures and diagnostic criteria used (Table 6). In total, 21 studies found subsidence to be associated with poor bone health as assessed by DXA (6 studies), vBMD (4 studies), lumbar HU (10 studies), and VBQ scores (2 studies). A forest plot of all reporting relevant statistics is shown in Figure 3. Four [[26], [27], [28], [29]] of 5 studies with comparative data found HU to be more predictive of complications than traditional T-scores. Amorim-Barbosa et al. [26] showed that patients with HU<135 had a 6-fold increased risk of CS after 1-2 level PLIF or TLIF; while CS was not associated with worse functional outcomes, lower HU predicted worse disability scores and less return to work postoperatively. The remaining study, published by Pu et al. [30], found nondominant forearm T-scores to more accurately predict subsidence than mean lumbar HU (AUC 0.840 vs. 0.744), though both were independent risk factors. Notably, there were no significant differences in lumbar T-scores. Several studies investigated BMD of the fusion-level endplates as a potential predictor, reporting mixed results.
Table 6.
Details and results of studies reporting on cage subsidence.
| Study | Sample size, No. patients | Surgery type Levels treated (No.) | Supplemental fixation | Radiographic follow-up | Complication rates (No. segments) | Summary of results | Associated Clinical Outcomes |
|---|---|---|---|---|---|---|---|
| Alan et al. [31] | 55 (97 levels) | LLIF | 16 BPS, 39 SA * | 6 wk, 3, 6, and 12 months | 8.25% (8/97), severe (3) grade I (5), II (2), III (1) † |
|
N/A |
| Amorim-Barbosa et al. [26] | 165 (208 levels) | TLIF (122) or PLIF (43) L2/3 (3), L3/4 (20), L4/5 (74), L5/S1 (68) |
Yes | NR (minimum 6 months) |
50% (83/165*), 22% (36) severe |
|
|
| Cho et al. [34] | 86 | PLIF L3/4 (13), L4/5 (60), L5/S1 (13) |
Yes | Mean time to CS: Osteoporosis: 6.3±3.4 mo Normal BMD: 6.2±3.6 mo |
30% (26/86) >2mm |
|
|
| Guha et al. [27] | 89 (150 levels) | LLIF | 84 BPS, 66 SAa | NR (minimum 6 months) |
17.3% (26/89) grade: I (18), II (4), III (4) |
|
N/A |
| Hiyama et al. [120] | 59 | LLIF L2/3 (2), L3/4 (16), L4/5 (41) |
10 UPS, 49 BPS | Immediately postoperative (within 2 wk) and 1 year | 33.9% (20/59) grade I 55% (11), II 25% (5), III 20% (4) - 15.3% (9/59) early, 18.6% (11/59) delayed |
|
|
| Hu et al. [39] | 242 | TLIF L1/2 (1), L2/3 (1), L3/4 (9), L4/5 (175), L5/S1 (56) |
Yes | 2 wk, 3, 6, 12, and 24 mo | 45.87% (111/242) grade I (102), II (6), III (3) |
|
|
| Jones et al. [122] | 347 (567 levels) | LLIF L1/2 (34), L2/3 (111), L3/4 (186), L4/5 (236) |
239 BPS, 108 SA | Between 5 and 14 mo | 28.2% (160/567) grade I (124), II (24), III (12) |
|
|
| Jones et al. [123] | 89 (205 levels) | LLIF L1/2 (3), L2/3 (40), L3/4 (79), L4/5 (83) |
No | Between 5 and 14 mo | 56.6% (116/205), severe 24.4% (50) |
|
N/A |
| Jung et al. [32] | 84 | DLIF L1/2 (1), L2/3 (4), L3/4 (12), L4/5 (67) |
Yes | 1, 3, 6, 12, and 24 mo | 22.61% (19/84), >3mm 13.0% (11) |
|
|
| Kim et al. [33] | 104 (122 levels) | MI-TLIF L2/3 (2), L3/4 (8), L4/5 (72), L5/S1 (40) |
Yes | Mean time to CS 7.2±8.5 mo (1–25) | 32.8% (40/122): >2mm 14.8% (18), >4mm 6.6% (8) |
|
N/A |
| Kotheeranurak et al. [126] | 107 (137 levels) | OLIF L2/3 (26), L3/4 (43), L4/5 (68) |
Yes | Mean time to CS 3.7±2.2 mo | 41.6% (57/137) |
|
|
| Mi et al. [129] | 36 | TLIF L4/5 (36) |
UPS | NR (minimum 6 mo) |
Case (n=18), control (n=18) |
|
N/A |
| Oh et al. [131] | 102 (139 levels) | PLIF L2/3 (7), L3/4 (32), L4/5 (86), L5/S1 (14) |
Yes | 1 year | >1mm 59.0% (82/139); >3mm 15.8% (22) |
|
|
| Okano et al. [132] | 96 (210 levels) | LLIF L1/2 (11), L2/3 (53), L3/4 (74), L4/5 (72) |
198 SA, 12 lateral plate ‡ | Between 6 and 12 mo | Severe CS 27.6% (58/210) |
|
N/A |
| Park et al. [81] | 784 (881 levels) | TLIF L1/2 (8), L2/3 (25), L3/4 (181), L4/5 (560), L5/S1 (124) |
Yes | 1.5 y | CM 6.4% (56/881), CS 4.1% (36), CR 1.9% (17) § |
|
|
| Pisano et al. [134] | 89 | TLIF | Yes | NR (minimum 1 year) |
>2mm 50.6% (45/89 a) |
|
|
| Pu et al. [30] | 71 | PLIF L4/5 (71) |
Yes | NR | ≥2mm 23.9% (17/71) |
|
|
| Ran et al. [135] | 70 | OLIF L4/5 (70) |
Yes | 3 days, 3, 6, and 12 mo | >2mm 25.7% (18/70) |
|
|
| Rentenberger et al. [65] | 133 (258 levels) | LLIF T12/L1 (3), L1/2 (14), L2/3 (64), L3/4 (93), L4/5 (84) |
No | Mean time to CS 203 days (160-371) | Severe CS 26.7% (69/258) |
|
|
| Xi et al. [139] | 68 | LLIF L1/2 (2), L2/3 (9), L3/4 (26), L4/5 (31) |
Yes | 1 year | 41.1% (28/68): grade I (15), II (9), III (4) |
|
|
| Xie et al. [28] | 279 | TLIF L3/4 (8), L4/5 (161), L5/S1 (110) |
Yes | NR | >2mm 29.4% (82/279) |
|
|
| Yao et al. [143] | 93 (126 levels) | MI-TLIF L3/4 (19), L4/ 5 (80), L5/S1 (27) |
Yes | 6 wk, 3, 6, 12, and 24 mo | >2mm 34.1% (43/126), >3mm 15.9% (20) |
|
|
| Zhao et al. [144] | 242 | OLIF L4/5 (242) |
Yes | 1, 3, 6, and 12 mo; all cases identified within 3 mo | >2mm 32.6% (79/242), >4mm (31) |
|
|
| Zhou et al. [29] | 76 (84 levels) | OLIF L2/3 (4), L3/4 (24), L4/5 (56) |
No | NR (minimum 6 mo) |
21.2% (16/76 *), ≥2mm (7) |
|
|
Study acronyms are explained in the first footnote to Table 3. Abbreviations: AF, anterior fixation; BPS, bilateral pedicle screw; UPS, unilateral pedicle screw; OR, odds ratio; UOR, unit odds ratio; CI, 95% confidence interval; VAS, visual analog scale; ODI, Oswestry disability index; JOA, Japanese Orthopaedic Association score; AUC, area under curve; NRS, numerical rating scale.
Given as number of patients (rather than number of cages) only.
Unless otherwise indicated, complication rates defined as number of cages with any amount of subsidence, with severe CS referring to Grade II or Grade III CS as defined by Marchi et al.
Statistical analysis performed excluding levels with lateral plate fixation.
Cage migration (CM): horizontal migration >2mm, cage subsidence: diagonal or vertical migration >2mm, cage retropulsion (CR): any migration into the canal or foramen.
Regression analyses performed separately with BMD-f and BMD-L as independent predictors; OR for mean HU were 1.068 (CI 1.044–1.092, p<.01) and 1.076 (CI 1.054–1.098, p<.01) in these analyses, respectively. However, only 23.7% of study participants had preoperative femoral neck DXA data and remainder obtained postoperatively, though unclear what proportion of patients ultimately had DXA data available.
Fig. 3.
Forest plot showing relationship between osteoporosis and cage subsidence.
Three studies did not find a significant association between BMD and CS [[31], [32], [33]]. Among these, Alan et al. [31] observed no difference in T-scores (p=.78) or fusion-level HU (p=.26) between patients with and without subsidence. However, the authors noted their study was likely underpowered given a relatively low complication rate (8 of 97 cages) and prevalence of osteoporosis (3 of 55 patients). Furthermore, all patients with low BMD were referred for preoperative endocrinology consultation with initiation of anti-osteoporosis therapy if recommended. Another potential confounder discussed was how osteoporosis status may have altered the surgical plan in favor of using supplemental pedicle screw fixation due to a presumed higher risk of subsidence. In this study, all instances of CS occurred in standalone fusions.
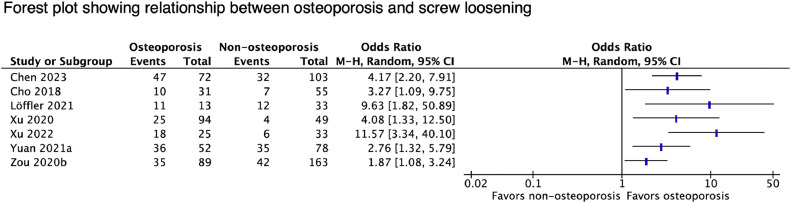
Screw loosening
All 14 studies reporting on screw loosening found an association between bone density and complications (Table 7). A forest plot of all contributing relevant statistics is shown in Figure 4. Three studies found higher SL rates in patients with DXA-diagnosed osteoporosis [[34], [35], [36]]. SL was also associated with HU (9 studies), vBMD (1 study), and VBQ scores (1 study). All 5 studies with comparative data found alternative metrics to be more predictive than DXA T-scores [[37], [38], [39], [40], [41]]. Zou et al. [41] demonstrated that among patients with non-osteoporotic T-scores, SL rates were significantly higher for those with HU ≤ 110 (44.4% vs. 18.6%, p<.001), suggesting that HU may be a more sensitive metric for predicting SL. Four studies measured HU along the screw trajectory, which was found to be the best predictor of loosening at those levels [39,40,42,43].
Table 7.
