Dear Editor,
Herpes zoster is a common viral infection caused by the varicella zoster virus (VZV).1 Following a previous varicella episode, the latent VZV infection persists in sensory ganglia and may reactivate to cause herpes zoster, which presents with unilateral radicular pain and vesicular eruptions restricted to two or three dermatomes.1,2 This typically affects the thoracic and lumbar spine, but it also causes sacral zoster in about 4% of cases.3 Sphincter dysfunction can occur in sacral zoster to result in symptoms such as bladder or bowel dysfunction.4,5,6 This report discusses an uncommon case of sacral zoster reactivation in the S2 and S3 dermatomes in an 80-year-old male, leading to acute urinary retention with minimal preceding urinary obstructive symptoms.
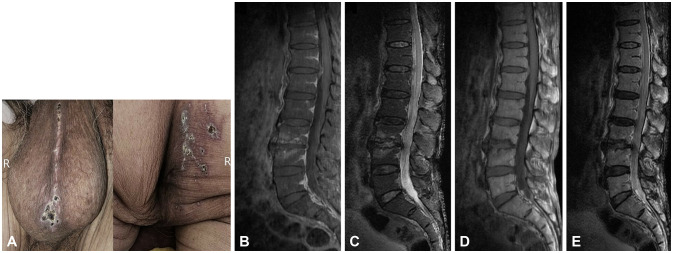
An 80-year-old male patient with diabetes mellitus presented with acute urinary retention after experiencing skin rashes. He had been clinically diagnosed with sacral zoster 12 days previously, and was treated with oral acyclovir for 12 days. He was afebrile, and a physical examination revealed erythematous vesicular and crusted lesions over the right S2 and S3 dermatomes and the perineal raphe (Fig. 1A). A neurological examination identified intact light-touch sensation, but decreased anal tone and a tingling sensation in the right posterior thigh. Along with normal deep tendon reflexes, there were no lateralized deficits, cranial nerve dysfunction, or neck stiffness. Hemoglobin A1c on the day of admission was 7.7%. The findings of the complete blood cell count and biochemical blood analyses, including blood urea nitrogen, creatinine, serum electrolytes, and C-reactive protein, were within the normal ranges. The patient’s acute urinary retention had improved at admission to allow self-voiding. However, urination did not return to its state prior to the zoster infection, and he experienced ongoing discomfort during voiding. Urine analysis was normal, but a urodynamic study revealed mild hypotonic detrusor (bladder areflexia). Lumbosacral spine magnetic resonance imaging (MRI) (Fig. 1B-E) and a cerebrospinal fluid (CSF) study were conducted to identify potential central nervous system (CNS) involvement. There were uncertain signal alterations around the whole spinal cord and canal that warranted a differential diagnosis between an artifact and true myelopathy or intraspinal pathology. CSF analysis showed an opening pressure of 7 cm H2O, white blood cells at 23/mm3 with a lymphocyte predominance (87%), 53 mg/dL protein, 78 mg/dL glucose (136 mg/dL in serum), and negative results for viruses, fungi, tuberculosis, cryptococcus, syphilis, and mycoplasma. Although the polymerase chain reaction produced negative results for VZV DNA from CSF, the temporal relationship between urinary dysfunction and skin rashes supported a sacral zoster diagnosis. Intravenous (IV) acyclovir therapy dosing at 10 mg/kg three times daily was started for 7 days, and IV ceftriaxone dosing at 2 g/day for 5 days was also administered to prevent skin and urinary infections. An oral corticosteroid was given at 5 mg twice daily due to the possibility of true myelopathy. All of the symptoms had improved on day 8 of hospitalization, and he was safely discharged without any additional follow-up examinations.
Fig. 1. Dermatologic examination and lumbosacral spine magnetic resonance imaging (MRI). Unilateral vesicular rashes in its healing stage distributed over the S2 and S3 dermatomes on the right buttocks and perineal raphe (A). Contrast-enhanced sagittal fat-suppressed T1-weighted image (B), sagittal fat-suppressed T2-weighted image (C), sagittal T1-weighted image (D), and sagittal T2-weighted image of the lumbosacral spine in MRI (E). There was no definite abnormal enhancement along the cauda equina, except for questionable signal alterations around the whole spinal cord and spinal canal, which required a differential diagnosis between an artifact and true myelopathy.
