Abstract
Neutrophils dominate acute inflammatory responses that generally evolve into chronic inflammatory reactions mediated by monocyte/macrophages and lymphocytes. The latter cell types also serve as major targets for human immunodeficiency virus type 1 (HIV-1). In this study we have investigated the role of neutrophil products, particularly cathepsin G, in HIV infection. Cathepsin G induced chemotaxis and production of proinflammatory cytokines by macrophages but not CD4+ T cells. Pretreatment with cathepsin G markedly increased susceptibility of macrophages but not CD4+ T cells to acute HIV-1 infection. When macrophages were exposed to pertussis toxin prior to cathepsin G treatment, the cathepsin G-mediated effect was almost abrogated, suggesting that enhancement of HIV-1 replication by cathepsin G requires Gi protein-mediated signal transduction. Although prolonged exposure to cathepsin G suppressed HIV infection of macrophages, serine protease inhibitors, which are exuded from the bloodstream later during inflammatory processes, neutralized the inhibitory effect. Neutrophil extracts or supernatants from neutrophil cultures, which contain cathepsin G, had effects similar to purified cathepsin G. Thus, cathepsin G, and possibly other neutrophil-derived serine proteases, may have multiple activities in HIV-1 infection of macrophages, including chemoattraction of monocyte/macrophages (HIV-1 targets) to inflamed tissue, activation of target cells, and increase in their susceptibility to acute HIV-1 infection.
Serine proteases constitute a gene superfamily of proteolytic enzymes that are characterized by a unique reactive serine side chain and that maintain critical and diverse biological functions. In particular, it has recently been demonstrated that proteases are capable of transducing outside-in signals of leukocyte activation that influence a wide array of leukocyte effector functions, such as cytotoxicity, chemotaxis, and costimulation of cellular proliferation (reviewed in reference 1). A specific family of lysosomal serine proteases (also called serprocidins), including cathepsin G, elastase, and proteinase 3, are expressed predominantly in neutrophils and, to a lesser extent, in cells of the monocyte/macrophage lineage (6, 24, 29).
Neutrophils dominate acute inflammatory responses induced by microbial infections or tissue damage. Subsequently, monocyte/macrophages and lymphocytes mediate subacute and chronic inflammatory reactions. Recently, cathepsin G, a neutrophil-derived serine protease, was identified as a chemoattractant for monocytes (7). Cathepsin G was also shown to stimulate lymphocytes (12). Since lymphocytes and monocytes are major targets of human immunodeficiency virus type 1 (HIV-1), we sought to determine whether cathepsin G-induced stimulation of these cells could modulate HIV infection.
In this study we demonstrate that (i) cathepsin G induces the expression of a number of cytokines as well as chemotactic activity in macrophages but not in CD4+ T cells; (ii) pretreatment of macrophages with cathepsin G markedly increases susceptibility to HIV-1 infection, while cathepsin G has minimal effects on HIV-1 infection of CD4+ T cells; (iii) prolonged exposure of macrophages to cathepsin G suppressed HIV expression, but α1-antichymotrypsin (ACT), an inhibitor of cathepsin G present in the plasma, neutralizes the inhibitory effect mediated by prolonged exposure to cathepsin G; and (iv) neutrophil extracts or supernatants from neutrophil cultures containing cathepsin G have similar effects on HIV infection of macrophages as does purified cathepsin G. These results suggest that cathepsin G, and possibly other serine proteases, plays a critical role in HIV infection of cells of the monocyte/macrophage lineage.
MATERIALS AND METHODS
Reagents.
Human neutrophil cathepsin G, elastase, and ACT were purchased from ICN Pharmaceuticals Inc. (Costa Mesa, Calif.); the purity of these reagents was >98% as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (according to the manufacturer's instructions). Human secretary leukocyte protease inhibitor (SLPI) (purity, >97%) was purchased from R&D Systems (Minneapolis, Minn.). Human macrophage-derived chemokine (MDC) was purchased from Pepro Tech EC Ltd. (London, England). Pertussis toxin (PTx) was purchased from Sigma Chemical (St. Louis, Mo.). Endotoxin was undetected in these reagents by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.).
Cells.
Monocyte-derived macrophages (MDM) were isolated from healthy volunteers (Department of Transfusion Medicine [DTM], Warren Grant Magnusson Clinical Center [WGMCC], National Institutes of Health [NIH], Bethesda, Md.) and propagated in Dulbecco's minimal essential medium (DMEM) supplemented with 10% human male AB serum (HS; Sigma Chemical Co.), as described previously (17), and either unstimulated or stimulated with lipopolysaccharide (LPS) for 5 to 7 days before experiments. CD4+ T cells were prepared from peripheral blood lymphocytes (PBL) as described previously (19) and either unstimulated or stimulated with phytohemagglutinin (PHA) for 3 days before experiments.
Granulocyte packs obtained from healthy donors were provided by the DTM (WGMCC, NIH), and neutrophils were isolated as described previously (5). The neutrophils were resuspended in AIM-V serum-free medium at 2 × 106 cells/ml and either unstimulated or stimulated with LPS (100 ng/ml). After incubation at 37°C for 24 h, cell-free supernatants were collected and kept at −80°C until use. Neutrophil pellets were washed three times in phosphate-buffered saline (PBS), resuspended in AIM-V medium at 2 × 106 cells/ml, subjected to three cycles of freezing-thawing, and centrifuged to remove cell debris, and crude cell extracts were kept at −80°C until use.
