Abstract
Deep eutectic solvents (DES) are a generation of ionic liquids that benefit from low cost, good stability, and environmental-friendly features. In this research, a porous silica gel was impregnated with a eutectic Choline Chloride-Monoethanolamine solvent (ChCl-MEA) to greatly improve its CO2 capture performance. In the impregnation, the weight percentages of ChCl-MEA were used in the range of 10–60 wt% at a temperature of 25 °C. The effect of ChCl-MEA loading on the structural properties of the DES-modified silica samples was studied by BET, FTIR, and TGA analyses. Investigation of the CO2 adsorption performance at different operational conditions showed that the modified silica gel with 50 wt% ChCl-MEA (Silica-CM50) presents the highest CO2 capture capacity of 89.32 mg/g. In the kinetic modeling, the fractional order model with a correlation coefficient of 0.998 resulted in the best fit with the experimental data. In addition, the isotherm data for Silica-CM50 were well-fitted with the Dual site Langmuir isotherm model with a correlation coefficient of 0.999, representing two distinct sites for the adsorption process. Moreover, the thermodynamic parameters including Enthalpy, Entropy, and Gibbs free energy at 25 °C were obtained to be − 2.770, − 0.005 and − 1.162, respectively. The results showed the exothermic, spontaneous and feasibility of the adsorption process.
Keywords: Deep eutectic solvent, CO2, Adsorption, Choline chloride, Monoethanolamine
Subject terms: Environmental chemistry, Environmental impact, Chemical engineering
Introduction
With increasing levels of air pollutants from fossil fuel combustion and industrial sources and the significance of the greenhouse effect1, scientists have predicted that the average global temperature will rise by several degrees2. Due to the effects of increasing temperature on the environment and ecosystems of different countries, a lot of research has been done in this field, which has led to the presentation of different technologies for the capture and storage of carbon dioxide3–5. The Intergovernmental Panel on the Nobel Prize in Climate Change (IPCC) reports that CO2 emissions will be reduced by about 50–80 percent by 2050 as a result of progress in controlling carbon dioxide emissions6–8. An example of significant progress in this area is the use of green solvents, which are cheap, non-toxic, and degradable9 to solve the problems caused by the use of aqueous alkanolamines (corrosion and high energy consumption)10–13. Generally, three techniques were used for CO2 capture including pre-combusition, post-combusition, and oxy-fuel14. Post-combustion CO2 capture, which adsorbs CO2 molecules after composition by dry/wet adsorbs, is seen as a promising technique for trapping and storing CO2 molecules15.
Among traditional organic amines, ionic liquids (ILs) are considered environmentally friendly adsorbents used in different processes like gas separation because of their excellent selectivity and high thermal stability. ILs can absorb CO2 effectively due to their easy alkylation in alkaline conditions. A notable IL is the deep eutectic solvent (DES) made up of a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), leading to liquid blends with lower melting points than the individual compounds16.
Smith et al.17, described DES that are deemed as an attractive alternative to ionic liquids. DESs, unlike ionic liquids, have very low cost, simple preparation methods, and better biodegradability, so are known as green solvents18. A DES is synthesized by mixing a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), the resulting solution has a melting point lower than the pure compounds of which the DES is prepared19,20. Pressure, temperature, nature, symmetry of the salt, alkyl chain length in HBA and HBD, nature of the HBD and molar ratio affect the solubility of CO2 in the DES21.
However, due to its high viscosity and the need for special operating conditions (high pressure 5 bar and low temperature), there are limitations to the use of these solvents in carbon dioxide capture22–24. One of the suggestions to overcome this problem is to fix DESs on the porous solid materials with large surface area and pore volume25,26. Due to the high thermal stability, uniform particle size, ecfriendly, moisture tolerance, and excellent regeneration process, silica gel can be a good choice to solve this problem27–29. According to the studies, to enhance adsorption capacity of silica gel, increasing both selectivity and stability of it, silica gel is better to increase the adsorption rate by functionalization of the silica gel with DES30.
ChCl-MeA is an abbreviation for choline chloride-methanol adduct, a deep eutectic solvent (DES) created by combining choline chloride and methanol in a specific ratio. DES, a type of ionic liquid, has lower melting points and is considered more environmentally friendly than traditional solvents. When combined with silica gel, ChCl-MeA could form a solid adsorbent for CO2 capture. Silica gel offers a porous structure to trap CO2 molecules, while ChCl-MeA can improve selectivity and efficiency. This composite material might be utilized in carbon capture and storage technologies to reduce CO2 emissions from industrial processes or power plants31–33.
Some successful research has been done on these adsorbents to check out their properties and capacity to adsorb carbon dioxide as shown in Tables 1 and 2. Jiao et al. studied CO2 adsorption capacity by amine-functionalized mesoporous silica and showed the high CO2 adsorption capacity and the ability to overcome toxicity, corrosion, and high energy for desorption of amine solution34. Ren et al. investigated that by confinement of IL into PMMA bead, CO2 adsorption capacity was improved35. Moreover, Zulkurnai et al. measured CO2 adsorption capacity by DES-functionalized activated carbon and reported higher CO2 adsorption capacity than the non-functionalized activated carbon36.
Table 1.
Solubility of CO2 in different DESs at different molar ratios, temperatures and pressures.
| Researchers | Year | DES | Molar ratio | T (K) | P (MPa) | qCO2 (mg/g) | References |
|---|---|---|---|---|---|---|---|
| Serrano et al | 2012 | ChCl: Urea | 1:2 | 303.15 | 0.29 | 12.240 | 22 |
| Leron et al | 2013 | ChCl: Glycerol | 1:2 | 303.15 | 0.19 | 6.370 | 23 |
| Leron et al | 2013 | ChCl: Ethylene glycol | 1:2 | 303.15 | 0.24 | 4.720 | 37 |
| Ma et al | 2017 | TPAC:EA: AC | 1:2:2 | 298.15 | 2.00 | 140.000 | 38 |
| Benzyltriphenylphosphonium chloride: Glycerol | 1:12 | 0.021 | |||||
| Ali et al | 2014 | Methyl triphenyl phosphonium bromide: Ethanol Amine | 1:6 | 298.15 | 1.00 | 0.072 | 39 |
| Tetra Butyl Ammonium Bromide: Ethanol Amine | 1:6 | 0.059 |
Table 2.
CO2 adsorption capacity at different samples.
| Basis adsorbent | Solvent | CO2 adsorption capacity (mg/g) | T (°C) | P (atm) | Gas | References |
|---|---|---|---|---|---|---|
| Silica-gel | PEI | 44.0 | 35 | 1.0 | CO2 | 40 |
| Silica-gel | PEI | 55.0 | 45 | 0.1 | CO2 | 41 |
| Bentonite | PEI | 47.0 | 75 | 1.0 | CO2 | 42 |
| Nanoporous silica | ChCl:U | 23.0 | – | – | CO2 | 43 |
| Nanoporous silica | ChCl:Gly | 20.1 | 25 | – | CO2 | 44 |
| ChCl:EG | 18.8 | |||||
| Silica-gel | ChCl:U:PEI | 51.0 | 25 | 1.0 | CO2 | 45 |
| ChCl:PEI | 43.0 | |||||
| PEI | 48.0 | |||||
| PHE | CNT-DES | 298.0 | – | – | CO2 | 46 |
| CRV | CNT-DES | 394.0 | ||||
| Activated carbon | DES ( ChCl and Glycerol) | 9. 8 | – | – | CO2 | 36 |
| 9.5 | N2 |
This study aimed to compare the CO2 adsorption capacities of bare Silica gel and Silica_CMx for CO2 capture. Initially, our team impregnated silica gel with Choline chloride_MEA in varying ratios to find the best ratio between Silica gel and Choline Chloride-MEA. The distinctive aspect of our study was the impregnation process with Choline chloride_MEA, which enhanced the CO2 adsorption properties of the silica gel. This innovative approach differentiates our study from prior research on CO2 capture using silica materials. The primary goals of our experiment were to assess the impact of Choline chloride_MEA impregnation on enhancing CO2 adsorption capacity and to compare the efficacy of silica_CMx with bare silica gel in CO2 capture. The characterization of prepared adsorbents was determined by X-ray diffraction (XRD), Emission Scanning Microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and Thermogravimetric (TGA) analysis. The kinetic, isotherm, and thermodynamic studies were performed for both adsorbents and their results were compared.