Details and results of studies reporting on screw loosening.
| Study | Surgical procedure Treated levels (No.) | Radiographic Follow-up | Complication Rates, (No. patients) | Summary of Results | Associated clinical outcomes |
|---|---|---|---|---|---|
| Bokov et al., [119] | Short-segment fusion±TLIF (162) or ALIF/D-LIF (50) 1 (153), 2 (70), 3 (21), 4 (5), 5 (1) |
6, 12, and 18 mo | 38.8% (97/250) |
|
|
| Chen et al. [37] | Short-segment fusion with PLIF 1 (97), 2 (57), 3 (12), 4 (8) |
Median 10 mo (8-18) | 29.88% (52/174), 9.18% (83/904) of screws |
|
|
| Cho et al., [34] | Single-level PLIF L3/4 (13), L4/5 (60), L5/S1 (13) |
Mean time to SL: 6.3±3.4 mo (osteoporosis) vs. 7.3±3.0 mo (normal BMD) | 19.76% (17/86) |
|
|
| Kim et al., [38] | Single or multilevel lumbosacral fusion | NR | 30.97% (35/113) |
|
N/A |
| Li et al., [42] | L4/5 OLIF | 1 year | 35.71% (40/112 levels) in 56 patients |
|
N/A |
| Löffler et al. [128] | Short-segment posterior fusion L1-5 (2), L2-5 (16), L2-S1 (8), L3-S1(18), L4-S1 (2) |
Case: 185 days (71-1359) Control: 229 days (8-2679) |
Case (n=23), control (n=23) |
|
N/A |
| Matsukawa et al., [39] | Single-level PLIF, instrumented using CBT | 1 year | 13% (12/92), 4.6% (16/351) of screws |
|
N/A |
| Sakai et al. [40] | Single-level PLIF L1/2 (1), L2/3 (2), L3/4 (8), L4/5 (30), L5/S1 (12) |
3 mo | 23% (12/52), 12% (24/206) of screws |
|
N/A |
| Shin et al. [137] | Short-segment fusion with PLIF 1 (300), 2 (140), 3 (36), 4 (2) |
1 year | 22.59% (108/478) |
|
N/A |
| Xu et al. [43] | L3-5 Posterolateral fusion | 1 year | L3 SL 20.3% (29/143) |
|
N/A |
| Xu et al. [35] | Long posterior fusion to sacrum Median 6 (range 3-12) |
NR | S1 SL 41.0% (32/78) |
|
|
| Yuan et al. [36] | Long posterior fusion SL 6.28±1.98, no SL 5.81±1.33 |
NR | 54.6% (71/130), 9.4% (168/1784) of screws |
|
|
| Zou et al. [145] | PLF to L5 or S1±PLIF (323) 1 (170), 2 (210), 3 (90), 4 (3) |
1 year | 30.0% (151/503) |
|
|
| Zou et al., [41] | Short-segment fusion to L5 or S1±PLIF (169) 1 (78), 2 (112), 3 (45), 4 (17) |
3, 6, and 12 mo | 30.6% (77/252); Most (96.1%, 172/179) at LIV or UIV |
|
|
Study acronyms are explained in the first footnote to Table 3. Abbreviations: OR, odds ratio; UOR, unit odds ratio; VAS, visual analog scale; ODI, Oswestry disability index; (SRS)-22 score, scoliosis research society; AUC, area under curve.
Fig. 4.
Forest plot showing relationship between osteoporosis and screw loosening.
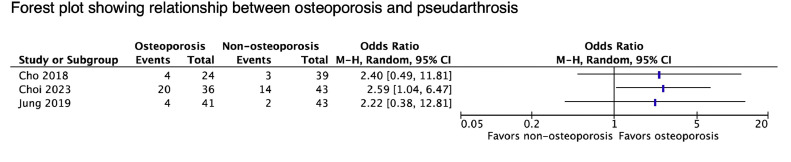
Pseudarthrosis
Seven studies evaluated fusion failure in relation to bone density. Criteria used to identify pseudarthrosis varied and commonly included: (1) dynamic motion at the fusion site, (2) absence of bridging trabecular bone, and 3) evidence of implant loosening (Table 8). Three studies found a significant relationship between BMD and fusion rates. A forest plot of all contributing relevant statistics is shown in Figure 5. Choi et al. [44] uniquely used 2 different CT-based radiographic criteria to investigate time to fusion after single-level TLIF and showed that osteoporosis (HU<90) was an independent predictor of slower fusion.
Table 8.
Details and results of studies reporting on pseudarthrosis.
| Study | Surgical procedure Levels treated (No.) | Method to assess fusion | Radiographic Follow-up | Complication Rates | Summary of Results | Associated Clinical Outcomes |
|---|---|---|---|---|---|---|
| Cho et al. [34] | Single-level PLIF L3/4 (13), L4/5 (60), L5/S1 (13) |
1) segmental angulation ≤2° on dynamic x-ray, 2) absence of bridging trabecular bone or peripheral cortication on CT | 1 year (XR and CT) and 2 y (XR) | 6.17% (5/86) |
|
|
| Choi et al. [44] | Single-level TLIF L3/4 (7), L4/5 (47), L5/S1 (25) |
Grade 0 (nonunion): lucency visible at one or both endplates on CT Grade 1 (fusion): absence of peri-graft radiolucency Grade 2 (fusion): trabecular bone bridging |
Annually to 5 y | * See footnotes |
|
N/A |
| ng , 2019 [32] | Single-level D-LIF L1/2 (1), L2/3 (4), L3/4 (12), L4/5 (67) |
1) segmental motion (<3° or 3mm) on dynamic x-ray, 2) intervertebral bridging bone on CT, and 3) no revision or evidence of implant loosening | 6 mo (XR and CT), re-evaluation at 12 and 24 mo if nonunion | 5.95% (5/84) |
|
|
| Lee et al., [127] | Long posterior fusion [T10-L1 to L5/S1] with ALIF (44) or PLIF (15) Mean 7.4±1.3 |
3D-CT to assess for presence of trabecular bridging | 3, 6, 9, 12, and 24 mo | L5/S1 38.98% (23/59) |
|
|
| Liu et al., [84] | Single-level PLIF L4/5 (63), L5/S1 (42) |
3D-CT to assess for presence of trabecular bridging | At last follow-up (minimum 2 y) | 12.38% (13/105) |
|
|
| Nguyen et al. [130] | L4-S1 Posterolateral fusion | Cases identified by intractable pain with either radiographic or intraoperative evidence of nonunion | 1 year | Case (n=10), control (n=10) |
|
N/A |
| Otsuki et al. [133] | L4/5 TLIF | 1) Segmental dynamic motion ≤3°, 2) visible gap between cage and endplate on CT, 3) no screw loosening | 1 year | 26% (19/85) |
|
|
Study acronyms are explained in the first footnote to Table 3. Abbreviations: OR, odds ratio; UOR, unit odds ratio; VAS, visual analog scale; ODI, Oswestry disability index; JOA, Japanese Orthopaedic Association score; AUC, area under curve; HR, hazard ratio.
At 2 years: percentage of patients demonstrating fusion with normal BMD, low BMD, and osteoporosis based on criteria of peri-graft lucency (77.1% vs. 57.2% vs. 44.6%, p=.029) and trabecular bridging (22.7% vs. 11.1%, vs. 4.0%, p=.037), respectively
Fig. 5.
Forest plot showing relationship between osteoporosis and pseudarthrosis.
Vertebral fracture
Six studies evaluated the relationship between osteoporosis and new vertebral fractures. Complications were identified using X-ray, CT, MRI, and/or bone scans, however, no study detailed explicit diagnostic criteria (Table 9). All 5 studies of proximal fractures found low BMD, defined by T-score [45,46] or junctional HU [[47], [48], [49]], to be an independent risk factor for new VCF. Yao et al. [49] showed not only that HU < 120 at the planned UIV strongly predicted bony PJF (OR 5.74, 95% CI 1.01-32.54, p=.04), but that there was a significant linear correlation between HU and PJK angles (r=-0.475). Due to lack of available raw data, it was not possible to create a forest plot for this outcome.
Table 9.
Details and results of studies reporting on new vertebral fractures.
| Study | Surgical procedure Levels treated (No.) |
Radiographic Follow-up | Complication rates | Fracture location level (no.) | Summary of results | Associated clinical outcomes |
|---|---|---|---|---|---|---|
| Ha et al. [45] | Long posterior fusion to L5 or S1 UIV fracture 6.2±0.4, UIV+1 fracture 5.8±1.6, no PJF 6.6±1.5 |
Mean time to fracture: UIV 1.5 mo (1-4.5), UIV+1 36 mo (11-88) |
7.0% (11/157) bony PJF | UIV (5), UIV+1 (6) |
|
|
| Kurra et al. [47] | Long posterior fusion to pelvis Mean 10.7 (5-17) |
NR | 11.95% (11/92) new VCF without PJK | UIV-1, UIV, or UIV+1 |
|
N/A |
| Luo et al. [46] | Short-segment fusion with PLIF | 1, 3, 6, and 12 mo, annually thereafter | 3.86% (27/669) adjacent VCF | T12 (8), T12 + L1 (1), L1 (8), L2 (5), L3 (1), L4 (2), L5 (2) |
|
N/A |
| Meredith et al. [48] | Long posterior or AP fusion Posterior (range 2-16 levels): mean fracture 6.6, control 7.3 Anterior (range 0-7 levels): mean fracture 3.1, control 3 |
Mean time to fracture 14.2 wk (2.3–45.1) | Adjacent VCF (n=20), control (n=20) | All proximal, no distal |
|
N/A |
| Salzmann et al. [136] | Long posterior fusion to S1 Fracture 5.6±3.0, control 5.1±2.4 |
Mean time to fracture 87 days; 76% within 3 mo | Sacral VCF (n=21), control (n=42) | All sacral |
|
|
| Yao et al. [49] | Long posterior fusion Bony PJK 9.7±4.3, no PJK 10.75±3.9 |
6 wk, 6 mo, 1 year | 11.11% (7/63) bony PJK |
|
N/A |
Study acronyms are explained in the first footnote to Table 3. Abbreviations: OR, odds ratio; HR, hazard ratio; PJA, proximal junctional angle.
Adjacent segment disease
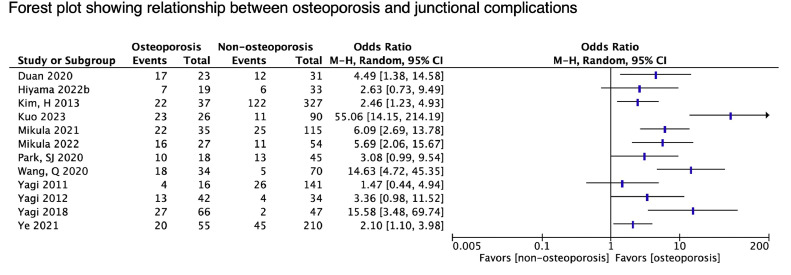
23 studies reported on adjacent-segment complications (Table 10). A forest plot of all providing relevant data is shown in Figure 6. Most (20 studies) discussed outcomes of PJK [50] or PJF after long-segment deformity correction. Index procedures varied in terms of primary versus revision, number of fused levels, location of end-instrumented vertebrae, osteotomy, interbody fusion, and fusion to the pelvis. Studies inconsistently commented on the use of cement augmentation, proximal hooks, or other modifications to improve fixation. All studies of junctional kyphosis found low BMD to be a risk factor. Yuan et al. [51] showed that osteoporotic patients had a 14-fold increased risk of PJK (p=.028); at final follow-up, those with PJK had significantly worse back pain and disability scores. Park et al. [52] found a combination of 3 factors to highly predict PJF after multilevel fusion to the sacrum: age ≥70, osteoporosis, and PJA ≥0°. PJF developed in 55.6%, 73.3%, and 100% of patients with 1, 2, and all 3 characteristics, compared to none of patients without any risk factors. Of 11 studies using HU, 10 showed that values from junctional levels best predicted complications [45,47,49,[52], [53], [54], [55], [56], [57], [58]] Kuo et al.[59] performed opportunistic screening using MRI. They observed a strong linear correlation between postoperative change in PJK angles and VBQ scores (r=0.786), which was the only independent risk factor for PJK.
