Highlights
-
•
Epilepsy surgery was highly effective for both FCD and tumor.
-
•
Completeness of resection and temporal location predicted seizure freedom.
-
•
Temporal SOZ predicted seizure freedom in patients with FCD.
-
•
Concordant pre-diagnostic imaging may contribute to higher seizure control rates.
Keywords: Epilepsy, Surgery, Pediatric, Seizure Outcomes, Low-Grade Tumors, FCD
Abstract
Epilepsy may be drug-resistant in a third of patients necessitating alternative treatments, such as surgery. Among refractory epilepsy patients, the most common etiologies are tumors and focal cortical dysplasia (FCD). Surgical management of tumor-related epilepsy has one of the highest rates of seizure freedom, whereas FCD represents some of the lowest success rates in epilepsy treatment. This study investigates the pre-operative characteristics associated with differences in postsurgical seizure outcomes in patients with FCD and tumors. We completed a retrospective cross-sectional review of epilepsy surgery patients with tumors (n = 29) or FCD (n = 44). Participants had a minimum medical follow-up at least 6 months after surgery (FCD M = 2.1 years; Tumors M = 2.0 years). Patients with FCD trended toward an earlier age of onset (t = -4.19, p = 0.058) and longer epilepsy duration (t = 3.75, p < 0.001). Epilepsy surgery is highly effective in reducing seizures in patients with FCD or tumors with over 70 % of all patients achieving seizure freedom. We found a higher rate of seizure freedom in patients with tumors than FCD, but this difference did not reach significance (79 vs. 66 %). Predictive factors of outcomes for FCD and tumors differ. Findings indicate that diagnostic tests may be differentially sensitive to patients with tumors, and future research is needed.
Introduction
Epilepsy is the most common neurological disease affecting children and is primarily treated using anti-seizure medications (ASMs) to reduce the frequency of seizures and improve quality of life [1]. However, in up to a third of patients epilepsy can become drug-resistant, typically defined as after two failed medications, necessitating additional treatment options. Epilepsy surgery, neuro-stimulation devices, and dietary modifications are all treatment alternatives. Surgical resection of the epileptogenic zone can be highly effective in certain refractory populations, with many patients’ seizure freedom rates much higher than additional medical therapy [2], [3]. Among pediatric epilepsy surgery, low-grade epilepsy-associated tumors and focal cortical dysplasia (FCD) are the most common causes of intractable epilepsy [4]. This study aims to investigate the pre-operative characteristics that explain the difference in post-surgical seizure outcomes in patients with FCD and tumor.
Low-grade tumors among pediatric patients primarily consist of glioneuronal tumors, such as ganglioglioma and DNET [5]. Surgical treatment of such tumors historically has one of the highest favorable post-operative outcomes in epilepsy surgery, with 70–83 % of patients achieving seizure freedom [4], [6], [7], [8]. Similarly, surgical resection of FCD is highly effective but has worse overall seizure outcomes, with 53–59 % of pediatric patients achieving seizure freedom [2], [9], [10], [11]. Among pediatric epilepsy, strong predictive factors for favorable seizure outcomes following surgery are complete resection of the epileptogenic zone [6], [9], [12] and an abnormal preoperative MRI [12], [13]. Less favorable factors include earlier age of onset, longer duration of epilepsy, multilobed epileptogenic zone, and presence of FCD type I [14], [15]. FCD potentially has worse outcomes than tumor because the disease can appear as non-lesional or subtle on MRIs, with a normal MRI reported in 39–58 % of children with FCD [16]. Normal MRIs make complete resection more difficult and may delay the time to surgery. Furthermore, FCD tends to have an earlier age of onset and a multilobed epileptogenic zone in pediatric patients more often than those with low-grade tumors [7], [16], [17], [18].
There is abundant research on FCD and low-grade tumors among pediatric populations; however, the difference in pre-surgical workup has not been well defined. This comparison will highlight important considerations when deciding the extent of surgical work-up and the predictive value of diagnostic studies based on etiology. As such, this study will explore known predictive factors for seizure freedom between patients that receive surgery for tumors and those with FCD. By comparing how these factors differ between etiologies, we aim to elucidate why surgical resection for tumors has better outcomes and inform better surgical strategies.
Methods
Study population
We retrospectively reviewed 73 patients with a postoperative diagnosis of either FCD or Low-grade tumor treated with resective surgery at Boston Children’s Hospital between 2003–2020. Institutional IRB reviewed and approved all study procedures. All patients were younger than eighteen at surgery and were followed for at least 6 months. Patients also had no prior epileptic surgeries in the reviewed data. A comprehensive review of their medical records included their demographics (gender, age at seizure onset, duration of epilepsy, age at epilepsy surgery), epilepsy details (epileptogenic zone/lateralization), pre-operative tests (PET, MEG, and SPECT concordance), and post-operative outcomes (complete resection and seizure freedom).
Pathology
Based on a medical record chart review, we determined patient etiology from postsurgical pathology reports. FCD determination was made using the current ILAE classification [19]. Most patients with FCD were determined to have FCD2, with FCD2a as the most common subtype. Of the patients with tumors, low-grade glioma was the most common. Other low-grade tumor types included DNET, low-grade glioma, ganglioma, and glioneural tumors, refer to Table 1.