Experimental methods
Materials
Choline chloride(ChCl), Monoethanolamine (MEA), Silica gel and Methanol, all with mass fraction purity ≥ 98.0%, were purchased from Merck chemical company. The pure carbon dioxide and nitrogen (purity > 99.0%) were supplied from Hamta Gas Co.
Preparation of deep eutectic solvent
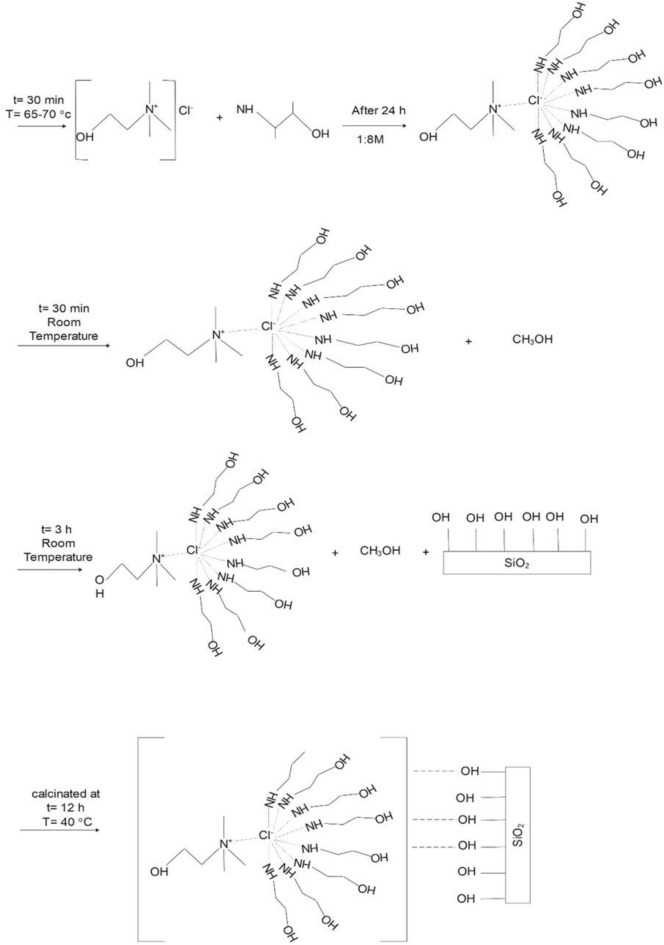
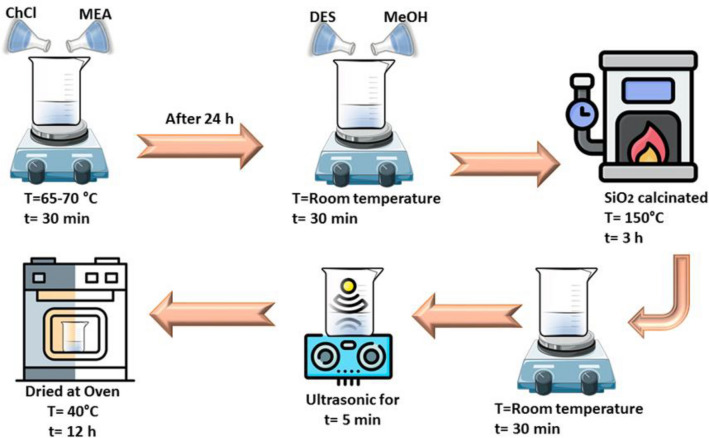
To prepare a deep eutectic solvent consisting of choline chloride and Monoethanolamine with a molar ratio of 1:8 (ChCl: MEA), the desired amounts of these substances were placed in a closed bath using a closed vial (Fig. 2). To homogenize the solvent, the solution is stirred at a temperature of 60–70 °C for 30 min using a heater to obtain a uniform solution. The resulting solution was left at the ambient temperature for 24 h and after that used to modify the adsorbent.
Figure 2.
Formulation of the synthesis reaction.
Preparation of DES impregnated silica gel
Due to the importance of using porous adsorbents in the CO2 capture, as shown in Figs. 1 and 2. In this research silica gel were modified by the impregnation method, with eutectic solvent. First, silica gel was dried at 150 °C for 3 h in a vacuum oven to remove its moisture. After that, different weight percentages of eutectic solvent (10–60 wt%) were stirred with methanol for 30 min at room temperature. After complete dissolution, each solution was poured into a vial with silica gel and placed in ultrasonic for 5 min and then stirred at room temperature for 3 h. The methanol solvent was removed at room temperature and finally, the resulting samples were dried in an oven at 40 °C for 24 h to obtain the desired adsorbent powder. The obtained DES-modified samples were named as Silicon Varbide-Carbon–Metal x (Silica-CMx), where x refers to the weight percent of ChCl-MEA. All procedures and formulation of the synthesis reaction are presented in Fig. 2.
Figure 1.
Schematic diagram of adsorbent fabrication.
Characterization methods
The presence of surface functional groups was determined using a Fourier transformed-infrared 8400S spectrometer (Shimadzu Corporation, Japan). The surface area and porosity parameters of the samples were specified using N2 physisorption at 77 K using a Micromeritics ASAP 2020 analyzer. Brunauer, Emmet and Teller (BET) method was used to determine the surface area of the adsorbents. Pore volumes and pore size distribution were calculated by the Barret–Joyner–Halenda (BJH) method. In addition, Thermogravimetric analysis (TGA) was used for thermal analysis of the adsorbent. The surface morohology of samples was studied by scanning electron microscopy (SEM) analysis.
CO2 adsorption experiments and setup
Carbon dioxide adsorption was performed on bare silica gel and modified silica gel at room temperature using a system consisting of a stainless-steel batch reactor, which includes inlets and outlets for gas to pass through the adsorbent surface13. As shown in Fig. 3, this experimental set-up was equipped with a pressure regulator and a thermocouple to adjust the pressure and desired temperature, respectively. The adsorbents, which were prepared in different weight percentages, were placed inside the reactor using a mesh-shaped chamber. At this stage, the temperature of the device is adjusted and the reactor door is completely closed to isolate the system so that the thermal balance between the adsorbent and the system can be achieved.
Figure 3.
Setup of CO2 adsorption.
As the gas enters the reactor, the adsorption operation begins. It must be ensured that the reactor outlet valve is closed at this stage. At the start of the operation, information about the pressure and temperature of the reactor can be seen by the system monitor. By checking the observed pressure drop when this value is almost constant, it can be considered as the equilibrium time and the end of the adsorption operation. All required data including temperature, pressure and adsorption time during the adsorption process were recorded by the system.
CO2 adsorption calculations
The moles of CO2 adsorbed into the DES-based sorbent () was calculated as follows:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
where Ptotal is the total inner pressure of the reactor, is the vapor pressure of the aqueous alkanolamine solution at the given temperature, R is the gas constant, Tc is the critical temperature, Tr is the reduced temperature, Pc is the critical pressure and is the acentric factor, Z is the compressibility factor and calculated using Soave–Redlich–Kwong (SRK) equation of the state. Superscripts G and L refer to the gas and liquid phases, respectively47.
Result and discussion
Characterization of DES-modified silica gel
FTIR spectra of the experimental samples of silica gel modified by eutectic solvent with six different weight percentages and a sample of bare silica gel were examined. An infrared spectrometer (Shimadzu Corporation, Japan) was used for spectral analysis. The FTIR spectra of the samples can be seen in Fig. 4. Peaks at 800.74 cm−1 and 1098.11 cm−1 corresponded to Si–O and Si–O–Si of silica gel, respectively48. The peak at 3438.51 cm−1 was assigned to the N–H bonding from choline chloride49. The peak at 1100 cm−1, which shifted to 1070 cm−1, can be linked to the C–N bonding48. Haider et al. reported that due to the formation of hydrogen bonds in the DES, peaks have the same wavenumbers, therefore, through the formation of different types of hydrogen bonds there is some physical interaction50. The presence of amine in the silica gel can be detected with the presence of the peak at 2900 cm−1, which i intensified as the DES load is increased.
Figure 4.