Table 10.
Details and results of studies reporting on junctional complications.
| Study | Surgical procedure Levels treated (No.) |
Complications considered | Radiographic follow-up | Complication rates | Summary of results | Associated Clinical Outcomes |
|---|---|---|---|---|---|---|
| Barton et al. [118] | Posterior or A-P (36) fusion + osteotomy Median posterior 8 (2-17), anterior 2 (1-6) |
PJF: fracture or spondylolisthesis of UIV or UIV+1 | Between 24 and 60 mo | 11.7% (11/94) |
|
|
| Chen et al., 2011 [60] | L4/5 PLIF | Progression of L3/4 degeneration: 1) disc height >3mm; 2) dynamic angulation >5°; 3) L3 slippage >3mm | Between 24 and 52 mo (at final follow-up) | 22.01% (24/109) |
|
|
| Duan et al. [53] | Long posterior fusion from T9-12 to sacrum | PJK* | < 1 month and at final follow-up | 53.7% (29/54) |
|
N/A |
| Ha et al. [45] | Long posterior fusion to L5 or S1 PJF 6.1±1.1 no PJF, 6.6±1.5 |
Acute PJF b | Mean time to PJF 23.4±29.9 mo, median 8 mo (1–88) | 11.5% (18/157) |
|
|
| Hiyama et al. [54] | Staged: 1) 2-4 level LLIF, 2) long posterior fusion with L5/S1 TLIF Mean 9.7±2.5 |
PJF: any symptomatic PJK requiring revision | 1 year; mean time to revision 18.4±13.9 mo | 25% (13/52) |
|
N/A |
| Hyun et al. [121] | Long posterior or AP (20) fusion with T9-L2 UIV PJK 5.6±1.4, no PJK 5.6±1.3 |
PJK | NR | 38.6% (17/44) |
|
|
| Kim et al. [33] | Long posterior or AP (218) fusion | PJK: PJA > 10° | 1-2 mo, 2 y, and at final follow-up | 39.5% (144/364) |
|
|
| Kim et al. [125] | Long posterior or AP (32) fusion from T10-L2 to L5 or S1 | PJK: angle change of >10° on dynamic x-rays | NR | 32.65% (16/49) |
|
N/A |
| Kuo et al. [59] | Thoracolumbar fusion | PJK and PJF requiring revision | NR | 29.3% (34/116): PJK 24.1% (28), PJF 8.6% (10) |
|
NA |
| Kurra et al. [47] | Long fusion to pelvis Mean 10.7 (5-17) |
PJK | NR | 35.8% (33/92): PJK 23.9% (22), VCF excluding PJK (11) |
|
N/A |
| Mikula et al. [55] | Long instrumented fusion from T10-L2 to pelvis | PJF: PJK requiring revision | Mean time to PJK 22±18 mo and PJF 19±18 mo | PJK/PJF 31.33% (47/150) |
|
N/A |
| Mikula et al. [56] | Long instrumented fusion from T1-T6 to pelvis | PJF: PJK requiring revision | Mean time to PJK 22 mo, PJF 14 mo | PJK/PJF 33% (27/81): PJK 26% (21), PJF 19% (15) |
|
N/A |
| Park et al., [52] | Long posterior (24) or AP (39) fusion from T11-L1 to sacrum | PJF: PJA >20°, UIV or UIV+1 fracture, UIV fixation failure, myelopathy, or need for proximal extension | Mean time to PJF 9.3±14.1 mo (1.2–55) | 36.5% (23/63) |
|
|
| Wang H et al., 2016 [57] | Long posterior fusion from T9-L3 to L4-S1 | PJK or spontaneous adjacent VCF | NR | 17.3% (17/98) |
|
N/A |
| Wang et al. [61] | TLIF (98) or PLIF (139) 1 (176), 2 (59) |
Symptomatic adjacent segment degeneration | NR | 6.3% (15/237) |
|
N/A |
| Wang et al. [138] | Long instrumented fusion Median levels: PJF 5 (4-8), control 7 (4-12) |
PJF: UIV or UIV+1 fracture, screw loosening or pullout at UIV | Median time to PJF 10 mo (2-45); 86.95% occurred within 2 y | 22.1% (23/104) |
|
N/A |
| Yagi et al., [140] | Anterior (14), posterior (82) or AP (61) fusion Mean 10.7 (6-15) |
PJK | Final follow-up (mean 4.3 y); 75% occurred within 2 y |
20% (32/157) |
|
|
| Yagi et al. [141] | Anterior (4), posterior (35), or AP (37) fusion PJK 10.8±3.9, no PJK 11.2±3.6 |
PJK | 2-3 mo, 2 and 5 y, and at final follow-up; 76% occurred within 3 mo, none after 5 y | 22.4% (17/76) |
|
|
| Yagi et al., 142] | Long thoracolumbar fusion S-group 10.2±2.3, M-group 9.8±2.4 |
PJF: PJA increase ≥20° with deterioration of 1+ SRS-Schwab sagittal modifier grade, or any PJK requiring revision | Within 2 y | 25% (29/113) PJK, 19% (22) PJF |
|
|
| Yao et al. [49] | Long posterior fusion Bony PJK 9.7±4.3, non-bony PJK 11.9±4.2, no PJK 10.75±3.9 |
PJK | 6 wk, 6 mo, 1 year; 65% and 87% occurred within 6 wk and 6 mo, respectively | 36.5% (23/63) |
|
|
| Ye et al. [62] | TLIF 1 (988), 2 (270) |
Symptomatic adjacent level disease requiring revision | Mean time to presentation 68.3±25.1 mo (20–123) | 6.5% (65/1258) |
|
|
| Yuan et al. [51] | Long posterior fusion with T9-L2 UIV PJK 6.47±2.10, No PJK 5.87±1.27 |
PJK | Within 6 wk and at final follow-up | 20.24% (17/84) |
|
|
| Zhang et al. [58] | Posterior thoracolumbar fusion PJK 4.3±1.7, No PJK 3.8 ± 1.3 |
PJK | 1, 3, 6, 12, 24, and 36 mo | 32.4% (108/333) |
|
N/A |
Study acronyms are explained in the first footnote to Table 3. Abbreviations: OR, odds ratio; UOR, unit odds ratio; VAS, visual analog scale; ODI, Oswestry disability index; SRS-22 score, Scoliosis Research Society; JOA, Japanese Orthopaedic Association score; AUC, area under curve; AP, anterior-posterior combined approach.
PJK defined as proximal junctional angle (PJA), measured as the sagittal Cobb between the inferior endplate of the UIV and superior endplate of UIV+2, that is both >10° and at least 10° greater than the preoperative measurement [50].
Fig. 6.
Forest plot showing relationship between osteoporosis and junctional complications.
Three studies reported on adjacent segment degeneration after 1 to 2 level LIF [[60], [61], [62]]. Ye et al. [62] observed a higher incidence of osteoporosis among symptomatic patients requiring revision (30.7% vs. 17.5%; p=.069). The other 2 studies compared T-scores between those with and without complications. While Chen et al. [60] found affected patients had slightly lower T-scores (−1.23±0.23 vs. −1.12±0.19; p=.08), Wang et al. [61] reported no differences (-1±0.2 vs. -1.2±-0.3, p=.413). Notably, these studies were comprised of younger patients (mean ages 53.4 and 53.2, respectively) with relatively narrow BMD ranges.
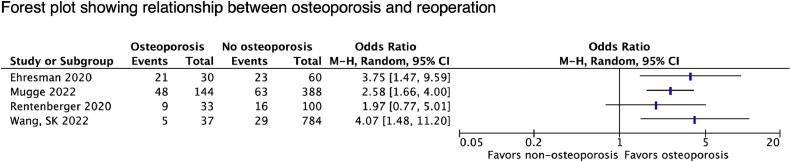
Reoperation
Five studies evaluated bone health as a predictor of reoperation, the timing and indications for which varied (Table 11). A forest plot of all contributing relevant data is shown in Figure 7. Wang et al. [63] showed osteoporotic patients had a 3.6-fold increased risk of reoperation within 3 months, most commonly for surgical site infection (32.3%), hematoma (23.5%), or hardware failure (20.6%). Mugge et al. [64] also observed higher revision rates with osteoporosis (33.3% vs. 16.2%; OR 2.93, 95% CI 1.68–5.12, p<.001), particularly for implant failures (OR 2.21, 95% CI 1.12–3.18, p=.022). Guha et al. [27] found fusion-level HU to independently predict reoperation after single or multilevel LLIF. Rentenberger et al. [65] also observed a trend towards lower vBMD in patients who required revision after SA-LLIF. In a case-control study, Ehresman et al. [66] found significantly higher VBQ scores among patients undergoing revision for symptomatic pseudarthrosis or instrumentation failure (3.29±0.68 vs. 2.92±0.46, p=.01).
Table 11.
Details and results of studies reporting on reoperation
| Study | Index surgical procedure Levels treated (No.) |
Complications considered | Timing of reoperation | Reported rates of revision surgery | Summary of results | Associated clinical outcomes |
|---|---|---|---|---|---|---|
| Ehresman et al. [66] | Multilevel lumbar fusion Case 3.6±1.1, control 3.3±0.9 (p=.106) |
Clinical or radiographic adjacent level disease or symptomatic hardware failure | Mean time to revision 3.3±2.6 y | Case (n=30), control (n=60) |
|
N/A |
| Guha et al. [27] | Instrumented (84) or SA-LLIF (66) 1 (54), 2+ (35) |
Revisions within 1 level of index surgery and not strictly for debridement*[] | NR | 22.4% (20/89) in 28/150 levels |
|
N/A |
| Mugge et al., [64] | Long thoracolumbar fusion Osteoporosis 6.7±3.6, no Osteoporosis 6.1±3.5 |
Infection, neurological deficit, disease progression, construct failure (radiographic implant loosening, displacement, or fracture) | Mean time to revision 32.2±64.1 mo (osteoporosis) vs. 24.2±36.6 mo (no osteoporosis) | 20.9% (111/532) |
|
N/A |
| Rentenberger et al. [65] | SA-LLIF 1 (33), 2 (55), 3 (39), 4 (5), 5(1) |
Involving index and/or adjacent level: pain or neurologic deficit (68%), radiographic adjacent segment disease (16%), pseudarthrosis (16%), hardware failure (8%) | Within 1 year | 18.79% (25/133), including 21 revised and 4 recommended |
|
- Revision surgery not predicted by BMD or CS |
| Wang SK et al. [63] | Short-segment fusion with TLIF 1-2 (607), 3-5 (214) |
Early: infection (32.3%), hematoma (23.5%), implant failure (20.6%), pain (11.7%), adjacent segment disease (8.8%), CSF leak (3%) Late: adjacent segment disease (38.9%), implant failure (36.1%), infection (16.7%), pain (8.3%) |
4.1% at 3 mo, 6.2% at 1 year, 8.2% at 3 y | Early (<3 mo): 4.1% (34/821) Late (>3 mo): 4.3% (36/821) |
|
- Worse VAS back pain at final follow-up in those who underwent revision (p=.01) |
Study acronyms are explained in the first footnote to Table 3. Additional abbreviations: odds ratio (OR), unit odds ratio (UOR), visual analog scale (VAS), cerebrospinal fluid (CSF)
Indications for revision noted to be not completely recorded, but included diagnoses related to CS, pseudarthrosis, and adjacent segment disease
Fig. 7.