Viruses and infection.
T-cell-tropic (X4) NL4-3 and macrophage-tropic (R5) ADA8 virus strains were propagated by transfecting 293T cells with the respective molecular clone as described previously (16), except that the cells were maintained in serum-free AIM-V medium. Approximately 2 × 105 MDM were extensively washed in PBS, resuspended in serum-free AIM-V medium (Life Technologies, Inc., Grand Island, N.Y.) with or without cathepsin G, incubated for 30 min at 37°C, and then infected with the indicated molecular clone stock at a multiplicity of infection of approximately 0.05. After a 3-h incubation at 37°C, the infected cells were extensively washed in PBS, resuspended in AIM-V with or without cathepsin G, and plated on a 96-well tissue culture plate. Approximately half of the cell culture supernatants was collected for the reverse transcriptase (RT) assay every 3 to 4 days.
Single-round virus replication assays.
Single-round virus replication assays were performed using replication-incompetent luciferase reporter viruses that were pseudotyped by Env derived from either T-tropic (X4) HIV-1 HXB2, M-tropic (R5) HIV-1 ADA, or amphotropic murine leukemia virus (AMV), as described previously (10, 16). In brief, 5 × 105 cells were extensively washed in PBS, resuspended in AIM-V with and without the indicated serine protease, incubated for 30 min at 37°C, and then infected with the indicated virus stock (50,000 cpm of RT activity). Three days later, the infected cells were lysed and subjected to luciferase assays, using commercially available reagents (Promega, Madison, Wis.).
Transient-expression assays.
Freshly isolated monocytes were plated on 60-mm-diameter tissue culture dishes and allowed to differentiate into macrophages with DMEM supplemented with 10% HS for 5 to 7 days. The cells were transfected with 15 μg of pHIV-1 LTR-luc (encoding the luciferase gene under the control of HIV-1 long terminal repeat [LTR]) or 3 μg of pCMV-luc (encoding the luciferase gene under the control of human cytomegalovirus [CMV] major immediate-early gene promoter [MIEP]) by the modified calcium phosphate method (20), washed, and incubated with AIM-V medium in the presence of cathepsin G with and without ACT. Luciferase activity was measured for cell lysates 2 days after transfection, as described previously (18).
Flow cytometric analyses.
Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies to CCR5 and CXCR4 were purchased from PharMingen (San Diego, Calif.), while FITC- and PE-conjugated isotype controls were purchased from Becton-Dickinson (San Jose, Calif.). Cells were stained with the indicated antibodies and analyzed in a FACScan (Becton-Dickinson), as described previously (16).
ELISA.
For the enzyme-linked immunosorbent assays (ELISA), levels of tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β in the cell culture supernatants were measured using commercially available ELISA kits (R&D Systems).
Chemotaxis assays.
Macrophage migration was evaluated using a 96-well microchamber method, as described previously (16). In brief, various amounts of either cathepsin G, elastase, or MDC were added to 27.5 μl of RPMI 1640 supplemented with bovine serum albumin (2 mg/ml) (RPMI-BSA) and placed in the lower wells of the chemotaxis chamber (Neuro Probe Inc., Cabin John, Md.), while cells were suspended in 25 μl of RPMI-BSA at 2 × 106 cells/ml and placed in the upper wells. After 2 h of incubation at 37°C, the Nucleopore polycarbonate filter (5-μm pore size) that separated the two compartments was removed, fixed, and stained, and the number of cells migrating to the lower surface of the filter was counted under the microscope. The mean number of migrating cells was calculated from four high-power fields (HPF) (×400).
RESULTS
Cathepsin G is a chemoattractant for and inducer of cytokine production by macrophages.
Certain members of the serine protease family are capable of stimulating leukocytes to exert a wide array of effector functions, such as cytotoxicity, chemotaxis, and costimulation of cellular proliferation (reviewed in reference 1). Therefore, we investigated the effects of cathepsin G, a neutrophil-derived serine protease, on macrophages and lymphocytes, two major targets for HIV-1.