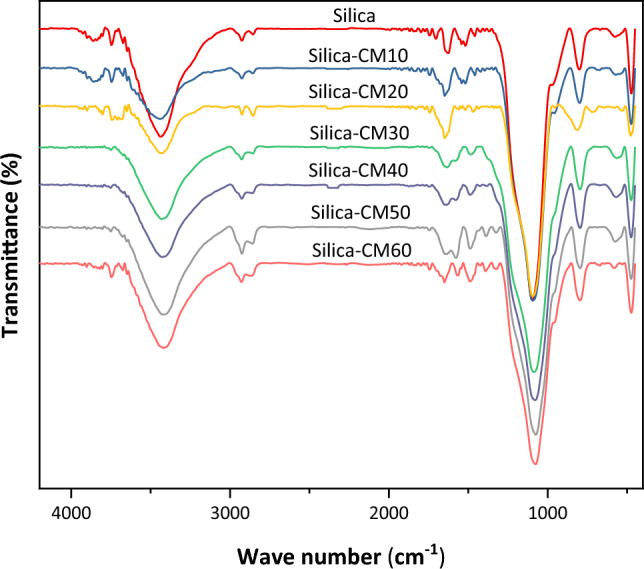
FTIR spectra for pure silica gel and DES-modified silica with six different weight percent.
Nitrogen adsorption and desorption analysis were used to show the surface properties and porosity of silica gel modified by eutectic solvent and pure silica gel. Samples were placed in a vacuum oven for resuscitation before analysis. In this analysis, the samples of pure silica gel and modified silica gel with 30, 50 and 60% have been used. Also, the analysis has been done using a Micromeritics ASAP 2020. To explain the isotherms, Brunauer, Emmett and Teller (BET) classification was provided51,52. According to Fig. 5, the adsorption and desorption isotherms for all the samples belong to type IV, which according to IOPAC classification indicates the mesoporous of the adsorbent samples53. The results of the sample analysis are given in Fig. 5. Nitrogen adsorption and desorption isotherm for the bare silica and DES-modified silica.
Figure 5.
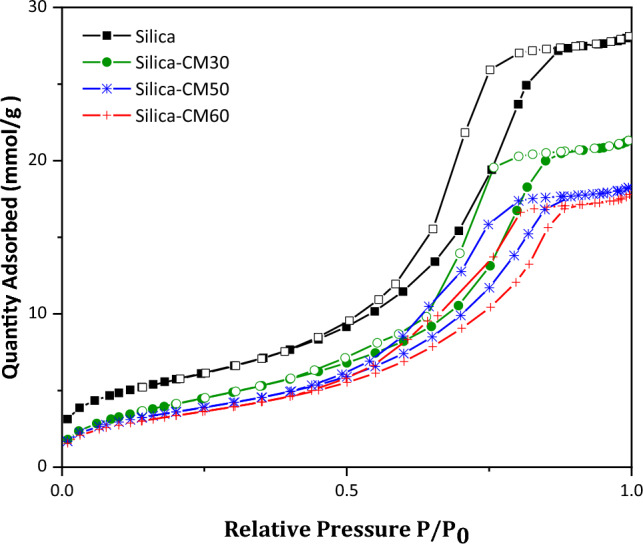
Nitrogen adsorption and desorption isotherm for the bare silica and DES-modified silica.
Table 3. According to the results, it can be seen that the specific surface area of the adsorbent is reduced and the pore size is increased with increasing the weight percentage of the eutectic solvent, which may be due to the pore blocking, pore filling, and thickening of ChCl-MEA layer onto the silica gel25,52,54.
Table 3.
BET Analysis Results for the bare silica and DES-modified silica samples.
| Samples | Specific surface area (m2 g-1) | Pore volume (m3 g-1) | Average pore diameter (nm) |
|---|---|---|---|
| Silica | 466.91 | 0.964 | 8.260 |
| Silica-CM30 | 350.75 | 0.729 | 8.315 |
| Silica-CM50 | 299.43 | 0.624 | 8.338 |
| Silica-CM60 | 279.93 | 0.605 | 8.640 |
Figure 6 illustrates temperature decomposition of bare silica and Silica-CM50. According to the Fig. 6, bare Silica did not display any weight loss at high temperature. That is because Silica nano-particles had high thermal stability55. However, by increasing temperature to 200 °C, moisture of both sample disappeared. In the TGA analysis of Silica_CM50, weight loss happens in two stages. Initially, as the temperature rises to 300 °C, the sample's moisture evaporates. Subsequently, as the temperature climbs to 400 °C, the impregnated Choline chloride_MEA decomposes and gets removed from the silica gel matrix56. The weight loss seen in the TGA analysis stems from the thermal breakdown of the impregnated material when exposed to higher temperatures. This decomposition results in the liberation of gases and volatile substances, leading to a reduction in the sample’s 35% overall weight38.
Figure 6.
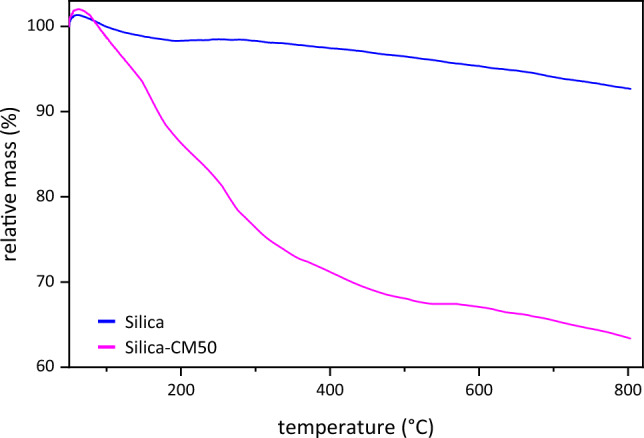
TGA Analysis results for the bare silica and Silica-CM50.
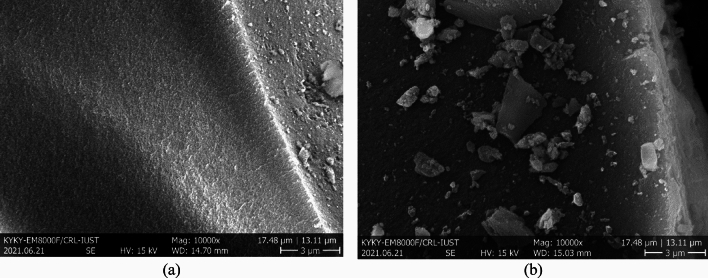
Figure 7 shows the Silica-CM50 sample before exposure with CO2 and bare Silica morphology. There were unclear changes in the sample structure after the modification with Choline Chloride-Monoethanolamine. The Silica surface was covered with ChCl: MEA, therefore, the presence of the pore on the surface of silica could not be clearly seen.
Figure 7.
SEM micrograph for (a) bare Silica, (b)Silica-CM50 sample.
CO2 capture capacity
To compare the CO2 adsorption capacity of the bare silica and Silica-CMx samples, the adsorption experiments were performed at 25 °C and an initial pressure of 6 bar. As can be seen in Fig. 8, the CO2 adsorption capacity increases with increasing DES loading on silica up to 50 wt% and then the CO2 uptake decreases for the silica-CM60 sample. Modification of silica gel with ChCl-MEA enhances the CO2 capacity from 31.02 mg/g to 35.77, 38.72, 43.69, 45.54, 65.78, and 56.628 mg/g for 10 to 60 wt% DES loading, respectively. According to Malherbe et al.57, Bare silica mainly adsorbs CO2 through physical interactions such as van der Waals forces and electrostatic interactions. On the other hand, DES-modified silica can enhance CO2 adsorption due to the functional groups in the DES that can chemically interact with CO2 molecules, leading to stronger adsorption. Additionally, specific functional groups in the DES, like amino groups or hydroxyl groups, can assist in the chemisorption of CO2 through mechanisms like hydrogen bonding or acid–base interactions. Understanding these interactions at a molecular level is crucial for elucidating the CO2 adsorption process of DES-modified silica and improving its performance for practical applications58. Hence, despite a significant drop in surface area with DES loading, the higher CO2 uptake of Silica-CMx samples could be attributed to the strong interactions between CO2 and Silica-CM50.
Figure 8.
Experimental equilibrium data for CO2 adsorption at bare silica and Silica-CM50.