Herpes zoster typically presents with vesicular skin eruptions, indicating an inflammatory process that affects the related dorsal nerve roots and ganglia, and occasionally even the anterior horn.2,7 Bladder dysfunction is an atypical complication of sacral zoster that usually has a benign prognosis with recovery over 4–8 weeks.7,8 It can occur via three primary mechanisms: 1) direct VZV invasion along visceral and somatic nerves, leading to ipsilateral herpetic hemicystitis, 2) neuritis-associated voiding dysfunction, caused by VZV invasion from the dorsal root ganglion into sacral motoneurons, roots, or peripheral nerves, disrupting the detrusor reflex, and 3) myelitis-associated dysfunction, resulting in spastic bladder due to suprasacral spinal cord injury with predominant dorsal and ventral horn involvement.9 The present case is an unusual instance of sacral zoster reactivation with pleocytosis, causing neuritis in S2 and S3 dermatomes resulting in acute urinary retention with detrusor areflexia. The patient seemed to have progressed from sacral zoster to neuritis, with a potential risk of further CNS invasion that can lead to conditions such as meningitis, myelitis, and encephalitis. However, he had been taking oral acyclovir for 12 days before visiting our hospital, which appeared to have prevented disease progression. This also led to uncertain MRI signals and pleocytosis in the CSF, with no prominent CNS symptoms. It is plausible that rare CNS complications such as meningitis, myelitis, and encephalitis can follow VZV infection.10 Also, the patient had risk factors for herpes zoster that could decrease cellular immunity, such as being elderly and having diabetes.10 These factors may have played a role in the progression of sacral zoster. Thus, if patients present with sacral zoster, they should be informed about the potential risk of urinary dysfunction, and physicians should recognize it to ensure appropriate diagnosis and management.
Footnotes
Ethics Statement: This study was conducted adhering to the declaration of Helsinki. The CARE guidelines were followed to enhance the quality and standardization of the reported cases. The written conformed consent for the clinical records was not required because of the guaranteed anonymity and de-identification. The study was approved by the Institutional Review Board of CHA Bundang Medical Center (IRB No. 2023-10-230).
- Conceptualization: Won Chan Kim.
- Data curation: Taeho Seo.
- Formal analysis: Taeho Seo.
- Investigation: Taeho Seo.
- Methodology: Taeho Seo.
- Project administration: Taeho Seo.
- Resources: Taeho Seo.
- Software: Taeho Seo.
- Supervision: Won Chan Kim.
- Validation: Taeho Seo, Yoo Jeong Roh, Dong Hyun Shin.
- Visualization: Taeho Seo, Yoo Jeong Roh, Dong Hyun Shin.
- Writing—original draft: Taeho Seo.
- Writing—review & editing: Won Chan Kim.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
References
- 1.Marques SA, Hortense J. Herpes zoster-associated acute urinary retention in immunocompetent patient. An Bras Dermatol. 2014;89:985–987. doi: 10.1590/abd1806-4841.20143185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erol B, Avci A, Eken C, Ozgok Y. Urinary retention, erectile dysfunction and meningitis due to sacral herpes zoster: a case report and review of the literature. Urol Int. 2009;82:238–241. doi: 10.1159/000200807. [DOI] [PubMed] [Google Scholar]
- 3.Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Cohen LM, Fowler JF, Owen LG, Callen JP. Urinary retention associated with herpes zoster infection. Int J Dermatol. 1993;32:24–26. doi: 10.1111/j.1365-4362.1993.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 5.Hur J. Sacral herpes zoster associated with voiding dysfunction in a young patient with scrub typhus. Infect Chemother. 2015;47:133–136. doi: 10.3947/ic.2015.47.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grekin JA, Mehregan DA. Sacral zoster with a primary complaint of difficulty voiding. JAAD Case Rep. 2017;3:509–511. doi: 10.1016/j.jdcr.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addison B, Harvey M. Herpes zoster-induced acute urinary retention. Emerg Med Australas. 2013;25:279–281. doi: 10.1111/1742-6723.12079. [DOI] [PubMed] [Google Scholar]
- 8.Acheson J, Mudd D. Acute urinary retention attributable to sacral herpes zoster. Emerg Med J. 2004;21:752–753. doi: 10.1136/emj.2003.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen PH, Hsueh HF, Hong CZ. Herpes zoster-associated voiding dysfunction: a retrospective study and literature review. Arch Phys Med Rehabil. 2002;83:1624–1628. doi: 10.1053/apmr.2002.34602. [DOI] [PubMed] [Google Scholar]
- 10.Takami K, Kenzaka T, Kumabe A, Fukuzawa M, Eto Y, Nakata S, et al. Varicella-zoster virus-associated meningitis, encephalitis, and myelitis with sporadic skin blisters: a case report. World J Clin Cases. 2022;10:717–724. doi: 10.12998/wjcc.v10.i2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the study are included in this published article.