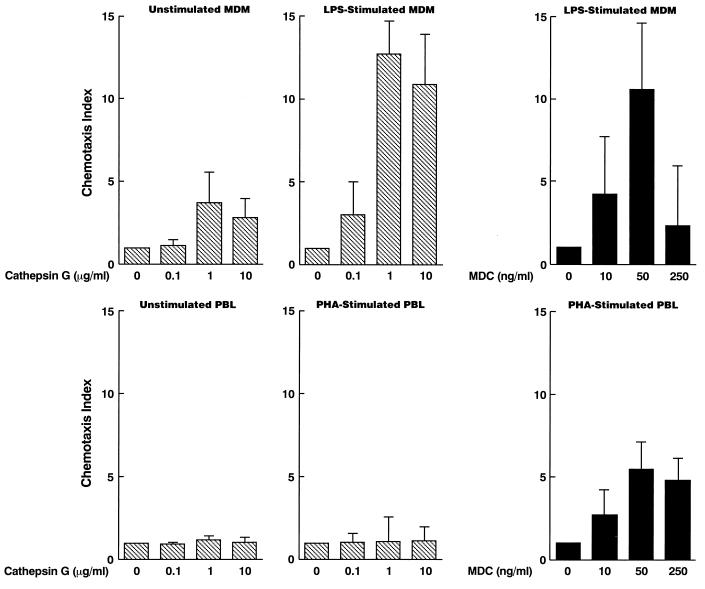
First, chemotaxis assays showed that cathepsin G weakly chemoattracted MDM (Fig. 1) and barely chemoattracted monocytes (data not shown). The chemotactic activity of cathepsin G was also reported by Chertov et al. (7). Furthermore, we have demonstrated that cathepsin G attracted MDM stimulated with LPS, a gram-negative bacterial cell wall component, much more efficiently than unstimulated MDM (Fig. 1). These results suggest that the inflammatory products of bacteria and neutrophils may synergize to attract MDM. In contrast, cathepsin G did not efficiently chemoattract lymphocytes whether unstimulated or stimulated with PHA (Fig. 1). Thus, the chemotactic activity of these serine proteases appears to be specific for MDM.
FIG. 1.
Cathepsin G is a chemoattractant for macrophages. MDM were either unstimulated or stimulated with LPS for 3 days, while PBL were either unstimulated or stimulated with PHA for 3 days. Cell migration was evaluated using a 96-well microchamber technique as described in Materials and Methods. The results are expressed as the chemotaxis index, which represents the ratio of the number of migrating cells in response to cathepsin G or MDC to those in control medium. Baseline migration for unstimulated MDM, LPS-stimulated MDM, unstimulated PBL, and PHA-stimulated PBL was 68 (29 to 89), 15 (5 to 29), 9 (2 to 18), and 6 (2 to 11) cells per HPF, respectively [mean (range)]. Data represent means ± standard error of the mean (SEM) for three independent experiments.
Next, we investigated whether stimulation of MDM with cathepsin G can induce production of the proinflammatory cytokines TNF-α and IL-1β, which have been shown to induce expression of HIV (reviewed in reference 23). To this end, 106 MDM were extensively washed in PBS, resuspended in AIM-V with or without cathepsin G, incubated for 30 min at 37°C, washed in PBS, resuspended in 1 ml of DMEM–10% HS, and incubated at 37°C. The supernatants were collected 16 h after mock or cathepsin G treatment, and levels of cytokines secreted from MDM were measured by ELISA. Exposure to cathepsin G induced production of these cytokines by MDM but not by CD4+ T cells (Table 1). Thus, cathepsin G is a chemoattractant for and inducer of cytokine production by macrophages.
TABLE 1.
Induction of proinflammatory cytokines in macrophages stimulated with cathepsin Ga
| Treatment | Concn (pg/ml)
|
|||
|---|---|---|---|---|
| Macrophages
|
CD4+ T cells
|
|||
| TNF-α | IL-1β | TNF-α | IL-1β | |
| None | 15.9 | 18.2 | 12.2 | 12.8 |
| ACT | 17.3 | 16.9 | ND | ND |
| PTx | 12.0 | 15.2 | ND | ND |
| Cathepsin G | 120.2 | 100.1 | 14.5 | 12.0 |
| Cathepsin G + ACT | 30.9 | 32.8 | ND | ND |
| Cathepsin G + PTx | 20.4 | 16.9 | ND | ND |
MDM or CD4+ T cells were propagated from PBL derived from healthy donors, washed in PBS, resuspended in AIM-V serum-free medium, and either left untreated or treated with ACT (20 μg/ml), PTx (300 ng/ml), or cathepsin G (5 μg/ml) alone or in combination, as shown. Cell-free supernatants were collected 16 h after treatment, and cytokine concentrations were measured by ELISAs. Results are representative of three independent experiments. ND, not determined.
Macrophages treated with cathepsin G become highly susceptible to acute HIV infection.
Macrophages migrate to infected and inflamed tissues and are exposed to microbial and neutrophil products, including neutrophil-derived serine proteases such as cathepsin G. As indicated above, cathepsin G can influence macrophage functions in various ways. Thus, we sought to determine whether cathepsin G has an effect on HIV infection of macrophages.