Decrease in CO2 adsorption capacity can be attributed to several factors. Initially, bare silica primarily adsorbs CO2 through physical adsorption, where CO2 molecules are attracted to the surface of the silica via weak van der Waals forces. Conversely, DES-modified silica can demonstrate improved CO2 adsorption performance due to the presence of DES molecules, which can interact with CO2 through specific chemical interactions like hydrogen bonding or Lewis acid–base interactions. Moreover, the presence of DES molecules on the silica surface can enhance CO2 adsorption by offering additional adsorption sites and facilitating specific interactions with CO2 molecules. Nevertheless, at high DES loadings, these advantages may be offset by factors such as pore blockage or competitive adsorption, resulting in a reduction in CO2 uptake59,60. Figure 9 shows mechanism for CO2 capture by Silica-CM50.
Figure 9.

Mechanism of CO2 adsorption using Silica-CM50.
Adsorption kinetics
Three widely applied kinetic models, including pseudo first, second, and fractional order models were applied to study the kinetic performance of the Silica-CMx samples for CO2 capture. The kinetic models and their integrated forms after applying the boundary condition (qt = 0 at t = 0 and qt = qe at t = ∞) are written as follows:
Pseudo first order61:
| 8 |
| 9 |
Pseudo second order62:
| 10 |
| 11 |
Fractional order63:
| 12 |
| 13 |
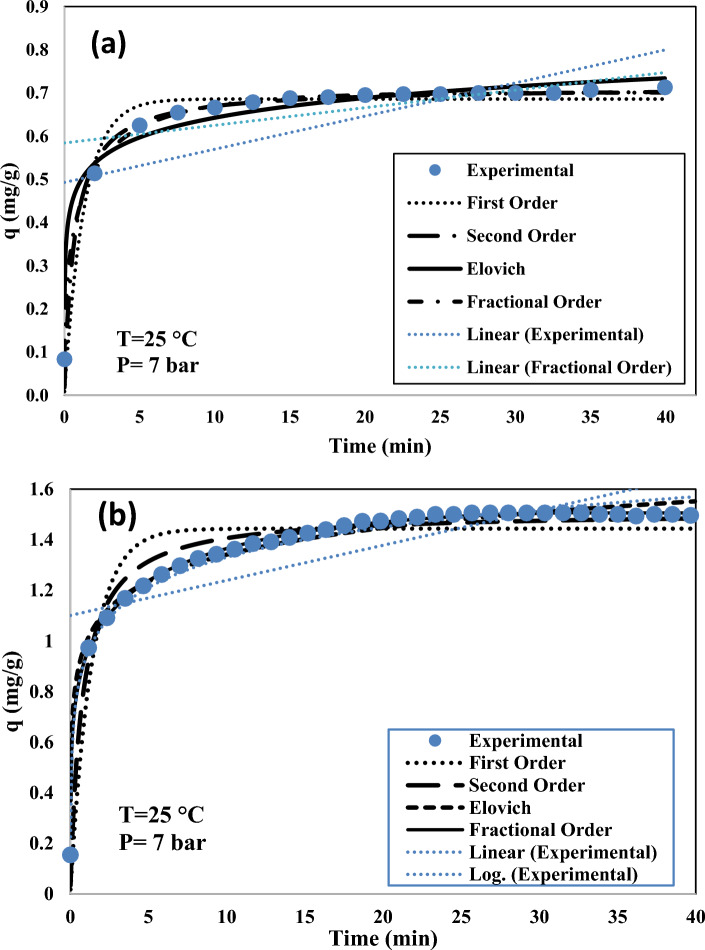
where qt and qe are the adsorption capacity at the time t and at equilibrium, respectively, k1 and k2 are the pseudo first order and pseudo second order adsorption rate constants, respectively, and kn, m, and n are the constants for the fractional order kinetic model. To obtain kinetic information about CO2 adsorption onto Silica-CMx samples pervious mentioned kinetic models were fitted to the experimental data. For instance, the curves generated by kinetic models as well as the experimental data for bare silica and silica-CM50 are depicted in Fig. 10a,b.
Figure 10.
Comparison of pseudo first, second, Elovich and fractional order kinetic models for (a) silica gel, (b) Silica-CM50.
To enhance the adsorption process, kinetic modeling was employed to specify residence time and adsorption rate. The kinetic models assist to measure adsorption mechanism and adsorption rate as well. All experimental runs were performed at 25 °C and 7 bar64,65. The corresponding kinetic parameters, coefficient of determination (R2) for regressions and error are summarized in Table 4. The pseudo first-order model is often employed to estimate the kinetic of CO2 physisorption on solid sorbents66,67. This model does not properly fit with the experimental data at the initial and final stage of adsorption, especially at higher ChCl-MEA loadings. In contrast, the pseudo-second-order model presented a better agreement with the experimental data. Other previous studies have also reported some limitations for the pseudo-first and second-order kinetic models to predict CO2 adsorption on amine-modified silica10,63. The fractional order model, being put forward by Heydari-Gorji and Sayari63 to describe CO2 capture on polyethylenimine-impregnated mesoporous silica, offers the best description for the CO2 adsorption behavior onto Silica-CMx samples over the entire adsorption range. The parameter m of this model refers to diffusion resistance, and n demonstrates the effect of the driving force (number of unoccupied sites)63,68. In general, m decreases with increasing ChCl-MEA loading because the pores blocked by excess deep eutectic solvent lead to slower CO2 diffusion. In addition, small values of the parameter n indicate that the adsorption rate is less dependent on the driving force at higher ChCl-MEA loading69. In the case of CO2 adsorption by Silica_CM50, both bare silica and deep eutectic solvent (DES) modified silica may fit the fractional order model better for several reasons: The adsorption of CO2 on silica surfaces can involve multiple, simultaneous reaction pathways that are not adequately described by simple integer-order kinetics. The fractional order model allows for the representation of these complex pathways more accurately. Both bare and DES-modified silica have heterogeneous surfaces with a variety of active sites. The fractional order model can account for the different reactivities of these sites, which is not possible with integer-order models. The process of CO2 adsorption by silica may be limited by diffusion, especially in the pores of the silica matrix. Fractional order kinetics can incorporate the effects of anomalous diffusion, which is more representative of the actual process. The interaction between CO2 and the silica surface might involve the formation of intermediate complexes. The kinetics of these complex formations and decompositions can be better described by fractional orders due to their non-integer stoichiometry. The energy barrier for CO2 adsorption varies across the surface of the silica. The fractional-order model can accommodate the distribution of these energy barriers better than integer-order models. These reasons contribute to the better fit of the fractional order model for describing the kinetics of CO2 adsorption by both bare silica and DES modified silica. It's important to note that the specific reasons can vary based on the characteristics of the silica used and the conditions of the adsorption process70.
Table 4.
Results of CO2 adsorption kinetic models from experimental data of bare silica gel and DES-modified silica gel with different ratios.