Table 1.
Demographic and epilepsy characteristics for the whole groups and broken down by sub-groups. Post-surgical pathology for Tumor and FCD.
| Sex |
Whole Group (%) |
FCD (%) |
Tumors (%) |
|---|---|---|---|
| 37 males, 36 females | 19 males, 25 females | 18 males, 11 females | |
| Temporal Epilepsy | 35/73 (48) | 20/44 (45) | 15/29 (52) |
| Left Lateralization | 42/73 (58) | 27/44 (61) | 15/29(52) |
| Complete Resection | 35/73 (48) | 20/44 (45) | 15/29 (52) |
| Phase 2 | 23/73 (32) | 22/44 (50) | 1/29 (3) |
| ECOG | 71/73 (97) | 42/44 (95) | 29/29 (1 0 0) |
| FCD type | − | FCD 1a – 6 1c – 1 FCD 2a – 20 2b – 12 FCD 3a – 4 3d – 1 |
− |
| Tumor Type | − | − | DNET – 6 Ganglioma – 7 Low Grade Glioma – 13 Low Grade Glioneural – 1 Spindle Cell – 2 |
Presurgical evaluation
Epilepsy surgery evaluation at Boston Children’s hospital follows a multi-phase approach. As part of phase 1, all patients are referred to the Epilepsy Monitoring Unit and receive presurgical testing including electroencephalogram (EEG)/Long Term Monitoring (LTM) and 3T Magnetic resonance imaging (MRI). Additional tests can be performed to assist in surgical planning, such as Transcranial Magnetic Stimulation (TMS), Function Magnetic Resonance Imaging (fMRI), Single photon Emission Computerized Tomography (SPECT), Positron Emission Tomography (PET), and Magnetoencephalography (MEG). Additional testing typically occurs if there is uncertainty of the epileptogenic zone or if the MRI is non-lesional. After the initial presurgical evaluation, each patient case is presented and discussed during a conference attended by neurologists, neurosurgeons, and neuropsychologists. Of the sample, only four patients had non-lesional MRIs, all of which had post-surgery pathologies of FCD.
Surgery
All patients underwent resective surgery for the treatment of their epilepsy. Patients who required invasive recordings underwent a planned two-phase procedure involving invasive EEG monitoring with either subdural grids and strips or stereoEEG. After identifying and mapping the epileptogenic focus and the adjacent functional cortex, patients return to the OR for electrode removal and a definitive resection. Patients receive pre-resection ECoG with subdural grids, which is used as a baseline during surgery. Resection is then based on pre-surgical MRI lesional data, non-invasive mapping, and data from invasive EEG monitoring if applicable. Once an initial resection is made, ECoG is performed again and interpreted by seeing if any interictal epileptiform activity is present. If spike discharges are still present, the surgeons then determine if it can be safely resected to remove all potentially epileptogenic tissue. The goal of surgical treatment is complete resection of the epileptogenic zone. However, this is not always possible. Incomplete surgical resection occurred in patients where the epileptogenic zone overlapped with the eloquent cortex. Patients were retrospectively reviewed and classified as incomplete if residual FCD or tumor recurrence was noted on post-operative MRI reports.
Seizure outcome classification
Postoperative seizure outcome was classified according to Engel's classification. Engel scores are classified as follows: (I) seizure-free or auras only or convulsions with ASM discontinuation only, (II) rare disabling seizures (<2 seizures/year or ≥90 % seizure reduction), (III) worthwhile seizure reduction (reduction of seizure frequency ≥75 %), (IV) no worthwhile improvement (reduction of seizure frequency <75 %). However, for the current study, patients were classified as seizure-free (Engel I) or not seizure-free (Engel II, III, IV).
Statistical analysis
The Statistical Package for the Social Sciences, version 27.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for analysis. Descriptive statistics were used for data expressed as means (±SD) and percentages. Continuous data were analyzed by comparing group means with independent sample t-tests, while categorical data were evaluated using logistic regressions and chi-square tests. To evaluate the effect of variables on postoperative seizure outcome, variables with a P value < 0.5 on univariate analysis were included in an interaction logistic regression analysis. Interactions were analyzed further with a chi-square test analysis, where a P-value < 0.05 was considered statistically significant.
Results
Descriptive characteristics
From 2003 to 2020, 73/424 children underwent first-time resective surgery for epilepsy-related FCD or low-grade tumors at Boston Children’s Hospital with a minimum 6 months surgical follow up. In this sample, 44 patients had a pathology diagnosis of FCD and 29 had low-grade tumors. The mean age at surgery for the whole group was 10.71 ± 5.97 years. The mean postoperative follow-up time was 2.02 ± 1.79 years with the shortest follow-up time being 6 months, refer to Table 2. At a minimum, patients underwent a preoperative evaluation by a multidisciplinary epilepsy team and received an MRI and video EEG. Due to limited sample size across locations, patients were divided up as extratemporal seizure focus and temporal seizure focus, refer to supplementary table 1. A temporal lobe seizure focus was observed in 35 patients (48 %) and complete resections were performed in 35 patients (48 %).