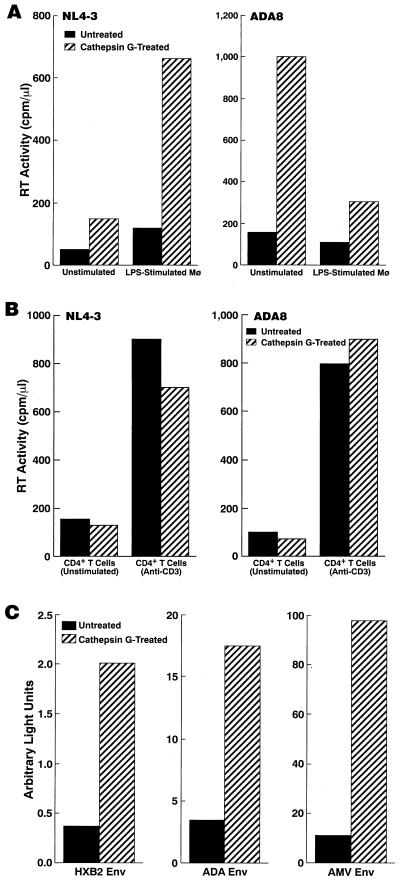
MDM were treated with cathepsin G and infected with HIV-1; HIV-1 replication was monitored by RT assays. As previously reported (21), replication of T-tropic (X4) HIV-1 was enhanced, while that of M-tropic (R5) HIV-1 was suppressed in LPS-stimulated MDM compared to unstimulated MDM (Fig. 2A). Replication of both R5 and X4 HIV-1 was markedly enhanced in cathepsin G-treated MDM compared to untreated MDM (Fig. 2A). In contrast, cathepsin G had no effect on HIV infection of CD4+ T cells (Fig. 2B). We also examined whether cathepsin G treatment of virus stocks instead of target cells has any effect. 293T cells were transfected with pNL4-3 or pAD8 and incubated with AIM-V serum-free medium. The culture supernatants containing NL4-3 or ADA8 virus were either mock treated or treated with cathepsin G for 30 min and then mixed with RPMI 1640 supplemented with 20% FBS to neutralize the enzymatic activity. Macrophages were infected with either mock-treated or cathepsin G-treated virus stocks. The infectability of the virus was not increased by cathepsin G treatment (data not shown). These results indicate that cathepsin G interacts with a host factor(s), not virus itself, to mediate its effects on HIV infection of macrophages.
FIG. 2.
Cathepsin G treatment renders macrophages (Mø) highly susceptible to acute HIV-1 infection. MDM (A) and CD4+ T cells (B) were either untreated or treated with cathepsin G (5 μg/ml) for 30 min before infection with the indicated strain of HIV-1. Peak RT titers on day 12 postinfection are shown. Results are representative of seven independent experiments. (C) MDM either untreated or treated with cathepsin G were infected with replication-incompetent luciferase reporter NL4-3luc-R−E− virus that had been pseudotyped by Env from either T-tropic HIV-1 HXB2, M-tropic HIV-1 ADA, or AMV. Luciferase activity was measured for the infected cell lysates 3 days after infection. Results are representative of seven independent experiments.
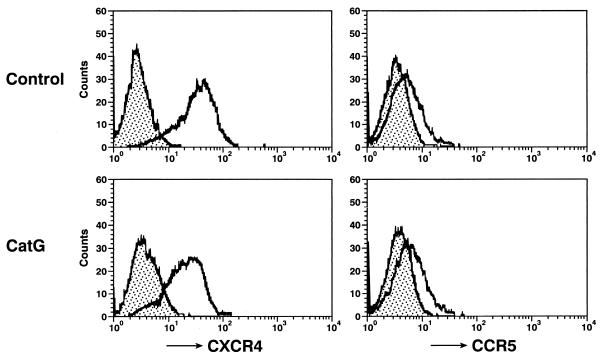
In order to further investigate the effects on HIV infection mediated by cathepsin G, single-round virus replication assays were performed. Similar to standard infection assays described above, pretreatment of macrophages with cathepsin G enhanced the infectivity of all HIV-1 strains, including a strain pseudotyped by Env from AMV (Fig. 2C). These results suggest that cathepsin G treatment of macrophages may have multiple effects on HIV-1 infection during the viral replicative cycle and that this effect appears to be independent of HIV coreceptor-mediated entry, since infection even by amphotropic virus was enhanced by treatment of macrophages with cathepsin G. Flow cytometric analysis showed that cathepsin G treatment does not increase expression of HIV coreceptors CCR5 and CXCR4 (Fig. 3).
FIG. 3.
Cathepsin G (CatG) treatment does not increase cell surface expression of HIV-1 coreceptors. MDM were either unstimulated (control) or stimulated with cathepsin G (5 μg/ml) for 30 min, washed twice, stained with anti-CXCR4 or anti-CCR5, and analyzed in a FACScan as described in Materials and Methods. Filled areas indicate isotype control staining, while open areas under solid lines indicate staining with anti-CXCR4 or anti-CCR5. Results are representative of three independent experiments.
Chemotactic activity or effect of cathepsin G on HIV-1 infection of macrophages is dependent on its catalytic activity and Gi protein signal transduction.
Some of the serine proteases are able to transduce outside-in signals of leukocyte activation that influence a wide array of leukocyte effector functions (reviewed in reference 1). A prototypic serine protease thrombin is capable of transducing signals through the interaction with thrombin receptors that are members of the seven-transmembrane-domain, G-protein-coupled receptor family (reviewed in reference 1). Proteolytic modification of thrombin receptors by thrombin appears to be critical for the events mediated by thrombin (1). Although a receptor(s) for cathepsin G has not been well described, by analogy with thrombin, it is possible that cathepsin G mediates its biological activities through interaction with a seven-transmembrane-domain, G-protein-coupled receptor(s).