| Kinetic model | Parameters | Units | Model qt | Experimental qt | |||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | ||||
| Pseudo first order | qt | mg/g | 30.184 | 34.45 | 37.44 | 42.15 | 44 | 63.54 | 55 |
| k1 | 1/min | 0.758 | 0.673 | 0.767 | 0.543 | 0.871 | 0.724 | 0.905 | |
| R2 | – | 0.943 | 0.923 | 0.883 | 0.886 | 0.897 | 0.835 | 0.851 | |
| Pseudo second order | qt | mg/g | 31.42 | 36.04 | 39 | 44.4 | 45.67 | 66.53 | 56.41 |
| k2 | g/mg min | 2.104 | 1.57 | 1.682 | 1.02 | 1.641 | 0.879 | 1.327 | |
| R2 | 0.988 | 0.982 | 0.963 | 0.955 | 0.974 | 0.938 | 0.954 | ||
| Elovich | α | – | 25,608 | 7906 | 15,948 | 2557 | 31,254 | 4471 | 20,644 |
| β | – | 0.066 | 0.083 | 0.084 | 0.111 | 0.092 | 0.152 | 0.115 | |
| R2 | – | 0.93065 | 0.964 | 0.9711 | 0.9741 | 0.9589 | 0.9843 | 0.9776 | |
| Fractional order | qt | mg/g | 30.844 | 35.95 | 38.72 | 43.6 | 45.76 | 66 | 56.76 |
| kn | gn-1/mmoln-1 minm | 0.367 | 0.37 | 0.231 | 0.191 | 0.385 | 0.22 | 0.273 | |
| n | – | 0.832 | 0.946 | 0.601 | 0.438 | 0.914 | 0.397 | 0.647 | |
| m | – | 0.437 | 0.424 | 0.293 | 0.281 | 0.389 | 0.233 | 0.284 | |
| R2 | 0.998 | 0.998 | 0.998 | 0.996 | 0.997 | 0.995 | 0.997 | ||
Adsorption isotherms
The adsorption isotherms can be used to describe the nature of the CO2-adsorbent interactions. With that end in view, the experimental equilibrium data were fitted according to the most common isotherm models: Langmuir, Dual site Langmuir, and Freundlich models. Langmuir, the simplest theoretical model, describes a monolayer physisorption or chemisorption over a homogenous surface as follow71:
| 14 |
where b and qm are Langmuir parameters representing the affinity constant (1/bar) and the maximum monolayer uptake capacity (mg/g). P is the equilibrium CO2 partial pressure (bar) and qe refers to the amount of gas adsorbed at this pressure (mg/g). Dual site Langmuir predicts the adsorption over a heterogeneous surface, assuming two distinct adsorption sites would be available to gas molecules:
| 15 |
where the subscripts refer to sites 1 and 2. Freundlich isotherm is the earliest empirical model describing the adsorption process. Freundlich equation can be applied to study the multilayer adsorption of gas molecules on heterogeneous surfaces72:
| 16 |
where k is the Freundlich constant and n is an indicator of the surface heterogeneity. The accuracy of each model was assessed by coefficient of determination (R2) and an average relative error (ARE):
| 17 |
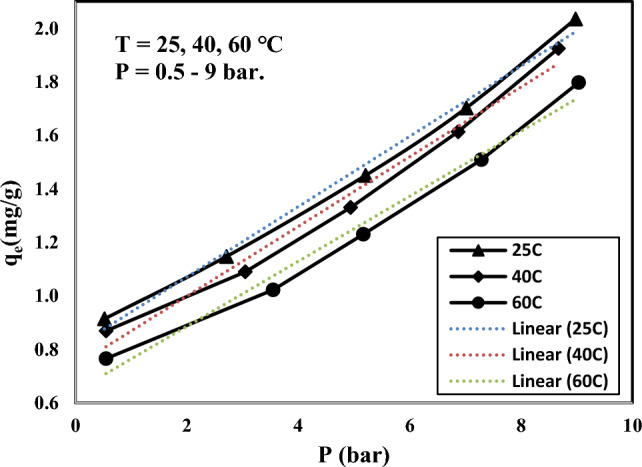
where qexp and qmod denote the experimental and model predicted values of adsorption uptakes, respectively, n is the number of experimental data. To find out the temperature influence on the CO2 uptake capacity, the equilibrium isotherm data were measured for the Silica-CM50 sample, which exhibited substantially higher CO2 capacity value among all studied samples. Figure 13 shows the adsorption isotherms of CO2 on Silica-CM50 at 25, 40 and 60 °C and 7 bar; the fitting of isotherm data with Dual site Langmuir model is illustrated as well. In the studied range of pressure, the maximum CO2 capture is 89.32 mg/g, obtained at 25 °C. As can be seen from Fig. 11, at higher temperatures, the CO2 adsorption capacity decreases, indicating the exothermic nature of the adsorption process. This could be because with rising temperature, the interaction between CO2 molecules and sorbent surface becomes weak and the desorption process is initialized.
Figure 13.
Adsorption isotherm of CO2 on Silica-CM50 at differentj temperatures.
Figure 11.
Isotherm data of Silica-CM50 at 25, 40, and 60 °C and in pressure of 7 bar.
To figure out the surface characteristics of Silica-CM50, Langmuir, Dual site Langmuir, and Freundlich isotherm models have been fitted to the obtained equilibrium data and the corresponding parameters along with the R2 and error values are listed in Table 5. Langmuir model showed a significant deviation from the experimental data which could be because this model assumes a monolayer CO2 uptake and does not consider the surface heterogeneity. Freundlich isotherm follows the experimental data more accurately. However, the higher R2 and lower error values confirm that the dual-site Langmuir model fits best with the isotherm data. This reflects the heterogeneous nature of the surface which is supported by values greater than one obtained for the n parameter in Freundlich isotherms. Also, the affinity constant b indicates a reducing trend with temperature increasing, verifying the exothermic nature of the CO2 uptake process73,74.
Table 5.
Results of CO2 adsorption isotherm models from experimental data of Silica-CM50.
| Isotherm model | Parameters | Units | Temperature | ||
|---|---|---|---|---|---|
| 25 °C | 40 °C | 60 °C | |||
| Langmuir | qm | mg/g | 83.6 | 79.2 | 74.8 |
| b | 1/bar | 1.13 | 1.09 | 0.96 | |
| R2 | – | 0.9665 | 0.9650 | 0.9645 | |
| ARE | % | 11.33 | 15.41 | 15.72 | |
| Freundlich | qm | mg/g | 40.48 | 37.4 | 33.44 |
| n | – | 3.07 | 2.96 | 2.81 | |
| R2 | – | 0.9881 | 0.9869 | 0.9868 | |
| ARE | % | 5.60 | 10.10 | 10.26 | |
| Dual site Langmuir | qm,1 | mg/g | 46.76 | 44.29 | 40.43 |
| b1 | 1/bar | 0.0029 | 0.0030 | 0.0031 | |
| qm,2 | mg/g | 0.81 | 0.74 | 0.65 | |
| b2 | 1/bar | 161.90 | 181.07 | 178.35 | |
| R2 | – | 0.9989 | 0.9984 | 0.9982 | |
| ARE | % | 2.70 | 3.62 | 3.62 | |
Adsorption thermodynamic
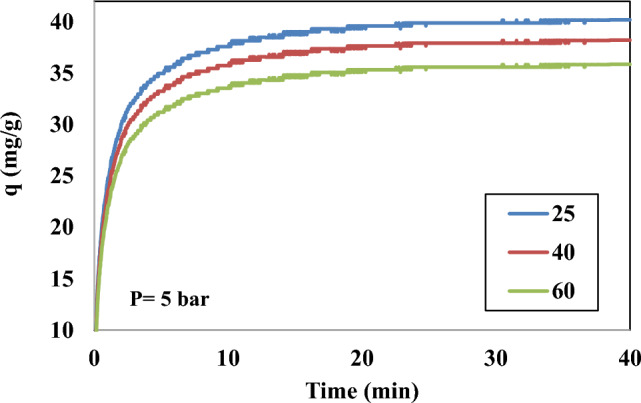
To find out the effects of adsorption temperature on DES-modified adsorbent, CO2 adsorption curves of Silica-CM50 at various adsorption temperatures are tested, as appeared in Fig. 12. It can be seen that by raising the temperature from 298 to 343 K, a decrease in the uptake ability of CO2 can be observed. The thermodynamic parameters are obtained from the adsorption experiments at 298, 313, 328 and 343 K and 5 bar. Thermodynamic parameters, including ΔG, ΔS and ΔH, are used to evaluate the thermodynamic feasibility of the process between gas molecules and Silica-CM50 and to confirm the nature of the uptake process. The laws of thermodynamics shared with the experimentally attained adsorption data obtained from the Langmuir isotherm can be used to evaluate the thermodynamic limitations according to Eqs. (18) and (19):
| 18 |
| 19 |
Figure 12.
Effect of temperature on uptake of CO2 on Silica-CM50 at different temperatures at 5 bar.
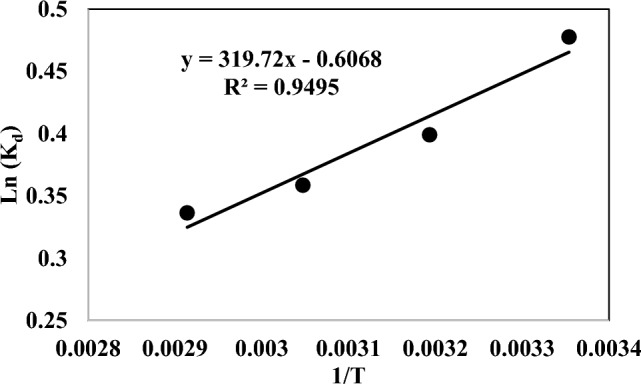
The ΔS and ΔH were determined from the intercept and slope of the linear variation of ln Kd against 1/T as shown in Fig. 13, with Eq. (20):
| 20 |
ΔG is used to understand the spontaneity of the adsorption process, which is also a vital factor. The negative ΔG values were acquired for CO2 at the three above-mentioned temperatures, reflecting the gas uptake was thermodynamically spontaneous and favourable at low temperatures. The positive or negative ΔS values lead to an increase or a decrease in the randomness at the gas–solid interface during the adsorption process, respectively. The negative ΔS value (0.00541 kj/mol K) demonstrates the decrease in entropy during the adsorption process, which indicates the high order of adsorbent molecules in the adsorption process.