Forest plot showing relationship between osteoporosis and reoperation.
Discussion
There has been a dramatic rise in the number of elective lumbar fusions performed over the past few decades, with the most significant increases occurring in patients over 65 [67]. As these procedures are associated with relatively high complication rates, particularly in elderly patients [68,69], surgeons must be aware of modifiable risk factors to allow for identification of those who may benefit from medical or surgical optimization. Biomechanically, osteoporotic bone offers poor support for instrumentation, which may predispose to failures at the implant-bone interface. In the present study, we reviewed the literature on osteoporosis as a risk factor for different mechanical complications of lumbar fusion.
An increasing number of studies have reported osteoporosis as a risk factor for cage subsidence, a finding supported by our review and others [70,71]. In osteoporotic patients, compromised vertebral strength, decreased endplate failure loads, and increased stress concentration within the surgical segment all contribute to failure at the cage-endplate interface. Surgical variables like implant design (size, shape, material properties), cage positioning on the endplate, and use of supplemental fixation are other important predictors of endplate stress and subsidence [72,73]. Selecting implants with greater endplate contact, positioning over stronger regions of the endplate, and using supplemental fixation can all help prevent subsidence [74].
Screw loosening is another common complication of lumbar fusion often associated with poor bone stock [75]. Concerns for adequate fixation in osteoporosis has prompted investigation of a number of technique modifications including cement augmentation of high-risk levels to enhance screw purchase and prevent complications [76,77]. Use of alternative screw trajectories that take advantage of stronger regions of the vertebra is also an option. BMD has a well-established association with regional vertebral strength and pedicle screw stability in vitro [78]. More specifically, BMD measurements made along a screw's trajectory can provide a particularly accurate prediction of mechanical performance and are commonly used in biomechanical investigations of fixation strength and stability [79,80]. Similar metrics are increasingly being investigated for predicting complications clinically.
Pseudarthrosis is a common consequence of implant malfunction and may be more likely in patients with osteoporosis. Among studies in this review, pseudarthrosis was most frequently reported as a secondary outcome in relation to cage subsidence or screw loosening. Park et al. [81] reported coexistence of all 3 complications: pseudarthrosis occurred in 2.9% of cages without migration compared to 45%, 58.3%, and 82.4% with migration, subsidence, and retropulsion, respectively; concomitant SL rates were 4.7%, 10%, 61.1%, and 70.6%, respectively. Unfortunately, we found relatively limited and inconclusive data regarding a direct association between bone density and fusion. One study observed that fusion took significantly longer in osteoporotic patients [44], which is consistent with findings reported by meta-analyses of randomized controlled trial data showing osteoporosis treatment improves fusion rates after lumbar instrumentation [82,83]. Liu et al. [84] published the only study in our review using micro-CT. Ex-vivo analysis of spinous process specimens obtained during index surgery revealed higher trabecular number and lower trabecular separation with greater bone surface/total volume (BS/TV) among patients ultimately achieving solid fusion. Low BS/TV was the only independent predictor of pseudarthrosis and was strongly associated with worse low back pain and disability outcomes. Although micro-CT cannot be used for preoperative risk stratification, these results can help strengthen the evidence associating bony structural deficiencies with complications.
Thoracolumbar fragility fractures are a hallmark complication of osteoporosis, usually occurring after a fall or low-energy trauma [85]. Patients who undergo long-segment fusions may be particularly susceptible to new junctional fractures under the increased stress of instrumentation. These classically occur as either (1) simple, usually chronic, compression of the first uninstrumented vertebra (UIV+1), or (2) acute UIV collapse, followed by ligamentous failure and adjacent vertebral subluxation [45,86]. The latter case is thought to be directly precipitated by significant mechanical stress from substantial alignment correction and tends to result in a more severe kyphotic deformity associated with higher rates of neurologic deficits, PJF, and revision surgery. Our review confirms osteoporosis to be a significant risk factor for PJK and PJF, particularly secondary to fracture [45,49]. In addition to considering alternative alignment targets for correction in osteoporotic patients, choosing an appropriate UIV can help minimize junctional stresses. For patients still thought to be high-risk for bony failures, prophylactic augmentation of the UIV and UIV+1 may be performed during the index procedure. There is also an abundance of evidence that perioperative anti-osteoporosis therapy can help prevent PJK. [87] Yagi et al. [88] showed that 6 months of teriparatide following thoracolumbar fusion significantly increased BMD at the UIV+1 and was associated with lower rates of bony PJK at 2 years compared to untreated controls (4.6% vs. 15.2%; p=.02).
In terms of functional outcomes, mechanical complications in this review ranged from asymptomatic radiographic findings to disabling events requiring additional surgery. In addition to construct revision for mechanical failures, several studies also found osteoporotic patients were more likely to undergo unplanned reoperations for other indications as well [64,63]. Quality of life metrics were inconsistently evaluated and represent an area of future research needed.
Implications for practice
Collectively, our findings support the utility of osteoporosis screening prior to elective lumbar fusion. The International Society for Clinical Densitometry (ISCD) recommends preoperative bone health assessment for all females aged ≥65 years and males ≥70 years, as well as those with prior fragility fracture or other risk factors for osteoporosis [89]. However, relatively high rates of poor bone quality have been reported in younger surgical spine patients [[90], [91], [92]]. Williamson et al. [93] further demonstrated the clinical significance of this, showing that in patients under 65 with minimal deformity, osteoporosis was the most significant risk factor for major mechanical (33% vs. 7% without osteoporosis, p=.025; OR 5.9, p=.048) and major radiological (29% vs. 6%, p=.001; OR 7, p=.003) complications, trends not observed in their overall cohort. Moreover, our review suggests that patients with poor bony strength despite a non-osteoporotic BMD may also be at increased risk for mechanical complications. Thus, it may be necessary to consider alternative screening recommendations in adults presenting for elective spinal fusion [91,94,95].
Additionally, there are no current guidelines for how BMD values should be used to inform treatment in elective lumbar fusions. In particular, there has been growing interest in using site-specific HU obtained in opportunistic screening to guide risk assessment, for example using values from the UIV and adjacent levels to predict PJK. [96] However, although individual surgeons may currently employ these techniques to inform their own decision-making processes, methodologies have not been standardized and optimal thresholds for predicting complications remain unknown. Ultimately, clinical care could benefit from standardized evidence-based recommendations that reference specific BMD cutoff values and related implications for treatment.
Limitations and future directions
The findings of this review should be interpreted in the context of its limitations. Current literature on osteoporosis and surgical outcomes is largely retrospective, which introduces a number of concerns for bias [97] Unfortunately, due to insufficient practices of osteoporosis screening, there is a relative lack of available DXA data for analysis [98,99]. As a result, many retrospective cohort studies will use International Classification of Diseases (ICD) codes to obtain information about osteoporosis status from medical records or other healthcare databases. However, it has been well-established that even among patients with documented fragility fractures, osteoporosis is profoundly underdiagnosed in both electronic medical records [[100], [101], [102]] and administrative databases [103,104]. Evaluations of osteoporosis reporting patterns in claims data have revealed the magnitude of these deficiencies, leading to recommendations against using this data in place of BMD-based reference standards [103]. In light of these limitations, the authors therefore felt it necessary to employ a more restrictive selection criteria with respect to study methodologies, focusing on comparative evaluations that explicitly investigated BMD as a predictor of mechanical complications. This excluded studies using age as a proxy for poor bone health, including those performed in elderly populations where many were likely osteoporotic but lacked attention to this diagnosis [105,106]. We also eliminated all studies in which osteoporosis status was assigned solely based on ICD code, as the absence of an osteoporosis diagnosis alone cannot be considered a reliable indicator of good bone health. Nevertheless, even among included studies, many still had potential for selection bias due to the use of imaging-based patient selection criteria, which did lower the strength of this evidence. Consideration of these limitations highlights the need for greater attention to the screening, diagnosis, and documentation of conditions like osteoporosis.
Another limitation of included studies is the potential that patients’ presumed osteoporosis status influenced the treatment they received, which could have dramatically reduced the apparent impact of osteoporosis on patient outcomes. Unfortunately, confounding variables related to the initiation, type, and duration of pharmacologic therapy as well as the use of surgical technique modifications were infrequently addressed.
Finally, studies varied considerably in patient selection criteria, surgical indications and procedures, imaging modality and anatomical site(s) of BMD assessment, and the diagnostic study, criteria, and timing used to define clinical endpoints. Together, these factors likely resulted in significant differences in both the number of complications identified and proportion attributed to osteoporosis. Use of variable and non-standardized thresholds for statistical analyses further limited any ability for direct comparison and precluded meta-analyses. Recognition of this heterogeneity is critical as it reflects the lack of consensus among surgeons and researchers regarding how to best screen for spinal osteoporosis and predict related surgical complications, concepts that are not necessarily synonymous.
The current gold-standard for assessing bony strength, skeletal fragility, and fracture risk relies on BMD, measured at the spine or hip, using DXA [25]. The anatomical site of BMD measurement is also important, as T-scores obtained from different locations are not necessarily interchangeable [107]. In a population defined by the coexistence of spinal osteoporosis and surgical degenerative pathology, measurement of BMD in the lumbar spine would theoretically be ideal for predicting focal osteoporosis-related mechanical failures [91]. However, these degenerative changes also can falsely elevate lumbar T-scores, making them paradoxically less accurate for predicting regional bony strength [108,109].
Given these shortcomings, there has been recent interest in opportunistic screening using CT or MRI, which may improve access to clinically relevant information on bone health for surgical spine patients [91,110] CT-based methods have been shown to generate reliable measures of volumetric BMD that correlate well with vertebral biomechanical properties, fracture risk, and outcomes of lumbar fusion [[111], [112], [113]]. A notable advantage of CT lies in the ability to obtain measurements from customizable regions of interest, usually located in trabecular bone and excluding osteophytes or degenerated facet joints, making them less susceptible to error from degenerative changes [114]. These measurements can also be isolated to surgically-relevant areas like pedicles, endplates, and vertebral bodies of planned instrumented levels, which may allow for personalized risk stratification and surgical decision-making. Less extensively investigated, MRI-determined VBQ scores have also shown promise for identifying osteoporosis and predicting postoperative complications [37,66,115]. As overall bony strength is determined by both bone density and quality, these investigations may provide important supplemental information to inform risk assessment [116].