Table 2.
Clinical characteristics and seizure freedom for the whole group and subgroups. Included are mean scores, standard deviations, sums, T-scores, X2-scores with associated p-values.
| Whole Group | FCD | Tumors | T | X2 | P values | |
|---|---|---|---|---|---|---|
| Follow up time | 2.02(±1.79) | 2.07(±1.76) | 1.96 (±1.87) | 0.324 | −- | 0.927 |
| Age at surgery | 10.71(±5.97) | 10.34(±6.34) | 11.25(±5.40) | −0.683 | −- | 0.332 |
| Age of Onset | 6.21 (±5.47) | 4.28(±4.74) | 9.13(±5.62) | −4.092 | −- | 0.058+ |
| Disease Duration | 4.50 (±4.85) | 6.06(±5.27) | 2.18 (±2.79) | 3.748 | −- | <0.001** |
| Engle 1 Outcome | 52/73 | 29/44 | 23/29 | −- | 1.532 | 0.216 |
| Engle Ia | 45/73 | 26/44 | 19/29 | −- | 0.546 | 0.265 |
Note. +p = 0.05, **p < 0.001.
Surgical data
Phase II
In the whole sample, 23 patients had a Phase II surgery workup (i.e., invasive monitoring), and of those the vast majority (n = 22/23) had an FCD. Patients without a Phase II were more likely to be seizure free with 74 % seizure free compared 65 % of those who underwent Phase II surgical workup (refer to supplemental table 3).
ECoG
In our sample, 71/73 patients received ECoG consisting of subdural grids during surgery. Those who did not have ECoG (n = 2) had an FCD. These patients received subdural grid, strip, and/or depth electrodes, and did not achieve seizure freedom. For FCD, ECoG was performed in the majority of patients (n = 42/44), 29 of which were seizure free post-surgery. For tumor, ECoG was performed in all patients, 23 of which were seizure free post surgery (refer to supplemental Table 4). At our facility, hippocampal sparing resections are determined through a multitude of clinical factors, including intraoperative ECoG and risk to functional outcome. Our sample included 4 patients with hippocampal sparing resections, all of whom achieved seizure freedom. In both the temporal lobe FCD and tumor groups, hippocampal resection was not related to seizure outcomes.
Complications
Complications from surgery occurred in 8 patients, 7 of which were in the FCD group. Complications included bleeding and swelling during surgery, post operative infections, and surgical wound drainage. Two patients returned to the operating room to treat post-surgical infections.
Seizure outcome
Seizure freedom was high for the whole group, with 71 % of the sample achieving seizure freedom (Engel 1) at the last follow-up, refer to Table 2. Patients with tumors had a higher rate of favorable outcomes with 79 % of the sample achieving seizure freedom compared to 66 % of patients with FCD. However, Chi-square test analysis indicated no statistical difference between seizure freedom for patients with FCD and those with tumors, (X2 (1,73) = 1.532, p = 0.216), refer to Table 2.
FCD clinical data
In the FCD group, the most common type was FCD2 which was present in 72 % of the sample with FCD2a as the most common subtype. The mean age at seizure onset was 4.28 years, and the mean epilepsy duration was 6.06 years. Of the patients with FCD that underwent resective surgery, 66 % were seizure-free, and 34 % were not (4 Engel Class 2, 5 Engel class 3, 2 Engel class 4).
Tumors clinical data
In the tumor group, the most common type was low-grade glioma which was present in 13 patients (45 %). Patients with tumors had an older mean age of onset than those with FCD at 9.13 years and a shorter mean epilepsy duration at 2.12 years. Of the patients with tumors that underwent resective surgery, 23 (79 %) were seizure-free, and 6 (21 %) were not (5 Engel Class 2, 2 Engel Class 3, 1 Engel Class 4).
Pre-diagnostic testing
Patients with FCD were likely to be seizure free with either a concordant or discordant SPECT and EEG. Conversely, in the FCD group, concordant PET and EEG were more likely to be seizure free than those with discordant data, with 71 % of the group classified as Engle 1. However, only 3 patients had a discordant PET, of which only 1 achieved seizure freedom. In the tumor group, SPECT was performed on 12 patients, where those with concordant data SPECT and EEG were more likely to be seizure-free. Of these patients, 88 % achieved seizure freedom. PET was performed on 9 patients with tumors. All 3 patients with a discordant PET achieved seizure freedom, but only 50 % of patients with concordant PET achieved seizure freedom. Additionally, MEG was performed in 14 patients with FCD, 7 of which were concordant with EEG. Patients with concordant EEG and MEG were more likely to be seizure free post-surgery (refer to supplemental table 2).
Predictive factors for post-operative seizure freedom
Using an independent samples t-test, only duration was found to be statistically different and age of onset trended towards significance between patients with FCD and those with tumors (refer to Table 2). Patients with FCD had an earlier age of onset and a longer seizure duration than those with tumors. See (Table 3).
Table 3.