ΔH is a critical parameter to examine whether the adsorption process is exothermic or endothermic according to its negative or positive value. The negative ΔH value (− 2.774 kJ/mol) indicated the exothermic nature of CO2 uptake on this synthesized sample, which was the explanation for the reduction in uptake amount at higher temperatures. These results can be attributed to the higher temperatures, the interaction between the adsorbent surface and gas molecules becomes weak and the results in the initialization of the desorption process68. The summary of the calculated thermodynamic parameters is listed in Table 6.
Table 6.
Thermodynamic parameters for CO2 adsorption on Silica-CM50.
| ΔG (kj/mol) | ΔS (kj/mol K) | ΔH (kj/mol) | − ΔH/R | ΔS/R | R (kJ/ mol K) | |||
|---|---|---|---|---|---|---|---|---|
| 55 °C | 45 °C | 35 °C | 25 °C | − 0.0054 | − 2.7747 | − 319.72 | − 0.6505 | 0.00831 |
| − 0.919 | − 1.000 | − 1.081 | − 1.162 | |||||
Desorption analysis
Adsorbent regeneration and activation are the most important issues in the design of adsorbents for practicable applications75. The regeneration studies was performed at 25 °C and 9 bar. The feasibility of regeneration was determined by desorption studies for the adsorbent using nitrogen gas. Figure 14 shows the adsorption capacity (q) diagram after 5 steps of CO2 adsorption and the nitrogen gas was used to reduce the adsorbent with each adsorption step. The decrease in silica_CM50's ability to regenerate for CO2 capture could be due to several reasons. The silica may deteriorate or disintegrate from frequent exposure to high temperatures during regeneration, leading to a decrease in surface area and pore volume, which affects CO2 adsorption. Deactivation of active sites by CO2 molecules and performance decline caused by material degradation from repeated adsorption-regeneration cycles may also play a role in reducing efficiency over time.
Figure 14.
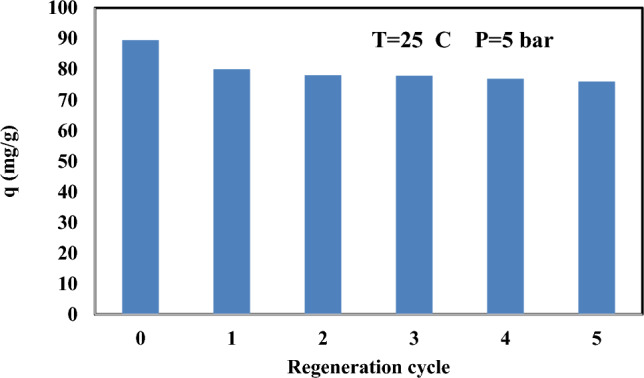
CO2 adsorption capacity of bare silica and DES-modified silica after 5 adsorption and regeneration step.
Conclusion
The primary objective of this investigation is to analyze the CO2 adsorption capabilities of untreated silica gel and silica_CMx with varying ratios for CO2 capture. Initially, silica gel was impregnated with Choline chloride_MEA in various proportions in order to accomplish this goal. Our research team observed through experimentation that silica_CM50 demonstrated a superior CO2 adsorption capacity in comparison to untreated silica gel. The distinguishing feature of our study is the utilization of the impregnation technique with Choline chloride_MEA, which facilitated the enhancement of the CO2 adsorption characteristics of the silica gel. This novel methodology differentiates our study from prior investigations in the realm of CO2 capture utilizing silica-based materials. Also, by examining the structural properties of the adsorbent, it was founded that the confinement of DES reduced the BET surface area. The presence of surface functional groups was determined using Fourier transformed-infrared 8400S spectrometer and Thermogravimetric analysis was performed for samples. In addition, experiments were performed to investigate isothermal and kinetic models of the adsorption process. It was observed that the isotherm model is consistent with the dual Langmuir isotherm model, which refers to heterogeneous adsorption. The study of kinetic models showed that the fractional order model had the best fit with the adsorption data. The thermodynamic parameters indicated the exothermic nature of CO2 uptake on this synthesized sample which was the explanation for the reduction in uptake amount at higher temperature. Silica-CM50 may not be the top performer in CO2 adsorption compared to other materials, but it has notable advantages as a CO2 adsorbent. These include a high adsorption capacity of 89.32 mg/g at 25 °C, selectivity for CO2, stability under various conditions, ease of regeneration for multiple cycles of CO2 capture, and the ability to be produced in large quantities at a reasonable cost. Evaluating its potential for cost-effective CO2 capture should consider these strengths. Further research may reveal more benefits and optimization strategies for Silica-CM50 in CO2 capture applications.
Author contributions
A. G: Supervision and writing review & editing, methodology, software, visualization, writing original draft preparation and writing review & editing. M. J: Conceptualization, data curation, formal analysis and investigation, methodology, software, validation, visualization, writing original draft preparation, and writing review and editing. M. H. formal analysis and investigation, methodology, software, validation, visualization, and writing review & editing.
Data availability
The data used and analyzed during the current work is available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang G, et al. Low-price MnO2 loaded sepiolite for Cd2+ capture. Adsorption. 2019;25(6):1271–1283. doi: 10.1007/s10450-019-00132-3. [DOI] [Google Scholar]
- 2.Masoumi H, Ghaemi A, Gilani HG. Evaluation of hyper-cross-linked polymers performances in the removal of hazardous heavy metal ions: a review. Sep. Purif. Technol. 2021;260:118221. doi: 10.1016/j.seppur.2020.118221. [DOI] [Google Scholar]
- 3.Mondal MK, Balsora HK, Varshney P. Progress and trends in CO2 capture/separation technologies: a review. Energy. 2012;46(1):431–441. doi: 10.1016/j.energy.2012.08.006. [DOI] [Google Scholar]
- 4.Kamarudin KSN, Alias N. Adsorption performance of MCM-41 impregnated with amine for CO2 removal. Fuel Process. Technol. 2013;106:332–337. doi: 10.1016/j.fuproc.2012.08.017. [DOI] [Google Scholar]
- 5.Mohammad NK, Ghaemi A, Tahvildari K. Hydroxide modified activated alumina as an adsorbent for CO2 adsorption: experimental and modeling. Int. J. Greenh. Gas Control. 2019;88:24–37. doi: 10.1016/j.ijggc.2019.05.029. [DOI] [Google Scholar]
- 6.Karimi M, et al. In-situ capture and conversion of atmospheric CO2 into nano-CaCO3 using a novel pathway based on deep eutectic choline chloride-calcium chloride. J. Environ. Manag. 2018;206:516–522. doi: 10.1016/j.jenvman.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Fashi F, Ghaemi A, Moradi P. Piperazine-modified activated alumina as a novel promising candidate for CO2 capture: experimental and modeling. Greenh. Gases Sci. Technol. 2019;9(1):37–51. doi: 10.1002/ghg.1829. [DOI] [Google Scholar]
- 8.Ramezanipour Penchah H, et al. Characterization of hypercrosslinked polymer adsorbent based on carbazole to achieve higher CO2 capture. Environ. Progress Sustain. Energy. 2021;40:e13586. doi: 10.1002/ep.13586. [DOI] [Google Scholar]
- 9.Hu X, et al. Low-cost novel silica@polyacrylamide composites: fabrication, characterization, and adsorption behavior for cadmium ion in aqueous solution. Adsorption. 2020;26(7):1051–1062. doi: 10.1007/s10450-020-00225-4. [DOI] [Google Scholar]
- 10.Serna-Guerrero R, Belmabkhout Y, Sayari A. Modeling CO2 adsorption on amine-functionalized mesoporous silica: 1. A semi-empirical equilibrium model. Chem. Eng. J. 2010;161(1–2):173–181. doi: 10.1016/j.cej.2010.04.024. [DOI] [Google Scholar]