Large-scale prospective evaluations will be necessary to evaluate the utility of these different methodologies for bone health assessment and determine which imaging study and threshold value(s) best predict mechanical complications and patient outcomes. In the meantime, it is important to consider the mounting evidence that osteoporosis is a significant risk factor for complications after lumbar fusion, and the crucial role that preoperative bone health assessment can play in mitigating these risks. Expanding access to tools like DXA, which has been widely validated and remains the current gold-standard for diagnosing osteoporosis and initiating pharmacologic therapy, will be important for identifying and treating at-risk patients [25,107,117].
Conclusion
This systematic review provides a comprehensive summary of osteoporosis and mechanical complications of lumbar fusion. Our results demonstrate that poor bone health is an important risk factor for implant-related failure, pseudarthrosis, VCF, junctional deformity, and reoperation after elective lumbar fusion. These findings strongly support the role of preoperative screening to identify high-risk patients and allow implementation of low-risk management strategies. Our review also highlights current challenges in the evaluation and management of osteoporotic patients undergoing lumbar fusion, including a paucity of relevant and complete clinical data, variability in methods of bone health assessment and reporting of complications, and the use of heterogeneous definitions that limit the interpretation, generalizability, and meta-analysis of available evidence. As we move towards addressing these gaps, it is important to consider the mounting evidence that osteoporotic patients with degenerative spinal disease may represent a unique population in which bone health is of utmost importance, but current practices of identifying high-risk patients are inadequate. The authors therefore suggest a collaborative, multidisciplinary Academic Consortium specifically dedicated to addressing the unique challenges of treating spinal disease in patients with osteoporosis. The goals of such a consortium would begin with development of consensus criteria for best practices of bone health assessment and uniform definitions for clinical endpoint evaluation. Establishment of standardized metrics will facilitate the consistency of data collection and minimize ambiguity in a way that enables direct comparison and meta-analysis, which will be essential for adequately investigating these relationships. Ongoing integration of evolving evidence will be necessary to identify unmet needs, advance targeted research, and guide clinical decision-making towards evidence-based practice, ultimately leading to better patient outcomes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge an NSF Industry/University Cooperative Research Program called the Center for Disruptive Musculoskeletal Innovations (IIP-1916629) for support of this work. This work was also substantially supported by the UCSF Skeletal Health Service and Department of Orthopaedic Surgery.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: AF: Nothing to disclose. AB: Nothing to disclose. ARBN: Nothing to disclose. KH: Nothing to disclose. AA: Nothing to disclose. AX: Nothing to disclose. DH: Nothing to disclose. CC: Nothing to disclose. IT: Nothing to disclose. JR: Nothing to disclose. TL: Grants: NIH / NIAMS (H). SB: Grants: Center for Disruptive Musculoskeletal Innovation (D); Royalties: Stryker (D); Stock Ownership: Novapproach (1%), Green Sun Medical (1%); Consulting: Medtronic (D), Globus (C), Kuros (B), SI Bone (C); Scientific Advisory Board/Other Office: Medtronic (none). AS: Grants: CDMI/NSF (D); Royalties: Elsevier: Book copy (not yet received); Stock Ownership: Advisor to Ouva (1.5% company interest); Scientific Advisory Board/Other Office: Scripps Research Institute (B); Research Support (Investigator Salary); MCRI (F); Grants: TRISH/Baylor (9/30/20 - 0/30/21) (F).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2024.100327.
Appendix. Supplementary materials
References
- 1.Cummings SR, Iii LJM. Epidemiology and outcomes of osteoporotic fractures. THE LANCET. 2002;359:7. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 2.Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105–117. doi: 10.1093/bmb/ldaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan ZQ, Yan XA, Li BF, et al. Prevalence of osteoporosis in spinal surgery patients older than 50 years: a systematic review and meta-analysis. PloS One. 2023;18(5) doi: 10.1371/journal.pone.0286110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varshneya K, Bhattacharjya A, Jokhai RT, et al. The impact of osteoporosis on adult deformity surgery outcomes in Medicare patients. Eur Spine J. 2022;31(1):88–94. doi: 10.1007/s00586-021-06985-z. [DOI] [PubMed] [Google Scholar]
- 5.Sharma M, John K, Dietz N, et al. Factors impacting outcomes and health care utilization in osteoporotic patients undergoing lumbar spine fusions: a MarketScan database analysis. World Neurosurg. 2020;141:e976–e988. doi: 10.1016/j.wneu.2020.06.107. [DOI] [PubMed] [Google Scholar]
- 6.Lee CK, Choi SK, An SB, et al. Influence of osteoporosis following spine surgery on reoperation, readmission, and economic costs: an 8-year nationwide population-based study in Korea. World Neurosurg. 2021;149:e360–e368. doi: 10.1016/j.wneu.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 7.St. Jeor JD, Jackson TJ, Xiong AE, et al. Average lumbar hounsfield units predicts osteoporosis-related complications following lumbar spine fusion. Glob Spine J. 2022;12(5):851–857. doi: 10.1177/2192568220975365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjerke BT, Zarrabian M, Aleem IS, et al. Incidence of osteoporosis-related complications following posterior lumbar fusion. Glob Spine J. 2018;8(6):563–569. doi: 10.1177/2192568217743727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo CC, Soliman MAR, Aguirre AO, et al. Risk factors of early complications after thoracic and lumbar spinal deformity surgery: a systematic review and meta-analysis. Eur Spine J. 2023;32(3):899–913. doi: 10.1007/s00586-022-07486-3. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Cha T, Schwab J, et al. Osteoporosis increases the likelihood of revision surgery following a long spinal fusion for adult spinal deformity. Spine J. 2021;21(1):134–140. doi: 10.1016/j.spinee.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Formby PM, Kang DG, Helgeson MD, Wagner SC. Clinical and radiographic outcomes of transforaminal lumbar interbody fusion in patients with osteoporosis. Glob Spine J. 2016;6(7):660–664. doi: 10.1055/s-0036-1578804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain N, Labaran L, Phillips FM, et al. Prevalence of osteoporosis treatment and its effect on post-operative complications, revision surgery and costs after multi-level spinal fusion. Glob Spine J. 2022;12(6):1119–1124. doi: 10.1177/2192568220976560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernatz JT, Winzenried AE, Hare KJ, et al. Effect of bone health optimization on osteoporosis screening and treatment before thoracolumbar fusion. JAAOS Glob Res Rev. 2022;6(3):e21. doi: 10.5435/JAAOSGlobal-D-21-00253. 00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dipaola CP, Bs JEB, Biswas D, Dipaola M, Grauer JN, Rechtine GR. Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. Spine J. 2009;8 doi: 10.1016/j.spinee.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Díaz-Romero R, Henríquez MS, Melián KA, Balhen-Martin C. Practice patterns of spine surgeons regarding osteoporosis: an International survey. Int J Spine Surg. 2021;15(2):376–385. doi: 10.14444/8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantoja S, Molina M. Surgeon management of osteoporosis in instrumented spine surgery: AOSpine Latin America Survey. Glob Spine J. 2019;9(2):169–172. doi: 10.1177/2192568218785369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezae F, Kelly A, Dey S, Moles R, Carter S. Healthcare professionals’ perspectives and experiences of osteoporosis medication treatment: a qualitative systematic review. Arch Osteoporos. 2024;19(1):8. doi: 10.1007/s11657-023-01359-y. [DOI] [PubMed] [Google Scholar]
- 18.Choksi P, Gay BL, Haymart MR, Papaleontiou M. Physician-reported barriers to osteoporosis screening: a nationwide survey. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2023;29(8):606–611. doi: 10.1016/j.eprac.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoggard TM, Jeray KJ. Osteoporosis management in the United States. OTA Int. 2022;5(3 Suppl):e184. doi: 10.1097/OI9.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik AT, Retchin S, Phillips FM, et al. Declining trend in osteoporosis management and screening following vertebral compression fractures – a national analysis of commercial insurance and medicare advantage beneficiaries. Spine J. 2020;20(4):538–546. doi: 10.1016/j.spinee.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Chapter 25: assessing risk of bias in a non-randomized study. Accessed December 28, 2022. https://training.cochrane.org/handbook/current/chapter-25.