In order to address this hypothesis, the following experiments were performed. First, cathepsin G-induced migration of macrophages was determined following treatment of macrophages with PTx, an inhibitor of Gi protein signal transduction (22), or pretreatment of cathepsin G with ACT, a serine protease inhibitor present in plasma. As shown in Fig. 4A, both PTx and ACT markedly attenuated the chemotactic activity of cathepsin G.
FIG. 4.
Chemotactic activity and effect of cathepsin G (CatG) on HIV-1 infection of macrophages is dependent on its catalytic activity and Gi protein signal transduction. (A) Chemotaxis assay. Where indicated, macrophages were preincubated with PTx (300 ng/ml) for 15 min or cathepsin G was pretreated with ACT (10 μg/ml) prior to treatment of macrophages. As controls, macrophages were treated with ACT alone or PTx alone. Data represent mean ± SEM for three independent experiments. (B) Infection assay. Where indicated, macrophages were preincubated with PTx (300 ng/ml) for 15 min and treated with cathepsin G (5 μg/ml) for 30 min. Cathepsin G was either untreated or pretreated with ACT (10 μg/ml). Macrophages were then infected with replication-incompetent luciferase reporter NL4-3luc-R−E− virus that had been pseudotyped by Env from M-tropic HIV-1 ADA. Luciferase activity was measured for the infected cell lysates 3 days after infection. Data represent mean ± SEM for five independent experiments.
Next, macrophages were exposed to ACT or PTx prior to cathepsin G treatment, and levels of proinflammatory cytokines in the culture supernatants were determined. As shown in Table 1, pretreatment of macrophages with ACT or PTx abolished induction of proinflammatory cytokine (TNF-α and IL-1β) production.
Finally, macrophages were exposed to PTx prior to cathepsin G treatment followed by infection with a replication-incompetent luciferase reporter virus that had been pseudotyped by HIV-1 ADA Env. Pretreatment of macrophages with cathepsin G markedly enhanced infectability by ADA Env-bearing virus, and PTx treatment alone had no or minimal effect on the infectivity; however, PTx treatment almost abrogated the cathepsin G-mediated effect (Fig. 4B). Pretreatment of cathepsin G with ACT also abrogated induction of viral replication by cathepsin G (Fig. 4B). Taken together, these results suggest that cathepsin G mediates the above biological functions through signal-transducing events following its catalytic interaction with a seven-transmembrane-domain, G-protein-coupled receptor(s).
Prolonged exposure to cathepsin G diminishes HIV-1 expression from infected macrophages, but serine protease inhibitors in the plasma neutralize the inhibitory effect.
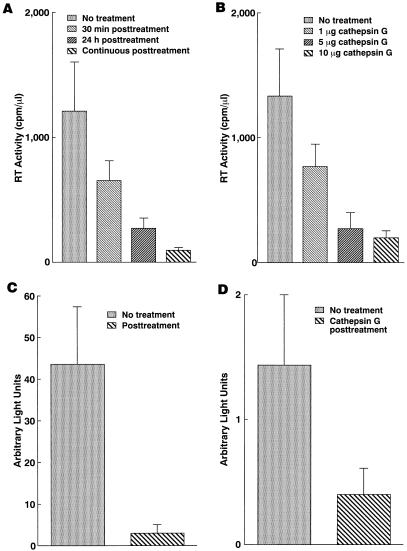
The above experiments have demonstrated that pretreatment of macrophages with cathepsin G enhanced HIV-1 replication. In order to investigate the effect of prolonged exposure to cathepsin G on HIV-1 infection, macrophages were treated with cathepsin G at various time points and durations after infection with HIV-1. In contrast to pretreatment with cathepsin G, HIV-1 infection of macrophages was suppressed by posttreatment with cathepsin G, and the inhibitory effect was dose and duration dependent (Fig. 5A and B). Single-round virus replication assays indicate that the inhibitory effect of cathepsin G is independent of coreceptor-mediated cellular entry of the virus, since infection even by amphotropic virus was suppressed by posttreatment of macrophages with cathepsin G (Fig. 5C). Furthermore, transient-expression assays indicate that cathepsin G treatment downregulates HIV-1 LTR activity (Fig. 5D), while it had little effect on transcription from CMV MIEP (data not shown).
FIG. 5.
Prolonged exposure to cathepsin G downregulates HIV expression. The inhibitory effect by postexposure to cathepsin G is duration (A) and dose (B) dependent. (A) Macrophages were either mock treated or treated with cathepsin G (5 μg/ml) immediately after or for 30 min prior to infection with ADA8. Cathepsin G was washed out after the indicated period (30 min or 24 h) of incubation or continuously supplied by replacing approximately half of the medium containing the same amount of cathepsin G every 4 days. Data represent means ± SEM for three parallel experiments. Similar results were obtained for three independent experiments. (B) Macrophages were treated with the indicated amount of cathepsin G immediately after infection with ADA8. Data represent means ± SEM for three parallel experiments. Similar results were obtained for three independent experiments. (C) The inhibitory effect of cathepsin G is independent of coreceptor-mediated cellular entry of the virus. Macrophages were either mock treated or treated with cathepsin G (5 μg/ml) for 24 h immediately after infection with NL4-3luc-R−E− virus that had been complemented with AMV Env. Data represent means ± SEM for five independent experiments. (D) Postexposure to cathepsin G downregulates HIV-1 LTR activity. Macrophages were either mock treated or treated with cathepsin G for 24 h immediately after transfection with pHIV-1 LTR-luc or pCMV-luc. Luciferase activity in the pHIV-1 LTR-luc-transfected cell lysates is shown. Data represent means ± SEM for five independent experiments.