- 11.Nematollahi MH, Carvalho PJ. Green solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2019;18:25–30. doi: 10.1016/j.cogsc.2018.11.012. [DOI] [Google Scholar]
- 12.Taheri FS, et al. High CO2 adsorption on amine-functionalized improved mesoporous silica nanotube as an eco-friendly nanocomposite. Energy Fuels. 2019;33(6):5384–5397. doi: 10.1021/acs.energyfuels.9b00703. [DOI] [Google Scholar]
- 13.Saeidi M, et al. Exploiting response surface methodology (RSM) as a novel approach for the optimization of carbon dioxide adsorption by dry sodium hydroxide. J. Chin. Chem. Soc. 2018;65(12):1465–1475. doi: 10.1002/jccs.201800012. [DOI] [Google Scholar]
- 14.Hu X, et al. A review of N-functionalized solid adsorbents for post-combustion CO2 capture. Appl. Energy. 2020;260:114244. doi: 10.1016/j.apenergy.2019.114244. [DOI] [Google Scholar]
- 15.Liu L, et al. Low-cost DETA impregnation of acid-activated sepiolite for CO2 capture. Chem. Eng. J. 2018;353:940–948. doi: 10.1016/j.cej.2018.07.086. [DOI] [Google Scholar]
- 16.Zhang Y, et al. CO2 absorption performance of ChCl-MEA deep eutectic solvent in microchannel. J. Environ. Chem. Eng. 2022;10(6):108792. doi: 10.1016/j.jece.2022.108792. [DOI] [Google Scholar]
- 17.Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014;114(21):11060–11082. doi: 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- 18.Kussainova D, Shah D. Structure of monoethanolamine based type III DESs: insights from molecular dynamics simulations. Fluid Phase Equilibria. 2019;482:112–117. doi: 10.1016/j.fluid.2018.11.017. [DOI] [Google Scholar]
- 19.Haider MB, et al. Modelling and simulation of CO2 removal from shale gas using deep eutectic solvents. J. Environ. Chem. Eng. 2019;7(1):102747. doi: 10.1016/j.jece.2018.10.061. [DOI] [Google Scholar]
- 20.Ren H, et al. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Cleaner Prod. 2018;193:802–810. doi: 10.1016/j.jclepro.2018.05.051. [DOI] [Google Scholar]
- 21.Sarmad S, et al. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: from solubility to viscosity. New J. Chem. 2017;41(1):290–301. doi: 10.1039/C6NJ03140D. [DOI] [Google Scholar]
- 22.Serrano MC, et al. Synthesis of novel lidocaine-releasing poly (diol-co-citrate) elastomers by using deep eutectic solvents. Chem. Commun. 2012;48(4):579–581. doi: 10.1039/C1CC15284J. [DOI] [PubMed] [Google Scholar]
- 23.Leron RB, Li M-H. Solubility of carbon dioxide in a eutectic mixture of choline chloride and glycerol at moderate pressures. J. Chem. Thermodyn. 2013;57:131–136. doi: 10.1016/j.jct.2012.08.025. [DOI] [Google Scholar]
- 24.Bhattacharyya S, Filippov A, Shah FU. High CO2 absorption capacity by chemisorption at cations and anions in choline-based ionic liquids. Phys. Chem. Chem. Phys. 2017;19(46):31216–31226. doi: 10.1039/C7CP07059D. [DOI] [PubMed] [Google Scholar]
- 25.Ruckart KN, et al. Porous solids impregnated with task-specific ionic liquids as composite sorbents. J. Phys. Chem. C. 2015;119(35):20681–20697. doi: 10.1021/acs.jpcc.5b04646. [DOI] [Google Scholar]
- 26.Du Y, et al. Carbon dioxide adsorbent based on rich amines loaded nano-silica. J. Colloid Interface Sci. 2013;409:123–128. doi: 10.1016/j.jcis.2013.07.071. [DOI] [PubMed] [Google Scholar]
- 27.Zulfiqar U, Subhani T, Husain SW. Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J. Sol-Gel Sci. Technol. 2016;77(3):753–758. doi: 10.1007/s10971-015-3950-7. [DOI] [Google Scholar]
- 28.Mohamed M. Effect of molecular weight on the properties of liquid epoxidised natural rubber acrylate (LENRA)/silica hybrid composites. Sains Malays. 2011;40(7):743–748. [Google Scholar]
- 29.Abdullah, N.A., N.S.M. Tahiruddin, and R. Othaman. Effects of silica content on the formation and morphology of ENR/PVC/Silica composites beads. in AIP Conference Proceedings. 2017. AIP Publishing LLC.
- 30.Yu B, et al. Carbon dioxide capture by dendrimer-modified silica nanoparticles. Adsorpt. Sci. Technol. 2011;29(8):781–788. doi: 10.1260/0263-6174.29.8.781. [DOI] [Google Scholar]
- 31.Haider J, et al. Biogas upgrading through blends of deep eutectic solvents and monoethanol amine: 4 E analysis (energy, exergy, environmental, and economic) Green Chem. 2021;23(16):6076–6089. doi: 10.1039/D1GC00714A. [DOI] [Google Scholar]
- 32.Wibowo H, et al. Experimental study on the effect of water addition to ChCl-MEA DES towards its performance in CO2 removal from syngas. Biomass Convers. Biorefinery. 2022;12:1–11. doi: 10.1007/s13399-021-01673-w. [DOI] [Google Scholar]
- 33.Yan M, et al. Effect of operating parameters on CO2 capture from biogas with choline chloride—monoethanolamine deep eutectic solvent and its aqueous solution. Biomass Convers. Biorefinery. 2024;14(1):283–297. doi: 10.1007/s13399-021-02246-7. [DOI] [Google Scholar]
- 34.Jiao J, et al. Improvement of adsorbent materials for CO2 capture by amine functionalized mesoporous silica with worm-hole framework structure. Chem. Eng. J. 2016;306:9–16. doi: 10.1016/j.cej.2016.07.041. [DOI] [Google Scholar]
- 35.Ren J, et al. Supported ionic liquid sorbents for CO2 capture from simulated flue-gas. Chin. J. Chem. Eng. 2018;26(11):2377–2384. doi: 10.1016/j.cjche.2018.04.025. [DOI] [Google Scholar]
- 36.Zulkurnai, N., et al. Carbon dioxide (CO2) adsorption by activated carbon functionalized with deep eutectic solvent (DES). in IOP Conference Series: Materials Science and Engineering. 2017. IOP Publishing.
- 37.Leron RB, Li M-H. Solubility of carbon dioxide in a choline chloride–ethylene glycol based deep eutectic solvent. Thermochimica Acta. 2013;551:14–19. doi: 10.1016/j.tca.2012.09.041. [DOI] [Google Scholar]
- 38.Tanzifi M, et al. Artificial neural network optimization for methyl orange adsorption onto polyaniline nano-adsorbent: kinetic, isotherm and thermodynamic studies. J. Mol. Liq. 2017;244:189–200. doi: 10.1016/j.molliq.2017.08.122. [DOI] [Google Scholar]
- 39.Ali E, et al. Solubility of CO2 in deep eutectic solvents: experiments and modelling using the Peng-Robinson equation of state. Chem. Eng. Res. Design. 2014;92(10):1898–1906. doi: 10.1016/j.cherd.2014.02.004. [DOI] [Google Scholar]
- 40.Zhu T, et al. Adsorption of carbon dioxide using polyethyleneimine modified silica gel. Korean J. Chem. Eng. 2010;27(6):1910–1915. doi: 10.1007/s11814-010-0284-6. [DOI] [Google Scholar]
- 41.Bollini P, Didas SA, Jones CW. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 2011;21(39):15100–15120. doi: 10.1039/c1jm12522b. [DOI] [Google Scholar]
- 42.Chen C, Park D-W, Ahn W-S. Surface modification of a low cost bentonite for post-combustion CO2 capture. Appl. Surf. Sci. 2013;283:699–704. doi: 10.1016/j.apsusc.2013.07.005. [DOI] [Google Scholar]
- 43.Ghazali Z, et al. New green adsorbent for capturing carbon dioxide by choline chloride: urea-confined nanoporous silica. Arab. J. Sci. Eng. 2020;45:1–14. doi: 10.1007/s13369-019-04306-7. [DOI] [Google Scholar]
- 44.Ghazali, Z., M.A. Yarmo, R. Othaman. Confinement and characterization of deep eutectic solvent based on choline chloride: alcohol into nanoporous silica for CO2 capture. in AIP Conference Proceedings. 2019. AIP Publishing LLC.