- 22.West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Agency for Healthcare Research and Quality (US) 2002 [PMC free article] [PubMed] [Google Scholar]
- 23.Norvell DC, Dettori JR, Skelly AC, Riew KD, Chapman JR, Anderson PA. Methodology for the systematic reviews on an adjacent segment pathology. Spine. 2012;37:S10. doi: 10.1097/BRS.0b013e31826cd9c8. [DOI] [PubMed] [Google Scholar]
- 24.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 25.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 26.Amorim-Barbosa T, Pereira C, Catelas D, et al. Risk factors for cage subsidence and clinical outcomes after transforaminal and posterior lumbar interbody fusion. Eur J Orthop Surg Traumatol Orthop Traumatol. 2022;32(7):1291–1299. doi: 10.1007/s00590-021-03103-z. [DOI] [PubMed] [Google Scholar]
- 27.Guha D, Mushlin HM, Muthiah N, et al. Computed 1ar Interbody Fusion. World Neurosurg. 2022;161:e417–e426. doi: 10.1016/j.wneu.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Xie F, Yang Z, Tu Z, et al. The value of Hounsfield units in predicting cage subsidence after transforaminal lumbar interbody fusion. BMC Musculoskelet Disord. 2022;23:882. doi: 10.1186/s12891-022-05836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Yuan C, Liu C, Zhou L, Wang J. Hounsfield unit value on CT as a predictor of cage subsidence following stand-alone oblique lumbar interbody fusion for the treatment of degenerative lumbar diseases. BMC Musculoskelet Disord. 2021;22(1):960. doi: 10.1186/s12891-021-04833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pu H yu, Chen Q, Huang K, Zeng R, Wei P. Forearm T-score as a predictor of cage subsidence in patients with degenerative lumbar spine disease following posterior single-segment lumbar interbody fusion. BMC Musculoskelet Disord. 2022;23:1058. doi: 10.1186/s12891-022-05930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alan N, Vodovotz L, Muthiah N, et al. Subsidence after lateral lumbar interbody fusion using a 3D-printed porous titanium interbody cage: single-institution case series. J Neurosurg Spine. 2022;37(5):663–669. doi: 10.3171/2022.4.SPINE2245. [DOI] [PubMed] [Google Scholar]
- 32.Jung J myung, Chung CK, Kim CH, Yang SH. Clinical and radiologic outcomes of single-level direct lateral lumbar interbody fusion in patients with osteopenia. J Clin Neurosci. 2019;64:180–186. doi: 10.1016/j.jocn.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Bridwell KH, Lenke LG, et al. Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients. Spine. 2013;38(11):896–901. doi: 10.1097/BRS.0b013e3182815b42. [DOI] [PubMed] [Google Scholar]
- 34.Cho JH, Hwang CJ, Kim H, Joo YS, Lee DH, Lee CS. Effect of osteoporosis on the clinical and radiological outcomes following one-level posterior lumbar interbody fusion. J Orthop Sci. 2018;23(6):870–877. doi: 10.1016/j.jos.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Zhou S, Zou D, Li W, Sun Z, Jiang S. The relationship between S1 screw loosening and postoperative outcome in patients with degenerative lumbar scoliosis. BMC Musculoskelet Disord. 2022;23(1):186. doi: 10.1186/s12891-022-05107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan L, Zhang X, Zeng Y, Chen Z, Li W. Incidence, Risk, and outcome of pedicle screw loosening in degenerative lumbar scoliosis patients undergoing long-segment fusion. Global Spine J. 2023;13(4):1064–1071. doi: 10.1177/21925682211017477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Lei F, Ye F, Zhang H, Yuan H, Li S, Feng D. Prediction of pedicle screw loosening using an MRI-based vertebral bone quality score in patients with lumbar degenerative disease. World Neurosurg. 2023;171:e760–e767. doi: 10.1016/j.wneu.2022.12.098. [DOI] [PubMed] [Google Scholar]
- 38.Kim KH, Kim TH, Kim SW, et al. Significance of measuring lumbar spine 3-dimensional computed tomography hounsfield units to predict screw loosening. World Neurosurg. 2022;165:e555–e562. doi: 10.1016/j.wneu.2022.06.104. [DOI] [PubMed] [Google Scholar]
- 39.Matsukawa K, Abe Y, Yanai Y, Yato Y. Regional Hounsfield unit measurement of screw trajectory for predicting pedicle screw fixation using cortical bone trajectory: a retrospective cohort study. Acta Neurochir (Wien) 2018;160(2):405–411. doi: 10.1007/s00701-017-3424-5. [DOI] [PubMed] [Google Scholar]
- 40.Sakai Y, Takenaka S, Matsuo Y, et al. Hounsfield unit of screw trajectory as a predictor of pedicle screw loosening after single level lumber interbody fusion. J Orthop Sci. 2018;23(5):734–738. doi: 10.1016/j.jos.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Zou D, Sun Z, Zhou S, Zhong W, Li W. Hounsfield units value is a better predictor of pedicle screw loosening than the T-score of DXA in patients with lumbar degenerative diseases. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2020;29(5):1105–1111. doi: 10.1007/s00586-020-06386-8. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Zhang Z, Xie T, Song Z, Song Y, Zeng J. The preoperative Hounsfield unit value at the position of the future screw insertion is a better predictor of screw loosening than other methods. Eur Radiol. 2023;33(3):1526–1536. doi: 10.1007/s00330-022-09157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu F, Zou D, Li W, et al. Hounsfield units of the vertebral body and pedicle as predictors of pedicle screw loosening after degenerative lumbar spine surgery. Neurosurg Focus. 2020;49(2):E10. doi: 10.3171/2020.5.FOCUS20249. [DOI] [PubMed] [Google Scholar]
- 44.Choi TY, Chang MY, Lee SH, Park Y, Ha JW, Park JH. Differences in time-to-fusion based on “absence of peri-graft radiolucency” and “trabecular bone bridging” criteria after transforaminal lumbar interbody fusion in patients with low and normal bone density. Skeletal Radiol. 2022;52(4):733–742. doi: 10.1007/s00256-022-04219-x. [DOI] [PubMed] [Google Scholar]
- 45.Ha KY, Kim YH, Oh IS, et al. Clinical and radiographic features of subtypes of acute proximal junctional failures following correction surgery for degenerative sagittal imbalance. World Neurosurg. 2019;125:e304–e312. doi: 10.1016/j.wneu.2019.01.069. [DOI] [PubMed] [Google Scholar]
- 46.Jie Luo P, Chao Tang Y, Peng Zhou T, et al. Risk factor analysis of the incidence of subsequent adjacent vertebral fracture after lumbar spinal fusion surgery with instrumentation. World Neurosurg. 2020;135:e87–e93. doi: 10.1016/j.wneu.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Kurra S, Farhadi HF, Metkar U, et al. CT based bone mineral density as a predictor of proximal junctional fractures. North Am Spine Soc J. 2022;11 doi: 10.1016/j.xnsj.2022.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meredith DS, Schreiber JJ, Taher F, Cammisa FPJ, Girardi FP. Lower preoperative Hounsfield unit measurements are associated with adjacent segment fracture after spinal fusion. Spine. 2013;38(5):415–418. doi: 10.1097/BRS.0b013e31826ff084. [DOI] [PubMed] [Google Scholar]
- 49.Yao YC, Elysee J, Lafage R, et al. Preoperative Hounsfield units at the planned upper instrumented vertebrae may predict proximal junctional kyphosis in adult spinal deformity. Spine. 2021;46(3):E174. doi: 10.1097/BRS.0000000000003798. [DOI] [PubMed] [Google Scholar]
- 50.Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards CI. Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine. 2005;30(14):1643. doi: 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 51.Yuan L, Zeng Y, Chen Z, Li W, Zhang X, Mai S. Degenerative lumbar scoliosis patients with proximal junctional kyphosis have lower muscularity, fatty degeneration at the lumbar area. Eur Spine J. 2021;30(5):1133–1143. doi: 10.1007/s00586-020-06394-8. [DOI] [PubMed] [Google Scholar]
- 52.Park SJ, Lee CS, Park JS, Lee KJ. Should thoracolumbar junction be always avoided as upper instrumented vertebra in long instrumented fusion for adult spinal deformity?: Risk factor analysis for proximal junctional failure. Spine. 2020;45(10):686–693. doi: 10.1097/BRS.0000000000003364. [DOI] [PubMed] [Google Scholar]
- 53.Duan PG, Mummaneni PV, Rivera J, et al. The association between lower Hounsfield units of the upper instrumented vertebra and proximal junctional kyphosis in adult spinal deformity surgery with a minimum 2-year follow-up. Neurosurg Focus. 2020;49(2):E7. doi: 10.3171/2020.5.FOCUS20192. [DOI] [PubMed] [Google Scholar]
- 54.Hiyama A, Sakai D, Katoh H, Sato M, Watanabe M. Relationship between Hounsfield units of upper instrumented vertebrae, proximal junctional failure, and global alignment and proportion score in female patients with adult spinal deformity. World Neurosurg. 2022;164:e706–e717. doi: 10.1016/j.wneu.2022.05.030. [DOI] [PubMed] [Google Scholar]
- 55.Mikula AL, Fogelson JL, Lakomkin N, et al. Lower Hounsfield units at the upper instrumented vertebrae are significantly associated with proximal junctional kyphosis and failure near the thoracolumbar junction. Oper Neurosurg. 2021;21(4):270. doi: 10.1093/ons/opab236. [DOI] [PubMed] [Google Scholar]
- 56.Mikula AL, Lakomkin N, Pennington Z, et al. Association between lower Hounsfield units and proximal junctional kyphosis and failure at the upper thoracic spine. J Neurosurg Spine. 2022;37(5):694–702. doi: 10.3171/2022.3.SPINE22197. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Ma L, Yang D, et al. Incidence and risk factors for the progression of proximal junctional kyphosis in degenerative lumbar scoliosis following long instrumented posterior spinal fusion. Medicine (Baltimore) 2016;95(32):e4443. doi: 10.1097/MD.0000000000004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.tong Zhang T, zhe Ding J, Kong C, guo Zhu W, kang Wang S, bao Lu S. Paraspinal muscle degeneration and lower bone mineral density as predictors of proximal junctional kyphosis in elderly patients with degenerative spinal diseases: a propensity score matched case–control analysis. BMC Musculoskelet Disord. 2022;23:1010. doi: 10.1186/s12891-022-05960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo Cathleen, Soliman Mohamed, Aguirre Alexander, et al. Vertebral bone quality score independently predicts proximal junctional kyphosis and/or failure after adult spinal deformity surgery. Neurosurgery. 2023;92(5):945–954. doi: 10.1227/neu.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 60.Chen BL, Wei FX, Ueyama K, Xie DH, Sannohe A, Liu SY. Adjacent segment degeneration after single-segment PLIF: the risk factor for degeneration and its impact on clinical outcomes. Eur Spine J. 2011;20(11):1946–1950. doi: 10.1007/s00586-011-1888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Ma L, Yang D, et al. Incidence and risk factors of adjacent segment disease following posterior decompression and instrumented fusion for degenerative lumbar disorders. Medicine (Baltimore) 2017;96(5):e6032. doi: 10.1097/MD.0000000000006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye J, Yang S, Wei Z, et al. Incidence and risk factors for adjacent segment disease after transforaminal lumbar interbody fusion in patients with lumbar degenerative diseases. Int J Gen Med. 2021;14:8185–8192. doi: 10.2147/IJGM.S337298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang SK, Wang P, Li XY, Kong C, Niu JY, Lu SB. Incidence and risk factors for early and late reoperation following lumbar fusion surgery. J Orthop Surg. 2022;17(1):385. doi: 10.1186/s13018-022-03273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mugge L, DeBacker Dang D, Caras A, et al. Osteoporosis as a risk factor for intraoperative complications and long-term instrumentation failure in patients with scoliotic spinal deformity. Spine. 2022;47(20):1435. doi: 10.1097/BRS.0000000000004418. [DOI] [PubMed] [Google Scholar]