Comparison of pre-surgical SPECT and PET outcomes for FCD and Tumor with corresponding percent seizure freedom (Engle 1).
|
FCD (%) |
Tumors (%) |
|||
|---|---|---|---|---|
| Concordant | Discordant | Concordant | Discordant | |
| PET | 20/28 (71) | 1/3 (33) | 3/6 (50) | 3/3 (1 0 0) |
| SPECT | 16/25 (64) | 5/7 (71) | 7/8 (88) | 2/4 (50) |
Using Simple Logistic Regression with simultaneous entry, complete resection status and temporal seizure focus were found to be predictors of positive seizure outcome in Model 1. Age of onset, epilepsy duration, and age at surgery were not predictors of seizure freedom (see Table 4).
Table 4.
Regression Models.
| Model 1 | B | SE | Wald | df | P Value | Exp(B) | 95 %CI |
|---|---|---|---|---|---|---|---|
| Constant | −0.733 | 0.767 | 0.915 | 1 | 0.339 | 0.480 | − |
| Resection Status | 1.233 | 0.618 | 4.032 | 1 | 0.045* | 3.433 | (1.030, 11.439) |
| Temporal Location | −1.225 | 0.6018 | 3.929 | 1 | 0.047* | 0.294 | (0.088, 0.986) |
| Age at Surgery | −0.029 | 0.063 | 0.216 | 1 | 0.642 | 0.971 | (0.859, 1.098) |
| Age of Onset | 0.021 | 0.083 | 0.065 | 1 | 0.799 | 1.021 | (0.868, 1.201) |
| Etiology | −0.652 | 0.767 | 0.861 | 1 | 0.353 | 0.521 | (0.132, 2.064) |
| Model 2 | |||||||
| Resection * Location | 0.799 | 0.259 | 9.528 | 1 | 0.002* | 2.224 | (1.339, 3.694) |
| Temporal Complete | −2.303 | 0.742 | 10.816 | 3 | 0.013* | 0.100 | − |
| Extratemporal complete | 1.003 | 0.987 | 1.033 | 1 | 0.309 | 2.727 | (0.394–18.875) |
| Temporal incomplete | 1.003 | 0.987 | 1.033 | 1 | 0.309 | 2.727 | (0.394–18.875) |
| Extratemporal incomplete | 2.47 | 0.847 | 8.597 | 1 | 0.004 | 11.818 | (2.246–62.191) |
|
Model 3 | |||||||
| Etiology * Location | 0.022 | 0.241 | 0.009 | 1 | 0.926 | 1.023 | (0.638, 1.640) |
| FCD Temporal | −2.197 | 0.745 | 8.690 | 3 | 0.015* | 0.111 | − |
| FCD Extratemporal | 2.364 | 0.851 | 7.727 | 1 | 0.005* | 10.636 | (2.008–56.332) |
| Tumor Temporal | 0.811 | 0.986 | 0.676 | 1 | 0.411 | 2.250 | (0.326–15.541) |
| Tumor Extratemporal | 0.898 | 0.990 | 0.823 | 1 | 0.364 | 2.455 | (0.353–17.082) |
Note: Interactions were analyzed by multiplying two variables of interest together to create a measure of how these variables interacted with each other and influenced seizure freedom.
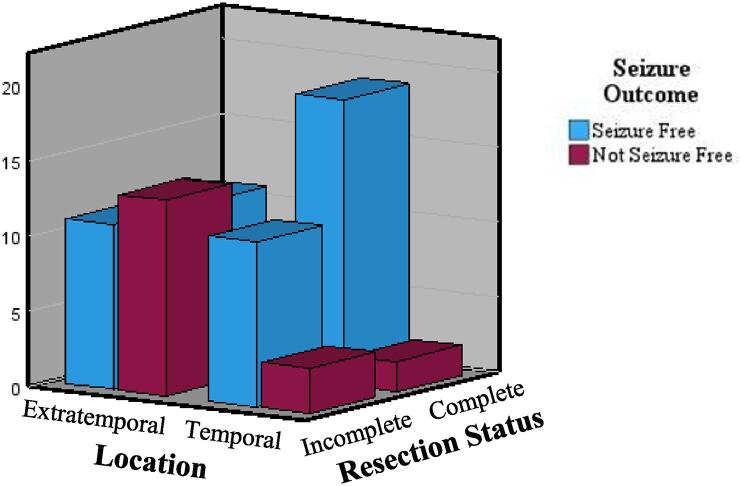
For Model 2, we used the same method (simultaneous entry logistical regression). We computed an interaction term between resection (complete/incomplete) and location (temporal/extratemporal) which was dummy coded for analysis. The interaction term (Resection * Location) was significant, and the interaction was probed. Individuals with extratemporal and incomplete resections were more likely to have seizures when compared to the temporal complete group (see Table 4). To further examine interactions, Chi-square test analysis indicated that individuals with extratemporal lesions had a higher chance of seizure freedom with complete resections than incomplete (X2 (1,38) = 3.88, p = 0.049), refer to Table 5 and Fig. 2.
Table 5.