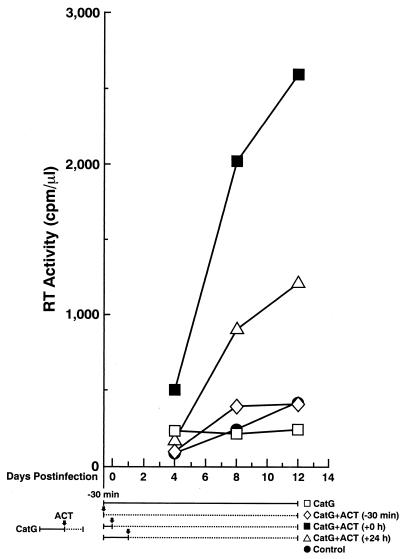
As the inflammatory process proceeds, plasma is exuded into the inflamed tissue, secondary to increased capillary permeability. A number of serine protease inhibitors are present in the plasma and other body fluids and play a critical role in the regulation of serine protease activity (reviewed in reference 27). In order to mimic the in vivo inflammatory process during which serine protease activity is likely to be neutralized by serine protease inhibitors in plasma, ACT, a serine protease inhibitor present in plasma, was added at different time points to HIV-1-infected macrophage cultures that had been treated with cathepsin G. Cathepsin G was added 30 min before and immediately after infection and replaced on days 4 and 8 postinfection. ACT was added either 30 min before and immediately after infection [CatG+ACT (−30 min)], immediately after infection [CatG+ACT (+0 h)], or 24 h after infection [CatG+ACT (+24 h)] and replaced on days 4 and 8 postinfection in either case. When cathepsin G alone was added before and after infection in the absence of ACT (CatG in Fig. 6), HIV replication was modestly higher on day 4 than in the control, probably reflecting the enhancing effect of cathepsin G; however, HIV replication was not increased thereafter, indicating that cathepsin G posttreatment inhibited HIV replication. When ACT was added for the whole period with cathepsin G [CatG+ACT (−30 min) in Fig. 6], ACT neutralized both the enhancing and inhibitory effects on HIV infection of macrophages by cathepsin G, and there was no or minimal effect on infectability by HIV-1 compared to the control. When ACT was added immediately after virus absorption [CatG+ACT (+0 h) in Fig. 6], only the inhibitory effect by cathepsin G was neutralized, and HIV replication was markedly enhanced. When ACT was added 24 h after infection [CatG+ACT (+24 h) in Fig. 6], HIV replication was also enhanced, although to a lesser degree, probably because the inhibitory effect of cathepsin G during 24 h after infection reduced its enhancing effect on HIV replication. We have obtained similar results using other serine protease inhibitors such as SLPI (data not shown). Therefore, it is likely that the inflammatory process in vivo provides a favorable environment for HIV infection: first, cathepsin G and other inflammatory products (i.e., proinflammatory cytokines) accelerate establishment of acute HIV infection, and subsequently serine protease inhibitors neutralize the inhibitory effect by cathepsin G.
FIG. 6.
Serine protease inhibitor ACT neutralizes the effects mediated by cathepsin G. Macrophages were treated with cathepsin G (CatG, 5 μg/ml) for 30 min before and immediately after infection with the ADA8 strain of HIV-1. ACT was added either 30 min before and immediately after infection [CatG+ACT (−30 min)], immediately after infection [CatG+ACT (+0 h)], or 24 h after infection [CatG+ACT (+24 h)]. Approximately half of the medium was replaced with AIM-V medium containing the same amounts of cathepsin G and ACT every 4 days, and RT activities in the cell-free supernatants were measured. Arrows indicate time points when ACT was added. Solid and broken lines indicate durations of cathepsin G treatment in the absence and presence of ACT, respectively. Results are representative of four independent experiments.
Neutrophil extracts or supernatants from neutrophil cultures had similar effects on HIV infection of macrophages as did cathepsin G.
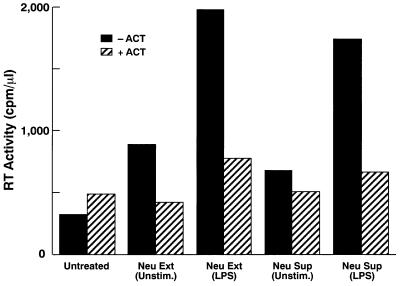
We next investigated whether crude extracts from neutrophils or supernatants from neutrophil cultures which contain cathepsin G and other serine proteases similarly modulate HIV infection. Neutrophils were either unstimulated or stimulated with LPS, which is known to induce the production and release of cathepsin G. Pretreatment of macrophages with these neutrophil-derived products enhanced HIV-1 replication (Fig. 7), while posttreatment suppressed HIV-1 infection of macrophages (data not shown). In the supernatant transfer experiments, LPS added to neutrophil cultures might be transferred to macrophage cultures and could influence HIV-1 ADA8 infection of macrophages (see Fig. 2), neutrophil-derived factors appeared to overcome possibly inhibitory effects of LPS. Serine protease inhibitors neutralized both enhancing and inhibitory effects by neutrophils (Fig. 7; data not shown). These results indicate that neutrophils can modulate HIV infection by producing serine proteases, which include but may not be limited to cathepsin G.