- 65.Rentenberger C, Okano I, Salzmann SN, et al. Perioperative risk factors for early revisions in stand-alone lateral lumbar interbody fusion. World Neurosurg. 2020;134:e657–e663. doi: 10.1016/j.wneu.2019.10.164. [DOI] [PubMed] [Google Scholar]
- 66.Ehresman J, Ahmed AK, Lubelski D, et al. Vertebral bone quality score and postoperative lumbar lordosis associated with need for reoperation after lumbar fusion. World Neurosurg. 2020;140:e247–e252. doi: 10.1016/j.wneu.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 67.Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44(5):369. doi: 10.1097/BRS.0000000000002822. [DOI] [PubMed] [Google Scholar]
- 68.Cloyd JM, Acosta FL, Jr, Ames CP. Complications and outcomes of lumbar spine surgery in elderly people: a review of the literature. J Am Geriatr Soc. 2008;56(7):1318–1327. doi: 10.1111/j.1532-5415.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 69.Alvarez Reyes A, Jack AS, Hurlbert RJ, Ramey WL. Complications in the elderly population undergoing spinal deformity surgery: a systematic review and meta-analysis. Glob Spine J. 2022;12(8):1934–1942. doi: 10.1177/21925682221078251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pu X, Wang D, Gu S. Advances in Hounsfield units value for predicting cage subsidence on spinal interbody fusion surgery. Eur Spine J. 2023;32(9):3149–3157. doi: 10.1007/s00586-023-07805-2. [DOI] [PubMed] [Google Scholar]
- 71.Wu Hao, Shan Zhi, Zhao Fengdng, Cheung Jason Pui Yin. Poor bone quality, multilevel surgery, and narrow and tall cages are associated with intraoperative endplate injuries and late-onset cage subsidence in lateral lumbar interbody fusion: a systematic review. Clin Orthop Relat Res. 2021;480(1):163–188. doi: 10.1097/CORR.0000000000001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palepu V, Helgeson MD, Molyneaux-Francis M, Nagaraja S. The effects of bone microstructure on subsidence risk for ALIF, LLIF, PLIF, and TLIF spine cages. J Biomech Eng. 2019;141(3):031002. doi: 10.1115/1.4042181. [DOI] [PubMed] [Google Scholar]
- 73.Zou D, Yue L, Fan Z, et al. Biomechanical analysis of lumbar interbody fusion cages with various elastic moduli in osteoporotic and non-osteoporotic lumbar spine: a finite element analysis. Glob Spine J. 2023 doi: 10.1177/21925682231166612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Y, Robinson DL, Ackland DC, Yang Y, Lee PVS. Influence of the geometric and material properties of lumbar endplate on lumbar interbody fusion failure: a systematic review. J Orthop Surg. 2022;17:224. doi: 10.1186/s13018-022-03091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marie-Hardy L, Pascal-Moussellard H, Barnaba A, Bonaccorsi R, Scemama C. Screw loosening in posterior spine fusion: prevalence and risk factors. Glob Spine J. 2020;10(5):598–602. doi: 10.1177/2192568219864341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rometsch E, Spruit M, Zigler JE, et al. Screw-related complications after instrumentation of the osteoporotic spine: a systematic literature review with meta-analysis. Glob Spine J. 2020;10(1):69–88. doi: 10.1177/2192568218818164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yagi M, Ogiri M, Holy CE, Bourcet A. Comparison of clinical effectiveness of fenestrated and conventional pedicle screws in patients undergoing spinal surgery: a systematic review and meta-analysis. Expert Rev Med Devices. 2021;18(10):995–1022. doi: 10.1080/17434440.2021.1977123. [DOI] [PubMed] [Google Scholar]
- 78.Weiser L, Huber G, Sellenschloh K, et al. Insufficient stability of pedicle screws in osteoporotic vertebrae: biomechanical correlation of bone mineral density and pedicle screw fixation strength. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2017;26(11):2891–2897. doi: 10.1007/s00586-017-5091-x. [DOI] [PubMed] [Google Scholar]
- 79.Liu D, Kahaer A, Wang Y, et al. Comparison of CT values in traditional trajectory, traditional cortical bone trajectory, and modified cortical bone trajectory. BMC Surg. 2022;22:441. doi: 10.1186/s12893-022-01893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikeura A, Kushida T, Oe K, et al. Correlation between the computed tomography values of the screw path and pedicle screw pullout strength: an experimental study in porcine vertebrae. Asian Spine J. 2020;14(3):265–272. doi: 10.31616/asj.2019.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park MK, Kim KT, Bang WS, et al. Risk factors for cage migration and cage retropulsion following transforaminal lumbar interbody fusion. Spine J. 2019;19(3):437–447. doi: 10.1016/j.spinee.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 82.He XY, Chen HX, Zhao ZR. Efficacy and safety of different anti-osteoporotic drugs for the spinal fusion surgery: a network meta-analysis. World J Clin Cases. 2023;11(30):7350–7362. doi: 10.12998/wjcc.v11.i30.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buerba RA, Sharma A, Ziino C, Arzeno A, Ajiboye RM. Bisphosphonate and teriparatide use in thoracolumbar spinal fusion: a systematic review and meta-analysis of comparative studies. Spine. 2018;43(17):E1014–E1023. doi: 10.1097/BRS.0000000000002608. [DOI] [PubMed] [Google Scholar]
- 84.Liu P, Zhou B, Chen F, Dai Z, Kang Y. Effect of trabecular microstructure of spinous process on spinal fusion and clinical outcomes after posterior lumbar interbody fusion: bone surface/total volume as independent favorable indicator for fusion success. World Neurosurg. 2020;136:e204–e213. doi: 10.1016/j.wneu.2019.12.115. [DOI] [PubMed] [Google Scholar]
- 85.Keller TS, Harrison DE, Colloca CJ, Harrison DD, Janik TJ. Prediction of osteoporotic spinal deformity. Spine. 2003;28(5):455–462. doi: 10.1097/01.BRS.0000048651.92777.30. [DOI] [PubMed] [Google Scholar]; https://oce.ovid.com/article/00007632-200303010-00009/PDF.
- 86.Watanabe K, Lenke LG, Bridwell KH, Kim YJ, Koester L, Hensley M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: analysis of morphological features. Spine. 2010;35(2):138–145. doi: 10.1097/BRS.0b013e3181c8f35d. [DOI] [PubMed] [Google Scholar]
- 87.Echt M, Ranson W, Steinberger J, Yassari R, Cho SK. A systematic review of treatment strategies for the prevention of junctional complications after long-segment fusions in the osteoporotic spine. Glob Spine J. 2021;11(5):792–801. doi: 10.1177/2192568220939902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yagi M, Ohne H, Konomi T, et al. Teriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformity. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2016;27(12):3495–3502. doi: 10.1007/s00198-016-3676-6. [DOI] [PubMed] [Google Scholar]
- 89.Anderson PA, Morgan SL, Krueger D, et al. Use of bone health evaluation in orthopedic surgery: 2019 ISCD official position. J Clin Densitom. 2019;22(4):517–543. doi: 10.1016/j.jocd.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Chin DK, Park JY, Yoon YS, et al. Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int. 2007;18(9):1219–1224. doi: 10.1007/s00198-007-0370-8. [DOI] [PubMed] [Google Scholar]
- 91.Zou D, Jiang S, Zhou S, et al. Prevalence of osteoporosis in patients undergoing lumbar fusion for lumbar degenerative diseases: a combination of DXA and Hounsfield Units. Spine. 2020;45(7):E406. doi: 10.1097/BRS.0000000000003284. [DOI] [PubMed] [Google Scholar]
- 92.Paz RDR, Henríquez MS, Melián KA, Martin CB. Prevalence of poor bone quality in patients undergoing spine surgery: a comprehensive approach. Glob Spine J. 2022;12(7):1412–1419. doi: 10.1177/2192568221989684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williamson TK, Passfall L, Ihejirika-Lomedico R, et al. Assessing the influence of modifiable patient-related factors on complication rates after adult spinal deformity surgery. Bone Jt J. 2022;104-B(11):1249–1255. doi: 10.1302/0301-620X.104B11.BJJ-2022-0574.R1. [DOI] [PubMed] [Google Scholar]
- 94.Sardar ZM, Kim Y, Lafage V, et al. State of the art: proximal junctional kyphosis—diagnosis, management and prevention. Spine Deform. 2021;9(3):635–644. doi: 10.1007/s43390-020-00278-z. [DOI] [PubMed] [Google Scholar]
- 95.Kuprys TK, Steinmetz LM, Fischer CR, et al. Preoperative assessment of bone quality in spine deformity surgery: correlation with clinical practice and published recommendations. Spine. 2019;44(12):E735. doi: 10.1097/BRS.0000000000002956. [DOI] [PubMed] [Google Scholar]
- 96.Chen JW, McCandless MG, Bhandarkar AR, et al. The association between bone mineral density and proximal junctional kyphosis in adult spinal deformity: a systematic review and meta-analysis. J Neurosurg Spine. 2023;39(1):82–91. doi: 10.3171/2023.2.SPINE221101. [DOI] [PubMed] [Google Scholar]
- 97.Systematic reviews of prognostic factor studies - systematic reviews in health research - Wiley Online Library. Accessed January 28, 2024 (pp. 324–346). doi: 10.1002/9781119099369.ch17
- 98.Alarkawi D, Ali MS, Bliuc D, Center JR, Prieto-Alhambra D. The challenges and opportunities of pharmacoepidemiology in bone diseases. JBMR Plus. 2018;2(4):187–194. doi: 10.1002/jbm4.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang J, Yun H, Wright NC, Kilgore M, Saag KG, Delzell E. Potential and pitfalls of using large administrative claims da ta to study the safety of osteoporosis therapies. Curr Rheumatol Rep. 2011;13(3):273–282. doi: 10.1007/s11926-011-0168-8. [DOI] [PubMed] [Google Scholar]
- 100.Allin Sonya, Munce Sarah, Jaglal Susan, Butt Debra, Young Jacqueline, Tu Karen. Capture of osteoporosis and fracture information in an electronic medical record database from primary care. AMIA Annu Symp Proc. 2014;2014:240–248. [PMC free article] [PubMed] [Google Scholar]
- 101.M T Löffler, M Kallweit, E Niederreiter, et al. Epidemiology and reporting of osteoporotic vertebral fractures in patients with long-term hospital records based on routine clinical CT imaging. Osteoporos Int. 33(3):685-694. doi:10.1007/s00198-021-06169-x [DOI] [PMC free article] [PubMed]
- 102.Barton DW, Behrend CJ, Carmouche JJ. Rates of osteoporosis screening and treatment following vertebral fracture. Spine J. 2019;19(3):411–417. doi: 10.1016/j.spinee.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Cheng H, Gary LC, Curtis JR, et al. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2009;20(9):1507–1515. doi: 10.1007/s00198-009-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weaver J, Sajjan S, Lewiecki EM, Harris ST. Diagnosis and treatment of osteoporosis before and after fracture: a side-by-side analysis of commercially insured and medicare advantage osteoporosis patients. J Manag Care Spec Pharm. 2017;23(7):735–744. doi: 10.18553/jmcp.2017.23.7.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine. 2006;31(19S):S144. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 106.Agarwal N, Faramand A, Alan N, et al. Lateral lumbar interbody fusion in the elderly: a 10-year experience: presented at the 2018 AANS/CNS joint section on disorders of the spine and peripheral nerves. J Neurosurg Spine. 2018;29(5):525–529. doi: 10.3171/2018.3.SPINE171147. [DOI] [PubMed] [Google Scholar]
- 107.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 108.von der Recke1 P, Hansen MA, Overgaard K, Christiansen C. The impact of degenerative conditions in the spine on bone mineral density and fracture risk prediction. Osteoporos Int. 1996;6(1):43–49. doi: 10.1007/BF01626537. [DOI] [PubMed] [Google Scholar]