Examining the interaction effects between etiology, location, and resection status. Included are seizure outcomes for each group, Chi-Square test, and Cramer’s V.
| Engle I (%) | Engle II-IV (%) | Chi-SquareTest | Cramer’s V | |
|---|---|---|---|---|
| FCD | 0.002* | 0.464 | ||
| Temporal (n = 20) | 18 (90) | 2 (10) | ||
| Extratemporal (n = 24) | 11 (46) | 13 (54) | ||
| Tumors | 0.924 | 0.018 | ||
| Temporal (n = 15) | 12 (80) | 3 (20) | ||
| Extratemporal (n = 14) | 11 (79) | 3 (21) | ||
| Temporal | 0.324 | 0.167 | ||
| Complete(n = 21) | 19 (90) | 2 (10) | ||
| Incomplete(n = 14) | 11 (79) | 3 (21) | ||
| Extratemporal | 0.049* | 0.320 | ||
| Complete (n = 14) | 11 (79) | 3 (21) | ||
| Incomplete (n = 24) | 11 (46) | 13 (54) | ||
Fig. 2.
Displays seizure outcomes for patients based on epilepsy lesion location/SOZ and surgical resection status.
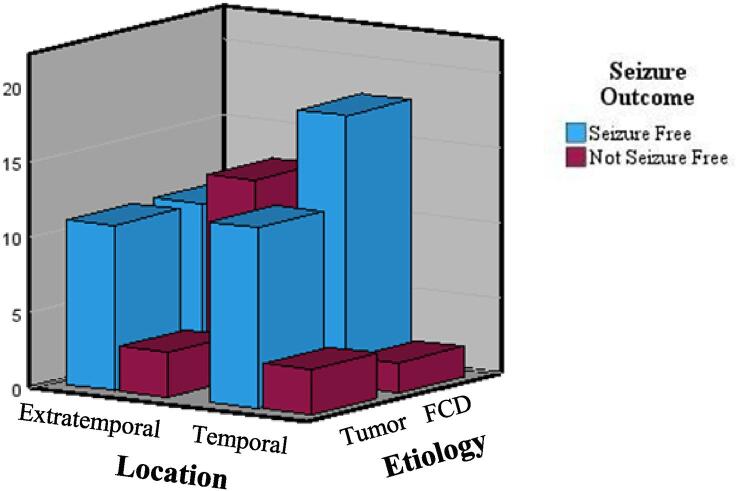
For Model 3, we used the same method to explore an interaction between seizure etiology and location (see Table 4). Results indicate that extratemporal FCD has worse seizure outcomes when compared to temporal FCDs. Chi-square test analysis indicated that individuals with temporal FCD are more likely to be seizure free than those with extratemporal FCD (X2 (1,44) = 9.471, p = 0.002), refer to Table 5 and Fig. 1
Fig. 1.
Displays seizure outcomes for patients based on epilepsy lesion location/SOZ and etiology.
Discussion
At our center, epilepsy surgery for FCD (66 %) was nearly as effective as surgery for tumors (79 %) at an average 2 year follow up, (range: 0.5 – 8.58 years). This is notably better than other studies that have reported seizure freedom rates for FCD as low as 50 % [9], [20] and consistent with strong seizure freedom outcomes in tumor groups [8], [21], [7], [22]. Overall, about 11 % of patients had complications. Of those with complications, they were more likely to be FCD and less likely to achieve seizure freedom.
It has been established that temporal lobe focus is a strong predictor of seizure freedom [2], [7], [24], [25], [26]. Consistent with prior research, we found temporal lobe seizure focus was a predictor of seizure freedom. Further, we extend the literature by revealing that the combination of location and etiology are important. We found that FCD benefited more from temporal lobe resection when compared to the tumor group. In contrast, extratemporal FCD had a less than 50 % chance of being seizure free. Previous studies on pediatric epilepsy surgery report that 29–57 % of patients have focal temporal lobe epilepsy [4], [11], [26]. Similarly, studies on FCD have reported that 12–75 % of patients with FCD have temporal lesions, with worse overall outcomes seen in studies with a lower proportion of TLE FCD [14], [20], [27]. With nearly half of the FCD sample having a temporal SOZ, the higher rate of seizure freedom for this group is likely driving the overall seizure outcomes for the whole FCD group. For the tumor group, seizure freedom was high regardless of temporal vs. extratemporal location. Epilepsy surgery is a valuable tool for achieving seizure freedom for patients with FCD, and this study suggests that this benefit is even higher for those with temporal lobe FCD.
Unsurprisingly, completion of surgical resection was much more likely to result in seizure freedom (86 %) when compared to incomplete resection (58 %). However, this finding was even more pronounced for patients with extratemporal lobe epilepsy (78 % vs. 46 %, respectively). Whereas, complete or incomplete resection was less relevant for temporal lobe epilepsy (90 % vs. 79 %, respectively). This is particularly relevant as the decision to do a hippocampal sparing resection may spare function and have a small impact on change of seizure outcomes.