- 45.Ghazali Z, et al. Impregnating deep eutectic solvent choline chloride: urea: polyethyleneimine onto mesoporous silica gel for carbon dioxide capture. J. Mater. Res. Technol. 2020;9(3):3249–3260. doi: 10.1016/j.jmrt.2020.01.073. [DOI] [Google Scholar]
- 46.Lawal IA, et al. Theoretical and experimental adsorption studies of phenol and crystal violet dye on carbon nanotube functionalized with deep eutectic solvent. J. Mol. Liq. 2019;288:110895. doi: 10.1016/j.molliq.2019.110895. [DOI] [Google Scholar]
- 47.Alivand MS, et al. Preparation of nanoporous carbonaceous promoters for enhanced CO2 absorption in tertiary amines. Engineering. 2020;6(12):1381–1394. doi: 10.1016/j.eng.2020.05.004. [DOI] [Google Scholar]
- 48.Schaber PM, et al. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochimica Acta. 2004;424(1–2):131–142. doi: 10.1016/j.tca.2004.05.018. [DOI] [Google Scholar]
- 49.Tang B, Park HE, Row KH. Preparation of chlorocholine chloride/urea deep eutectic solvent-modified silica and an examination of the ion exchange properties of modified silica as a Lewis adduct. Anal. Bioanal. Chem. 2014;406(17):4309–4313. doi: 10.1007/s00216-014-7817-5. [DOI] [PubMed] [Google Scholar]
- 50.Haider MB, et al. Thermodynamic and kinetic studies of CO2 capture by glycol and amine-based deep eutectic solvents. J. Chem. Eng. Data. 2018;63(8):2671–2680. doi: 10.1021/acs.jced.8b00015. [DOI] [Google Scholar]
- 51.Singh MP, Singh RK, Chandra S. Properties of ionic liquid confined in porous silica matrix. ChemPhysChem. 2010;11(9):2036–2043. doi: 10.1002/cphc.200900983. [DOI] [PubMed] [Google Scholar]
- 52.Tian Z, Dai S, Jiang D-E. Confined ionic liquid in an ionic porous aromatic framework for gas separation. ACS Appl. Polym. Mater. 2018;1(1):95–102. doi: 10.1021/acsapm.8b00089. [DOI] [Google Scholar]
- 53.S. Brunauer, P.E.a.E.T., J. Amer. Chem. Soc., (1938).
- 54.Shi F, et al. Silica-gel-confined ionic liquids: a new attempt for the development of supported nanoliquid catalysis. Chem. A Eur. J. 2005;11(18):5279–5288. doi: 10.1002/chem.200500107. [DOI] [PubMed] [Google Scholar]
- 55.Chong MY, et al. Enhancing the performance of green solid-state electric double-layer capacitor incorporated with fumed silica nanoparticles. J. Phys. Chem. Solids. 2018;117:194–203. doi: 10.1016/j.jpcs.2018.02.030. [DOI] [Google Scholar]
- 56.Zhang C, et al. Porous core-membrane microstructured nanomaterial composed of deep eutectic solvents and MOF-808 for CO2 capture. ChemSusChem. 2023;16(24):e202300864. doi: 10.1002/cssc.202300864. [DOI] [PubMed] [Google Scholar]
- 57.Roque-Malherbe R, Polanco-Estrella R, Marquez-Linares F. Study of the interaction between silica surfaces and the carbon dioxide molecule. J. Phys. Chem. C. 2010;114(41):17773–17787. doi: 10.1021/jp107754g. [DOI] [Google Scholar]
- 58.Sarmad S, Nikjoo D, Mikkola J-P. Amine functionalized deep eutectic solvent for CO2 capture: measurements and modeling. J. Mol. Liq. 2020;309:113159. doi: 10.1016/j.molliq.2020.113159. [DOI] [Google Scholar]
- 59.Bisht M, et al. Deep eutectic solvent-modified mesoporous silica for CO2 capture: a new generation of hybrid sorbents. J. Mol. Liq. 2023;384:122203. doi: 10.1016/j.molliq.2023.122203. [DOI] [Google Scholar]
- 60.Hussin F, Aroua MK, Yusoff R. Adsorption of CO2 on palm shell based activated carbon modified by deep eutectic solvent: breakthrough adsorption study. J. Environ. Chem. Eng. 2021;9(4):105333. doi: 10.1016/j.jece.2021.105333. [DOI] [Google Scholar]
- 61.Lagergren SK. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898;24:1–39. [Google Scholar]
- 62.Espinoza-Sánchez MA, et al. Cr (VI) adsorption from aqueous solution by fungal bioremediation based using Rhizopus sp. J. environ. Manag. 2019;251:109595. doi: 10.1016/j.jenvman.2019.109595. [DOI] [PubMed] [Google Scholar]
- 63.Heydari-Gorji A, Sayari A. CO2 capture on polyethylenimine-impregnated hydrophobic mesoporous silica: experimental and kinetic modeling. Chem. Eng. J. 2011;173(1):72–79. doi: 10.1016/j.cej.2011.07.038. [DOI] [Google Scholar]
- 64.Helmi M, et al. Facile synthesis of Persian gum–graphene oxide composite as a novel adsorbent for CO2 capture: characterization and optimization. Sci. Rep. 2024;14(1):5511. doi: 10.1038/s41598-024-56070-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helmi M, et al. Synthesis, characterization and performance evaluation of NaOH@ Chitosan-Fe3O4 as an adsorbent for CO2 capture. Fuel. 2023;338:127300. doi: 10.1016/j.fuel.2022.127300. [DOI] [Google Scholar]
- 66.Álvarez-Gutiérrez N, et al. Kinetics of CO2 adsorption on cherry stone-based carbons in CO2/CH4 separations. Chem. Eng. J. 2017;307:249–257. doi: 10.1016/j.cej.2016.08.077. [DOI] [Google Scholar]
- 67.Gao J, et al. Amine-containing nanogel particles supported on porous carriers for enhanced carbon dioxide capture. Appl. Energy. 2019;253:113567. doi: 10.1016/j.apenergy.2019.113567. [DOI] [Google Scholar]
- 68.Rahmati A, Ghaemi A, Samadfam M. Kinetic and thermodynamic studies of uranium (VI) adsorption using Amberlite IRA-910 resin. Ann. Nuclear Energy. 2012;39(1):42–48. doi: 10.1016/j.anucene.2011.09.006. [DOI] [Google Scholar]
- 69.Fashi F, Ghaemi A, Behroozi AH. Piperazine impregnation on Zeolite 13X as a novel adsorbent for CO2 capture: experimental and modeling. Chem. Eng. Commun. 2020;208:1–17. [Google Scholar]
- 70.Castellano M, et al. Dependence of surface properties of silylated silica on the length of silane arms. Adsorption. 2012;18(3):307–320. doi: 10.1007/s10450-012-9402-6. [DOI] [Google Scholar]
- 71.Mathias PM, et al. Correlation of multicomponent gas adsorption by the dual-site Langmuir model. Application to nitrogen/oxygen adsorption on 5A-zeolite. Ind. Eng. Chem. Res. 1996;35(7):2477–2483. doi: 10.1021/ie950291y. [DOI] [Google Scholar]
- 72.Freundlich H. Over the adsorption in solution. J. Phys. Chem. 1906;57(385471):1100–1107. [Google Scholar]
- 73.Pashaei H, Ghaemi A, Nasiri M. Modeling and experimental study on the solubility and mass transfer of CO2 into aqueous DEA solution using a stirrer bubble column. RSC Adv. 2016;6(109):108075–108092. doi: 10.1039/C6RA22589F. [DOI] [Google Scholar]
- 74.Khajeh M, Ghaemi A. Exploiting response surface methodology for experimental modeling and optimization of CO2 adsorption onto NaOH-modified nanoclay montmorillonite. J. Environ. Chem. Eng. 2020;8(2):103663. doi: 10.1016/j.jece.2020.103663. [DOI] [Google Scholar]
- 75.Helmi M, Hemmati A. Synthesis of magnetically solid base catalyst of NaOH/Chitosan-Fe3O4 for biodiesel production from waste cooking oil: Optimization, kinetics and thermodynamic studies. Energy Convers. Manag. 2021;248:114807. doi: 10.1016/j.enconman.2021.114807. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analyzed during the current work is available from the corresponding author upon reasonable request.