- 109.Bao J, Zou D, Li W. Characteristics of the DXA measurements in patients undergoing lumbar fusion for lumbar degenerative diseases: a retrospective analysis of over 1000 patients. Clin Interv Aging. 2021;16:1131–1137. doi: 10.2147/CIA.S300873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pennington Z, Ehresman J, Lubelski D, et al. Assessing underlying bone quality in spine surgery patients: a narrative review of dual-energy X-ray absorptiometry (DXA) and alternatives. Spine J. 2021;21(2):321–331. doi: 10.1016/j.spinee.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 111.Zaidi Q, Danisa OA, Cheng W. Measurement techniques and utility of Hounsfield unit values for assessment of bone quality prior to spinal instrumentation: a review of current literature. Spine. 2019;44(4):E239. doi: 10.1097/BRS.0000000000002813. [DOI] [PubMed] [Google Scholar]
- 112.Gehweiler D, Schultz M, Schulze M, Riesenbeck O, Wähnert D, Raschke MJ. Material properties of human vertebral trabecular bone under compression can be predicted based on quantitative computed tomography. BMC Musculoskelet Disord. 2021;22(1):709. doi: 10.1186/s12891-021-04571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y, Dash A, Krez A, et al. Low volumetric bone density is a risk factor for early complications after spine fusion surgery. Osteoporos Int. 2020;31(4):647–654. doi: 10.1007/s00198-019-05245-7. [DOI] [PubMed] [Google Scholar]
- 114.Kulkarni AG, Thonangi Y, Pathan S, et al. Should Q-CT be the gold standard for detecting spinal osteoporosis? Spine. 2022;47(6):E258. doi: 10.1097/BRS.0000000000004224. [DOI] [PubMed] [Google Scholar]
- 115.Hu YH, Yeh YC, Niu CC, et al. Novel MRI-based vertebral bone quality score as a predictor of cage subsidence following transforaminal lumbar interbody fusion. J Neurosurg Spine. 2022;37(5):654–662. doi: 10.3171/2022.3.SPINE211489. [DOI] [PubMed] [Google Scholar]
- 116.Chen Z, Lei F, Ye F, Yuan H, Li S, Feng D. MRI-based vertebral bone quality score for the assessment of osteoporosis in patients undergoing surgery for lumbar degenerative diseases. J Orthop Surg. 2023;18:257. doi: 10.1186/s13018-023-03746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lewiecki EM, Ortendahl JD, Vanderpuye-Orgle J, et al. Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the United States. JBMR Plus. 2019;3(9):e10192. doi: 10.1002/jbm4.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burger EL, Kleck C, Cain CMJ, Patel VV, Noshchenko A, Barton C. Different types of mechanical complications after surgical correction of adult spine deformity with osteotomy. World J Meta-Anal. 2017;5(6):132–149. doi: 10.13105/wjma.v5.i6.132. [DOI] [Google Scholar]
- 119.Bokov A, Bulkin A, Aleynik A, Kutlaeva M, Mlyavykh S. Pedicle screws loosening in patients with degenerative diseases of the lumbar spine: potential risk factors and relative contribution. Glob Spine J. 2019;9(1):55–61. doi: 10.1177/2192568218772302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hiyama A, Sakai D, Katoh H, Nomura S, Sato M, Watanabe M. Comparative study of cage subsidence in single-level lateral lumbar interbody fusion. J Clin Med. 2022;11(5):1374. doi: 10.3390/jcm11051374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hyun SJ, Kim YJ, Rhim SC. Patients with proximal junctional kyphosis after stopping at thoracolumbar junction have lower muscularity, fatty degeneration at the thoracolumbar area. Spine J. 2016;16(9):1095–1101. doi: 10.1016/j.spinee.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 122.Endplate volumetric bone mineral density is a predictor for cage subsidence following lateral lumbar interbody fusion: a risk factor analysis. Spine J. 2021;21(10):1729–1737. doi: 10.1016/j.spinee.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 123.Jones C, Okano I, Arzani A, et al. The predictive value of a novel site-specific MRI-based bone quality assessment, endplate bone quality (EBQ), for severe cage subsidence among patients undergoing standalone lateral lumbar interbody fusion. Spine J. 2022;22(11):1875–1883. doi: 10.1016/j.spinee.2022.07.085. [DOI] [PubMed] [Google Scholar]
- 124.Kim MC, Chung HT, Cho JL, Kim DJ, Chung NS. Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2013;26(2):87–92. doi: 10.1097/BSD.0b013e318237b9b1. [DOI] [PubMed] [Google Scholar]
- 125.Kim DK, Kim JY, Kim DY, Rhim SC, Yoon SH. Risk factors of proximal junctional kyphosis after multilevel fusion surgery : more than 2 years follow-up data. J Korean Neurosurg Soc. 2017;60(2):174–180. doi: 10.3340/jkns.2016.0707.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kotheeranurak V, Jitpakdee K, Lin GX, et al. Subsidence of interbody cage following oblique lateral interbody fusion: an analysis and potential risk factors. Glob Spine J. 2021 doi: 10.1177/21925682211067210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee KY, Lee JH, Kang KC, et al. Strategy for obtaining solid fusion at L5–S1 in adult spinal deformity: risk factor analysis for nonunion at L5–S1. J Neurosurg Spine. 2020;33(3):323–331. doi: 10.3171/2020.2.SPINE191181. [DOI] [PubMed] [Google Scholar]
- 128.Löffler MT, Sollmann N, Burian E, et al. Opportunistic osteoporosis screening reveals low bone density in patients with screw loosening after lumbar semi-rigid instrumentation: a case-control study. Front Endocrinol. 2021;11 doi: 10.3389/fendo.2020.552719. https://www.frontiersin.org/articles/10.3389/fendo.2020.552719 Accessed August 30, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body Hounsfield units are associated with cage subsidence after transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Clin Spine Surg. 2017;30(8):E1130. doi: 10.1097/BSD.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 130.Nguyen HS, Shabani S, Patel M, Maiman D. Posterolateral lumbar fusion: relationship between computed tomography Hounsfield units and symptomatic pseudoarthrosis. Surg Neurol Int. 2015;6(Suppl 24):S611–S614. doi: 10.4103/2152-7806.170443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oh KW, Lee JH, Lee JH, Lee DY, Shim HJ. The correlation between cage subsidence, bone mineral density, and clinical results in posterior lumbar interbody fusion. Clin Spine Surg. 2017;30(6):E683–E689. doi: 10.1097/BSD.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 132.Okano I, Jones C, Salzmann SN, et al. Endplate volumetric bone mineral density measured by quantitative computed tomography as a novel predictive measure of severe cage subsidence after standalone lateral lumbar fusion. Eur Spine J. 2020;29(5):1131–1140. doi: 10.1007/s00586-020-06348-0. [DOI] [PubMed] [Google Scholar]
- 133.Otsuki B, Fujibayashi S, Tanida S, Shimizu T, Murata K, Matsuda S. Possible association of pedicle screw diameter on pseudoarthrosis rate after transforaminal lumbar interbody fusion. World Neurosurg. 2021;150:e155–e161. doi: 10.1016/j.wneu.2021.02.117. [DOI] [PubMed] [Google Scholar]
- 134.Pisano AJ, Fredericks DR, Steelman T, Riccio C, Helgeson MD, Wagner SC. Lumbar disc height and vertebral Hounsfield units: association with interbody cage subsidence. Neurosurg Focus. 2020;49(2):E9. doi: 10.3171/2020.4.FOCUS20286. [DOI] [PubMed] [Google Scholar]
- 135.Ran L, Xie T, Zhao L, Huang S, Zeng J. Low Hounsfield units on computed tomography are associated with cage subsidence following oblique lumbar interbody fusion (OLIF) Spine J. 2022;22(6):957–964. doi: 10.1016/j.spinee.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 136.Salzmann SN, Miller CO, Carrino JA, et al. BMI and gender increase risk of sacral fractures after multilevel instrumented spinal fusion compared with bone mineral density and pelvic parameters. Spine J. 2019;19(2):238–245. doi: 10.1016/j.spinee.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 137.Shin HK, Koo HW, Kim KH, Yoon SW, Sohn MJ, Lee BJ. The usefulness of trabecular CT attenuation measurement at L4 level to predict screw loosening after degenerative lumbar fusion surgery: consider number of fused levels and postoperative sagittal balance. Spine. 2022;47(10):745. doi: 10.1097/BRS.0000000000004330. [DOI] [PubMed] [Google Scholar]
- 138.Wang Q, Wang C, Zhang X, et al. Correlation of vertebral trabecular attenuation in Hounsfield units and the upper instrumented vertebra with proximal junctional failure after surgical treatment of degenerative lumbar disease. J Neurosurg Spine. 2020;34(3):456–463. doi: 10.3171/2020.7.SPINE20920. [DOI] [PubMed] [Google Scholar]
- 139.Xi Z, Mummaneni PV, Wang M, et al. The association between lower Hounsfield units on computed tomography and cage subsidence after lateral lumbar interbody fusion. Neurosurg Focus. 2020;49(2):E8. doi: 10.3171/2020.5.FOCUS20169. [DOI] [PubMed] [Google Scholar]
- 140.Yagi M, Akilah KB, Boachie-Adjei O. Incidence, risk factors and classification of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Spine. 2011;36(1):E60–E68. doi: 10.1097/BRS.0b013e3181eeaee2. [DOI] [PubMed] [Google Scholar]
- 141.Yagi M, King AB, Boachie-Adjei O. Incidence, Risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. minimum 5 years of follow-up. Spine. 2012;37(17):1479–1489. doi: 10.1097/BRS.0b013e31824e4888. [DOI] [PubMed] [Google Scholar]
- 142.Yagi M, Fujita N, Tsuji O, et al. Low bone-mineral density is a significant risk for proximal junctional failure after surgical correction of adult spinal deformity: a propensity score-matched analysis. Spine. 2018;43(7):485–491. doi: 10.1097/BRS.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 143.Yao YC, Chou PH, Lin HH, Wang ST, Liu CL, Chang MC. Risk factors of cage subsidence in patients received minimally invasive transforaminal lumbar interbody fusion. Spine. 2020;45(19):E1279. doi: 10.1097/BRS.0000000000003557. [DOI] [PubMed] [Google Scholar]
- 144.Zhao L, Xie T, Wang X, et al. Clinical and radiological evaluation of cage subsidence following oblique lumbar interbody fusion combined with anterolateral fixation. BMC Musculoskelet Disord. 2022;23(1):214. doi: 10.1186/s12891-022-05165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zou D, Muheremu A, Sun Z, Zhong W, Jiang S, Li W. Computed tomography Hounsfield unit–based prediction of pedicle screw loosening after surgery for degenerative lumbar spine disease. J Neurosurg Spine. 2020;32(5):716–721. doi: 10.3171/2019.11.SPINE19868. [DOI] [PubMed] [Google Scholar]
- 146.Shuhart CR, Yeap SS, Anderson PA, et al. Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom. 2019;22(4):453–471. doi: 10.1016/j.jocd.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 147.Yu JS, Krishna NG, Fox MG, et al. ACR Appropriateness Criteria® osteoporosis and bone mineral density: 2022 update. J Am Coll Radiol. 2022;19(11, Supplement):S417–S432. doi: 10.1016/j.jacr.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 148.Jang S, Graffy PM, Ziemlewicz TJ, Lee SJ, Summers RM, Pickhardt PJ. Opportunistic osteoporosis screening at routine abdominal and thoracic CT: normative L1 trabecular attenuation values in more than 20 000 adults. Radiology. 2019;291(2):360–367. doi: 10.1148/radiol.2019181648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pinto EM, Neves JR, Teixeira A, et al. Efficacy of Hounsfield units measured by lumbar computer tomography on bone density assessment: a systematic review. Spine. 2022;47(9):702. doi: 10.1097/BRS.0000000000004211. [DOI] [PubMed] [Google Scholar]
- 151.Ahern DP, McDonnell JM, Riffault M, et al. A meta-analysis of the diagnostic accuracy of Hounsfield units on computed topography relative to dual-energy X-ray absorptiometry for the diagnosis of osteoporosis in the spine surgery population. Spine J. 2021;21(10):1738–1749. doi: 10.1016/j.spinee.2021.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.