Based on our small sample, concordant pre-operative SPECT, PET, and MEG for identifying epileptic foci [28], [29] possibly contributed to higher seizure control rates. For patients with FCD, concordant PET and SPECT were strongly associated with seizure freedom. We also examined MEG outcomes for the FCD group and found that MEG concordance in patients with FCD was associated with better seizure outcomes. Patients with tumors were less likely to receive additional testing since these tests occur when there is uncertainty of the epileptogenic zone or more discrete lesions, which occurs less often in patients with tumors. However, only SPECT concordance was associated with seizure freedom for the tumor patients who received this test (concordant n = 8; discordant n = 4), indicating that concordant prediagnostic imaging potentially contributed to higher seizure control rates, but seizure control may vary between etiologies. It is important to note that FCD and tumor can co-occur, although that was not present in our cohort. Furthermore, in the presurgical workup, this dual pathology is not always evident on neuroimaging. However, this is an important area of future research, particularly given that FCDs associated with low-grade tumors have better outcomes than FCD1a alone [27], [30].
In this study, completeness of the resection and temporal lobe epileptogenic zone were the most significant predictors of seizure freedom following surgery. We observed differences in predictive factors for seizure freedom following surgery between patients with low-grade tumors and those with FCD. Our results highlight the importance of tailored presurgical planning based on research that considers differences due to pathology.
Limitations
Seizure freedom for FCD was higher in our sample than previous studies potentially due to the low proportion of FCD1, which has been observed to have the lowest seizure control of the subtypes [9]. Furthermore, studies show that non-lesional MRIs is a strong predictor of negative seizure outcome [2], [9], [23], but only four patients in our sample had an MRI-negative FCD and seizure freedom was still likely, with only one continuing to have seizures. Additionally, this study cohort only included patients with resective surgeries. At our institution, patients with MRI negative FCD are more likely to pursue alternative therapies or different surgical methods, as such this population may not generalize to all FCD pediatric patients. Furthermore, our sample had a high rate of temporal seizures which may have led to an increase in seizure success rate for FCD surgeries in this cohort.
Additionally, despite trends in other studies, age of onset and age at surgery were not predictive factors for either FCD or tumors. A recent meta-analysis of pediatric surgery postulated that age of onset and age at surgery seemed to be the most predictive of seizure freedom for patients with mixed pathologies and surgical locations [2]. Furthermore, patients with non-lesional MRIs tend to have better outcomes with an older age of onset [31]. In this current study, participants were defined by a single pathology, and few had a normal MRI, which may explain why these relationships were not observed.
Another limitation was the variable intervals between surgery and medical follow-up (range: 6 months – 8.58 years). Many patients only had a 6-month post-operative follow-up with an average of 2 years, which may not reflect their long-term seizure outcomes. Future longitudinal studies with a longer follow-up period are indicated. Additionally, small sample sizes across extratemporal locations made it challenging to look at more specific location categories (i.e., frontal, parietal). This study lacked homogeneity of FCD type within each group, specifically with the high prevalence of FCD2a. As a result, this study cannot be generalized to all FCD subtypes. While we present results on pre-surgical imaging (i.e., SPECT, PET, MEG), our sample was limited in size and these results should be replicated with larger studies. While we attempted to look at invasive recoding and ECoG, sample size limited our analyses, and future research should investigate how extra or intraoperative invasive recordings impact the completeness of resection and seizure outcomes. Lastly, this study was retrospective in design and limited to one pediatric epilepsy center, reducing the external validity of our findings.
Future directions
Future research is needed to identify factors contributing to seizure outcomes based on location. While this study identified that extratemporal lesions had worse overall outcomes among those with FCD, more research is needed to identify what underlying factors may contribute to this outcome, including the role of pathology. It is important to consider genetic factors underlying FCD and how they may contribute to seizure outcomes. For example, patients with GATOR1related FCD have been observed to have poorer seizure control from surgery than those with other related genetic pathways [32]. Future studies should examine the genetic etiologies of FCD, including the mTOR pathway, which could explain some of the heterogeneity seen in FCD populations and seizure outcomes. Additionally, other studies should include larger sample sizes to examine differences in seizure outcomes between the different types of FCD and tumors. Specifically, while not seen in this sample, FCD can cooccur with tumor defined as FCD3b. However, it is difficult to differentiate dual pathology using neuroimaging, and FCD3b is not always evident until after surgery. Studies show that outcomes are improved for FCD associated with tumors compared to FCD alone, and research on whether certain tests may be more or less sensitive to identifying the seizure foci and predicting outcomes based upon pathology is needed. Specific tests can be burdensome to patients and families, and a better understanding of the benefit of these tests specific to pathology could aid in pre-surgical counseling.
Ethics statement
Institutional IRB reviewed and approved all study procedures (IRB-P00009381).