FIG. 7.
Neutrophil extracts (Neu Ext) or crude supernatants from neutrophil cultures (Neu Sup) render macrophages highly susceptible to acute HIV-1 infection. Macrophages were either mock treated (Unstim.) or treated with the indicated neutrophil derivatives for 30 min and then infected with ADA8 virus. The infected cells were washed and resuspended in AIM-V serum-free medium, and approximately half of the culture supernatant was collected every 3 to 4 days for RT assays. Peak titers on day 12 postinfection are shown. Results are representative of four independent experiments.
DISCUSSION
Bacterial infections have been associated with increased HIV transmission and replication (reviewed in references 4, 8, and 28). Since HIV infection is associated with immune activation (reviewed in reference 11), stimulation of lymphocytes and macrophages, which serve as major target cells for HIV, has been considered to be responsible for disease progression as well as efficient transmission (reviewed in references 4, 8, and 28). In this regard, we have recently demonstrated that exposure to bacterial cell wall components renders macrophages highly susceptible to T-tropic or dual-tropic HIV-1 infection (21), which predominates during the later stages of HIV disease in certain individuals (9, 25).
Neutrophils are recruited to the site of most infections and dominate acute inflammatory responses. Therefore, although neutrophils themselves do not serve as host cells for HIV, we hypothesize that neutrophils or their products may play a role in the pathogenesis of HIV disease, especially in patients with microbial coinfections. In this regard, a recent study by Ho et al. (13) has demonstrated that neutrophils are capable of increasing HIV replication by the generation of reactive oxygen intermediates or by proinflammatory cytokines; however, other neutrophil products may also influence HIV infection. Among such neutrophil products is cathepsin G, which has been shown to chemoattract macrophages (7), to stimulate lymphocytes (12), and to bind to the V3 loop of HIV-1 gp120 (3). Therefore, we examined the role of cathepsin G in HIV infection of macrophages and lymphocytes, which dominate subacute to chronic inflammatory responses. We have confirmed that cathepsin G is an efficient chemoattractant for macrophages and have also shown that macrophages stimulated with LPS, a bacterial product, migrate much more efficiently in response to cathepsin G than do unstimulated macrophages. We have also demonstrated that cathepsin G induces expression of proinflammatory cytokines by macrophages and, more importantly, increases the susceptibility of these cells to acute HIV infection. Although prolonged exposure to cathepsin G suppressed HIV expression, introduction of serine protease inhibitors counteracted the inhibitory effect, a scenario that likely occurs during the inflammatory process in vivo. These results indicate that cathepsin G, a neutrophil product, may play a critical role in HIV infection of macrophages in inflamed tissue: it chemoattracts macrophages (HIV targets), activates them, and renders them highly susceptible to acute HIV infection. We have obtained similar but less pronounced results for another neutrophil-derived serine protease, elastase (data not shown). Taken together, these observations suggest that some neutrophil-derived serine proteases may play a critical role in HIV-1 infection of macrophages in infected or inflamed tissue.
Although we have shown that cathepsin G-mediated signal-transducing events appear to influence a postfusion/entry early event(s) during the HIV replicative cycle, the precise mechanisms whereby cathepsin G modulates HIV infection of macrophages remain unknown. While previous studies have indicated that cathepsin G can interact with the V3 loop of HIV-1 gp120, the present study suggests that interaction of cathepsin G with a host factor(s) but not with virus itself is critical for the effects mediated by the enzyme. Interestingly, SLPI, a natural inhibitor of serine proteases including cathepsin G (26), has been shown to suppress HIV infection of monocytes in vitro (14). Although its mechanism of action remains to be determined, SLPI appears to inhibit HIV replication at an early step(s) during the viral replicative cycle by binding specifically to a host cell molecule(s) other than CD4 (14, 15). Therefore, it is possible that SLPI suppresses HIV infection of monocytes by counteracting a serine protease(s) which is capable of enhancing HIV replication in these cells. Increased infectability of murine retrovirus by protease treatment of host cells has also been reported (2). Taken together, these studies suggest that proteases and protease inhibitors may play an important role in the pathogenesis of retroviral diseases. Identification of the host factor(s) responsible for the protease-mediated effects is currently under investigation.
ACKNOWLEDGMENTS
We thank M. Martin (NL4-3), T. Theodore (AD8), and N. Landau (NL4-3luc-R−E− virus) for reagents (in parentheses) and J. Weddle for graphic work.