CRediT authorship contribution statement
Alena Hornak: Writing – review & editing, Writing – original draft, Project administration, Formal analysis, Data curation. Jeffery Bolton: Writing – review & editing, Supervision, Resources, Methodology, Data curation, Conceptualization. Melissa Tsuboyama: Supervision, Conceptualization. Phillip L. Pearl: Writing – review & editing, Supervision, Resources, Funding acquisition. Song Dam: Data curation. Trey Moore: Data curation. Brigitte Wilson: Data curation. Scellig Stone: Writing – review & editing, Supervision, Conceptualization. Alyssa Ailion: Writing – review & editing, Writing – original draft, Visualization, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2024.100680.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sharma P, Hussain A, Greenwood R. Precision in pediatric epilepsy. F1000Research. 2019;8. [DOI] [PMC free article] [PubMed]
- 2.Widjaja E., Jain P., Demoe L., Guttmann A., Tomlinson G., Sander B. Seizure outcome of pediatric epilepsy surgery: systematic review and meta-analyses. Neurology. 2020 Feb 18;94(7):311–321. doi: 10.1212/WNL.0000000000008966. [DOI] [PubMed] [Google Scholar]
- 3.West S., Nevitt S.J., Cotton J., Gandhi S., Weston J., Sudan A., et al. Surgery for epilepsy. Cochrane Database Syst Rev. 2019;6 doi: 10.1002/14651858.CD010541.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kan P., Van Orman C., Kestle J.R. Outcomes after surgery for focal epilepsy in children. Childs Nerv Syst. 2008 May;24:587–591. doi: 10.1007/s00381-007-0545-9. [DOI] [PubMed] [Google Scholar]
- 5.Ko A., Lee J.S. Factors associated with seizure and cognitive outcomes after epilepsy surgery for low-grade epilepsy-associated neuroepithelial tumors in children. Clinical and Experimental Pediatrics. 2020 May;63(5):171. doi: 10.3345/kjp.2019.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M.T., Boop F.A. Epilepsy surgery for pediatric low-grade gliomas of the cerebral hemispheres: neurosurgical considerations and outcomes. Childs Nerv Syst. 2016 Oct;32:1923–1930. doi: 10.1007/s00381-016-3162-7. [DOI] [PubMed] [Google Scholar]
- 7.Uliel-Sibony S., Kramer U., Fried I., Fattal-Valevski A., Constantini S. Pediatric temporal low-grade glial tumors: epilepsy outcome following resection in 48 children. Childs Nerv Syst. 2011 Sep;27:1413–1418. doi: 10.1007/s00381-011-1454-5. [DOI] [PubMed] [Google Scholar]
- 8.Faramand A.M., Barnes N., Harrison S., Gunny R., Jacques T., Tahir M.Z., et al. Seizure and cognitive outcomes after resection of glioneuronal tumors in children. Epilepsia. 2018 Jan;59(1):170–178. doi: 10.1111/epi.13961. [DOI] [PubMed] [Google Scholar]
- 9.Choi S.A., Kim S.Y., Kim H., Kim W.J., Kim H., Hwang H., et al. Surgical outcome and predictive factors of epilepsy surgery in pediatric isolated focal cortical dysplasia. Epilepsy Res. 2018 Jan;1(139):54–59. doi: 10.1016/j.eplepsyres.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Kimura N., Takahashi Y., Shigematsu H., Imai K., Ikeda H., Ootani H., et al. Developmental outcome after surgery in focal cortical dysplasia patients with early-onset epilepsy. Epilepsy Res. 2014 Dec 1;108(10):1845–1852. doi: 10.1016/j.eplepsyres.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Ka A., Taher A., D'Souza S., Barnes E.H., Gupta S., Troedson C., et al. Predictors of longitudinal seizure outcomes after epilepsy surgery in childhood. Epilepsy & Behavior Reports. 2022 Jan;1(19) doi: 10.1016/j.ebr.2022.100561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Gonzalez M.A., Gonzalez-Martinez J.A., Jehi L., Kotagal P., Warbel A., Bingaman W. Epilepsy surgery of the temporal lobe in pediatric population: a retrospective analysis. Neurosurgery. 2012 Mar 1;70(3):684–692. doi: 10.1227/NEU.0b013e318235183d. [DOI] [PubMed] [Google Scholar]
- 13.Zupanc M.L., dos Santos Rubio E.J., Werner R.R., Schwabe M.J., Mueller W.M., Lew S.M., et al. Epilepsy surgery outcomes: quality of life and seizure control. Pediatr Neurol. 2010 Jan 1;42(1):12–20. doi: 10.1016/j.pediatrneurol.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Hirfanoglu T., Serdaroglu A., Kurt G., Erdem A., Capraz I., Bilir E., et al. Outcomes of resective surgery in children and adolescents with focal lesional epilepsy: the experience of a tertiary epilepsy center. Epilepsy Behav. 2016 Oct;1(63):67–72. doi: 10.1016/j.yebeh.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 15.Giulioni M., Marucci G., Pelliccia V., Gozzo F., Barba C., Didato G., et al. Epilepsy surgery of “low grade epilepsy associated neuroepithelial tumors”: a retrospective nationwide Italian study. Epilepsia. 2017 Nov;58(11):1832–1841. doi: 10.1111/epi.13866. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan R., Leach J.L., Mangano F.T., Gelfand M.J., Rozhkov L., Miles L., et al. Prospective detection of cortical dysplasia on clinical MRI in pediatric intractable epilepsy. Pediatr Radiol. 2016 Sep;46:1430–1438. doi: 10.1007/s00247-016-3623-x. [DOI] [PubMed] [Google Scholar]