REFERENCES
- 1.Altieri D C. Proteases and protease receptors in modulation of leukocyte effector functions. J Leukocyte Biol. 1995;58:120–127. doi: 10.1002/jlb.58.2.120. [DOI] [PubMed] [Google Scholar]
- 2.Anderson K B, Skov H. Retrovirus-induced cell fusion is enhanced by protease treatment. J Gen Virol. 1989;70:1921–1927. doi: 10.1099/0022-1317-70-7-1921. [DOI] [PubMed] [Google Scholar]
- 3.Avril L E, Di Martino-Ferrer M, Pignede G, Seman M, Gauthier F. Identification of the U-937 membrane-associated proteinase interacting with the V3 loop of HIV-1 gp120 as cathepsin G. FEBS Lett. 1994;345:81–86. doi: 10.1016/0014-5793(94)00410-2. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard A, Montagnier L, Gougeon M-L. Influence of microbial infections on the progression of HIV disease. Trends Microbiol. 1997;5:326–331. doi: 10.1016/S0966-842X(97)01089-5. [DOI] [PubMed] [Google Scholar]
- 5.Boyum A. Separation of leukocytes from blood and bone marrow. Scand J Clin Lab Investig. 1968;21(Suppl.):77–89. [PubMed] [Google Scholar]
- 6.Campbell E J, Silverman E K, Campbell M A. Elastase and cathepsin G of human monocytes: quantification of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity. J Immunol. 1989;143:2961–2968. [PubMed] [Google Scholar]
- 7.Chertov O, Ueda H, Xu L L, Tani K, Murphy W J, Wang J M, Howard O M, Sayers T J, Oppenheim J J. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med. 1997;186:739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen M S. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351:sIII5–sIII7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 9.Connor R L, Sheridan K E, Ceradini C, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 12.Hase-Yamaguchi T, Aoki Y. Stimulation of human lymphocytes by cathepsin G. Cell Immunol. 1995;160:24–35. doi: 10.1016/0008-8749(95)80005-4. [DOI] [PubMed] [Google Scholar]
- 13.Ho J L, He S, Hu A, Geng J, Basile F G, Almeida M G, Saito A Y, Laurence J, Johnson W D., Jr Neutrophils from human immunodeficiency virus (HIV)-seronegative donors induce HIV replication from HIV-infected patients' mononuclear cells and cell lines: an in vitro model of HIV transmission facilitated by Chlamydia trachomatis. J Exp Med. 1995;181:1493–1505. [PMC free article] [PubMed] [Google Scholar]
- 14.McNeely T B, Dealy M, Dripps D J, Orenstein J M, Eisenberg S P, Wahl S M. Secretary leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Investig. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeely T B, Shugars D C, Rosendahl M, Tucker C, Eisenberg S P, Wahl S M. Inhibition of human immunodeficiency virus type 1 infectivity by secretary leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 16.Moriuchi H, Moriuchi M, Arthos J, Hoxie J, Fauci A S. Promonocytic U937 subclones expressing CD4 and CXCR4 are resistant to infection with and cell-to-cell fusion by T-cell-tropic human immunodeficiency virus type 1. J Virol. 1997;71:9664–9671. doi: 10.1128/jvi.71.12.9664-9671.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriuchi H, Moriuchi M, Combadiere C, Murphy P M, Fauci A S. CD8+ T-cell-derived factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriuchi H, Moriuchi M, Fauci A S. Nuclear factor-κB potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- 19.Moriuchi H, Moriuchi M, Fauci A S. Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1. J Exp Med. 1998;187:1689–1697. doi: 10.1084/jem.187.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriuchi M, Moriuchi H, Straus S E, Cohen J I. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology. 1994;200:297–300. doi: 10.1006/viro.1994.1190. [DOI] [PubMed] [Google Scholar]
- 21.Moriuchi M, Moriuchi H, Turner W, Fauci A S. Exposure to bacterial products renders macrophages highly susceptible to T-tropic human immunodeficiency virus type 1: implications for in vivo coinfections. J Clin Investig. 1998;109:1540–1550. doi: 10.1172/JCI4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy P M. Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine Growth Factor Res. 1996;7:47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 23.Poli G, Fauci A S. A role of cytokine in the pathogenesis of human immunodeficiency virus infection. In: Aggarwahl B, Puri R, editors. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Science; 1995. pp. 421–449. [Google Scholar]
- 24.Takahashi H, Nukiwa T, Basset P, Crystal R G. Myelomonocytic cell lineage expression of the neutrophil elastase gene. J Biol Chem. 1988;263:543–547. [PubMed] [Google Scholar]
- 25.Tersmette M, de Goede R E, Al B J, Winkel I N, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson R C, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretary leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci USA. 1986;83:6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travis J, Salvesen G S. Human plasma protease inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 28.Wahl S M, Orenstein J M. Immune stimulation and HIV-1 viral replication. J Leukocyte Biol. 1997;62:67–71. doi: 10.1002/jlb.62.1.67. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer M, Medcalf R L, Fink T M, Mattmann C, Lichter P, Jenne D E. Three human elastase-like genes coordinately expressed in the myelomonocyte lineage are organized as a single genetic locus on 19pter. Proc Natl Acad Sci USA. 1992;89:2228–2232. doi: 10.1073/pnas.89.17.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]