- 17.Mühlebner A., Gröppel G., Dressler A., Reiter-Fink E., Kasprian G., Prayer D., et al. Epilepsy surgery in children and adolescents with malformations of cortical development—outcome and impact of the new ILAE classification on focal cortical dysplasia. Epilepsy Res. 2014 Nov 1;108(9):1652–1661. doi: 10.1016/j.eplepsyres.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Babini M., Giulioni M., Galassi E., Marucci G., Martinoni M., Rubboli G., et al. Seizure outcome of surgical treatment of focal epilepsy associated with low-grade tumors in children. J Neurosurg Pediatr. 2013 Feb 1;11(2):214–223. doi: 10.3171/2012.11.PEDS12137. [DOI] [PubMed] [Google Scholar]
- 19.Najm I., Lal D., Alonso Vanegas M., Cendes F., Lopes-Cendes I., Palmini A., et al. The ILAE consensus classification of focal cortical dysplasia: an update proposed by an ad hoc task force of the ILAE diagnostic methods commission. Epilepsia. 2022 Aug;63(8):1899–1919. doi: 10.1111/epi.17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloss S., Pieper T., Pannek H., Holthausen H., Tuxhorn I. Epilepsy surgery in children with focal cortical dysplasia (FCD): results of long-term seizure outcome. Neuropediatrics. 2002 Feb;33(01):21–26. doi: 10.1055/s-2002-23595. [DOI] [PubMed] [Google Scholar]
- 21.Ranger A., Diosy D. Seizures in children with dysembryoplastic neuroepithelial tumors of the brain—a review of surgical outcomes across several studies. Childs Nerv Syst. 2015 Jun;31:847–855. doi: 10.1007/s00381-015-2675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessling C., Bartels S., Sassen R., Schoene-Bake J.C., von Lehe M. Brain tumors in children with refractory seizures—a long-term follow-up study after epilepsy surgery. Childs Nerv Syst. 2015 Sep;31:1471–1477. doi: 10.1007/s00381-015-2825-0. [DOI] [PubMed] [Google Scholar]
- 23.Rowland N.C., Englot D.J., Cage T.A., Sughrue M.E., Barbaro N.M., Chang E.F. A meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg. 2012 May 1;116(5):1035–1041. doi: 10.3171/2012.1.JNS111105. [DOI] [PubMed] [Google Scholar]
- 24.Englot D.J., Rolston J.D., Wang D.D., Sun P.P., Chang E.F., Auguste K.I. Seizure outcomes after temporal lobectomy in pediatric patients: a systematic review. J Neurosurg Pediatr. 2013 Aug 1;12(2):134–141. doi: 10.3171/2013.5.PEDS12526. [DOI] [PubMed] [Google Scholar]
- 25.Englot D.J., Breshears J.D., Sun P.P., Chang E.F., Auguste K.I. Seizure outcomes after resective surgery for extra–temporal lobe epilepsy in pediatric patients: a systematic review. J Neurosurg Pediatr. 2013 Aug 1;12(2):126–133. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- 26.Englot D.J., Han S.J., Rolston J.D., Ivan M.E., Kuperman R.A., Chang E.F., et al. Epilepsy surgery failure in children: a quantitative and qualitative analysis. J Neurosurg Pediatr. 2014 Oct 1;14(4):386–395. doi: 10.3171/2014.7.PEDS13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J., Huang Z., Li L., Ren L., Wang Y. Histological type of focal cortical dysplasia is associated with the risk of postsurgical seizure in children and adolescents. Ther Clin Risk Manag. 2019 Jul;11:877–884. doi: 10.2147/TCRM.S203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Oertzen T.J. PET and ictal SPECT can be helpful for localizing epileptic foci. Curr Opin Neurol. 2018 Apr 1;31(2):184–191. doi: 10.1097/WCO.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra P.S., Vaghania G., Bal C.S., Tripathi M., Kuruwale N., Arora A., et al. Role of concordance between ictal-subtracted SPECT and PET in predicting long-term outcomes after epilepsy surgery. Epilepsy Res. 2014 Dec 1;108(10):1782–1789. doi: 10.1016/j.eplepsyres.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Cossu M., Fuschillo D., Bramerio M., Galli C., Gozzo F., Pelliccia V., et al. Epilepsy surgery of focal cortical dysplasia–associated tumors. Epilepsia. 2013 Dec;54:115–122. doi: 10.1111/epi.12455. [DOI] [PubMed] [Google Scholar]
- 31.Arya R., Leach J.L., Horn P.S., Greiner H.M., Gelfand M., Byars A.W., et al. Clinical factors predict surgical outcomes in pediatric MRI-negative drug-resistant epilepsy. Seizure. 2016 Oct;1(41):56–61. doi: 10.1016/j.seizure.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Benova B., Sanders M.W., Uhrova-Meszarosova A., Belohlavkova A., Hermanovska B., Novak V., et al. GATOR1-related focal cortical dysplasia in epilepsy surgery patients and their families: a possible gradient in severity? Eur J Paediatr Neurol. 2021 Jan;1(30):88–96. doi: 10.1016/j.ejpn.2020.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.